Abstract
We determined the sequence and genetic organization of plasmid pIP823, which contains the dfrD gene; dfrD confers high-level trimethoprim resistance to Listeria monocytogenes BM4293 by synthesis of dihydrofolate reductase type S2. pIP823 possessed all the features of the pUB110/pC194 plasmid family, whose members replicate by the rolling-circle mechanism. The rep gene encoded a protein identical to RepU, the protein required for initiation of the replication of plasmids pTB913 from a thermophilic Bacillus sp. and pUB110 from Staphylococcus aureus. The mob gene encoded a protein with a high degree of amino acid identity with the Mob proteins involved in conjugative mobilization and interplasmidic recombination of pTB913 and pUB110. The host range of pIP823 was broad and included L. monocytogenes, Enterococcus faecalis, S. aureus, Bacillus subtilis, and Escherichia coli. In all these species, pIP823 replicated by generating single-stranded DNA and was stable. Conjugative mobilization of pIP823 was obtained by self-transferable plasmids between L. monocytogenes and E. faecalis, between L. monocytogenes and E. coli, and between strains of E. coli, and by the streptococcal conjugative transposon Tn1545 from L. monocytogenes to E. faecalis, and from L. monocytogenes and E. faecalis to E. coli. These data indicate that the gene flux observed in nature from gram-positive to gram-negative bacteria can occur by conjugative mobilization. Our results suggest that dissemination of trimethoprim resistance in Listeria spp. and acquisition of other antibiotic resistance determinants in this species can be anticipated.
Most small multicopy plasmids from gram-positive bacteria replicate by an asymmetric rolling-circle (RC) mechanism, producing a single-stranded DNA (ssDNA) intermediate. Recently, plasmids which replicate by the same mechanism have been detected in gram-negative bacteria (11). Three structural modules are required for RC replication: (i) the rep gene, which encodes the Rep protein involved in initiation of plasmid replication; (ii) the double-stranded origin (dso or plus origin); and (iii) the single-stranded origin (sso or minus origin) (12). The Rep protein introduces a strand- and site-specific nick in supercoiled plasmid DNA at the dso domain (21, 32). Replication proceeds around the whole plasmid, until the entire dso is synthesized and the parental plus strand is released as an ssDNA intermediate. The last stage of RC replication involves the conversion of plasmid ssDNA to double-stranded DNA (dsDNA) by synthesis of the lagging strand (8), initiated at the sso domain. On the basis of homology in the DNA sequence and the genetic organization of the replication region, these plasmids have been classified into four families represented by pUB110/pC194, pT181, pLS1/pE194, and pSN2 (12). Some of these plasmids have a broad host range and can replicate in both gram-positive and gram-negative bacteria (11).
Frequently, RC replication plasmids contain two other structural elements: (i) a mob or pre gene, which encodes a protein involved in plasmid conjugative mobilization and site-specific interplasmidic recombination, and (ii) an antibiotic resistance determinant (12, 24). Mobilization of these small non-self-transferable plasmids by conjugative plasmids or transposons has been reported for gram-positive bacterial genera such as Bacillus and Staphylococcus (24, 25, 28). The mobilization process seems to occur by donation, as has been observed in gram-negative bacteria: the trans-acting Mob protein produces a nick in the plasmid DNA at the RSA, site which functions as a cis-acting origin of transfer (24). The RC replication plasmids are highly recombinogenic, a feature that may favor their intra- and intergeneric dissemination (12). Both the simplicity of the replication mechanism and the dependence on host functions for their replication may explain the widespread interspecies transfer of plasmids by the RC replication mode (11). RC replication plasmids conferring resistance to tetracycline, chloramphenicol, neomycin, bleomycin, erythromycin, and kanamycin have been reported (11).
Listeria monocytogenes is a ubiquitous gram-positive, rod-shaped species causing perinatal infections, meningitis, meningoencephalitis, and septicemia (26). Penicillin G or ampicillin, alone or in combination with gentamicin or cotrimoxazole, constitutes standard treatment for listerial infections (13). It has been shown that self-transferable plasmids and conjugative transposons can confer multiple-antibiotic resistance in L. monocytogenes (4, 6). Several studies have demonstrated that antibiotic resistance in L. monocytogenes emerged recently worldwide, probably as the result of acquisition of these two types of mobile genetic elements from enterococci (4, 6).
We previously isolated plasmid pIP823, which carries the dfrD gene; this gene confers high-level trimethoprim resistance to L. monocytogenes BM4293 (3, 4). In this study, we report the entire sequence and structural organization of pIP823, which belongs to the pUB110/pC194 family of RC replication plasmids. Conjugative mobilization of pIP823 is shown to be mediated by self-transferable plasmids between L. monocytogenes and Enterococcus faecalis, between Escherichia coli and L. monocytogenes, and among strains of E. coli, and by the conjugative transposon Tn1545 from L. monocytogenes to E. faecalis and from L. monocytogenes and E. faecalis to E. coli.
MATERIALS AND METHODS
Bacterial strains, plasmids, transposon, and growth conditions.
The main characteristics of the bacterial strains, plasmids, and transposon used in this study are listed in Table 1. Bacillus subtilis BM4150; E. coli DH5α, HB101, and K802N; E. faecalis JH2-2 and BM4110; L. monocytogenes BM4293, EGDSmR, and LO17RF; and Staphylococcus aureus 80CR5RF, 80CR5Str, and RN4220 were used as recipients in conjugation experiments. E. coli DH5α and SM10, E. faecalis JH2-2, L. monocytogenes LO17RF, and S. aureus RN4220 were used in electrotransformation experiments. B. subtilis 168 and E. coli HB101 and JM83 were used in transformation experiments. Bacteria were grown in brain-heart infusion broth (Difco Laboratories, Detroit, Mich.) and on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) at 37°C.
TABLE 1.
Bacterial strains, plasmids, and transposons
| Strain, plasmid, or transposon | Relevant characteristic(s)a | Reference |
|---|---|---|
| Strains | ||
| Bacillus subtilis | ||
| BM4150 | Strr | 7 |
| 168 | trpC2 Lvs+ Prt+ | 17 |
| Escherichia coli | ||
| DH5α | F− (φ80ΔlacZΔM15) endA1 recA1 hsdR17 supE44 thi-1 λ− gyrA96 relA1 Δ(lacZYA-argF)U169 | 37 |
| HB101 | F−hsdS20 (rB− mB−) Δ(gpt-proA)62 leu supE44 λ− ara-14 galK2 lacY1 Δ(merC-mrr) rpsL20 (Strr) xyl-5 mtl-1 recA13 | 27 |
| JM83 | F−ara Δ(lac-proAB) rpsL (Strr) φ80lacZΔM15 | 38 |
| K802N | hsdR mutant, hsdM+ F−gal met supE Nalr Rifr | 27 |
| 14R525 | Prototroph, Nalr | 31 |
| SM10 | RP4-2-Tc::Mu Kmrthi thr leu suIII | 30 |
| Enterococcus faecalis | ||
| JH2-2 | Fusr Rifr, spontaneous mutant of JH2 | 14 |
| BM4110 | Strr, spontaneous mutant of JH2 | 7 |
| Listeria monocytogenes | ||
| BM4293 | pIP823 | 4 |
| LO17RF | Fusr Rifr, spontaneous mutant of LO17 | 4 |
| EGDSmR | Strr, spontaneous mutant of EGD | 4 |
| Staphylococcus aureus | ||
| RN4220 | Restriction-deficient derivative of 8325-4 | 16 |
| 80CR5RF | Rifr Fusr Res−, spontaneous mutant of 80CR5 | 10 |
| 80CR5Str | Strr Res−, spontaneous mutant of 80CR5 | 10 |
| Plasmids | ||
| pIP823 | Tpr (dfrD) Tra− Mob+ | 4 and this study |
| pUC18 | Plasmid vector, AprlacZα Tra− Mob− | 38 |
| pAT459 | pIP823 linearized with HindIII cloned into pUC18 | This study |
| pAMβ1 | Tra+ Emr | 5 |
| pAT191 | oriR pAMβ1 oriR pBR322 tra pAMβ1 oriT RK2 KmrlacZα | 34 |
| RP4 | Tra+ IncP Apr Kmr Tcr | 1 |
| R64 | Tra+ IncI1 Smr Tcr | 1 |
| pIP55-1 | Tra+ IncA-C Cmr Sur | 1 |
| pOX38-Km | Tra+ IncF1 Kmr | 2 |
| pBR322 | Tra− Apr Tcr | 1 |
| pSC101 | Tra− Tcr | 1 |
| Transposon Tn1545 | Tra+ Emr Kmr Tcr | 7 |
Cmr, resistance to chloramphenicol; Emr, resistance to erythromycin; Fusr, resistance to fusidic acid; Kmr, resistance to kanamycin; Nalr, resistance to nalidixic acid; Rifr, resistance to rifampin; Smr, plasmid-mediated resistance to streptomycin; Strr, chromosomal resistance to streptomycin; Sur, resistance to sulfonamide; Tcr, resistance to tetracycline; Tpr, resistance to trimethoprim.
Preparation of DNA, DNA techniques, and sequencing.
Total DNA was prepared as described previously (4). Plasmid pIP823 DNA was purified according to a modification of the alkaline-sodium dodecyl sulfate extraction procedure (4). Recombinant DNA techniques, including cleavage of DNA with restriction endonucleases and ligation with T4 DNA ligase, were performed by standard methods (27). Sequencing reactions were performed on both strands of DNA by the dideoxynucleotide chain-termination method (27) with a Sequenase version 2.0 kit, modified T7 DNA polymerase, and [α-35S]dATP as recommended by the manufacturers.
Computer analysis of sequence data.
DNA and amino acid sequence analyses were performed with Genetics Computer Group programs (9).
Transformation and electrotransformation.
Transformation was performed as described previously (17, 27). Electrotransformation was carried out by using a Gene Pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) (18, 27). The antibiotics and the concentrations used for selection of the transformants were as follows: ampicillin, 100 μg/ml; fusidic acid, 20 μg/ml; nalidixic acid, 25 μg/ml; rifampin, 20 μg/ml; streptomycin, 200 μg/ml; and trimethoprim, 5 μg/ml.
Mating experiments.
Filter matings were performed as described previously (4, 34, 35). Transfer frequencies were expressed as the number of transconjugants per donor CFU after the mating period. The antibiotics and the concentrations used for selection of transconjugants were as follows: chloramphenicol, 15 μg/ml; erythromycin, 10 μg/ml; fusidic acid, 20 μg/ml; kanamycin, 20 μg/ml for E. coli and L. monocytogenes and 1,000 μg/ml for E. faecalis; nalidixic acid, 25 μg/ml for E. coli DH5α and 50 μg/ml for E. coli K802N; rifampin, 20 μg/ml; streptomycin, 200 μg/ml for E. coli HB101 and 500 μg/ml for B. subtilis BM4150, E. faecalis BM4110, L. monocytogenes EGDSmR, and S. aureus 80CR5Str; tetracycline, 10 μg/ml; and trimethoprim, 5 μg/ml.
PCR amplification.
The PCR mixture was submitted to a denaturation step (3 min at 94°C), followed by 35 cycles of amplification (50 s of denaturation at 94°C, 50 s of annealing at 50°C, and 1 min 30 s of elongation at 72°C). The primers used were D1 (5′ ATTTCTTTAATTGTTGC 3′OH), D2 (5′ GACATAAGGCAAGAACA 3′OH), M1 (5′ ATTACTTGTCACGTCTG 3′OH), M2 (5′ TTCTTTTGCTCGATCCC 3′OH), R1 (5′ CGAGTCTTTCTATTCTT 3′OH), and R2 (5′ TTTATTGTCACTTCCGT 3′OH). The amplified DNA fragments were separated by agarose gel electrophoresis and transferred to Nytran membranes (27). Plasmid pIP823 DNA was labeled with [α-32P]dCTP with a Nick-Translation kit according to the instructions of the manufacturer. Prehybridization and hybridization under stringent conditions were carried out as described previously (4, 27).
Detection of pIP823 ssDNA.
Strains harboring pIP823 were grown to mid-logarithmic phase and treated for 2 h with (i) no additions, (ii) erythromycin (100 μg/ml), or (iii) erythromycin plus rifampin (100 μg/ml each). Total DNA was prepared (31), electrophoresed on a 1% agarose gel, and transferred to a Nytran membrane by diffusion with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) according to the instructions of the manufacturer. Membranes were prehybridized and hybridized at 68°C with radiolabeled pIP823 dsDNA as described previously (27).
Characterization of ssDNA and determination of plasmid pIP823 copy number.
Replication intermediates of RC replication plasmids can be detected in crude extracts from host cells as a band that migrates faster than supercoiled plasmid DNA during electrophoresis of agarose gels containing ethidium bromide (33). If pIP823 replicates by an RC mechanism, then ssDNA intermediates should accumulate in the various bacterial hosts. Erythromycin inhibits protein synthesis and thus prevents initiation of plasmid replication. In the presence of erythromycin, ssDNA molecules are converted to dsDNA by the host RNA polymerases that are still efficient. Since rifampin inhibits the activity of RNA polymerases in the presence of both erythromycin and rifampin, the conversion of ssDNA to dsDNA is inhibited, resulting in the accumulation of ssDNA molecules. Whole-cell lysates were prepared as described previously (33). Appropriate dilutions of DNA were run on 1% agarose gels containing 0.5 μg of ethidium bromide per ml. The plasmid copy number was estimated by comparing the intensity of plasmid DNA in each strain with those of plasmids pSC101, pBR322, and pUC18.
Analysis of plasmid pIP823 segregational stability.
Bacterial strains harboring plasmid pIP823 were grown in Mueller-Hinton medium containing trimethoprim (5 μg/ml). Overnight saturated cultures were used to inoculate the same medium without antibiotic and were incubated at 37°C. Ten successive transfers (approximately 100 generations) were performed in antibiotic-free medium. The cells were then plated on nonselective Mueller-Hinton agar after serial dilutions. Approximately 100 colonies from each sample were transferred onto nonselective and selective agar plates to determine the proportion of bacteria containing plasmid DNA.
Enzymes and chemicals.
Restriction endonucleases, T4 DNA ligase, T7 DNA polymerase, and Taq DNA polymerase (Pharmacia Biotech SA, Saint Quentin en Yvelines, France) were used according to the recommendations of the manufacturer. Lysozyme and lysostaphin were obtained from Sigma Chemical Co. (St. Louis, Mo.) and Applied Microbiology Inc. (Tarrytown, N.Y.), respectively. A Sequenase version 2.0 DNA sequencing kit was provided by United States Biochemical Corporation (Cleveland, Ohio). The Nick-Translation reagent kit was supplied by Amersham International plc (Little Chalfont, Buckinghamshire, England). Nytran NY13N was obtained from Schleicher and Schuell (Duren, Germany). [α-35S]dATP and [α-32P]dCTP were purchased from Amersham Radiochemical Center (Amersham, England). The 1-kb-ladder molecular weight marker and the supercoiled-DNA marker were obtained from Life Technologies Gibco BRL (Eragny, France). The following antibiotics were provided by the indicated laboratories: ampicillin, Panpharma (Fougères, France); chloramphenicol, Roussel-Uclaf (Romainville, France); erythromycin, Abbott (Rungis, France); fusidic acid, Leo (Montigny-Le-Bretonneux, France); kanamycin, Bristol (Paris-La Défense, France); nalidixic acid, Sanofi Winthrop (Gentilly, France); rifampin, Marion Merrel SA (Levallois-Perret, France); streptomycin, Diamant (Puteaux, France); tetracycline, Rhône-Poulenc Rorer (Vitry-sur-Seine, France); and trimethoprim, Roche (Fontenay-sous-Bois, France).
Nucleotide sequence accession number.
The nucleotide sequence of plasmid pIP823 has been deposited in the GenBank database under accession no. u0997.
RESULTS AND DISCUSSION
Nucleotide sequence of pIP823.
Plasmid pIP823 DNA was digested with HindIII, cloned into HindIII-linearized pUC18, and transformed into E. coli JM83. The resulting plasmid, pAT459, conferred trimethoprim resistance to this new host. The sequence of the pAT459 insert of 3,712 bp was determined on both strands (Fig. 1). Analysis of the sequence revealed the presence of two open reading frames (ORFs) and of a third ORF, which appeared to be disrupted at the HindIII cloning site. In order to confirm this assumption and prove that no segment of pIP823 was lost during cloning, two primers (5′ ATTACTTGTCACGTCTG 3′OH) and (5′ TTTCTTTTGCTCGATCC 3′OH) were designed to be complementary to the pAT459 insert at 261 bp upstream and 245 bp downstream from the HindIII site while allowing amplification of a 514-bp fragment with pIP823 DNA as a template. The sequence of the amplification product corresponded to that predicted for the insert of pAT459 circularized at the HindIII site. The third ORF started 735 bp upstream from the HindIII site in the 3′ part of the insert and ended at 504 bp downstream from this site in the 5′ part of the insert. Thus, the 3,712-bp HindIII insert of pAT459 corresponded to pIP823 linearized at this site.
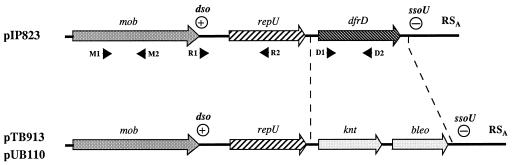
FIG. 1.
Schematic representation of the relationship between pIP823 from L. monocytogenes (3,712 bp), pUB110 from S. aureus (4,525 bp), and pTB913 from Bacillus stearothermophilus (4,525 bp). bleo, gene conferring resistance to bleomycin; dfrD, gene encoding trimethoprim-resistant S2DHFR; dso, double-stranded origin or plus origin of replication; knt, gene conferring resistance to kanamycin; mob, gene encoding the mobilization protein Mob; repU, gene encoding the replication protein RepU; RSA, palindromic site involved in conjugative mobilization and recombination; ssoU, single-stranded origin or minus origin of replication. The positions of the PCR primers in dfrD (D), mob (M), dso (R1), and repU (R2) are indicated by filled arrowheads.
pIP823 organization and similarities to RC plasmids.
The first ORF, from positions 2159 to 2675, corresponds to the dfrD gene, which encodes trimethoprim-resistant dihydrofolate reductase type S2 (S2DHFR) (Fig. 1) (3). The second ORF, from positions 970 to 1975, corresponds to the Rep protein with motifs characteristic of RC replication proteins (Fig. 1). The sequence of Rep was found to be identical to that of RepU of plasmids pUB110 from S. aureus and pTB913 from a thermophilic Bacillus sp. (19, 23), which belong to the pC194/pUB110/pBC16 RC replication plasmid family (12). The degree of amino acid identity of the deduced Rep protein of pIP823 with Rep proteins of the other plasmids of this family ranged from 31.8 to 52.6%. The predicted Rep protein of pIP823 contains a set of three conserved sequence motifs that are typical of initiator Rep proteins of RC replication replicons related to the E. coli bacteriophage φX174, and this Rep is closely related to those of the pC194 family (12). A sequence similar to that of dso of the RC replication plasmids was detected 449 bp upstream from the rep gene. This sequence, from positions 796 to 821, differs by a single nucleotide from the dso of pTB913 (Fig. 1). We also identified in plasmid pIP823, between nucleotides 2775 and 3046 upstream from the mob gene, a conversion signal identical to that of the palU-type sso of pTB913 (8). Therefore, it is likely that both plasmids have the same mechanism of replication initiation and termination. The product of the third ORF, from positions 3206 to 742, corresponds to a Mob protein possessing 97.6% identity with the Pre protein of plasmid pTB913, 90.4% identity with that of pTB53, but only 60.5% identity with the Mob protein of pUB110. Proteins Pre and Mob are involved in site-specific recombination and conjugative mobilization of these plasmids (23). The high level of similarity between the product of the ORF of pIP823 and the Pre and Mob proteins of pTB913 and pUB110 makes it likely that this ORF is responsible for recombination or mobilization of pIP823. A sequence similar to that of the RSA site of pUB110 was identified between positions 3127 and 3150 upstream from the mob gene. The RSA site of pUB110 has been shown to be involved in conjugative mobilization, and it has been suggested that the potential palindromic structure of this site might have a function similar to that of the origins of transfer of gram-negative bacteria (24, 28).
Host range, stability, and copy number of pIP823.
Plasmid pIP823 was introduced by transformation into B. subtilis 168, E. coli HB101, E. faecalis JH2-2, L. monocytogenes LO17RF, S. aureus RN4220, and E. coli DH5α. The plasmid conferred high-level resistance to trimethoprim (MICs from 1,028 to 2,056 μg/ml) and was stably maintained in the various hosts, with 100% of the cells retaining the plasmid after growth for ca. 100 generations in the absence of trimethoprim. The copy number of pIP823 was estimated to vary from 2 to 20 copies per chromosome equivalent, depending on the host.
Identification of ssDNA pIP823 replication intermediates.
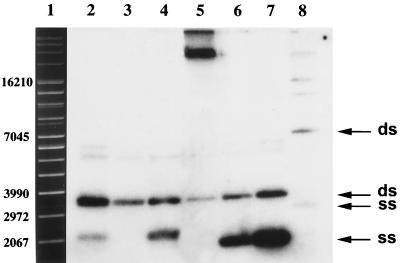
Numerous RC replication plasmids from gram-positive bacteria accumulate ssDNA molecules (32, 33). We screened for the presence of pIP823 ssDNA intermediates in lysates of L. monocytogenes BM4293, E. faecalis JH2-2 (pIP823), S. aureus RN4220 (pIP823), B. subtilis 168 (pIP823), and E. coli HB101 (pIP823). As shown in Fig. 2 (lane 2), in the absence of rifampin and erythromycin L. monocytogenes BM4293 accumulated ssDNA molecules, suggesting that sso of pIP823 is not efficient in this host. In the presence of erythromycin (lane 3), ssDNA was converted to dsDNA. In the presence of erythromycin and rifampin, ssDNA molecules accumulated in L. monocytogenes BM4293 (lane 4) but also in B. subtilis (pIP823) (lane 7), S. aureus (pIP823) (lane 6), and E. coli HB101 (pIP823) (lane 8), confirming that conversion of ssDNA to dsDNA is dependent on RNA polymerase activity in these hosts. Under similar culture conditions, multimer ssDNA intermediates were detected in E. faecalis JH2-2 (pIP823) lysates (lane 5). In contrast to pUB110, which does not replicate in E. coli, pIP823 replicated and accumulated ssDNA in this species. The sso of pIP823 was RNA polymerase dependent (Fig. 2 and data not shown). Usually sso shows activity only in a limited number of hosts (11, 12). The amount of ssDNA molecules accumulated during RC replication is plasmid and host dependent since the conversion rate of ssDNA to dsDNA depends on the efficiency of the host machinery to recognize a given plasmid’s sso (36).
FIG. 2.
Detection of single-stranded pIP823 DNA. Bacterial cultures were grown to mid-logarithmic phase and treated for 2 h with (i) no addition, (ii) erythromycin (100 μg/ml), or (iii) erythromycin plus rifampin (100 μg/ml each). Total DNA was prepared, and equivalent amounts of samples were run on a 0.9% agarose gel, transferred to a Nytran membrane, and hybridized to an in vitro 32P-labeled pIP823 probe. Lanes: 1, supercoiled-DNA ladder; 2 to 4, L. monocytogenes BM4293: 2, no addition; 3, erythromycin added; 4, erythromycin and rifampin added; 5 to 8, erythromycin and rifampin added: 5, E. faecalis JH2-2 (pIP823); 6, S. aureus RN4220 (pIP823); 7, B. subtilis 168 (pIP823); 8, E. coli HB101 (pIP823).
pIP823 was not self-transferable by conjugation.
In a previous study, we proposed that transferable plasmid-mediated multiple-antibiotic resistance and transposon-borne tetracycline resistance in L. monocytogenes result from acquisition of these genetic elements from enterococci (4). Based on the identity of dfrD of L. monocytogenes BM4293 with the corresponding gene in Staphylococcus haemolyticus, we also suggested that trimethoprim resistance in Listeria may have originated in Staphylococcus (3). The data obtained since then show that trimethoprim resistance in strain BM4293 is associated with an RC replicon, a plasmid type common in staphylococci. It is thus possible that trimethoprim resistance is due to plasmid transfer, and we therefore studied the transferability of pIP823. As expected, attempts to transfer pIP823 by conjugation between L. monocytogenes, E. faecalis, S. aureus, B. subtilis, and E. coli were unsuccessful, suggesting that pIP823 was not self-transferable (data not shown). The ability of pIP823 to be mobilized by various conjugative plasmids and transposons between these gram-positive and gram-negative species in which it replicates was then studied (Table 2).
TABLE 2.
Conjugative mobilization of pIP823 by self-transferable plasmids and conjugative transposon Tn1545 between gram-positive bacteria, between E. coli strains, from gram-positive bacteria to E. coli, and from E. coli to L. monocytogenes
| Donor | Recipient | Antibiotic selectiona | Mobilization frequencyb (avg ± SD) |
|---|---|---|---|
| L. monocytogenes BM4293 (pAMβ1) | L. monocytogenes LO17RF | RIF, FUS, TP | (4.3 ± 4.7) × 10−5 |
| E. faecalis JH2-2 | RIF, FUS, TP | (4.1 ± 5.5) × 10−5 | |
| S. aureus 80CR5RF | RIF, FUS, TP | <10−9 | |
| B. subtilis BM4150 | SM, TP | <10−9 | |
| E. coli HB101 | SM, TP | <10−9 | |
| E. coli DH5α | NAL, TP | <10−9 | |
| L. monocytogenes LO17RF (pAMβ1 plus pIP823) | L. monocytogenes EGDSmR | SM, TP | (7.8 ± 2) × 10−6 |
| E. faecalis BM4110 | SM, TP | (2.7 ± 1.4) × 10−5 | |
| E. faecalis JH2-2 (pAMβ1 plus pIP823) | L. monocytogenes EGDSmR | SM, TP | (2.9 ± 1.7) × 10−5 |
| S. aureus RN4220 (pAMβ1 plus pIP823) | L. monocytogenes EGDSmR | SM, TP | <10−9 |
| L. monocytogenes LO17RF | RIF, FUS, TP | <10−9 | |
| S. aureus 80CR5RF | RIF, FUS, TP | <10−9 | |
| L. monocytogenes LO17RF (pAT191 plus pIP823) | E. faecalis BM4110 | SM, TP | 2 × 10−7 |
| E. coli HB101 | SM, TP | 1.1 × 10−8 | |
| E. faecalis JH2-2 (pAT191 plus pIP823) | E. faecalis BM4110 | SM, TP | 2.9 × 10−5 |
| E. coli HB101 | SM, TP | (1.2 ± 1.6) × 10−8 | |
| E. coli HB101 (pAT191 plus pIP823) | E. coli K802N | NAL, TP | <10−9 |
| L. monocytogenes LO17RF | RIF, FUS, TP | <10−9 | |
| E. faecalis JH2-2 | RIF, FUS, TP | <10−9 | |
| L. monocytogenes LO17RF::Tn1545 (pIP823) | L. monocytogenes EGDSmR | SM, TP | 1.6 × 10−9 |
| E. faecalis BM4110 | SM, TP | <10−9 | |
| E. coli HB101 | SM, TP | (3.2 ± 1.5) × 10−9 | |
| E. faecalis JH2-2::Tn1545 (pIP823) | L. monocytogenes EGDSmR | SM, TP | (4.9 ± 0.5) × 10−8 |
| E. faecalis BM4110 | SM, TP | (2.1 ± 2.4) × 10−8 | |
| E. coli HB101 | SM, TP | (1.3 ± 9.1) × 10−9 | |
| E. coli HB101 (RP4 plus pIP823) | E. coli K802N | NAL, TP | 1 × 10−1 |
| E. coli K802N (RP4 plus pIP823) | L. monocytogenes EGDSmR | SM, TP | (7.5 ± 3.5) × 10−7 |
| E. faecalis BM4110 | SM, TP | <10−9 | |
| E. coli SM10 (pIP823) | E. coli K802N | NAL, TP | 4 × 10−1 |
| L. monocytogenes EGDSmR | RIF, FUS, TP | 3.3 × 10−7 | |
| E. faecalis BM4110 | RIF, FUS, TP | <10−9 | |
| E. coli HB101 (R64 plus pIP823) | E. coli K802N | NAL, TP | 3.2 × 10−2 |
| E. coli K802N (R64 plus pIP823) | L. monocytogenes EGDSmR | SM, TP | <10−9 |
| E. faecalis BM4110 | SM, TP | <10−9 | |
| E. coli HB101 (pIP55-1 plus pIP823) | E. coli K802N | NAL, TP | (4 ± 5) × 10−5 |
| E. coli K802N (pIP55-1 plus pIP823) | L. monocytogenes EGDSmR | SM, TP | <10−9 |
| E. faecalis BM4110 | SM, TP | <10−9 | |
| E. coli HB101 (pOX38-Km plus pIP823) | E. coli DH5α | NAL, TP | <10−9 |
| L. monocytogenes LO17RF | RIF, FUS, TP | <10−9 | |
| E. coli DH5α (pOX38-Km plus pIP823) | E. coli HB101 | SM, TP | 1.2 × 10−7 |
FUS, fusidic acid; NAL, nalidixic acid; RIF, rifampin; SM, streptomycin; TP, trimethoprim.
Results are the means obtained from results of a minimum of three independent matings.
Conjugative mobilization of pIP823 by self-transferable plasmids from gram-positive bacteria.
The broad-host-range enterococcal plasmid pAMβ1 was introduced by conjugation from E. faecalis BM4110 (pAMβ1) into L. monocytogenes BM4293 and S. aureus RN4220 (pIP823) at mobilization frequencies of 3.7 × 10−2 and 9.3 × 10−6, respectively. Transfer by conjugative mobilization of plasmid pIP823 was obtained from the L. monocytogenes BM4293 (pAMβ1) donor to L. monocytogenes LO17RF and E. faecalis JH2-2 recipients. From transconjugant L. monocytogenes LO17RF (pAMβ1 plus pIP823), pIP823 could be mobilized to L. monocytogenes EGDSmR and E. faecalis BM4110, and from transconjugant E. faecalis JH2-2 (pAMβ1 plus pIP823), it could be mobilized to L. monocytogenes EGDSmR. The resistance phenotypes of 100 transconjugants from each mobilization experiment indicated that pAMβ1 was cotransferred in all cases. Mobilization of pIP823 by pAMβ1 was not obtained with (i) L. monocytogenes BM4293 (pAMβ1) as a donor and the other gram-positive bacteria or E. coli as recipients or (ii) an S. aureus donor and either L. monocytogenes or S. aureus recipients. Nevertheless, pAMβ1 could be conjugated from L. monocytogenes BM4293 to S. aureus 80CR5RF and from S. aureus RN4220 to L. monocytogenes LO17RF at frequencies of 10−5 and 2 × 10−9, respectively. No mobilization of pIP823 by pAMβ1 could be detected from L. monocytogenes to S. aureus, B. subtilis, and E. coli (Table 2). The gram-negative and gram-positive shuttle plasmid pAT191 contains the origin of replication and the transfer functions of pAMβ1, the replication origin of pBR322, and the transfer origin of IncP plasmid RK2 from gram-negative bacteria (33). This bireplicon, which conjugates from E. faecalis to E. coli, was introduced by conjugation from E. faecalis BM4110 (pAT191) to L. monocytogenes BM4293 and E. faecalis JH2-2 (pIP823) with frequencies of 1.8 × 10−3 and 1.7 × 10−2, respectively. Conjugative mobilization of pIP823 by pAT191 was obtained from L. monocytogenes BM4293 and E. faecalis JH2-2 (pIP823) donors to E. faecalis BM4110 and E. coli HB101 recipients. Plasmid pIP823 could not be mobilized by conjugation by pAT191 from E. coli HB101 to E. coli K802N, L. monocytogenes LO17RF, or E. faecalis JH2-2.
Conjugative mobilization of pIP823 by the streptococcal conjugative transposon Tn1545.
Plasmid pIP823 was introduced by electrotransformation into E. faecalis JH2-2::Tn1545 and L. monocytogenes LO17RF::Tn1545. The plasmid was successfully mobilized by conjugative transposon Tn1545 from L. monocytogenes LO17RF::Tn1545 (pIP823) to L. monocytogenes EGDSmR and E. coli HB101 and from E. faecalis JH2-2::Tn1545 (pIP823) to L. monocytogenes EGDSmR (Table 2). None of the transconjugants contained Tn1545. No conjugative mobilization of pIP823 by Tn1545 from L. monocytogenes LO17RF::Tn1545 (pIP823) to E. faecalis BM4110 and E. coli HB101 and from E. faecalis JH2-2::Tn1545 (pIP823) to E. faecalis BM4110 was obtained.
Conjugative mobilization of pIP823 by self-transferable plasmids from gram-negative bacteria.
Plasmids RP4, R64, and pIP55-1 were introduced by conjugation into E. coli HB101 (pIP823) with frequencies of 1.4 × 10−4, 2.2 × 10−4, and 1.4 × 10−4, respectively. Plasmid pIP823 could be mobilized from E. coli HB101 to E. coli K802N by the three plasmids (Table 2). In the experiment with RP4 as a helper plasmid, of the 100 transconjugants analyzed, all had acquired both pIP823 and RP4 whereas R64 was cotransferred to only 32% of the 100 transconjugants studied. Plasmid pIP823 was mobilizable from E. coli K802N (RP4 plus pIP823) to L. monocytogenes EGDSmR but not to E. faecalis BM4110 or from E. coli K802N (RP64 plus pIP823) or K802N (pIP55-1 plus pIP823) to L. monocytogenes EGDSmR and E. faecalis BM4110.
Plasmid pOX38-Km, an F-factor derivative, was introduced by conjugation into E. coli HB101 (pIP823) and E. coli DH5α (pIP823) at frequencies of 1.2 × 10−4 and 1.1 × 10−1, respectively. Plasmid pIP823 was mobilized from E. coli DH5α (pOX38-Km plus pIP823) to E. coli HB101 but not from E. coli HB101 (pOX38-Km plus pIP823) to E. coli DH5α and L. monocytogenes LO17RF (Table 2). E. coli SM10, which contains the IncP group transfer functions of RK2 integrated into the chromosome, was electrotransformed with pIP823 DNA. Conjugative mobilization was obtained from E. coli SM10 (pIP823) to E. coli K802N and L. monocytogenes EGDSmR but not to E. faecalis BM4110. This result indicates that mobilization of pIP823 by RK2 occurs in the absence of cointegration.
The presence of pIP823 in transconjugants from all of the mobilization experiments described above was examined by amplification of total DNA with oligonucleotides specific for rep, dfrD, and mob followed by hybridization of the PCR products obtained with pIP823 DNA as a probe (data not shown). For each strain, fragments of the expected size which hybridized with the pIP823 probe were obtained. Small nonconjugative plasmids have been shown to be mobilized by conjugative plasmids among gram-positive bacteria. The S. aureus plasmid pC221 is mobilized by pG01 (25), whereas pLS20 can mediate transfer of pBC16 and pUB110 (15). The nonconjugative streptococcal plasmid pMV158 can be mobilized by both pAMβ1 and pIP501 (24). The mechanism of mobilization of relaxable plasmids in S. aureus appears to be analogous to that of mobilization by donation in gram-negative bacteria (25). Transfer by mobilization of pC194 and pUB110 by the conjugative transposons Tn925 and Tn916 between Bacillus species has been described previously (20, 29). In contrast to pIP823, pTB913 was reported to be nonmobilizable (22). This is surprising in view of the similarities between the Mob proteins and the RSA sites of the two plasmids. It would be interesting to examine transfer of pTB913 in the context developed here.
In conclusion, we have characterized the natural RC plasmid pIP823 from L. monocytogenes BM4293, which bears the gene that encodes trimethoprim-resistant S2DHFR. This plasmid belongs to the pC194/pUB110 family of RC replication plasmids. The similarities with pUB110 from Staphylococcus and pTB913 from Bacillus include all the features essential for this replication mode: (i) a dso sequence for initiation of plus-strand synthesis, (ii) a rep lication protein, and (iii) an sso sequence for initiation of minus-strand synthesis. Rep of pIP823 was identical to those of pTB913 and pUB110. Plasmid pIP823 bears the gene that encodes a Mob protein showing a high degree of identity with Mob of pTB913 and possesses a potential RSA site closely related to those of members of the pC194/pUB110 RC replication plasmid family. Plasmid pIP823 has an exceptionally broad host range of replication since it is maintained stably in L. monocytogenes, E. faecalis, S. aureus, B. subtilis, and E. coli, in which plasmid ssDNA intermediates accumulated. One of the main features of RC plasmids is their promiscuity, which is attributed to replication functions that appear to be active in a large variety of hosts. Other functions such as transferability by mobilization, cassette exchange, and recombination may also contribute to dissemination of these plasmids. The bleo and knt genes of pTB913 and pUB110 confer resistance to bleomycin and kanamycin, respectively. Plasmid pIP823 may have diverged from pTB913 by replacement of the bleomycin and kanamycin resistance cassettes by the trimethoprim resistance determinant at the recombination-specific site RSA (Fig. 1); the reverse filiation is as likely. However, it is noteworthy that pTB913 has been detected only in an integrated state as part of plasmid pTB19 of Bacillus. The high degree of similarity between the RC plasmids indicates easy horizontal transfer of these replicons between gram-positive cocci and bacilli, including Listeria. In addition, we have demonstrated conjugative mobilization of pIP823 by plasmids and a transposon between L. monocytogenes, E. faecalis, and E. coli. These findings raise, once more, the question of the so-called genetic barrier between gram-positive and gram-negative bacteria (6). Based on its properties, pIP823 might be attractive for the development of new broad-host-range cloning and expression gram-positive–gram-negative shuttle vectors. Characterization of pIP823 is also of interest in view of the possible dissemination of trimethoprim resistance in Listeria.
ACKNOWLEDGMENTS
E.C. was a recipient of a Pasteur-Weizmann fellowship during part of this work.
We are grateful to A. Gruss for helpful discussions and P. Trieu-Cuot for critical reading of the manuscript. We thank R. Gardan for the protocol of B. subtilis transformation.
REFERENCES
- 1.Bukhari A I, Shapiro J A, Adhya S L. DNA insertion elements, plasmids, and episomes. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1977. [Google Scholar]
- 2.Chandler M, Galas D J. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J Mol Biol. 1983;170:61–91. doi: 10.1016/s0022-2836(83)80227-7. [DOI] [PubMed] [Google Scholar]
- 3.Charpentier E, Courvalin P. Emergence of the trimethoprim resistance gene dfrD in Listeria monocytogenes BM4293. Antimicrob Agents Chemother. 1997;41:1134–1136. doi: 10.1128/aac.41.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charpentier E, Gerbaud G, Jacquet C, Rocourt J, Courvalin P. Incidence of antibiotic resistance in Listeria species. J Infect Dis. 1995;172:277–281. doi: 10.1093/infdis/172.1.277. [DOI] [PubMed] [Google Scholar]
- 5.Clewell D B, Yagi Y, Dunny G M, Schultz S K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974;117:283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courvalin P. Transfer of antibiotic resistance genes between gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 1994;38:1447–1451. doi: 10.1128/aac.38.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courvalin P, Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986;205:291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey L A, Zhao A C, Khan S A. Localization of the start sites of lagging-strand replication of rolling-circle plasmids from gram-positive bacteria. Mol Microbiol. 1995;15:679–687. doi: 10.1111/j.1365-2958.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 9.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel H W B, Soedirman N, Rost J A, van Leeuwen W J, van Embden J D A. Transferability of macrolide, lincomycin, and streptogramin resistances between group A, B, and D streptococci, Streptococcus pneumoniae, and Staphylococcus aureus. J Bacteriol. 1980;142:407–413. doi: 10.1128/jb.142.2.407-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa M, del Solar G, Rojo F, Alonso J C. Plasmid rolling circle replication and its control. FEMS Microbiol Lett. 1995;130:111–120. doi: 10.1111/j.1574-6968.1995.tb07707.x. [DOI] [PubMed] [Google Scholar]
- 12.Gruss A, Ehrlich S D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989;53:231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hale E, Habte-Gabr E, McQueen R, Gordon R. Co-trimoxazole for the treatment of listeriosis and its successful use in a patients with AIDS. J Infect Dis. 1994;28:110–113. doi: 10.1016/s0163-4453(94)94688-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehler T M, Thorne C B. Bacillus subtilis (natto) plasmid pLS20 mediates interspecies plasmid transfer. J Bacteriol. 1987;169:5271–5278. doi: 10.1128/jb.169.11.5271-5278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreiswirth B N, Löfdahl S, Betley M J, O’Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by prophage. Nature (London) 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 17.Kunst F, Rapoport G. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J Bacteriol. 1995;177:2403–2407. doi: 10.1128/jb.177.9.2403-2407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luchansky J B, Muriana P M, Klaenhammer T R. Application of electroporation for transfer of plasmid DNA to Lactobacillus, Lactococcus, Leuconostoc, Listeria, Pediococcus, Bacillus, Staphylococcus, Enterococcus and Propionibacterium. Mol Microbiol. 1988;2:637–646. doi: 10.1111/j.1365-2958.1988.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 19.McKenzie T, Hoshino T, Tanaka T, Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986;15:93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- 20.Naglich J G, Andrews R E., Jr Tn916-dependent conjugal transfer of PC194 and PUB110 from Bacillus subtilis into Bacillus thuringiensis subsp. israelensis. Plasmid. 1988;20:113–126. doi: 10.1016/0147-619x(88)90014-5. [DOI] [PubMed] [Google Scholar]
- 21.Noirot-Gros M F, Bidnenko V, Ehrlich S D. Active site of the replication protein of the rolling circle plasmid pC194. EMBO J. 1994;13:4412–4420. doi: 10.1002/j.1460-2075.1994.tb06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oskam L, Hillenga D J, Venema G, Bron S. The large Bacillus plasmid pTB19 contains two integrated rolling-circle plasmids carrying mobilization functions. Plasmid. 1991;26:30–39. doi: 10.1016/0147-619x(91)90034-t. [DOI] [PubMed] [Google Scholar]
- 23.Oskam L, Hillenga D J, Venema G, Bron S. The integrated state of the rolling-circle plasmid pTB913 in the composite Bacillus plasmid pTB19. Mol Gen Genet. 1992;233:462–468. doi: 10.1007/BF00265444. [DOI] [PubMed] [Google Scholar]
- 24.Priebe S D, Lacks S A. Region of the streptococcal plasmid pMV158 required for conjugative mobilization. J Bacteriol. 1989;171:4778–4784. doi: 10.1128/jb.171.9.4778-4784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Projan S J, Archer G L. Mobilization of the relaxable Staphylococcus aureus plasmid pC221 by the conjugative plasmid pG01 involves three pC22 loci. J Bacteriol. 1989;171:1841–1845. doi: 10.1128/jb.171.4.1841-1845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocourt J. Listeria monocytogenes: the state of the science. Dairy Food Environ Sanit. 1994;14:70–82. [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Selinger L B, McGregor N F, Khachatourians G G, Hynes M F. Mobilization of closely related plasmids pUB110 and pBC16 by Bacillus plasmid pXO503 requires trans-acting open reading frame β. J Bacteriol. 1990;172:3290–3297. doi: 10.1128/jb.172.6.3290-3297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Showsh S A, Andrews R E., Jr Functional comparison of conjugative transposons Tn916 and Tn925. Plasmid. 1996;35:164–173. doi: 10.1006/plas.1996.0019. [DOI] [PubMed] [Google Scholar]
- 30.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 31.Smith H R, Humphreys G O, Anderson E S. Genetic and molecular characterization of some non-transferring plasmids. Mol Gen Genet. 1974;129:229–242. doi: 10.1007/BF00267915. [DOI] [PubMed] [Google Scholar]
- 32.te Riele H, Michel B, Ehrlich S D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci USA. 1986;83:2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.te Riele H, Michel B, Ehrlich S D. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986;5:631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trieu-Cuot P, Carlier C, Courvalin P. Conjugative plasmid transfer from Enterococcus faecalis to Escherichia coli. J Bacteriol. 1988;170:4388–4391. doi: 10.1128/jb.170.9.4388-4391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trieu-Cuot P, Carlier C, Martin P, Courvalin P. Plasmid transfer by conjugation from Escherichia coli to gram-positive bacteria. FEMS Microbiol Lett. 1987;48:289–294. [Google Scholar]
- 36.van der Lelie D, Bron S, Venema G, Oskam L. Similarity of minus origins of replication and flanking open reading frames of plasmids pUB110, pTB913 and pMV158. Nucleic Acids Res. 1989;17:7283–7294. doi: 10.1093/nar/17.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodcock D M, Crowther P J, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith S S, Michael M Z, Graham M W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]