Abstract
This study aimed to compare the success rates of two approaches for seminal vesiculoscopy: through the interior of the prostatic utricle and through the neck of the prostatic utricle. The patients were divided into two groups based on the seminal vesiculoscopy used. Group A was an interior of the prostatic utricle group (152 cases), and group B was a neck of the prostatic utricle group (146 cases). The general clinical data, intraoperative conditions and surgical results of the two groups were compared. Compared with group A, group B had a higher surgical success rate (94.5% vs. 62.5%, p < .001), a shorter operation time (33 min vs. 45 min, p < .001), less blood loss (0.5 ml vs. 2 ml, p < .001), a higher pain relief rate (86.6% vs. 52.3%, p < .001), a higher remission rate of haemospermia (82.2% vs. 58.5%, p = .011), a lower recurrence rate of pain (10.4% vs. 35.4%, p < .001), a lower recurrence rate of haemospermia (15.6% vs. 37.7%, p = .014), a higher symptom remission rate of the lower urinary tract (90.9% vs. 50.0%, p = .030), a higher remission rate of scrotal moisture (84.6% vs. 45.5%, p = .042) and a higher remission rate of frequent spermatorrhea (80.0% vs. 55.6%, p = .033). Seminal vesiculoscopy undertaken through the neck of the prostatic utricle has the characteristics of high success rate, short operation time and good surgical effect and is worthy of promotion and application.
Keywords: seminal vesicle, haemospermia, pain, success rate
Introduction
Seminal vesiculoscopy has become an important examination and treatment method for seminal vesicle gland diseases in recent years. Seminal vesiculoscopy has been rapidly popularized in the field of andrology because of its clear effect and few surgical complications (Liao et al., 2017). However, the success rate of seminal vesiculoscopy has always been the bottleneck restricting the method’s application in andrological diseases, affecting their treatment.
Locating the ejaculatory duct or seminal vesicle gland during surgery is the key to the successful development of seminal vesiculoscopy. At present, the most commonly used method is to enter the seminal vesicle gland through the wall of the prostatic vesicle (Chen et al., 2018; Hu & Chen, 2018; Liao et al., 2019), but the success rate of this method limits the application of seminal vesiculoscopy. In April 2020, the Andrology Department of the First Affiliated Hospital of Zhengzhou University adopted the method of breaking the wall through the neck of the prostatic vesicle and entering the seminal vesicle gland through the ejaculatory duct with the help of a holmium laser, which has greatly improved the success rate of seminal vesiculoscopy. This study aimed to compare the success rates of these two approaches and provide theoretical basis for clinical application of seminal vesiculoscopy.
Materials and Methods
Clinical Data
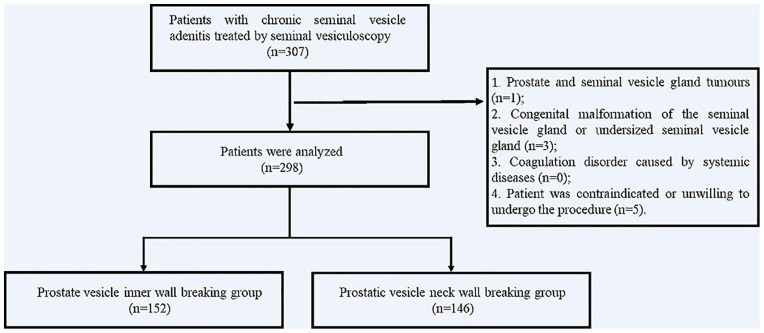
The data of 298 patients with chronic seminal vesicle adenitis treated by seminal vesiculoscopy in the Andrology Department of the First Affiliated Hospital of Zhengzhou University between January 2018 and February 2021 were analyzed retrospectively. In 152 of the cases (between January 2018 and April 2020), the seminal vesicle was entered through the wall of the prostatic vesicle, assisted by holmium laser (group A); in the other 146 cases (between April 2020 and February 2021), the seminal vesicle was entered through the ejaculatory duct via the wall of the neck of the prostatic vesicle, assisted by holmium laser (group B). The operators of our study were experienced in both the methods. All patients were treated with an SRM-H3B model yttrium aluminum garnet (YAG) laser treatment machine equipped with a 272-μm fiber and a seminal vesiculoscope with a diameter of F4.6-6.4 (see Figure 1).
Figure 1.
Flowchart of the Operation.
This study was approved by the ethics committee.
Inclusion Criteria
The inclusion criteria for patients in this study were as follows:
Symptoms of refractory haemospermia or recurrent pain and discomfort in the perineum, lower abdomen, or groin, damp scrotum, frequent spermatorrhea, and lower urinary tract symptoms; (2) transrectal color Doppler ultrasound or magnetic resonance imaging showed inflammatory changes, such as thickening of the seminal vesicle wall, dilatation of the seminal vesicle duct, or hemorrhage in the seminal vesicle; and (3) conservative treatments, such as oral drugs and physical therapy, had failed for more than 3 months.
Exclusion Criteria
The exclusion criteria for patients in this study were:
(1) Prostate and seminal vesicle gland tumors; (2) congenital malformation of the seminal vesicle gland or undersized seminal vesicle gland; (3) coagulation disorder caused by systemic diseases; and (4) patient was contraindicated or unwilling to undergo the procedure.
Surgical Methods
General anesthesia was used in both seminal vesiculoscopy methods. The lithotomy position was taken, routine disinfection and draping were performed, and a seminal vesiculoscope with a diameter of F4.6-6.4 was retrogradely inserted into the seminal caruncle of the posterior urethra through the external orifice of the urethra. The morphology of the seminal caruncle and whether there was an ejaculatory duct opening in the membranous urethral mucosa were observed. However, the approach through natural opening of the ejaculatory duct is very difficult and has a high failure rate, so opening of the prostatic vesicle was located at the seminal caruncle and used as the insertion point in our research.
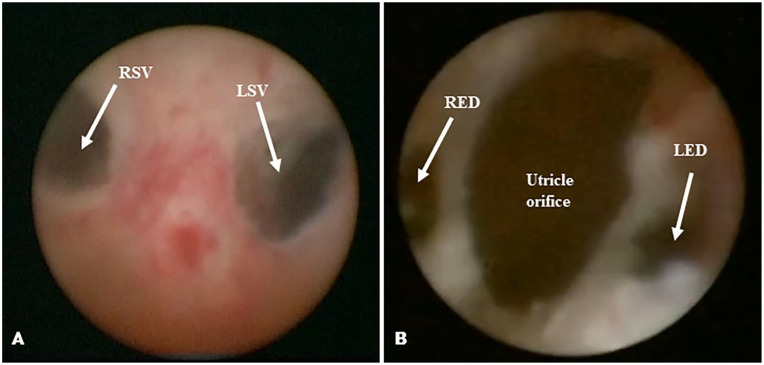
Group A (entrance through the wall of the prostatic vesicle): First, a small-diameter rigid vesiculoscope was inserted into the utricle orifice. Then, any presence of a weak area on the lateral wall of the vesicle was identified. If there was a weak area, it could be directly broken by a low-energy holmium laser in order to enter the seminal vesicle. If there was no weak area, negative pressure was created in a capsule, and the vibrations of the side wall, at 2–5 o’clock of the inner left wall, and at 7–10 o’clock of the right wall were observed. A 2.0 Hz, 1.2 J low-energy holmium laser was then used to break the identified point in the wall for 1 to 3 minutes, after which a 20 mL syringe was used to pressurize and flush the area with water in order to locate the opening of ejaculatory duct or seminal vesicle gland. Then the vesiculoscope was inserted into the seminal vesicle gland through the opening. If the procedure was repeatedly for 30 minutes without success, the surgery was abandoned (see Figure 2A).
Figure 2.
(A) Approach Through the Interior Wall of the Prostatic Vesicle. (B) Approach Through the Ejaculatory Duct Via the Neck of the Prostatic Vesicle.
Note. RSV = right seminal vesicle; LSV = left seminal vesicle; RED = right ejaculatory duct; LED = left ejaculatory duct.
Group B (entrance through the ejaculatory duct via the neck of the prostatic vesicle): The neck of the prostatic vesicle was lightly burned with a 2.0 Hz, 1.2 J low-energy holmium laser at 5 and 7 o’clock, after which a 20 ml syringe was used to pressurize and flush the area with water in order to locate the ejaculatory duct opening. The seminal vesicle glands on both sides were then slowly entered through the ejaculatory duct (see Figure 2B).
After the seminal vesiculoscope was inserted into the seminal vesicle gland, the seminal vesicle gland was repeatedly washed with normal saline until it was clean. If there was a stone, a holmium laser lithotripsy was performed. If there was bleeding, a holmium laser was used to stop it.
Postoperative Treatment
Routine antibiotics and haemostatic drugs were given for 2 weeks after surgery, and outpatient examinations were carried out 2 weeks after surgery and every month thereafter. Clinical symptoms and postoperative adverse events were recorded, and transrectal color Doppler ultrasound was rechecked. The follow-up time was 3–12 months, with an average of 4.9 months. We advised that patients ejaculate 1 to 2 times a week.
Observation Indicators
The operation success rate, operation time and bleeding volume were recorded during surgery. The time with urinary catheter, haemospermia relief rate, pain relief rate, lower urinary tract symptom relief rate, scrotal dampness relief rate, frequent spermatorrhea relief rate, pain recurrence rate and haemospermia recurrence rate were observed after surgery.
Evaluation Criteria
Haemospermia Treatment
Haemospermia treatment was considered completely effective if the semen color to the naked eye was white after operation; it was considered moderately effective if the semen color was very diluted. A combination of the two was considered effective. When the color of the semen was normal after surgery but became red again one month later, it signified recurrence.
Pain Treatment
The visual analogue scale (VAS) was adopted to evaluate pain, with a scale of 0–10. The higher the score, the more severe the pain and discomfort (Chen et al., 2018). If the VAS score decreased to less than 3, the pain treatment was considered effective. If the VAS score rose to more than 3 after 1 month, it signified recurrence.
Statistical Analysis
R 4.0.3 statistical software was used for analysis. Qualitative variables were presented as counts and percentages and the quantitative variables were expressed as mean ± standard deviation. The differences between two groups were compared using two independent samples t-test, Mann–Whitney U test, Chi-squared test, Fisher’s exact test. P ≤ 0.05 was considered statistically significant.
Results
Characteristics of Patients
The patients were 18 to 54 years old, with an average of 37.0 ± 13.2 years, and had an average course of 18.5 ± 16.2 months. The main symptoms of seminal vesicle adenitis were refractory haemospermia or recurrent pain and discomfort in the perineum, lower abdomen, or groin, ejaculatory pain, lower urinary tract urgency, frequent urination, damp scrotum, frequent spermatorrhea, and other symptoms. Prior to surgery, transrectal color Doppler ultrasound was used to determine the volume of the prostate and seminal vesicle gland. During the surgery, the longitudinal diameter of the prostate vesicle was measured with a thin ureteral catheter or a zebra guide wire. The clinical data of the two groups of patients are presented in Table 1.
Table 1.
Clinical Data of the Two Groups of Patients.
| Group | Prostate vesicle inner wall breaking group | Prostatic vesicle neck wall breaking group | p | |
|---|---|---|---|---|
| N | 152 | 146 | ||
| Age (years, ) | 38.1 ± 15.5 | 36.0 14.7 | 0.807 | .370 |
| Smoking, n (%) | 62 (40.8%) | 59 (38.8%) | 0.247 | .559 |
| Drinking, n (%) | 69 (45.4%) | 65 (42.8%) | 0.262 | .601 |
| Course of disease (months, ) | 18.8 ± 19.9 | 18.2 ± 18.7 | 3.702 | .055 |
| Body mass index (kg/m2, ) | 22.4 ± 7.1 | 23.5 ± 12.2 | 4.872 | .068 |
| Hemospermia, n (%) | 53 (34.9%) | 45 (30.8%) | 0.552 | .457 |
| Pain, n (%) | 65 (42.8%) | 67 (45.9%) | 0.295 | .587 |
| Perineum | 31 (20.4%) | 32 (21.9%) | 0.104 | .748 |
| Lower abdomen and groin | 22 (14.5%) | 21 (14.4%) | 0 | .982 |
| Ejaculation pain | 12 (7.9%) | 14 (9.6%) | 0.268 | .604 |
| Lower urinary tract symptoms, n (%) | 14 (9.2%) | 11 (7.5%) | 0.272 | .602 |
| Damp scrotum, n (%) | 11 (7.2%) | 13 (8.9%) | 0.280 | .597 |
| Frequent spermatorrhea, n (%) | 9 (5.9%) | 10 (6.8%) | 0.107 | .743 |
| Prostate volume (ml, ) | 19.2 ± 4.9 | 19.6 ± 5.1 | 0.019 | .890 |
| Seminal vesicle gland volume (ml, ) | ||||
| Left side | 4.2 ± 2.9 | 4.1 ± 2.7 | 0.007 | .933 |
| Right side | 4.3 ± 2.6 | 4.2 ± 2.5 | 1.194 | .275 |
| Prostate vesicle longitudinal diameter | ||||
| <5mm | 85 (55.9%) | 84 (57.5%) | 0.079 | .779 |
| >5mm | 67 (44.1%) | 62 (42.5%) | 0.079 | .779 |
Comparison of Success Rate
All 298 patients with seminal vesicle adenitis showed no abnormal openings in the prostatic vesicle, and all underwent holmium laser wall-breaking surgery. The surgical success rate of group A was 95/152 (62.5%), which was significantly lower than that of group B, which was 138/146 (94.5%), and the difference was statistically significant (p < .001, Table 2).
Table 2.
Comparison of Intraoperative and Postoperative Conditions Between the Two Groups.
| Group | Prostate vesicle inner wall breaking group | Prostatic vesicle neck wall breaking group | Statistics | p |
|---|---|---|---|---|
| n | 152 | 146 | ||
| Operation success rate (%) | 95 (62.5%) | 138 (94.5%) | 44.771 | <.001 |
| Operation time (minutes) | 45 ± 16 | 33 ± 11 | 54.854 | <.001 |
| Bleeding volume (ml) | 2 ± 0.5 | 0.5 ± 0.1 | 1,187.955 | <.001 |
| Time with urinary catheter (h) | 22 ± 4 | 20 ± 2 | 0.574 | .5661 |
| Surgical complications | 0 | 0 | NA | 1 |
| Pain relief rate (%) | 34/65 (52.3%) | 58/67 (86.6%) | 18.335 | <.001 |
| Hemospermia relief rate (%) | 31/53 (58.5%) | 37/45 (82.2%) | 6.453 | .011 |
| Pain recurrence rate (%) | 23/65 (35.4%) | 7 /67 (10.4%) | 11.682 | <.001 |
| Hematospermia recurrence rate (%) | 20/53 (37.7%) | 7/45 (15.6%) | 5.998 | .014 |
| Lower urinary tract symptom relief rate (%) | 7/14 (50.0%) | 10/11 (90.9%) | 4.738 | .030 |
| Scrotal dampness relief rate (%) | 5/11 (45.5%) | 11/13 (84.6%) | 4.112 | .042 |
| Frequent spermatorrhea relief rate (%) | 4/9 (55.6%) | 9/10 (80.0%) | 4.550 | .033 |
Comparison of Postoperative Situation
The intraoperative, postoperative, and follow-up conditions of the two groups of patients are presented in Table 2. Compare with prostate vesicle inner wall breaking group, the prostatic vesicle neck wall breaking group had shorter operation time (33 ± 11 min vs 45 ± 16 min), less blood loss (0.5 ± 0.1 ml vs 2 ± 0.5 ml), and higher relief rate of symptoms such as pain (86.6% vs 52.3%), hemospermia (82.2% vs 58.5%), lower urinary tract symptoms (90.9% vs 50.0%), scrotal dampness (84.6% vs 45.5%), and frequent spermatorrhea (80.0% vs 55.6%). In addition, the differences above were statistically significant because all P values were <.05.
Discussion
Seminal vesiculoscopy is the best option for the treatment of obstructive azoospermia and chronic seminal vesicle inflammation, especially chronic seminal vesicle adenitis manifested by haemospermia (Liao et al., 2017; R. Wang et al., 2016); however, the success rate of seminal vesiculoscopy is an important factor that prevents the promotion of seminal vesiculoscope technology. The key to improving the success rate of seminal vesiculoscopy is whether the seminal vesiculoscope can enter the seminal vesicle. This study reported that the success rate of using a holmium laser to break through the bilateral posterior wall of the seminal vesicle was significantly lower than that of using the laser to break through the neck of the vesicle, as the volume of the human prostate vesicle changes so much. M. S. Wang et al. (2015) studied 109 patients with benign prostatic hyperplasia and reported that only 47.8% (22/46) of men had prostate vesicles, with the rest only having a tiny crypt-like depression in the seminal area. In this study, more than half of the patients had prostate vesicles with a longitudinal diameter of less than 5 mm. The relative distance between the seminal vesicle glands, the double ejaculatory ducts, and the prostate vesicles is also not constant. The ejaculatory ducts enter the prostate parenchyma at an acute angle of 10° to 15° from the midline and open on the seminal caruncle. When the prostate vesicles become smaller, the relative distance between the vesicles and the ejaculatory ducts or seminal vesicle glands increases, which makes it difficult to break through the vesicles, especially when the prostate vesicles are too small or underdeveloped. In these cases, the failure rate of using a holmium laser to break through the posterior wall of the prostatic vesicle into the seminal vesicle gland also increases. In our study, the success rate of vesiculoscope access through the neck of the seminal vesicle was significant high (94.5%) and patients had greater intraoperative, postoperative, and follow-up conditions as we talked before. And our results are in consistence with previous study (Liao et al., 2019; Shao et al., 2018a; Zhao et al., 2016). There are three other reasons why it may be difficult to enter the seminal vesicle through the natural passage of the ejaculatory duct. First, even if the ejaculatory duct is reported under natural conditions, the opening of the ejaculatory duct in the natural state is only 0.1 to 0.3 mm (Nguyen et al., 1966; Shao et al., 2018b; M. S. Wang et al., 2015), much smaller than the diameter of the seminal vesicles. Second, about 60% of the ejaculatory duct openings are located outside the 45° angle from the apex of the prostate vesicle opening (Shao et al., 2018b), which makes it difficult for the seminal vesiculoscope to enter the seminal vesicle glands through the natural ducts. Third, under normal circumstances, the opening of the ejaculatory duct is covered with a large number of unidirectional villi (Li et al., 2019), which cause the direction of water injection of seminal vesiculoscope is against the unidirectional villi when pressurized water is injected into the vesiculoscope, so it is difficult to rush through the villi and expose the ejaculatory duct orifice. These adverse factors make it difficult to master the natural channel of the vesiculoscope (Chen et al., 2018; Hu & Chen, 2018; Liao et al., 2019).
The high success rate of vesiculoscope access through the neck of the seminal vesicle is influenced by two factors. First, the neck of the seminal vesicle is closest to the ejaculatory duct. Many scholars have reported that the openings of bilateral ejaculatory ducts are at 5 and 7 o’clock, 2 to 3 mm on both sides of the opening of the prostatic vesicle (Liao et al., 2017; Shao et al., 2018b; M. S. Wang et al., 2015). Li et al. (2019) dissected specimens of the normal ejaculatory duct area and reported that the horizontal distance from the apex of the seminal caruncle to the left and right openings of the ejaculatory duct is (0.87 ± 0.10) mm, while the vertical distance is (1.36 ± 0.16) mm and (1.36 ± 0.15) mm, respectively. Second, low-energy holmium laser burning at 5 and 7 o’clock on the neck of the vesicle can remove the unidirectional villi covering the opening of the ejaculatory duct, making it easy to reveal the ejaculatory duct opening during pressurized water injection.
A question can be raised as to whether the holmium laser burning the villi around the opening of the ejaculatory duct will cause damage to the ejaculatory duct, lead to urine reflux, or narrow the ejaculatory duct again (and other side effects) (Modgil et al., 2016). The ejaculatory duct can be divided into three segments: the proximal and middle duct walls have intact muscle layers, and, at the end of the ejaculatory duct, only a bundle of longitudinal musclefibres occasionally surround the distal segment, with no anti-reflux anatomical structure. Instead, the anti-reflux of the ejaculatory duct is that the ejaculatory duct, seminal vesicle gland and vas deferens are always in a state of high pressure. In this study, hydrostatic pressure measurements were performed at these three parts of the ejaculatory duct during surgery. The hydrostatic pressures of these three parts were all above 100 cm H2O, which greatly reduces the possibility of urine reflux into the seminal vesicle glands. In addition, in the follow-up observation of this group of cases for more than 2 years, there was no incidence of epididymitis after seminal vesiculoscopy, indicating that seminal vesiculoscope technology does not cause damage to the adjacent seminal vesicle glands or related organs, regardless of whether the wall is broken in the vesicle or through the neck.
As the success rate of breaking through the neck of the prostatic vesicle is much higher than that of breaking through the wall of the prostatic vesicle, the effective rate and symptom recurrence rate of breaking through the neck of the prostatic vesicle in the treatment of seminal vesicle adenitis are better than that of breaking through the wall of the prostatic vesicle. This is because treatment is limited to conventional antibacterial treatment in cases where the wall of the prostatic vesicle is not broken, which reduces the effective rate and increases the recurrence rate.
In this study, the dividing line of the size of the prostatic vesicle was set as 5 mm. This was based on existing studies that reported that the average size of the prostatic vesicle is 6.7 ± 1.7 mm (M. S. Wang et al., 2015) or that the maximum size of the prostatic vesicle is 9.3 mm, the minimum is 2.6 mm, and the average is 4.8 ± 2.0 mm (Zhu et al., 2018). However, the latter findings were the result of research conducted on cadavers and surgical specimens, and the volume may be slightly smaller than in vivo.
There are some limitations should be considered. First, our study only focused on the patients who received seminal vesicular endoscopic treatment for chronic seminal vesicular adenitis in our hospital, and there were certain limitations in the study population. In the future, a large number of multicenter studies are needed to confirm the conclusions of this study. Second, the surgeon’s experience and learning curve to some extent affects the success of surgery, resulting in some potential bias.
Conclusion
Compared with entering the seminal vesicle through the wall of the prostatic vesicle, the endoscopic method of entering via the neck of the prostatic vesicle has the characteristics of high success rate, short operation time, and good surgical effect. It is therefore worthy of promotion and application in the clinical practice of seminal vesiculoscopy.
Footnotes
Authors’ Contributions: Z.T. and Z.T.B. conceived of the study, and L.K.L., N.Y.H., L.Y.F., and H.Y.W. participated in its design and coordination. Z.J. and W.R. helped to draft the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
ORCID iD: Rui Wang  https://orcid.org/0000-0002-8784-8412
https://orcid.org/0000-0002-8784-8412
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
References
- Chen R., Wang L., Sheng X., Piao S. G., Nian X. W., Cheng X., Zhou T., Li H. Z., Liu Y. W., Chen G. H., Zhang C. L., Kong D. P., Xiao G. A., Lu X., Jia Z. Y., Liu Z. Y., Sun Y. H. (2018). Transurethral seminal vesiculoscopy for recurrent hemospermia: Experience from 419 cases. Asian Journal of Andrology, 20(5), 438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., Chen C. S. (2018). Transurethral seminal vesiculoscopy acts as a therapeutic investigation for intractable hemospermia: Step-by-step illustrations and single-surgeon experience. International Journal of Urology, 25(6), 589–595. [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Xu Y., Liu C., Xiao Z. M., Luo B. H., Xu G. W., Wu K. C., Zhong S. Z., Ouyang J. (2019). Anatomical study of the seminal vesicle system for transurethral seminal vesiculoscopy. Clinical Anatomy, 32(2), 244–252. [DOI] [PubMed] [Google Scholar]
- Liao L. G., Li Y. F., Zhang Y., Li K., Zhu T., Li B. J., Wang Q., Liu X. D., Luo Y., Zhou B., Jiang J. (2019). Etiology of 305 cases of refractory hematospermia and therapeutic options by emerging endoscopic technology. Scientific Reports, 9(1), 5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L. G., Li Y. F., Zhu T., Liu X. D., Zhang Y., Li B. J., Li K., Jiang J., Luo Y. (2017). Clinical analysis of 216 cases of refractory hematospermia diagnosed and treated by seminal duct endoscopy. Journal of Clinical Urology, 32(1), 26–38. [Google Scholar]
- Modgil V., Rai S., Ralph D. J., Muneer A. (2016). An update on the diagnosis and management of ejaculatory duct obstruction. Nature Reviews Urology, 13(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Etzell J., Turek P. J. (1996). Normal human ejaculatory duct anatomy: A study of cadaveric and surgical specimens. Urology Journal, 155(5), 1639–1642. [DOI] [PubMed] [Google Scholar]
- Shao J. C., Zeng Z. J., Wang X., Liao R. (2018. a). [Distribution of ejaculatory duct openings and the method of entering the vesiculoscope into the seminal vesicle]. Zhonghua Nan Ke Xue, 24(8), 686–689. [PubMed] [Google Scholar]
- Shao J. C., Zeng Z. J., Wang X., Liao R. (2018. b). A preliminary study on the distribution of ejaculatory duct openings and the approach of seminal vesicle endoscopy. Chinese Journal of Andrology, 24(8), 686–689. [PubMed] [Google Scholar]
- Wang M. S., Zhou T. Y., Zhang Y., Luo Y., Li K., Huang Z. M., Liu X. D., Sun Z. Y., Jiang J. (2015). Applied anatomy and MRI imaging characteristics of the distal region of the seminal duct. Journal of the Third Military Medical University, 37(23), 2373–2377. [Google Scholar]
- Wang R., Zhang W. X., Zhang T. B., Li R., Wang C. L., Song X. H., Yang Y. L., Li X. Y. (2016). Seminal vesiclescopy treatment of 64 cases of seminal vesiculitis manifested by hemospermia. Chinese Journal of Andrology, 22(4), 335–338. [PubMed] [Google Scholar]
- Zhao J., Zhai X. Q., Li H. C., Chong T. (2016). [Seminal vesiculoscopy in the treatment of refractory hemospermia and ejaculatory duct obstruction]. Zhonghua Nan Ke Xue, 22(7), 630–634. [PubMed] [Google Scholar]
- Zhu G. Y., Liu D. C., Zhou J. H., Dong B. Z., He H. G., Liang Q., Zhou X. J., Fan T. J., Chen B., Zhang J. J. (2018). Anatomical characteristics of the seminal vesicles and seminal duct and its guiding significance in clinical endoscopic surgery. Chinese Journal of Andrology, 24(9), 802–806. [Google Scholar]