Abstract
Current management of moderate-to-severe psoriasis may be heterogeneous between European countries, probably due to differences in the organization of care. The aim of this study was to compare the utilization of systemic treatments for psoriasis between 2 countries. All adults with psoriasis who were registered in the French (SNDS) and the Dutch (VEKTIS) national health insurance databases between 2012 and 2016 were eligible for inclusion. In France, 105,035 (15%) of 684,156 patients and, in the Netherlands, 37,405 (28.6%) of 130,822 patients received at least a systemic agent. In France, the proportion of patients treated with systemic agents was constant, while the type of drugs dispensed shifted from non-biological to biological agents. In the Netherlands, the first systemic treatment was methotrexate and, in France, acitretin. In France, the choice of the first biologic was much more variable than it was in the Netherlands, where a large proportion of patients were dispensed ustekinumab. This study highlights discrepancies between France and the Netherlands concerning the choice of first non-biologic agent and first biologic agent for patients with psoriasis. These discrepancies may be due to differences in the healthcare systems between the 2 countries.
Key words: chronic disease, psoriasis, therapeutic, national health insurance
Psoriasis is a chronic inflammatory disease of the skin, with a prevalence ranging from 0.9% (USA) to 8.5% (Norway) (1). The global level of severity of psoriasis usually fluctuates around the same level for a given patient (2). The therapeutic approach to psoriasis includes topical treatments as a single strategy in the management of minor forms (3). Nevertheless, approximately 10–20% of people with psoriasis have a moderate-to-severe form, requiring phototherapy, non-biologic systemic agents (such as ciclosporin, methotrexate, acitretin or fumaric acid esters (FAE)) or biologic agents (with a higher efficacy for biologic than non-biologic agents (4, 5)). Biologic agents and targeted therapies have been developed for psoriasis over the past 2 decades, and at least 10 have been approved by the US Food and Drug Administration (FDA): tumour necrosis factor (TNF) inhibitors, interleukin (IL)-12/23 and IL-17 inhibitors, apremilast and, more recently, IL-23 inhibitors. Taking into account the huge number of systemic agents available for psoriasis, the management of the disease must define the most effective therapeutic strategy with the best safety profile drug for a given patient. Until 2020, national or European guidelines did not include treatment algorithms, except for the National Institute for Health and Care Excellence (NICE) clinical guidelines in the UK, which proposed methotrexate as the first choice of non-biologic systemic agents (www.nice.org.uk) (6, 7). Moreover, in Europe, some eligibility criteria (selecting high-need patients) are needed before being considered for biological agents. In the absence of strong recommendations, the factors that may influence treatment choices are both intrinsic (e.g. age, sex, comorbidities) and extrinsic to patients (e.g. prescriptions habits, therapeutic innovation, organization of care, pharmaceutical marketing, and/or cultural factors) (8). At the population level, intrinsic factors might be the same between European countries. Physician’s knowledge regarding the benefit-to-risk balance of each drug also might not differ between European countries. However, this study hypothesized that current management of moderate-to-severe psoriasis is heterogeneous between European countries. Therefore, the aim of this study was to compare the choice of systemic treatments for patients with psoriasis between France (which had no available guidelines during the study period, and which dispensed biologic agents in private pharmacies) and the Netherlands (with guidelines available during the study period, and which had limited dispensing of biologic agents in hospital pharmacies).
SIGNIFICANCE
This study highlights important discrepancies between France and the Netherlands concerning the care of patients with psoriasis with non-biologic and biologic drugs. The implementation of national guidelines would be a key element in reaching evidence-based medicine in the field of psoriasis.
METHODS
Data sources
The French nationwide cohort study was based on health administrative data obtained from the French National Health Insurance scheme, covering approximately 67 million individuals linked with the national hospital discharge database (SNDS-PMSI) by means of a unique anonymous identifier, as described previously (9–11).
The Netherlands nationwide cohort study was based on health administrative data obtained from the Netherlands national health insurance scheme, covering approximately 17 million individuals (VEKTIS). In the Netherlands, inhabitants have an obligatory health insurance and immediate access to the healthcare system at all times. All diagnoses and treatments provided in the hospital are coded according to a national financial coding system. Insurance companies collect all hospital claims, which are registered centrally by the national centre for healthcare data and information from the national insurance companies (Vektis B.V., Zeist, The Netherlands).
Study population and follow-up procedures
All adults (≥ 18 years of age) with psoriasis registered in the SNIIRAM and in VEKTIS between 1 January 2012 and 31 December 2016, were eligible for inclusion. Psoriasis was defined as having at least 2 prescriptions of topical vitamin D derivatives (ATC D05AX, the recommended first-line treatment for psoriasis) within a 2-year period, as used robustly in previous studies (12, 13). The date of inclusion in the study cohort (index date) was defined as the date of the second fulfilment of the topical vitamin D prescription. The first prescription of topical vitamin D derivatives was also recorded between 1 January 2010 and 31 December 2011. Participants were followed up until 31 December 2016 or the censorship date (death).
Patients with at least one prescription of a systemic medication for psoriasis were identified. These treatments included non-biologic medications (acitretin – ATC D05BB02; cyclosporine – L04AD01; FAEs – D05BX51; phototherapy – UVA and UVB), and methotrexate – L01BA01 or L04AX03) and biologics/biosimilars (etanercept – L04AB01; infliximab – L04AB02; adalimumab – L04AB04; ustekinumab – L04AC05; ixekizumab – L04AC13;, and secukinumab – L04AC10) or apremilast (L04AA32).
Objectives and outcomes
This aims of this study were: (i) to compare the proportion of healthcare users with psoriasis over time and between France and the Netherlands; (ii) to compare the proportion of psoriasis healthcare users treated with systemic agents over time and between France and the Netherlands; (iii) to compare the first choice of non-biologic systemic agents and biologic agents for systemic therapy and biologic-naïve patients with psoriasis over time and between France and the Netherlands; and (iv) to compare the more frequent sequences allocated to each patients between France and the Netherlands.
Definitions
Systemic therapy-naïve patients were defined as those who had fulfilled a first prescription of systemic medication for psoriasis after 2 years without any treatments.
Biologic-naïve patients were defined as those who had fulfilled a first prescription of available biologic agents, i.e. etanercept, infliximab, adalimumab, ustekinumab, apremilast, ixekizumab and secukinumab, after 2 years without any biologics during the study period.
For each systemic therapy-naïve and biologic-naïve patient, all the different systemic treatment sequences were defined during the period of follow-up. The duration of a systemic treatment was defined as the length of time from initiation to discontinuation. Discontinuation of treatment was defined as a period of more than 90 days without fulfilment of a prescription for the same treatment after the period covered by the previous prescription. The treatment regimen differed markedly from one systemic treatment to another. In fact, the period covered by a prescription was 30 days for non-biologic systemic medications, etanercept, adalimumab, apremilast, ixekizumab and secukinumab, 56 days for infliximab, and 84 days for ustekinumab. Exposure to combinations of drugs (co-prescription) was defined as a period of less than 30 days between the prescription of 2 different systemic drugs and the fulfilment of another prescription for both drugs in the following 90 days.
Statistical analysis
For descriptions of the study population, qualitative variables were reported as the number (percentage). Quantitative variables were reported as the median and/or the mean ±standard deviation (SD) or were converted to qualitative variables.
The proportion of healthcare users with psoriasis was described over time in France and in the Netherlands, as well as the proportion of psoriasis healthcare users treated with systemic agents. First choice of systemic treatments was described in France and in the Netherlands. The top 5 sequences of non-biologic and biologic treatments, respectively, allocated to each patient with psoriasis in France and in the Netherlands were reported.
Data were analysed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patients with psoriasis
In the French cohort, a total of 684,156 patients were identified as having psoriasis (mean ± SD age: 54.8±17; males: 53.7%, mean follow-up time: 3.2 ± 1.5 years, death during the follow-up period: 6.3%); 105,035 (15%) of these patients had fulfilled at least one prescription for a systemic medication used to treat psoriasis (Table I, Fig. S11).
Table I.
Patients’ characteristics
| Patients’ characteristics | French SNDS data n = 684,156 | The Netherlands VEKTIS data n = 130,821 |
|---|---|---|
| Males, n (%) | 367,759 (53.7) | 68,980 (52.7) |
| Age, years (mean ± SD; median [IQR]) | 54.8 ± 16.9; 5.7 [42–67] | 53.9 ± 17; 55 [41–66] |
| Period of follow-up (mean ± SD; median [IQR]) | 3.2 ± 1.5; 3.6 [1.9–4.6] | 3.6 ± 1.6; 4.5 [2.5–5.0] |
| Death, n (%) | 42,944 (6.3) | 11,650 (8.9) |
| At least one systemic drug for psoriasis, n (%) | 105,035 (15) | 37,405 (29) |
| During the study period, n (%) | ||
| 1 systemic drug | 76,544 (11) | 27,460 (21) |
| 2 systemic drugs | 20,655 (3) | 7,047 (5.4) |
| 3 systemic drugs | 5,725 (1) | 2,081 (1.6) |
| ≥ 4 systemic drugs | 2,111 (0.3) | 623 (0.5) |
SD: standard deviation; IQR: interquartile range.
In the Netherlands cohort, a total of 130,822 patients were identified as having psoriasis (mean ±SD age: 53.9 ±17 years; males: 52.7%, mean follow-up time: 3.6 ±1.6 years, death during the follow-up period: 8.9%); 37,405 (28.6%) of these patients had fulfilled at least one prescription for a systemic medication used to treat psoriasis (Table I, Fig. S11).
During the study period, the proportion of healthcare users with psoriasis was constant over time in France and in the Netherlands, as well as the proportion of psoriasis healthcare users treated with systemic agents. In France, the proportion of psoriasis healthcare users treated with biologic agents increased by 70% within a 5-year period, whereas the proportion of psoriasis healthcare users treated with non-biologic agents decreased by 15%. Finally, the proportion of psoriasis healthcare users who used both non-biologic and biologic agents during the same period was multiplied by 3 (an increase of 200%). In the Netherlands, no change regarding the proportion of each type of systemic treatment was observed during the same period (Table II).
Table II.
Patients using systemic treatments each year and for the all study period
| Total n (%) | 2012 n (%) | 2013 n (%) | 2014 n (%) | 2015 n (%) | 2016 n (%) | |
|---|---|---|---|---|---|---|
| France | ||||||
| Psoriasis healthcare users | 684,156 | 338,950 | 339,109 | 350,042 | 353,700 | 329,992 |
| Patients receiving systemic treatments | 105,035 (15) | 27,116 (8) | 37,302 (11) | 42,005 (12) | 42,444 (12) | 42,899 (13) |
| Only non-biologic treatments | 85,778 (82) | 23,850 (88) | 31,471 (84) | 34,262 (82) | 34,022 (80) | 32,768 (76) |
| Both non- and biologic treatments | 11,450 (11) | 1,015 (3) | 1,826 (5) | 2,212 (5) | 2,469 (6) | 3,220 (9) |
| Only biologic treatments | 7,807 (7) | 225 (9) | 4,005 (11) | 5,164 (13) | 5,584 (13) | 6,614 (15) |
| The Netherlands | ||||||
| Psoriasis healthcare users | 130,822 | 62,885 | 64,565 | 63,166 | 60,804 | 58,112 |
| Patients receiving systemic treatments | 37,405 (29) | 10,671 (17) | 11,393 (18) | 11,848 (19) | 11,162 (18) | 11,149 (19) |
| Only non-biologic treatments | 30,672 (82) | 8,161 (77) | 8,695 (76) | 9,020 (76) | 8,329 (74) | 8,265 (74) |
| Both non- and biologic treatments | 5,237 (14) | 792 (7) | 803 (7) | 862 (7) | 839 (8) | 883 (8) |
| Only biologic treatments | 1,496 (4) | 1,718 (16) | 1,895 (17) | 1,966 (17) | 1,994 (18) | 2,001 (18) |
Non-biologic treatments: phototherapy, acitretin, ciclosporin, methotrexate. Biological treatments: Biologics and small molecules (etanercept, infliximab, adalimumab, ustekinumab, apremilast, ixekizumab and secukinumab).
At the drugs level, dispensing of acitretin decreased over time, both in France and in the Netherland (by 25% and 40%, respectively) as did the prescription of phototherapy (a decrease by 30% and 15%) (Table SI1). Dispensing of methotrexate increased by 25% in France, whereas it was constant in the Netherlands. The dispensing of biologic agents increased over time in France, by 25%, 50% and 250% for etanercept, adalimumab and ustekinumab. In the Netherlands, adalimumab dispensing was constant over time, whereas there was a decrease by 25% for etanercept and an increase by 230% for ustekinumab.
First choice of systemic treatments
The first choice of non-biologic systemic agents naïve-psoriasis patients, between 2012 and 2016, in France was acitretin (18,653/38,649, 48%; mean duration of the first sequence 167 days), then methotrexate (13,593/38,649, 35%; duration of the first sequence 314 days) (Table SI1). In the Netherlands, the first choice was methotrexate (4,330/7,141, 56%; duration of the first sequence 139 days), far ahead acitretin (1,251/7,141, 16%; duration of the first sequence 99 days) (Table III).
Table III.
First choice of non-biologic treatments for naïve-psoriasis patients
| Non-biologic treatments | Number | % | Duration (days) |
|---|---|---|---|
| France | |||
| Acitretin | 18,653 | 48 | 166.6 |
| Acitretin – Phototherapy | 1,391 | 4 | |
| Acitretin – Methotrexate | 82 | 0 | |
| Ciclosporin | 1,001 | 3 | 206.9 |
| Methotrexate | 13,593 | 35 | 314.0 |
| Methotrexate – Phototherapy | 103 | 0 | |
| Phototherapy | 3,826 | 10 | |
| The Netherlands | |||
| Acitretin | 1,251 | 16 | 98.6 |
| Acitretin – Phototherapy | 256 | 3 | 81.1 |
| Acitretin – Methotrexate | 11 | 0 | 110.1 |
| Ciclosporin | 594 | 8 | 112.8 |
| Fumaric acid ester | 41 | 0 | 43.9 |
| Methotrexate | 4,330 | 56 | 138.7 |
| Methotrexate – Phototherapy | 452 | 6 | 96.6 |
| Phototherapy | 206 | 3 | 25.7 |
The first choice of biologic agents naïve-psoriasis patients between 2012 and 2016 in France was adalimumab (3,850/11,257, 34%; duration of the first sequence 357 days), then etanercept (2,665/11,257, 24%; duration of the first sequence 363 days) and ustekinumab (2,074/11,257, 18%; duration of the first sequence 451 days) (Table IV). In the Netherlands, the first choice was ustekinumab (1,234/3,184, 39%; duration of the first sequence 117 days), then adalimumab (1,074/3,184, 34%; duration of the first sequence 94 days) in the same period.
Table IV.
First choice of biologic agents for naïve-psoriasis patients
| Biological agents | n (%) | Duration (days) | Co-prescription per molecule n (%) | Duration (days) |
|---|---|---|---|---|
| France | ||||
| Adalimumab | 3,850 (34) | 357 | 917 (24) | 367 |
| Apremilast | 1,403 (12) | 51 | 224 (16) | 54 |
| Etanercept | 2,665 (24) | 363 | 721 (27) | 356 |
| Infliximab | 930 (8) | 369 | 186 (19) | 388 |
| Ixekizumab | 4 | 28 | 0 | – |
| Secukinumab | 331 (3) | 92 | 36 (11) | 80 |
| Ustekinumab | 2,074 (18) | 451 | 417 (20) | 397 |
| The Netherlands | ||||
| Adalimumab | 1,077 (34) | 94 | 638 (59) | 120 |
| Apremilast | 18 (0.5) | 47 | 0 | – |
| Etanercept | 605 (19) | 108 | 319 (59) | 161 |
| Infliximab | 223 (7) | 126 | 63 (28) | 173 |
| Ixekizumab | 0 | – | – | – |
| Secukinumab | 27 (0.5) | 55 | 0 | – |
| Ustekinumab | 1,234 (39) | 117 | 108 (9) | 94 |
In France, approximately one-quarter of patients with psoriasis using biologic agents had a co-prescription, i.e. combinations of both non-biologic and biologic treatments, at baseline (from 20% for ustekinumab to 27% for etanercept), whereas the co-prescription was two-fold for anti-TNF agents in the Netherlands and less than 10% for ustekinumab (Table IV).
Sequences allocated to each patient
Figs 1 and 2 highlight the top 5 sequences of non-biologic and biologic treatments, respectively, allocated to each patient with psoriasis in France and in the Netherlands.
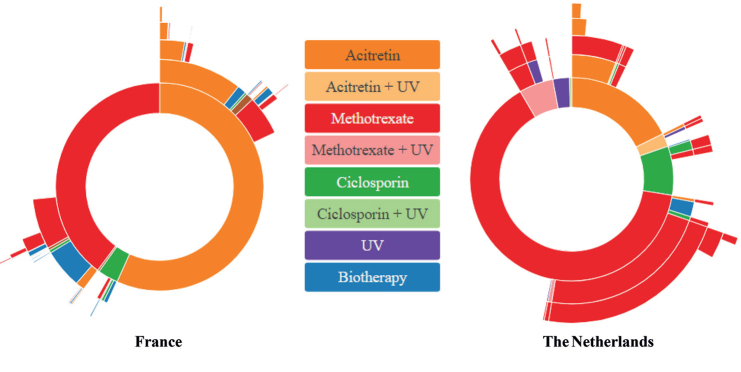
Fig. 1.
Top 5 sequences of non-biologic therapies allocated to each patient with psoriasis. For each country, France (left) and the Netherlands (right), the inner circle shows the first non-biologic systemic treatment that the patient took, the second circle shows the second medication, and so forth, until the fifth medication sequence. UV: ultraviolet.
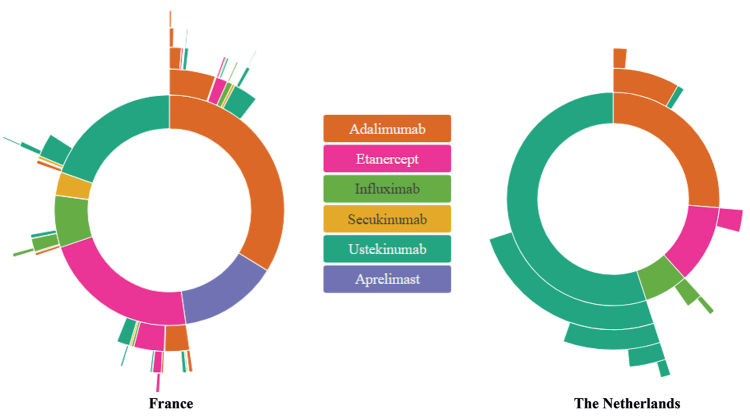
Fig. 2.
Top 5 sequences of biologic therapies allocated to each patient with psoriasis. For each country, France (left) and the Netherlands (right), the inner circle shows the first biologic systemic treatment that the patient took, the second circle shows the second medication, and so forth until the fifth medication sequence.
In the Netherlands, multiple sequences with the same drugs (methotrexate and ustekinumab for non-biologic and biologic treatments, respectively) are delivered to patients, whereas, in France, patients received fewer sequences during the same follow-up. The proportion of patients who switched from non-biologic to biologic agents was higher in France compared with the Netherlands (Fig. 1). In both countries, methotrexate was most often used prior to starting a biologic agent. However, in France, less than 10% of patients with psoriasis went on a biologic agent following acitretin only (Fig. 1). In France, the choice of the first sequence of biologics was much more disparate than in the Netherlands, and the following biologic drug is more likely to differ from the first drug (Fig. 2). Adalimumab was the first biologic agent in approximately one-quarter of patients in both countries. In contrast, ustekinumab represented more than half of the Dutch patients receiving their first biologic drug between 2012 and 2016 in The Netherlands. During the current study period, apremilast was not reimbursed in the Netherlands.
DISCUSSION
The current study identified important differences concerning the care of patients with psoriasis with non-biologic and biologic drugs between France and the Netherlands. Over a 5-year period, the proportion of patients treated with systemic agents was constant; however, the type of drugs dispensed shifted from non-biologic to more biologic agents in France, associated with a 3-fold increase in the proportion of patients who received both non-biologic and biologic agents during the study period. The first systemic treatment was methotrexate in the Netherlands, and it was constant over time; whereas in France, acitretin was the first systemic treatment prescribed for psoriasis event if the dispensing of methotrexate increased by 25% over time. In France, the choice of the first biologic was much more variable than it was in the Netherlands, where a large proportion of patients were dispensed ustekinumab.
Those treatment differences could, in part, be explained by the specific Dutch guideline on psoriasis, first published in 2003 (14) and updated at least twice (15, 16). In 2003, Dutch dermatologists proposed methotrexate and ciclosporin as the oral treatments for psoriasis, because of a higher efficacy compared with retinoids and fumarates. A specific French guideline was published for the first time in 2019, and proposed methotrexate as the first line of systemic treatment (17). The increase in dispensing of methotrexate in France found in the current study might be related to the labelling of biologics use for psoriasis in France (in 2004): prescription of methotrexate is mandatory to be reimbursed of biologics dispensing. This hypothesis has been discussed in a cross-sectional study from Denmark, where almost all patients receiving biologic agents had been treated with methotrexate (18). It could also be a consequence of updated guidelines, first in 2012 in UK and, secondly, in 2015 at the European level, which proposed methotrexate as the first-choice treatment. In 2011, Dutch guidelines proposed adalimumab or etanercept as first-line biologic agents, because the long-term efficacy and safety profile of ustekinumab were less known compared with those of anti-TNF agents (15). Despite an update of the Dutch guidelines in 2017, adalimumab and ustekinumab were the first choice of biologic agent, with equal proportions (16). Ustekinumab also showed an increase in use after it was introduced in the 2010s in other countries (19). In the Netherlands, biologics can be prescribed only by a medical specialist working in a hospital setting and dispensed by a hospital pharmacist, whereas, in France, prescription of biologics can be renewed by a medical specialist with a private practice and dispensed by a private pharmacist. This last point could, in part, explain the greater variability in the choice of the first sequence of biologics in France.
Implementation of Dutch guidelines was assessed in 2008 using an anonymous postal survey sent to 357 members of the Dutch Society for Dermatology and Venereology (20). A high self-reported awareness and familiarity with the Dutch psoriasis guidelines was confirmed (overall response rate 46%).
The British, Canadian, European, and American psoriasis guidelines were compared for the management of moderate-to-severe plaque psoriasis, and shared the majority of the recommendations (21). Moreover, the majority of recommendations for the use of a drug were strong recommendations based on high level of confidence in the studies’ design and a high level of evidence quality for each outcome (22). As an example, despite a high level of evidence for using methotrexate as a first-line of systemic treatment for psoriasis including an adherence to acitretin lower than methotrexate (23), acitretin was the first systemic treatment prescribed in France. The first choice of methotrexate was mandatory in 2003 according to the Dutch Society for Dermatology and Venereology, in 2012 according to the British Association of Dermatologists, in 2015 at the European level. Thus, national guidelines are needed, because they would probably have a higher impact on change in physicians’ practice.
Finally, this study highlighted a start and stop manner of treatment in the Netherlands, to minimize cumulative toxicity. This may explain why patients were re-using the drug they had used in the past. In contrast to non-biologic drugs, most Dutch hospitals have restricted budgets for the use of biologic agents and the budgets are often drug specific. The first consequence may be that Dutch patients responding well to the biologic drug are discontinued in order to minimize the impact on the financial budget. The second consequence might be that new drugs entering the market have not been negotiated in the running budget, which implies that patients are started on the same drug again.
Study strengths and limitations
Strengths of the present study include the large and unbiased sample size from 2 national representative databases, as discussed previously, and the 5-year study period. Among the study limitations is the absence of medical chart review to easily identify psoriasis cases. However, this study used a definition based on the dispensation of at least 2 topical vitamin D derivatives within a 2-year period. Topical vitamin D derivatives are the first-line treatment of psoriasis (24) and the current study definition has been used and validated in the Danish health insurance database (12). Thus, the number of psoriasis cases in this study is probably under-estimated; however, on the one hand this misclassification should not be differential between the type of drugs, and on the other hand, we are confident about the specificity of our definition. Another limitation of this study is the absence of prescriber characteristics (age, type of activity: only private, hospital or both, special interest for psoriasis or not), which did not allow us to perform sub-group analyses. Finally, this study did not include data on the amount of biologic treatment across the 2 countries.
Conclusion
These data highlight, in a real-life setting, important discrepancies between France and the Netherlands, concerning the care of patients with psoriasis with non-biologic and biologic drugs, especially regarding the choice of the first non-biologic, then biologic agent. A major difference between the 2 countries was the endorsement of national guidelines by the Dutch Society of Dermatology. The implementation of national guidelines would be a key element for acheiving evidence-based medicine in the field of psoriasis.
ACKNOWLEDGEMENTS
This study was approved by the French data protection agency Commission Nationale de l’Informatique et des Libertés (regulatory decision DE-2015-165).
ES was supported by the French Society of Dermatology and by the French Teachers College, section dermatology CEDEF.
CB, MM, AW, JR were employees of the French National Health Insurance Fund. The present paper represents the opinions of the authors and does not necessarily reflect the position of their employers.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 2.Nijsten T, Looman CWN, Stern RS. Clinical severity of psoriasis in last 20 years of PUVA study. Arch Dermatol 2007; 143: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 3.Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev 2013: CD005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI, et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris – Update 2015 – Short version – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29: 2277–2294. [DOI] [PubMed] [Google Scholar]
- 5.Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2020; 1: CD011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. J Invest Dermatol 2015; 135: 2641–2648. [DOI] [PubMed] [Google Scholar]
- 7.Nast A, Spuls PI, van der Kraaij G, Gisondi P, Paul C, Ormerod AD, et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris – update apremilast and secukinumab – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2017; 31: 1951–1963. [DOI] [PubMed] [Google Scholar]
- 8.Sbidian E, Giboin C, Bachelez H, Paul C, Beylot-Barry M, Dupuy A, et al. Factors associated with the choice of the first biologic in psoriasis: real life analysis from the Psobioteq cohort. J Eur Acad Dermatol Venereol 2017; 31: 2046–2054. [DOI] [PubMed] [Google Scholar]
- 9.Weill A, Dalichampt M, Raguideau F, Ricordeau P, Blotière P-O, Rudant J, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ 2016; 353: i2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuppin P, Rudant J, Constantinou P, Gastaldi-Ménager C, Rachas A, de Roquefeuil L, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique 2017; 65 Suppl 4: S149–S167. [DOI] [PubMed] [Google Scholar]
- 11.Tuppin P, Drouin J, Mazza M, Weill A, Ricordeau P, Allemand H. Hospitalization admission rates for low-income subjects with full health insurance coverage in France. Eur J Public Health 2011; 21: 560–566. [DOI] [PubMed] [Google Scholar]
- 12.Egeberg A, Mallbris L, Gislason GH, Skov L, Hansen PR. Risk of multiple sclerosis in patients with psoriasis: a Danish nationwide cohort study. J Invest Dermatol 2016; 136: 93–98. [DOI] [PubMed] [Google Scholar]
- 13.Sbidian E, Mezzarobba M, Weill A, Coste J, Rudant J. Persistence of treatment with biologics for patients with psoriasis: a real-world analysis of 16 545 biologic-naïve patients from the French National Health Insurance database (SNIIRAM). Br J Dermatol 2019; 180: 86–93. [DOI] [PubMed] [Google Scholar]
- 14.Spuls PI, Tuut MK, van Everdingen JJE, de Rie MA, Werkgroep Psoriasis van de Nederlandse Vereniging voor Dermatologie en Venereologie. [The practice guideline ‘Photo(chemo)therapy and systemic therapy in severe chronic plaque-psoriasis’]. Ned Tijdschr Geneeskd 2004; 148: 2121–2125 (in Dutch). [PubMed] [Google Scholar]
- 15.Zweegers J, de Jong EMGJ, Nijsten TEC, de Bes J, te Booij M, Borgonjen RJ, et al. Summary of the Dutch S3-guidelines on the treatment of psoriasis 2011. Dutch Society of Dermatology and Venereology. Dermatol Online J 2014; 20: doj_21769. [PubMed] [Google Scholar]
- 16.van der Kraaij GE, Balak DMW, Busard CI, van Cranenburgh OD, Chung Y, Driessen RJB, et al. Highlights of the updated Dutch evidence- and consensus-based guideline on psoriasis 2017. Br J Dermatol 2019; 180: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amatore F, Villani A-P, Tauber M, Viguier M, Guillot B, Psoriasis Research Group of the French Society of Dermatology (Groupe de Recherche sur le Psoriasis de la Société Française de Dermatologie). French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol 2019; 33: 464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loft N, Skov L, Bryld LE, Gislason G, Egeberg A. Treatment history of patients receiving biologic therapy for psoriasis – a Danish nationwide study. J Eur Acad Dermatol Venereol 2017; 31: e362–e363. [DOI] [PubMed] [Google Scholar]
- 19.Han JH, Lee JH, Han KD, Seo H-M, Bang CH, Park YM, et al. Epidemiology and medication trends in patients with psoriasis: a nationwide population-based cohort study from Korea. Acta Derm Venereol 2018; 98: 396–400. [DOI] [PubMed] [Google Scholar]
- 20.Wakkee M, Lugtenberg M, Spuls PI, de Jong EM, Thio HB, Westert GP, et al. Knowledge, attitudes and use of the guidelines for the treatment of moderate to severe plaque psoriasis among Dutch dermatologists. Br J Dermatol 2008; 159: 426–432. [DOI] [PubMed] [Google Scholar]
- 21.Ighani A, Partridge ACR, Shear NH, Lynde C, Gulliver WP, Sibbald C, et al. Comparison of management guidelines for moderate-to-severe plaque psoriasis: a review of phototherapy, systemic therapies, and biologic agents. J Cutan Med Surg 2019; 23: 204–221. [DOI] [PubMed] [Google Scholar]
- 22.Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol 2017; 177: 628–636. [DOI] [PubMed] [Google Scholar]
- 23.Dommasch ED, Lee MP, Joyce CJ, Garry EM, Gagne JJ. Drug utilization patterns and adherence in patients on systemic medications for the treatment of psoriasis: a retrospective, comparative cohort study. J Am Acad Dermatol 2018; 79: 1061–1068.e1. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev 2013; CD009481. [DOI] [PMC free article] [PubMed] [Google Scholar]