Abstract
Uraemic pruritus is one of the most bothersome symptoms in patients receiving haemodialysis. A total of 175 patients receiving maintenance haemodialysis, with 74 patients experiencing uraemic pruritus, were prospectively recruited to assess the influence of the phenotype of blood monocytes and various cytokines on uraemic pruritus. The phenotype of blood monocytes was determined by flow cytometry as classical (CD14++CD16−) monocytes, non-classical (CD14+CD16++) monocytes, and intermediate (CD14++CD16+) monocytes. Eight cytokines, including interleukin (IL)-2, interferon-γ, IL-12p70, IL-4, IL-5, IL-6, tumour necrosis factor-α, and IL-10, were simultaneously detected with a multiplex bead-based immunoassay. Multivariate linear regression analysis showed that a higher percentage of intermediate monocytes (effect estimate 0.08; 95% confidence interval 0.01–0.16) were independent predictors of a higher visual analogue scale score for pruritus intensity. No differences were noted for all 8 cytokines between patients with and without uraemic pruritus. The results of this study indicate that altered monocytic phenotypes could play a role in uraemic pruritus.
Key words: cytokines, CD14++CD16+ monocytes, haemodialysis, itch, intermediate monocytes, uraemic pruritus
Uraemic pruritus is one of the most common and bothersome symptoms in patients with end-stage renal disease (ESRD). It is usually severe and involves a large percentage of the body surface area, with chronic courses lasting for more than 1 year in most patients receiving haemodialysis (HD) (1–3). This persistent, debilitating symptom can significantly affect the quality of life in multiple aspects, including mood, sleep, and social relationships. However, the treatment options for uraemic pruritus are few (4–6).
The mechanisms of uraemic pruritus mostly remain unclear, and many hypotheses have been postulated, including xerosis, hyperparathyroidism, opioid system derangements, etc. (7–9). Evidence has indicated that immune dysregulation plays a central role in the pathophysiology of uraemic pruritus. Increased serum levels of high-sensitivity C-reactive protein, proinflammatory cytokines, and Th1-dominant immune activation have been reported to be associated with uraemic pruritus (10–14). Furthermore, uraemic pruritus is of prognostic importance for dialysis patients, and inflammation related to uraemic pruritus could lead to higher mortality (15, 16).
SIGNIFICANCE
Uraemic pruritus is one of the most common and bothersome symptoms in patients receiving haemodialysis, but its pathophysiology remains obscure. Altered human monocyte phenotypes have been implicated in many chronic inflammatory diseases. This study found that a higher percentage of intermediate monocytes (CD14++CD16+) independently predicts a higher visual analogue scale score for pruritus intensity. Eight cytokines, including interleukin-2, interferon-γ, interleukin-12p70, -4, -5, -6, tumour necrosis factor-α, and interleukin-10, showed no differences between patients with and without uraemic pruritus. The results indicate that altered monocytic phenotypes could play a role in uraemic pruritus.
Monocytes are key players in the immune system. Human peripheral blood monocytes can be classified into 3 subsets based on the surface expression of the lipopolysaccharide receptor (CD14) and the low-affinity Fcγ-III receptor (CD16); namely, classical (CD14++CD16−), intermediate (CD14++CD16+), and non-classical (CD14+ CD16++) monocytes (17, 18). Among them, intermediate monocytes have been reported to show the highest levels of major histocompatibility complex class II and produce the most tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 after being treated with lipopolysaccharides (19–21). In addition, intermediate monocytes are known to expand in multiple inflammatory and infectious conditions, including rheumatoid arthritis, asthma, peripheral arterial disease, and sepsis (22–25). However, the relationships between human monocyte phenotypes and uraemic pruritus have not been assessed.
The aims of this study were to assess the phenotype of blood monocytes using surface markers and to examine its relationship with the severity of uraemic pruritus in patients receiving HD. Furthermore, the study analysed 8 cytokines, including IL-2, interferon (IFN)-γ, IL-12p70, IL-4, IL-5, IL-6, TNF-α, and IL-10, using a multiplex bead-based immunoassay to elucidate the interactive behaviour of monocytes and cytokines to understand immune reactions in uraemic pruritus.
MATERIALS AND METHODS
Study participants
The immunity in ESRD (iESRD) study is a prospective multicentre cohort study investigating the effects of immunological factors on the long-term outcomes of patients receiving HD (26). The current study assessed the participants of the iESRD cohort who received HD in the Far Eastern Memorial Hospital, a tertiary medical centre in Taiwan. Eligible patients were at least 20 years old and had received maintenance HD for more than 3 months in December 2014. The patients received 3–5 h of HD 3 times a week with adequate dialysis doses. All study participants used biocompatible dialyzers. Exclusion criteria were: (i) primary skin disorders; (ii) active infection; (iii) malignancy; (iv) cholestatic liver disease; (v) acute hepatitis; or (vi) communication problems. The Institutional Review Board of the Far Eastern Memorial Hospital approved this study, and all participants provided written informed consent.
Pruritus evaluation
Uraemic pruritus was defined if the patient receiving HD had met any of the following criteria: (i) they were bothered by at least 3 episodes of pruritus within 2 weeks, with each episode of pruritus symptoms occurring a few times a day and lasting for a few minutes; or (ii) they had pruritus symptoms within 6 months, but with a lower frequency than in (i) (15, 27, 28). The participants marked the intensity of their itch on a 10-cm line, measuring the current general severity of pruritus using a visual analogue scale (VAS) from 0 to 10 (0=no pruritus, 10=worst pruritus imaginable) (29). The extent of the body surface area affected by pruritus was also evaluated (28).
Demographics and laboratory parameters
Demographic data, including age, sex, comorbid diseases, aetiology of ESRD, dialysis regimens, and duration of dialysis, were recorded for each participant. Venous blood samples were obtained in the fasting state before the patient’s midweek HD session, and were used to assess laboratory parameters, monocyte phenotypes, and blood levels of cytokines. All laboratory tests were performed in the central laboratory of the Far Eastern Memorial Hospital. The adequacy of dialysis doses, as assessed by Kt/V, was calculated using a single-compartment model (30, 31).
Monocyte differentiation panel
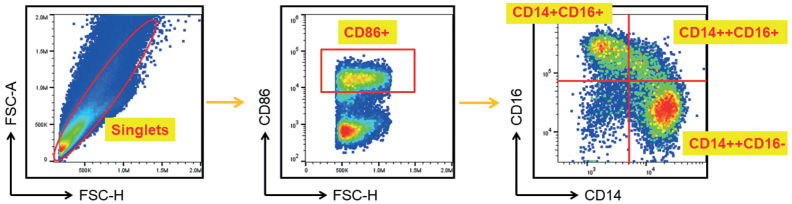
On the day of blood sampling, the mononuclear cell interphase was isolated by Ficoll-Paque PLUS gradient centrifugation according to the manufacturer’s instructions (GE Healthcare, South Plainfield, NJ, USA). Phycoerythrin-conjugated anti-CD86 antibody (clone IT2.2, eBioscience, San Diego, CA, USA) was used to gate the CD86+ monocytes. The surface expression of CD14 and CD16 on peripheral blood monocytes was analysed by staining with fluorescein isothiocyanate-conjugated anti-CD14 antibody (clone M5E2, BioLegend, San Diego, CA, USA) and allophycocyanin-conjugated anti-CD16 antibody (clone 3G8, eBioscience) for 30 min at 4°C in the dark. The gating strategy is shown in Fig. 1. Monocytes were classified into 3 subsets: classical (CD14++CD16−), intermediate (CD14++CD16+), and non-classical (CD14+CD16++) monocytes, according to the Nomenclature Committee of the International Union of Immunological Societies (17). All experiments were performed in the central laboratory of the Far Eastern Memorial Hospital and analysed using a Beckman Coulter MoFloTM-XDP multicolour flow cytometer (Beckman Coulter, Krefeld, Germany).
Fig. 1.
Flow cytometric analysis of monocyte subpopulations. Freshly isolated peripheral blood mononuclear cells were stained and analysed for monocyte subpopulations. The pan-monocyte marker CD86 was used to identify all monocytes. Subsequently, 3 monocyte subsets were identified based on the patterns of CD16 and CD14 expression. The following cell definitions were used: classical monocyte: CD14++CD16–; intermediate monocyte: CD14++CD16+; and non-classical monocyte: CD14+CD16+.
Multiplex bead-based cytokine analysis
Plasma samples were collected at the time of monocyte isolation and frozen at –80°C until cytokine analyses. The blood levels of the Th1/Th2 Essential Th1/Th2 Cytokine 6-Plex (IFN-γ, IL-12p70, IL-4, IL-5, IL-6, and TNF-α) with the addition of IL-2 and IL-10 were detected using Luminex-based multiplexed immunoassays (ProcartaPlex, eBioscience, USA) following the manufacturer’s protocol. The limit of detection was 0.8 pg/ml for IL-2, 0.2 pg/ml for IFN-γ, 0.04 pg/ml for IL-12p70, 1.5 pg/ml for IL-4, 0.3 pg/ml for IL-5, 0.4 pg/ml for IL-6, 0.4 pg/ml for TNF-α, and 0.1 pg/ml for IL-10. Acquisition was performed on a Luminex® 100/200™ analyzer (Luminex, Austin, TX, USA). Data analysis was performed using ProcartaPlex Analyst 1.0 (eBioscience, USA).
Statistical analysis
Statistical analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC, USA). The data are expressed as the means with standard deviations (SDs) for continuous variables and as numbers with percentages for categorical variables. The differences in the distributions of continuous variables and categorical variables were examined using the Wilcoxon rank-sum test and χ2 test, respectively. To examine the associations between the potential predictors and the intensity of uraemic pruritus, first, univariate linear regression models were used to obtain the effect estimate and 95% confidence intervals (95% CIs) of the influence of each variable on the VAS score for pruritus intensity. Then, age- and sex-adjusted multivariate linear regression models were used with the stepwise variable selection procedure to identify the predictors of uraemic pruritus. All the variables were initially included in the multivariate linear regression model, and the final regression model was then established by excluding variables with a p-value > 0.05 one at a time until the remaining variables were significant. A 2-sided p-value ≤ 0.05 was considered statistically significant.
RESULTS
A total of 175 patients receiving maintenance HD participated in the study. Table I shows the demographic characteristics of the study participants. The mean age of the participants was 60.5 years, and 43% were female. There were 74 patients (42.3%) who had uraemic pruritus, with a mean VAS score of 5.8. Among patients with uraemic pruritus, 68.9% (51/74) had more than 25% of their body surface area affected by pruritus. The median duration of pruritus symptoms was 12 months (first quartile, 3 months; third quartile, 60 months). The clinical characteristics and laboratory parameters of participants with and without pruritus are shown in Table II. Patients with uraemic pruritus had a lower percentage of classical monocytes than those without uraemic pruritus. Patients with uraemic pruritus also had higher blood levels of triglycerides and were more likely to have diabetes than those without pruritus symptoms.
Table I.
Demographic characteristics of study participants
| Baseline characteristics | |
|---|---|
| Patients, n | 175 |
| Sex (female: male) | 76:99 |
| Age, years, mean ± SD | 60.5 ± 11.0 |
| Duration of dialysis, years, mean± SD | 7.6 ± 6.0 |
| Aetiology of end-stage renal disease, n (%) | |
| Diabetes mellitus | 69 (39.4) |
| Glomerulonephritis | 59 (33.7) |
| Hypertension | 20 (11.4) |
| Systemic lupus erythematosus | 3 (1.7) |
| Interstitial nephritis | 1 (0.6) |
| Polycystic kidney disease | 4 (2.3) |
| Obstructive uropathy | 3 (1.7) |
| Others | 16 (8.9) |
SD: standard deviation.
Table II.
Clinical characteristics of participants with and without pruritus
| Variable | With pruritus | Without pruritus | p-value |
|---|---|---|---|
| Participants, n | 74 | 101 | |
| VAS score for pruritus intensity, mean ± SD | 5.8 ± 2.5 | 0.0 ± 0.00 | <0.0001 |
| Clinical characteristics | |||
| Age, years, mean ± SD | 61.4 ± 11.8 | 59.9 ± 10.47 | 0.27 |
| Female, n (%) | 34 (45.95) | 42 (41.58) | 0.57 |
| Duration of dialysis, years, mean ± SD | 7.08 ± 5.87 | 7.96 ± 6.10 | 0.25 |
| Kt/V, mean ± SD | 1.57 ± 0.24 | 1.54 ± 0.29 | 0.38 |
| Haematocrit, %, mean± SD | 35.56 ± 3.35 | 34.85 ± 4.01 | 0.20 |
| Creatinine, mg/dl, mean ± SD | 10.74 ± 2.11 | 10.78 ± 2.06 | 0.99 |
| Uric acid, mg/dl, mean± SD | 7.68 ± 1.48 | 7.57 ± 1.33 | 0.99 |
| Albumin, g/dl, mean ± SD | 4.14 ± 0.31 | 4.15 ± 0.37 | 0.82 |
| Fasting glucose, mg/dl, mean ± SD | 121.88 ± 62.70 | 116.67 ± 60.58 | 0.22 |
| Total cholesterol, mg/dl, mean ± SD | 166.93 ± 40.10 | 159.61 ± 39.78 | 0.22 |
| Triglyceride, mg/dl, mean ± SD | 180.61 ± 111.47 | 140.64 ± 108.86 | < 0.001* |
| Aspartate transaminase, U/l, mean ± SD | 17.11 ± 8.05 | 18.23 ± 9.80 | 0.45 |
| Alanine transaminase, U/l, mean ± SD | 14.82 ± 8.31 | 17.75 ± 19.60 | 0.72 |
| Alkaline phosphatase, U/l, mean ± SD | 110.85 ± 62.13 | 136.15 ± 192.01 | 0.98 |
| Total bilirubin, mg/dl, mean ± SD | 0.40 ± 0.14 | 0.42 ± 0.11 | 0.08 |
| Ferritin, ng/ml, mean± SD | 424.24 ± 254.42 | 408.18 ± 230.48 | 0.81 |
| Calcium, albumin adjusted, mg/dl, mean ± SD | 8.98 ± 0.76 | 8.92 ± 0.81 | 0.59 |
| Phosphorus, mg/dl, mean ± SD | 4.96 ± 1.30 | 4.78 ± 1.36 | 0.37 |
| Ca × P mg/dl × mg/dl, mean ± SD | 44.58 ± 12.06 | 42.93 ± 13.75 | 0.27 |
| Intact parathyroid hormone, pg/ml, mean ± SD | 358.54 ± 336.29 | 398.01 ± 462.25 | 0.84 |
| High-sensitivity C-reactive protein, mg/l, mean ± SD | 0.77 ± 0.97 | 0.84 ± 2.01 | 0.17 |
| Diabetes mellitus, n (%) | 43 (58.11) | 40 (39.6) | 0.02* |
| Hepatitis B, n (%) | 9 (12.16) | 18 (17.82) | 0.31 |
| Hepatitis C, n (%) | 5 (6.76) | 11 (10.89) | 0.35 |
| Monocytes subsets | |||
| Classical monocytes, %, mean ± SD | 69.19 ± 9.88 | 72.79 ± 8.47 | 0.01* |
| Intermediate monocytes, %, mean ± SD | 13.55 ± 7.86 | 11.78 ± 5.72 | 0.22 |
| Non-classical monocytes, %, mean ± SD | 10.15 ± 5.24 | 9.17 ± 4.49 | 0.21 |
| Cytokines | |||
| Interleukin-2, pg/ml, mean ± SD | 0.09 ± 0.58 | 0.31 ± 1.20 | 0.07 |
| Interferon-γ, pg/ml, mean ± SD | 0.88 ± 2.30 | 1.18 ± 4.53 | 0.92 |
| Interleukin-12p70, pg/ml, mean ± SD | 0.91 ± 1.14 | 1.00 ± 1.98 | 0.41 |
| Interleukin-4, pg/ml, mean ± SD | 0.69 ± 2.01 | 0.50 ± 1.99 | 0.17 |
| Interleukin-5, pg/ml, mean ± SD | 0.08 ± 0.36 | 0.06 ± 0.33 | 0.66 |
| Interleukin-6, pg/ml, mean ± SD | 6.40 ± 8.99 | 7.45 ± 20.29 | 0.83 |
| Tumour necrosis factor-a, pg/ml, mean ± SD | 0.52 ± 0.62 | 0.51 ± 0.66 | 0.95 |
| Interleukin-10, pg/ml, mean ± SD | 1.55 ± 1.91 | 1.74 ± 2.02 | 0.42 |
p ≤ 0.05.
SD: standard deviation; VAS: visual analogue scale; Ca × P: product of albumin-adjusted serum calcium (Ca) and serum phosphorus (P).
Effects of patient characteristics on the subsets of blood monocytes
Table III shows the influence of patient characteristics on the subsets of blood monocytes. Men had a higher percentage of classical monocytes than did women (72.48 ± 9.20% vs 69.68 ± 9.09%, p = 0.03). Patients with diabetes had a lower percentage of non-classical monocytes (8.80 ± 4.56% vs 10.29 ± 4.99%, p = 0.05). The subsets of blood monocytes did not differ significantly regarding viral hepatitis B, viral hepatitis C, or cigarette smoking.
Table III.
Univariate analysis of the association of patient characteristics with the monocyte subsets
| Participants | Classical monocytes, % | p-value | Intermediate monocytes, % | p-value | Non-classical monocytes, % | p-value | |
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Sex | 0.03* | 0.16 | 0.13 | ||||
| Female | 76 | 69.68 ± 9.09 | 13.25 ± 6.95 | 10.15 ± 4.79 | |||
| Male | 99 | 72.48 ± 9.20 | 11.97± 9.55 | 9.15 ± 4.84 | |||
| Diabetes mellitus | 0.30 | 0.97 | 0.05* | ||||
| With diabetes | 83 | 71.97 ± 9.56 | 12.53 ± 6.65 | 8.80 ± 4.56 | |||
| Without diabetes | 92 | 70.63 ± 8.94 | 12.53 ± 6.86 | 10.29 ± 4.99 | |||
| Hepatitis B | 0.33 | 0.43 | 0.46 | ||||
| With hepatitis B | 27 | 72.91 ± 7.66 | 11.59 ± 6.54 | 8.98 ± 4.69 | |||
| Without hepatitis B | 148 | 70.96 ± 9.49 | 12.70 ± 6.79 | 9.70 ± 4.86 | |||
| Hepatitis C | 0.84 | 0.10 | 0.09 | ||||
| With hepatitis C | 16 | 71.81 ± 8.66 | 15.82 ± 8.43 | 7.74 ± 4.08 | |||
| Without hepatitis C | 159 | 71.21 ± 9.32 | 12.20 ± 6.49 | 9.77 ± 4.87 | |||
| Cigarette smoking | 0.33 | 0.45 | 0.92 | ||||
| Smoker | 13 | 72.25 ± 6.23 | 11.01 ± 4.14 | 9.90 ± 4.81 | |||
| Non-smoker | 131 | 70.21 ± 9.62 | 13.05 ± 7.17 | 9.93 ± 4.91 |
p ≤ 0.05.
SD: standard deviation
Effects of the subsets of monocytes and plasma cytokines on pruritus intensity
Linear regression models were used to explore the effects of blood monocytes, proinflammatory cytokines (IL-2, IFN-γ, IL-12p70, IL-4, IL-5, IL-6, TNF-α), and anti-inflammatory cytokine IL-10 on uraemic pruritus. In the univariate linear regression models, a higher VAS score for pruritus intensity was significantly associated with a higher percentage of intermediate monocytes (effect estimate 0.072; 95% CI, 0.0002 to 0.144; p = 0.05), a lower percentage of classical monocytes (effect estimate –0.067; 95% CI –0.119 to –0.015; p =0.01), and the presence of diabetes (effect estimate 1.111; 95% CI 0.149 to 2.073; p =0.02) (Table IV). In addition, none of the 8 cytokines was associated with the VAS score for pruritus intensity (Table IV). Table V shows the results of the multivariate linear regression model for assessing the predictors of pruritus intensity. After adjusting for age and sex, a higher percentage of intermediate monocytes (effect estimate 0.08; 95% CI 0.01 to 0.16; p =0.03) and the presence of diabetes (effect estimate 1.52; 95% CI 0.42 to 2.61; p = 0.01) were independent predictors of a higher VAS score for pruritus intensity (Table V). In addition, cigarette smoking (effect estimate 1.88; 95% CI –0.07 to 3.83, p = 0.06) was borderline significantly associated with higher pruritus intensity (Table V).
Table IV.
Univariate linear regression models for the effects of covariates on the visual analogue scale score for pruritus intensity
| Covariates | Effect estimate | 95% CI | p-value |
|---|---|---|---|
| Age, years | –0.006 | –0.050˜0.039 | 0.81 |
| Men | –0.544 | –1.524˜0.436 | 0.28 |
| Cigarette smoking | 1.061 | –0.816˜2.937 | 0.27 |
| Kt/V | 0.495 | –1.326˜2.316 | 0.59 |
| Duration of dialysis | –0.026 | –0.107˜0.056 | 0.54 |
| Diabetes mellitus | 1.111 | 0.149˜2.073 | 0.02* |
| White blood cell, 103/μl | 0.029 | –0.209˜0.267 | 0.81 |
| Aspartate transaminase, U/l | 0.004 | –0.050˜0.058 | 0.89 |
| Total bilirubin, mg/dl | –2.133 | –6.072˜1.807 | 0.29 |
| Total cholesterol, mg/dl | 0.012 | –0.001˜0.024 | 0.06 |
| Triglyceride, mg/dl | 0.004 | –0.0003˜0.008 | 0.07 |
| Fasting glucose, mg/dl | 0.001 | –0.007˜0.009 | 0.77 |
| Ca × P, mg/dl×mg/dl | 0.014 | –0.024˜0.051 | 0.48 |
| Ferritin, ng/ml | 0.0005 | –0.002˜0.002 | 0.66 |
| Tranferrin saturation, % | –0.023 | –0.063˜0.018 | 0.27 |
| Intact parathyroid hormone, pg/ml | –0.0003 | –0.001˜0.001 | 0.67 |
| hsCRP, mg/l | –0.028 | –0.324˜0.269 | 0.86 |
| Hepatitis B | –0.759 | –2.104˜0.585 | 0.27 |
| Hepatitis C | –0.280 | –1.971˜1.410 | 0.75 |
| Classical monocytes, % | –0.067 | –0.119˜–0.015 | 0.01* |
| Intermediate monocytes, % | 0.072 | 0.0002˜0.144 | 0.05* |
| Non-classical monocytes, % | 0.075 | –0.025˜0.176 | 0.15 |
| IL-2, pg/ml | –0.214 | –0.709˜0.281 | 0.40 |
| IFN-γ, pg/ml | –0.069 | –0.201˜0.062 | 0.30 |
| IL-12p70, pg/ml | –0.060 | –0.354˜0.235 | 0.69 |
| IL-4, pg/ml | 0.115 | –0.132˜0.361 | 0.36 |
| IL-5, pg/ml | –0.120 | –1.549˜1.308 | 0.87 |
| IL-6, pg/ml | –0.011 | –0.041˜0.019 | 0.49 |
| TNF-α, pg/ml | –0.160 | –0.933˜0.614 | 0.69 |
| IL-10, pg/ml | –0.167 | –0.415˜0.082 | 0.19 |
p ≤ 0.05.
CI: confidence interval; Ca × P: product of albumin-adjusted serum calcium (Ca) and serum phosphorus (P); hsCRP, high-sensitivity C-reactive protein; IL: interleukin; IFN: interferon; TNF: tumour necrosis factor.
Table V.
Multivariate linear regression analysis of predictors of the visual analogue scale score for pruritus intensity
| Covariate | Parameter estimate | 95% CI | p-value |
|---|---|---|---|
| Age (years) | –0.01 | –0.06˜0.04 | 0.67 |
| Men | –0.63 | –1.81˜0.56 | 0.30 |
| Cigarette smoking | 1.88 | –0.07˜3.83 | 0.06 |
| Diabetes mellitus | 1.52 | 0.42˜2.61 | 0.01* |
| Intermediate monocytes (%) | 0.08 | 0.01˜0.16 | 0.03* |
p ≤ 0.05.
CI: confidence interval.
DISCUSSION
This is the first study to investigate the association of monocyte phenotypes and uraemic pruritus. Patients receiving HD who had uraemic pruritus were found to have altered monocytic phenotypes. Among the subsets of monocytes, a higher percentage of intermediate monocytes was independently associated with a higher VAS score for pruritus intensity. The results may indicate the subset-specific involvement of monocytes and the distinct role of intermediate monocytes in the pathogenesis of uraemic pruritus.
Patients with maintenance HD are subject to chronic low-grade inflammation owing to multifactorial causes, including uraemic toxins, oxidative stress, underlying comorbidities, and the dialysis process, etc. (32). Compared with non-dialysis patients, patients receiving HD showed a higher prevalence of hypoalbuminaemia, hyperferritinaemia, and elevated C-reactive protein levels (32, 33). There is increasing evidence that immune dysregulation plays a critical role in uraemic pruritus. Kimmel et al. (11) showed that patients with uraemic pruritus had an increased proportion of Th1 cells, as measured by intracytoplasmic cytokines and the expression of chemokine receptors. Evidence also showed that the serum levels of histamine, IL-2, IL-6, and IL-31 were elevated in patients receiving HD with uraemic pruritus (10, 11, 14). However, there is debate regarding whether uraemic pruritus is a primary inflammatory itch, as it is not uncommon for dialysis patients to report severe itching without obvious clinical inflammation of the skin (34). Based on our findings that altered monocytic phenotypes are associated with the severity of uraemic pruritus, further investigations are needed to identify the role of monocytes in itch signalling and to explore the immunopathogenic mechanisms that underlie pruritus occurrence and severity.
Monocytes represent a heterogenic and dynamic cell population continuum of diverse differentiation stages. Intermediate CD14++CD16+ monocytes comprise approximately 11–14% of circulating monocytes in patients receiving HD, which is higher than the 2–8% of intermediate monocytes in normal subjects (35). Our previous study also reported that patients receiving HD, compared with healthy individuals of the same age, had significantly increased levels of intermediate and non-classical monocytes, and both monocyte subsets increased with the duration of dialysis (26). In patients receiving HD, intermediate monocytes are known to be associated with an increased risk of cardiovascular disease, but the association between intermediate monocytes and pruritus has not been reported (36). As dialysis adequacy is an independent predictor of pruritus intensity in patients on maintenance HD (7), and uraemic toxins induce DNA epigenetic changes involving the differentiation of monocytes toward intermediate monocytes (37), the accumulation of uraemic toxins may link monocyte phenotypes with uraemic pruritus.
The current study did not find a significant association between uraemic pruritus and cytokines, including IL-2, IFN-γ, IL-12p70, IL-4, IL-5, IL-6, TNF-α, and IL-10. In contrast to our findings, some previous studies have indicated that IL-2 or IL-6 cytokine levels are elevated in patients with uraemic pruritus compared with those without uraemic pruritus (10, 11). A probable reason for the discrepancy is that, in previous studies, the analysis was not adjusted for comorbid conditions, such as diabetes or cigarette smoking, which could also enhance the status of inflammation. Individual heterogeneity and the complex pathogenesis of uraemic pruritus could also cause the discrepancies.
Among the monocyte subsets, the intermediate monocytes are the major source of lipopolysaccharides-induced IL-6 and TNF-α, and have been reported to produce the highest level of TNF-α in response to T cell contact (19, 38). In this study, relevant cytokines were not elevated in patients with higher intensity of pruritus. One possible reason is that intermediate monocytes are not the only cell sources to secrete these cytokines. IL-6 is also produced by endothelial cells, macrophages, monocytes, lymphocytes, and keratinocytes; while TNF-α is secreted by activated macrophages, monocytes, CD4+ T cells, B cells, neutrophils, etc. (39). Another explanation is that this cross-sectional study assessed the proportions of monocytes and multiple cytokines at the same time, in contrast to the in vitro studies, which used lipopolysaccharides and other Toll-like receptor ligands to stimulate cytokine production (19, 40). Further longitudinal studies on the complex interactions between cytokines and monocytes in an in vivo environment are required.
This study has several limitations. First, the study design was cross-sectional in nature, so the causality and temporality between subsets of monocytes and uraemic pruritus cannot be clarified. Secondly, because the participants were patients receiving HD enrolled from a single medical centre in Taiwan, the generalizability to other races or patients with peritoneal dialysis or chronic kidney disease is limited. Thirdly, the study sample size was relatively small. However, the number of participants was still able to provide sufficient power to identify the association between monocyte subsets and uraemic pruritus. In addition, clinical characteristics, monocyte phenotypes, and proinflammatory and anti-inflammatory cytokines were considered in the multivariate linear regression model to minimize influences from confounding factors.
In conclusion, altered monocytic phenotypes are associated with the pruritus intensity of uraemic pruritus in patients receiving HD, and a higher percentage of intermediate monocytes independently predicts higher VAS scores for pruritus intensity. No significant association was found between uraemic pruritus and cytokines, including IL-2, IFN-γ, IL-12p70, IL-4, IL-5, IL-6, TNF-α, and IL-10. The results indicate that altered monocytic phenotypes could play a role in uraemic pruritus.
ACKNOWLEDGEMENTS
The authors thank the Far Eastern Memorial Hospital Biobank for providing additional human plasma samples as technical controls for measuring cytokine levels. This study was supported by research grants to Dr Hon-Yen Wu from the National Health Research Institutes, Taiwan (NHRI-EX110-11026PI) and the Far Eastern Memorial Hospital, New Taipei City, Taiwan (FEMH-EX110-11026PI), to Dr Mei-Ju Ko from the Ministry of Science and Technology, Taiwan (MOST 109-2635-B-532-001) and the Department of Health, Taipei City Government, Taipei City, Taiwan (10801-62-007, 10901-62-004), and to Dr Yen-Ling Chiu from the Ministry of Science and Technology, Taiwan (MOST 104-2314-B-418-017, MOST 105-2314-B-418-002, MOST 109-2314-B-418-011-MY3). The funders had no role in study design, data collection, data analysis, manuscript preparation or publication decisions.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Wu HY, Peng YS, Chen HY, Tsai WC, Yang JY, Hsu SP, et al. A comparison of uremic pruritus in patients receiving peritoneal dialysis and hemodialysis. Medicine (Baltimore) 2016; 95: e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathur VS, Lindberg J, Germain M, Block G, Tumlin J, Smith M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisoni RL, Wikstrom B, Elder SJ, Akizawa T, Asano Y, Keen ML, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006; 21: 3495–3505. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen E, Komenda P, Lerner B, Askin N, Bohm C, Shaw J, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis 2017; 70: 638–655. [DOI] [PubMed] [Google Scholar]
- 5.Ko MJ, Huang JW, Wu HY, Tsai WC, Hsu LY, Tsai PH, et al. Risk of skin cancer among patients with chronic kidney disease treated with ultraviolet B phototherapy for uraemic pruritus: a nationwide cohort Study. Acta Derm Venereol 2021; 101: adv00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko MJ, Yang JY, Wu HY, Hu FC, Chen SI, Tsai PJ, et al. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol 2011; 165: 633–639. [DOI] [PubMed] [Google Scholar]
- 7.Ko MJ, Wu HY, Chen HY, Chiu YL, Hsu SP, Pai MF, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One 2013; 8: e71404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol 2005; 16: 3742–3747. [DOI] [PubMed] [Google Scholar]
- 9.Wieczorek A, Krajewski P, Koziol-Galczynska M, Szepietowski JC. Opioid receptors expression in the skin of haemodialysis patients suffering from uraemic pruritus. J Eur Acad Dermatol Venereol 2020; 34: 2368–2372. [DOI] [PubMed] [Google Scholar]
- 10.Fallahzadeh MK, Roozbeh J, Geramizadeh B, Namazi MR. Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrol Dial Transplant 2011; 26: 3338–3344. [DOI] [PubMed] [Google Scholar]
- 11.Kimmel M, Alscher DM, Dunst R, Braun N, Machleidt C, Kiefer T, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 2006; 21: 749–755. [DOI] [PubMed] [Google Scholar]
- 12.Balaskas EV, Bamihas GI, Karamouzis M, Voyiatzis G, Tourkantonis A. Histamine and serotonin in uremic pruritus: effect of ondansetron in CAPD-pruritic patients. Nephron 1998; 78: 395–402. [DOI] [PubMed] [Google Scholar]
- 13.Chiu YL, Chen HY, Chuang YF, Hsu SP, Lai CF, Pai MF, et al. Association of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patients. Nephrol Dial Transplant 2008; 23: 3685–3689. [DOI] [PubMed] [Google Scholar]
- 14.Ko MJ, Peng YS, Chen HY, Hsu SP, Pai MF, Yang JY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol 2014; 71: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 15.Wu HY, Huang JW, Tsai WC, Peng YS, Chen HY, Yang JY, et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: a prospective cohort study. PLoS One 2018; 13: e0203474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HY, Chiu YL, Hsu SP, Pai MF, Lai CF, Yang JY, et al. Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM 2010; 103: 837–846. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116: e74–e80. [DOI] [PubMed] [Google Scholar]
- 18.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011; 118: e16–e31. [DOI] [PubMed] [Google Scholar]
- 19.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthrit Rheum 2012; 64: 671–677. [DOI] [PubMed] [Google Scholar]
- 20.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell R-R, Fliser D, et al. SuperSAGE evidence for CD14++ CD16+ monocytes as a third monocyte subset. Blood 2011; 118: e50–e61. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Tam H, Adler L, Ilstad-Minnihan A, Macaubas C, Mellins ED. The MHC class II antigen presentation pathway in human monocytes differs by subset and is regulated by cytokines. PLoS One 2017; 12: e0183594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukamoto M, Seta N, Yoshimoto K, Suzuki K, Yamaoka K, Takeuchi T. CD14(bright)CD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res Ther 2017; 19: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung CH, Wang CC, Suen JL, Sheu CC, Kuo CH, Liao WT, et al. Altered pattern of monocyte differentiation and monocyte-derived TGF-β1 in severe asthma. Sci Rep 2018; 8: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, et al. The “Intermediate” CD14(++)CD16(+) monocyte subset increases in severe peripheral artery disease in humans. Sci Rep 2016; 6: 39483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hortova-Kohoutkova M, Laznickova P, Bendickova K, De Zuani M, Andrejcinova I, Tomaskova V, et al. Differences in monocyte subsets are associated with short-term survival in patients with septic shock. J Cell Mol Med 2020; 24: 12504–12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu YL, Shu KH, Yang FJ, Chou TY, Chen PM, Lay FY, et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: the iESRD study. Immun Ageing 2018; 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mistik S, Utas S, Ferahbas A, Tokgoz B, Unsal G, Sahan H, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol 2006; 20: 672–678. [DOI] [PubMed] [Google Scholar]
- 28.Yosipovitch G, Zucker I, Boner G, Gafter U, Shapira Y, David M. A questionnaire for the assessment of pruritus: validation in uremic patients. Acta Derm Venereol 2001; 81: 108–111. [DOI] [PubMed] [Google Scholar]
- 29.Furue M, Ebata T, Ikoma A, Takeuchi S, Kataoka Y, Takamori K, et al. Verbalizing extremes of the visual analogue scale for pruritus: a consensus statement. Acta Derm Venereol 2013; 93: 214–215. [DOI] [PubMed] [Google Scholar]
- 30.Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther 1995; 2: 295–304. [DOI] [PubMed] [Google Scholar]
- 31.KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930. [DOI] [PubMed] [Google Scholar]
- 32.Jofré R, Rodriguez-Benitez P, López-Gómez JM, Pérez-Garcia R. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 2006; 17 (12 Suppl 3): S274–S280. [DOI] [PubMed] [Google Scholar]
- 33.Stenvinkel P, Gillespie IA, Tunks J, Addison J, Kronenberg F, Drueke TB, et al. Inflammation modifies the paradoxical association between body mass index and mortality in hemodialysis patients. J Am Soc Nephrol 2016; 27: 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong LS, Wu T, Lee CH. Inflammatory and noninflammatory itch: implications in pathophysiology-directed treatments. Int J Mol Sci 2017; 18: 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampath P, Moideen K, Ranganathan UD, Bethunaickan R. Monocyte subsets: phenotypes and function in tuberculosis infection. Front Immunol 2018; 9: 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, et al. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int 2008; 73: 622–629. [DOI] [PubMed] [Google Scholar]
- 37.Zawada AM, Schneider JS, Michel AI, Rogacev KS, Hummel B, Krezdorn N, et al. DNA methylation profiling reveals differences in the 3 human monocyte subsets and identifies uremia to induce DNA methylation changes during differentiation. Epigenetics 2016; 11: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010; 33: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol 2016; 138: 984–1010. [DOI] [PubMed] [Google Scholar]
- 40.Lachmandas E, Boutens L, Ratter JM, Hijmans A, Hooiveld GJ, Joosten LA, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol 2016; 2: 16246. [DOI] [PubMed] [Google Scholar]