Abstract
Over the past few years, the cancer-related disease has had a high mortality rate and incidence worldwide, despite clinical advances in cancer treatment. The drugs used for cancer therapy, have high side effects in addition to the high cost. Subsequently, to reduce these side effects, many studies have suggested the use of natural bioactive compounds. Among these, which have recently attracted the attention of many researchers, quercetin has such properties. Quercetin, a plant flavonoid found in fresh fruits, vegetables and citrus fruits, has anti-cancer properties by inhibiting tumor proliferation, invasion, and tumor metastasis. Several studies have demonstrated the anti-cancer mechanism of quercetin, and these mechanisms are controlled through several signalling pathways within the cancer cell. Pathways involved in this process include apoptotic, p53, NF-κB, MAPK, JAK/STAT, PI3K/AKT, and Wnt/β-catenin pathways. In addition to regulating these pathways, quercetin controls the activity of oncogenic and tumor suppressor ncRNAs. Therefore, in this comprehensive review, we summarized the regulation of these signalling pathways by quercetin. The modulatory role of quercetin in the expression of various miRNAs has also been discussed. Understanding the basic anti-cancer mechanisms of these herbal compounds can help prevent and manage many types of cancer.
Keywords: Quercetin, Malignant tumors, Pharmacology, Signalling pathways, Molecular targets
Introduction
Cancer is a set of diseases in which cells develop abnormally and have the ability to invade and spread (metastasize) to other parts of the body [1, 2]. Chemotherapy drugs on the market today are effective in treating cancer, but they are expensive and come with a long list of undesirable side effects [3, 4]. Chemopreventive properties of natural bioactive substances have been increasingly important to researchers in the field of medicine in recent years [5, 6]. More and more research has demonstrated the anti-cancer potential and usefulness of natural ingredients in the treatment of cancer [7–10]. Quercetin, a plant flavonol produced from the polyphenol family, is an example of a useful, accessible, and highly effective natural substance. It is found at great levels in fruits, vegetables, leaves, and seeds [11]. Quercetin has many physiological activities such as antioxidant and free radical scavenger [12], iNOS synthase inhibitor [13], xanthine oxidase inhibitor [14, 15], reduction of leukocyte immobilization [16], and modulation of gene expression [17, 18]. Numerous studies have shown that quercetin is used to treat a variety of diseases including coronary heart diseases [19], diabetes [20] and cancers [21, 22].
The anti-cancer effects of quercetin have been investigated in many studies and the role of that in preventing the growth, proliferation and progression of cancer through cellular signalling pathways such as Wnt/β-catenin signalling, phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway, Janus kinase (JAK)/signal transducer and transcription activator (STAT) signalling pathway, mitogen-activated protein kinase (MAPK) pathway, p53 signalling and nuclear factor kappa B (NF-κB) pathway [23]. It was also demonstrated that quercetin can impact and specifically target tumour suppressor and oncogenic miRNAs and lncRNAs [24–26].
An important therapeutic limitation is due to the low absorption of quercetin; therefore, researchers have been looking for solutions to increase plasma concentrations such as incorporation into nanoparticles or structural chemical changes [27]. The anticancer effects of nanoparticles have been confirmed in a recent study conducted by Lou et al., The results showed that quercetin nanoparticles induced cell death in human neuroglioma cells in a dose-and time-dependent manner; it also markedly promoted apoptosis in the cells [28]. Besides, the results revealed the positive correlation of ERK, LC3, cleaved Caspase-3, cytoplasm p53, and PARP expressions with quercetin nanoparticle concentrations [28]. The results also showed the induction of apoptosis and autophagy by quercetin nanoparticles is at least partly through the activation of LC3/ERK/Caspase-3 and the inhibition of AKT/mTOR signalling pathways [28]. Another possibility of increasing the bioavailability of quercetin is encapsulation in phytosomes. Phytosomes are derived from phospholipids, which are made from the same cell membrane material. When encapsulated in a phytosome, quercetin can cross the cell membrane and is delivered directly inside the cell. By combining this compound with a unique phospholipid delivery system, the quercetin in this formula is more bioavailable. The technology used is similar to liposomal encapsulation technology [29].
Isoquercitrin is an enzymatically modified form of Quercetin with increased bioavailability, a bioflavonoid that has significant antiallergic effects and contributes to immune function. Enzymatically modified isoquercitrin is prepared using a natural enzymatic process that attaches polysaccharides to convert quercetin, which has poor bioavailability, into a water-soluble form (Alpha-Glycosyl Isoquercitrin). These are the top benefits of quercetin, such as high absorption and increased bioavailability. According to pharmacokinetic data, the absorption of isoquercetin is up to 40 times higher (Cmax) than that of quercetin and reaches maximum levels in the bloodstream in just 15 min [30]. Isoquercitrin has been used in a few clinical studies studying the treatment of kidney cancer, renal cell carcinoma, advanced renal cell carcinoma, and venous thromboembolism in pancreatic cancer and colorectal cancer. It has antineoplastic activity and has been shown to reduce the rate of polymerization of red blood cells. It acts as an antineoplastic agent, bone density preservative, osteogenesis regulator, antioxidant, histamine antagonist and antipruritic drug. As a result, isoquercitrin is much more active than quercetin and has a wider range of therapeutic activities [31]. Another limitation of this update is represented by a lack of clinical studies that support its anticancer effect. A clinical study on 144 adenocarcinoma (AD) and 120 squamous cell carcinoma (SQ) patients has revealed that a quercetin-rich diet can highly influence the expression of the miRs. Let-7 family and miR-146a which act as tumor suppressors have significantly higher expression in more quercetin consumer lung cancer patients. On the other side, miR-17 oncogenic shows downregulation in 67% of its members [32].
The purpose of this review is to examine quercetin's function in cancer through influencing cellular signalling pathways, miRNA and lncRNA expression. With a thorough understanding of the mechanism behind quercetin's anti-cancer actions, it is possible to conclude that this combination might be employed clinically in cancer therapy.
Review methodology
To conduct this comprehensive review, we searched numerous specialized electronic databases for research on the anticancer and chemopreventive properties of quercetin, including PubMed/Medline, DynaMed Plus, Embase, ScienceDirect, and the TRIP database. The following MeSH terms were used in the literature search: "antineoplastic agents," "phytogenic/pharmacology," "antioxidants/pharmacology," "cell line," "tumour," "diet," "humans," "neoplasms/drug treatment," and "quercetin/pharmacology." There were no constraints on language or publication type, and the reference lists of chosen papers were carefully examined, omitting research including homoeopathic medications.
Inclusion criteria include the following: (i) preclinical studies on quercetin's anticancer effects; (ii) in vitro studies on cancer cells with evidence of molecular processes, and (iii) in vivo animal studies using well-defined experimental dosages.
Exclusion criteria include the following: (i) abstracts, incomplete articles, and duplicate articles; (ii) studies that did not establish a direct correlation between the anticancer effect and the observed results; and (iii) studies that did not use quercetin alone as an intervention group and instead used it in combination with other cytostatic drugs.
Anti-cancer effects of quercetin: modulation of cancer signalling pathways
Wnt/β-catenin pathway
Various physiological processes such as cell proliferation, differentiation, stemness, migration, and apoptosis are controlled by the Wnt signalling system, also known as APC/β-catenin/Tcf pathway [33].
There are two different Wnt/β-catenin signalling pathways: canonical and non-canonical. Canonical pathway employ β-catenin regulating gene expression, in contrast, non-canonical is a β-catenin-independent pathway [34]. To be more specific, in the canonical pathway, Wnt ligand (Wnt1 and Wnt3a) binds to Frizzled and Lrp5/6 receptor complexes which lead to stabilization and transcriptional activation of cytoplasmic β-catenin while non-canonical Wnt signalling pathway is represented as a response to Wnt ligands which is independent of β-catenin stabilization [35]. The loss of Wnt signalling regulation is frequently correlated with tumor progression and metastasis [36]. Various studies have demonstrated that the anti-tumor effect of quercetin on the Wnt/β-catenin pathway has a multifunctional impact. Cells from the NT2/D1 line were studied by Mojsin et al. (human in vitro model of teratocarcinoma). In NT2/D1 cells, they discovered that quercetin inhibits SOX2, Nanog, and Oct4 expression, as well as β-catenin nuclear translocation, resulting in a decrease in β-catenin-dependent transcriptional activity [37]. In research by Haesung et al., quercetin caused apoptosis in 4T1 cells and showed dose-dependent anticancer efficacy (murine mammary cancer cell line). After treatment with 20 µM quercetin, Dickkopf-related protein 1 (DKK1), 2 and 3, which are regulators of Wnt signalling, are increased and cell viability is reduced, according to the researchers [38]. The Wnt signalling pathway was studied in human colon cancer cells (SW480) by Shan et al., who found that quercetin inhibits the production of cyclin D1 and survivin, which are involved in the cell cycle regulation and death, in the cells [39]. Quercetin's influence on GSK3 has been examined in a recent study on HT29 colon cancer cells (key elements of the Wnt pathway). Quercetin at doses of 10 to 75 µM did not significantly inhibit GSK3 and GSK3, and as a result, the total β-catenin level in HT29 cells was mostly unaffected. Various experimental setups and biological settings can produce different kinds of reactions [40].
The PI3K/AKT pathway
For AKT to translocation to the plasma membrane, phosphatidylinositol-3-kinase (PI3K) has to be present in the cell. Biochemical processes such as cell cycle progression, differentiation, cell survival, and cell proliferation are all influenced by the PI3K/AKT pathway [41]. The Bcl-2 protein family and Bax are controlled by the PI3K/AKT pathway, which has anti-apoptotic properties (a pro-apoptotic gene) [42]. Deregulation of this pathway could be a key event in cancer pathogenesis [41]. Several studies explored the quercetin effect on PI3K/AKT pathway. Using the HCC1937 PTEN-null cancer cell line, Gulati et al. observed that 25 µM quercetin may decrease active AKT/PKB phosphorylation and dramatically limit cell proliferation of PTEN-null cancer cells, which is consistent with previous studies [43].
In a study on HL-60 leukaemia cell lines, Yuan et al. investigated the quercetin mechanism and discovered that quercetin decreased p-AKT and Bcl-2 levels, as well as triggered apoptosis, in a manner that was associated with the inhibitory actions of PI3K/AKT. It was revealed that at 150 µM quercetin, the inhibitory effects were the most pronounced [42, 44, 45].
JAK/STAT signalling pathway
In interconnected networks in a cell, the JAK/STAT (Janus kinase/signal transducers and activators of transcription) signalling pathway detects stimulus signals from outside the cell and transmits its message to the cell nucleus and activates several transcription factors [46–48]. The JAK/STAT pathway is involved in regulating processes such as immune maintenance, cell division and growth, cell death, and tumor formation. JAK/STAT pathway components are regulated by other paths such as ERK MAPK and PI3K [49–51]. Skin, immune system, and cancer disorders are caused by JAK/STAT pathway disruptions [52].
In line with this, Qin et al. examined the effect of quercetin on leptin and its receptor in MGC-803 cells via the JAK/STAT signalling pathway. They found that quercetin caused the arrest of cells in the G2/M stage of the cell cycle through the p-STAT3 pathway and celled to apoptosis and necrosis. On the other hand, the flavonoid compound reduces the expression of leptin and its receptor [53].
It has been shown that quercetin can inhibit IL-6-induced glioblastoma cell growth and migration by regulating the STAT3 signalling pathway, which affects the expression of this protein in glioblastoma cells. In T98G and U-87 cell lines, quercetin blocked the IL-6-induced STAT3 pathway, reducing the expression of GP130, JAK1, and STAT3. As a result, there is a reduction in the proliferation and migration of cancer cells [54].
The effects of quercetin and epigalactochin-3-gallate (EGCG) on cholangiocarcinoma cells were investigated by Senggunprai et al. IL-6 and IFN-gamma were found to regulate JAK/STAT (STAT1/3 phosphorylation) pathways in cholangiocarcinoma cells, and the results suggested that these two chemicals can be employed as chemopreventive agents against these cells. Lastly, these compounds were able to suppress KKU100 cancer cell proliferation and migration [55, 56].
Quercetin suppresses clonogenic survival in BT-474 cells, triggers apoptosis via caspase 3, 8, and PARP cleavage, and causes cell cycle arrest in the sub-G0/G1 phase, according to a study by Seo et al., which also found that quercetin reduces p-JAK1 and p-STAT3 expression and inhibits MMP-9 secretion. When it comes to HER-2-expressing breast cancer, this flavonoid can both prevent and treat the disease [57]. Luo et al. examined the processes of apoptosis, autophagy, and quercetin proliferation in cervical cancer cells. Using quercetin-conjugated gold nanoparticles, they found that the complex shows a similar role in suppressing JAK2, a protein expressed in cervical cancer cells, which suppresses proliferation, invasion, and migration processes. They also found that apoptosis and autophagy processes occurred through caspase-3 in cancer cells inhibited by JAK2, and that cyclin D1 and mTOR were suppressed by the STAT3/5 and PI3K/AKT signalling pathways [58, 59].
The MAPK signalling pathway
Mitogen-Activated Protein Kinase (MAPK) fall into three main categories: ERKs (stimulated by mitogens and growth factors), JNK/SAPK, and p38s (stimulated by cellular stress and inflammatory cytokines) [60–62]. Cell proliferation, differentiation, gene expression, cell survival, mitosis, and apoptosis are all affected by MAPK, which has been found in MAPK14, MAPK 7, and MAPK 12 [63]. Due to the association between the MAPK pathway and many types of cancer, some bioactive natural compounds, such as quercetin, have been found to prevent cancer by altering the MAPK signalling pathway. Chen et al. observed that stimulating the p38 MAPK signalling pathway boosted the expression of ABCG2, MDR1, and pHSP27 genes in multidrug-resistant spheres of oral cancer cells. Through the suppression of pHSP27 expression and the progressive modification of EMT, they discovered that quercetin might promote apoptosis in cancer spheres. Quercetin and cisplatin, when combined, can inhibit the development of oral cancers and diminish the resistance of the malignancies to treatment [64].
The isocourestine chemical isolated from Bidens bipinnata L. was examined by Huang et al. and was proven to inhibit liver tumour growth and progression. Activation of caspases 3, 8, and 9 leads to an increase in apoptosis, which in turn triggers JNK phosphorylation, suppresses the production of ERK and p38 MAPK and lowers the level of PKC, all of which lead to the cell cycle arrest at G1. Isoquercetin, on the other hand, inhibits the development of transplanted tumours in naked mice when tested in vivo [65–67]. Zhu et al., studied the molecular effect of a quercetin derivative called 7-O-granuestine quercetin (GQ) on gastric cancer cells (SGC-7901 and MGC-803). They found that the compound induced cancer cell apoptosis and cell arrest in the G2/M phase. Its molecular mechanism is that GQ increases ROS production, activates the p38 and JNK pathways, disrupts the regulation of Bcl-2, Bcl-xl, and Bax proteins, releases cytochrome c, and ultimately induces apoptosis [68, 69]. ERK and AKT were shown to be connected with the advancement of esophageal squamous cell carcinoma in a study conducted by Zhao et al. The phosphorylated form of these proteins was found to be associated with the progression of the disease. Quercetin-3-methyl ether was employed to limit the kinase activity of these proteins, which in turn inhibited the proliferation and advancement of esophageal cancer cells, according to the researchers [70].
Another study on prostate cancer (PC3 and LNCaP cell line) by Erdogan et al., showed that quercetin inhibited the proliferation of PC3 and CD44+ / CD133+ cells. On the other hand, co-administration of siRNA and quercetin induces apoptosis and induces cell entry into G1 and cell arrest, and finally inhibits the phosphorylation of AKT, PI3K, and ERK1/2 proteins and the expression of p38, ABCG2 proteins, and NF-κB was reduced [71–73].
The apoptotic effects of quercetin on canine osteosarcoma cells (D17 and DSN) were examined by Ryu et al., Apoptosis, cell cycle, ROS content, mitochondrial membrane depolarization, intracellular calcium concentrations, and quercetin's antiproliferative effect were all shown to be affected by quercetin. S6, AKT, and p70S6K proteins can be inhibited by quercetin whereas ERK1/2, p38, and c-JNK proteins can be elevated. Osteosarcoma cells die as a result of the combination of these events [74].
The p53, NF-ĸB, and apoptotic pathways
In human CRC cell lines obtained from patients with microsatellite instability (MSI), quercetin stimulated 5-fluorouracil-induced apoptosis in a p53-dependent way [75]. Bcl-2 protein expression was reduced in CO115 cells when quercetin and 5-FU were combined. This suggests that quercetin and 5-FU synergism is dependent on the apoptotic mitochondrial pathway [75]. HCT15 (p53 mutant) cells, on the other hand, lacked this synergy, indicating that quercetin's effects are p53-dependent [75]. Quercetin also accelerated the apoptosis generated by doxorubicin (DOX) in hepatoma cells in a p53-dependent manner by downregulating Bcl-xl expression [76]. In line with this result, the quercetin impact on DOX-mediated apoptosis was reduced upon the applying pifithrin-a (p53 inhibitor), Z-VAD fmk (caspase inhibitor), or Bcl-xl expression vector [76]. Moreover, when Bcl-xl expression is inhibited, quercetin promoted p53 protein expression, Bax translocation, transcriptional activity and DOX-induced PUMA (p53 upregulated modulator of apoptosis) expression [76]. Quercetin promoted the expressions of cell death-related genes including Bax, p53, caspase-3 and cytochrome-c in cervical cancer cells, while significantly down-regulated AKT and Bcl-2 expression [77]. Quercetin promoted mitochondrial cytochrome-c release and the accumulation of ROS, resulting in apoptosis and cell cycle arrest at G2/M phases [77]. It has been shown that quercetin strongly promoted p53, Sestin 2, and activated AMPK protein expressions while suppressing mTOR in a dose-dependent manner [78]. Quercetin generated intracellular ROS, leading to the induction of apoptosis and the regulation of the Sestrin 2/AMPK/mTOR pathway in a p53- independent manner [78]. It has been reported that quercetin significantly enhanced Trichostatin A (TSA)-induced apoptosis and growth arrest in A549 cells; the transfection of p53 siRNA reduced its enhancing impacts [79]. However, since p53 silencing does not entirely limit quercetin's effect on TSA-induced apoptosis in A549 cells, it has been postulated that the p53-independent route may potentially contribute to quercetin's boosting effect [79]. Quercetin promoted the antitumor effect of TSA in a xenograft mouse model of lung cancer [79]. The combination of TSA and quercetin treatment of tumors in mice increased p53 and apoptosis levels [79]. It has been reported that quercetin, regardless of p53 status, markedly suppresses cell proliferation, and promotes sub-G1 and apoptotic cell populations in lung cancer cells [80]. Besides, upon treatment of H460 cells with quercetin, genes related to death pathways such as the c-Jun Nterminal kinase (JNK) pathway (JNK, MEKK1, MKK4), death receptors (TNFR1, TRAILR, FAS), the interleukin-1 receptor pathway (IRAK, IL1, IL1R), the NF-κB pathway (IκBα) and the caspase cascade (dFF45, caspase-10) were upregulated, while genes associated with cell survival (NF-κB, AKT, IKK) and genes involved in cell proliferation (SCF, CdKs, SKP2, cyclins) were downregulated [80, 81]. Moreover, quercetin promoted the expression of GAdd45, involved in cell cycle arrest [80].
Quercetin generates intracellular ROS production, decreases mitochondrial membrane potential, and promotes sestrin 2 expression via the AMPK/p38 pathway, thus leading to apoptosis in HT-29 colon cancer cells in a p53-independent pathway [82]. Nano-quercetin (NQ) has been shown to activate p53-ROS crosstalk and cause apoptosis in HepG2 cells; in addition, it triggers epigenetic modifications that lead to cell cycle arrest in the sub-G phase, decreased cell populations in the mitotic and synthetic phases, and suppression of proliferation [83]. In addition, MET containing a variety of phenolics such as quercetin, homoorientin, naringin, and isorhamnetin has been found to induce apoptosis in HCCSCs without relying on p53 by increasing the Bax/Bcl-2 ratio; cleaved PARP protein expression was used to indicate apoptosis [84, 85].
Quercetin caused a marked cell cycle arrest in HT-29 cells in the S-phase [86]. A reduction in phosphorylated-AKT and CSN6 protein expression, as well as a decrease in Bcl-2 and Myc protein expression, was seen after quercetin administration [86]. Moreover, CSN6 overexpression omitted the impacts of quercetin treatment on HT-29 cells, indicating the involvement of the AKT-CSN6-Myc signalling axis in quercetin-induced apoptosis in the cells [86]. The synergistic effect of rutin (a glycoside with 5-FU( in the induction of apoptosis in PC3 cells line has been reported [87]. The combination of rutin/5-FU promoted p53 gene expression and decreased Bcl-2 protein expression [87]. A quercetin-supplemented diet promoted the antitumor effect of TSA in nude mice which bears lung cancer in a dose-dependent manner via the upregulation of p53 [88].
Cell cycle arrest and apoptosis have been seen in MDA-MB-231 breast cancer cells after treatment with quercetin, which was decreased by knocking down the protein Foxo3a [89]. Furthermore, quercetin-induced Foxo3a activity was eliminated by using a JNK inhibitor [89]. The activities of p21, p53 and GADD45 signalling pathways, activated by quercetin, were decreased as a result of Foxo3a knockdown and the suppression of JNK activity [89]. Quercetin exhibited a time- and dose-dependent impact on cell survival in prostate cancer cells [90]. Disturbing the ROS homeostasis and affecting mitochondrial integrity, quercetin caused apoptotic and necrotic cell death in the cells [90]. DU-145 prostate cancer cells with mutated p53 and increased ROS levels displayed a marked decrease in pro-survival AKT pathway activation even though Raf/MEK were stimulated, upon quercetin treatment [90]. However, in the PC-3 prostate cancer cell line, which lacks p53 and PTEN, and bears low ROS levels, vivid activation of AKT and NF-κB pathways were found [90]. Through controlling ROS, AKT, and NF-κB pathways, quercetin employs its anti-cancer impact [90].
Pretreatment with quercetin could make ovarian cancer cells susceptible to radiation-induced cell death in p53 dependent manner [91]; the combinational treatment of irradiation and quercetin could exacerbate DNA damages, cause apoptotic cell death, promote Bax and p21 expressions and reduce Bcl-2 expression in the cells [91]. However, all of these events could be reversed upon knocking down of p53 [91]. The combinational treatment of irradiation and quercetin in the human ovarian cancer xenograft model could restrict tumor growth while activating p53, γ-H2AX and CCAAT/enhancer-binding protein homologous protein [91].
As Caspase-3 is activated in the cancer cells, quercetin ruthenium complex dramatically up-regulates the p53 and Bax, and Caspase-3 expression downregulates the Bcl-2 proteins in HT-29 cell lines [92]. The results of in vivo study showed that the complex treatment could significantly decrease the Bcl-2 expression in colon cancerous tissues while significantly increasing p53 and Bax expression [92]. The results also revealed that the complex caused apoptosis in colon cancer cells via p53-mediated activation of Bax, caspase-3, and Bcl-2 downregulation as well as the suppression of the mTOR/AKT pathway [92]. The vanadium–quercetin combination increased the expression of p53, caspase-9, caspase-3, and Bax and decreased the expression of Bcl-2, mTOR, VEGF, and AKT, resulting in cell cycle arrest and death in rat mammary carcinogenesis and the MCF-7 cell line [93].
When AGS human gastric cancer cells were treated with quercetin, anti-apoptotic Bcl-2, Mcl-1, and Bcl-x protein expression were decreased, and pro-apoptotic proteins such as Bad, Bid, and Bax protein expression was increased [94]. In addition, CDK10 (cyclin-dependent kinase 10), VEGFB (vascular endothelial growth factor B), and KDELC2 (KDEL [Lys-Asp-Glu-Leu] containing 2) gene expressions, associated with apoptosis pathways, were decreased after quercetin treatment [94]. However, quercetin promoted TP53INP1 (tumor protein p53 inducible nuclear protein 1), TNFRSF10D (Tumor necrosis factor receptor superfamily, member 10d, decoy with truncated death domain), and JUNB (jun B proto-oncogene) gene expressions [94, 95].
Quercetin has been shown to promote trichostatin A (TSA) induced apoptosis in H1299 cells, and p53 null cancer cells, in p53-independent manners, which may be less effective than the p53-dependent pathway [79, 96]. The protein expression of p53 significantly was increased in cervical cancer cells including in HeLa, HFF, and SiHa cells after quercetin treatment. Besides, quercetin has also been shown to increase the p53 nuclear signal in SiHa and HeLa cells [97]. Quercetin also increased the transcript level of p21 and the level of Bax protein in HeLa and SiHa cells, famous target genes of p53, suggesting the induction of the transcriptional activity of p53 by quercetin [97]. While increasing p53 and its nuclear signal, quercetin caused apoptosis and cell cycle arrest in the G2 phase in HeLa and SiHa cells [97].
Tamoxifen and quercetin have been reported to control several apoptosis-related gene expressions including p53, Bax, p21, and Bcl-2 in breast cancer cells, leading to cell apoptosis regulation [98]. The results revealed that the impact of quercetin on tamoxifen-induced cell apoptosis is via the p53 signalling pathways [98]. Through suppression of NF-κB and phosphorylation of AKT, the combination of quercetin and curcumin synergistically reduced cell growth and proliferation in K562 chronic myeloid leukemia cells [99]. Additionally, the combination via stimulation of the p53 signalling pathway and FasL triggered apoptotic cell death [99]. Upon exposure to the increasing concentrations of quercetin, p53 expression was increased while cyclin D1 expression was decreased in HepG2 and PANC-1 cells [100]. Furthermore, Quercetin triggered cell cycle arrest in the S phase in GEM-resistant cell lines [100].
Quercetin has been shown to enhance the efficacy of anti-cancer medications such as gemcitabine and doxorubicin, as measured by an increase in the proportion of dead cells [101]. In addition, the results revealed that the combinational treatment of quercetin with anti-cancer drugs decreased the expression of HIF-1α and promoted the expression of cleaved caspase 3 and p53, the regulator of apoptosis [101].
Table 1 presents numerous case studies demonstrating the impact of quercetin on various malignancies via signalling pathways.
Table 1.
The role of quercetin in various cancers mediated by signalling pathways—evidence from preclinical studies
| Signalling pathways | Subfamily involved in the signalling pathway | Cancer types | Quercetin IC50 |
Target genes | Cell line (s)/in vitro model | Possible mechanisms | Refs. |
|---|---|---|---|---|---|---|---|
| MAPK (family) signalling | p38 | Oral cancer | 100 µM | MDR1, ABCG2 Hsp27 | SCC25 |
↓ Hsp70 expression changes in EMT ↑apoptosis in drug-resistant cells |
[64] |
|
p38 ERK JNK |
Hepatocellular carcinoma | 400 µM | – | HepG2 Hep3B |
↓growth, ↓proliferation ↑apoptosis cell cycle arrest in the G1 phase |
[65] | |
|
p38 JNK ERK |
Gastric cancer | 267 μM | TRPM7 | AGS |
↓growth, ↓proliferation, TRPM7 channel inhibition ↑apoptosis |
[66] | |
|
p38 ERK1/2 JNK |
Choriocarcinoma | 20, 50, 100 μM | – | JAR JEG3 |
↓proliferation cell cycle arrest in the sub-G1 phase ↑ROS, ↑MMP |
[67] | |
|
p38 JNK |
Gastric cancer | 20 and 40 µM |
Bcl-2 Bcl-xl Bax |
SGC-7901 MGC-803 |
↓cell viability ↑apoptosis cell cycle arrest in the G2/M phase ↑ROS |
[68] | |
|
p38 JNK |
Retinoblastoma | 0, 25, 75, and 100 µM |
p27 p21 Caspase-3 Caspase -9 |
Y79 |
↓cell viability cell cycle arrest in the G1 phase ↑apoptosis |
[69] | |
| ERK | Esophageal cancer | 0–10 µM |
AP-1 NF-κB, p65 COX-2 |
ESCC |
↓growth ↓proliferation ↓inflammation ↓pre-neoplastic lesion formation by NMBA |
[70] | |
| ERK1/2 | Prostate cancer | 40 μM | p38, ABCG2, NF-κB | PC3, LNCaP ARPE-19 |
↓ cell viability ↑apoptosis cell cycle arrest in G1 phase ↓cell migration |
[71] | |
| ELK1 MEKK/MAP3K5 | Cervical cancer | 25, 50, 100 µM | Caspases, pro-apoptotic genes | HeLa |
↓growth ↓proliferation ↓colony formation ↑apoptosis ↑cell DNA damage cell cycle arrest in G2/M phase ↓cell migration |
[72] | |
|
p38 JNK ERK1/2 |
Melanoma | 0–200 µM | Apoptotic genes | A375SM A375P |
↓cell viability ↓growth ↓proliferation ↑morphological and histological changes ↑apoptosis |
[73] | |
|
p38 JNK ERK1/2 |
Canine osteosarcoma | 0–100 µM | – | D‐17, DSN |
↓proliferation, ↑ MMP, ↑ROS ↓free cytosolic calcium cell cycle arrest in G1 phase |
[74] | |
| JAK/STAT (family) | STAT3 | Gastric cancer | 40 μmol/L | Leptin receptor gene | MGC-803 |
↑apoptosis ↑necrosis cell cycle arrest in G2/M phase |
[53] |
| JAK1/STAT3 | Glioblastoma | 0–100 µM |
IL-6 cyclin D1, MMP2 |
U87, T98G |
↓ cancer cells growth ↓ IL-6 ↓Rb phosphorylation, ↓cyclin D1 ↓MMP2 ↓cell migration |
[54] | |
|
STAT1/3 JAK1/2 |
Cholangiocarcinoma | 20–100 µM | iNOS, ICAM-1 | KKU100, KKU-M139 KKU-M213 |
↓STAT1/3 phosphorylation ↓iNOS, ↓ICAM-1 ↓growth, ↓migration ↓activity |
[55] | |
| STAT3 | Non-small-cell Lung-cancer | 10–100 μM |
NF-κB, Bcl2 Bax |
A549 H460 |
↓growth ↑apoptosis cell cycle arrest in sub-G1 phase |
[56] | |
| JAK1/STAT3 | Breast cancer | 0–100 µM | HER-2, MMP-9 | BT474 |
↓ growth and ↓clonogenic ↑apoptosis ↑STAT3 |
[57] | |
|
JAK2 STAT3/5 |
Cervical cancer | - | Cyclin D1 Apoptotic proteins | Caski, Hela Siha |
↓ cancer cells proliferation, ↓ migration, ↓invasion, ↑apoptosis, ↑autophagy, ↓xenograft growth and development, cell cycle arrest in G2/M phase |
[58] | |
| JAK2/STAT3 | Hepatocellular carcinoma | 80, 120 μmol/L | – | LM3 |
↓tumor cell growth ↓viability ↓migration, ↓invasion ↑autophagy cell cycle arrest in S and G2/M phases |
[59] | |
| Wnt/β-catenin | β-catenin/Tcf | Teratocarcinoma | 70 µM | β-catenin, SOX2, Nanog, Oct4 | NT2/D1 | ↓β-catenin nuclear translocation, ↓transcription factors expression | [37] |
| DKK1, 2 and 3 | Breast cancer | 10, 20, 40 µM | Apoptotic genes | 4T1 |
↑apoptosis ↓ cancer cell viability |
[38] | |
| β-catenin/Tcf | Colon cancer | 40, 80 µmol/L | Cyclin D1, survivin | SW480 |
↑Wnt/β-catenin ↓ cyclin D1, ↓survivin |
[39] | |
|
β-catenin/ TCF/LEF |
Colon cancer | 10–75 µM | GSK3 α ,GSK3 β | HT29 | the level of β-catenin in HT29 cells remained unaffected | [40] | |
| PI3K/Akt | p-Akt | Breast cancer | 25 µM | PTEN | HCC1937 |
↓Akt/PKB phosphorylation ↓cell proliferation |
[43] |
|
p-Akt PI3K |
Leukaemia | 150 µM |
Bcl-2, Bax, caspase-2 caspase -3 poly (ADP-ribose) polymerase cleavage |
HL-60 |
cell cycle arrest in G (0)/G (1) phase ↑apoptosis |
[44] | |
| p-Akt | Gastric cancer stem cell | 20, 100 µM |
Caspase-3 Caspase-9, Bcl2, Cyt-c |
MGC803 | ↑ apoptosis via mitochondrial-dependent pathway and mediated PI3K-Akt signalling pathway | [45] | |
|
PI3K p-Akt |
Cervical cancer | 25, 50, 100 µM | Bcl-2, Bax | HeLa |
cell cycle arrest in G (0)/G (1) phase, anti-proliferative ↑apoptosis |
[42] |
The autophagy pathway
Autophagy is the process by which cells, under conditions of starvation and lack of energy, synthesize new macromolecules and ATP through a series of reactions, and maintain normal metabolism and cell survival, respectively [102–104]. Autophagosome formation is an essential step in autophagy and is based on the positive regulator of the ATG1/ULK complex consisting of ATG1, ATG13, and ATG17. The class III PI3K complex is then activated, ATG5-12 conjugated with 16 promoting autophagy membrane elongation, followed by autophagosome LC3II marker formation [105]. Dual membrane autophagosome formation, characterized by PI3 kinase cascade type III-Atg6/Beclin 1, is a major feature of autophagy [106]. Autophagous expansion is performed by two conjugate systems such as ubiquitin: the Atg12-Atg5 conjugate system and the Atg8/LC3-phosphatidyl ethanolamine conjugate system [107]. Signalling autophagy can be combined with multiple signalling pathways in response to various types of cell stress including hypoxia, radiation, starvation, or active chemical insults [108]. From them, the AKT-mTOR classic signalling path is considered as a normal negative regulator to start the formation of two-sided membranes [109], while according to signalling positively, the accumulation of HIF-1α typically activates the progression of autophagy by suppressing the mTOR1 complex or induction of BNIP3/ BNIP3L that can disrupt the interaction of Beclin 1 with Bcl-2/Bcl-xL [110, 111]. The molecular interference between the autophagic signalling pathway and apoptotic is complex and both routes are similar or attached genes that are very important for their respective performance [112]. It has been reported that Atg5, which is essential in the fusion system such as autophagic can be broken in response to death stimuli and then change autophagy to apoptosis [113]. In addition, Bcl-2 and Bcl-xL, two negative apoptosis regulators can connect to the BH3 domain of Beclin 1 and ensure the progress of autophagy [114].
Treatment of stomach cancer cells with quercetin, autophagic vacuoles and acids vaginal organs (AVOs) was developed, LC3I, and A focus on the autophagosome-recruiting LC3II led to the induction of protective autophagy in stomach cancer cells. By decreasing AKT-mTOR signalling and increasing HIF-expression, quercetin prevents the growth of gastric cancer cells [115]. LC3II accumulation and AVOs formation were also found in quercetin-treated glioblastoma cells and colorectal cancer, all of which induced quercetin-protective tumor cell autophagy [116–118]. However, before treatment with chloroquine, an autophagy inhibitor, it can increase apoptosis and inhibit quercetin proliferation [115, 116].
Based on studies, the development of some tumor diseases is strongly related to autophagy, and there are changes in autophagy in a large number of tumor cells. In the oncology field, autophagy initially appears to be a tumor development inhibitor while further research shows that autophagy can upgrade the progression of the tumor [119, 120].
The Hedgehog signalling pathway
The Discovery of PTCH1 gene mutation in people with Gorlin syndrome and sporadic forms of carcinoma basal cell carcinoma (CBC) has led to the importance of the hedgehog (HH) signalling pathway in human carcinogenesis [121]. Mutations in the PTCH1 gene or one of the components in the hedgehog (HH) signalling pathway is involved in the etiology and the development of some forms of cancers such as—medulloblastoma, breast cancer, colon cancer, pancreatic cancer, esophageal adenocarcinomas and in small cell lung cancer [122]. The hedgehog (HH) signalling pathway is one of the regulatory pathways found in humans preserved since Drosophila Melanogaster. Three counterparts of Drosophila hedgehog (HH) have been identified: Sonic Hedgehog (SHH), Desert Hedgehog (DHH) and Indian Hedgehog (IHH) [123]. Sonic Hedgehog Protein (SHH) is the most important in the development of basal cell carcinoma [124]. PTCH1 is the receptor for all forms of hedgehog (HH) in humans and is part of a cell surface receptor complex consisting of two transmembrane proteins: PTCH1 and SMO (smoothened) [123]. The mechanism by which activation of the hedgehog (HH) pathway leads to carcinogenesis is unknown. Later, hedgehog (HH) is a secretory protein that binds to PTCH1 to activate the signalling pathway. When the hedgehog (HH) binds to the PTCH1 receptor complex, SMO inhibition occurs and the signal is transduced. This is happening through the interaction of a series of proteins, including SUFU (suppressor of fused) which leads to the activation of gli transcription (glioma-associated oncogene) [125]. Gli1 always leads to transcriptional activation, while Gli2 and Gli3 may cause activation or suppression. The regulators of the cell cycle are the target genes WNT, TGF-b, PTCH1 and Gli1. Hedgehog interacting protein (HIP) binds to the hedgehog (HH) and acts as a negative regulator in the signalling pathway [125].
Only a few pharmacological studies are proving the anticancer mechanisms of Quercetin by acting on the hedgehog signalling pathway. In a recent study by Mousavi et al., quercetin nanoparticles were tested in vitro at concentrations of 10, 20, 40, 80, and 100 μM) on LNCaP prostate cancer cells. The results showed that activation of the hedgehog signalling pathway at 40 mM concentrations is the mechanism underlying the anticancer and antiproliferative action [126]. In another study by Slusarz et al., the anticancer effect of Quercetin on PC3 human prostate cancer lines and TRAMP-C2 mouse lines at IC50 1–25 mol/L was demonstrated by inhibiting Gli1 mRNA and hedgehog signalling, respectively [127].
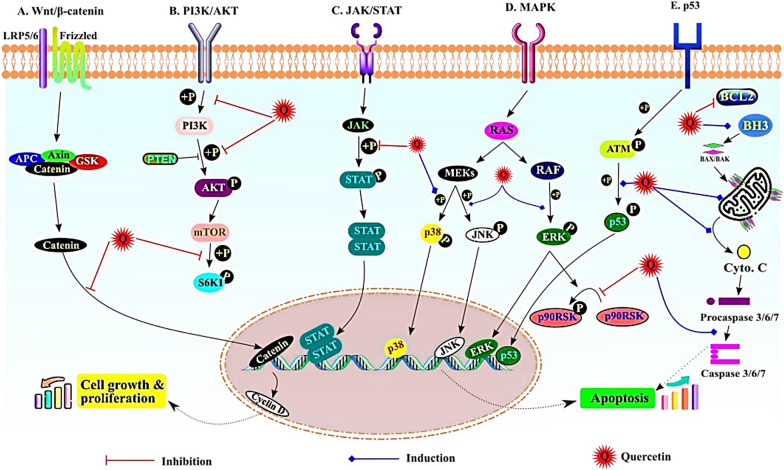
The most representative signalling pathways affected by quercetin during cancer prevention are illustrated in Fig. 1.
Fig. 1.
The most important signalling pathways affected by quercetin during cancer prevention. A) Wnt/β catenin pathway; quercetin inhibits the β-catenin translocation in nucleus, B) PI3K/Akt pathway; inhibition of phosphorylation of PI3k, Akt and S6K, C) JAK/STAT pathway; inhibit the p-STAT formation; D) MAPK pathway; induced phosphorylation of p38, JNK and ERK, E) p53 pathway; induced phosphorylation of p53 and induced the apoptosis pathway
Epigenetic modulation by quercetin
Regulation of miRNAs in different cancer types: effects on cancer cell progression and proliferation
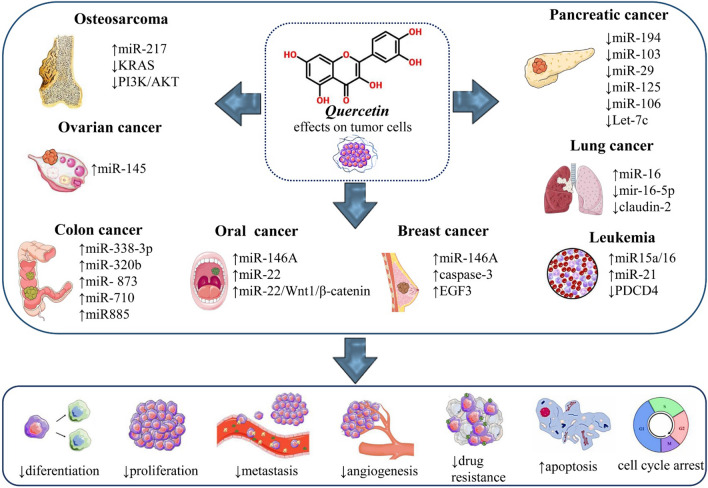
Part of the quercetin anticancer effect is exerted by regulating the expression of miRNAs (Additional file 1) and the interaction between quercetin and miRs is an important subject that has been investigated in many studies (Fig. 2).
Fig. 2.
Regulation of miRNAs by quercetin and its role in various cancer types. Abbreviations and symbols: ↑ increase, ↓decrease
Pancreatic cancer
A recent study on the effect of quercetin on pancreatic cancer shows that the expression of 105 miRNAs changed after treatment with quercetin, including the miR-194, miR-103, miR-29, miR-125, miR-106, and let-7 family, a crucial function in preventing proliferation, invasion, and metastasis as well as promoting cell death is played by these miRNAs. Let-7c is one of the most important miRNAs among them. Numbl is a downstream target of let-7c that is regulated by this miRNA after transcription. The Numbl further antagonizes Notch and therefore prevents the progression of cancer [128]. Numbl and Notch levels are also affected by miR-200b-3p in pancreatic ductal adenocarcinoma (PDA) treatment with quercetin downregulates the miR-200b-3p expression and inhibits self-renewal reduces the cancer stem cells’(CSC) aggressiveness [129]. MiR-142-3p is another example of quercetin-regulated miRs in PDA cell lines [130].
Lung cancer
Mir-16 is also influenced by quercetin treatment in lung cancer. Quercetin can elevate the expression level of miR-16. MiR-16 inhibits the expression of claudin-2 in a promoter-independent way by reducing claudin-2 mRNA stability. Claudin-2 is a proliferation driver so this regulation leads to cancer inhibition [131]. Moreover, quercetin treatment increases the radiosensitivity of non-small cell lung cancer (NSCLC) cells by downregulating miR-16-5p and interfering with the miR-16-5p/WEE1 axis [132].
Oral cancer
Oral cancer appears as a lesion on the oral mucosa, and is caused by the chaotic division and development of cells; it can develop in any area of the oro-maxillo-facial area, but it most often occurs in the area of the tongue and the floor of the mouth [133]. In oral cancer, quercetin increases miR-16 expression levels, which in turn targets MMP-9, MMP-2 and HOXA10, so quercetin can prevent cell proliferation, migration, and survival in oral cancer [134]. It also has a similar effect on oral squamous cell carcinoma (OSCC). Quercetin has been shown to reduce cell viability and induce apoptosis by positively regulating miR-22 and thus regulating the miR-22/Wnt1/β-catenin axis in OSCC [135].
Breast cancer
In a study by Tao at el., it was found that quercetin could positively regulate miR-146a, which in turn, through processes such as regulating the expression of cleaved-caspase-3 and EGFR can induce apoptosis and prevent invasion in breast cancer cells [136].
Ovarian cancer
In ovarian cancer cell lines, including SKOV-3 and A2780, quercetin induces apoptosis by rearranging miR-145 [137].
Colon cancer
Colon cancer is a type of cancer whose starting point is in the colon, an important segment of the digestive tract [3]. In colon cancer HCT-116 cell line, miR-338-3p -CRC, miR-320b, miR-320c, and miR-320d, miR-125b-2-3p, miR873, miR-710, miR-20, and miR-885 have all been significantly adjusted due to quercetin treatment [138].
Osteosarcoma
Quercetin can also regulate the cancer cells' resistance to chemotherapy and radiotherapy. Quercetin-treated osteosarcoma 143B cells show less resistance to cisplatin due to quercetin-induced positive regulation of miR-217. This upregulation decreases one of the miR-217 targets, KRAS level both in mRNA and protein states and inhibits the PI3K/AKT oncogenic pathway and subsequently inhibits the cisplatin resistance [139].
Chronic lymphocytic leukemia
Quercetin increases the expression level of miR15a/16 chronic lymphocytic leukemia (CLL) and reduces the radio-resistant B-1 cells’ ability to survive [140]. In addition to the above, quercetin can be effective in counteracting the effects of carcinogens on cells. A 2017 study on BEAS-2B and mouse model showed that quercetin can reverse the upregulation of onco miR-21 and inhibition of its target, tumor suppressor gene programmed cell death 4 (PDCD4), which was induced by hexavalent chromium [Cr(VI)] carcinogen exposure [141] (Fig. 2).
Quercetin as bioactive molecule in cancer
Plants are important sources for the treatment of cancers because they contain secondary plant metabolites [133, 142, 143]. Quercetin is considered for chemical prophylaxis that acts as a modulator in signal transduction pathways to prevent, inhibit or reverse carcinogenesis [144, 145]. To assess anticancer activities of quercetin in biological systems various studies have been done. Thus, these investigations indicated that quercetin and its metabolites, which exist in diverse plants, have an essential function in protecting against cancer and oxidative stress. As an anti-oxidant and anti-inflammatory, quercetin is widely used. These two quercetin effects have been commonly employed to cope with oxidative stress and inflammation and are shown to be the major source of supplements for individuals suffering from this condition [146].
Structural activity relationship of quercetin with anticancer mechanism
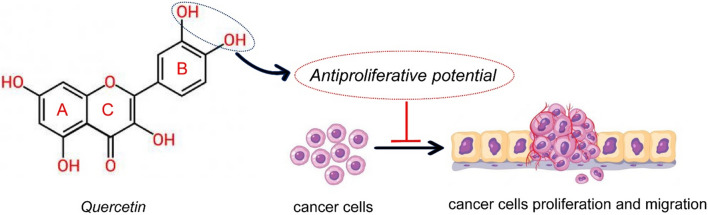
Quercetin is a flavonoid and chemically it is a phenyl-substituted chromone composed of a basic skeleton of fifteen carbon atoms, composed of a chromium nucleus formed by the benzo ring A and the heterocyclic ring C, and in the aromatic ring B has a phenyl substitution [147]. Data have shown that the different substituents in rings A and B are responsible for the pharmacological activities of quercetin [148].
Although many researchers have attempted to highlight and understand the possible structure-anticancer activity correlation of quercetin, there is a scarcity of data to explain the relationship between chemical structure-cytotoxic and antitumor effect [149]. As a potential correlation, the hydroxylation pattern of the B ring in the chemical structure of quercetin would be responsible for its antiproliferative effects and inhibition of protein kinase B (AKT) [148, 150] (Fig. 3).
Fig. 3.
The chemical structure of the flavonol quercetin (3,3′,4′,5,7-pentahydroxyflavone) and potential structure-anticancer activity relationship. Symbol: ⊥ inhibition
Synergistic mechanisms of quercetin with anticancer drugs and other phytochemicals
Many studies show that quercetin in combination with anticancer drugs and other anti-cancer bioactive compounds can have a greater effect [151].
The use of quercetin in combination with temozolomide, a standard care chemotherapy treatment for brain tumors, significantly improved the inhibitory effect of temozolomide on human glioblastoma/brain cancer cells and suppressed the survival of glioblastoma cells [152].
The benefits of quercetin in a study by Singhal et al. [153], showed its effects on the management of recurrent cases of breast cancer that is not surgical. In breast cancer cells, quercetin with doxorubicin was administered, and it became clear that the anti-tumor effects of synthetic drugs strengthen. In addition, quercetin combines the two substances to reduce the side effects of synthetic drugs on non-tumor cells. Therefore, it is considered a promising factor in the development of chemotherapy compounds in the treatment of breast cancer [154].
Another study showed that the use of quercetin may increase the anticancer effects of doxorubicin chemotherapy on liver cancer cells while protecting normal liver cells [76]. Li at el. evaluated the impact of the use of quercetin in combination with cisplatin chemotherapy in human oral squamous cell carcinoma (OSCC) cell lines, as well as in oral cancer-induced mice. The study found that the combination of quercetin and cisplatin increased apoptosis in human oral cancer cells, as well as inhibited the growth of cancer in mice, suggesting the therapeutic potential of the combination of quercetin and cisplatin in oral cancer [155].
A combination of hyperoside and quercetin (QH; 1:1) is shown to have synergic anticancer effects. QH treatment in PC3 prostate cancer cell line can downregulate miR-21 expression and therefore, can reduce the inhibitory effect of this miR on its targets PDCD4 and MARCKS, which ultimately leads to decreased invasion and increased apoptosis [156–158]. In addition, QH treatment in renal cancer cells can inhibit the expression of specificity protein (Sp) transcription factors and survive in expression which is controlled by SPs. Further studies show that this inhibition is exerted via down-regulating the oncomiR-27 and up-regulating the zinc finger protein ZBTB10 [157].
Singh et al. demonstrated in vivo in mice with transgenic prostate adenocarcinoma TRAMP that the combination of supplements of quercetin and resveratrol, two antioxidants found abundantly in grapes, had anti-cancer benefits in this mouse model of prostate cancer [159].
The combination of sulforaphane or quercetin with green tea catechins (GTCs) prevents the progression of PDA by upregulating miR-let-7a and downregulating K-ras. The combinations have much more anti-cancer benefits than any of these components alone [160]. Combination of resveratrol and quercetin (RQ) has a similar effect on colon cancer [161].
The synergistic combination of arctigenin and quercetin has been studied in two prostate cancer cell lines, LAPC-4 and LNCaP. The results indicate that this combination can inhibit the expression of oncogenic miRs miR21, miR-19b and miR-148a in LAPC-4 and miR21 and miR-148a in LNCaP cell line way more effectively than quercetin alone [162–164].
Quercetin and resistance mechanism of anticancer drugs
Multidrug resistance (MDR) is the most important cause of cancer treatment failure. Deciphering the mechanisms of multidrug resistance could contribute to the efficiency of therapeutic strategies for the treatment of neoplastic diseases, and also mitigate the side effects of drugs. [8, 165]. The reason for this is the increased activity of ATP-binding cassette family transporters (ABC) [166].
Quercetin can reverse the resistance mechanism of anticancer drugs through the inhibition of the group P function and ABCB1 gene expression in many cell lines [167, 168]. However, many studies have focused on the anti-cancer properties of this bioactive compound. Several pathways have been identified which can affect quercetin in various cancers [169, 170].
Therapeutic perspectives
Despite numerous advances in cancer treatment, it is still a life-threatening disease [171–173]. Today, natural compounds due to their predictable function, high therapeutic potential and low toxicity, are significant in preventing various types of cancer [174, 175]. Flavonoids derived from fruits and vegetables are known to be important compounds because of their positive effects on cancer prevention [176].
Various preclinical pharmacological studies have been performed to investigate the anticancer effects of quercetin, which has been shown to reduce the growth rate of cancer cells and also to induce apoptosis [177]. Inducing apoptosis in cancer cells is a vital step to develop a new anticancer drug [146]. In addition, in vivo studies have shown that quercetin is involved in the prevention of several types of cancer, especially colon cancer [178]. Mechanisms responsible for quercetin cancer prevention by eliminating free radicals [179], inhibiting enzymes that activate carcinogens, modifying signal transduction pathways, interacting with estrogen receptors [180], transcription factors [181], and other proteins are affected [182]. Anti-cancer activity of quercetin such as induction of apoptosis [183], fatty acid synthase (FAS) [184], inhibition of cell proliferation [185, 186], reduction of metalloproteinase-2 (MMP-2), and metalloproteinase-9 (MMP-9) expression [187] in prostate cancer cells were studied.
Suppression of carcinogenesis may be due to its radical inhibitory activity [188] and quercetin has been reported to reduce the CYP450 family of enzymes, which plays a key role in the activation of several suspected human carcinogens. Quercetin can demethylase the promoter of the gene p161NK4a, which is hypermethylated in human colon cancer cells [189]. Quercetin regulates the expression of tumor suppressor genes, inhibits the expression of cell cycle genes, and regulates oncogenes expression in the prostate cancer cell line [190]. Quercetin also activates the enzymatic activity of histone deacetylase in which the decreased histone H3 acetylation may be responsible for inhibiting the viable expression and subsequent susceptibility to TRAIL-induced apoptosis.
Studies have revealed that quercetin increases the stability of and promotes the apoptotic effects of the p53 gene through quercetin's ability to phosphorylate and stabilize the p53 gene [191]. In HepG2 cells, quercetin disrupts the cell cycle and induces apoptosis by p53 phosphorylation and by stabilizing p53 in both mRNA and protein levels [192].
Using molecular dynamics simulations, Joshi et al., [193] demonstrated the anti-inflammatory, antioxidant, and analgesic effect observed in the Q-Cl analogue, which indicates good activity in HepG2 cell lines, compared to other cell lines and in particular a decrease in anti-inflammatory activity in structural modification.
Quercetin has been studied in several animal models and human cancer cell lines and found to be present in different cell types including leukemia, colon [194], breast [153], osteosarcoma cells [195], pancreatic [196], gastric [197], and melanoma [198] has anti-proliferative properties. Quercetin has also been tested for leukemia malignancy and has been shown to have an anti-leukemic ability because it acts as an inducer of apoptosis and can also cause allergies [194]. ErbB2 and ErbB3 are tyrosine kinase receptors that have been linked to the growth of human colon cancer and are highly expressed in HT-29 cells. In a study by Kim et al., [194] quercetin reduced the levels of these two enzymes in a dose-dependent manner, and prevented the cell growth of colon cancer cells causing apoptosis in these cells.
Quercetin has been reported to be beneficial against U2OS/MTX300 human osteosarcoma cells by inhibiting proliferation and apoptosis. Inhibition of parathyroid hormone receptor 1 also reduces the invasion, adhesion, proliferation, and migration of osteosarcoma cells [195]. Zhou et al., [196] investigated the effect of quercetin on pancreatic cancer and found that resistance to apoptosis was reversed and that proliferation, angiogenesis, and expression of cancer stem cell markers were reduced by treatment with quercetin, a dietary polyphenol. Quercetin, found freely in foods in addition to forms of β-glycosides such as rutin and quercetin, has been reported to inhibit G1 in human gastric cancer cells [197]. Furthermore, in human gastric cancer cells AGS, quercetin caused morphological changes and reduced total survival through apoptotic cell death, decreased anti-apoptotic proteins Mcl-1, Bcl-2, and Bcl-x and increased the pro-apoptotic proteins Bad, Bax, and Bid [94]. In addition, quercetin has been shown to have anti-cancer effects in melanoma [198]. Inhibition of tumor growth was assessed by quercetin when used as a dietary supplement for experimental models [199].
Clinical therapeutic gaps may result from some potential interactions between quercetin and certain drugs. Oncological patients with various comorbidities who are being treated with antibiotics, anticoagulants, corticosteroids, cyclosporine, digoxin or those on chemotherapy should not take natural supplements with quercetin unless their doctor agrees [200]. Excessive intake of quercetin can lead to side effects, such as digestive effects: nausea, abdominal discomfort or interfering with thyroid function [201].
Concluding remarks
The plant derivative is an appealing source of alternative anti-tumor drugs. Quercetin, polyphenolic flavonoids, possesses some significant anticancer activities. It has been shown that quercetin uses multiple mechanisms to exert its anticancer effects by modulating different dysregulated signalling pathways which implicated apoptosis and autophagy. Particularly, quercetin exerts its anti-cancer effects via modulating numerous signalling pathways like PI3K/AKT, NF-κB, P53, Wnt/β-catenin, MAPK, JAK/STAT and Hedgehog pathway. Quercetin interferes with numerous intracellular signalling molecules such as TNF-α, Bax, Bcl-2, caspases, and VEGF. The anticancer effects of quercetin have been studied in various types of cancers including breast cancer, prostate cancer, ovarian cancer, lung cancer, colon cancer, hepatocellular carcinoma, lymphoma, and pancreatic cancer. However, the majority of the recent anticancer evidence of quercetin is focused on cancer in vitro models. Due to the lack of in vivo studies, there is an urgent need for different in vivo studies in this field to analyze the therapeutic activities and safety of quercetins. Among natural plant extracts, quercetin shows several significant biological anti-tumor effects. ncRNA (including miRNA and lncRNA) plays a key role in cancer development. Quercetin regulates the expression of ncRNAs, which in turn affects the related signalling pathway genes/proteins expression, suppresses the cancer cell growth, promotes cell apoptosis, and enhances sensitivity to chemotherapy agents. These shed the light on the molecular mechanism between quercetin and ncRNAs which can be considered for its application in clinical adjuvant treatment. Taken together, quercetin is a natural compound with a potential anticancer effect in the adjuvant treatment of cancers.
Acknowledgements
We would like to thank the Clinical Research Development Unit of Sina Educational, Research and Treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research.
Abbreviations
- PI3K
Phosphoinositide 3-kinase
- AKT
Protein kinase B
- JAK
Janus kinase
- STAT
Signal transducer and transcription activator
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa B
- DKK1
Dickkopf-related protein 1
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- ERKs
Stimulated by mitogens and growth factors
- GQ
7-O-Granuestine quercetin
- MSI
Microsatellite instability
- DOX
Doxorubicin
- TSA
Trichostatin A
- JNK
C-Jun Nterminal kinase
- NQ
Nano-quercetin
- MET
Methanol extract of triphala
- CDK10
Cyclin-dependent kinase 10
- VEGFB
Vascular endothelial growth factor B
- KDELC2
KDEL [Lys-Asp-Glu-Leu] containing 2
- TP53INP1
Tumor protein p53 inducible nuclear protein 1
- TNFRSF10D
Tumor necrosis factor receptor superfamily, member 10d, decoy with truncated death domain
- JUNB
Jun B proto-oncogene
- YYQFT
Yang-Yin-Qing-Fei-Tang
- AVOs
Acids vaginal organs
- lncRNAs
Long noncoding RNAs
- snoRNA
Small Nucleolar RNA
- HOTAIR
HOX antisense intergenic RNA
- NORAD
Noncoding RNA activated by DNA damage
- SAMMSON
The survival associated mitochondrial melanoma specific oncogenic non-coding RNA
- PVT1
Plasmacytoma variant translocation 1
- MINCR
MYC-induced long non-coding RNA
- PCAT1
Prostate cancer associated transcript 1
- CCAT1 and CCAT2
Colon and cancer associated transcripts 1 and 2 respectively
- TARID
TCF21 antisense RNA inducing demethylation
- GAS5
Growth arrest-specific transcript 5
- hnRNPK
Heterogeneous nuclear ribonucleoprotein K
- BARD1
BRCA1-associated RING domain protein 1
- LED
LncRNA activator of enhancer domains
- NEAT1
LncRNA nuclear enriched abundant transcript 1
- MALAT1
Metastasis associated with lung adenocarcinoma transcript
- lncRNA-ATB
LncRNA-activated by TGF-β
- SChLAP1
Second chromosome locus associated with prostate-1
- PDA
Pancreatic ductal adenocarcinoma
- CSC
Cancer stem cells’
- NSCLC
Non-small cell lung cancer
- OSCC
Oral squamous cell carcinoma
- CLL
Chronic lymphocytic leukemia
- PDCD4
Programmed cell death 4
- QH
Hyperoside and quercetin
- Sp
Specificity protein
- GTCs
Green tea catechins
- RQ
Resveratrol and quercetin
- AD
Adenocarcinoma
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. That is revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
This work was supported by Tabriz University of Medical Sciences of Iran. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Yes.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parina Asgharian and Abbas Pirpour Tazekand contributed equally to this work
Contributor Information
Parina Asgharian, Email: parina.asgharian@gmail.com.
Abbas Pirpour Tazekand, Email: abbaspirpour94@gmail.com.
Kamran Hosseini, Email: kamran_hosseini2015@yahoo.com.
Haleh Forouhandeh, Email: hforouha@gmail.com.
Tohid Ghasemnejad, Email: Tohidgasemnejad@gmail.com.
Maryam Ranjbar, Email: maryam.ranjbar14@yahoo.com.
Muzaffar Hasan, Email: muzaffarhassan88@gmail.com.
Manoj Kumar, Email: manojkumarpuniya114@gmail.com.
Sohrab Minaei Beirami, Email: minaei.sohrab@yahoo.com.
Vahideh Tarhriz, Email: t.tarhriz@yahoo.com.
Saiedeh Razi Soofiyani, Email: saeedeh.razi@gmail.com.
Latipa Kozhamzharova, Email: erasl2006@mail.ru.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk
References
- 1.Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, et al. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. 2021;2021:3687700. doi: 10.1155/2021/3687700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ianoși SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, et al. Non-invasive imaging techniques for the in vivo diagnosis of Bowen's disease: three case reports. Oncol Lett. 2019;17(5):4094–4101. doi: 10.3892/ol.2019.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitrut P, Docea AO, Kamal AM, Mitrut R, Calina D, Gofita E, Padureanu V, Gruia C, Streba L. Colorectal cancer and inflammatory bowel disease; 2016.
- 4.Hosseini K, Jasori S, Delazar A, Asgharian P, Tarhriz V. Phytochemical analysis and anticancer activity of Falcaria vulgaris Bernh growing in Moghan plain, northwest of Iran. BMC Complement Med Ther. 2021;21(1):1–10. doi: 10.1186/s12906-021-03464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifi-Rad J, Bahukhandi A, Dhyani P, Sati P, Capanoglu E, Docea AO, Al-Harrasi A, Dey A, Calina D. Therapeutic potential of neoechinulins and their derivatives: an overview of the molecular mechanisms behind pharmacological activities. Front Nutr. 2021;8:664197. doi: 10.3389/fnut.2021.664197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharifi-Rad J, Quispe C, Herrera-Bravo J, Akram M, Abbaass W, Semwal P, Painuli S, Konovalov DA, Alfred MA, Kumar NVA, et al. Phytochemical constituents, biological activities, and health-promoting effects of the Melissa officinalis. Oxid Med Cell Longev. 2021;2021:6584693. doi: 10.1155/2021/6584693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Goyal S, Gupta N, Chatterjee S, Nimesh S. Natural plant extracts as potential therapeutic agents for the treatment of cancer. Curr Top Med Chem. 2017;17(2):96–106. doi: 10.2174/1568026616666160530154407. [DOI] [PubMed] [Google Scholar]
- 8.Sharifi-Rad J, Quispe C, Kumar M, Akram M, Amin M, Iqbal M, Koirala N, Sytar O, Kregiel D, Nicola S, et al. Hyssopus essential oil: an update of its phytochemistry, biological activities, and safety profile. Oxid Med Cell Longev. 2022;2022:8442734. doi: 10.1155/2022/8442734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasanbeiglu S, Hosseini K, Molavi O, Asgharian P, Tarhriz V. Eryngium billardieri extract and fractions induces apoptosis in cancerous cells. Anti Cancer Agents Med Chem. 2021;22(11):2189–2201. doi: 10.2174/1871520621666211201151736. [DOI] [PubMed] [Google Scholar]
- 10.Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, Szopa A, Sharifi-Rad J, Docea AO, Mardare I, et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22(1):206. doi: 10.1186/s12935-022-02624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossain R, Sarkar C, Hassan SMH, Khan RA, Arman M, Ray P, Islam MT, Daştan SD, Sharifi-Rad J, Almarhoon ZM, et al. In silico screening of natural products as potential inhibitors of SARS-CoV-2 using molecular docking simulation. Chin J Integr Med. 2021;28:1–8. doi: 10.1007/s11655-021-3504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam MS, Quispe C, Hossain R, Islam MT, Al-Harrasi A, Al-Rawahi A, Martorell M, Mamurova A, Seilkhan A, Altybaeva N, et al. Neuropharmacological effects of quercetin: a literature-based review. Front Pharmacol. 2021;12:665031. doi: 10.3389/fphar.2021.665031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shutenko Z, Henry Y, Pinard E, Seylaz J, Potier P, Berthet F, Girard P, Sercombe R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem Pharmacol. 1999;57(2):199–208. doi: 10.1016/S0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 14.Chang W-S, Lee Y, Lu F, Chiang H-C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13(6A):2165–2170. [PubMed] [Google Scholar]
- 15.Iio M, Ono Y, Kai S. FUKUMOTO M: Effects of flavonoids on xanthine oxidation as well as on cytochrome c reduction by milk xanthine oxidase. J Nutr Sci Vitaminol. 1986;32(6):635–642. doi: 10.3177/jnsv.32.635. [DOI] [PubMed] [Google Scholar]
- 16.Friesenecker B, Tsai A, Allegra C, Intaglietta M. Oral administration of purified micronized flavonoid fraction suppresses leukocyte adhesion in ischemia-reperfusion injury: in vivo observations in the hamster skin fold. J Vasc Res. 1994;14(1–2):50–55. doi: 10.1159/000178206. [DOI] [PubMed] [Google Scholar]
- 17.Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88(6):1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 18.Rahman A, Hadi SM, Parish JH. Complexes involving quercetin, DNA and Cu(II) Carcinogenesis. 1990;11(11):2001–2003. doi: 10.1093/carcin/11.11.2001. [DOI] [PubMed] [Google Scholar]
- 19.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130(9):2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 20.Costantino L, Rastelli G, Gamberini MC, Vinson JA, Bose P, Iannone A, Staffieri M, Antolini L, Del Corso A, Mura U. 1-Benzopyran-4-one antioxidants as aldose reductase inhibitors. J Med Chem. 1999;42(11):1881–1893. doi: 10.1021/jm980441h. [DOI] [PubMed] [Google Scholar]
- 21.Soobrattee MA, Bahorun T, Aruoma OI. Chemopreventive actions of polyphenolic compounds in cancer. BioFactors. 2006;27(1–4):19–35. doi: 10.1002/biof.5520270103. [DOI] [PubMed] [Google Scholar]
- 22.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Can Res. 1997;57(14):2916–2921. [PubMed] [Google Scholar]
- 23.Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8(9):529. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvi NK, Rizvi RY, Hadi SM. Interaction of quercetin with DNA. Biosci Rep. 1986;6(10):861–868. doi: 10.1007/BF01116239. [DOI] [PubMed] [Google Scholar]
- 25.Rahman A, Hadi SM, Parish JH, Ainley K. Strand scission in DNA induced by quercetin and Cu(II): role of Cu(I) and oxygen free radicals. Carcinogenesis. 1989;10(10):1833–1839. doi: 10.1093/carcin/10.10.1833. [DOI] [PubMed] [Google Scholar]
- 26.Fazal F, Rahman A, Greensill J, Ainley K, Hadi SM, Parish JH. Strand scission in DNA by quercetin and Cu(II): identification of free radical intermediates and biological consequences of scission. Carcinogenesis. 1990;11(11):2005–2008. doi: 10.1093/carcin/11.11.2005. [DOI] [PubMed] [Google Scholar]
- 27.Zang X, Cheng M, Zhang X, Chen X. Quercetin nanoformulations: a promising strategy for tumor therapy. Food Funct. 2021;12(15):6664–6681. doi: 10.1039/D1FO00851J. [DOI] [PubMed] [Google Scholar]
- 28.Lou M, Zhang L-N, Ji P-G, Feng F-Q, Liu J-H, Yang C, Li B-F, Wang L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed Pharmacother. 2016;84:1–9. doi: 10.1016/j.biopha.2016.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P. Improved oral absorption of quercetin from quercetin Phytosome®, a new delivery system based on food grade lecithin. Eur J Drug Metab Pharmacokinet. 2019;44(2):169–177. doi: 10.1007/s13318-018-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Ma J, Han J, Li M, Shang J. Pharmacokinetic comparison of quercetin, isoquercitrin, and quercetin-3-O-β-d-glucuronide in rats by HPLC-MS. PeerJ. 2019;7:e6665–e6665. doi: 10.7717/peerj.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentová K, Vrba J, Bancířová M, Ulrichová J, Křen V. Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol. 2014;68:267–282. doi: 10.1016/j.fct.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Lam TK, Shao S, Zhao Y, Marincola F, Pesatori A, Bertazzi PA, Caporaso NE, Wang E, Landi MT. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2176–2184. doi: 10.1158/1055-9965.EPI-12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):1–16. doi: 10.1186/s13045-016-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23(2):111–116. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 35.Many AM, Brown AM. Both canonical and non-canonical Wnt signaling independently promote stem cell growth in mammospheres. PLoS ONE. 2014;9(7):e101800. doi: 10.1371/journal.pone.0101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polakis P. Wnt signaling and cancer. Gene Dev. 2000;14(15):1837–1851. doi: 10.1101/gad.14.15.1837. [DOI] [PubMed] [Google Scholar]
- 37.Mojsin M, Vicentic JM, Schwirtlich M, Topalovic V, Stevanovic MJ. Quercetin reduces pluripotency, migration and adhesion of human teratocarcinoma cell line NT2/D1 by inhibiting Wnt/β-catenin signaling. Food Funct. 2014;5(10):2564–2573. doi: 10.1039/C4FO00484A. [DOI] [PubMed] [Google Scholar]
- 38.Kim H, Seo E-M, Sharma AR, Ganbold B, Park J, Sharma G, Kang Y-H, Song D-K, Lee S-S, Num J-S. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. Int J Oncol. 2013;43(4):1319–1325. doi: 10.3892/ijo.2013.2036. [DOI] [PubMed] [Google Scholar]
- 39.Shan B-E, Wang M-X, Li QR. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β-catenin signaling pathway. Cancer Investig. 2009;27(6):604–612. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- 40.Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54(19):7075–7082. doi: 10.1021/jf0612530. [DOI] [PubMed] [Google Scholar]
- 41.Osaki M, Oshimura MS, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9(6):667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 42.Xiang T, Fang Y, Wang S-X. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway. J Huazhong Univ Sci Technol. 2014;34(5):740–744. doi: 10.1007/s11596-014-1345-6. [DOI] [PubMed] [Google Scholar]
- 43.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26(2A):1177–1181. [PubMed] [Google Scholar]
- 44.Yuan Z, Long C, Junming T, Qihuan L, Youshun Z, Chan Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol Biol Rep. 2012;39(7):7785–7793. doi: 10.1007/s11033-012-1621-0. [DOI] [PubMed] [Google Scholar]
- 45.Shen X, Si Y, Wang Z, Wang J, Guo Y, Zhang X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int J Mol Med. 2016;38(2):619–626. doi: 10.3892/ijmm.2016.2625. [DOI] [PubMed] [Google Scholar]
- 46.Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 47.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 48.Harrison DA. The jak/stat pathway. Cold Spring Harb Perspect Biol. 2012;4(3):a011205. doi: 10.1101/cshperspect.a011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature. 2009;461(7265):819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li WX. Canonical and non-canonical JAK–STAT signaling. Trends Cell Biol. 2008;18(11):545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin Y, He L, Chen Y, Wang W, Zhao X, Wu M. Quercetin affects leptin and its receptor in human gastric cancer MGC-803 cells and JAK-STAT pathway. Chin J Cell Mol Immunol. 2012;28(1):12–16. [PubMed] [Google Scholar]
- 54.Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318(8):925–935. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28(6):841–848. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee A, Khuda-Bukhsh AR. Quercetin down-regulates IL-6/STAT-3 signals to induce mitochondrial-mediated apoptosis in a nonsmall-cell lung-cancer cell line, A549. J Pharmacopuncture. 2015;18(1):19. doi: 10.3831/KPI.2015.18.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo H-S, Ku JM, Choi H-S, Choi YK, Woo J-K, Kim M, Kim I, Na CH, Hur H, Jang BH. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncol Rep. 2016;36(1):31–42. doi: 10.3892/or.2016.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo C-L, Liu Y-Q, Wang P, Song C-H, Wang K-J, Dai L-P, Zhang J-Y, Ye H. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed Pharmacother. 2016;82:595–605. doi: 10.1016/j.biopha.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 59.Wu L, Li J, Liu T, Li S, Feng J, Yu Q, Zhang J, Chen J, Zhou Y, Ji J. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019;8(10):4806–4820. doi: 10.1002/cam4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu J, Sun X, Goie JYG, Zhang Y. Regulation of host immune responses against influenza A virus infection by mitogen-activated protein kinases (MAPKs) Microorganisms. 2020;8(7):1067. doi: 10.3390/microorganisms8071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun S-C, Shi J. TRAF regulation of NF-κB and MAPK pathways. Front Immunol. 1849;2018:9. doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearson G, Robinson F, Beers Gibson T, Xu B-E, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 63.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 64.Chen S-F, Nieh S, Jao S-W, Liu C-L, Wu C-H, Chang Y-C, Yang C-Y, Lin Y-S. Quercetin suppresses drug-resistant spheres via the p38 MAPK–Hsp27 apoptotic pathway in oral cancer cells. PLoS ONE. 2012;7(11):e49275. doi: 10.1371/journal.pone.0049275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang G, Tang B, Tang K, Dong X, Deng J, Liao L, Liao Z, Yang H, He S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol Rep. 2014;31(5):2377–2384. doi: 10.3892/or.2014.3099. [DOI] [PubMed] [Google Scholar]
- 66.Kim MC, Lee HJ, Lim B, Ha K-T, Kim SY, So I, Kim BJ. Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells. Int J Mol Med. 2014;33(6):1657–1663. doi: 10.3892/ijmm.2014.1704. [DOI] [PubMed] [Google Scholar]
- 67.Lim W, Yang C, Park S, Bazer FW, Song G. Inhibitory effects of quercetin on progression of human choriocarcinoma cells are mediated through PI3K/AKT and MAPK signal transduction cascades. J Cell Physiol. 2017;232(6):1428–1440. doi: 10.1002/jcp.25637. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, Jiang Y, Shi L, Du L, Xu X, Wang E, Sun Y, Guo X, Zou B, Wang H. 7-O-Geranylquercetin induces apoptosis in gastric cancer cells via ROS-MAPK mediated mitochondrial signaling pathway activation. Biomed Pharmacother. 2017;87:527–538. doi: 10.1016/j.biopha.2016.12.095. [DOI] [PubMed] [Google Scholar]
- 69.Liu H, Zhou M. Antitumor effect of Quercetin on Y79 retinoblastoma cells via activation of JNK and p38 MAPK pathways. BMC Complement Altern Med. 2017;17(1):1–8. doi: 10.1186/s12906-016-1505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao S, Jiang Y, Zhao J, Li H, Yin X, Wang Y, Xie Y, Chen X, Lu J, Dong Z. Quercetin-3-methyl ether inhibits esophageal carcinogenesis by targeting the AKT/mTOR/p70S6K and MAPK pathways. Mol Carcinog. 2018;57(11):1540–1552. doi: 10.1002/mc.22876. [DOI] [PubMed] [Google Scholar]
- 71.Erdogan S, Turkekul K, Dibirdik I, Doganlar O, Doganlar ZB, Bilir A, Oktem G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed Pharmacother. 2018;107:793–805. doi: 10.1016/j.biopha.2018.08.061. [DOI] [PubMed] [Google Scholar]
- 72.Kedhari Sundaram M, Raina R, Afroze N, Bajbouj K, Hamad M, Haque S, Hussain A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci Rep. 2019;39(8):BSR20190720. doi: 10.1042/BSR20190720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim S-H, Yoo E-S, Woo J-S, Han S-H, Lee J-H, Jung S-H, Kim H-J, Jung J-Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur J Pharmacol. 2019;860:172568. doi: 10.1016/j.ejphar.2019.172568. [DOI] [PubMed] [Google Scholar]
- 74.Ryu S, Park S, Lim W, Song G. Quercetin augments apoptosis of canine osteosarcoma cells by disrupting mitochondria membrane potential and regulating PKB and MAPK signal transduction. J Cell Biochem. 2019;120(10):17449–17458. doi: 10.1002/jcb.29009. [DOI] [PubMed] [Google Scholar]
- 75.Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. 2011;68(6):1449–1457. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 76.Wang G, Zhang J, Liu L, Sharma S, Dong Q. Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PLoS ONE. 2012;7(12):e51764. doi: 10.1371/journal.pone.0051764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda-Bukhsh A. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Prolif. 2013;46(2):153–163. doi: 10.1111/cpr.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim GT, Lee SH, Kim YM. Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 colon cancer cells. J Cancer Prev. 2013;18(3):264. doi: 10.15430/JCP.2013.18.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan S-T, Yang N-C, Huang C-S, Liao J-W, Yeh S-L. Quercetin enhances the antitumor activity of trichostatin A through upregulation of p53 protein expression in vitro and in vivo. PLoS ONE. 2013;8(1):e54255. doi: 10.1371/journal.pone.0054255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Youn H, Jeong J-C, Jeong YS, Kim E-J, Um S-J. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H460 lung cancer cells. Biol Pharm Bull. 2013;36(6):944–951. doi: 10.1248/bpb.b12-01004. [DOI] [PubMed] [Google Scholar]
- 81.Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H, Hejazi MS. Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem. 2019;120(11):18854–18861. doi: 10.1002/jcb.29204. [DOI] [PubMed] [Google Scholar]
- 82.Kim GT, Lee SH, Kim JI, Kim YM. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int J Mol Med. 2014;33(4):863–869. doi: 10.3892/ijmm.2014.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bishayee K, Khuda-Bukhsh AR, Huh S-O. PLGA-loaded gold-nanoparticles precipitated with quercetin downregulate HDAC-Akt activities controlling proliferation and activate p53-ROS crosstalk to induce apoptosis in hepatocarcinoma cells. Mol Cells. 2015;38(6):518. doi: 10.14348/molcells.2015.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vadde R, Radhakrishnan S, Reddivari L, Vanamala JK. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/Cyclin D1 and elevation of Bax/Bcl-2 ratio. BioMed Res Int. 2015;2015:649263. doi: 10.1155/2015/649263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan Y, Lu H, Ma H, Feng F, Hu X, Zhang Q, Wang J, Xu Y, Zhao Q. Bioactive compounds of Eriocaulon sieboldianum blocking proliferation and inducing apoptosis of HepG2 cells might be involved in Aurora kinase inhibition. Food Funct. 2015;6(12):3746–3759. doi: 10.1039/C5FO00371G. [DOI] [PubMed] [Google Scholar]
- 86.Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu H, Wang H, Guo S, Hisamitsu T. Quercetin-induced apoptosis of HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis. Mol Med Rep. 2016;14(5):4559–4566. doi: 10.3892/mmr.2016.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satari A, Amini SA, Raeisi E, Lemoigne Y, Heidarian E. Synergetic impact of combined 5-fluorouracil and rutin on apoptosis in pc3 cancer cells through the modulation of p53 gene expression. Adv Pharm Bull. 2019;9(3):462. doi: 10.15171/apb.2019.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan S-T, Chuang C-H, Lin Y-C, Liao J-W, Lii C-K, Yeh S-L. Quercetin enhances the antitumor effect of trichostatin A and suppresses muscle wasting in tumor-bearing mice. Food Funct. 2018;9(2):871–879. doi: 10.1039/C7FO01444A. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen LT, Lee Y-H, Sharma AR, Park J-B, Jagga S, Sharma G, Lee S-S, Nam J-S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J Physiol Pharmacol. 2017;21(2):205. doi: 10.4196/kjpp.2017.21.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J Surg Oncol. 2018;16(1):1–12. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gong C, Yang Z, Zhang L, Wang Y, Gong W, Liu Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. Onco Targets Ther. 2018;11:17. doi: 10.2147/OTT.S147316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roy S, Das R, Ghosh B, Chakraborty T. Deciphering the biochemical and molecular mechanism underlying the in vitro and in vivo chemotherapeutic efficacy of ruthenium quercetin complex in colon cancer. Mol Carcinog. 2018;57(6):700–721. doi: 10.1002/mc.22792. [DOI] [PubMed] [Google Scholar]
- 93.Roy S, Banerjee S, Chakraborty T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, Akt/mTOR pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals. 2018;31(4):647–671. doi: 10.1007/s10534-018-0117-3. [DOI] [PubMed] [Google Scholar]
- 94.Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, Lin YF, Chung JG. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ Toxicol. 2018;33(11):1168–1181. doi: 10.1002/tox.22623. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Tan L, Zhang J-W, Chen H, Liang B, Qiu T, Li Q-S, Cai M, Zhang Q-H. Quercetin is the active component of Yang-Yin-Qing-Fei-Tang to induce apoptosis in non-small cell lung cancer. Am J Chin Med. 2019;47(04):879–893. doi: 10.1142/S0192415X19500460. [DOI] [PubMed] [Google Scholar]
- 96.Chuang C-H, Chan S-T, Chen C-H, Yeh S-L. Quercetin enhances the antitumor activity of trichostatin A through up-regulation of p300 protein expression in p53 null cancer cells. Chem Biol Interact. 2019;306:54–61. doi: 10.1016/j.cbi.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Clemente-Soto AF, Salas-Vidal E, Milan-Pacheco C, Sánchez-Carranza JN, Peralta-Zaragoza O, González-Maya L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol Med Rep. 2019;19(3):2097–2106. doi: 10.3892/mmr.2019.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu Z, Zhao D, Zheng X, Huang B, Xia X, Pan X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ Toxicol. 2020;35(11):1179–1193. doi: 10.1002/tox.22983. [DOI] [PubMed] [Google Scholar]
- 99.Mutlu Altundağ E, Yılmaz AM, Koçtürk S, Taga Y, Yalçın AS. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells. Nutr Cancer. 2018;70(1):97–108. doi: 10.1080/01635581.2018.1380208. [DOI] [PubMed] [Google Scholar]
- 100.Liu Z-J, Xu W, Han J, Liu Q-Y, Gao L-F, Wang X-H, WLi X-L. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anti Cancer Drugs. 2020;31(7):684–692. doi: 10.1097/CAD.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 101.Hassan S, Peluso J, Chalhoub S, Idoux Gillet Y, Benkirane-Jessel N, Rochel N, Fuhrmann G, Ubeaud-Sequier G. Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3D culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1α and MDR1. PLoS ONE. 2020;15(10):e0240676. doi: 10.1371/journal.pone.0240676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136. doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zlatian OM, Comanescu MV, Rosu AF, Rosu L, Cruce M, Gaman AE, Calina CD, Sfredel V. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol. 2015;56(1):175–181. [PubMed] [Google Scholar]
- 104.Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, et al. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. 2021;2021:5905357. doi: 10.1155/2021/5905357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hurley JH, Young LN. Mechanisms of autophagy initiation. Annu Rev Biochem. 2017;86:225–244. doi: 10.1146/annurev-biochem-061516-044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 107.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008;9(9):859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283(16):10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29(10):2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo S, Rubinsztein D. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 2007;14(7):1247. doi: 10.1038/sj.cdd.4402149. [DOI] [PubMed] [Google Scholar]
- 113.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon H-U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8(10):1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 114.Levine B, Sinha SC, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4(5):600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang K, Liu R, Li J, Mao J, Lei Y, Wu J, Zeng J, Zhang T, Wu H, Chen L. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR-and hypoxia-induced factor 1α-mediated signaling. Autophagy. 2011;7(9):966–978. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 116.Kim H, Moon JY, Ahn KS, Cho SK. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev. 2013;2013:596496. doi: 10.1155/2013/596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Carcinogenesis. 2007;28(5):1021–1031. doi: 10.1093/carcin/bgl232. [DOI] [PubMed] [Google Scholar]
- 118.Bi Y, Shen C, Li C, Liu Y, Gao D, Shi C, Peng F, Liu Z, Zhao B, Zheng Z. Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumor Biol. 2016;37(3):3549–3560. doi: 10.1007/s13277-015-4125-4. [DOI] [PubMed] [Google Scholar]
- 119.White E. The role for autophagy in cancer. J Clin Investig. 2015;125(1):42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chai JY, Sugumar V, Alshawsh MA, Wong WF, Arya A, Chong PP, Looi CY. The role of smoothened-dependent and -independent hedgehog signaling pathway in tumorigenesis. Biomedicines. 2021;9(9):1188. doi: 10.3390/biomedicines9091188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci. 2018;18(1):8–20. doi: 10.17305/bjbms.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qi X, Li X. Mechanistic insights into the generation and transduction of hedgehog signaling. Trends Biochem Sci. 2020;45(5):397–410. doi: 10.1016/j.tibs.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42(2):263–279. doi: 10.1007/s40264-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doheny D, Manore SG, Wong GL, Lo HW. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells. 2020;9(9):2114. doi: 10.3390/cells9092114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mousavi N, Rahimi S, Emami H, Kazemi AH, Mohammad Taghi Kashi R, Heidarian R. The effect of quercetin nanosuspension on prostate cancer cell line LNCaP via Hedgehog signaling pathway. Rep Biochem Mol Biol. 2021;10(1):69–75. doi: 10.52547/rbmb.10.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ślusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Can Res. 2010;70(8):3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 128.Nwaeburu CC, Bauer N, Zhao Z, Abukiwan A, Gladkich J, Benner A, Herr I. Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget. 2016;7(36):58367–58380. doi: 10.18632/oncotarget.11122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer. 2017;16(1):23. doi: 10.1186/s12943-017-0589-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.MacKenzie TN, Mujumdar N, Banerjee S, Sangwan V, Sarver A, Vickers S, Subramanian S, Saluja AK. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol Cancer Ther. 2013;12(7):1266–1275. doi: 10.1158/1535-7163.MCT-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sonoki H, Sato T, Endo S, Matsunaga T, Yamaguchi M, Yamazaki Y, Sugatani J, Ikari A. Quercetin decreases claudin-2 expression mediated by up-regulation of microRNA miR-16 in lung adenocarcinoma A549 cells. Nutrients. 2015;7(6):4578–4592. doi: 10.3390/nu7064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Q, Chen Y, Lu H, Wang H, Feng H, Xu J, Zhang B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life. 2020;72(5):1012–1022. doi: 10.1002/iub.2242. [DOI] [PubMed] [Google Scholar]
- 133.Salehi B, Jornet PL, Lopez EPF, Calina D, Sharifi-Rad M, Ramirez-Alarcon K, Forman K, Fernandez M, Martorell M, Setzer WN, et al. Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, aloe vera, green tea and coffee properties. Biomolecules. 2019;9(3):23. doi: 10.3390/biom9030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun M, Qiao B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur J Pharmacol. 2019;847:11–18. doi: 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Zhang C, Hao Y, Sun Y, Liu P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J Pharmacol Sci. 2019;140(2):128–136. doi: 10.1016/j.jphs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 136.Tao SF, He HF, Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol Cell Biochem. 2015;402(1–2):93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 137.Zhou J, Gong J, Ding C, Chen G. Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA-145. Mol Med Rep. 2015;12(2):3127–3131. doi: 10.3892/mmr.2015.3679. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Z, Li B, Xu P, Yang B. Integrated whole transcriptome profiling and bioinformatics analysis for revealing regulatory pathways associated with quercetin-induced apoptosis in HCT-116 cells. Front Pharmacol. 2019;10:798. doi: 10.3389/fphar.2019.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X, Guo Q, Chen J, Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS axis. Mol Cells. 2015;38(7):638–642. doi: 10.14348/molcells.2015.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramos YAL, Souza OF, Novo MCT, Guimarães CFC, Popi AF. Quercetin shortened survival of radio-resistant B-1 cells in vitro and in vivo by restoring miR15a/16 expression. Oncotarget. 2021;12(4):355–365. doi: 10.18632/oncotarget.27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pratheeshkumar P, Son YO, Divya SP, Wang L, Turcios L, Roy RV, Hitron JA, Kim D, Dai J, Asha P, et al. Quercetin inhibits Cr(VI)-induced malignant cell transformation by targeting miR-21-PDCD4 signaling pathway. Oncotarget. 2017;8(32):52118–52131. doi: 10.18632/oncotarget.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sani TA, Mohammadpour E, Mohammadi A, Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M, Etemad L, et al. Cytotoxic and apoptogenic properties of dracocephalum kotschyi aerial part different fractions on calu-6 and mehr-80 lung cancer cell lines. Farmacia. 2017;65(2):189–199. [Google Scholar]
- 143.Salehi B, Shetty MS, Kumar NVA, Zivkovic J, Calina D, Docea AO, Emamzadeh-Yazdi S, Kilic CS, Goloshvili T, Nicola S, et al. Veronica plants-drifting from farm to traditional healing, food application, and phytopharmacology. Molecules. 2019;24(13):35. doi: 10.3390/molecules24132454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, Bertoncelli L, Cooper EL, Cossarizza A. Quercetin and cancer chemoprevention. Evid Based Complement Altern Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alper M, Güneş H. The anticancer and anti-inflammatory effects of Centaurea solstitialis extract on human cancer cell lines. Turkish J Pharm Sci. 2019;16(3):273. doi: 10.4274/tjps.galenos.2018.27146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumar R, Vijayalakshmi S, Nadanasabapathi S. Health benefits of quercetin. Def Life Sci J. 2017;2(2):142–151. doi: 10.14429/dlsj.2.11359. [DOI] [Google Scholar]
- 147.Mohsin NUA, Irfan M, Hassan SU, Saleem U. Current strategies in development of new chromone derivatives with diversified pharmacological activities: a review. Pharm Chem J. 2020;54(3):241–257. doi: 10.1007/s11094-020-02187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Michala A-S, Pritsa A. Quercetin: a molecule of great biochemical and clinical value and its beneficial effect on diabetes and cancer. Diseases. 2022;10(3):37. doi: 10.3390/diseases10030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.López-Lázaro M. Flavonoids as anticancer agents: structure-activity relationship study. Curr Med Chem Anticancer Agents. 2002;2(6):691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- 150.Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci. 2018;13(1):12–23. doi: 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20(13):3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Persano F, Gigli G, Leporatti S. Natural compounds as promising adjuvant agents in the treatment of gliomas. Int J Mol Sci. 2022;23(6):3360. doi: 10.3390/ijms23063360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singhal RL, Yeh YA, Prajda N, Olah E, Sledge G, Weber G. Quercetin down-regulates signal transduction in human breast carcinoma cells. Biochem Biophys Res Commun. 1995;208(1):425–431. doi: 10.1006/bbrc.1995.1355. [DOI] [PubMed] [Google Scholar]
- 154.Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol. 2011;68(5):1161–1172. doi: 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- 155.Li X, Guo S, Xiong XK, Peng BY, Huang JM, Chen MF, Wang FY, Wang JN. Combination of quercetin and cisplatin enhances apoptosis in OSCC cells by downregulating xIAP through the NF-κB pathway. J Cancer. 2019;10(19):4509–4521. doi: 10.7150/jca.31045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang FQ, Liu M, Li W, Che JP, Wang GC, Zheng JH. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol Med Rep. 2015;11(2):1085–1092. doi: 10.3892/mmr.2014.2813. [DOI] [PubMed] [Google Scholar]
- 157.Li W, Liu M, Xu Y-F, Feng Y, Che J-P, Wang G-C, Zheng J-H. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncol Rep. 2014;31(1):117–124. doi: 10.3892/or.2013.2811. [DOI] [PubMed] [Google Scholar]
- 158.Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, Khan AA, Rahmani AH. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules. 2021;26(5):1315. doi: 10.3390/molecules26051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh CK, Chhabra G, Ndiaye MA, Siddiqui IA, Panackal JE, Mintie CA, Ahmad N. Quercetin-resveratrol combination for prostate cancer management in TRAMP Mice. Cancers. 2020;12(8):2141. doi: 10.3390/cancers12082141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int J Oncol. 2014;45(4):1391–1400. doi: 10.3892/ijo.2014.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr Cancer. 2013;65(3):494–504. doi: 10.1080/01635581.2012.725194. [DOI] [PubMed] [Google Scholar]
- 162.Wang P, Phan T, Gordon D, Chung S, Henning SM, Vadgama JV. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol Nutr Food Res. 2015;59(2):250–261. doi: 10.1002/mnfr.201400558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, et al. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid Med Cell Longev. 2022;2022:2910411. doi: 10.1155/2022/2910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, et al. Cucurbita plants: from farm to industry. Appl Sci-Basel. 2019;9(16):21. [Google Scholar]
- 165.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22(47):7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 167.Borska S, Sopel M, Chmielewska M, Zabel M, Dziegiel P. Quercetin as a potential modulator of P-glycoprotein expression and function in cells of human pancreatic carcinoma line resistant to daunorubicin. Molecules. 2010;15(2):857–870. doi: 10.3390/molecules15020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J Chemother. 2005;17(1):86–95. doi: 10.1179/joc.2005.17.1.86. [DOI] [PubMed] [Google Scholar]
- 169.Gates MA, Vitonis AF, Tworoger SS, Rosner B, Titus-Ernstoff L, Hankinson SE, Cramer DW. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2009;124(8):1918–1925. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song NR, Chung M-Y, Kang NJ, Seo SG, Jang TS, Lee HJ, Lee KW. Quercetin suppresses invasion and migration of H-Ras-transformed MCF10A human epithelial cells by inhibiting phosphatidylinositol 3-kinase. Food Chem. 2014;142:66–71. doi: 10.1016/j.foodchem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 171.Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, et al. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus bulbophyllum. Evid Based Complement Altern Med. 2022;2022:6727609. doi: 10.1155/2022/6727609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, et al. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus bulbophyllum. Evid Based Complement Alternat Med. 2022;2022:6727609. doi: 10.1155/2022/6727609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buga AM, Docea AO, Albu C, Malin RD, Branisteanu DE, Ianosi G, Ianosi SL, Iordache A, Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol Lett. 2019;17(5):4170–4175. doi: 10.3892/ol.2019.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6(1):1–13. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taheri Y, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash Mishra A, Sener B, et al. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2022;2022:4024331. doi: 10.1155/2022/4024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharifi-Rad J, Quispe C, Shaheen S, El Haouari M, Azzini E, Butnariu M, Sarac I, Pentea M, Ramírez-Alarcón K, Martorell M, et al. Flavonoids as potential anti-platelet aggregation agents: from biochemistry to health promoting abilities. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1924612. [DOI] [PubMed] [Google Scholar]
- 177.Ahmed MS, Ainley K, Parish JH, Hadi SM. Free radical-induced fragmentation of proteins by quercetin. Carcinogenesis. 1994;15(8):1627–1630. doi: 10.1093/carcin/15.8.1627. [DOI] [PubMed] [Google Scholar]
- 178.Rather RA, Bhagat M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: molecular mechanisms and implications in human health. Cancer Med. 2020;9(24):9181–9192. doi: 10.1002/cam4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bors W, Saran M. Radical scavenging by flavonoid antioxidants. Free Radical Res Commun. 1987;2(4–6):289–294. doi: 10.3109/10715768709065294. [DOI] [PubMed] [Google Scholar]
- 180.Choi J-A, Kim J-Y, Lee J-Y, Kang C-M, Kwon H-J, Yoo Y-D, Kim T-W, Lee Y-S, Lee S-J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19(4):837–844. doi: 10.3892/ijo.19.4.837. [DOI] [PubMed] [Google Scholar]
- 181.Chen J-C, Ho F-M, Chao P-DL, Chen C-P, Jeng K-CG, Hsu H-B, Lee S-T, Wu WT, Lin W-W. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521(1–3):9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 182.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 183.Shen F, Herenyiova M, Weber G. Synergistic down-regulation of signal transduction and cytotoxicity by tiazofurin and quercetin in human ovarian carcinoma cells. Life Sci. 1999;64(21):1869–1876. doi: 10.1016/S0024-3205(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 184.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280(7):5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 185.Daker M, Ahmad M, Khoo AS. Quercetin-induced inhibition and synergistic activity with cisplatin–a chemotherapeutic strategy for nasopharyngeal carcinoma cells. Cancer Cell Int. 2012;12(1):1–8. doi: 10.1186/1475-2867-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhong L, Chen F, Wang H, Ten Y, Wang C, Ouyang R. Effects of quercetin on morphology and VEGF secretion of leukemia cells NB4 in vitro. Zhonghua zhong liu za zhi [Chin J Oncol] 2006;28(1):25–27. [PubMed] [Google Scholar]
- 187.Vijayababu M, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3) Mol Cell Biochem. 2006;287(1):109–116. doi: 10.1007/s11010-005-9085-3. [DOI] [PubMed] [Google Scholar]
- 188.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 189.Tan S, Wang C, Lu C, Zhao B, Cui Y, Shi X, Ma X. Quercetin is able to demethylate the p16INK4a gene promoter. Chemotherapy. 2009;55(1):6–10. doi: 10.1159/000166383. [DOI] [PubMed] [Google Scholar]
- 190.Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22(3):409–414. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 191.Tanigawa S, Fujii M, Hou D-X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem. 2008;72(3):797–804. doi: 10.1271/bbb.70680. [DOI] [PubMed] [Google Scholar]
- 192.Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Vaccine Immunol. 2004;11(1):63–69. doi: 10.1128/CDLI.11.1.63-69.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Joshi U, Gadge A, D’Mello P, Sinha R, Srivastava S, Govil G. Anti-inflammatory, antioxidant and anticancer activity of quercetin and its analogues. Int J Res in Pharma and Biomed Sci. 2011;2:1756–1766. [Google Scholar]
- 194.Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, Cho HJ, Park JH. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J Nutr Biochem. 2005;16(3):155–162. doi: 10.1016/j.jnutbio.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 195.Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed Pharmacother. 2019;114:108839. doi: 10.1016/j.biopha.2019.108839. [DOI] [PubMed] [Google Scholar]
- 196.Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Büchler MW. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37(3):551–561. doi: 10.3892/ijo_00000704. [DOI] [PubMed] [Google Scholar]
- 197.Yoshida M, Sakai T, Hosokawa N, Marui N, Matsumoto K, Fujioka A, Nishino H, Aoike A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990;260(1):10–13. doi: 10.1016/0014-5793(90)80053-L. [DOI] [PubMed] [Google Scholar]
- 198.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87(4):595–600. doi: 10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 199.Yang CS, Landau JM, Huang M-T, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21(1):381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 200.Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. 2018 doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 201.Giuliani C, Bucci I, Di Santo S, Rossi C, Grassadonia A, Piantelli M, Monaco F, Napolitano G. The flavonoid quercetin inhibits thyroid-restricted genes expression and thyroid function. Food Chem Toxicol. 2014;66:23–29. doi: 10.1016/j.fct.2014.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.