Abstract
Gastrointestinal cancer is the most common human malignancy characterized by high lethality and poor prognosis. Emerging evidences indicate that N6-methyladenosine (m6A), the most abundant post-transcriptional modification in eukaryotes, exerts important roles in regulating mRNA metabolism including stability, decay, splicing, transport, and translation. As the key component of the m6A methyltransferase complex, methyltransferase-like 14 (METTL14) catalyzes m6A methylation on mRNA or non-coding RNA to regulate gene expression and cell phenotypes. Dysregulation of METTL14 was deemed to be involved in various aspects of gastrointestinal cancer, such as tumorigenesis, progression, chemoresistance, and metastasis. Plenty of findings have opened up new avenues for exploring the therapeutic potential of gastrointestinal cancer targeting METTL14. In this review, we systematically summarize the recent advances regarding the biological functions of METTL14 in gastrointestinal cancer, discuss its potential clinical applications and propose the research forecast.
Keywords: Gastrointestinal cancer, RNA modification, m6A, METTL14
Introduction
Increasing studies demonstrated that epigenetics plays a crucial role in cancer occurrence and progression [1, 2]. Different to genetic alterations, epigenetic modifications are reversible and inheritable processes which regulate gene expression without DNA sequences changes [3, 4]. Although the scope of epigenetics is not fully explored, it is commonly defined as chemical modifications, including chromatin rearrangement, DNA and RNA methylation, non-coding RNA and histone modification [5]. Previous reports mainly focused on the biological functions of DNA methylation, non-coding RNAs regulation and histone modification [6–8]. Recently, mounting studies have identified more than 100 kinds of chemical modifications in RNA, which exploits a new research field of epigenetic regulation controlled by RNA modification [9–11]. RNA methylation is the main form of RNA modifications, including N6-methyladenosine (m6A), m1A, 1-methylguanosine (m1G), m2G, m6G, m7G, 5-methylcytosine (m5C), 2ʹ-O-methylation (Nm), pseudouridine (Ψ) and Inosine (I), among which m6A modification is the most abundant kind accounting for approximately half of all RNA methylation modifications [5, 12–14]. m6A modification exists in nearly all eukaryotes and in a part of viruses, yeasts, bacteria, and plants [12]. m6A binding sites are found in the RRACH sequence (R = A/G, H = A/C/U) and are mainly enriched in the 3’ untranslated regions (UTRs) near the stop codon of mRNA exon [12, 15]. Remarkably, m6A mediated-RNA epigenetics modification plays an important role in controlling physiological activities, such as embryonic stem cell differentiation, DNA repair, meiosis, tissue remodeling, and circadian rhythm, etc. [11, 16]. Dysregulation of m6A modification gives rise to multiple pathological processes, including tumorigenesis and development [14, 17, 18].
Composition of m6A
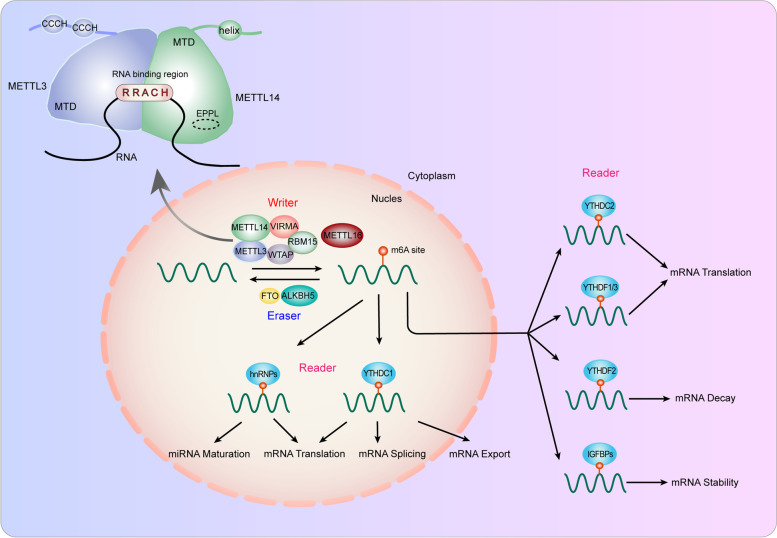
As a dynamic and reversible process, m6A modification can be catalyzed by m6A methyltransferases (“writer”) and eliminated by demethylases (“eraser”) [19–21]. Moreover, RNA-binding proteins (“reader”) specifically recognize and bind to m6A sites to regulate fate of RNAs (Fig. 1) [16, 19, 22].
Fig. 1.
The composition and function of m6A modification. The m6A modification is installed by writers, including METTL3, METTL14, WTAP, RBM15, VIRMA and METTL16. FTO and ALKBH5 are m6A erasers that remove m6A modifications. Readers are required to recognize m6A and exert post-transcriptional regulation. Writers, erasers, and readers synergistically regulate RNA splicing, export, translation, decay, and stability
Writer
“Writer” regulators traditionally consist of methyltransferase-like 3/14/16 (METTL3/14/16), WT1-associated protein (WTAP), zinc finger CCCH-type containing 13 (ZC3H13), virlike m6A methyltransferase-associated (VIRMA/KIAA1429), RNA-binding motif protein 15/15B (RBM15/15B), and Fl(2)d-associated complex component (Flacc) [15, 22–29]. Among them, METTL3 is the first identified m6A methyltransferase and exerts catalytic function with the assistance of METTL14, which stabilizes METTL3 and recognizes target RNAs [30–33]. METTL3 and METTL14 form a stable methyltransferase complex, while WTAP interacts with the heterodimer complex and ensures it to be localized in the nuclear spots and triggers catalytic activity [34–36]. METTL16 can function alone and control m6A modification in mRNAs, U6-snRNA and long noncoding RNAs [27, 29, 37–39]. RBM15, VIRMA and ZC3H13 modulate region-selective m6A methylation modification by binding to methyltransferase complex and localizing it to special RNA sites [40–42].
Eraser
m6A methylation can be eliminated via demethylation, which is mediated by demethylases, also called “eraser”, including Fat mass and obesity-associated protein (FTO) and AlkB homolog 3/5 RNA demethylase (ALKBH3/5) [17, 43]. FTO is identified as the first “eraser” and mainly influence mRNA stability, translation and splicing by regulating m6A demethylation [44, 45]. As the homologue of FTO, ALKBH3 and ALKBH5 principally mediate the transport, metabolism, and assembly of mRNA [46–48]. These erasers promote the transformation of m6A into N6-hydroxymethyladeosine and N6-formyladenosine successively, which is finally hydrolyzed into adenosine [12, 17].
Reader
In addition, another essential group of regulators of m6A modification is “reader”, which can recognize and bind to m6A methylated targets to induce various biological phenotypes. The “reader” mainly consists of YTH domain family of proteins (YTHDC1/2, YTHDF1/2/3) [49–55], IGF2 mRNA binding protein (IGF2BP1/2/3) [56–59], the heterokaryotic nuclear RNA protein family (HNRNPC, HNRNPG) [60–62], and eukaryotic initiation factor 3 (eIF3) [63, 64], which affect m6A methylation by modulating RNA metabolism [16].
Function of m6A
"Writers", "erasers" and “readers” work together to effectively catalyze, remove and recognize m6A methylation and establish a reversible and dynamic balance of m6A modification. mRNA, miRNAs, and long noncoding RNAs can all be regulated by m6A methylation, which controls RNA stability, decay, translation, splicing, transport, localization, and RNA–protein interactions (Fig. 1) [20, 65–67].
Splicing
m6A modification can modulate pre-mRNA splicing by interacting with different splicing factors. FTO preferentially binds adjacent to the alternative splicing exon and polyA sites, thus depresses recruitment of serine/arginine-rich splicing factor 2 (SRSF2) and induces exon 6 skipping [44, 68]. ALKBH5 can promote the phosphorylation of ASF/SF2, and the hyper-phosphorylated ASF/SF2 participates in splicing [46]. It has been reported that downregulation of m6A writers interfered splicing and gene expression [69, 70]. Also, loss of hnRNPC/hnRNPG can change the splicing pattern in an m6A-dependent way [62].
Nuclear export
Previous studies confirmed that ALKBH5 could restrain nuclear export, determining the subcellular location of mRNAs. Mechanistically, ALKBH5 can reduced the hypo-phosphorylated form of ASF/SF2, which promotes mRNA export mediated by TAP-p15 complex [71]. YTHDC1 facilitates nuclear export by promoting the binding of RNA to nuclear RNA export factor 1 (NXF1) and export adaptor protein SRSF3 [72]. Fragile X mental retardation protein (FMRP), another reader, was identified to be indispensable in CRM1-mediated nuclear export [73].
Translation
METTL3 can regulate translation via different readers specifically recognizing m6A sites. It can also exert regulatory role independent on methyltransferase activity, by interacting with eIF3h to promote translation [74, 75]. Recently, METTL16 has also been confirmed to regulate translation in both methyltransferase activity-dependent and -independent manner [27]. Notably, YTHDF proteins play important roles in modulating translation. YTHDF1 can promote the cap-dependent translation initiation by participating in the formation of loop structure with eIF4G and eIF3 and recruitment of ribosomes [49]. Besides, YTHDF1 can facilitate the expression of eIF3C in an m6A-dependent way [76]. In synergism with YTHDF1, YTHDF3 promotes translation via interacting with 40S and 60S ribosome subunits [77]. YTHDF3 also improves the translation efficiency of ITGA6 and promotes malignant progression of bladder cancer [78]. YTHDC2 can boost translation with its helicase activity, independent on m6A modification, which is enhanced by 5' → 3' exoribonuclease XRN1 [79].
Stability
m6A modification serves as a double-edged sword in regulating mRNA stability. YTHDF2 plays a vital role in RNA degradation by recruiting the deadenylase complex CCR4-NOT [80]. And YTHDF3 cooperates with YTHDF2 to facilitate mRNA degradation [81]. For instance, YTHDF2 recognizes the methylation of suppressor of cytokine signaling 2 (SOCS2) and arrestin domain-containing protein 4 (ARRDC4) and induces their mRNA degradation thus enhances metastasis and dissemination of cancer cells [82, 83]. YTHDF1 can induce the degradation of MAT2A mRNA by binding to m6A sites in the 3’-UTR [84]. Another group of readers, IGF2BP1/2/3 can enhance mRNAs stability via KH domain binding to target m6A sites [56]. Moreover, FTO is reported to increase the stability of MYC mRNA by depressing the YTHDF2-mediated decay [85].
METTL14
As a key allosteric activator of METTL3, METTL14 functions as the major m6A methyltransferase to regulate m6A modification on mRNA and non-coding RNA. Advances have been achieved in exploring the crucial roles and molecular mechanisms of METTL14 in multiple types of cancer, especially in gastrointestinal cancer, including liver cancer, colorectal cancer, gastric cancer, and pancreatic cancer. In this review, we will summarize the biological functions and underlying mechanisms of METTL14 in gastrointestinal cancer determined by the latest research progresses of our and other research teams, discuss the potential clinical applications and propose future research directions of METTL14 in gastrointestinal cancer.
The structural basis of METTL14
METTL3 and METTL14 form a stable heterodimer in 1:1 ratio, the N-terminal extension of METTL14 interacts with METTL3 via loops and helixes (Fig. 1) [32, 33]. METTL14 has the homologous methyltransferases domain (MTD) as METTL3, but it owns a closed conformation of catalytic chamber without SAM binding sites. And METTL14 lacks the two CYS-CYS-HIS (CCCH)-type zinc binding motifs of METTL3, which also deprives its catalytic activity [33]. However, it exerts an essential structural role to support METTL3's catalytic function and METTL3 alone merely exhibits weak activity. Significantly, METTL14 provides an RNA-binding scaffold that plays a crucial role in recognizing and binding substrate RNAs [86]. The RGG repeats of the METTL14 C-terminus are supposed to contribute to the recognition of RNAs [87]. Similar to METTL3, METTL14 preferentially recognizes RNA with the “RRACH” (R = A/G, H = A/U/C) sequences [30], but the priority mechanism remains unclear.
The function role of METTL14 in gastrointestinal cancer
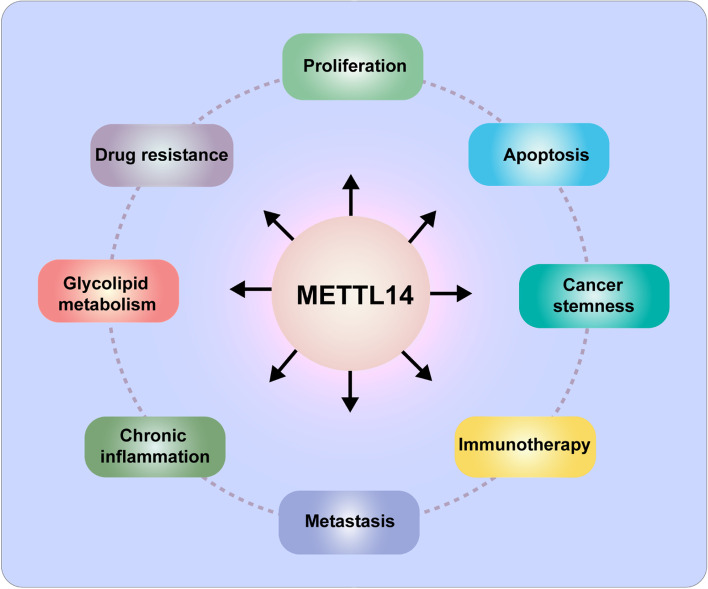
Recent researches have demonstrated that dysregulation of METTL14 is tightly relative to the phenotypes involved in the malignant development of various cancer, including proliferation [88–90], metastasis [91–96], apoptosis [97–101], drug resistance [102–105], cancer stem cell like characteristic [106, 107], immunotherapy [21, 108, 109], chronic inflammation [110] and glycolipid metabolism (Fig. 2) [95]. Herein, we systematically summarize the recent advances of METTL14 in gastrointestinal cancer (Table 1).
Fig. 2.
The biological function of METTL14 in cancer. METTL14 is involved in various processes of tumor development, including proliferation, metastasis, apoptosis, drug resistance, cancer stem cell like characteristic, immunotherapy, chronic inflammation, and glycolipid metabolism
Table 1.
Role of METTL14 in gastrointestinal cancer
| Cancer type | Role | Target | Upstream | Reader | Functions |
|---|---|---|---|---|---|
| HCC | Tumor suppressor |
EGFR PI3K/AKT |
Suppresses migration and invasion [111] | ||
| Tumor suppressor | miR-126 | Suppresses metastasis [92] | |||
| Tumor suppressor | SLC7A11 | HIF-1α | YTHDF2 | Suppresses hypoxia blocked-ferroptosis [112] | |
| Tumor suppressor |
USP48 SIRT6 |
Attenuate glycolysis and malignancy [113] | |||
| Tumor suppressor | HNF3γ | Suppresses proliferation and sorafenib resistance [103] | |||
| Oncogene |
ACLY SCD1 |
Promotes FA synthesis and lipid accumulation [114] | |||
| CRC | Tumor suppressor |
miR-375 YAP1/SP1 |
Suppresses migration, invasion, proliferation [94] | ||
| Tumor suppressor | KLF4 | IGF2BP2 | Suppresses migration and invasion [115] | ||
| Tumor suppressor | ARRDC4 |
HuR TCF4 |
YTHDF2 | Suppresses metastasis [83] | |
| Tumor suppressor | SOX4 |
KDM5C H3K4me3 |
YTHDF2 | Suppresses metastasis [116] | |
| Tumor suppressor | LincR XIST | YTHDF2 | Suppresses proliferation and metastasis [93] | ||
| Tumor suppressor | miR-149-3p | Suppresses inflammation and malignancy [110] | |||
| Tumor suppressor |
IFN-c/ STAT1/IRF1 |
YTHDF2 | Promotes immune responses to anti-PD-1 therapy [108] | ||
| Tumor suppressor | EBI3 | Promotes antitumor response and CD8 + T cell infiltration [109] | |||
| Tumor suppressor | ANKLE1 | YTHDF1 | Suppresses proliferation, and colony formation [117] | ||
| Oncogene | PHLDB2 | Promotes Cetuximab Resistance [105] | |||
| GC | Tumor suppressor | circORC5 | Suppresses proliferation and invasion [118] | ||
| Tumor suppressor |
Wnt/ PI3K‐Akt |
Suppresses proliferation and invasion [119] | |||
| Tumor suppressor |
PI3K/AKT/ mTOR |
Suppresses proliferation and invasion [120] | |||
| Oncogene | LINC01320 | Promotes migration, invasion, proliferation [121] | |||
| PC | Oncogene | PERP | Suppresses growth and metastasis [91] | ||
| Oncogene | CDA | P65 | Promotes gemcitabine resistance [122] | ||
| Oncogene |
AMPKα/ ERK/mTOR |
Suppresses apoptosis and autophagy [101] | |||
| Tumor suppressor | PIK3CB | YTHDF2 | Suppresses proliferation and invasion [123] | ||
| Tumor suppressor |
CLK1 SRSF5 |
Suppresses proliferation and metastasis [96] |
Liver cancer
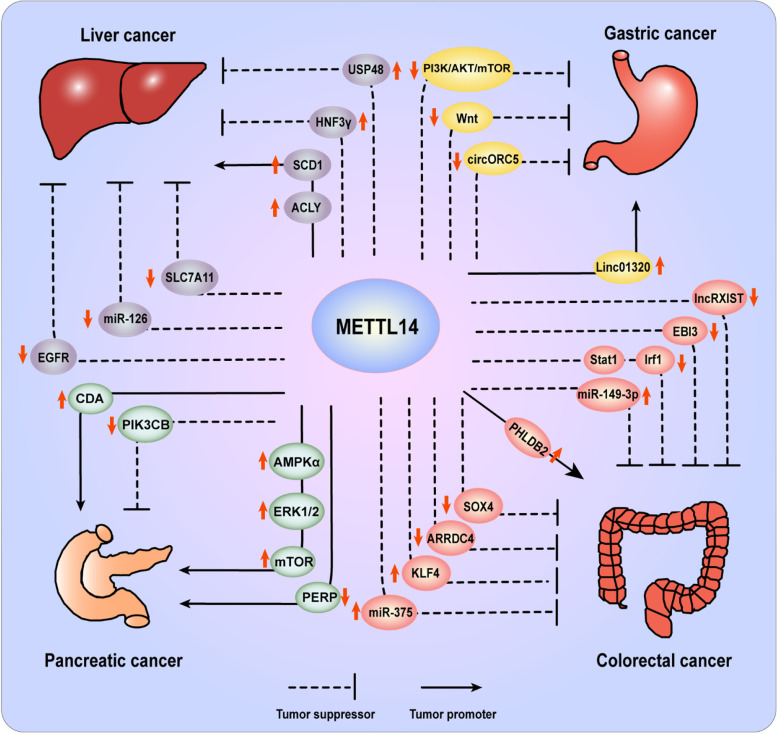
Liver cancer (LC) is a common malignancy with the fourth lethality in cancers worldwide. The predominant form of LC is hepatocellular carcinoma (HCC), which accounts for ~ 80% of primary LC and present an increasing incidence globally [124]. Emerging reports have confirmed the significance of m6A modification in LC, and continuous efforts have been put to investigate the complicated molecular mechanism of abnormal m6A modification and dysregulation of m6A regulators in HCC. First of all, the expression level of METTL14 was identified to be obviously decreased in HCC, which closely correlated with clinicopathological factors, including tumor stage and prognosis (Fig. 3) [125–128]. Based on the analysis of data from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO), Liu et al. showed the opposite expression level and prognostic value of METTL14 and METTL3 in HCC [127]. Similarly, METTL14 was predicted to participate in HCC malignant progression by modulating the m6A-modified transcripts, such as cysteine sulfonate decarboxylase (CSAD), glutamic oxalacetic transaminase (GOT2), and SOCS2 [126]. Through overlapping RNA-sequencing and m6A-sequencing data, epidermal growth factor receptor (EGFR) was identified as the direct target of METTL14. Knockdown of METTL14 activates EGFR/PI3K/AKT signaling and thus promotes epithelial-mesenchymal transition (EMT), migration and invasion of HCC cells [111]. In metastatic HCC, METTL14 interacts with the microprocessor protein DiGeorge syndrome critical region 8 (DGCR8) to suppress tumor metastasis. Mechanistically, METTL14 enhances the engagement of pri-miR126 by DGCR8 and promotes the subsequent processing into miRNA126, which was recognized as a metastasis suppressor. What’s more, the researchers verified the suppressive role of miR126 in HCC cell metastasis using miR126 mimic and inhibitor [92]. Beyond inhibiting metastasis, METTL14 serves as a tumor suppressor involved in various biological processes. METTL14 facilitates hypoxia-blocked ferroptosis of HCC cells by catalyzing m6A modification at the mRNA 5’UTR of solute carrier family 7 member 11 (SLC7A11), then promotes YTHDF2-dependent degradation of SCL7A11 transcripts [112]. METTL14-triggered m6A methylation also inhibits the degradation of ubiquitin specific peptidase 48 (USP48) mRNA, which can deubiquitylate and stabilize sirtuin 6 (SIRT6) to suppresses glycolysis and HCC tumorigenesis. The METTL14-USP48-SIRT6 signaling may be a potential therapeutic strategy for HCC in the future [113]. In addition, reduced METTL14 level in HCCs decreases the stability of m6A-modified hepatocyte nuclear factor-3γ (HNF-3γ) mRNA, since decreased m6A level impairs IGF2BPs-mediated stabilization of mRNA. Reduced HNF3γ expression not only leads to HCC proliferation by inhibiting the differentiation of HCC cells and liver cancer stem cells, but also downregulates organic anion-transporting polypeptide 1B1 (OATP1B1) and 1B3 (OATP1B3) expression and thus impedes sorafenib uptake, resulting in the decreased sensitivity of HCC cells to sorafenib [103]. However, Yang et al. proposed the opposite role of METTL14 in HCC, they detected upregulated level of METTL14 in both HCC cells and patient samples. It was demonstrated that overexpressed METTL14 stabilized m6A-modified ATP citrate lyase (ACLY) and stearoyl-CoA desaturase 1 (SCD1) mRNA to increase their expression, thereafter aggravated FA synthesis and lipid accumulation, which contributed to DNA damage, chronic inflammation, cell apoptosis, excessive compensatory cell proliferation in livers, further developing non-alcohol Fatty Liver Disease (NAFLD) and HCC [114]. In conclusion, these findings suggested the important impact of METTL14 on LC.
Fig. 3.
The targets and functions of METTL14 in gastrointestinal cancer. In liver cancer, METTL14 suppresses cancer development via downregulating EGFR, miR-126, SLC7A11 and upregulating USP48, HNF3γ, while promotes tumor progression via increasing SCD1 and ACLY expression. In GC, METTL14 suppresses cancer development via inhibiting circORC5, Wnt and PI3K/AKT/mTOR, while promotes tumor progression via upregulating Linc01320. In CRC, METTL14 suppresses cancer development via downregulating ARRDC4, SOX4, EBI3, STAT1/IRF1, LncRXIST and upregulating KLF4, miR-375, miR-149-3p, while promotes tumor progression via increasing PHLDB2 expression. In PC, METTL14 suppresses cancer development via downregulating PIK3CB, while promotes tumor progression via increasing CDA, AMPKα/ERK/mTOR, and downregulating PERP
Colorectal cancer
Colorectal cancer (CRC) is a malignant tumor worldwide with an increasingly high incidence and mortality. Recurrence and metastasis are stubbornly major barriers to the treatment of CRC patients. According to the statistics, there are approximately 945,000 new cases and about 700,000 deaths of CRC every year [129–131]. Despite the great research advances in CRC over the years, the molecular mechanisms underlying tumorigenesis and development are still elusive. Recently, growing evidences revealed that METTL14-mediated m6A modification plays a vital role in controlling the progression of CRC (Fig. 3). Experimental studies and bioinformatics confirmed that METTL14 is highly expressed in CRC compared with normal tissues, and high expression level of METTL14 is closely associated with the better prognosis of CRC patients [83, 132, 133]. METTL14 can inhibit CRC metastasis and proliferation through multiple pathways and mechanisms. For example, Chen et al. discovered that the overexpressed METTL14 dramatically enhanced m6A level of CRC cells and suppressed CRC proliferation and metastasis in vitro, while METTL14 loss exerts opposite roles. Mechanistically, METTL14 modulates the processing of pre-miR-375 by DGCR8 and increases miR-375 level via a m6A dependent manner, which subsequently inhibits CRC growth and metastatic capability through downregulating Yes-associated protein 1 (YAP1) and SP1 respectively [94]. Wang et al. found that METTL14 can upregulate the expression of tumor suppressor protein Kruppel-like factor 4 (KLF4) to inhibit the invasion and metastasis of CRC cells. During this process, IGF2BP2 was involved in identifying m6A methylation sites of KLF4 to stabilize its mRNA [115]. In addition, our recent study demonstrated that METTL14 served as an independent predictor of CRC survival and suppressed CRC metastasis in vivo and in vitro. Through transcriptomic sequencing (RNA-seq) and methylated RNA immunoprecipitation sequencing (MeRIP-seq), METTL14 was identified to downregulate ARRDC4 by promoting its mRNA degradation depending on YTHDF2 recognition. Furthermore, the EMT related transcriptional factor ZEB1 was elevated by increased ARRDC4 in METTL14 deficient CRC cells, promoting metastasis of CRC [83]. Chen et al. also indicated METTL14 as a prognostic factor in CRC. They proved that METTL14 and YTHDF2 synergistically regulated m6A methylation modification and decreased the expression of SRY-box transcription factor 4 (SOX4), thereby abrogated EMT and PI3K/AKT signaling pathway, ultimately inhibited migration, invasion, and metastasis of CRC both in vivo and on vitro [116]. Another research confirmed that METTL14 blocked the metastasis and proliferation of CRC by decreasing oncogenic lncRNA XIST expression relying on YTHDF2-mediated degradation [93]. Recently, Tian et al. showed that the variant rs8100241[A] of tumor suppressor ankyrin repeat and LEM domain-containing protein 1 (Ankle1) could be more efficiently catalyzed by METTL14 and recognized by YTHDF1, thus upregulating m6A methylation level and protein expression of ANKLE1, which correlates with a reduced risk of CRC by suppressing tumor malignant proliferation and maintaining the genomic stability [117]. Moreover, besides metastasis and proliferation, METTL14 can modulate other malignant phenotypes. Dong et al. proved that METTL14 depletion in CRC-associated macrophages can induce epstein-Barr virus induced 3 (EBI3) upregulation in an m6A dependent manner mediated by YTHDF2, contributing to CD8+ T cells dysfunction, thereafter accelerating malignant progression of CRC, which was verified by mouse models and clinical samples [109]. Nevertheless, Wang et al. observed that loss of METTL14 elevated the response of CRC to programmed cell death-1 (PD-1) therapy. Reduced METTL14 can promote the amount of CD8 + T cells to secrete interferon-γ (IFN-γ), Chemokine (C-X-C motif) ligand 19 (CXCL19) and CXCL10, via enhancing the stability of Signal transducer and activator of transcription 1(STAT1) and interferon regulatory factor 1 (IRF1) mRNA dependent on YTHDF2, both of which are involved in IFN-γ signaling and anti-PD1 response. This enlightened that METTL14 could be a potential therapeutic target in mismatch-repair-proficient or microsatellite instability-low (pMMR-MSI-L) CRC [108]. Furthermore, Cao et al. revealed that Enterotoxigenic Bacteroides fragilis (ETBF) inhibited METTL14 to reduce m6A modified splicing of pri-miR-149, leading to downregulation of miR-149-3p. Subsequently, the decreased miR-149-3p not only induced PHF5A-mediated KAT2A RNA alternative splicing to promote tumorigenesis of CRC, but also contributed to the differentiation of Th17 cells resulting in intestinal inflammation [110]. However, Luo et al. showed the proto-oncogene role of METTL14 in CRC. They found that oxidative stress induced by chemotherapeutic drug could upregulate METTL14, which elevated the expression of Pleckstrin homology-like dom ain family B member 2 (PHLDB2). Increased PHLDB2 enhanced EGFR stability, contributing to chemo-resistance of CRC to cetuximab [105]. In summary, these studies proved the close connection between METTL14 and CRC progression, suggesting that METTL14 may be a potential therapeutic target for CRC treatment.
Gastric cancer
Gastric cancer (GC) is a common malignant tumor worldwide. Although the incidence and mortality of GC display a downward trend in recent years, there are a mass of GC patients in China with the third mortality rate in cancer death, remarkably higher than other countries and regions [134–137]. The fact that epigenetics plays a significant role in GC progression has been widely confirmed. However, the clinicopathological functions and molecular mechanisms of m6A modification in GC remain largely unclear. To date, there has been no consensus on the role of METTL14 in GC (Fig. 3). Fan et al. showed that METTL14 was downregulated in GC tissues and associated with the poor survival in GC patients. METTL14 deficiency induced proliferation and metastasis of GC cells both in vivo and in vitro, while METTL14 overexpression harbored the opposite roles. Mechanistically, METTL14 triggered circORC5 m6A methylation modification to repress its expression, thus increased miR-30c-2-3p expression and whereafter downregulated AKT1 substrate 1 (AKT1S1) and eukaryotic translation initiation factor 4B (eIF4B), resulting in inhibition of GC tumorigenesis [118]. Liu et al. demonstrated that METTL14 was a tumor suppressor and potential biomarker of GC via bioinformatics analysis and clinical samples. METTL14 was downregulated in GC and exogenous expressed METTL14 repressed aggressive phenotype of GC by deactivating the PI3K/AKT/mTOR signaling axis [120]. In addition, Zhang et al. revealed that deficiency of METTL14 induced proliferation and invasion of GC cells by activating Wnt and PI3K‐Akt signal pathway in vitro. And they also found the potential correlation between m6A level and immunotherapy features and interferon signaling in METTL14-knockdown cells [119]. Nevertheless, Hu et al. expressed the opposite view that METTL14-mediated upregulation of long noncoding RNA Linc01320 can facilitate GC tumorigenesis in vitro. Linc01320 was found to downregulate miR-495-5p, leading to upregulated RAB19 in GC cells, which promotes GC cells proliferation, migration, and invasion in an unclear mechanism [121]. Conclusively, the biofunctions and regulation mechanisms of METTL14 in GC is rarely investigated and the research advances are limited. It is worth to extensively explore the value of METTL14 in GC in the future research.
Pancreatic cancer
Pancreatic cancer (PC) is one of the most malignant tumors with a five-year survival rate of only 8% and largely PC patients die within seven years after surgery treatment [138, 139]. Since the mortality rates are on the rise, PC is predicted to become the second most common cause of cancer death by 2030 [140]. However, the underlying mechanisms of PC’s high lethality are still not well determined. Therefore, screening and identifying the critical molecules that regulate PC progression is meaningful for performing possible therapeutic strategies. Recently, emerging studies have reported the important role of m6A modification in PC (Fig. 3). Bioinformatics projections showed that the expression of METTL14 was closely related to overall survival of pancreatic ductal adenocarcinoma (PDAC) [141]. Based on TCGA database, Xu et al. established an independent risk prognostic signature of PC consisted of 5 m6A regulating genes, including METTL14, METTL3, KIAA1429, ALKBH5 and YTHDF1 [142]. Wang et al. determined that METTL14 served as an oncogene in PC. METTL14 was highly expressed in PC tissue and associated with the poor survival of PC patients. Increased METTL14 expression induced the degradation of p53 effector related to PMP-22 (PERP) via m6A dependent manner, contributing to the proliferation and metastasis of PC [91]. Interestingly, Chen et al. found that Cdc2-like kinases 1 (CLK1)/SR-like splicing factors5 (SRSF5) axis mediated aberrant exon skipping of METTL14, which leads to dysregulated m6A methylation modification and promotes the proliferation and metastasis of PDAC [96]. Moreover, Tian et al. showed that the variant rs142933486[G] allele of oncogene phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta (PIK3CB) was correlated with high level of m6A modification, which was catalyzed by METTL14 and recognized by YTHDF2. This could promote mRNA decay and decrease PIK3CB expression, leading to a reduced risk of PC [123]. A recent study showed that the expression of METTL14 was increased in gemcitabine resistant PC cells, while inhibition of METTL14 significantly enhanced the gemcitabine sensitivity of resistant PC cells by downregulating cytidine deaminase (CDA), an enzyme which can inactivate gemcitabine. And the downregulation of CDA is supposed to be mediated by regulation on the mRNA stability in an m6A-dependent manner [122]. Similarly, METTL14 knockdown promoted apoptosis and autophagy and enhanced sensibility of PC cells to cisplatin by repressing AMPKα, ERK1/2 and mTOR signal pathways, but the regulatory mechanisms of METTL14 and the exact roles of AMPKα and ERK1/2 in this process need further explorations [101]. In general, the role of METTL14 in PC is distinct from that in HCC, CRC, and GC. Since METTL14 acts as an oncogene in PC, it may be an effective therapeutic target for PC.
Upstream regulators of METTL14
Considering the important role of METTL14 in cancer progression, emerging studies have pay emphasis on the upstream regulatory mechanisms involved in the aberrant expression of METTL14 in cancer (Fig. 4).
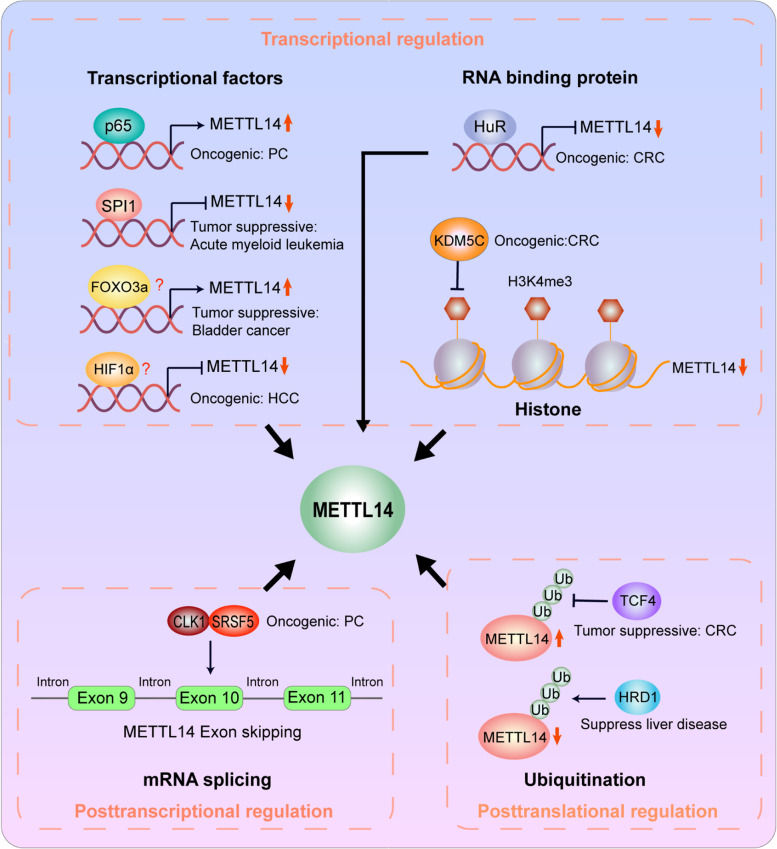
Fig. 4.
The upstream region of METTL14. KDM5C inhibits histone H3K4me3 and decrease METTL14 mRNA expression. The transcription factor SPI1 and HIF1α and RNA binding protein HuR inhibit METTL14 mRNA expression, while p65 and FOXO3a promote METTL14 mRNA expression. The SPI1, HuR and p65 can directly bind to the promoter of METTL14, however, the regulation mechanism of FOXO3a and HIF1α on METTL14 need to be further explored. In addition, CLK1 and SRSF5 can control METTL14 exon10 skipping. Moreover, TCF4 can inhibits ubiquitination of METTL14 and increases its expression. HRD1 is an E3 ubiquitin ligase of METTL14 and can promote ubiquitination of METTL14 and decreases its expression
Transcriptional regulation
It was universally recognized that transcriptional factors and histone modification play the key role in regulating METTL14. In bladder cancer cells, knockdown of transcriptional factor forkhead box O3a (FOXO3a) conspicuously decreased METTL14 expression [143]. A recent study revealed that the transcriptional factor p65 was involved in upregulating METTL14 by targeting its promoter site in PC cells [122]. Moreover, Weng et.al showed that transcription factor PU.1 (Putative oncogene Spi-1, SPI1) functioned as a direct transcriptional suppressor of METTL14. SPI1 knockdown led to upregulation of METTL14 mRNA and protein in both normal and malignant hematopoietic cells, while SPI1 overexpression harbored the opposite effect in these cells [107]. In CRC, our latest findings proved that RNA-binding protein human antigen R (HuR) directly bound to METTL14 promoter and thus suppressed its expression [83]. In addition, Chen et al. indicated that (K)-specific demethylase 5C (KDM5C)-mediated demethylation of H3K4me3 could repress the transcription of METTL14 [116].
Posttranscriptional regulation
Chen et al. found that CLK1/SRSF5 axis could regulate aberrant exon skipping of METTL14 [96]. Through transcriptome sequencing, METTL14 exon10 skipping regulated by the CLK1-SRSF5 axis was identified as the key alternative splicing event, promoting the m6A modification level and metastasis of PDAC cells.
Posttranslational regulation
Recently, we demonstrated that transcriptional factor 4 (TCF4) depletion downregulated METTL14 expression via promoting its ubiquitination-mediated degradation in CRC. Furthermore, Wei et al. revealed that endoplasmic reticulum (ER) proteotoxic stress selectively promoted METTL14 expression through inhibiting its ubiquitination-mediated degradation by repressing HMG-CoA reductase degradation protein 1 (HRD1), an important E3 ubiquitin ligase of METTL14 [100].
Taken together, these findings illustrate that METTL14 abnormal expression can be affected by a series of transcription factors, histone modification and ubiquitination-mediated degradation. Investigation on the upstream regulatory mechanisms of METTL14 dysregulation helps us to better comprehend the biological role of METTL14 in cancers and provides possible therapeutic targets for anti-tumor therapy.
Potential clinical application of METTL14
The above evidences emphasize that METTL14 is essential for tumorigenesis and development of gastrointestinal cancer, suggesting that METTL14 may be a promising biomarker for clinical diagnosis and potential therapeutic target of gastrointestinal cancer. The results from our and other laboratories showed that METTL14 was downregulated in CRC, HCC and GC, repressed tumor proliferation and metastasis and correlated negatively with tumor prognosis [83, 92, 93, 116, 118]. And we found that decreased METTL14 level was tightly associated with tumor stages of CRC. By the multivariate Cox regression analysis, METTL14 was identified as an independent prognostic factor for CRC patients [83]. Therefore. METTL14 may be a promising biomarker of aggressive CRC, HCC, and GC. However, it is obvious that more attention has been paid on exploring the functions and mechanisms of METTL14 in gastrointestinal cancer, while the expression of METTL14 in early stage of tumorigenesis need to be further investigated and confirmed. It is worthwhile to evaluate METTL14 as a biomarker for early diagnosis and prevention of gastrointestinal cancer in the future studies.
Immunotherapy has become one of the unprecedented therapeutic strategies for multiple malignant tumors by modulating the immune system of cancer patients. By targeting m6A modification, immune responses can be further significantly activated during antitumor immunotherapy. A recent study indicated that suppression of METTL14 mediated-m6A mRNA modification elevated the therapeutic effect of anti-PD-1 therapy in CRC. Inhibition of METTL14 not only promoted the proliferation and accumulation of cytotoxic tumor-infiltrating CD8 + T cells, but also induced the secretion of IFN-C, CXCL9, and CXCL10, thus enhanced immunotherapy efficacy and suppressed cancer proliferation [108]. In addition, METTL14 plays an important role in regulating chemoresistance, which seriously limited the efficacy of chemotherapy, the main clinical treatment for gastrointestinal cancer. In PC, METTL14 knockdown enhanced sensibility of cancer cells to cisplatin, promoting apoptosis and autophagy by repressing AMPKα, ERK1/2 and mTOR signal pathways [101]. Similarly, inhibition of METTL14 enhanced the gemcitabine sensitivity of PC cells by downregulating CDA [122]. METTL14 also mediated chemoresistance of CRC cells to cetuximab and HCC cell to sorafenib [103]. Given the crucial role of METTL14 in gastrointestinal cancer, it is urgently expected to screen, design, and develop effective METTL14 inhibitors and activators. Additionally, exploring drugs targeting upstream or downstream molecules of METTL14 may be also an effective measure for gastrointestinal cancer therapy. The combined applications of METTL14 inhibitor or activators with chemotherapy or immunotherapy show great potential as a promising treatment strategy and are anticipated to be investigated in the future.
Discussion
m6A methylation is the most abundant RNA modification and has become research hotspot in recent years. m6A methylation affects the processing of mRNA and non-coding RNA and is of great significance for gene expression regulation. Mounting evidence indicated that m6A modification plays a critical role in tumorigenesis and progression. The present review showed the expression, function, and the regulatory mechanism of the methyltransferase METTL14 in gastrointestinal cancer, suggesting that METTL14 might be a promising biomarker for clinical diagnosis and therapeutic target of gastrointestinal cancer. However, with breakthroughs made in various aspects, contradictions and uncertainties have also been exposed, which is mainly consist of the following situations. (1) In different cancers, METTL14 has a dual regulatory effect on tumors. It serves as an oncogene in PC, while plays a suppressive role in HCC, CRC, and GC. Such complexity highlights that attention should be given to the application of METTL14 activators or inhibitors in case of inducing other tumors. (2) In different cancers, the expression level of METTL14 varies a lot. Some upstream regulations have been identified in specific cellular context, at transcriptional, posttranscriptional, and posttranslational level. However, the underlying rationales of whether it is upregulated or downregulated remain elusive. (3) For the same cancer, different researchers hold the opposite conclusions of METTL14. For example, Fan et al. found that overexpressed METTL14 inhibited proliferation and metastasis of GC [118], but Hu and his colleagues proved that METTL14-mediated upregulation of Linc01320 promotes GC cells proliferation, metastasis [121]. (4) For the same cancer, different studies showed the inconsistent results of METTL14. For instance, Dong et al. showed that inhibition of METTL14 resulted in CD8+ T cells dysfunction and promoted malignant progression of CRC [109]. Nevertheless, Wang et al. indicated that METTL14 deletion enhanced the efficiency of CD8 + T cells and elevated the immune response of CRC [108]. To sum up, multi-center large-scale studies are extremely required to further determine the role of METTL14 in gastrointestinal cancer, which could lay a foundation for precise individualized treatment.
Although great advances have been achieved in revealing the functions and regulatory mechanisms of METTL14, some problems need to be further explored. (1) As an integrity of m6A methyltransferase complex, METTL14 and METTL3 are supposed to have synergistic effects. However, many findings have demonstrated the opposite expression and functions between them in various cancers. Also, different targets and regulatory mechanisms of METTL3 and METTL14 have been proved. We hypothesized that they have biological functions independent of the methyltransferase complex, with their own bias towards targets. Therefore, the structural basis and regulatory roles of the METTL3/14 complex, and their respective functional mechanisms require further experimental verification. (2) As a promising biomarker for tumor clinical diagnosis, the sensitivity and specificity of METTL14 need to be further clarified. (3) Previous studies suggested that METTL14 may be a potential therapeutic target for gastrointestinal cancer, but insufficient attention was paid to drug development and no specific chemotherapeutic agent targeting METTL14 has been reported in both experimental researches and clinical practice so far. It is worth to determine the validity and feasibility of METTL14-targeted agents alone or in combination with existing therapies for treating tumors in the future. Importantly, special emphasis shall be given on the development of METTL14 inhibitors or activators in cancer treatment due to the double-edged sword roles of METT14 in gastrointestinal cancer.
Conclusion
In summary, METTL14 plays an important role in gastrointestinal cancer and it may serve as a promising diagnostic/prognostic biomarker and a potential therapeutic target. We anticipate more future researches to further explore the therapeutic potential of METTL14 for feasible application in clinical practice.
Acknowledgements
Not applicable.
Abbreviations
- 3’UTRs
3’ Untranslated regions
- ACLY
ATP citrate lyase
- ALKBH3/5
AlkB homolog 3/5 RNA demethylase
- Ankle1
Ankyrin repeat and LEM domain-containing protein 1
- AKT1S1
AKT1 substrate 1
- ARRDC4
Arrestin domain-containing protein 4
- CLK1
Cdc2-like kinases 1
- CRC
Colorectal cancer
- CSAD
Cysteine sulfonate decarboxylase
- CXCL19
Chemokine (C-X-C motif) ligand 19
- DGCR8
DiGeorge syndrome critical region 8
- EBI3
Epstein-Barr virus induced 3
- EGFR
Epidermal growth factor receptor
- eIF
Eukaryotic initiation factor
- EMT
Epithelial-mesenchymal transition
- ER
Endoplasmic reticulum
- Flacc
Fl(2)d-associated complex component
- FOXO3a
Forkhead box O3a
- FTO
Fat mass and obesity-associated protein
- GC
Gastric cancer
- GEO
Gene Expression Omnibus
- GOT2
Glutamic oxalacetic transaminas
- HCC
Hepatocellular carcinoma
- HNF-3γ
Hepatocyte nuclear factor-3γ
- HNRNP
Heterokaryotic nuclear RNA protein
- HRD1
HMG-CoA reductase degradation protein 1
- HuR
Human antigen R
- IFN-c
Interferon-c
- IGF2BP
IGF2 mRNA binding protein
- KDM5C
(K)-specific demethylase 5C
- LC
Liver cancer
- m1G
1-Methylguanosine
- m5C
5-Methylcytosine
- m6A
N6-methyladenosine
- METTL3/14/16
Methyltransferase-like 3/14/16
- NAFLD
Non-alcohol Fatty Liver Disease
- OATP1B
Organic anion-transporting polypeptide 1B
- PC
Pancreatic cancer
- PIK3CB
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta
- RBM15/15B
RNA-binding motif protein 15/15B
- SCD1
Stearoyl-CoA desaturase 1
- SIRT6
Sirtuin 6
- SLC7A11
Solute carrier family 7 member 11
- SOCS2
Suppressor of cytokine signaling 2
- SOX4
SRY-box transcription factor 4
- SPI1
Transcription factor PU.1
- SRSF5
SR-like splicing factors 5
- TCF4
Transcriptional factor 4
- TCGA
The Cancer Genome Atlas
- USP48
Ubiquitin specific peptidase 48
- VIRMA/KIAA1429
Virlike m6A methyltransferase-associated
- WTAP
WT1-associated protein
- YTHD
YTH domain family of protein
- ZC3H13
Zinc finger CCCH-type containing 13
Authors’ contributions
Bin Shi and Ke Yang collected the related literature. Hao Wang, Ke Yang and Bin Shi wrote the manuscript. Wei-Wei Liu revised the manuscript. Hao Wang and Guan-min Jiang participated in the design of the review and revised the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by the Youth Innovation Project of University of Science and Technology of China (WK9110000203), the Natural Science Foundation of Anhui Province (2008085MH241), the National Natural Science Foundation of China (No. 82072365) and Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515011666).
Availability of data and materials
Not Applicable.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bin Shi, Wei-Wei Liu and Ke Yang contributed equally to this work.
Contributor Information
Guan-Min Jiang, Email: jianggm3@mail.sysu.edu.cn.
Hao Wang, Email: demo@ustc.edu.cn.
References
- 1.Li S, Kuo HD, Yin R, Wu R, Liu X, Wang L, Hudlikar R, Peter RM, Kong AN. Epigenetics/epigenomics of triterpenoids in cancer prevention and in health. Biochem Pharmacol. 2020;175:113890. doi: 10.1016/j.bcp.2020.113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin YT, Wu KJ. Epigenetic regulation of epithelial-mesenchymal transition: focusing on hypoxia and TGF-beta signaling. J Biomed Sci. 2020;27:39. doi: 10.1186/s12929-020-00632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou S, Treloar AE, Lupien M. Emergence of the Noncoding Cancer Genome: A Target of Genetic and Epigenetic Alterations. Cancer Discov. 2016;6:1215–1229. doi: 10.1158/2159-8290.CD-16-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550–563. doi: 10.1053/j.gastro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Hu BB, Wang XY, Gu XY, Zou C, Gao ZJ, Zhang H, Fan Y. N(6)-methyladenosine (m(6)A) RNA modification in gastrointestinal tract cancers: roles, mechanisms, and applications. Mol Cancer. 2019;18:178. doi: 10.1186/s12943-019-1099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nebbioso A, Tambaro FP, Dell'Aversana C, Altucci L. Cancer epigenetics: Moving forward Plos Genet. 2018;14:e1007362. doi: 10.1371/journal.pgen.1007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Kelly AD, Issa JJ. The promise of epigenetic therapy: reprogramming the cancer epigenome. Curr Opin Genet Dev. 2017;42:68–77. doi: 10.1016/j.gde.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Traube FR, Carell T. The chemistries and consequences of DNA and RNA methylation and demethylation. Rna Biol. 2017;14:1099–1107. doi: 10.1080/15476286.2017.1318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He PC, He C. m(6) A RNA methylation: from mechanisms to therapeutic potential. Embo J. 2021;40:e105977. doi: 10.15252/embj.2020105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Q, Lin H, Miao L, Guo H, Chen Y, Zhuo Z, He J. Functions, mechanisms, and therapeutic implications of METTL14 in human cancer. J Hematol Oncol. 2022;15:13. doi: 10.1186/s13045-022-01231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Geng X, Li Q, Xu J, Tan Y, Xiao M, Song J, Liu F, Fang C, Wang H. m6A modification in RNA: biogenesis, functions and roles in gliomas. J Exp Clin Cancer Res. 2020;39:192. doi: 10.1186/s13046-020-01706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Liang L, Yang Y, Li X, Ma Y. N(6)-methyladenosine as a biological and clinical determinant in colorectal cancer: progression and future direction. Theranostics. 2021;11:2581–2593. doi: 10.7150/thno.52366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, Yang C, Chen Y. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. 2020;19:88. doi: 10.1186/s12943-020-01204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Lv J, Yu H, Han J, Yang X, Feng D, Wu Q, Yuan B, Lu Q, Yang H. Mechanism of RNA modification N6-methyladenosine in human cancer. Mol Cancer. 2020;19:104. doi: 10.1186/s12943-020-01216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu ZM, Huo FC, Pei DS. Function and evolution of RNA N6-methyladenosine modification. Int J Biol Sci. 2020;16:1929–1940. doi: 10.7150/ijbs.45231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai D, Wang H, Zhu L, Jin H, Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018;9:124. doi: 10.1038/s41419-017-0129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou X, Wang JJ, Wei YQ, Sun JJ. Emerging role of RNA modification N6-methyladenosine in immune evasion. Cell Death Dis. 2021;12:300. doi: 10.1038/s41419-021-03585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 23.Guo T, Duan H, Chen J, Liu J, Othmane B, Hu J, Li H, Zu X. N6-Methyladenosine Writer Gene ZC3H13 Predicts Immune Phenotype and Therapeutic Opportunities in Kidney Renal Clear Cell Carcinoma. Front Oncol. 2021;11:718644. doi: 10.3389/fonc.2021.718644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, Chen ZH, Zeng ZL, Wang F, Zheng J, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18:112. doi: 10.1186/s12943-019-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie S, Chen W, Chen K, Chang Y, Yang F, Lin A, Shu Q, Zhou T, Yan X. Emerging roles of RNA methylation in gastrointestinal cancers. Cancer Cell Int. 2020;20:585. doi: 10.1186/s12935-020-01679-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan Q, Liu PY, Haase J, Bell JL, Huttelmaier S, Liu T. The Critical Role of RNA m(6)A Methylation in Cancer. Cancer Res. 2019;79:1285–1292. doi: 10.1158/0008-5472.CAN-18-2965. [DOI] [PubMed] [Google Scholar]
- 27.Su R, Dong L, Li Y, Gao M, He PC, Liu W, Wei J, Zhao Z, Gao L, Han L, et al. METTL16 exerts an m(6)A-independent function to facilitate translation and tumorigenesis. Nat Cell Biol. 2022;24:205–216. doi: 10.1038/s41556-021-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruszkowska A. METTL16, Methyltransferase-Like Protein 16: Current Insights into Structure and Function. Int J Mol Sci. 2021;22(4):2176. doi: 10.3390/ijms22042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK. The U6 snRNA m(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell. 2017;169:824–835. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117. doi: 10.1186/s13045-020-00951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Peng C, Chen J, Chen D, Yang B, He B, Hu W, Zhang Y, Liu H, Dai L, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127. doi: 10.1186/s12943-019-1053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, Li Y, Zhi S, Ding Z, Wang W, Peng Y, Huang Y, Zheng R, Yu H, Wang J, et al. WTAP promotes osteosarcoma tumorigenesis by repressing HMBOX1 expression in an m(6)A-dependent manner. Cell Death Dis. 2020;11:659. doi: 10.1038/s41419-020-02847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satterwhite ER, Mansfield KD. RNA methyltransferase METTL16: Targets and function. Wiley Interdiscip Rev RNA. 2022;13:e1681. doi: 10.1002/wrna.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendel M, Chen KM, Homolka D, Gos P, Pandey RR, McCarthy AA, Pillai RS. Methylation of Structured RNA by the m(6)A Writer METTL16 Is Essential for Mouse Embryonic Development. Mol Cell. 2018;71:986–1000. doi: 10.1016/j.molcel.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hobartner C, Sloan KE, Bohnsack MT. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. Embo Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L, et al. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villasenor R, Hess D, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuidhof HR, Calkhoven CF. Oncogenic and tumor-suppressive functions of the RNA demethylase FTO. Cancer Res. 2022;82(12):2201–2212. doi: 10.1158/0008-5472.CAN-21-3710. [DOI] [PubMed] [Google Scholar]
- 46.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueda Y, Ooshio I, Fusamae Y, Kitae K, Kawaguchi M, Jingushi K, Hase H, Harada K, Hirata K, Tsujikawa K. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, Lu Z, Zheng Z, Dai Q, Wang H. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaccara S, Jaffrey SR. A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell. 2020;181:1582–1595. doi: 10.1016/j.cell.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu Y, Zhuang X. m(6)A-binding YTHDF proteins promote stress granule formation. Nat Chem Biol. 2020;16:955–963. doi: 10.1038/s41589-020-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Gao M, He J, Wu K, Lin S, Jin L, Chen Y, Liu H, Shi J, Wang X, et al. The RNA m(6)A reader YTHDC1 silences retrotransposons and guards ES cell identity. Nature. 2021;591:322–326. doi: 10.1038/s41586-021-03313-9. [DOI] [PubMed] [Google Scholar]
- 55.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller S, Glass M, Singh AK, Haase J, Bley N, Fuchs T, Lederer M, Dahl A, Huang H, Chen J, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47:375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu X, Peng WX, Zhou H, Jiang J, Zhou X, Huang D, Mo YY, Yang L. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2020;27:1782–1794. doi: 10.1038/s41418-019-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Z, Wang T, Wu D, Min Z, Tan J, Yu B. RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J Exp Clin Cancer Res. 2020;39:203. doi: 10.1186/s13046-020-01714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang XT, Li JH, Zhu XX, Huang CS, Gao ZX, Xu QC, Zhao W, Yin XY. HNRNPC impedes m(6)A-dependent anti-metastatic alternative splicing events in pancreatic ductal adenocarcinoma. Cancer Lett. 2021;518:196–206. doi: 10.1016/j.canlet.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Wang LC, Chen SH, Shen XL, Li DC, Liu HY, Ji YL, Li M, Yu K, Yang H, Chen JJ, et al. M6A RNA Methylation Regulator HNRNPC Contributes to Tumorigenesis and Predicts Prognosis in Glioblastoma Multiforme. Front Oncol. 2020;10:536875. doi: 10.3389/fonc.2020.536875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN, He C, Parisien M, Pan T. Regulation of Co-transcriptional Pre-mRNA Splicing by m(6)A through the Low-Complexity Protein hnRNPG. Mol Cell. 2019;76:70–81. doi: 10.1016/j.molcel.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee AS, Kranzusch PJ, Cate JH. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature. 2015;522:111–114. doi: 10.1038/nature14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin Y, Li F, Huang L, Polte C, Duan H, Fang J, Sun L, Xing X, Tian G, Cheng Y, et al. eIF3 Associates with 80S Ribosomes to Promote Translation Elongation, Mitochondrial Homeostasis, and Muscle Health. Mol Cell. 2020;79:575–587. doi: 10.1016/j.molcel.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Peng Y, Li J, Chen Z, Chen F, Tu J, Lin S, Wang H. N(6)-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat Commun. 2020;11:2578. doi: 10.1038/s41467-020-16306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang H, Weng H, Chen J. m(6)A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell. 2020;37:270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng X, Su R, Weng H, Huang H, Li Z, Chen J. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3'-end processing. Nucleic Acids Res. 2017;45:11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, Doki Y, Mori M, Ishii H, Ogawa K. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52:621–629. doi: 10.3892/ijo.2017.4219. [DOI] [PubMed] [Google Scholar]
- 70.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Manley JL. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017;6:e31311. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edens BM, Vissers C, Su J, Arumugam S, Xu Z, Shi H, Miller N, Rojas RF, Ming GL, He C, et al. FMRP Modulates Neural Differentiation through m(6)A-Dependent mRNA Nuclear Export. Cell Rep. 2019;28:845–854. doi: 10.1016/j.celrep.2019.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ianniello Z, Sorci M, Ceci GL, Iaiza A, Marchioni M, Tito C, Capuano E, Masciarelli S, Ottone T, Attrotto C, et al. New insight into the catalytic -dependent and -independent roles of METTL3 in sustaining aberrant translation in chronic myeloid leukemia. Cell Death Dis. 2021;12:870. doi: 10.1038/s41419-021-04169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, Cheng C, Li L, Pi J, Si Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–3831. doi: 10.1093/nar/gkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin H, Ying X, Que B, Wang X, Chao Y, Zhang H, Yuan Z, Qi D, Lin S, Min W, et al. N(6)-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine. 2019;47:195–207. doi: 10.1016/j.ebiom.2019.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li L, Krasnykov K, Homolka D, Gos P, Mendel M, Fish RJ, Pandey RR, Pillai RS. The XRN1-regulated RNA helicase activity of YTHDC2 ensures mouse fertility independently of m(6)A recognition. Mol Cell. 2022;82:1678–1690. doi: 10.1016/j.molcel.2022.02.034. [DOI] [PubMed] [Google Scholar]
- 80.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–2270. doi: 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Wei W, Zhang ZY, Liu Y, Shi B, Zhong W, Zhang HS, Fang X, Sun CL, Wang JB, Liu LX. TCF4 and HuR mediated-METTL14 suppresses dissemination of colorectal cancer via N6-methyladenosine-dependent silencing of ARRDC4. Cell Death Dis. 2021;13:3. doi: 10.1038/s41419-021-04459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shima H, Matsumoto M, Ishigami Y, Ebina M, Muto A, Sato Y, Kumagai S, Ochiai K, Suzuki T, Igarashi K. S-Adenosylmethionine Synthesis Is Regulated by Selective N(6)-Adenosine Methylation and mRNA Degradation Involving METTL16 and YTHDC1. Cell Rep. 2017;21:3354–3363. doi: 10.1016/j.celrep.2017.11.092. [DOI] [PubMed] [Google Scholar]
- 85.Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C, et al. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m(6)A/MYC/CEBPA Signaling. Cell. 2018;172:90–105. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou H, Yin K, Zhang Y, Tian J, Wang S. The RNA m6A writer METTL14 in cancers: Roles, structures, and applications. Biochim Biophys Acta Rev Cancer. 2021;1876:188609. doi: 10.1016/j.bbcan.2021.188609. [DOI] [PubMed] [Google Scholar]
- 87.Scholler E, Weichmann F, Treiber T, Ringle S, Treiber N, Flatley A, Feederle R, Bruckmann A, Meister G. Interactions, localization, and phosphorylation of the m(6)A generating METTL3-METTL14-WTAP complex. RNA. 2018;24:499–512. doi: 10.1261/rna.064063.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun T, Wu Z, Wang X, Wang Y, Hu X, Qin W, Lu S, Xu D, Wu Y, Chen Q, et al. LNC942 promoting METTL14-mediated m(6)A methylation in breast cancer cell proliferation and progression. Oncogene. 2020;39:5358–5372. doi: 10.1038/s41388-020-1338-9. [DOI] [PubMed] [Google Scholar]
- 89.Zhang BY, Han L, Tang YF, Zhang GX, Fan XL, Zhang JJ, Xue Q, Xu ZY. METTL14 regulates M6A methylation-modified primary miR-19a to promote cardiovascular endothelial cell proliferation and invasion. Eur Rev Med Pharmacol Sci. 2020;24:7015–7023. doi: 10.26355/eurrev_202006_21694. [DOI] [PubMed] [Google Scholar]
- 90.Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–1083. doi: 10.1038/s41556-018-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M, Liu J, Zhao Y, He R, Xu X, Guo X, Li X, Xu S, Miao J, Guo J, et al. Upregulation of METTL14 mediates the elevation of PERP mRNA N(6) adenosine methylation promoting the growth and metastasis of pancreatic cancer. Mol Cancer. 2020;19:130. doi: 10.1186/s12943-020-01249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, Wang TT, Xu QG, Zhou WP, Sun SH. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 93.Yang X, Zhang S, He C, Xue P, Zhang L, He Z, Zang L, Feng B, Sun J, Zheng M. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46. doi: 10.1186/s12943-020-1146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X, Xu M, Xu X, Zeng K, Liu X, Sun L, Pan B, He B, Pan Y, Sun H, et al. METTL14 Suppresses CRC Progression via Regulating N6-Methyladenosine-Dependent Primary miR-375 Processing. Mol Ther. 2020;28:599–612. doi: 10.1016/j.ymthe.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Zhang C, Chen L, Liu Y, Huang J, Liu A, Xu Y, Shen Y, He H, Xu D. Downregulated METTL14 accumulates BPTF that reinforces super-enhancers and distal lung metastasis via glycolytic reprogramming in renal cell carcinoma. Theranostics. 2021;11:3676–3693. doi: 10.7150/thno.55424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen S, Yang C, Wang ZW, Hu JF, Pan JJ, Liao CY, Zhang JQ, Chen JZ, Huang Y, Huang L, et al. CLK1/SRSF5 pathway induces aberrant exon skipping of METTL14 and Cyclin L2 and promotes growth and metastasis of pancreatic cancer. J Hematol Oncol. 2021;14:60. doi: 10.1186/s13045-021-01072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H, Yuan J, Dang X, Shi Z, Ban W, Ma D. Mettl14-mediated m6A modification modulates neuron apoptosis during the repair of spinal cord injury by regulating the transformation from pri-mir-375 to miR-375. Cell Biosci. 2021;11:52. doi: 10.1186/s13578-020-00526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Z, Liu N, Huang Z, Wang W. METTL14 Overexpression Promotes Osteosarcoma Cell Apoptosis and Slows Tumor Progression via Caspase 3 Activation. Cancer Manag Res. 2020;12:12759–12767. doi: 10.2147/CMAR.S284273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pang P, Qu Z, Yu S, Pang X, Li X, Gao Y, Liu K, Liu Q, Wang X, Bian Y, et al. Mettl14 Attenuates Cardiac Ischemia/Reperfusion Injury by Regulating Wnt1/beta-Catenin Signaling Pathway. Front Cell Dev Biol. 2021;9:762853. doi: 10.3389/fcell.2021.762853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J, Harada BT, Lu D, Ma R, Gao B, Xu Y, Montauti E, Mani N, Chaudhuri SM, Gregory S, et al. HRD1-mediated METTL14 degradation regulates m(6)A mRNA modification to suppress ER proteotoxic liver disease. Mol Cell. 2021;81:5052–5065. doi: 10.1016/j.molcel.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kong F, Liu X, Zhou Y, Hou X, He J, Li Q, Miao X, Yang L. Downregulation of METTL14 increases apoptosis and autophagy induced by cisplatin in pancreatic cancer cells. Int J Biochem Cell Biol. 2020;122:105731. doi: 10.1016/j.biocel.2020.105731. [DOI] [PubMed] [Google Scholar]
- 102.Qiao X, Zhu L, Song R, Shang C, Guo Y. METTL3/14 and IL-17 signaling contribute to CEBPA-DT enhanced oral cancer cisplatin resistance. Oral Dis. 2021;00:1–15. doi: 10.1111/odi.14083. [DOI] [PubMed] [Google Scholar]
- 103.Zhou T, Li S, Xiang D, Liu J, Sun W, Cui X, Ning B, Li X, Cheng Z, Jiang W, et al. m6A RNA methylation-mediated HNF3gamma reduction renders hepatocellular carcinoma dedifferentiation and sorafenib resistance. Signal Transduct Target Ther. 2020;5:296. doi: 10.1038/s41392-020-00299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiang M, Liu W, Tian W, You A, Deng D. RNA N-6-methyladenosine enzymes and resistance of cancer cells to chemotherapy and radiotherapy. Epigenomics-Uk. 2020;12:801–809. doi: 10.2217/epi-2019-0358. [DOI] [PubMed] [Google Scholar]
- 105.Luo M, Huang Z, Yang X, Chen Y, Jiang J, Zhang L, Zhou L, Qin S, Jin P, Fu S, et al. PHLDB2 Mediates Cetuximab Resistance via Interacting With EGFR in Latent Metastasis of Colorectal Cancer. Cell Mol Gastroenterol Hepatol. 2022;13:1223–1242. doi: 10.1016/j.jcmgh.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Z, Wu K, Gu S, Wang W, Xie S, Lu T, Li L, Dong C, Wang X, Zhou Y. A methyltransferase-like 14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer stem cell persistence and radioresistance via histone deacetylase 2-mediated epigenetic modulation in esophageal squamous cell carcinoma. Clin Transl Med. 2021;11:e545. doi: 10.1002/ctm2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, et al. METTL14 Inhibits Hematopoietic Stem/Progenitor Differentiation and Promotes Leukemogenesis via mRNA m(6)A Modification. Cell Stem Cell. 2018;22:191–205. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L, Hui H, Agrawal K, Kang Y, Li N, Tang R, Yuan J, Rana TM. m(6) A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. Embo J. 2020;39:e104514. doi: 10.15252/embj.2020104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong L, Chen C, Zhang Y, Guo P, Wang Z, Li J, Liu Y, Liu J, Chang R, Li Y, et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–957. doi: 10.1016/j.ccell.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 110.Cao Y, Wang Z, Yan Y, Ji L, He J, Xuan B, Shen C, Ma Y, Jiang S, Ma D, et al. Enterotoxigenic Bacteroidesfragilis Promotes Intestinal Inflammation and Malignancy by Inhibiting Exosome-Packaged miR-149-3p. Gastroenterology. 2021;161:1552–1566. doi: 10.1053/j.gastro.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Shi Y, Zhuang Y, Zhang J, Chen M, Wu S. METTL14 Inhibits Hepatocellular Carcinoma Metastasis Through Regulating EGFR/PI3K/AKT Signaling Pathway in an m6A-Dependent Manner. Cancer Manag Res. 2020;12:13173–13184. doi: 10.2147/CMAR.S286275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan Z, Yang G, Zhang W, Liu Q, Liu G, Liu P, Xu L, Wang J, Yan Z, Han H, et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2-dependent silencing of SLC7A11. J Cell Mol Med. 2021;25:10197–10212. doi: 10.1111/jcmm.16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, Wang Y, Wang Y, Tang B. USP48 Is Upregulated by Mettl14 to Attenuate Hepatocellular Carcinoma via Regulating SIRT6 Stabilization. Cancer Res. 2021;81:3822–3834. doi: 10.1158/0008-5472.CAN-20-4163. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y, Cai J, Yang X, Wang K, Sun K, Yang Z, Zhang L, Yang L, Gu C, Huang X, et al. Dysregulated m6A modification promotes lipogenesis and development of non-alcoholic fatty liver disease and hepatocellular carcinoma. Mol Ther. 2022;30(6):2342–2353. doi: 10.1016/j.ymthe.2022.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang S, Gan M, Chen C, Zhang Y, Kong J, Zhang H, Lai M. Methyl CpG binding protein 2 promotes colorectal cancer metastasis by regulating N(6) -methyladenosine methylation through methyltransferase-like 14. Cancer Sci. 2021;112:3243–3254. doi: 10.1111/cas.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, Li C, Sun L, Qin J, Xu T, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19:106. doi: 10.1186/s12943-020-01220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tian J, Ying P, Ke J, Zhu Y, Yang Y, Gong Y, Zou D, Peng X, Yang N, Wang X, et al. ANKLE1 N(6) -Methyladenosine-related variant is associated with colorectal cancer risk by maintaining the genomic stability. Int J Cancer. 2020;146:3281–3293. doi: 10.1002/ijc.32677. [DOI] [PubMed] [Google Scholar]
- 118.Fan HN, Chen ZY, Chen XY, Chen M, Yi YC, Zhu JS, Zhang J. METTL14-mediated m(6)A modification of circORC5 suppresses gastric cancer progression by regulating miR-30c-2-3p/AKT1S1 axis. Mol Cancer. 2022;21:51. doi: 10.1186/s12943-022-01521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J, Gong J, Shen L. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. 2019;8:4766–4781. doi: 10.1002/cam4.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X, Xiao M, Zhang L, Li L, Zhu G, Shen E, Lv M, Lu X, Sun Z. The m6A methyltransferase METTL14 inhibits the proliferation, migration, and invasion of gastric cancer by regulating the PI3K/AKT/mTOR signaling pathway. J Clin Lab Anal. 2021;35:e23655. doi: 10.1002/jcla.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu N, Ji H. N6-methyladenosine (m6A)-mediated up-regulation of long noncoding RNA LINC01320 promotes the proliferation, migration, and invasion of gastric cancer via miR495-5p/RAB19 axis. Bioengineered. 2021;12:4081–4091. doi: 10.1080/21655979.2021.1953210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang C, Ou S, Zhou Y, Liu P, Zhang P, Li Z, Xu R, Li Y. m(6)A Methyltransferase METTL14-Mediated Upregulation of Cytidine Deaminase Promoting Gemcitabine Resistance in Pancreatic Cancer. Front Oncol. 2021;11:696371. doi: 10.3389/fonc.2021.696371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tian J, Zhu Y, Rao M, Cai Y, Lu Z, Zou D, Peng X, Ying P, Zhang M, Niu S, et al. N(6)-methyladenosine mRNA methylation of PIK3CB regulates AKT signalling to promote PTEN-deficient pancreatic cancer progression. Gut. 2020;69:2180–2192. doi: 10.1136/gutjnl-2019-320179. [DOI] [PubMed] [Google Scholar]
- 124.Chen M, Wong CM. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol Cancer. 2020;19:44. doi: 10.1186/s12943-020-01172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gu Z, Du Y, Zhao X, Wang C. Diagnostic, Therapeutic, and Prognostic Value of the m(6)A Writer Complex in Hepatocellular Carcinoma. Front Cell Dev Biol. 2022;10:822011. doi: 10.3389/fcell.2022.822011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Z, Li F, Peng Y, Fang J, Zhou J. Identification of three m6A-related mRNAs signature and risk score for the prognostication of hepatocellular carcinoma. Cancer Med. 2020;9:1877–1889. doi: 10.1002/cam4.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X, Qin J, Gao T, Li C, Chen X, Zeng K, Xu M, He B, Pan B, Xu X, et al. Analysis of METTL3 and METTL14 in hepatocellular carcinoma. Aging (Albany NY) 2020;12:21638–21659. doi: 10.18632/aging.103959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu X, Zhang X, Tao L, Dai X, Chen P. Prognostic Value of an m6A RNA Methylation Regulator-Based Signature in Patients with Hepatocellular Carcinoma. Biomed Res Int. 2020;2020:2053902. doi: 10.1155/2020/2053902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J, Zhang Y, Song H, Yin H, Jiang T, Xu Y, Liu L, Wang H, Gao H, Wang R, Song J. The circular RNA circSPARC enhances the migration and proliferation of colorectal cancer by regulating the JAK/STAT pathway. Mol Cancer. 2021;20:81. doi: 10.1186/s12943-021-01375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 131.Brody H. Colorectal cancer. Nature. 2015;521:S1. doi: 10.1038/521S1a. [DOI] [PubMed] [Google Scholar]
- 132.Liu X, Liu L, Dong Z, Li J, Yu Y, Chen X, Ren F, Cui G, Sun R. Expression patterns and prognostic value of m(6)A-related genes in colorectal cancer. Am J Transl Res. 2019;11:3972–3991. [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Q, Cai Y, Kurbatov V, Khan SA, Lu L, Zhang Y, Johnson CH. Gene Alterations of N6-Methyladenosine (m(6)A) Regulators in Colorectal Cancer: A TCGA Database Study. Biomed Res Int. 2020;2020:8826456. doi: 10.1155/2020/8826456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 135.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 136.Siegel RL, Miller KD, Goding SA, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 137.Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 138.Valle S, Alcala S, Martin-Hijano L, Cabezas-Sainz P, Navarro D, Munoz ER, Yuste L, Tiwary K, Walter K, Ruiz-Canas L, et al. Exploiting oxidative phosphorylation to promote the stem and immunoevasive properties of pancreatic cancer stem cells. Nat Commun. 2020;11:5265. doi: 10.1038/s41467-020-18954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma GG, Okada Y, Von Hoff D, Goel A. Non-coding RNA biomarkers in pancreatic ductal adenocarcinoma. Semin Cancer Biol. 2021;75:153–168. doi: 10.1016/j.semcancer.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 141.Geng Y, Guan R, Hong W, Huang B, Liu P, Guo X, Hu S, Yu M, Hou B. Identification of m6A-related genes and m6A RNA methylation regulators in pancreatic cancer and their association with survival. Ann Transl Med. 2020;8:387. doi: 10.21037/atm.2020.03.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu F, Zhang Z, Yuan M, Zhao Y, Zhou Y, Pei H, Bai L. M6A Regulatory Genes Play an Important Role in the Prognosis, Progression and Immune Microenvironment of Pancreatic Adenocarcinoma. Cancer Invest. 2021;39:39–54. doi: 10.1080/07357907.2020.1834576. [DOI] [PubMed] [Google Scholar]
- 143.Zhang N, Hua X, Tu H, Li J, Zhang Z, Max C. Isorhapontigenin (ISO) inhibits EMT through FOXO3A/METTL14/VIMENTIN pathway in bladder cancer cells. Cancer Lett. 2021;520:400–408. doi: 10.1016/j.canlet.2021.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.