Abstract
Background and aim
Metabolic dysfunction and associated systemic inflammation are risk factors for chronic obstructive pulmonary disease (COPD) and COPD is highly prevalent in men. We investigated the impact of metabolic-associated fatty liver disease (MAFLD) and MAFLD-related systemic inflammation on COPD in men.
Methods
We enrolled 2,041 men with fatty liver. Patients were classified into the COPD (n = 420/2041) and non-COPD (n = 1621/2041) groups. COPD and its high-risk group were diagnosed using the Japanese Respiratory Society Disease statement. Systemic inflammation was evaluated using the C-reactive protein (CRP)/albumin ratio. Independent factors for COPD were investigated by multivariate analysis and decision-tree analysis.
Results
The prevalence of MAFLD was significantly higher in the COPD group than in the non-COPD group. In multivariable analysis, in addition to heavy smoking and aging, MAFLD was identified as an independent factor for COPD (OR 1.46, 95% CI 1.020–2.101, P = 0.0385). Decision-tree analysis showed that MAFLD, rather than heavy smoking, was the most influential classifier for COPD in non-elderly men (14% in MAFLD vs 6% in non-MAFLD groups). MAFLD was also the second most influential factor in elderly men who were not heavy smokers. In both groups, the CRP/albumin ratio was the first classifier for COPD (16% in the high CRP/albumin ratio group vs 3% in the low CRP/albumin ratio group of non-elderly men).
Conclusions
MAFLD is an independent predictor of COPD in men. MAFLD had a significant impact on COPD through systemic inflammation in men of all ages who were not heavy smokers. MAFLD may be useful to broadly identify COPD in men.
Keywords: Metabolic associated fatty liver disease, Chronic obstructive pulmonary disease, Systemic inflammation, CRP/albumin ratio, Steatosis
Introduction
In patients with fatty liver disease, the prevalence of extra-hepatic diseases is high, emphasizing the systemic involvement of metabolic dysfunctions [1]. Non-alcoholic fatty liver disease (NAFLD) has been used as a concept of fatty liver, however, NAFLD excludes other chronic diseases and moderate amounts of alcohol consumption. More importantly, NAFLD does not require the presence of metabolic dysfunction, resulting in the metabolic heterogeneity of NAFLD. This metabolic heterogeneity is thought to cause a mixture of patients at low and high risk of extra-hepatic disease [2, 3].
Recently, an international expert panel proposed a new concept for fatty liver disease: metabolic (dysfunction) associated with fatty liver disease (MAFLD) [2]. Since the presence of metabolic dysregulation is mandatory for the diagnosis of MAFLD, MAFLD captures patients with a high risk for extrahepatic complications, including chronic kidney disease and colorectal adenomas [4]. In addition, we previously reported that MAFLD identifies patients at a high risk of atherosclerotic cardiovascular disease [5]. Moreover, the changing NAFLD to MAFLD had been supported by various associations including the European Liver Patient's Association [6].
The prevalence of chronic obstructive pulmonary disease (COPD) is high in patients with NAFLD [7]. NAFLD is also associated with the severity of COPD, and the risk of COPD is particularly high in adult men [8]. Smoking and aging are well-established risk factors for COPD [9]. However, one-fourth of adults with COPD have never smoked, indicating the presence of other risk factors [10]. Metabolic syndrome is also common in patients with COPD, with a prevalence ranging from 23 to 53% [11, 12]. A PRISMA-compliant meta-analysis demonstrated that metabolic syndrome was significantly associated with a 1.53-fold increased risk of COPD [13]. Through systemic inflammation, metabolic syndrome is associated with various conditions, including interleukin-6 levels [14], which is also a characteristic of COPD [15].
Various parameters are associated with systemic inflammation. C-reactive protein (CRP) is a popular inflammatory molecule related to the exacerbation of COPD [16]. Similarly, serum albumin concentrations and negative acute phase response protein levels are lower in patients with COPD [17]. The CRP/albumin ratio has been widely used as a biomarker of systemic inflammation [18]. The CRP/albumin ratio is strongly associated with more severe metabolic dysfunction in premenopausal women [19]. The CRP/albumin ratio is also higher in patients with type 2 diabetes and diabetic nephropathy [20]. Recently, the CRP/albumin ratio has been reported as a novel biomarker to predict rehospitalization and frequent exacerbations in patients with acute COPD exacerbations [21].
This study aimed to investigate the impact of MAFLD on COPD in men. We also investigated the impact of metabolic dysfunction and systemic inflammation on COPD in patients with MAFLD.
Patients and methods
Study design and ethics
This was a single-center observational cohort study in Japan. The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the prior approval from the institutional review board of Kurume University School of Medicine (ID 20,050). An opt-out approach was used to obtain informed consent from the patients, and personal information was protected during data collection.
Study population and selection of patients for analysis
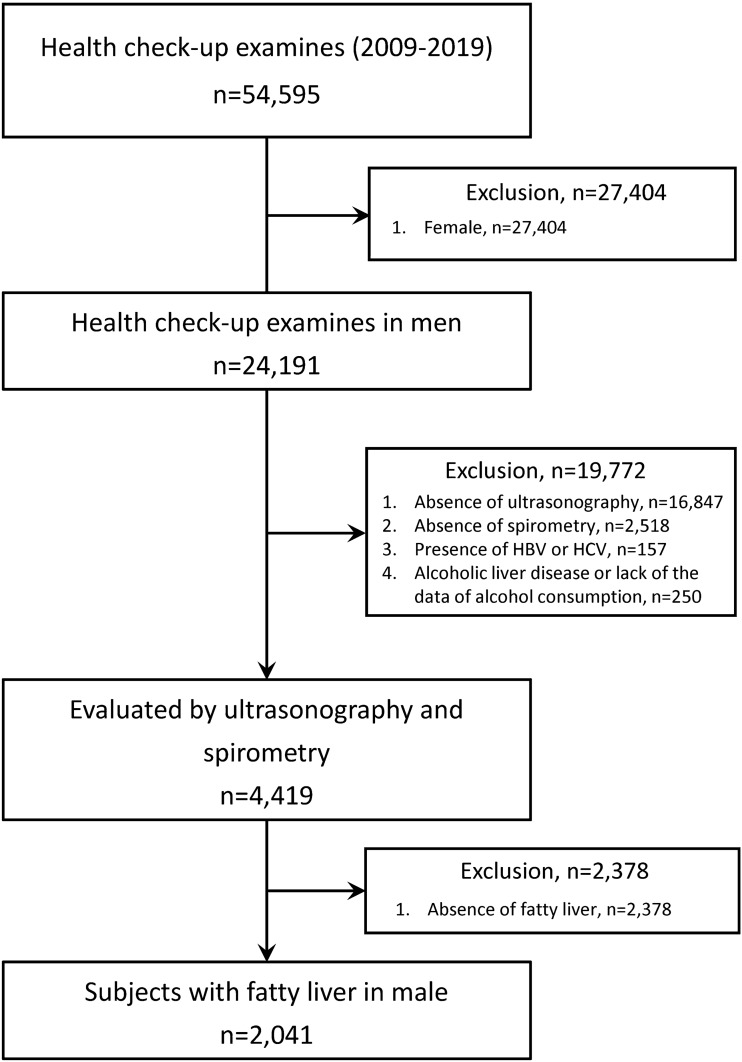
We enrolled 54,595 Asian participants who underwent health check-up examinations at the Saga Health and Clinical Examination Centre in Japan from January 2009 to March 2019 (Fig. 1). We excluded 27,404 women, and we excluded 19,772 of the 24,191 men for the following reasons: absence of ultrasonography, absence of spirometry test, presence of hepatitis B surface antigen, presence of anti-hepatitis C virus antibody, presence of alcoholic liver disease (pure ethanol ≥ 60 gms/day), and a lack of alcohol consumption data. From the remaining 4419 participants, we excluded 2378 participants because of the absence of fatty liver. Finally, we examined 2,041 men with fatty liver disease (Fig. 1).
Fig. 1.
Flow chart of patient selection
Data collection
All data were collected prospectively at the time of the medical check-up [5]. The following information was obtained using a self-reported questionnaire: age, sex, current smoking habits, alcohol consumption, comorbidities, and medication use. In the clinical review, we obtained the following data: body mass index (BMI), visceral adiposity (waist circumference ≥ 90 cm), blood pressure, presence/absence of diabetes, hypertension, and dyslipidemia, which were diagnosed according to standard criteria [22–24].
Biochemical analysis
Blood samples were obtained after an overnight fast, and the following biochemical parameters were measured: complete blood cell count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, γ-glutamyl transpeptidase (GGT), lactate dehydrogenase, total protein, albumin, total bilirubin, total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein (LDL) cholesterol, amylase, blood urea nitrogen, creatinine, estimated glomerular filtration rate, CRP, uric acid, electrolytes, fasting glucose, hemoglobin A1c (HbA1c), and cholinesterase.
Calculation of FIB-4 index
The FIB-4 index was calculated using age, serum levels of AST and ALT, and platelet count [25].
CRP/albumin ratio
The serum levels of CRP and albumin were measured simultaneously during the health check examination. The CRP/albumin ratio was calculated by dividing the serum CRP level by the serum albumin level [26].
Diagnosis of fatty liver
Fatty liver was diagnosed by abdominal ultrasonography as previously described [27]. All tests were performed by medical sonographers certified by the Japan Society of Ultrasonics in Medicine.
Diagnosis of MAFLD
MAFLD was diagnosed according to the criteria [2]. Briefly, the criteria include evidence of fatty liver, in addition to one of the following: overweight/obesity, presence of type 2 diabetes mellitus, or lean/normal weight with evidence of metabolic dysfunction. Overweight/obesity was defined as BMI ≥ 23 kg/m2, and type 2 diabetes mellitus was defined as HbA1c ≥ 6.5% or specific drug treatment. Metabolic dysfunction was defined as the presence of at least two metabolic risk abnormalities: 1) waist circumference ≥ 90 cm, 2) systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, or specific drug treatment; 3) plasma TG ≥ 150 mg/dL or specific drug treatment; 4) plasma HDL cholesterol < 40 mg/dL or specific drug treatment; and 5) prediabetes (fasting glucose levels 100–125 mg/dL or HbA1c 5.7%–6.4%) [2]. Homeostasis model assessment-insulin resistance score and plasma high-sensitivity C-reactive protein level are metabolic risk abnormalities [2]; however, these were not available in our dataset.
Definition of heavy and non-heavy smoking
We used pack years to evaluate the amount of cigarette smoking. It is calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked. We classified individuals into the following two groups according to their smoking habits: 1) heavy smoking; pack-years ≥ 60; 2) non-heavy smoking; 0 ≤ pack-years < 60, as previously described [28].
Spirometry measurement
Lung function was evaluated by spirometry with an electronic diagnostic spirometer (SP-770 COPD, FUKUDA DENSHI CO., LTD., Japan) for all participants, according to the American Thoracic Society quality criteria [29]. The results were expressed as a percentage of the predictive values based on age, height, and sex. All tests were performed during the early hours of the day. Three to six trials were performed for each subject. All tests were performed by a clinical laboratory technician.
Diagnosis of COPD
COPD is a chronic irreversible disease [30]; therefore, it is important to detect COPD in the high-risk group as recommended by the Japanese Respiratory Society [31]. Therefore, we integrated COPD with its high-risk group as a COPD group.
Airway obstruction was evaluated by the result of spirometry. COPD was defined as the forced expiratory volume % in one second (FEV1%) less than 70% according to the COPD management guidelines [32].
COPD high-risk group was defined by the percentage of predicted forced expiratory volume in 1 s (%FEV1) which was calculated by the FEV1 to predictive FEV adjusted by age, sex, and height [33]. COPD's high-risk group was defined by a %FEV1 less than 80% [31].
Statistical analysis
Continuous variables are expressed as medians, ranges, or numbers. Categorical variables are expressed as frequencies and percentages. Differences between the two groups were analyzed using the Mann–Whitney U test. Logistic regression analysis was used to identify independent factors associated with the presence of COPD. Since metabolic syndrome, visceral obesity, and diabetes are inclusion criteria for MAFLD, these metabolic factors were not used as explanatory variables in multivariable analysis because they are co-founding factors. Explanatory variables were selected in a stepwise manner, minimizing the Bayesian information criterion [34].
Data are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). A decision-tree algorithm was constructed to show profiles associated with the presence of COPD [35]. All P-values were two-tailed, and a value < 0.05 was considered statistically significant. Multivariate stepwise analysis and decision tree analysis were performed using JMP Pro16 (SAS Institute, Cary, NC, USA).
Results
Differences in patient characteristics between the COPD and non-COPD groups
The participant characteristics are summarised in Table 1. The COPD group comprised 20.6% of enrolled patients. Age was significantly higher in the COPD group than in the non-COPD group. The prevalence of smoking habits and pack years was significantly higher in the COPD group than in the non-COPD group. (Table 1).
Table 1.
The difference in patients’ characteristics between the COPD and non-COPD groups
| COPD group | Non-COPD group | P | |||
|---|---|---|---|---|---|
| Median (IQR) | Range (min–max) | Median (IQR) | Range (min–max) | ||
| Number | 20.6% (420/2041) | N/A | 79.4% (1621/2041) | N/A | N/A |
| Age | 58 (50–64.8) | 27–84 | 51 (43–60) | 21–85 | < 0.0001 |
| Presence or past smoking habit (Yes/No) | 86.0%/14.0% (361/59) | N/A | 71.9%/28.1% (1166/455) | N/A | < 0.0001 |
| Pack years (SUM of past and present) | 30 (0–40) | 0–160 | 14 (0–28) | 0–111 | < 0.0001 |
| Pack years ≥ 60 (Yes/No) | 10.5%/89.5% (44/376) | N/A | 3.5%/96.5% (57/1564) | N/A | < 0.0001 |
| Alcohol intake habit (None/Yes) | 32.9%/67.1% (138/282) | N/A | 31.2%/68.8% (1115/506) | N/A | 0.5188 |
| Dairy alcohol consumption (none/1–19 g/20–39 g/40–59 g) | 32.9%/16.2%/36.0%/15.0% (138/68/151/63) | N/A | 31.3%/23.1%/31.2%/14.4% (507/375/506/233) | N/A | 0.0178 |
| MAFLD/non-MAFLD | 90.5%/9.5% (380/40) | N/A | 85.9%/14.1% (1393/228) | N/A | 0.0148 |
| Body mass index (kg/m2) | 25.2 (23.2–27.7) | 15.7–43.2 | 24.9 (23.1–26.9) | 15.6–40.6 | 0.1282 |
| Waist circumference (cm) | 91 (86.5–98) | 70–137.5 | 89 (84.5–95) | 67–123.5 | < 0.0001 |
| Visceral adiposity (Presence/Absence) | 57.9%/42.1% (243/177) | N/A | 46.9%/53.1% (760/861) | N/A | < 0.0001 |
| Systolic blood pressure (mmHg) | 124 (114–132) | 84–197 | 120 (112–130) | 80–190 | 0.0006 |
| Diastolic blood pressure (mmHg) | 80 (70–86) | 50–118 | 78 (70–84) | 48–134 | 0.2075 |
| Type 2 diabetes mellitus (Presence/Absence) | 11.4%/88.6% (48/372) | N/A | 8.3%/91.7% (135/1486) | N/A | 0.0475 |
| Hypertension (Presence/Absence) | 46.2%/53.8% (194/226) | N/A | 38.8%/61.2% (629/992) | N/A | 0.0063 |
| Hypertriglyceridemia (Presence/Absence) | 48.1/51.9% (202/218) | N/A | 40.8%/59.2% (661/960) | N/A | 0.0068 |
| Depressed HDL-cholesterol (Presence/Absence) | 16.2%/83.8% (68/352) | N/A | 11.2%/88.8% (181/1440) | N/A | 0.0050 |
| %VC | 96.0 (86.8–110.4) | 50.1–149.2 | 108.4 (100–117.2) | 77.3–157.7 | < 0.0001 |
| FEV1% | 69.5 (65.7–75.3) | 40.3–90.9 | 79.7 (76.5–83.1) | 70–98.1 | < 0.0001 |
| %FEV1 | 75.9 (70.4–79.9) | 43.2–91.5 | 96.1 (89.2–103.6) | 80.0–110.8 | < 0.0001 |
| Red blood cell count (× 104/µL) | 487 (464–514) | 384–610 | 495 (469–520) | 258–657 | 0.0019 |
| Hemoglobin (g/dL) | 15.2 (14.5–15.9) | 12.7–19.1 | 15.3 (14.6–15.9) | 8.1–18.7 | 0.3179 |
| Hematocrit (%) | 44.5 (42.5–46.6) | 36.8–54.2 | 44.7 (42.9–46.4) | 28.5–56.2 | 0.4685 |
| White blood cell count (/µL) | 5700 (5100–7375) | 2700–15,100 | 5700 (4900–6800) | 2200–15,400 | 0.0446 |
| Platelet count (× 104/µL) | 22.7 (19.5–26.5) | 7.3–75.9 | 23.4 (20.3–26.7) | 6.4–51.5 | 0.0437 |
| AST (U/L) | 23 (19–29) | 10–90 | 23 (19–25) | 6–164 | 0.0928 |
| ALT (U/L) | 28 (20–38) | 6–277 | 28 (20–40) | 6–290 | 0.8053 |
| Lactate dehydrogenase (U/L) | 168 (153–188) | 70–311 | 164 (149–182) | 56–431 | 0.0081 |
| ALP (U/L) | 213 (178–256) | 73–502 | 209 (179–249) | 73–1409 | 0.2035 |
| GGT (U/L) | 43 (30–71) | 12–381 | 38((26–61) | 8–609 | 0.0002 |
| Total protein (g/dL) | 7.2 (7.0–7.4) | 6.2–8.4 | 7.2 (7.0–7.4) | 5.7–8.5 | 0.5728 |
| Cholinesterase (U/L) | 367 (322–404) | 188–532 | 369 (331–408) | 166–641 | 0.0883 |
| Albumin (g/dL) | 4.4 (4.3–4.6) | 3.1–5.1 | 4.5 (4.3–4.6) | 3.2–5.3 | < 0.0001 |
| Total bilirubin (mg/dL) | 0.7 (0.6–0.9) | 0.2–2.2 | 0.8 (0.6–1.0) | 0.2–3.3 | 0.0630 |
| Total cholesterol (mg/dL) | 202(183–223) | 122–331 | 207(186–229) | 120–357 | 0.0107 |
| HDL-cholesterol (mg/dL) | 48 (41–60) | 24–116 | 52 (45–60) | 26–164 | < 0.0001 |
| LDL-cholesterol (mg/dL) | 124 (107–141) | 39–260 | 129 (111–150) | 54–262 | 0.0011 |
| Triglycerides (mg/dL) | 136 (98–206) | 31–990 | 123 (90–178) | 33–1474 | 0.0005 |
| Fasting glucose (mg/dL) | 103 (96–113) | 81–220 | 99 (94–109) | 77–330 | < 0.0001 |
| HbA1c (%) | 5.8 (5.5–6.1) | 4.3–11.1 | 5.7 (5.5–6.0) | 4.4–12.8 | < 0.0001 |
| CRP (mg/dL) | 0.1 (0.1–0.17) | 0.01–3.99 | 0.1 (0.08–0.12) | 0.01–3.52 | < 0.0001 |
| BUN (mg/dL) | 13.7 (11.6–15.9) | 6.6–37.5 | 13.6 (11.7–15.8) | 6.4–30.3 | 0.8189 |
| Creatinine (mg/dL) | 0.8 (0.7–0.9) | 0.43–1.64 | 0.8 (0.74–0.9) | 0.48–2.4 | 0.0193 |
| eGFR (mL/min/1.73 m2) | 78.4 (69.7–89.8) | 34.9–149.4 | 79.8 (70.4–89.1) | 25.3–142.6 | 0.1243 |
| Uric acid (mg/dL) | 6.4 (5.6–7.1) | 3.4–11.9 | 6.3 (5.4–7.1) | 0.7–10.8 | 0.3569 |
| Sodium (mmol/L) | 141 (140–143) | 136–145 | 141 (140–142 | 134–147 | 0.6563 |
| Potassium (mmol/L) | 4.2 (4.0–4.4) | 3.3–5.3 | 4.2 (4.0–4.3) | 3.3–5.3 | 0.3399 |
| Chloride (mmol/L) | 105 (104–107) | 99–111 | 105 (104–107) | 98–110 | 0.1171 |
| CRP/albumin ratio | 0.023 (0.021–0.038) | 0.002–1.05 | 0.022 (0.018–0.027) | 0.002–0.892 | < 0.001 |
| FIB-4 index | 1.135 (0.847–1.484) | 0.225–6.985 | 0.937 (0.702–1.300) | 0.281–9.404 | < 0.001 |
COPD chronic obstructive pulmonary disease, MAFLD metabolic associated fatty liver disease, VC vital capacity, HDL-cholesterol high-density lipoprotein-cholesterol, FEV forced expiratory volume, FVC forced vital capacity, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyl transpeptidase, LDL-cholesterol low-density lipoprotein-cholesterol, HbA1c hemoglobin A1c, CRP C-reactive protein, BUN blood urea nitrogen, GFR glomerular filtration rate, FIB-4 fibrosis-4
The prevalence of MAFLD was greater than 90% in the COPD group and significantly higher than that in the non-COPD group. The serum albumin level was significantly lower in the COPD group than in the non-COPD group. Furthermore, the CPR/albumin ratio was significantly higher in the COPD group than in the non-COPD group. The FIB-4 index was significantly higher in the COPD group than in the non-COPD group (Table 1).
Multivariate analyses of independent factors for the COPD
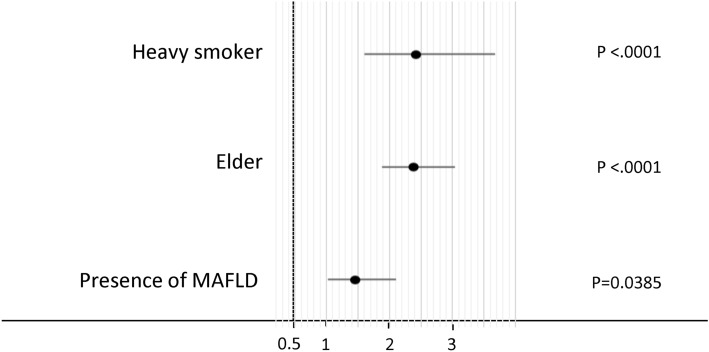
In the stepwise selection procedure, heavy smoking, aging, and MAFLD were selected for logistic regression analysis. Age was categorized by the cut-off value of 50 years old as previously described [36]. In multivariate analysis, the independent factors for COPD were MAFLD (OR 1.46, 95% CI 1.020–2.101, P = 0.0385), heavy smoking (OR 2.43, 95% CI 1.599–3.581, P < 0.0001), and aging (OR 2.39, 95% CI 1.878–3.037, P < 0.0001) (Fig. 2).
Fig. 2.
Independent factors for the presence of chronic obstructive pulmonary disease in all subjects. MAFLD metabolic-associated fatty liver disease
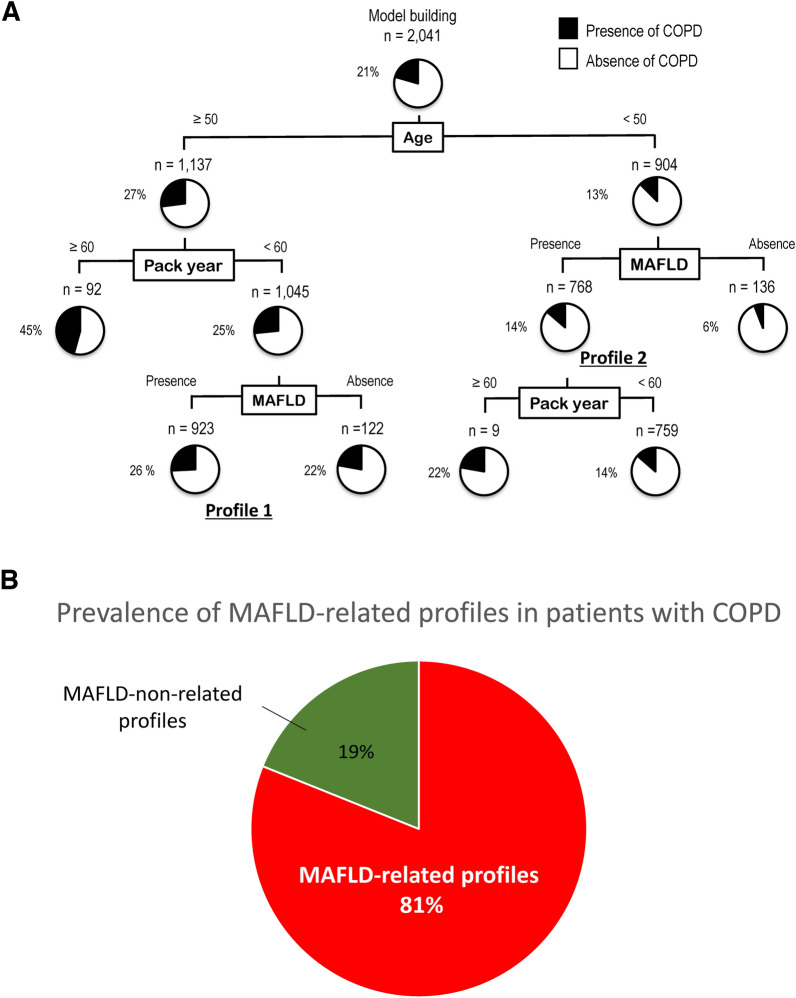
Impact of COPD according to decision tree analysis
We investigated the profiles of the presence of COPD using decision tree analysis (Fig. 3). The initial classifier is aging. Of the subjects aged ≥ 50 years, 27% had COPD. The second and third classifiers were smoking and MAFLD, respectively. In the subjects with < 60 pack-years and MAFLD, 26% had COPD (Profile 1). Of the subjects aged < 50 years, 13% had COPD. Of these, the second classifier was MAFLD rather than smoking (Profile 2). The prevalence of COPD was 14% in subjects aged < 50 years who had MAFLD. On the other hand, the prevalence was 6% in subjects aged < 50 years with non-MAFLD.
Fig. 3.
A Profiles associated with chronic obstructive pulmonary disease were evaluated by decision tree analysis (B) Prevalence of metabolic-associated fatty liver disease-related profiles in patients with chronic obstructive pulmonary disease. COPD chronic obstructive pulmonary disease, MAFLD metabolic-associated fatty liver disease
Thus, decision tree analysis demonstrated two MAFLD-related profiles. The prevalence of patients with MAFLD-related COPD profiles in all patients with COPD was calculated by the following formula. A) All patients with COPD: n = 420 (Table 1), B) MAFLD-related COPD: 923 by 0.26 (Profile 1) and 768 by 0.14 (Profile 2) = 347.5 Thus, subjects with these MAFLD-related COPD profiles were calculated as B divided by A (347.5/420), resulting in 81.1%. (Fig. 3).
Characteristics of patients with MAFLD-related profiles
As a sub-analysis, we investigated the characteristics of patients with COPD in subjects with two MAFLD-related profiles: Profile 1, ≥ 50 years old, less than 60 pack-years, and MAFLD) (Table 2), and Profile 2, < 50 years old and MAFLD (Table 3).
Table 2.
The patients’ characteristics in subjects with MAFLD-related Profiles 1
| Profile 1 ≥ 50 years old, less than 60 pack-years, and MAFLD | ||
|---|---|---|
| Median (IQR) | Range (min–max) | |
| Number | 45.2% (923/2041) | N/A |
| Age | 60 (55–65) | 50–85 |
| Body mass index (kg/m2) | 25.1 (23.6–26.8) | 15.7–36.8 |
| Waist circumference (cm) | 90 (86.5–95) | 69.5–123 |
| Visceral adiposity (Presence/Absence) | 53.6%/46.4% (495/428) | N/A |
| Systolic blood pressure (mmHg) | 126 (118–134) | 84–197 |
| Diastolic blood pressure (mmHg) | 80 (72–86) | 50–118 |
| Type 2 diabetes mellitus (Presence/Absence) | 12.2%/87.8% (113/810) | N/A |
| Hypertension (Presence/Absence) | 52.0%/48.9% (471/452) | N/A |
| Hypertriglyceridemia (Presence/Absence) | 45.0%/55.0% (416/507) | N/A |
| Depressed HDL-cholesterol (Presence/Absence) | 12.2%/87.8% (113/810) | N/A |
| Alcohol intake habit (None/Yes) | 30.0%/70.0% (277/646) | N/A |
| Dairy alcohol consumption (None/1–19 g/20–39 g/40–59 g) | 30.0%/23.4%/32.9%/13.7% (277/216/304/126) | N/A |
| Presence or past smoking habit (Yes/No) | 76.5%/23.5% (706/217) | N/A |
| Pack years (sum of past and present) | 20 (0.8–34.5) | 0–58.8 |
| Pack year ≥ 60 (Yes/No) | 10.5%/89.5% (44/376) | N/A |
| Pulmonary functions | ||
| %VC | 105.4 (96.3–114.9) | 50.1–151.4 |
| FEV1% | 76.9 (72.3–80.5) | 48.4–90.5 |
| %FEV1 | 92.7 (83.2–101.6) | 43.6–151.2 |
| Prevalence of COPD (Presence/Absence) | 25.8%/74.2% (238/685) | N/A |
| Biochemical examinations | ||
| Red blood cell count (× 104/µL) | 487 (461–509) | 370–603 |
| Hemoglobin (g/dL) | 15.1 (14.4–15.8) | 8.9–19.1 |
| Hematocrit (%) | 44.3 (42.3–46.2) | 30.1–75.9 |
| White blood cell count (/µL) | 5500 (4800–6700) | 2,200–15,400 |
| Platelet count (× 104/µL) | 22.2 (18.9–25.2) | 8.8–75.9 |
| AST (U/L) | 23 (19–28) | 12–154 |
| ALT (U/L) | 26 (19–35) | 6–290 |
| Lactate dehydrogenase (U/L) | 167 (151–187) | 56–330 |
| ALP (U/L) | 207 (174–250) | 73–1409 |
| GGT (U/L) | 38 (27–62) | 12–609 |
| Total protein (g/dL) | 7.2 (6.9–7.4) | 6.0–8.4 |
| Cholinesterase (U/L) | 360 (322–396) | 180–641 |
| Albumin (g/dL) | 4.4 (4.3–4.6) | 3.4–5.1 |
| Total bilirubin (mg/dL) | 0.8 (0.6–1.0) | 0.2–3.0 |
| Total cholesterol (mg/dL) | 205(186–227) | 122–313 |
| HDL-cholesterol (mg/dL) | 51 (44–60) | 26–123 |
| LDL-cholesterol (mg/dL) | 126 (109–147) | 55–225 |
| Triglycerides (mg/dL) | 130 (95–181) | 33–1474 |
| Fasting glucose (mg/dL) | 103 (97–115) | 78–330 |
| HbA1c (%) | 5.9 (5.6–6.2) | 4.3–12.8 |
| CRP (mg/dL) | 0.1 (0.09–0.13) | 0.01–3.99 |
| BUN (mg/dL) | 14.1 (12.2–16.3) | 8.0–37.5 |
| Creatinine (mg/dL) | 0.8 (0.74–0.9) | 0.43–1.7 |
| eGFR (mL/min/1.73 m2) | 75.1 (66.6–83.3) | 31.6–149.4 |
| Uric acid (mg/dL) | 6.3 (5.4–7.0) | 0.7–11.0 |
| Sodium (mmol/L) | 141 (140–143) | 134–146 |
| Potassium (mmol/L) | 4.2 (4.0–4.4) | 3.3–5.3 |
| Chloride (mmol/L) | 105 (104–107) | 98–111 |
| CRP/albumin ratio | 0.227 (0.02–0.429) | 0.002–1.05 |
| FIB-4 index | 1.267 (0.966–1.628) | 0.225–6.985 |
COPD chronic obstructive pulmonary disease, MAFLD metabolic associated fatty liver disease, VC vital capacity, HDL-cholesterol high-density lipoprotein-cholesterol, FEV forced expiratory volume, FVC forced vital capacity, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyl transpeptidase, LDL-cholesterol low-density lipoprotein-cholesterol, HbA1c hemoglobin A1c, CRP C-reactive protein, BUN blood urea nitrogen, GFR glomerular filtration rate, FIB-4 fibrosis-4
Table 3.
The patients’ characteristics in subjects with MAFLD-related Profiles 2
| Profile 2 < 50 years old and MAFLD | ||
|---|---|---|
| Median (IQR) | Range (min–max) | |
| Number | 37.6% (768/2041) | N/A |
| Age | 43 (39–47) | 21–49 |
| Body mass index (kg/m2) | 25.9 (24.4–28.2) | 18.4–137.5 |
| Waist circumference (cm) | 91.5 (87–97.5) | 71–123.5 |
| Visceral adiposity (Presence/Absence) | 59.0%/41.0% (453/315) | N/A |
| Systolic blood pressure (mmHg) | 120 (110–128) | 89–190 |
| Diastolic blood pressure (mmHg) | 78 (70–84) | 48–134 |
| Type 2 diabetes mellitus (Presence/Absence) | 7.9%/92.1% (61/707) | N/A |
| Hypertension (Presence/Absence) | 36.1%/63.9% (277/491) | N/A |
| Hypertriglyceridemia (Presence/Absence) | 48.9%/51.1% (375/393) | N/A |
| Depressed HDL-cholesterol (Presence/Absence) | 15.8%/84.2% (121/647) | N/A |
| Alcohol intake habit (None/Yes) | 33.4%/66.6% (257/511) | N/A |
| Dairy alcohol consumption (None/1–19 g/20–39 g/40–59 g) | 33.5%/19.9%/29.8%/16.8% (257/153/229/129) | N/A |
| Presence or past smoking habit (Yes/No) | 70.2%/29.8% (539/229) | N/A |
| Pack years (Sum of past and present) | 10 (0–22.5) | 0–100 |
| Pack year ≥ 60 (Yes/No) | 3.5%/96.5% (57/1564) | N/A |
| Pulmonary functions | ||
| %VC | 107.5 (98.0–117.2) | 70.6–155.5 |
| FEV1% | 80.7 (77.0–84.0) | 54.4–98.1 |
| %FEV1 | 93.2 (85.6–101.2) | 48.6–134.4 |
| Prevalence of COPD (Presence/Absence) | 13.6%/86.4% (105/663) | N/A |
| Biochemical examinations | ||
| Red blood cell count (× 104/µL) | 508 (483–531) | 396–657 |
| Hemoglobin (g/dL) | 15.5 (14.8–16.1) | 12.0–18.7 |
| Hematocrit (%) | 45.2 (43.5–46.9) | 36.4–56.2 |
| White blood cell count (/µL) | 6,050 (5100–7000) | 3000–13,600 |
| Platelet count (× 104/µL) | 24.4 (21.5–27.6) | 7.3–51.5 |
| AST (U/L) | 24 (19–30) | 6–164 |
| ALT (U/L) | 34 (24–51) | 8–277 |
| Lactate dehydrogenase (U/L) | 165 (151–182) | 84–431 |
| ALP (U/L) | 209 (182–248) | 73–506 |
| GGT (U/L) | 43 (29–68) | 10–449 |
| Total protein (g/dL) | 7.2 (7.0–7.4) | 5.9–8.4 |
| Cholinesterase (U/L) | 384 (346–427) | 208–613 |
| Albumin (g/dL) | 4.6 (4.4–4.7) | 3.1–5.2 |
| Total bilirubin (mg/dL) | 0.7 (0.6–0.9) | 0.2–3.0 |
| Total cholesterol (mg/dL) | 207 (186–229) | 120–357 |
| HDL-cholesterol (mg/dL) | 49 (43–57) | 24–164 |
| LDL-cholesterol (mg/dL) | 131 (114–152) | 54–262 |
| Triglycerides (mg/dL) | 136 (93–201) | 31–1233 |
| Fasting glucose (mg/dL) | 98 (93–104) | 77–312 |
| HbA1c (%) | 5.6 (5.4–5.8) | 4.5–12.6 |
| CRP (mg/dL) | 0.1 (0.08–0.15) | 0.01–3.39 |
| BUN (mg/dL) | 12.8 (11.0–14.9) | 6.5–26.3 |
| Creatinine (mg/dL) | 0.8 (0.73–0.9) | 0.48–2.4 |
| eGFR (mL/min/1.73 m2) | 85.1 (76.3–94.1) | 25.3–142.6 |
| Uric acid (mg/dL) | 6.5 (5.6–7.3) | 0.8–11.9 |
| Sodium (mmol/L) | 141 (140–142) | 136–146 |
| Potassium (mmol/L) | 4.2 (4.0–4.3) | 3.3–5.3 |
| Chloride (mmol/L) | 105 (104–107) | 98–110 |
| CRP/Albumin ratio | 0.0222 (0.020–0.032) | 0.002–0.892 |
| FIB-4 index | 0.725 (0.578–0.911) | 0.281–5.214 |
COPD chronic obstructive pulmonary disease, MAFLD metabolic associated fatty liver disease, VC vital capacity, HDL-cholesterol high-density lipoprotein-cholesterol, FEV forced expiratory volume, FVC forced vital capacity, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyl transpeptidase, LDL-cholesterol low-density lipoprotein-cholesterol, HbA1c hemoglobin A1c, CRP C-reactive protein, BUN blood urea nitrogen, GFR glomerular filtration rate, FIB-4 fibrosis-4
Subjects with MAFLD-related Profile 1 accounted for 45.2% of all subjects; their median age was 60 years, and their median BMI was 25.1. Their smoking habit was 76.5%, the median pack years was 20, and the prevalence of COPD was 25.8%. In addition, the prevalence of visceral adiposity, hypertension, diabetes, and hypertriglyceridemia was 59.0%, 36.1%, 7.9%, and 48.9%, respectively. The median FIB-4 index and CRP/albumin ratio were 1.267 and 0.227, respectively.
Subjects with MALFD-related Profile 2 accounted for 37.6% of all subjects, the median age was 43 years, and the median BMI was 25.1 (Table 3). The prevalence of visceral adiposity, hypertension, diabetes, and hypertriglyceridemia was 53.6%, 52.0%, 12.2%, and 45.0%, respectively. The median FIB-4 index and CRP/albumin ratio were 0.725 and 0.0222, respectively.
The reference values for CRP/albumin ratio are 0.002–0.0025 based on the reference values of CRP and albumin of < 0.01 mg/dL and 4–5 g/dL, respectively. Therefore, the CRP/albumin ratio in Profiles 1 and 2 were higher than the reference value.
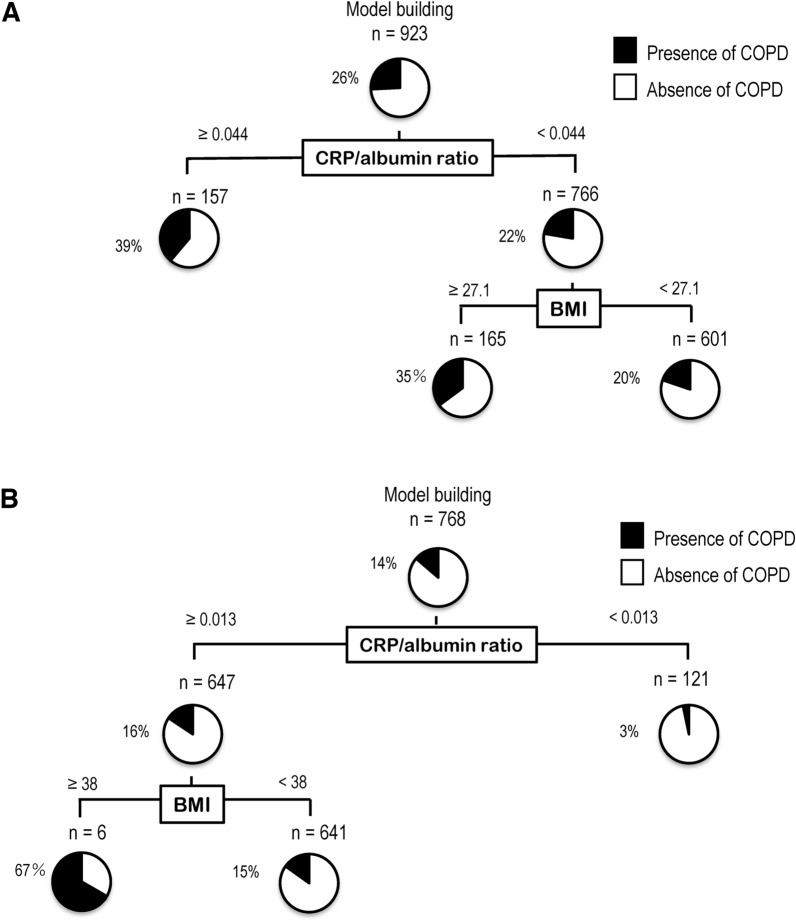
Decision-tree analysis of factors associated with COPD in subjects with MAFLD-related profiles
We investigated factors associated with COPD in subjects with MAFLD-related Profile 1 using the following explanatory variables: BMI, HbA1c, FIB-4 index, and CRP/albumin ratio. In the decision tree analysis, the initial classifier was CRP/albumin ratio. Among the subjects with a CRP/albumin ratio ≥ 0.044, 39% had COPD (Fig. 4A). On the other hand, in the subjects with a CRP/albumin ratio < 0.044, 22% had COPD.
Fig. 4.
A Decision tree analysis for factors associated with COPD in patients with metabolic-associated fatty liver disease-related Profile 1. B Decision tree analysis for factors associated with chronic obstructive pulmonary disease in patients with metabolic-associated fatty liver disease-related Profile 2. COPD chronic obstructive pulmonary disease, CRP C-reactive protein, BMI body mass index
In subjects with MAFLD-related Profile 2, the initial factor for COPD was the CRP/albumin ratio (Fig. 4B). Of the subjects with a CRP/albumin ratio ≥ 0.013, 16% had COPD. In contrast, in subjects with a CRP/albumin ratio < 0.013, 3% had COPD.
Discussion
In this study, we first investigated whether MAFLD was an independent factor for the presence of COPD in men. In particular, MAFLD had a significant association with subjects with the following profiles:1) ≥ 50 years old with less than 60 pack-years of smoking and 2) < 50 years old, which accounted for 89% of patients with COPD. Decision-tree analysis revealed that CRP/albumin, an index of systemic inflammation, was the most important factor for COPD.
This cohort data, obtained from 2009 to 2019, was based on health check examinees. The prevalence of fatty liver was 46.1% (2,041/4,419) for all male subjects, which is similar to its prevalence in the general population according to Japanese guidelines [37]. We enrolled only men because the association between NAFLD and COPD is stronger in men than that in women [7]. Furthermore, the Nippon COPD Epidemiology (NICE) Study which examined the general Japanese population revealed that the prevalence of COPD in females was very low, less than 1/3 of that in men. [38] The prevalence of MAFLD was 40.1% (1,773/4,419), which is also in good agreement with previous reports (30.2% to 46.7%) [39, 40]. Moreover, the prevalence of COPD was 21% in all subjects, which is in good accordance with previous reports (14.6% to 41%) of patients with NAFLD [41, 42]. In addition, aging and long-term smoking were risk factors for COPD in previous studies [9], and our study showed the same trend that median age, smoking rate, and heavy smoking were all higher in the COPD group than in the non-COPD group. Thus, our database appears to correspond with the general characteristics of the population in Japan.
We demonstrated that the presence of MAFLD was an independent factor for the presence of COPD independent of aging and heavy smoking. In previous studies, the association between COPD and NAFLD remains controversial. Several studies reported that NAFLD is highly prevalent in patients with COPD [7, 43]. In contrast, one Japanese study reported a low prevalence of NAFLD in patients with COPD [44]. These differences may be due to the heterogeneous metabolic features in patients with NAFLD. Metabolic abnormalities, including obesity and type 2 diabetes mellitus, are reportedly associated with the development and progression of COPD [7]. Comorbidity of metabolic abnormalities is an inclusion criterion for the diagnosis of MAFLD. Thus, MAFLD may be an independent factor in COPD.
We employed decision-tree analysis to examine the profiles associated with COPD. MAFLD was identified as a classifier in subjects who were ≥ 50 years old and had a < 60 pack-year smoking history. MAFLD was also a classifier in subjects < 50 years of age, regardless of smoking status. These profiles accounted for greater than 80% of the patients with COPD. Thus, we have revealed that MAFLD is extensively associated with COPD. The reason for the extensive impact of MAFLD on COPD remains unclear. However, one would think that changes in the clinical phenotypes of COPD may be a possible reason. It is now emerging that up to 50% of patients with COPD have metabolic dysfunction as a comorbidity [45]. Furthermore, recent studies have shown a direct association between metabolic dysfunction and progressive lung pathology in patients with COPD [45, 46]. In fact, in our cohort, patients with COPD had a higher prevalence of metabolic dysfunction, including visceral adiposity, type 2 diabetes mellitus, hypertension, and dyslipidemia compared to patients without COPD. Thus, MAFLD may be extensively associated with COPD because of the emerging impact of metabolic dysfunction in patients with COPD.
We also performed a sub-analysis to investigate the factors associated with COPD in patients with MAFLD-related profiles. In this analysis, we included various metabolic abnormalities and inflammatory indexes, such as the CRP/albumin ratio. We would like to investigate the impact of IL-6 on COPD as a biomarker of systemic inflammation. However, this information was not available because all participants were health check examinees and there was no preserved serum. Thus, we employed the CRP/albumin ratio instead of the IL-6.
In both MAFLD-related Profiles 1 and 2, the CRP/albumin ratio was the initial classifier for COPD. COPD can be caused by various pathogeneses, including systemic inflammation and reactive oxygen species. CRP is positively correlated with IL-6 levels, a major inflammatory cytokine [47]. IL-6 has been reported to be inversely correlated with FEV1% and is associated with increased mortality in patients with COPD [48, 49]. In addition, serum albumin is known to scavenge reactive oxygen species by a free cysteine residue [50]. A meta-analysis showed that serum albumin levels are even lower in patients with stable COPD, suggesting the importance of a deficit in systemic inflammation in COPD [17]. Thus, CRP/albumin may be the most important factor for COPD morbidity in patients with MAFLD.
This study had some limitations. First, this was a cross-sectional study conducted in a single center in Japan. Second, the cohort comprised only Asians. Third, we could not evaluate dietary and exercise habits. Further international multicentre prospective studies with an evaluation of lifestyle habits should be conducted.
In conclusion, we showed that MAFLD was an independent factor for the presence of COPD in men. MAFLD was associated with elderly non-heavy smokers and non-elderly individuals, accounting for 80% of COPD cases. Furthermore, CRP/albumin had the greatest impact on COPD. Thus, MAFLD may be widely associated with COPD via systemic inflammation in men.
Acknowledgements
We thank Dr. Hideo Ikeda (Public Utility Foundation Saga Prefectural Health Promotion Foundation) and Yumi Doi for providing health check-up data.
Disclosure
TK received lecture fees from Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, and Otsuka Pharmaceutical Co., Ltd. The other authors have no conflicts of interest relevant to this publication.
Abbreviations
- MAFLD
Metabolic associated fatty liver disease
- COPD
Chronic obstructive pulmonary disease
- NAFLD
Non-alcoholic fatty liver disease
- IL-6
Interleukin-6
- CRP
C-reactive protein
- BMI
Body mass index
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- GGT
γ-Glutamyl transpeptidase
- TG
Triglycerides
- HDL-cholesterol
High-density lipoprotein-cholesterol
- LDL-cholesterol
Low-density lipoprotein-cholesterol
- HbA1c
Hemoglobin A1c
- FIB-4 index
Fibrosis-4 index
- FEV
Forced expiratory volume
- FVC
Forced vital capacity
- OR
Odds ratio
- CI
Confidence interval
Author contributions
Conceptualization, TT, TK; methodology, SY, MK; software, DN, RH; validation, SY, DN, and MK; formal analysis, MK, HR; investigation, TT, TK, and SY; resources, SY; data curation, TT; writing—original draft preparation, TT, SY and TK; writing—review and editing, HT, KA; visualization, DN, RH; supervision, HT, and TK; project administration, TT; funding acquisition, TK. All authors read and approved the final manuscript.
Funding
This research was supported by the Research Program on Hepatitis from the Japan Agency for Medical Research and Development, AMED under Grant Number JP21fk0210090.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. Data sharing statement: no additional data available.
Declarations
Ethics approval and consent to participate
The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the prior approval from the institutional review board of Kurume University School of Medicine (ID 20050). An opt-out approach was used to obtain informed consent from the patients, and personal information was protected during data collection.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawaguchi T, Tsutsumi T, Nakano D, Torimura T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res. 2022;52(5):422–432. doi: 10.1111/hepr.13706. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, Koseki M, Yoshinaga S, Takahashi H, Anzai K, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res. 2021;51:1115–1128. doi: 10.1111/hepr.13685. [DOI] [PubMed] [Google Scholar]
- 6.Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Hogstrom S, Richardson B, Munoz C, Sigurethardottir S, Coulibaly A, et al. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6:73–79. doi: 10.1016/S2468-1253(20)30294-6. [DOI] [PubMed] [Google Scholar]
- 7.Viglino D, Jullian-Desayes I, Minoves M, Aron-Wisnewsky J, Leroy V, Zarski JP, Tamisier R, Joyeux-Faure M, Pepin JL. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur Respir J. 2017 doi: 10.1183/13993003.01923-2016. [DOI] [PubMed] [Google Scholar]
- 8.Soriano JB, Maier WC, Egger P, Visick G, Thakrar B, Sykes J, Pride NB. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax. 2000;55:789–794. doi: 10.1136/thorax.55.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie AI, Baker JR, Parekh TM, Allinson JP, Bhatt SP, Donnelly LE, Donaldson GC. Update in chronic obstructive pulmonary disease 2020. Am J Respir Crit Care Med. 2021;204:14–22. doi: 10.1164/rccm.202102-0253UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease C Prevention: chronic obstructive pulmonary disease among adults–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938–943. [PubMed] [Google Scholar]
- 11.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake G, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 12.Cebron Lipovec N, Beijers RJ, van den Borst B, Doehner W, Lainscak M, Schols AM. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13:399–406. doi: 10.3109/15412555.2016.1140732. [DOI] [PubMed] [Google Scholar]
- 13.Ye L, Huang X, Wang Q, Yang H, Cai D, Wang Z. PRISMA-compliant meta-analysis: association of metabolic syndrome and its components with the risk of chronic obstructive pulmonary disease. Biosci Rep. 2018;38:BSR20181199. 10.1042/BSR20181199. [DOI] [PMC free article] [PubMed]
- 14.Zhou W, Li CL, Cao J, Feng J. Metabolic syndrome prevalence in patients with obstructive sleep apnea syndrome and chronic obstructive pulmonary disease: relationship with systemic inflammation. Clin Respir J. 2020;14:1159–1165. doi: 10.1111/crj.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naseem S, Baneen U. Systemic inflammation in patients of chronic obstructive pulmonary disease with metabolic syndrome. J Family Med Prim Care. 2019;8:3393–3398. doi: 10.4103/jfmpc.jfmpc_404_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinellu E, Fois AG, Sotgiu E, Mellino S, Mangoni AA, Carru C, Zinellu A, Pirina P. Serum albumin concentrations in stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Clin Med. 2021;10(2):269. doi: 10.3390/jcm10020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starzer AM, Steindl A, Mair MJ, Deischinger C, Simonovska A, Widhalm G, Gatterbauer B, Dieckmann K, Heller G, Preusser M, et al. Systemic inflammation scores correlate with survival prognosis in patients with newly diagnosed brain metastases. Br J Cancer. 2021;124:1294–1300. doi: 10.1038/s41416-020-01254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalyan S, Hitchcock CL, Sirrs S, Pudek M, Prior JC. Cardiovascular and metabolic effects of medroxyprogesterone acetate versus conjugated equine estrogen after premenopausal hysterectomy with bilateral ovariectomy. Pharmacotherapy. 2010;30:442–452. doi: 10.1592/phco.30.5.442. [DOI] [PubMed] [Google Scholar]
- 20.Bilgin S, Kurtkulagi O, Atak Tel BM, Duman TT, Kahveci G, Khalid A, Aktas G. Does C-reactive protein to serum albumin ratio correlate with diabetic nephropathy in patients with type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes. 2021;15:1071–1074. doi: 10.1016/j.pcd.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Ma Y, Xue J, He C, Zhan Z, Liu X, Chen P, Cai S, Zeng Y, Wu Q, et al. C-Reactive protein to serum albumin ratio as a novel biomarker to predict prognosis in patients with chronic obstructive pulmonary disease. Clin Lab. 2021 doi: 10.7754/Clin.Lab.2020.200630. [DOI] [PubMed] [Google Scholar]
- 22.2018 Practice guidelines for the management of arterial hypertension of the European society of hypertension and the European society of cardiology: ESH/ESC task force for the management of arterial hypertension: erratum. J Hypertens. 2019; 37:456. [DOI] [PubMed]
- 23.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 24.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 25.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Mark SS, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 26.Zhou T, Zhan J, Hong S, Hu Z, Fang W, Qin T, Ma Y, Yang Y, He X, Zhao Y, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep. 2015;5:10481. doi: 10.1038/srep10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018–3030. doi: 10.1111/liv.14675. [DOI] [PubMed] [Google Scholar]
- 28.COPD guideline committee of the Japanese Respiratory Society . The guidelines for the diagnosis and treatment for COPD. Tokyo: Medical Review Co Ltd; 2018. [Google Scholar]
- 29.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, et al. Standardization of spirometry 2019 update an official american thoracic society and european respiratory society technical statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brassington K, Selemidis S, Bozinovski S, Vlahos R. Chronic obstructive pulmonary disease and atherosclerosis: common mechanisms and novel therapeutics. Clin Sci. 2022;136:405–423. doi: 10.1042/CS20210835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsumune T, Senoh E, Nishikawa H, Adachi M, Kajii E. The effect of obesity and smoking status on lung age in Japanese men. Respirology. 2009;14:757–760. doi: 10.1111/j.1440-1843.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- 32.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 33.Haruki T, Nakamura H, Taniguchi Y, Miwa K, Adachi Y, Fujioka S. 'Lung age' predicts post-operative complications and survival in lung cancer patients. Respirology. 2010;15:495–500. doi: 10.1111/j.1440-1843.2010.01708.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamamura S, Kawaguchi T, Nakano D, Tomiyasu Y, Yoshinaga S, Doi Y, Takahashi H, Anzai K, Eguchi Y, Torimura T, et al. Profiles of advanced hepatic fibrosis evaluated by FIB-4 index and shear wave elastography in health checkup examinees. Hepatol Res. 2020;50:199–213. doi: 10.1111/hepr.13436. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi T, Honda A, Sugiyama Y, Nakano D, Tsutsumi T, Tahara N, Torimura T, Fukumoto Y. Association between the albumin-bilirubin (ALBI) score and severity of portopulmonary hypertension (PoPH): a data-mining analysis. Hepatol Res. 2021;51:1207–1218. doi: 10.1111/hepr.13714. [DOI] [PubMed] [Google Scholar]
- 36.Colak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. The copenhagen general population study. Am J Respir Crit Care Med. 2020;201:671–680. doi: 10.1164/rccm.201908-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Yoneda M, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. 2021;56:951–963. doi: 10.1007/s00535-021-01796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, Kuriyama T, Takahashi K, Nishimura K, Ishioka S, Aizawa H, et al. COPD in Japan: the nippon COPD epidemiology study. Respirology. 2004;9:458–465. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, Targher G, Byrne CD, Yuan WJ, Zheng MH. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi: 10.1016/j.metabol.2020.154433. [DOI] [PubMed] [Google Scholar]
- 40.Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, Yang C, Zong G, Wu J, Jia W. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 46-year cohort study in China. J Clin Endocrinol Metab. 2022;107:88–97. doi: 10.1210/clinem/dgab641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong KS, Kim MC, Ahn JH. Sarcopenia is an independent risk factor for NAFLD in COPD: a nationwide survey (KNHANES 2008–2011) Int J Chron Obstruct Pulmon Dis. 2020;15:1005–1014. doi: 10.2147/COPD.S249534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonardo A, Nascimbeni F, de Ponz Leon M. Nonalcoholic fatty liver disease and COPD: is it time to cross the diaphragm. Eur Respir J. 2017 doi: 10.1183/13993003.00546-2017. [DOI] [PubMed] [Google Scholar]
- 43.Moon SW, Kim SY, Jung JY, Kang YA, Park MS, Kim YS, Chang J, Ro JS, Lee YH, Lee SH. Relationship between obstructive lung disease and non-alcoholic fatty liver disease in the Korean population: Korea national health and nutrition examination survey, 2007–2010. Int J Chron Obstruct Pulmon Dis. 2018;13:2603–2611. doi: 10.2147/COPD.S166902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minakata Y, Ueda H, Akamatsu K, Kanda M, Yanagisawa S, Ichikawa T, Koarai A, Hirano T, Sugiura H, Matsunaga K, et al. High COPD prevalence in patients with liver disease. Intern Med. 2010;49:2687–2691. doi: 10.2169/internalmedicine.49.3948. [DOI] [PubMed] [Google Scholar]
- 45.Chan SMH, Selemidis S, Bozinovski S, Vlahos R. Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies. Pharmacol Ther. 2019;198:160–188. doi: 10.1016/j.pharmthera.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, Lopez Garcia F, Montero L, Soriano JB. Working group on C, Spanish Society of internal M: COPD patients with and without metabolic syndrome: clinical and functional differences. Intern Emerg Med. 2014;9:419–425. doi: 10.1007/s11739-013-0945-7. [DOI] [PubMed] [Google Scholar]
- 47.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon AE, Raymond DM, Suratt BT, Bourassa LM, Irvin CG. Lower airway disease in asthmatics with and without rhinitis. Lung. 2008;186:361–368. doi: 10.1007/s00408-008-9119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, Calverley P, Coxson H, Crim C, Edwards LD, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 50.Alcaraz-Quiles J, Casulleras M, Oettl K, Titos E, Flores-Costa R, Duran-Guell M, Lopez-Vicario C, Pavesi M, Stauber RE, Arroyo V, et al. Oxidized albumin triggers a cytokine storm in leukocytes through P38 mitogen-activated protein kinase: role in systemic inflammation in decompensated cirrhosis. Hepatology. 2018;68:1937–1952. doi: 10.1002/hep.30135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. Data sharing statement: no additional data available.