Abstract
A Bacillus subtilis mutant with a deletion in the citC gene, encoding isocitrate dehydrogenase, the third enzyme of the tricarboxylic acid branch of the Krebs cycle, exhibited reduced growth yield in broth medium and had greatly reduced ability to sporulate compared to the wild type due to a block at stage I, i.e., a failure to form the polar division septum. In early stationary phase, mutant cells accumulated intracellular and extracellular concentrations of citrate and isocitrate that were at least 15-fold higher than in wild-type cells. The growth and sporulation defects of the mutant could be partially bypassed by deletion of the major citrate synthase gene (citZ), by raising the pH of the medium, or by supplementation of the medium with certain divalent cations, suggesting that abnormal accumulation of citrate affects survival of stationary-phase cells and sporulation by lowering extracellular pH and chelating metal ions. While these genetic and environmental alterations were not sufficient to allow the majority of the mutant cell population to pass the stage I block (lack of asymmetric septum formation), introduction of the sof-1 mutant form of the Spo0A transcription factor, when coupled with a reduction in citrate synthesis, restored sporulation gene expression and spore formation nearly to wild-type levels. Thus, the primary factor inhibiting sporulation in a citC mutant is abnormally high accumulation of citrate, but relief of this metabolic defect is not by itself sufficient to restore competence for sporulation.
Nutritional limitation leads Bacillus subtilis cells to cease normal cell division and initiate a highly organized developmental program, culminating in production of a dormant, environmentally resistant spore (11, 39). During the initiation of sporulation, cells undergo a modified cell division (asymmetric septation) in which a septum forms near one of the cell poles, creating a small forespore compartment and a larger mother cell compartment. This compartmentalization of sporulating cells restricts the activities of sporulation-specific sigma factors, causing each compartment to have a different program of gene expression (48). The ordered sequence of sporulation events is strictly governed by regulatory networks which coordinate nutritional, transcriptional, and morphological signals (47, 48). One aspect of physiological control of these networks is the metabolic and signalling role of the Krebs citric acid cycle. A strong linkage between Krebs cycle function and sporulation is reflected by the fact that Krebs cycle enzymes are maximally induced just before the onset of sporulation and by the finding that the absence of these enzymatic activities causes sporulation deficiency (8, 15, 18, 22, 24, 27, 28, 43, 53). Induction of the Krebs cycle at the end of the exponential growth phase presumably allows stationary-phase cells to fully metabolize by-products of glycolysis, such as pyruvate, lactate, acetate, and acetoin, yielding energy, reducing power, and biosynthetic intermediates for the synthesis of the RNA, protein, peptidoglycan, and lipid needed to form a spore (19).
As part of an assessment of the roles of individual citric acid cycle enzymes in sporulation, we have determined that a mutant defective in isocitrate dehydrogenase (ICDH) (the product of citC [24]), the third enzyme of the Krebs cycle, initiates sporulation but becomes blocked at stage I (28). That is, sporulating cells of the mutant assemble a ring of the cell division protein FtsZ at the sites at which polar septation would normally occur, but the septum does not form (28). This morphological block is reflected in a complementary block in gene expression. Sporulation-specific genes expressed before septation are transcribed normally in a citC mutant, but those genes that depend on the septation-dependent, forespore-specific, ςF-containing form of RNA polymerase (e.g., spoIIQ [33]) are not (28). The progression from polar localization of the FtsZ ring to formation of the asymmetric septum is mediated in part by an unknown gene whose expression is dependent on the earliest functioning sporulation sigma factor, ςH (32). Since ςH is active in a citC mutant (28), the mechanism by which the absence of ICDH activity interferes with asymmetric septation is not known. As shown in the accompanying paper (36), further analysis of the stage I block in a citC mutant has revealed that inactivation of the spoVG gene (42, 44, 46) allows the mutant cells to overcome in part their defect in asymmetric septation and activate forespore-specific, ςF-dependent genes. SpoVG, which is induced at the onset of stationary phase (54), acts as a transient negative regulator of the pathway leading to asymmetric septum formation (36).
Other recent studies on the sporulation deficiency of Krebs cycle mutants (8, 23, 24) have indicated that these mutants have blockages at multiple steps in the sporulation pathway. Surprisingly, a citrate synthase mutant is blocked at stage III (23), while an aconitase mutant is blocked at stage 0 (8). Since the absence of any of the first three enzymes of the Krebs cycle should cause equivalent defects in production of ATP, reducing power, and biosynthetic intermediates, these consequences of enzyme deficiency cannot explain the different phenotypes of the various mutants. To couple the developmental block in a citC mutant with the specific metabolic and physiological changes caused by the absence of ICDH activity, we sought to identify environmental conditions and compensatory mutations that would restore sporulation competence. The results suggest that accumulation of citrate is a major contributor to the mutant phenotype. Moreover, we have found that the inhibitory effects of overabundant citrate on asymmetric septation and activation of the compartment-specific sigma factors are mainly due to lowering of extracellular pH and depletion of manganese.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains of B. subtilis used in this work are listed in Table 1. Use of DS medium for growth and sporulation of B. subtilis has been described elsewhere (24). Growth was monitored by measuring the absorbance (or optical density) at 600 nm. To quantitate spore formation, 1-ml culture samples were heated to 80°C for 10 min, diluted in sterile water, and assayed for colony formation on DS plates. Escherichia coli JM107 (51) was used for propagation of plasmids.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Source, reference, or construction |
|---|---|---|
| JH642 | trpC2 pheA1 | J. A. Hoch |
| AG918 | sof-1 cat trpC2 pheA1 | A. D. Grossman |
| AG1431 | spo0F ΔS spo0BΔPstI trpC2 | A. D. Grossman |
| MO1657 | ΔamyE::[Φ(sspE′-lacZ) cat] trpC2 pheA1 | 17 |
| MO2051 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] trpC2 pheA1 | 33 |
| SC432 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] | S. Cutting |
| SJB219 | ΔcitC::spc trpC2 pheA1 | 28 |
| SJB225 | Φ(spoIID′-lacZ) cat trpC2 pheA1 | 28 |
| SJB229 | Φ(spoIID′-lacZ) cat ΔcitC::spc trpC2 pheA1 | 28 |
| SJB231 | ΔcitZC::spc trpC2 pheA1 | pCS66→JH642a |
| SJB256 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] trpC2 pheA1 | 27 |
| SJB294 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] trpC2 pheA1 | 28 |
| SJB295 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitC::spc trpC2 pheA1 | 28 |
| SJB2192 | ΔcitC::spc trpC2 pheA1 sof-1 cat | AG918 DNA→SJB219 |
| SR10 | Φ(spoIID′-lacZ) cat | 41 |
| AS1 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitC::spc sof-1 cat trpC2 pheA1 | SJB219 DNA→KMB424 |
| KMB119 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitZC::spc trpC2 pheA1 | MO2051 DNA→SJB231 |
| KMB154 | ΔamyE::[(Pspac-icdSmb) cat] ΔcitC::spc trpC2 pheA1 | pKM54→SJB219 |
| KMB165 | ΔamyE::[Φ(sspE′-lacZ) cat] ΔcitC::spc trpC2 pheA1 | MO1657 DNA→SJB219 |
| KMB170 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitC::spc trpC2 pheA1 | SC432 DNA→SJB219 |
| KMB174 | Φ(spoIID′-lacZ) ΔcitZC::spc trpC2 pheA1 | SR10 DNA→SJB231 |
| KMB175 | ΔamyE::[Φ(sspE′-lacZ) cat] ΔcitZC::spc trpC2 pheA1 | MO1657 DNA→SJB231 |
| KMB176 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitZC::spc trpC2 pheA1 | SC432→SJB231 |
| KMB252 | citC::pKM112 trpC2 pheA1 | pKM112→JH642 |
| KMB415 | sof-1 cat trpC2 pheA1 | AG918 DNA→JH642 |
| KMB416 | spo0A+ cat trpC2 pheA1 cat | AG918 DNA→JH642 |
| KMB424 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] sof-1 cat trpC2 pheA1 | SC432 DNA→KMB415 |
| KMB425 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] spo0A+ cat trpC2 pheA1 | SC432 DNA→KMB416 |
| KMB429 | ΔcitZC::spc sof-1 cat trpC2 pheA1 | SJB231 DNA→KMB415 |
| KMB430 | ΔcitZC::spc spo0A+ cat trpC2 pheA1 | SJB231 DNA→KMB416 |
| KMB435 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitZC::spc sof-1 cat trpC2 pheA1 | SC432 DNA→KMB429 |
| KMB436 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitZC::spc spo0 A+ cat trpC2 pheA1 | SC432 DNA→KMB430 |
Plasmid pCS66 was introduced into strain JH642.
The icdSm gene encodes S. mutans ICDH.
To verify the presence of the sof-1 allele of spo0A in various strains, DNA from the strain in question was used to transform strain AG1431 (spo0F spo0B), selecting for a chloramphenicol resistance (Camr) marker closely linked to spo0A. In transformants produced by DNA from sof-1 mutants, about 50% of the Camr transformants have a Spo+ phenotype on DS plates.
Enzymatic assays of citrate and isocitrate.
Cultures (30 ml) of wild-type or mutant cells were grown in DS medium, harvested 1 h after the entry into stationary phase (T1), and separated by centrifugation into supernatant fluid and cell pellet fractions. The supernatant fluid was neutralized by addition of KOH. The cell pellet was washed with 20 mM Tris-HCl buffer (pH 8) containing 1 mM EDTA and then subjected to deproteinization as follows. Cells were resuspended in 4 ml of 0.3 M perchloric acid, kept on ice for 10 min, and centrifuged. A 2-ml sample of the resulting supernatant fluid was mixed with 1 ml of 0.75 M potassium carbonate and kept on ice for 15 min. After the mixture was centrifuged, the resulting supernatant fluid was used as a cell extract for enzymatic assays.
Citrate was measured by using a citric acid assay kit (Boehringer Mannheim) as described previously (8). For measurement of isocitrate, a 50-μl sample was added to 950 μl of ICDH assay buffer (50 mM Tris-HCl [pH 7.5], 1 mM MnCl2, 1 mM NADP+). After purified B. subtilis ICDH (0.014 unit) (35) was added to the reaction mixture, NADPH production, coupled to conversion of isocitrate to 2-ketoglutarate, was monitored at 340 nm. The concentration of isocitrate was calculated from a standard curve based on commercially prepared isocitrate. One unit of enzymatic activity was defined as the amount of enzyme that produces 1 μmol of NADPH per min with a saturating concentration (0.2 mM) of dl-isocitrate.
Measurements of intracellular metabolite pools by HPLC analysis.
For each time point, a 5-ml sample of a culture in DS medium was removed and the cells were collected rapidly by filtration through a Millipore HA filter (pore size, 0.45 μm) that had previously been boiled for 10 min in 1 liter of water to remove contaminants. The filter was washed with ice-cold ultrapure water and plunged into 5 ml of boiling ultrapure water. When the volume was reduced to about 0.5 ml, the liquid was cooled, transferred to a microcentrifuge tube, and stored at −80°C. For analysis, each sample was thawed, centrifuged to remove debris, and passed through a 0.22-μm-pore-size high-performance liquid chromatography (HPLC) filter. The HPLC analyses (4) were performed on a Dionex DX-500-Microbore system equipped with the Pulsed Electrochemical Detector (ED40), which was operated in conductivity mode with an Anion Self-Regenerating Suppressor (ASRS-1) and the variable wavelength detector (AD20) set at 260 nm. The Dionex IonPac AS11 column (2 by 250 mm), equipped with a guard column, was employed with the following sodium hydroxide gradient elution profile. The column was first equilibrated for 10 min with 2 mM NaOH. Following sample injection, the NaOH gradient was increased linearly from 2 to 14 mM from 11 to 20 min, then increased with a slightly concave curve from 14 to 28 mM NaOH from 20 to 28 min, increased linearly from 28 to 80 mM NaOH from 28 to 35 min, and finally was held at 80 mM NaOH from 35 to 40 min. The flow rate was set at 0.5 ml/min, and a 10-μl sample injection loop was used. Data were analyzed by using PeakNet, release 4.11A, chromatography software program (Dionex Corp.). In an independent run, each sample was mixed with a set of chromatography standards and analyzed.
To calculate intracellular metabolite concentrations, a standard curve that relates cell number to optical density at 600 nm (OD600) was prepared and an internal volume of 1.1 × 10−9 μl per cell was assumed (50).
Deletion-insertion mutation of citZC.
Plasmid pCS66 (23) contains the first 30 amino acids of the citZ gene and the last 130 amino acids of the citC gene, with the spectinomycin cassette (spc) inserted in-between. The orientation of the spc gene in pCS66 is such that transcription is in the same direction as that of the citZ and citC genes. S. Jin introduced linearized pCS66 by transformation into B. subtilis JH642 to create strain SJB231. Transformants that were spectinomycin resistant and chloramphenicol sensitive were shown by Southern blot analysis to have undergone double-crossover recombination.
Expression of the Streptococcus mutants icd gene in B. subtilis.
The S. mutans icd gene, encoding ICDH, was amplified by PCR, using plasmid pJG400 (9) as the template and oligonucleotide primers that allowed introduction of a B. subtilis consensus ribosome binding site (RBS) just upstream of the icd coding sequence. The PCR product (1.2 kb) was inserted at the modified EcoRV site of pT7Blue(R) (Novagen, Inc.) (generating pKM35), and then subcloned between the SalI and SacI sites of pBluescript SK(−) (pSK−) (Stratagene, Inc.), to create pKM42. To eliminate possible PCR-derived errors, we replaced the 3′ end of the PCR-amplified icd gene (a HindIII-SacI fragment of about 1.2 kb) with the corresponding region of the original icd gene from pKM34 (see below), to create pKM49. The SalI (blunt-ended with the Klenow enzyme)-SacI fragment of pKM49, which contains the S. mutans icd gene preceded by the B. subtilis RBS, was inserted between the HindIII (blunt-ended) and SacI sites of pAF3 (16), creating pKM54. After transformation of B. subtilis ΔcitC mutant cells with pKM54 and selection for chloramphenicol resistance, we isolated clones in which icd was integrated at the amyE locus and its expression was under the control of the spac promoter (52).
pKM34 was constructed by ligating a 3.5-kb HindIII-EcoRI fragment (′acn citZ icd) of pJG400 to pSK− which had been cut with the same enzymes.
Cloning of the citC gene.
Plasmid pKM3 (a pSK- derivative) has a HindIII-EcoRI fragment (2.2 kb) of pCS60 (24), which contains the 3′ end of the citC gene (about 0.7 kb), the citH gene, and the 5′ end of the phoP gene. The entire citC gene was cloned by joining the SacII (blunt-ended)-HindIII fragment (0.7 kb) of pCS71 (24) to pKM3 which had been cut with SalI (blunt ended) and HindIII, resulting in pKM4. Plasmid pKM11 was constructed by inserting a 1.4-kb DraI-EarI fragment, which contains the entire citC gene without its gene-specific promoter, into the SmaI site of pBB544, a B. subtilis integrative vector (2). The orientation of the citC gene in pKM11 was the same as that of the lacZ gene in pBB544.
Site-directed mutagenesis.
An essential serine (residue 103) in the active site of B. subtilis ICDH (35) was changed to aspartic acid by PCR-mediated mutagenesis (20) with pKM11 as the template and the following oligonucleotide primers: primer a, 5′-GACACCTGTCGGCGGCGGTATCCGTGATTTGAACGTAGCGCTC; primer b, 5′-GAGCGCTACGTTCAAATCACGGATACCGCCGCCGACAGGTGT; primer c, 5′-GGAAACAGCTATGACCATG (vector-derived sequence, upstream of citC); and primer d, 5′-GTAAAACGACGGCCAGT (vector-derived sequence, downstream of citC). The mutation is underlined in the complementary mutagenic primers a and b. In brief, initial PCRs using oligonucleotide primers a and d or b and c produced fragments with overlapping ends. The entire citC gene containing the mutation was generated in a subsequent PCR using oligonucleotides primers c and d. In all PCR reactions, the Expand High Fidelity PCR system (Boehringer Mannheim) was used. After the amplified mutant citC gene was digested with BamHI and EcoRI, the DNA fragment was cloned between the BamHI and EcoRI sites of pBB544 (Neor) (2), creating pKM94. To remove other possible mutations before direct DNA sequencing, a 0.7-kb HindIII-ClaI (blunt-ended) fragment of pKM94 (3′ end of citC, downstream of the active-site mutation) was replaced with a HindIII-EcoRV fragment of pKM11, resulting in pKM112.
Plasmid pKM69, a derivative of the amyE integration vector pAF3 (16) was constructed as follows. The region of the PspacA(1)G-E. coli icd gene on the chromosome of strain KMB15 (28) was amplified by PCR, cloned in the modified EcoRV site of pT7Blue(R), and after cleavage with EcoRI and SacI, ligated to pAF3 that had been cleaved with the same enzymes, creating pKM67. The PCR-amplified E. coli icd gene preceded by the B. subtilis consensus RBS (HindIII-SacI fragment) in pKM67 was replaced with the modified icd gene (HindIII-SacI fragment) cloned in pKM12B (28), to create pKM69.
To test the function of B. subtilis ICDH(S103D), we constructed a plasmid (pKM118) having the mutated citC gene fused to the spacA(1)G promoter (28) in pKM69 and integrated this plasmid at the amyE locus in a ΔcitC mutant (SJB219). The resulting strain was a glutamate auxotroph, indicating that the point mutation disrupts ICDH enzymatic activity. Next, strain JH642 was transformed with pKM112. After selection for Neor and screening for Glt− clones, a single-crossover mutant (KMB252) was obtained for further study.
Protein analysis.
Cells were harvested from an 80-ml culture, washed with 20 mM Tris-HCl buffer (pH 7.4) containing 1 mM citrate, 5 mM MnCl2, 5 mM β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride, and 10% glycerol, and resuspended in 2 ml of the same buffer. Cells were disrupted by sonication and centrifuged to remove cell debris. The supernatant fluid was used as a crude extract in this study. Crude extract (3 μg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were then electrotransferred to Immobilon-P membranes (Millipore Corp.) with a Mini Trans-Blot transfer cell (Bio-Rad Laboratories) according to the supplier’s instructions. ICDH was detected by exposure to rabbit antibodies against B. subtilis ICDH (37) and subsequent alkaline phosphatase staining (26).
Other methods.
DNA manipulations were performed as described previously (24, 36). Transformation of cells of E. coli and B. subtilis and β-galactosidase assays were performed as described previously (24). Thin-section electron microscopic analysis was performed as described previously (28), with the assistance of A. Brown-Cormier, Electron Microscopy Unit, Department of Anatomy and Cellular Biology, Tufts University. All oligonucleotides used in this study were synthesized by M. Berne, Tufts University Protein and Nucleic Acid Analysis Facility.
RESULTS
Growth and sporulation of a citC mutant.
In nutrient broth sporulation (DS) medium, strain SJB219 (ΔcitC::spc) grew at about the same rate as did wild-type cells (Fig. 1) but reached stationary phase at a lower cell density (∼1 × 108 per ml versus ∼5 × 108 per ml). Moreover, while wild-type cultures lost 20 to 40% of their cells to lysis in stationary phase, the citC mutant cultures lost 60 to 90% of their cells. As a result of these effects, the titer of viable citC mutant cells at T20 was ≤10% of the wild-type titer. Of the surviving cells, only 0.1 to 1% formed heat-resistant spores and total spore yield in the mutant was 0.01 to 0.1% of that in the isogenic wild-type strain (Table 2). As shown previously, the specific stage of blockage of sporulation in the citC mutant is stage I, that is, the step during which the asymmetric septum forms (28). As a result, the citC mutant does not express genes whose transcription is dependent on ςF (e.g., spoIIQ) or any sporulation genes expressed later (28). Despite the fact that the ΔcitC mutant does not grow to as high a cell density as the wild type does, it is able to initiate sporulation, as shown by the normal levels of expression of Spo0A-phosphate-dependent genes and bipolar localization of FtsZ rings seen in the mutant (28).
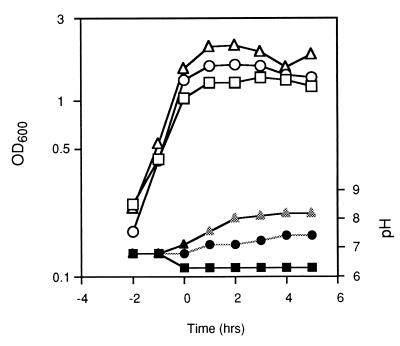
FIG. 1.
Growth and pH in DS medium. Strains JH642 (cit+; triangles), SJB219 (ΔcitC; squares), and SJB231 (ΔcitZC; circles) were grown in DS medium, and samples were removed at the indicated times for measurement of OD600 and, after removal of cells by centrifugation, for determination of the pH of the medium. The time of onset of stationary phase is designated as T0.
TABLE 2.
Sporulation of tricarboxylic acid cycle mutants
| Addition to mediuma | Genotype | Maximum OD600b | Maximum titerc | Titer at T20d | Spore titer at T20d | Sporulation efficiencye | Sporulated fractionf | Relative sporulation frequencyg |
|---|---|---|---|---|---|---|---|---|
| None | cit+ | 1.9 | 8.5 × 108 | 4.7 × 108 | 4.6 × 108 | 0.54 | 0.98 | (1.0) |
| ΔcitC | 1.2 | 1.4 × 108 | 1.4 × 107 | 5.7 × 104 | 0.0004 | 0.004 | 0.0001 | |
| ΔcitZC | 1.5 | 4.7 × 108 | 7.7 × 107 | 1.3 × 107 | 0.028 | 0.17 | 0.03 | |
| ΔcitZC sof-1 | 1.5 | 1.4 × 108 | 1.1 × 108 | 0.78 | 0.24 | |||
| MnCl2 | cit+ | 2.0 | 3.5 × 108 | 3.3 × 108 | 3.0 × 108 | 0.85 | 0.91 | (1.0) |
| ΔcitC | 1.1 | 1.6 × 108 | 5.3 × 107 | 1.5 × 106 | 0.009 | 0.028 | 0.005 | |
| HEPES | cit+ | 1.6 | 5.8 × 108 | 6.9 × 108 | 6.4 × 108 | 1.10 | 0.93 | (1.0) |
| ΔcitC | 1.1 | 3.1 × 108 | 4.4 × 107 | 3.3 × 106 | 0.001 | 0.075 | 0.005 | |
| Glt | cit+ | 2.3 | 5.4 × 108 | 5.2 × 108 | 0.96 | (1.0) | ||
| ΔcitC | 1.6 | 7.7 × 107 | 7.2 × 106 | 0.09 | 0.012 | |||
| Glucose | citC+ sof-1 | 2.1 | 7.2 × 108 | 8.2 × 108 | 8.2 × 108 | 1.13 | 1.00 | (1.0) |
| ΔcitC sof-1 | 2.2 | 2.1 × 108 | 7.0 × 106 | 1.2 × 105 | 0.006 | 0.017 | 0.0001 |
Final concentrations of additions to DS medium were as follows: 0.75 mM MnCl2, 50 mM HEPES (pH 8), 0.2% glutamate (Glt), and 0.1% glucose.
Maximum OD600 was defined as the highest OD600 in early stationary phase.
Maximum titer was the concentration of viable cells at the highest cell density.
At T20, viable-cell titer was determined and samples of cultures were heated at 80°C for 10 min to test for heat-resistant spore formation.
Sporulation efficiency is defined as the ratio of heat-resistant CFU at T20 to maximum titer in early stationary phase.
Sporulated fraction is defined as the ratio of heat-resistant CFU to total viable cell titer at T20.
Relative sporulation frequency is defined as heat-resistant spore titer in cit mutant cultures relative to that in cit+ cultures.
During growth of the wild-type cells in DS medium, the pH of the culture medium dropped transiently from 7.1 to about 6.8 and then rose during stationary phase to 8.0 to 8.5 (Fig. 1). In the citC mutant culture, however, the pH dropped to 6.3 to 6.5 and was not reversed (Fig. 1). This result would be consistent with the inability of these cells to import and oxidize acidic metabolites of pyruvate generated and excreted during exponential growth phase (17a). In DS medium, the primary carbon and energy sources are amino acids and glycerol (17b).
Effect of a citC missense mutation.
To know whether the requirement for the citC gene during sporulation is a reflection of the enzymatic activity of ICDH or some other role of the protein, we created a mutant strain having a single amino acid alteration (S103D) in the active-site serine (see Materials and Methods). Immunoblotting analysis indicated that ICDH(S103D) is stable in B. subtilis (data not shown). The mutant strain was totally devoid of ICDH activity, formed spores at 0.01% of the frequency of the wild-type strain, and failed to express a spoIIQ′-lacZ fusion, as is the case for the ΔcitC mutant, implying that the enzymatic activity of B. subtilis ICDH is the only function necessary for sporulation.
In previous work, we showed that the E. coli gene for ICDH could fully compensate for the absence of the B. subtilis citC gene with respect to growth and sporulation (28). To extend this analysis, we expressed in a B. subtilis ΔcitC mutant the icd gene from S. mutans (9). Again, the foreign gene for ICDH was fully capable of compensating for the absence of B. subtilis ICDH (data not shown). Taken together, these results support the idea that it is the enzymatic activity of ICDH, rather than some other hypothetical function of the protein, that is critical for sporulation.
Metabolic defects in a citC mutant.
In addition to producing 2-ketoglutarate, B. subtilis ICDH has the specific function of generating NADPH, a substrate for synthesis of fatty acids and some amino acids. To see whether this role of ICDH is important in sporulation, we supplemented the medium with the branched-chain, unsaturated fatty acids 13-methylmyristic acid and 12-methyltetradecanoic acid (each at 16 μg per ml) and a mixture of cysteine, arginine, lysine, methionine, and isoleucine (each at a concentration of 50 μg per ml). No increase in cell survival or sporulation was seen. As noted above, the S. mutans ICDH fully compensates for the absence of B. subtilis ICDH. Since S. mutans ICDH produces NADH rather than NADPH (9), the NADPH-producing function of ICDH does not appear to be essential for growth or sporulation in DS broth.
As a component of the Krebs cycle, ICDH participates in production of ATP and in gluconeogenesis. To assess the extent to which these roles of ICDH explain the stage I block of a citC mutant, we first measured ATP levels in mutant and wild-type cells (Table 3). At T0, the time of transition from exponential growth to stationary phase, the ATP level in both mutant and wild-type cells was approximately 1.5 mM (Table 3). One hour later, at T1, when the asymmetric septum is formed, the ATP concentration was higher in mutant cells than in wild-type cells (Table 3). Next, we added glucose to mutant and wild-type cells to provide an alternative source of ATP and to obviate the need for gluconeogenesis. Because glucose is normally a potent inhibitor of sporulation, we used a strain carrying a spo0A mutation, sof-1, that prevents inhibition of sporulation by glucose (21). Addition of glucose to citC+ sof-1 and ΔcitC sof-1 cultures allowed growth of the ΔcitC mutant to a higher cell density but caused only a very small increase in sporulation (Table 2) or spoIIQ′-lacZ expression (data not shown) by the citC mutant strain. We conclude that the block at stage I seen in citC mutant cells is not solely caused by a deficiency in ATP or gluconeogenesis or both.
TABLE 3.
Metabolite pools in ΔcitC mutant and wild-type cells determined by HPLC analysisa
| Strain (relevant genotype) | Time | AMP concn (mM) | ADP concn (mM) | ATP concn (mM) | Phosphoenol-pyruvate concn (mM) |
|---|---|---|---|---|---|
| JH642 (cit+) | T0 | 4.8 | 4.9 | 1.5 | 0.94 |
| T1 | 3.1 | 3.5 | 3.0 | 1.3 | |
| SJB219 (ΔcitC) | T0 | 7.9 | 1.5 | 1.5 | 1.5 |
| T1 | 4.8 | 6.3 | 3.9 | 2.3 |
HPLC analysis of metabolite pools was performed as described in Materials and Methods.
Metabolite accumulation.
A defect in any metabolic pathway can be predicted to cause not only a deficiency in the products of the pathway but also accumulation to abnormal levels of one or more intermediates upstream of the interrupted step. We therefore examined the concentrations of citrate and isocitrate in citC mutant cells. The measurement of these metabolites by enzymatic assays (see Materials and Methods) showed that the culture medium of the citC mutant cells at T1 contained both citrate and isocitrate at 10- to 15-fold-higher levels than those found in the wild-type culture medium (Table 4). The intracellular concentration of citrate was also 10-fold higher in the citC mutant than in a wild-type extract, but the intracellular concentration of isocitrate was too low to be measured accurately by this assay (Table 4). Therefore, we conducted a more-detailed analysis of intracellular metabolites by HPLC (see Materials and Methods). The HPLC analysis indicated that the citC mutant had 15- to 20-fold-higher intracellular concentrations of citrate and isocitrate than did wild-type cells (Table 4). This result fits with the absence of ICDH activity and the fact that the equilibrium of aconitase greatly favors conversion of isocitrate to citrate. It is noteworthy that all other pools examined by HPLC, including ATP, ADP, AMP, and phosphoenolpyruvate were at least as high in the citC mutant as in wild-type cells (Table 3). We do not know whether variations in the ratios of the nucleotides in any given extract are significant.
TABLE 4.
Measurements of citrate and isocitratea
| Strain | Intracellular concn by:
|
Extracellular concn by enzyme assay
|
||||
|---|---|---|---|---|---|---|
| Enzyme assay
|
HPLC analysis
|
Citrate (mM) | Isocitrate (mM) | |||
| Citrate (μmol/ OD600)b | Isocitrate (μmol/ OD600)b | Citrate (mM) | Isocitrate (mM) | |||
| cit+ | <0.073 | <0.024 | 1.3 | 0.17 | 0.062 | <0.02 |
| ΔcitC | 0.86 | <0.042 | 22 | 3.6 | 0.89 | 0.29 |
| ΔcitZC | 0.45 | <0.029 | 0.12 | <0.02 | ||
Cells were grown to stationary phase (T1) in DS medium, and samples for measurements of citrate and isocitrate were prepared by the methods described in Materials and Methods.
Total citrate or isocitrate (in micromoles) in cell extracts prepared from 30-ml cultures was normalized by dividing by the OD600 of the culture at the time of harvesting.
Phenotype of a citZ citC mutant.
To see whether the excessive accumulation of citrate and isocitrate is responsible for the lower growth yield and sporulation deficiency of the citC mutant, we constructed a mutant strain from which the major citrate synthase gene (citZ) and the citC gene were deleted simultaneously. Concentrations of citrate and isocitrate in the culture medium were reduced to near-wild-type levels (Table 4). Note that this strain continues to produce citrate at a low rate due to the activity of the citA gene product, a second isozyme of citrate synthase (24), but fails to convert citrate or isocitrate to 2-ketoglutarate because of the citC mutation. This ΔcitZC strain (SJB231) grew to a higher cell density than did the citC mutant and survived better in stationary phase (final titer of 7.7 × 107 per ml [Table 2]), supporting the idea that accumulation of citrate is in some way harmful to cell survival in stationary phase. One of the consequences of accumulation of citrate might be to lower the pH of the culture medium. In fact, the pH of the culture medium of the citZC mutant reached about 7.5 (about 1 unit higher than that of the citC mutant but still 1 unit lower than in the wild-type strain) in stationary phase (Fig. 1). The double-mutant cells produced 100 times more spores per ml than did the citC mutant cells (Table 2), but the majority of the double-mutant cells were still blocked at stage I of the developmental pathway as determined by measurements of spoIIQ′-lacZ expression (see below).
The finding that substantial reduction in citrate synthesis partially suppresses the defect in growth and sporulation of a citC mutant was confirmed by the isolation of spontaneous variants of SJB219. Colonies of SJB219 on DS plates are translucent due to substantial lysis of the cells. After several days of incubation, less-translucent papillae arose on many of the colonies. One such spontaneous variant was analyzed in detail. The mutation responsible for altered colony morphology was approximately 90% linked to ΔcitC::spc by transformation, suggesting that it was likely to lie within the citZCH operon. The double mutant grew and formed spores at the same frequency as SJB231 (ΔcitZC) (Table 2). Extracts of the variant strain showed that it had the same level of malate dehydrogenase (CitH) activity as in SJB219 but essentially no citrate synthase (CitZ) activity. Thus, the mechanism of suppression is apparently a reduction in accumulation of citrate due to inactivation of citZ. A mutant strain with deletions of both citrate synthase genes (citA and citZ) and of citC had a phenotype very similar to that of the citZC deletion strain (data not shown). Reduction of citrate synthase activity also serves to cure in part the growth defect of an ICDH mutant of E. coli (31).
Effects of divalent cations.
Accumulation of citrate in the citC mutant might also interfere with sporulation by chelating divalent cations and inhibiting the activity of one or more metal ion-dependent enzymes. We therefore added excess MnCl2, MgSO4, and FeSO4, separately or together, to DS medium. Raising the MnCl2 concentration in the medium from 0.01 mM (the standard concentration) to 0.5 or 0.75 mM had no significant effect on wild-type cells but increased sporulation of the citC mutant by 100- to 1,000-fold (Table 2). Higher concentrations inhibited sporulation. Similarly, increasing the FeSO4 concentration from 0.001 to 0.05 mM increased spore formation by the citC mutant by 50- to 100-fold. Supplementation of MnCl2 and FeSO4 simultaneously had no additive effect. Addition of supplementary MgSO4 did not increase sporulation efficiency. Thus, sporulation of the citC mutant seems to be limited in part by availability of certain divalent cations, presumably because of excess citrate accumulation. While supplementation with divalent cations did not restore expression of spoIIQ′-lacZ to the majority of the cells, it did allow a detectable increase in expression of both spoIIQ and spoIID (Table 5).
TABLE 5.
Expression of sporulation genes in tricarboxylic acid cycle mutants
| Relevant genotype | Supplement to mediumb | Relative expression of sporulation genes (%)a
|
|||
|---|---|---|---|---|---|
| spoIIQ | spoIID | sspE | cotA | ||
| ΔcitC | None | <10 | <10 | <10 | <10 |
| HEPES | <10 | <10 | <10 | <10 | |
| MnCl2 | 25 | 22 | <10 | <10 | |
| HEPES + MnCl2 | 20 | 13 | <10 | <10 | |
| ΔcitC sof-1 | None | <10 | <10 | ||
| ΔcitZC | None | 26 | 13 | <10 | <10 |
| ΔcitZC sof-1 | None | 110 | |||
B. subtilis strains carrying a promoter of a sporulation gene (ςF-dependent spoIIQ, ςE-dependent spoIID, ςG-dependent sspE, or ςK-dependent cotA) fused to the E. coli lacZ gene were grown in DS broth and harvested every hour after onset of stationary phase for measurements of β-galactosidase activity. The levels of expression of the lacZ fusions in mutants were estimated relative to the maximum level of expression of the same fusions in the wild-type strain. The average β-galactosidase activities (in Miller units) for the wild-type strains were as follows: spoIIQ-lacZ, 120; spoIID-lacZ, 27; sspE-lacZ, 250; and cotA-lacZ, 100.
In some experiments, 50 mM HEPES (pH 8) or 0.75 mM MnCl2 or both were added to DS medium.
Effect of pH alteration.
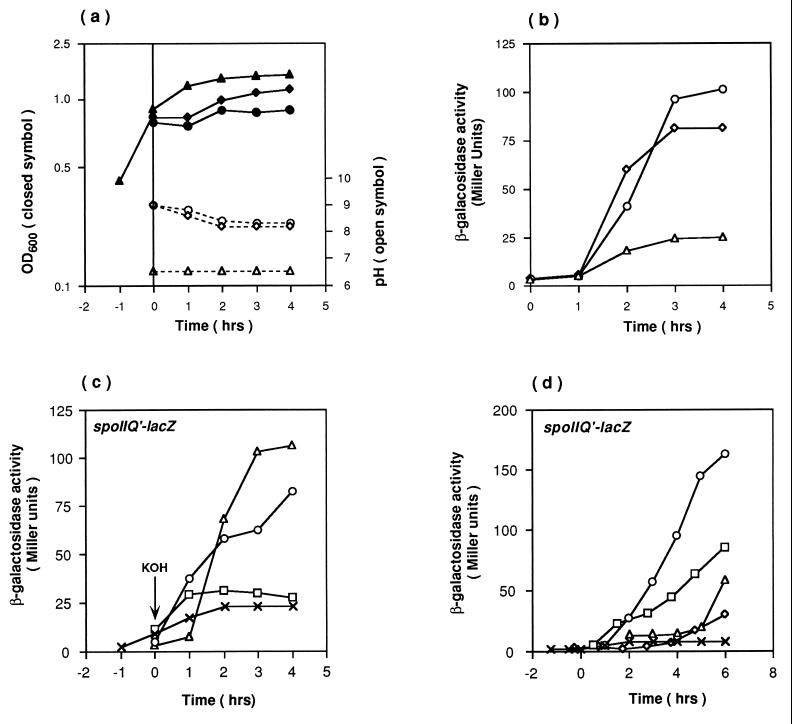
While searching for physiological conditions that would restore spoIIQ′-lacZ expression to a citC mutant, we unexpectedly found that addition of KOH to unbuffered DS medium has a significant stimulatory effect on spoIIQ expression. As shown in Fig. 2b, when KOH was added to the citC mutant culture to give a final concentration of 5 mM, high-level spoIIQ expression was observed, despite the fact that the growth rate dropped slightly over the same period. The pH of the culture medium just after KOH addition was almost 9 and then decreased to 8.0 to 8.5 over 4 h (Fig. 2a). The optimum concentration of KOH was determined to be 5 to 6.25 mM (Fig. 2c); a higher concentration of KOH (for example, 7.5 mM) caused aggregation of the cells, and no spoIIQ expression was observed. Altering the time of addition of KOH showed that KOH had its strongest activating effect on spoIIQ′-lacZ expression when it was added to the mutant culture at around T0 (Fig. 2d), while such addition caused delayed expression of spoIIQ′-lacZ in the wild-type strain (data not shown). The restoration of spoIIQ′-lacZ expression in the citC mutant is apparently due to raising the pH rather than increasing availability of K+ ions, since NaOH had the same effect as KOH (Fig. 2b) and KCl had no stimulatory effect (data not shown). These results suggest that raising the pH at T0 allows the citC mutant to bypass the stage I block in sporulation.
FIG. 2.
Effect of KOH on spoIIQ′-lacZ expression in a ΔcitC mutant. (a and b) Cells of strain SJB295 (ΔcitC) were grown in DS broth (triangles). After 5 mM KOH (circles) or NaOH (diamonds) was added to the culture at T0, the OD600 (panel a, closed symbols), pH of the medium (panel a, open symbols) and spoIIQ′-lacZ expression (panel b) were measured. (c) Different concentrations of KOH were added to the culture at T0 (×, no addition; □, 2.5 mM; ○, 5 mM; ▵, 6.25 mM). (d) KOH (5 mM) was added to the culture at T−1.25 (◊), T−0.5 (□), T0 (○), or T1 (▵). ×, control (no KOH added).
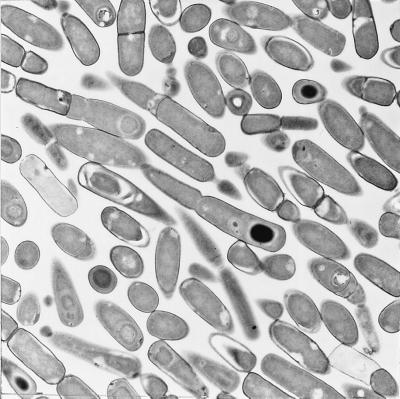
Under these conditions, however, the sporulation frequency of the citC mutant was not improved. Electron microscopy showed that about 50% of the cells were able to pass the stage I block in sporulation and that about half of these cells reached the engulfed forespore stage by T6 (Fig. 3). Mother cell-specific, ςE-dependent spoIID expression (41) and forespore-specific, ςG-dependent sspE expression (12) were partially activated by KOH addition, but ςK-dependent expression of the cotA gene (45) was still at a very low level (data not shown).
FIG. 3.
Thin-section electron microscopic analysis. Cells of a ΔcitC mutant strain (SJB219) were grown in DS medium to the end of exponential growth phase (T0), and 5 mM KOH was added to the culture. KOH-treated cells were harvested 6 h after the addition of KOH, and samples were prepared for transmission electron microscopy.
To assess further the importance of extracellular pH control in growth and sporulation of the citC mutant, we added HEPES (50 to 100 mM) or Tris (50 to 100 mM) buffers to DS medium to maintain a constant pH of 8.0 to 8.5. In such media, neither growth nor survival of the citC mutant was significantly improved, but the fraction of surviving cells that formed spores increased substantially (Table 2). Expression of spoIIQ′-lacZ was not recovered, however (Table 5), even when the buffer was added at T0 (data not shown). Simultaneous addition of HEPES or Tris (50 mM) and excess MnCl2 (0.75 mM) had no additive effect on heat-resistant spore formation (data not shown) or on spoIIQ′-lacZ expression (Table 5).
Effects of supplementation of metabolites.
The failure of citC mutant cells to grow to high titer is probably due primarily to the failure to utilize fully the by-products of pyruvate metabolism (acetate, lactate, and citrate). The effect might also be due in part to the failure to synthesize adequate 2-ketoglutarate. If so, addition to the medium of metabolites produced from 2-ketoglutarate might alleviate the defect. When the citC mutant cells were grown in DS medium supplemented with 0.2% glutamate, they grew to a higher density (as did the wild-type cells) and, as a result, achieved a high titer in late stationary phase. Moreover, 9% of the surviving cells were heat-resistant spores (≤2% of the wild-type spore production) (Table 2). Two types of explanations for this result can be imagined. On the one hand, citC mutants in DS medium might be limited for glutamate or 2-ketoglutarate. This seems very unlikely, however, since nutrient broth, the only organic constituent of DS medium, is made from boiled meat and contains substantial quantities of most amino acids, including glutamate. In fact, DS medium contains sufficient glutamate to permit normal growth and sporulation of a glutamate synthase mutant, which is an absolute glutamate auxotroph (3). In addition, glutamate dehydrogenase activity is induced in DS medium (3), allowing conversion of glutamate to 2-ketoglutarate. On the other hand, the addition of glutamate might have indirect effects on cell physiology. In fact, glutamate addition caused the pH of the culture medium to rise during growth so that even in citC mutant cells, it was >8.0 in stationary phase. Moreover, the effect of glutamate was mimicked by and additive with supplementation with succinate or aspartate (data not shown). Although addition of each of these compounds may help the citC mutant to sporulate for a different reason, the simplest interpretation is that they all work by causing extracellular pH to rise.
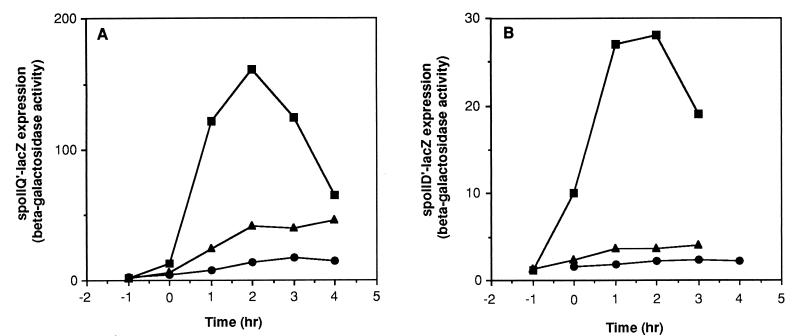
Synergistic effects of mutations in citZ and spo0A.
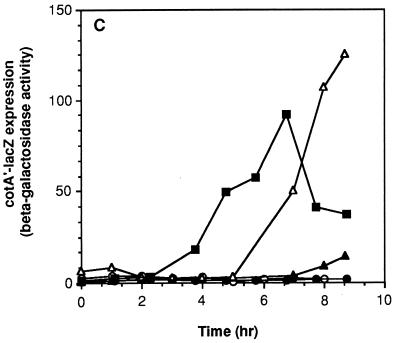
A mutant strain with both citZ and citC deleted was able to pass through stage I of sporulation and express, to a significant extent, genes dependent on ςF and, to a lesser extent, ςE (Fig. 4 and Table 5). The double mutant did not express genes dependent on ςG or ςK, however (Fig. 4 and Table 5). In an attempt to find conditions that would allow this double-mutant strain to progress further in the sporulation pathway, we tested the effects of introducing additional mutations. Surprisingly, complete restoration of ςK-dependent cotA′-lacZ expression and nearly complete restoration of spore formation were seen when the sof-1 allele of the spo0A gene was introduced into the ΔcitZC strain (Fig. 4C and Tables 2 and 5). This was the only combination of conditions or mutations that had such a strongly suppressive effect on the absence of ICDH. As shown above, the sof-1 mutation had no suppressive effect on a ΔcitC single mutant strain; its ability to suppress seems dependent on a reduction in citrate synthesis and accumulation.
FIG. 4.
Effects of citZ and sof-1 mutations on spo gene expression in a citC mutant. All strains were grown in DS medium and sampled for assays of β-galactosidase activity at the indicated times after the end of exponential growth (T0). (A) Strains SJB294 (cit+) (■), SJB295 (ΔcitC) (●), and KMB119 (ΔcitZC) (▴) were tested for spoIIQ′-lacZ expression. (B) Strains SJB225 (cit+) (■), SJB229 (ΔcitC) (●), and KMB174 (ΔcitZC) (▴) were assayed for spoIID′-lacZ expression. (C) Strains KMB425 (cit+) (■), KMB170 (ΔcitC) (●), KMB436 (ΔcitZC) (▴), AS1 (ΔcitC sof-1) (○), and KMB435 (ΔcitZC sof-1) (▵) were assayed for cotA′-lacZ expression.
DISCUSSION
Abnormal accumulation of citrate and isocitrate seems to be responsible for the earliest sporulation defect, i.e., lack of asymmetric septation, in a citC mutant. Citrate and isocitrate accumulate to unusually high levels because they cannot be further metabolized in the absence of ICDH. No citrate lyase or isocitrate lyase or other citrate- or isocitrate-degrading activity has been found in B. subtilis (13, 37), and examination of the B. subtilis genome sequence (30) revealed no homolog of E. coli citrate lyase, isocitrate lyase, or malate synthase. Such accumulation of citrate and isocitrate apparently has two negative effects on sporulation. First, the pH of the medium remains low (at 6.5) as cells enter stationary phase. By contrast, the pH of the culture medium of wild-type cells rises in stationary phase to 8.5 due, at least in part, to uptake of previously excreted organic acids, their conversion to citrate, and their subsequent oxidation. Asymmetric septation occurs at a time (approximately T1) when the extracellular pH is 7.5 to 8.0. In the citC mutant, extracellular organic acids, such as acetate and lactate, can presumably be taken up and converted to citrate and isocitrate, but no further metabolism ensues and citrate and isocitrate are excreted, maintaining low environmental pH. We have not measured the effect of the absence of ICDH on intracellular pH. In wild-type cells, internal pH is kept almost constant during growth and during most of sporulation (6, 7, 29, 34).
Raising external pH restored sporulation gene expression to a significant fraction of the citC mutant cells only when it was accomplished by the addition of inorganic alkali or certain Krebs cycle-derived compounds (glutamate, succinate, and aspartate); organic buffers affected only a small minority of the cell population even though they raised the extracellular pH to 8.0 or higher. We do not know either the basis for this difference or the mechanism that seems to link extracellular pH and asymmetric septation. It is possible that efficient uptake of the pH-altering agent is critical.
An effect of low external pH on sporulation gene expression has also been seen by others (6, 7). In DS medium supplemented with glucose and glutamine, the pH of the medium drops to 5.5, at which point the accumulation of ςH and expression of ςH-dependent genes is inhibited. The same mechanism is unlikely to be responsible for the phenotype of a citC mutant, since the pH does not reach 5.5 and expression of ςH-dependent genes is not inhibited (28).
The second effect of excess citrate and isocitrate appears to be chelation of Mn2+ (and perhaps Fe2+). Since the block in asymmetric septation can be bypassed in part of the cell population by addition of KOH or NaOH, we suggest that low pH is the primary deterrent to asymmetric septation in citC mutant cells. However, Mn2+ must also contribute to asymmetric septation, since expression of septation-dependent genes in a citC spoVG double mutant is raised significantly by supplementation with excess Mn2+ even though the pH remains low (36). The mechanistic basis of the cation effect is unknown. At least five enzymes of B. subtilis, aconitase (14), fructose-1,6-diphosphate aldolase (14), ICDH (37, 40), phosphoglycerate phosphomutase (38), and SpoIIE, a phosphatase that activates ςF by helping to liberate it from its inhibitor protein (10), are known to be Mn2+ or Fe2+ dependent and required for early sporulation events. SpoIIE is a particularly intriguing candidate for the Mn2+ target, since it localizes to the septum, is important for septum assembly or stability (28a), and is needed to activate ςF in the forespore (1).
Surprisingly, the roles of ICDH in ATP production, gluconeogenesis, and NADPH synthesis do not appear to be crucial for asymmetric septation, at least in DS medium. No defect in ATP synthesis was found in the citC mutant grown in DS medium at least until T1 and addition of glucose did not permit citC mutant cells to septate. Replacement of the B. subtilis citC gene by an S. mutans gene that expresses an NADH-producing ICDH completely restored sporulation to wild-type levels.
No single physiological condition or single suppressing mutation was able to restore sporulation completely to the citC mutant. Although addition of buffer, divalent cations, or certain metabolites to DS medium increased the fraction of the citC mutant population that survived and sporulated, it did not allow the vast majority of the population to advance beyond the original stage of blockage in the developmental program. Only the combination of reduction in citrate synthesis caused by a citZ mutation and alteration of the Spo0A transcription factor by the sof-1 mutation was able to restore late spo gene expression to the citC mutant. These results have two implications. First, the defects caused by the absence of Krebs cycle enzymes are manifold and affect multiple stages of sporulation. Even when early blockages are overcome by alterations in the environment or by a suppressing mutation, the cells are still blocked at a late stage of sporulation. Although neither energy nor reducing power appears to be limiting at early stages, one or the other may eventually become limiting as mutant cells try to complete the sporulation process. Second, the cell population is heterogeneous in its response to the additions, an idea consistent with a mechanism in which threshold concentrations of key metabolites determine whether any given cell will get past its genetically determined stage of blockage (5).
The mechanism of the synergistic suppression of the citC defect by mutations in citZ and spo0A (sof-1) is unclear. Since the sof-1 mutation by itself has no suppressive effect and since the citZ mutation is epistatic to the citC mutation, the most likely explanation is that an alteration in the Spo0A phosphorelay allows the need for CitZ to be bypassed. The phenotype of a citrate synthase mutant is to be blocked at morphological stage III (23), but expression of even early classes of spo genes is delayed (49). Perhaps the sof-1 mutation, which allows activation of Spo0A independently of the phosphorelay and normal developmental signals, overcomes a timing defect caused by the citZ mutation. In combination with a reduction in citrate synthesis and accumulation, the deleterious effects of the absence of ICDH are largely overcome.
In the accompanying paper (36), we show that loss-of-function mutations in the spoVG gene also cause a partial bypass of the citC mutant phenotype. The suppressive effect of a spoVG mutation is enhanced by deletion of citZ, especially in combination with a sof-1 mutation. SpoVG is suspected to affect asymmetric septation by altering the expression or inhibiting the activity of a Spo0A-dependent gene.
Interestingly, mutants blocked in each of the first three steps in the Krebs cycle have different sporulation phenotypes. A citA citZ mutant, deficient in both citrate synthases, proceeds as far as stage III in sporulation (23, 49), while an aconitase (citB) null mutant is blocked at stage 0 (8). The citrate synthase mutant makes no citrate, and the pH of its medium (7.5) is considerably higher than that of the other two mutants, consistent with the idea that asymmetric septation and other early stages of sporulation are very pH sensitive and divalent cation dependent. In fact, the aconitase mutant may have the earliest block because it accumulates the greatest amount of extracellular citrate (8), and its extracellular pH is even lower (by about 0.4 pH unit) than that of the ICDH mutant (37). Not surprisingly, citrate synthase mutations are epistatic to both aconitase and ICDH mutations (reference 8 and this work).
It was previously reported that a mutant (ΔcitA::neo ΔcitZ471 citC7) lacking the major and minor citrate synthases and ICDH was blocked in sporulation at stage 0 (22). In the present study, we examined a strain (ΔcitA::neo ΔcitZC::spc) lacking the same three enzymes but created with different mutations and found that its sporulation phenotype was different than that of the previous strain. Cells of the newer strain were able to express Spo0A-phosphate-, ςF- and ςE-dependent genes. We assume that the specific mutations carried by the previous strain caused an unusual phenotype or that the strain had acquired an unrecognized, additional mutation that caused a block at stage 0.
The multiple deleterious changes in cellular physiology caused by the absence of ICDH activity provide compelling reasons for regulating synthesis of this enzyme during sporulation. The fact that the citC gene is transcribed from its own promoter as well as from the citZCH operon promoter (25) may allow the cell to adjust citC expression to the specific environmental conditions in which cells find themselves.
ACKNOWLEDGMENTS
We thank D. Cvitkovitch and P. Stragier for making plasmids and strains available, S. Jin for construction of strain SJB231, B. Belitsky for discussions and criticism of the manuscript, D. de Mendoza for advice on fatty acid supplementation, and A. Brown-Cormier for assistance with electron microscopy.
This work was supported in part by research grants from the U.S. Public Health Service to A.L.S. (GM42219) and from the U.S. National Science Foundation to T.C. (MCB-9723593) and to T.M.H. (MCB-9723091).
REFERENCES
- 1.Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- 2.Belitsky B R, Gustafsson M C U, Sonenshein A L, Wachenfeldt C V. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J Bacteriol. 1997;179:5448–5457. doi: 10.1128/jb.179.17.5448-5457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belitsky, B. R. Personal communication.
- 4.Bhattacharya M, Fuhrman L, Ingram A, Nickerson K W, Conway T. Single-run separation and detection of multiple metabolic intermediates by anion-exchange high-performance liquid chromatography and application to cell pool extracts prepared from Escherichia coli. Anal Biochem. 1995;232:98–106. doi: 10.1006/abio.1995.9954. [DOI] [PubMed] [Google Scholar]
- 5.Chung J D, Stephanopoulos G, Ireton K, Grossman A D. Gene expression in single cells of Bacillus subtilis: evidence that a threshold mechanism controls the initiation of sporulation. J Bacteriol. 1994;176:1977–1984. doi: 10.1128/jb.176.7.1977-1984.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosby W M, Zuber P. Regulation of Bacillus subtilis ςH (Spo0H) and AbrB in response to changes in external pH. J Bacteriol. 1997;179:6778–6787. doi: 10.1128/jb.179.21.6778-6787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosby W M, Vollenbroich D, Lee O H, Zuber P. Altered srf expression in Bacillus subtilis resulting from changes in culture pH is dependent on the Spo0K oligopeptide permease and the ComQX system of extracellular control. J Bacteriol. 1998;180:1438–1445. doi: 10.1128/jb.180.6.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig J E, Ford M J, Blaydon D C, Sonenshein A L. A null mutation in the Bacillus subtilis aconitase gene causes a block in Spo0A-phosphate-dependent gene expression. J Bacteriol. 1997;179:7351–7359. doi: 10.1128/jb.179.23.7351-7359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvitkovitch D G, Gutierrez J A, Bleiweis A S. Role of the citrate pathway in glutamate biosynthesis by Streptococcus mutans. J Bacteriol. 1997;179:650–655. doi: 10.1128/jb.179.3.650-655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 11.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajardo-Cavazos P, Tovar-Rojo F, Setlow P. Effect of promoter mutations and upstream deletions on the expression of genes coding for small, acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1991;173:2011–2016. doi: 10.1128/jb.173.6.2011-2016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, S. Personal communication.
- 14.Fortnagel P, Freese E. Inhibition of aconitase by chelation of transition metals causing inhibition of sporulation in Bacillus subtilis. J Biol Chem. 1968;243:5289–5295. [PubMed] [Google Scholar]
- 15.Fortnagel P, Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968;95:1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouet A, Sonenshein A L. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990;172:835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frandsen N, Stragier P. Identification and characterization of the Bacillus subtilis spoIIP locus. J Bacteriol. 1995;177:716–722. doi: 10.1128/jb.177.3.716-722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Freese E, Fortnagel U. Growth and sporulation of Bacillus subtilis mutants blocked in the pyruvate dehydrogenase complex. J Bacteriol. 1969;99:745–756. doi: 10.1128/jb.99.3.745-756.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b.Freese E, Ichikawa T, Oh Y K, Freese E B, Prasad C. Deficiencies or excesses of metabolites interfering with differentiation. Proc Natl Acad Sci USA. 1974;71:4188–4193. doi: 10.1073/pnas.71.10.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freese E B, Marks C L. Developmental block in citric acid cycle mutants of Bacillus subtilis. J Bacteriol. 1973;116:1466–1468. doi: 10.1128/jb.116.3.1466-1468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hederstedt L. The Krebs citric acid cycle. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 181–197. [Google Scholar]
- 20.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 21.Hoch J A, Trach K, Kawamura F, Saito H. Identification of the transcriptional suppressor sof-1 as an alteration in the spo0A protein. J Bacteriol. 1985;161:552–555. doi: 10.1128/jb.161.2.552-555.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ireton K, Jin S, Grossman A D, Sonenshein A L. Krebs cycle function is required for activation of the Spo0A transcription factor in Bacillus subtilis. Proc Natl Acad Sci USA. 1995;92:2845–2849. doi: 10.1073/pnas.92.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin S. Ph.D. thesis. Boston, Mass: Tufts University; 1995. [Google Scholar]
- 24.Jin S, Sonenshein A L. Identification of two distinct Bacillus subtilis citrate synthase genes. J Bacteriol. 1994;176:4669–4679. doi: 10.1128/jb.176.15.4669-4679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin S, Sonenshein A L. Transcriptional regulation of Bacillus subtilis citrate synthase genes. J Bacteriol. 1994;176:4680–4690. doi: 10.1128/jb.176.15.4680-4690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin S, Sonenshein A L. Characterization of the major citrate synthase of Bacillus subtilis. J Bacteriol. 1996;178:3658–3660. doi: 10.1128/jb.178.12.3658-3660.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S, de Jesús-Berríos M, Sonenshein A L. A Bacillus subtilis malate dehydrogenase gene. J Bacteriol. 1996;178:560–563. doi: 10.1128/jb.178.2.560-563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin S, Levin P A, Matsuno K, Grossman A D, Sonenshein A L. Deletion of the Bacillus subtilis isocitrate dehydrogenase gene causes a block at stage I of sporulation. J Bacteriol. 1997;179:4725–4732. doi: 10.1128/jb.179.15.4725-4732.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Khvorova A, Zhang L, Higgins M L, Piggot P J. The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J Bacteriol. 1998;180:1256–1260. doi: 10.1128/jb.180.5.1256-1260.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krulwich T A, Agus R, Schneier M, Guffanti A A. Buffering capacity of bacilli that grow at different pH ranges. J Bacteriol. 1985;162:768–772. doi: 10.1128/jb.162.2.768-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature (London) 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmi T M, Helling R B. Selection for citrate synthase deficiency in icd mutants of Escherichia coli. J Bacteriol. 1976;127:76–83. doi: 10.1128/jb.127.1.76-83.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 33.Londoño-Vallejo J-A, Fréhel C, Stragier P. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- 34.Magill N G, Cowan A E, Koppel D E, Setlow P. The internal pH of the forespore compartment of Bacillus megaterium decreases by about 1 pH unit during sporulation. J Bacteriol. 1994;176:2252–2258. doi: 10.1128/jb.176.8.2252-2258.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuno K, Miller S, LaPorte D C, Sonenshein A L. Abstracts of the 12th International Spores Conference. 1996. Biochemical studies of B. subtilis isocitrate dehydrogenase, abstr. II-3. [Google Scholar]
- 36.Matsuno K, Sonenshein A L. Role of SpoVG in asymmetric septation in Bacillus subtilis. J Bacteriol. 1999;181:3392–3401. doi: 10.1128/jb.181.11.3392-3401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuno, K., and A. L. Sonenshein. Unpublished data.
- 38.Oh Y K, Freese E. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J Bacteriol. 1976;127:739–746. doi: 10.1128/jb.127.2.739-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piggot P J, Coote J G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaley R F, Hudock M O. Purification and properties of isocitrate dehydrogenase (NADP) from Thermus aquaticus YT-1, Bacillus subtilis-168 and Chlamydomonas reinhardti-2. Biochim Biophys Acta. 1973;315:22–36. doi: 10.1016/0005-2744(73)90125-3. [DOI] [PubMed] [Google Scholar]
- 41.Rong S, Rosenkrantz M S, Sonenshein A L. Transcriptional control of the Bacillus subtilis spoIID gene. J Bacteriol. 1986;165:771–779. doi: 10.1128/jb.165.3.771-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbluh A, Banner C D B, Losick R, Fitz-James P C. Identification of a new developmental locus in Bacillus subtilis by construction of a deletion mutation in a cloned gene under sporulation control. J Bacteriol. 1981;148:341–351. doi: 10.1128/jb.148.1.341-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutberg B, Hoch J A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970;104:826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandman K, Losick R, Youngman P. Genetic analysis of Bacillus subtilis spo mutations generated by Tn917-mediated insertional mutagenesis. Genetics. 1987;117:603–617. doi: 10.1093/genetics/117.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandman K, Kroos L, Cutting S, Youngman P, Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988;200:461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- 46.Segall J, Losick R. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell. 1977;11:751–761. doi: 10.1016/0092-8674(77)90289-6. [DOI] [PubMed] [Google Scholar]
- 47.Sonenshein A L. Metabolic regulation of sporulation and other stationary-phase phenomena. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C: American Society for Microbiology; 1989. pp. 109–130. [Google Scholar]
- 48.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 49.Swift, K., and A. L. Sonenshein. Unpublished data.
- 50.Whatmore A M, Reed R H. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor pressure regulation. J Gen Microbiol. 1990;136:2521–2526. doi: 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]
- 51.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 52.Yansura D G, Henner D J. Use of Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci USA. 1984;81:439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yousten A A, Hanson R S. Sporulation of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1972;109:886–894. doi: 10.1128/jb.109.2.886-894.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]