Abstract
Human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) significantly burden youth aged 13–24 years in the United States. Directly engaging youth in sexual health research is a public health priority and urgently needed to develop targeted, youth-friendly, and culturally relevant HIV/STI prevention interventions. Controversies arise, however, regarding informed assent and consent, parental permission or consent, and the definition of “child”/“minor” as it relates to medical, legal and ethical issues. In this paper, we describe challenges in the human subjects review processes that were undertaken before beginning an HIV/STI prevention research project with sexually active youth in an urban setting. These findings provide important contextual information to facilitate youth sexual health research and care, and Institutional Review Board approval processes with fewer delays.
Keywords: case study, consent, IRB, research participants, responsible conduct of research
Introduction
Incident sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), are estimated at 19.7 million cases in the United States (U.S.) annually. About half of cases (9.8 million) are among youth aged 15–24 years (Satterwhite, 2013); the United Nations defines “youth” as those persons between the ages of 15 and 24 years (United Nations Educational, Scientific and Cultural Organization, 2017). The cost burden of STIs for youth is estimated to be at least $6.5 million, with HIV accounting for more than 80% of the total cost burden (Chesson, 2004; Owosu-Edusei, 2013). In 2015, sexually active youth age 15–24 years comprised an estimated 41.2% of high school students (Kann, 2016) and an estimated 44–47% of home-sampled, never-married youth, including youth who may not attend school (Martinez, 2015). Recent data also suggest that about 60% of youth aged 13–24 years with HIV are unaware of their infection and therefore do not receive treatment; these youth can unknowingly pass HIV to others and remain undiagnosed until many years later (Whitmore, 2012). Considering these alarming statistics and the public health implications of HIV/STI transmission, research, education and prevention remain critical for U.S. youth who are disproportionately at risk for HIV/STIs (Centers for Disease Control and Prevention [CDC], 2016; Forhan, 2009; Satterwhite, 2013; Weinstock, 2004; Whitmore, 2012) and unintended pregnancies (Finer, 2010). Engaging youth in sexual health research efforts is vital and warranted to solicit their perspectives and opinions to effectively develop youth-friendly HIV/STI prevention interventions (Santelli, Rosenfeld, DuRant, Dubler, Morreale, English, & Smith Rogers, 1995; Santelli et al., 2003).
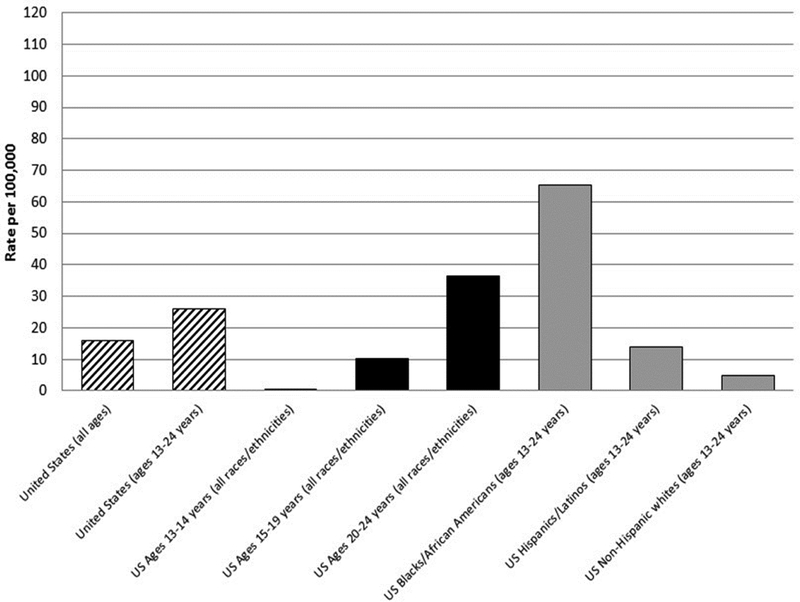
Efforts in domestic HIV prevention prioritize working with disproportionately affected groups, including youth as a priority group, to meet national goals to reduce new HIV infections, increase engagement of infected persons in care, and decrease HIV-related health disparities (White House Office of National AIDS Policy). National and local data suggest that among youth aged 13–24, Black/African American and Hispanic/Latino youth carry the largest burden of disease, especially during the time of this study (see Figure 1a; Whitmore, 2012). For example in 2015, Black youth only made up 14% of the 13–19 year old population in the U.S., yet these youth accounted for 64% of new HIV diagnoses (CDC, 2015). The causes of these disparities are multi-level, including the role of mental health and emotion regulation in youth’s sexual decision making and neighborhood-level poverty and disadvantage (Brawner et al., 2017; Bauermeister et al., 2015). These data underscore that research and program efforts with racial/ethnic minority youth, including individual and macro-level approaches (Prado, 2013), are urgently needed for domestic HIV prevention.
Figure 1a.
Rates of Diagnoses of HIV infection per 100,000 population, by young age groups (13–24 years) and race/ethnicity—United States, 2011
Data are from the US Centers for Disease Control and Prevention, HIV Surveillance Report, 2011.24
To facilitate routine HIV/STI testing and treatment and access for youth, all 50 states and the District of Columbia have laws in place that allow most minors (aged 12–17) to consent for their own sexual health care without parental permission—31 states explicitly include HIV testing and treatment as part of services accessible to youth (English & Kenney, 2010; Guttmacher Institute, 2013). Despite these supportive HIV/STI care access policies for youth, and an ethical context which supports HIV/STI prevention and control with at-risk populations (Semaan, 2007), controversy about parental permission and minors’ “maturity” remain in research. According to the United States Department of Health and Human Services (2009; see 45 CFR 46.402): “assent” is defined as a child’s affirmative agreement to participate in research; “permission” is defined as the agreement of parent(s) or guardian(s) to the participation of their child or ward in research; and “informed consent” occurs when someone who is legally able to consent (> age 18 years) gives permission after having the knowledge of the possible risks, probable consequences, and alternatives. Empirical data support minors’ competence to consent to research from the age of 12 and above, with recommendations for a dual child and parent consent only when the child has not reached the age where he/she is allocated rights for independent consent (Hein, De Vries, Troost, Meynen, Van Goudoever, & Lindauer, 2015). Before the age of 12, or if the child has not reached the age of independent consent, minors may assent to research along with their parents’/guardians’ consent for their participation (United States Department of Health and Human Services, 2009; see 45 CFR 46.408).
Valid medical, legal and ethical concerns exist regarding parental permission, waivers of permission, assent or consent by youth, and the definition of “child”/“minor” (someone under the age of majority, usually less than 18 years) in youth-focused practice (Coleman & Rosoff, 2013) and research (English & Kenney, 2010; Guttmacher Institute, 2013). These issues are complex and often challenging for investigators, institutional review boards (IRBs), parents, youth, and community advisors (Mammel, 1995; Santelli & Rogers, 2002). In addition, federal and local laws should be considered and balanced with the goals of protecting youth from risks. Understanding youths’ evolving maturity and autonomy and the importance of low-risk, biobehavioral and clinical research activities for youth-focused public health practice warrant consideration (Santelli et al., 2003).
At the intersection of ethics and law, two key areas potentially cause controversy in IRB determinations for sexual health research with youth: 1) level of risk, and 2) whether minors are emancipated for medical decision making under state or local law. Minimal risk studies are those in which the “probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests” (United States Department of Health and Human Services, 2009; see 45 CFR 46.102(i)). Wendler, Belsky, Thompson and Emanuel (2005) note that in the absence of empirical data on the risks of daily life and routine examinations among children, IRB members may default to intuitive judgement of these risks, which can lead to systematic errors in interpretation and implementation of federal regulations. An IRB’s assessment of minimal risk may also vary by institutional setting (e.g., academic center or hospital). In addition to risk determination, federal, state and local laws regulate issues such as the age of medical emancipation which determines whether minors can make medical decisions in the absence of a parent/guardian. From a research perspective, conflict can arise when IRBs disagree on the interpretation of state law in relation to a study’s level of risk.
During research collaborations, when multiple IRBs provide study oversight, the views and perspectives of IRB members may differ (Mammel, 1995; Molnar, 2013; Risjord, 2002). These IRB disagreements can be challenging if they potentially lead to research delays, especially if the research is addressing public health prevention priorities, such as sexual health. Risjord and Greenberg (2002) noted the potential negative implications of local and federal IRB disagreements and suggested lessons learned to facilitate future adolescent sexual health research. Our current experiences (2012–2013) suggest that more still needs to be done to improve timeliness of human subjects’ reviews, reduce IRB disagreements, and improve synergies among multiple entities in a youth-focused research team.
In this paper, we describe challenges and successes in the pre-research, human subjects’ local and federal review processes that were undertaken before beginning a youth-focused, HIV/STI prevention research project with sexually active youth. The case study explains the issues we faced when seeking approval for a minimal risk study involving Black youth of minority age, all of whom were receiving outpatient mental health care services. At center stage was the controversy surrounding whether or not a waiver of parental consent was legally or ethically necessary under the federal rules for human subjects protection. Final approval was received by the federal funding agency, the local health department, and the University after reaching an understanding about the level of risk of the study and the fact that the youth were medically emancipated minors who were competent to consent to research participation under applicable state law. We believe the lessons learned through working with two IRBs and federal-level reviewers, and seeking a non-IRB-affiliated youth-expert legal opinion, may provide important contextual information for others working with youth in HIV/STI prevention research. Additionally, given youth-related consent challenges in both clinical research and practice, this topic may have implications for broader health-related strategies with youth, which are especially important given the urgent context of HIV/STIs among racial/ethnic minority youth.
Local context—Philadelphia, Pennsylvania
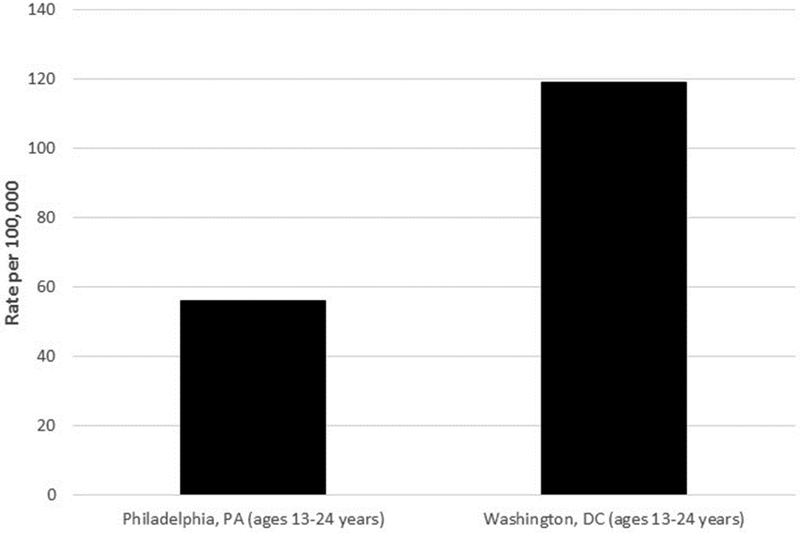
Philadelphia, Pennsylvania is a northeastern city whose large, urban school districts ranked second nationally (after Memphis, Tennessee) among similar school districts on percentage of high school students who ever had sexual intercourse (61.0%, 95% CI: 56.1–65.6%) and who first had sexual intercourse before 13 years of age (15.1%, 95% CI:13.0–17.5%; Eaton, 2012). Data from Philadelphia high school districts also ranked their high school students first among similar school districts regarding the percentage reporting four or more sex partners during their lifetime (27.2%, 95% CI:23.9–30.8%) and sexual activity during the most recent three-month period (44.9%, 95% CI: 40.4–49.6%; Eaton, 2012). At the time of our study (2011), the rate of diagnoses of new HIV infections among youth aged 13–24 in Philadelphia was 56.1 per 100,000 population (AIDS Activities Coordinating Office, 2011), over twice the national rate of 26 per 100,000 for persons aged 13–24 (CDC, 2013; see Figure 1b).
Figure 1b.
Rates of Diagnoses of HIV infection per 100,000 population, by young age groups (13–24 years)—Philadelphia, PA and Washington, DC, United States, 2011
Data are from the Philadelphia Department of Health,21 and the District of Columbia Department of Health.
Given the high rates of sexual risk behaviors among youth with mental illnesses and behavioral disorders (Brawner, Gomes, Jemmott, Deatrick, & Coleman, 2012; Brown et al., 2010; Donenberg, Emerson, Brown, Houck, & Mackesy-Amiti, 2012), our HIV/STI risk-reduction study focused on youth receiving care in outpatient mental health treatment programs who reported current sexual activity. At the time of the study, the most common psychiatric diagnoses among Black youth in mental health treatment in Philadelphia were: attention deficit/hyperactivity disorder (43%), adjustment disorder (36%), oppositional defiant disorder (23%), disruptive behavior disorder (14%), and depression (11%; Philadelphia Department of Behavioral Health, 2010); none of which are barriers to consenting for one’s own care if under age 18 and assessed as competent by the mental health care providers (Alderson, 2007; Geist & Opler, 2010; Shaw, 2001). Due to the local epidemiology in Philadelphia, with Black youth being disproportionately affected by HIV/STIs (AIDS Activities Coordinating Office, 2011), we sought to enroll youth aged 14–17 years who self-identified as Black (any racial or ethnic designation throughout the African diaspora) to develop and test an HIV/STI prevention intervention for at-risk Black youth receiving mental health treatment in an urban area.
Legal Context for Youth in Pennsylvania
In the state of Pennsylvania, minors (youth aged 14 to 17) can legally consent to both mental health treatment and HIV/STI testing and treatment without parental consent (Juvenile Law Center, 2006). This is especially important in the context of biobehavioral research, because under federal regulations, children are “persons who have not attained the legal age for consent to treatment or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted” (United States Department of Health and Human Services, 2009; see 45 CFR 46.402(a)). This has been interpreted to mean that if a minor is able to consent to medical health services under state law, the minor is also able to consent to participate in medical research (United States Department of Health and Human Services et al., 2009). Therefore, the informed consent of minors in Pennsylvania is legally sufficient for participation and no parental permission or waiver is required.
The “We are Kings and Queens” Study
We proposed to develop and test a HIV/STI risk-reduction intervention with sexually active Black youth receiving outpatient mental health treatment in Philadelphia. We established a youth-led community advisory board (CAB) and a youth advocate/ombudsman to ensure that the perspectives of the target population were infused through each aspect of the study. The youth CAB also created the study name: “We are Kings and Queens.”
For the group-level behavioral intervention (Authors), participants were randomized to either the HIV/STI risk-reduction program (treatment; n = 51) or general health promotion program (control; n = 57). The intervention consisted of a two-day, six- hour, skills-based, interactive educational program. The study procedures included focus group discussions (to inform the intervention content), electronic surveys (to collect demographic and behavioral data), diagnostic case ascertainment with the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998), urine specimen collection for Chlamydia and Gonorrhea testing, and an oral swab for OraQuick ADVANCE® Rapid HIV-1/2 antibody testing (unless declined by the participant). Laboratory testing was conducted for participants in both intervention conditions.
These research procedures were specific to the study and not part of routine care at the treatment programs. Two of the partnering agencies, however, did have relationships with outside providers to offer HIV testing onsite, reflecting moves toward the integration of HIV and behavioral health treatment (Substance Abuse and Mental Health Services Administration, 2016). The primary outcome was consistent condom use. Secondary outcomes included sexual activity (frequency of vaginal, anal and oral sex), the number of concurrent and sequential sex partners, and laboratory confirmed HIV/STIs. We performed assessments at baseline, immediately post-intervention, and at 3-, 6-, and 12-month follow-ups.
The Review and Approval Process
Before beginning the study, human subjects approval was required from three groups: 1) the IRB of the academic home institution; 2) the IRB of the local health department; and 3) the reviewers at the federal agency that funded the study. The study materials were submitted to each group and revised several times to clarify wording and address concerns. Changes made to meet the requirements of one agency were resubmitted to the other agencies for their review and approval. Full board review was required given the study’s complexity, which meant (at minimum) month-long waiting periods between review meetings. Some of the discussion points included: the appropriateness of mixed-gender focus groups for adolescent sexual health research, confidential recruitment of adolescent participants in clinics when parents/guardians are present, and actions to be taken for positive HIV/STI test results (e.g., linkage to care procedures). Additionally, given our target group of youth with mental illnesses, there was increased attention to human subjects’ protections due to concerns regarding competency to provide consent and whether parents/guardians would be notified if their child tested positive for HIV/STIs.
Through a series of face-to-face meetings and email exchanges, we worked to address the concerns. We advocated for mixed-gender focus groups to preserve the richness of data that stems from the interactive dialogue between heterosexually-active males and females. We emphasized the high caliber training our facilitators received and created a behavioral management plan, at the IRB’s request, to indicate how we would handle potentially challenging group dynamics. For participant recruitment when a parent/guardian was present, we agreed that recruiters would directly address both the parent/guardian and adolescent, identifying himself/herself as a research team member for a study on teen relationships. The adolescent was then informed that he/she could call the number on the business card for additional information. Adolescents were not screened in the presence of parents/guardians, and parents/guardians were not given details about the study. However, if a parent/guardian stated they did not want their child to participate in the study (at the time of approach or any time thereafter) we conceded to the IRBs request that we would not contact or enroll the adolescent in the study, even if he/she independently expressed interest in participation. Given that our target population of youth had all provided consent for their mental health treatment, the IRB approved our determination of competence to provide consent by study personnel as part of the screening process for enrollment. All participants were notified of their HIV/STI results. Those who tested positive for Chlamydia or Gonorrhea (n = 8, 7.4% at baseline) were notified of their results and referred to receive free treatment through the local department, or from their primary care provider. No participants tested positive for HIV, but positive cases would have been followed by the AIDS Service Organization that conducted the testing for confirmatory tests and linkage to care. While we were able to resolve these issues, there was a fundamental disagreement in the interpretation of the state law between the investigators and the review entities. The research team did not believe parental permission was warranted/applicable, while several local IRB members required parental permission, or at minimum, a solid justification for the appropriateness of a waiver.
To Waive or Not to Waive Parental Consent
Based on Pennsylvania minor consent laws, the participants in this study did not qualify as minors (they did not meet the regulatory definition of minor) for purposes of human subject review; when minors do not meet these qualifications, they can participate in research as adults (United States Department of Health and Human Services, 2009). Despite the documented legal guidance, obtaining approval became a challenge when the local and federal agencies disagreed about whether parental permission was required for the study. More specifically, the minors’ consent to medical health services was not viewed by all parties as sufficient to justify research consent. In the opinion of several IRB members, medical/mental health care was a necessity, while research involvement was a voluntary procedure that they believed would require parental involvement. In other words, at the intersection of ethics and law, some reviewers believed that while the minor was medically emancipated under state law to make decisions, the ethical risk associated with research participation outweighed the state regulations.
We had multiple discussions with the committee members to provide context and accurate data regarding the Health Information Portability and Accountability Act (HIPAA) and the protected confidentiality of medical data (United States Department of Health and Human Services, 2009), the urgency of the HIV epidemic among young people in Philadelphia (see Figure 1b), and the legal ramifications of sharing adolescent test results with parents without youth consent. We also provided information to the local and federal review agencies about the state laws and federal guidance in support of minors’ consent for their own mental health and sexual health services. For example, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978) stated that “…assent of such mature minors should be considered sufficient with respect to research about conditions for which they have legal authority to consent on their own treatment” (pg. 18). Thus, although parental permission was not technically applicable under state law and federal guidance (Juvenile Law Center, 2006; United States Department of Health and Human Services et al., 2009), our ethical review process required that we address the issue of parental permission/involvement.
We advocated to consent participants in the absence of parental involvement for several reasons: 1) to protect the youth participants’ privacy regarding their involvement in consensual sex; 2) to ensure youth receive HIV/STI testing and treatment (if needed); 3) to encourage participants to give honest answers to the study questions (without fear of parent/guardian reactions); and 4) to be in line with state and federal guidance on the issue. Previous research with youth suggests that requiring parental permission may decrease their interest in participating in biobehavioral research, especially when STI testing is involved (Brawner, Volpe, Stewart, & Gomes, 2013; Macapagal, Coventry, Arbeit, Fisher, & Mustanski, 2017). In addition, some parents/guardians may request to receive their child’s test results, which would be counter to the youth’s desires and legal precedent for maintaining their confidentiality. Requiring parental permission would also violate local and federal rights and breach confidentiality for youth whose parents/guardians did not know that they were receiving mental health treatment, engaging in consensual sexual activity and possibly seeking sexual health services (Juvenille Law Center, 2006; United States Department of Health and Human Services, 2009).
Despite eleven separate meetings and discussions, the local and federal approving agencies were unable to reach initial consensus about parental involvement. The federal funding agency waited to consider the decisions of the two local IRBs before completing its review. The local IRBs suggested obtaining a youth-focused third party review of the protocol to help support a decision. We contacted attorneys at the Juvenile Law Center, a non-IRB-affiliated youth-legal expert firm in Philadelphia (Juvenille Law Center, 2006). They reviewed the study materials and concluded that the study’s consent provisions were consistent with the Mental Health Procedures Act and HIPAA—a minor in Pennsylvania age 14–17 did not need parental consent to participate in the study (Juvenille Law Center, 2006). After several review and feedback sessions, both the academic institution and local health department IRBs approved written informed consent by the adolescent participants and waivers of parental permission for their parents. Approval by the local IRBs facilitated the green light by the federal agency to begin the study. The study was approved by all required local and federal bodies in March 2013; overall, a 14-month timeline for full review and approval of this protocol with associated suboptimal use of human and fiscal resources due to the lengthy delay.
Federal Guidance and Conflicting Perspectives
Even though parental permission was not technically applicable to our study population, we were still required to request a parental permission waiver by one local review board. Parental permission waivers are permitted under two sections of the U.S. Department of Health and Human Services’ Code of Federal regulations: 45 CFR 46.408(c) or 45 CFR 46.116 (d; United States Department of Health and Human Services et al., 2009). Thus we requested a parental permission waiver based on three premises: 1) our proposed study with sexually active youth who had already consented for mental health care involved minimal risk; 2) parental permission would be problematic for youth whose parents may have been unaware of their mental health services and sexual activity; and 3) the parental permission waiver request was not inconsistent with federal or state law (Juvenille Law Center, 2006; United States Department of Health and Human Services et al., 2009). Clear guidance in the interpretation and implementation of local and federal regulations is necessary to ensure adequate engagement of at-risk youth in research studies. The development of a formal strategy to engage multiple entities in conversations earlier in the research process, with a non-IRB-affiliated legal presence if available, may help address legal concerns about youth sexual health research and avoid costly delays. Using a collaborative strategy that provides researchers and community partners with an epidemiologic, legal and ethical context (English & Kenney, 2010; Moore, Paul, McGuire, & Majumder, 2016) is especially important to consider given the public health urgency for addressing youth sexual health needs and reducing alarming HIV/STI disparities.
Lessons Learned and Recommendations
Mustanski and Fisher (2016) state that “IRBs are complicit in the creation of health inequities when their disapproval of studies systematically prevent some communities from having the opportunity to receive the benefits of research” (pg., 249). Our experiences with the human subjects review processes for this study provided important lessons learned which may be helpful in advancing HIV/STI research and other areas of biobehavioral research with disproportionately affected youth. Local statutes and federal regulations are sometimes subject to varied interpretations; therefore, it is not uncommon for multiple IRBs to disagree. Developing clearer guidelines regarding collaborative authority processes for approval of funded protocols will be helpful for multidisciplinary research teams. Below we discuss lessons learned, including potential challenges that may arise as well as strategies to resolve them for others engaged in similar research.
Continued Training and Dialogue is Needed among IRB Members and Researchers
The research community could benefit from formal cross-IRB/regulatory body trainings and dialogue (Kaur, 2013). Several previous reports document IRB challenges with youth-focused research (English & Kenney, 2010; Rogers, Schwartz, Weissman, & English, 1999). For example, when Risjord and Greenberg (2002) applied for IRB approval for a study of high-risk sexual behavior in youth, initial local and federal IRB disagreement stalled the study for over nine months. Rogers et al. (1999) conducted a study of AIDS progression among HIV-positive youth. Of the eleven IRBs required for that study, each IRB had different interpretations regarding the parental permission waiver, which presented challenges for study implementation. Mammel and Kaplan (1995) surveyed 233 (39%) of 600 IRB chairs in the U.S. regarding research assent by minors; they found a wide range of interpretation among the IRBs. However, they found that more experienced IRBs, which reviewed more than 10 adolescent protocols annually, were less likely to require parental permission for minimal risk studies.
Having researchers and IRB members meet to review these data and receive training in the best way to move forward for their institutions may help avoid costly protocol delays while ensuring human subjects protections. Difficulty coordinating multiple schedules, or lack of interest, knowledge or a leader to champion the issue, could hinder these processes. However, perseverance, a commitment to reduce health disparities and a resolve to advocate for the inclusion of youth in sexual health research can help ensure the necessary activities occur. Additionally, starting on a small scale, such as hosting a brown bag lunch for both IRB members and researchers, can help facilitate the process to ensure that necessary training and dialogue occur.
Key Communities Must be invited to the Table for Discussions on Research-Related Topics
There is a need to have conversations with affected communities to discuss the local impact of HIV/STIs, particularly when the burden of disease is high. For HIV, which requires lifetime treatment to improve health outcomes and decrease the chance of ongoing transmission, helping all youth, parents and IRB members understand the local epidemiologic context may be vital for ongoing support of research that is geared toward finding solutions. The process of adolescent consent and parental permission and waivers (when applicable) for youth research should be explicitly discussed with the target community (where participants will be recruited) and all persons engaged in the human subjects process, so that participants and reviewers can fully understand the importance of the health issue being studied. Recent data are consistent with this concept and show that parents who consent for their youth to participate in sexuality research describe being motivated by potential benefits (Moilanen, 2016). Historical mistrust within communities toward research participation, particularly in predominantly racial/ethnic minority communities, may serve as a barrier to engagement (Scharff, Mathews, Jackson, Hoffsuemmer, Martin, & Edwards, 2010). However, having community representatives engage in discussions with researchers and IRB members is critical.
For example, consistent with federal regulations (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1978), we engaged a youth “ombudsman,” who was unrelated to the research, but able to explain the purpose of the research to prospective participants while also emphasizing that participation was unrelated to the provision of care. Such upfront, transparent conversations with youth and parents/guardians via town halls, open forums and online platforms (e.g., infographic distributed through social media) can help break down barriers to research participation and relieve concerns that researchers and/or IRBs are engaged in underhanded activities. We need continued dialogue on youth participation in sexual health research as a scientific community, in concert with the communities we intend to recruit from, to reach a resolution that will best serve the populations we represent while facilitating knowledge advancement.
Parental Concerns Should be Balanced with Adolescents’ Rights in Sexual Health Research
For decades, researchers and IRBs have navigated the complexities of parental involvement in sexual health research with youth. Valid concerns arise, such as parents/guardians wanting to be informed of their childs’ research test results, and youth wanting to maintain their right to confidentiality of their health data. This becomes particularly concerning when a diagnosis uncovered during a research study (e.g., HIV-positive status) could have financial implications for the parent/guardian (e.g., treatment expenses). However, even in this case, in many states youth can consent to HIV/STI testing without their parent’s/guardian’s permission, and receive independent treatment, and providers cannot violate a young person’s confidentiality by disclosing the positive diagnosis to a parent/guardian. Changes to care provisions have enabled autonomous decision-making for youth, increasing the number aware of their HIV/STI status and receiving treatment; some may not have pursued testing and/or treatment if their parent/guardian had to be involved for fear of punishment. Similarly, it is important to consider the negative consequences of requesting parental consent for adolescent research. There are data to suggest that obtaining parental consent for adolescent studies may reduce the participant pool (due to adolescent concerns about protection of confidentiality; Reed & Huppert, 2008; Reed, Thistlethwaite, & Huppert, 2007) and create less representative samples (racial/ethnic minority youth and medically underserved youth are less likely to participate in research studies; Anderman et al., 1995; Esbensen, Miller, Taylor, He, & Freng, 1999; Tigges, 2003). Research costs may also greatly increase in situations where active parental permission (all parents must return a permission form regardless of youth participation) is required and extensive follow-up conducted (Tigges, 2003).
While we stood our ground on several issues (e.g., mixed gender groups, waiver of parental consent), there were areas where we conceded to the IRB. In our study, we reached a middle ground with the IRB by agreeing that we would not approach or enroll youth whose parents/guardians indicated that they did not want their child to participate in the study. In this instance, the prospect of parental concern trumped the adolescents’ ability to choose whether or not they wanted to participate in the study. We learned through this process that we had to choose our battles to avoid further delays, and thus only pressed issues that we believed would compromise the study implications. Others working with this demographic may have to reach other concessions, and should be prepared to determine when they need staunch advocacy for their position versus making a required change.
Federal Guidelines Should be Clarified for Standardized Implementation
Some adolescent health experts have advocated for clearer adolescent consent and parental permission waiver guidance while underscoring the importance of protecting the minors’ legal and ethical rights related to HIV/STI service access (Santelli et al., 2003) and when children are research participants (Kopelman, 2006). The Society of Adolescent Medicine has published guidelines for adolescent health research that state waivers of parental permission should be strongly considered for adolescent research occurring in healthcare settings where youth can obtain healthcare without parental consent (Santelli et al., 2003). For our study, the additional context of mental health diagnoses and engagement of sexually active racial/ethnic minority youth warrants special attention to cultural context and sensitivity, which was considered by the racially-diverse research team at each step of study development and strengthened by the engagement of the local youth advisory board (which included members of the target youth population). The context of mental health diagnoses and inclusion of racial and ethnic minority groups does not negate upholding their legal and ethical rights when appropriate for minimal risk research (Fisher et al., 2002; Jenkins & Parron, 1995; Miller, Forte, Wilson, & Greene, 2006). Others working with populations deemed to be vulnerable or in need of additional protections may encounter similar challenges. Review of related research studies and legal cases can be used to educate regulatory bodies.
Conclusion
Advocating for at-risk youth, while also helping to improve and streamline the human subjects review process, is vital for sexual health research if we are to effectively reduce the unacceptably high burden of HIV/STI disease among young people in the United States. This requires urgent responses to develop evidence-based strategies to reduce sexual health risks among youth; youth involvement in this research is essential. The best interest of adolescent health and safe research environments for adolescent consent is of paramount importance and should be consistently conveyed to all persons in the human subjects review process; this may help reassure IRB members about the intentions of the research team members. We must continue to work to resolve IRB research barriers in a way that protects the rights and welfare of research participants without negating their ability to provide informed consent. Based on increasing strategies to promote equity and reduce disparities among racially/ethnically diverse youth (Barkley et al., 2013), our study well aligns with national HIV/STI prevention goals for youth (United States Department of Health and Human Services & Healthy People 2020) and is also strongly aligned with recent calls to action to address the disproportionate burden of HIV in young people (Koenig, Hoyer, Purcell, Zaza, & Mermin, 2016).
Acknowledgements:
This paper is supported in part by the Centers for Disease Control and Prevention, grant #1U01PS003304. The authors have no conflicts of interest relevant to this study.
Footnotes
Disclosure: The authors have no financial conflicts to report.
References
- AIDS Activities Coordinating Office. (2011). HIV/AIDS in Philadelphia: Cases reported through June 2011. Retrieved from Philadelphia, PA: http://www.phila.gov/health/pdfs/2010SurveillanceReportFinal.pdf [Google Scholar]
- Alderson P (2007). Competent children? Minors’ consent to health care treatment and research. Social Science & Medicine, 65(11), 2272–2283. [DOI] [PubMed] [Google Scholar]
- Anderman C, Cheadle A, Curry S, Diehr P, Shultz L, & Wagner E (1995). Selection bias related to parental consent in school-based survey research. Evaluation Review, 19(6), 663–674. [Google Scholar]
- Barkley L, Kodjo C, West KJ, Vo DX, Chulani VL, Svetaz MV, … Oscos Sanchez M (2013). Promoting equity and reducing health disparities among racially/ethnically diverse adolescents: A position paper of the Society for Adolescent Health and Medicine. Journal of Adolescent Health, 52(6), 804–807. [DOI] [PubMed] [Google Scholar]
- Bauermeister JA, Eaton L, Andrzejewski J, Loveluck J, VanHemert W, & Pingel ES (2015). Where you live matters: Structural correlates of HIV risk behavior among young men who have sex with men in Metro Detroit. AIDS and Behavior, 19(12), 2358–2369. doi: 10.1007/s10461-015-1180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner BM, Gomes MM, Jemmott LS, Deatrick JA, & Coleman CL (2012). Clinical depression and HIV risk-related sexual behaviors among African-American adolescent females: Unmasking the numbers. AIDS Care, 24(5), 618–625. doi: 10.1080/09540121.2011.630344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner BM, Jemmott LS, Wingood G, Reason J, Daly B, Brooks K, & Lanier Y (2017). Feelings matter: Depression severity and emotion regulation in HIV/STI risk-related sexual behaviors. Journal of Child and Family Studies, 26(6), 1635–1645. doi: 10.1007/s10826-017-0674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner BM, Volpe EM, Stewart JM, & Gomes MM (2013). Attitudes and beliefs toward biobehavioural research participation: Voices and concerns of urban adolescent females receiving outpatient mental health treatment. Annals of human biology, 40(6), 485–495. doi: 10.3109/03014460.2013.806590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, Hadley W, Stewart A, Lescano C, Whiteley L, Donenberg G, & DiClemente R (2010). Psychiatric disorders and sexual risk among adolescents in mental health treatment. Journal of Consulting and Clinical Psychology, 78(4), 590–597. doi: 10.1037/a0019632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). HIV Surveillance Report, 2011. Retrieved June 20, 2017 from http://www.cdc.gov/hiv/topics/surveillance/resources/reports/
- Centers for Disease Control and Prevention. (2015). HIV Surveillance- Adolescents and Young Adults Retrieved June 15, 2017, from https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-adolescents-young-adults-2015.pdf
- Centers for Disease Control and Prevention. (2016). Sexually Transmitted Disease Surveillance 2015. Retrieved from https://www.cdc.gov/std/stats11/default.htm
- Chesson HW, Blandford JM, Gift TL, Tao G, & Irwin KL (2004). The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspectives on Sexual and Reproductive Health, 36(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Coleman DL, & Rosoff PM (2013). The legal authority of mature minors to consent to general medical treatment. Pediatrics, 131(4), 786–793. doi: 10.1542/peds.2012-2470 [DOI] [PubMed] [Google Scholar]
- Donenberg GR, Emerson E, Brown LK, Houck C, & Mackesy-Amiti ME (2012). Sexual experience among emotionally and behaviorally disordered students in therapeutic day schools: An ecological examination of adolescent risk. Journal of Pediatric Psychology, 37(8), 904–913. doi: 10.1093/jpepsy/jss056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, et al. (2012). Youth risk behavior surveillance – United States 2011. Center for Disease Control and Prevention (MMWR), 61(SS04), 1–62. [PubMed] [Google Scholar]
- English A, & Kenney KE (2010). State Minor Consent Laws: A Summary (3rd ed.). Chapel Hill, NC: Center for Adolescent Health & the Law. [Google Scholar]
- Esbensen F-A, Miller MH, Taylor T, He N, & Freng A (1999). Differential attrition rates and active parental consent. Evaluation Review, 23(3), 316–335. [DOI] [PubMed] [Google Scholar]
- Finer LB (2010). Unintended pregnancy among U.S. adolescents: Accounting for sexual activity. Journal of Adolescent Health, 47(3), 312–314. [DOI] [PubMed] [Google Scholar]
- Fisher CB, Hoagwood K, Boyce C, Duster T, Frank DA, Grisso T, … Takanishi R (2002). Research ethics for mental health science involving ethnic minority children and youths. American Psychologist, 57(12), 1024. [DOI] [PubMed] [Google Scholar]
- Flicker S, & Guta A (2008). Ethical approaches to adolescent participation in sexual health research. Journal of Adolescent Health, 42(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Forhan SE, Gottlieb SL, Sternberg MR, et al. (2009). Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics, 124(6), 1505–1512. [DOI] [PubMed] [Google Scholar]
- Geist R, & Opler SE (2010). A guide for health care practitioners in the assessment of young people’s capacity to consent to treatment. Clinical Pediatrics, 49(9), 834–839. [DOI] [PubMed] [Google Scholar]
- Guttmacher Institute. Minors’ access to STI services as of June 1, 2013. Retrieved June 30, 2017 from http://www.guttmacher.org/statecenter/spibs/spib_MASS.pdf [Google Scholar]
- Hein IM, De Vries MC, Troost PW, Meynen G, Van Goudoever JB, & Lindauer RJ (2015). Informed consent instead of assent is appropriate in children from the age of twelve: Policy implications of new findings on children’s competence to consent to clinical research. BMC Medical Ethics, 16(1), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RR, & Parron D (1995). Guidelines for adolescent health research: Issues of race and class. Journal of Adolescent Health, 17(5), 314–322. [DOI] [PubMed] [Google Scholar]
- Juvenille Law Center. (2006). Consent to treatment and confidentiality provisions affecting minors in Pennsylvania. Retrieved from Philadelphia, PA. [Google Scholar]
- Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J,…Zaza S (2016). Youth risk behavior surveillance — United States, 2015. MMWR Surveill Summ 2016;65(SS-6):1–174. [DOI] [PubMed] [Google Scholar]
- Kaur S (2013). How IRBs make decisions: Should we worry if they disagree? Journal of Medical Ethics, 39(4), 230–230. [DOI] [PubMed] [Google Scholar]
- Koenig LJ, Hoyer D, Purcell DW, Zaza S, & Mermin J (2016). Young people and HIV: A call to action. American journal of public health, 106(3), 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman LM (2006). Children as research subjects: Moral disputes, regulatory guidance, and recent court decisions. The Mount Sinai Journal of Medicine, New York, 73(3), 596–604. [PubMed] [Google Scholar]
- Macapagal K, Coventry R, Arbeit MR, Fisher CB, & Mustanski B (2017). “I won’t out myself just to do a survey”: Sexual and gender minority adolescents’ perspectives on the risks and benefits of sex research. Archives of Sexual Behavior, 46(5), 1393–1409. doi: 10.1007/s10508-016-0784-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammel AK, & Kaplan DW. (1995). Research consent by adolescent minors and Institutional Review Boards. Journal of Adolescent Health, 17(5), 323–330. [DOI] [PubMed] [Google Scholar]
- Martinez G, & Abma JC (2015). Sexual activity, contraceptive use, and childbearing of teenagers aged 15 – 19 in the United States, 2011–2013. NCHS data brief, no 209. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Miller RL, Forte D, Wilson BDM, & Greene GJ (2006). Protecting sexual minority youth from research risks: Conflicting perspectives. American Journal of Community Psychology, 37(3–4), 341–348. [DOI] [PubMed] [Google Scholar]
- Molnar EE, Hensel DJ, Waltz AC, & Ott MA (2013). Investigator, IRB member, and staff assessment of ethics review of adolescent protocols. Journal of Adolescent Health, 52(2), S93–S94. [Google Scholar]
- Moilanen KL (2016). Why do parents grant or deny consent for adolescent participation in sexuality research? Journal of Youth and Adolescence, 45(5), 1020–1036. [DOI] [PubMed] [Google Scholar]
- Moore QL, Paul ME, McGuire AL, & Majumder MA (2016). Legal barriers to adolescent participation in research about HIV and other sexually transmitted infections. American journal of public health, 106(1), 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, & Fisher CB (2016). HIV rates are increasing in gay/bisexual teens. American Journal of Preventive Medicine, 51(2), 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1978). Report and recommendations: Research involving children. Report and recommendations of the National Commission for Human Subjects of Biomedical and Behavioral Research. Retrieved July 20, 2017, from https://archive.org/details/researchinvolvin01unit [Google Scholar]
- Owosu-Edusei K, Chesson HW, Gift TL, et al. (2013). The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sexually Transmitted Diseases, 40(3), 197–201. [DOI] [PubMed] [Google Scholar]
- Philadelphia Department of Behavioral Health (2010). [Psychiatric Diagnoses Ascertained from Outpatient Claims Database for Adolescents 13 to 17 Years Old].
- Prado G, Lightfoot M, & Brown CH (2013). Macro-level approaches to HIV prevention among ethnic minority youth. American Psychological Association, 68(4), 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, & Huppert JS (2008). Predictors of adolescent participation in sexually transmitted infection research: Brief report. Journal of Adolescent Health, 43(2), 195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Thistlethwaite JM, & Huppert JS (2007). STI research: Recruiting an unbiased sample. Journal of Adolescent Health, 41(1), 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risjord M, & Greenberg J (2002). When IRBs disagree: Waiving parental consent for sexual health research on adolescents. IRB: Ethics and Human Research, 24(2), 8–14. [PubMed] [Google Scholar]
- Rogers AS, Schwartz DF, Weissman G, & and Abigail English for the Adolescent Medicine HIV/AIDS Research Network. (1999). A case study in adolescent participation in clinical research: Eleven clinical sites, one common protocol, and eleven IRBs. IRB: Ethics and Human Research, 21(1), 6–10. [PubMed] [Google Scholar]
- Santelli JS, & Rogers AS (2002). Parental permission, passive consent, and “children” in research. Journal of Adolescent Health, 31(4), 303–304. [DOI] [PubMed] [Google Scholar]
- Santelli JS, Rosenfeld WD, DuRant RH, Dubler N, Morreale M, English A, & Rogers AS (1995). Guidelines for adolescent health research: A position paper of the Society for Adolescent Medicine. Journal of Adolescent Health, 17(5), 270–276. [DOI] [PubMed] [Google Scholar]
- Santelli JS, Rogers AS, Rosenfeld WD, DuRant RH, Dubler N, Morreale M, … & Schissel A (2003). Guidelines for adolescent health research. A position paper of the Society for Adolescent Medicine. Journal of Adolescent Health, 33(5), 396–409. [PubMed] [Google Scholar]
- Satterwhite CL, Torrone E, Meites E, et al. (2013). Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sexually Transmitted Diseases, 40(3), 187–193. [DOI] [PubMed] [Google Scholar]
- Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, & Edwards D (2010). More than Tuskegee: Understanding mistrust about research participation. Journal of Health Care for the Poor and Underserved, 21(3), 879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan S, & Leinhos M (2007). The ethics of public health practice for the prevention and control of STDs. In Aral SO, & Douglas JM (Ed.), Behavioral Interventions for Prevention and Control of Sexually Transmitted Diseases. New York, NY: Springer Science. [Google Scholar]
- Shaw M (2001). Competence and consent to treatment in children and adolescents. Advances in Psychiatric Treatment, 7(2), 150–159. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.); the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Supplement 20), 22–33. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2016). The Case for Behavioral Health Screening in HIV Care Settings Retrieved July 31, 2017, from http://store.samhsa.gov/shin/content/SMA16-4999/SMA16-4999.pdf
- Tigges BB (2003). Parental consent and adolescent risk behavior research. Journal of Nursing Scholarship, 35(3), 283–289. [DOI] [PubMed] [Google Scholar]
- United Nations Educational, Scientific and Cultural Organization (UNESCO). (2017). What do we mean by “youth”? Retrieved July 10, 2017, from http://www.unesco.org/new/en/social-and-human-sciences/themes/youth/youth-definition/
- United States Department of Health and Human Services. Health Information Privacy. Retrieved from http://www.hhs.gov/ocr/privacy/index.html
- United States Department of Health and Human Services, & Healthy People 2020. (April 10, 2013). Adolescent Health. Retrieved from July 30, 2017 http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicId=2
- United States Department of Health and Human Services. (2009). Code of Federal Regulations: Title 45-Public Welfare; Part 46: Protection of Human Subjects. Retrieved from http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.116
- Weinstock H, Berman S, & Cates W Jr. (2004). Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspectives on Sexual and Reproductive Health, 36(1), 6–10. [DOI] [PubMed] [Google Scholar]
- Wendler D, Belsky L, Thompson KM, & Emanuel EJ (2005). Quantifying the federal minimal risk standard: Implications for pediatric research without a prospect of direct benefit. JAMA, 294(7), 826–832. [DOI] [PubMed] [Google Scholar]
- White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Retrieved July 10, 2017 http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf
- Whitmore SK, Kann L, Prejean J, et al. (2012). Vital signs: HIV infection, testing, and risk behaviors among youths--United States. Centers for Disease Control and Prevention (MMWR), 61(47), 971–976. [PubMed] [Google Scholar]