ABSTRACT
Introduction:
Multiple studies have been published recently assessing feasibility of robot-assisted partial nephrectomy (RAPN) for moderate to highly complex renal masses. Some studies have even compared partial nephrectomy (PN) performed through various modalities such as open PN (OPN) versus RAPN and laparoscopic PN (LPN) versus OPN. The primary aim of this review was to analyze perioperative outcomes such as warm ischemia time (WIT), duration of surgery, estimated blood loss (EBL), complications, blood transfusion, length of stay, and margin status following RAPN for complex renal masses. Another objective was to compare perioperative outcomes following various surgical modalities, i.e., OPN, LPN, or RAPN.
Methods:
Literature search was conducted to identify studies reporting perioperative outcomes following RAPN for moderate (Radius, Endophytic/Exophytic, Nearness, Anterior/posterior location [RENAL] score 7–9 or Preoperative Aspects of Dimension used for anatomic classification [PADUA] score 8–9) to high complexity renal masses (RENAL or PADUA score ≥ 10). Meta-analysis of robotic versus OPN and robotic versus LPN was also performed. Study protocol was registered with PROPSERO (CRD42019121259).
Results:
In this review, 22 studies including 2,659 patients were included. Mean duration of surgery, WIT, and EBL was 132.5–250.8 min, 15.5–30 min, and 100–321 ml, respectively. From pooled analysis, positive surgical margin, need for blood transfusion, minor and major complications were seen in 3.9%, 5.2%, 19.3%, and 6.3% of the patients. No significant difference was noted between RAPN and LPN for any of the perioperative outcomes. Compared to OPN, RAPN had significantly lower EBL, complications rate, and need for transfusion.
Conclusions:
RAPN for moderate to high complexity renal masses is associated with acceptable perioperative outcomes. LPN and RAPN were equal in terms of perioperative outcomes for complex masses whereas, OPN had significantly higher blood loss, complications rate, and need for transfusion as compared to RAPN.
INTRODUCTION
Partial nephrectomy (PN) for small renal masses (T1a) is a well-established surgical treatment option. It is also favored over radical nephrectomy (RN) whenever feasible for T1brenal masses.[1] The current enthusiasm for PN has stemmed from demonstration of superior renal functional parameters without the compromise of surgical and oncological outcomes compared to RN.[2] With the advent of the robotic platform, with its numerous advantages such as ergonomically sound suturing due to wristed instruments, three-dimensional (3D) vision, and filtered hand movements, has made minimally invasive PN a feasible option even for highly complex renal masses.[3] There have been multiple studies describing the use of robot-assisted PN (RAPN) for complex renal masses. These studies have used various tumor-related factors to define complexity such as size,[4] endophytic nature,[5] multiple,[6] and hilar[7] location of the renal masses. To stratify patients into different complexity groups, various scoring methods have been introduced of which Radius, Endophytic/Exophytic, Nearness, Anterior/posterior location (RENAL), and preoperative aspects of dimension used for anatomic classification (PADUA) nephrometery scores are most frequently used.[8,9] Both of these scores have been correlated well with perioperative outcomes following PN. Experience regarding the management of complex renal masses (as defined by PADUA or RENAL score ≥7) with RAPN has been limited to various single or multicenter case series. As more and more data is emerging, it is worthwhile to critically analyze the perioperative outcomes for RAPN in patients with complex renal masses. In the present study, we performed a systematic literature review to identify studies reporting perioperative outcomes following RAPN for patients with complex renal masses. We performed pooled analysis of various perioperative outcomes such as warm ischemia time (WIT), duration of surgery, blood loss, complications, blood transfusion, length of stay (LOS), and margin status following RAPN for complex renal masses. Comparison of RAPN to laparoscopic PN (LPN) and open PN (OPN) was also performed for various perioperative outcomes. To the best of our knowledge, this is the first systematic review of its kind dealing with complex renal masses in RAPN.
Data acquisition
Study design
Systematic literature search was performed to identify all the relevant publications assessing the perioperative outcomes following RAPN for high complexity renal masses (RENAL or PADUA score ≥ 7). We also performed meta-analysis of RAPN versus OPN and RAPN versus LPN. A pre-specified study protocol was registered with PROPSERO (CRD42019121259) (Preferred reporting Items for Systematic reviews and Meta-analysis) guidelines were followed while conducting this review.[10]
Search strategy
Two review authors (GS and ST) independently conducted the systematic literature search for relevant papers on electronic databases PubMed/Medline, Embase, and Web of Science from their time of inception till the last search. Literature search was limited to English language only. We also performed hand searches of bibliography of articles selected for full review. Additional articles were sought from latest journal articles and conference papers. Last search was conducted on January 9, 2020.
(Patient/population/problem, Intervention, Control, and outcome) model was used to design search strategy:
Patient
Renal mass odds ratio (OR) kidney mass OR renal cell carcinoma OR renal cancer OR kidney cancer OR renal carcinoma OR renal tumor.
Intervention
High PADUA score OR high nephrometery score OR high RENAL score OR high RENAL nephrometery score OR complex OR hilar.
Control
Robot-assisted PN OR robotic PN OR robot-assisted nephron-sparing surgery OR robotic nephron-sparing surgery OR robot-assisted LPN OR robot-assisted laparoscopic nephron-sparing surgery.
Outcome
WIT OR complication OR margin OR trifecta.
Using combinations of above-mentioned keywords, all the electronic databases were then searched. Titles and abstracts of all thus generated articles were then screened for inclusion into the study by the two reviewers (GS and ST). In case of any discrepancy, help of other authors was sought (RSM, GSB). Search strategy used for PubMed has been provided in the supplementary file S1.
Supplementary file S1.
Table depicting search strategy employed for Pubmed
| Keywords | Results |
|---|---|
| Renal mass | 38,036 |
| Kidney mass | 47,082 |
| Renal cell carcinoma | 47,792 |
| Renal cancer | 124,869 |
| Kidney cancer | 117,426 |
| Renal carcinoma | 53,852 |
| Robot-assisted laparoscopic nephron-sparing surgery | 77 |
| Robot-assisted LPN | 343 |
| Robotic nephron-sparing surgery | 234 |
| Robot assisted nephron sparing surgery | 122 |
| Robotic PN | 1118 |
| RAPN | 567 |
| Hilar | 10,990 |
| Complex | 1258,502 |
| High renal nephrometry score | 108 |
| High PADUA score | 258 |
| High RENAL score | 4419 |
| WIT | 5123 |
| Complication | 258,770 |
| Margin | 58,038 |
| trifecta | 281 |
| WIT OR complication OR margin OR trifecta | 319,872 |
| High PADUA score OR high nephelometry score OR high renal score OR high renal nephelometry score OR complex OR hilar | 1273,504 |
| Renal mass OR kidney mass OR renal cell carcinoma OR renal cancer OR kidney cancer OR renal carcinoma OR renal tumor | 191,242 |
| RAPN OR robotic PN OR robot assisted nephron-sparing surgery OR robotic nephron-sparing surgery OR robot-assisted LPN OR robot-assisted laparoscopic nephron-sparing surgery | 1247 |
| WIT OR complication OR margin OR trifecta and high PADUA score OR high nephelometry score OR high renal score OR high renal nephelometry score OR complex OR hilar and renal mass OR kidney mass OR renal cell carcinoma OR renal cancer OR kidney cancer OR renal carcinoma OR renal tumor and RAPN OR robotic PN OR robot-assisted nephron-sparing surgery OR robotic nephron-sparing surgery OR robot-assisted LPN OR robot-assisted laparoscopic nephron-sparing surgery | 323 |
PN=Partial nephrectomy, RAPN=Robot assisted PN, LPN=Laparoscopic PN, WIT=Warm ischemia time, OR=Odds ratio, RENAL=Radius, endophytic/exophytic, nearness, anterior/posterior location, PADUA=Preoperative aspects of dimension used for anatomic classification
Selection criteria
To assess the eligibility of a study for inclusion in this review, initial title followed by abstract screening was performed and if required full-text review was performed by two review authors independently (GS and ST). For inclusion into the review, study should contain data regarding perioperative outcomes such as duration of surgery, WIT, surgical complications, estimated blood loss (EBL), need for blood transfusions LOS, and positive surgical margin in patients with moderate to high complexity renal tumors undergoing RAPN. Studies containing data on low or mixed complexity tumors, not reporting any of the above-mentioned perioperative outcomes, reviews, case reports, and conference abstracts were excluded. In case of discrepancy over inclusion/exclusion of a study, help of other review authors was sought (RSM and GSB) and final decision was reached after reaching consensus.
Outcomes
Primary outcomes
In this review, we planned to study perioperative outcomes such as duration of surgery, EBL, number of transfusions, LOS, positive surgical margins, surgical complications, and WIT. Meta-analysis of studies comparing these factors following RAPN, LPN, and OPN was also performed.
Quality assessment
This meta-analysis included retrospective case series and comparative cohort studies. For quality assessment of case series, we used institute of health economics (IHE) checklist for quality appraisal. This checklist consists of 20 items that assess study quality by assessing study objective, design, population, interventions, outcome measures used, statistical analysis, results, conclusions, competing interests, and sources of support. A study with ≥70% yes response is considered to be of good quality. For nonrandomized comparative cohort studies, we used Newcastle-Ottawa quality assessment scale (NOS). Using this scale, quality assessment of nonrandomized studies is done based upon selection and comparability of study groups and ascertainment of primary outcome in the two groups. A study can be awarded maximum of 9 stars; studies with >5 stars are considered to be of good quality.
Quality assessment was performed by two review authors (GS and ST) independently, thereafter, data were compared and any discrepancy was sorted out by arbitration with other review authors (RSM and GSB).
Data extraction and statistical analysis
Two review authors (GS and ST) independently performed full-text review of all the included articles to extract relevant data using a predefined template [Table 1]. Data were then cross-checked for consistency and in case of discrepancy, data were rechecked and help of other authors was sought when required (RSM and GSB). All the relevant data pertaining to the study were entered into a personal computer on Microsoft excel sheet. Quantitative meta-analysis of perioperative variables was done for studies comparing laparoscopic and open techniques with robotic techniques. For rest of the studies, narrative synthesis of data along with pooled analysis was done. Separate pooled analysis was conducted for the studies reporting data on high nephrometery score (RENAL or PADUA score ≥10).
Table 1.
Characteristics of the studies included in the review
| First author | Number of patients | Country | Years | Type of study | Duration of study | Number of institutions | Number of surgeons | Score used | Surgical approach | Median follow-up | Quality assessment score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abedali | 148 robot 74 open |
USA | 2020 | Retrospective | 2008-2016 | Single | Multiple | RENAL ≥7 | NS | NS | 8/9 stars |
| Beksac | 144 robot | USA | 2019 | Retrospective | 2006-2018 | Multiple | Multiple | RENAL ≥10 | NS | NS | 14/20 |
| Buffi | 255 robot | Italy | 2019 | Retrospective | 2010-2017 | Multiple | Multiple | PADUA ≥10 | Retro/transperitoenal | NS | 16/20 |
| Bora | 53 robot | India | 2017 | Retrospective | 2014-2016 | Single | Multiple | RENAL ≥7 | Transperitoneal | - | 15/20 |
| Deng | Robot 58 Laparoscopic 58 |
China | 2019 | Retrospective | 2010-2017 | Single | Single | RENAL ≥7 | Retro/transperitoenal | Robot 39.5 Laparoscopic 31 |
7/9 stars |
| Ellison | Robot 62 Laparoscopic 67 |
USA | 2012 | Retrospective | 2007-2011 | Single | Multiple | RENAL 7-10 | Retro/transperitoenal | - | 7/9 stars |
| Garisto | Open 76 Robot 203 |
USA | 2018 | Retrospective | 2006-2016 | Single | Multiple | RENAL ≥10 | Transperitoneal | 25 | 8/9 stars |
| Hennessey | 31 robot | Australia | 2017 | P | 2014-2016 | Three | Multiple | RENAL ≥10 | Transperitoneal | 12.5 | 14/20 |
| Jang | Laparoscopic 38 Robot 89 |
South Korea | 2014 | Retrospective | 2007-2013 | Single | Single | RENAL 7-10 | NS | - | 8/9 stars |
| Kim | Open 64 Robot 85 |
South Korea | 2019 | Retrospective | 2003-2017 | Single | NS | RENAL ≥10 | NS | 30 | 8/9 stars |
| Long | Laparoscopic 182 Robot 199 |
USA | 2012 | Retrospective | 2005-2009 | Single | Multiple | RENAL ≥7 | Transperitoneal | 8.3 | 7/9 stars |
| Png | 39 robot | USA | 2013 | Retrospective | 2003-2011 | Single | Single | RENAL ≥7 | NS | - | 14/20 |
| Raheem | 223 robot | South Korea | 2016 | Retrospective | 2006-2015 | Single | Single | PADUA>7 | Transperitoneal | 47 intermediate and 38.5 highly complex | 15/20 |
| Schiavina | Robot 165 | Italy | 2016 | Retrospective | 2010-2013 | Multicentre | Multiple | Both ≥7 | NS | - | 15/20 |
| Simhan | Robot 91 Open 190 |
USA | 2012 | Retrospective | 2007-2010 | Single | Multiple | RENAL ≥7 | NS | - | 6/9 stars |
| Tomaszewski | 210 robot | USA | 2014 | Retrospective | 2007-2012 | Single | Multiple | RENAL ≥7 | NS | - | 14/20 |
| Ubrig | 212 robot | Germany | 2018 | Retrospective | 2008-2016 | Multiple | Multiple | PADUA ≥10 | Transperitoneal except few cases | - | 16/20 |
| Volpe | 44 robot | Belgium | 2014 | Retrospective | 2006-2012 | Single | NS | PADUA ≥10 | Transperitoneal | - | 14/20 |
| Wang 2015 | 135 laparoscopic 81 robot |
China | 2015 | Retrospective | 2008-2014 | Two | Multiple | RENAL ≥7 | Transperitoneal | 16.5 | 7/9 stars |
| Wang 2016 | Open 190 Robot 190 |
China | 2016 | Retrospective | 2007-2014 | Two | Single | RENAL ≥7 | NS | 49 | 8/9 stars |
| White | Robot 67 | USA | 2011 | Retrospective | 2007-2010 | Single | Single | RENAL ≥7 | Transperitoneal | - | 16/20 |
| Zargar | Open 52 Robot 10 |
USA | 2014 | Retrospective | 2007-2013 | Multiple | Multiple | RENAL 9-12 | Transperitoneal | 4 | 6/9 stars |
NS=Not secified, RENAL=Radius, endophytic/exophytic, nearness, anterior/posterior location, PADUA=Preoperative aspects of dimension used for anatomic classification
Mean was estimated from the median and range using the formula reported by Hozo et al.[11] Statistical heterogeneity was tested using Chi-square and I2. A P < 0.10 was used to indicate heterogeneity and in the absence of statistical heterogeneity, the fixed-effects model (Mantel-Haenszel method) was used. In the presence of statistically significant heterogeneity, random effects model was used. A P < 0.05 indicates statistical significance. Statistical analysis was performed using the Cochrane Collaboration review manager software RevMan 5.2™.
Evidence synthesis
Search strategy and selection
Systematic literature search of Pubmed/Medline, Scopus, Web of Science, Embase databases yielded a total of 1,519 articles, of which 625 duplicate citations were removed. Rest of the 894 articles underwent title and abstract screening for possible inclusion into the study. After abstract and title screening, 861 articles were excluded and remaining 33 articles underwent full-text review. After full-text review, 11 articles were excluded due to lack of individualized data on perioperative outcomes for complex renal masses, for final analysis 22 studies were included[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Out of these 22 studies, 11 studies were included for quantitative data synthesis and all the 22 studies were included for narrative and pooled analysis [Figure 1].
Figure 1.
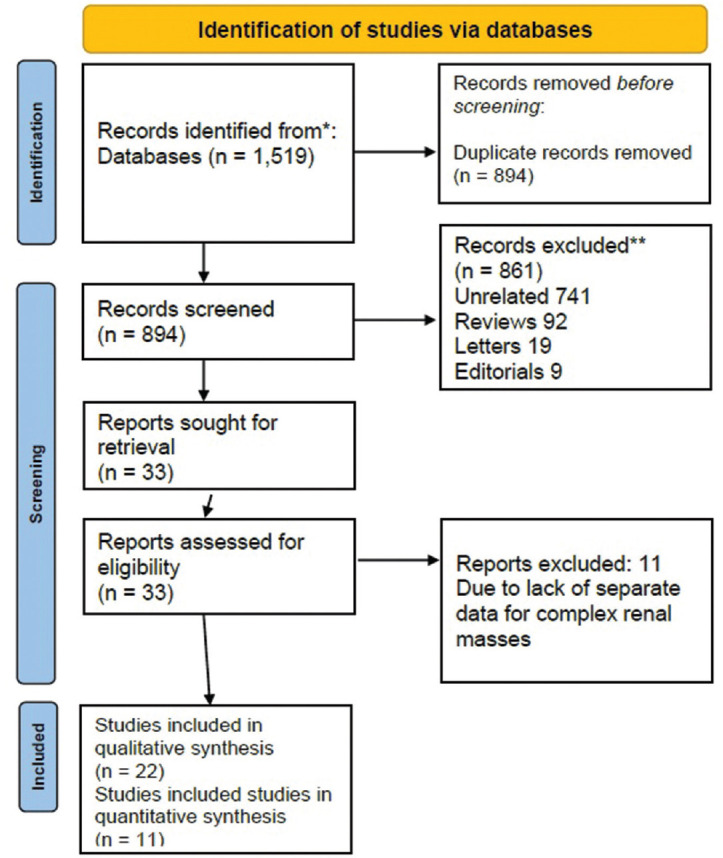
Preferred reporting items for systematic reviews and meta-analysis flow-chart depicting search strategy used for this review
Study characteristics
In this review, 22 studies including 2659 patients with complex renal masses who underwent RAPN were included. Of these 22 studies, 21 were retrospective (including retrospective review of prospectively maintained data) and one was a prospective study. RENAL score for defining the complexity of renal mass was used by 17 studies, 4 studies used PADUA score, and 1 study used both of these. Most of these studies were single-center retrospective case series describing their experience of managing patients with high nephrometery scores. Twelve out of these 22 studies separately reported data for highly complex renal masses, i.e., RENAL and PADUA score ≥10. Median duration of follow-up for RAPN ranged from 4 to 49 months. Complications in these studies were defined according to Clavien-Dindo classification.[34] Minor complications were defined as Grades I and II complications whereas major complications as Grades III and IV. Out of 11 studies used in meta-analysis, 5 studies compared LPN and RAPN and 6 studies compared OPN and RAPN [Table 2].
Table 2.
Comparison of robotic partial nephrectomy with open and laparoscopic for various clinical variables
| Variable | Number of studies | χ 2 | I2 (%) | Model | Pooled MD/OR | 95% CI | P |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Robotic versus laparoscopic | |||||||
| Duration of surgery | 5 | 54.08 | 93 | IV, random | −11.74 | −38.17-14.69 | 0.38 |
| EBL | 5 | 19.52 | 80 | IV, random | −16.98 | −52.03-18.08 | 0.34 |
| WIT | 5 | 7.08 | 44 | IV, random | −1.04 | −2.49-0.42 | 0.16 |
| Minor complications | 5 | 6.27 | 36 | M-H random | 0.98 | 0.65-1.46 | 0.91 |
| Major complications | 5 | 0.94 | 0 | M-H fixed | 1.14 | 0.65-1.99 | 0.65 |
| Blood transfusion | 5 | 2.91 | 0 | M-H fixed | 0.77 | 0.50-1.19 | 0.24 |
| Intraoperative complications | 3 | 1.04 | 0 | M-H fixed | 0.57 | 0.27-1.22 | 0.15 |
| LOS | 5 | 53.63 | 93 | IV, random | −0.36 | −1.04-0.32 | 0.30 |
| Positive surgical margin | 5 | 1.77 | 0 | M-H fixed | 0.89 | 0.35-2.27 | 0.81 |
|
| |||||||
| Robotic versus open | |||||||
|
| |||||||
| Duration of surgery | 7 | 14.69 | 59 | M-H random | 3.37 | −2.87-9.61 | 0.29 |
| EBL | 6 | 136.7 | 96 | IV, random | −82.4 | −133.8--30.9 | 0.002 |
| WIT | 7 | 439.1 | 99 | IV, random | −1.78 | −7.44-3.88 | 0.54 |
| Minor complications | 5 | 2.81 | 0 | M-H fixed | 0.58 | 0.43-0.79 | 0.0004 |
| Major complications | 6 | 7.97 | 37 | M-H fixed | 0.65 | 0.44-0.94 | 0.02 |
| Blood transfusion | 5 | 10.6 | 62 | M-H random | 0.33 | 0.14-0.81 | 0.02 |
| LOS | 7 | 7.21 | 17 | IV, fixed | −1.71 | −2.02-1.40 | <0.00001 |
| Positive surgical margin | 5 | 2.75 | 0 | M-H fixed | 0.65 | 0.36-1.19 | 0.16 |
M-H =Mantel-Haenszel, IV =Inverse variance, MD =Mean difference, OR =Odds ratio, WIT =Warm ischemia time, EBL =Estimated blood loss, LOS =Length of stay, CI =Confidence interval
Quality assessment
Quality assessment for 11 studies was done using IHE case series quality appraisal tool and for rest of the studies using NOS for cohort studies. Overall quality assessment of all of these studies was satisfactory as all the case series obtained score of 14 or more on IHE scale and all the comparative studies obtained 6 or more stars on NOS [Table 1].
Pooled analysis
Pooled analysis of perioperative outcomes for patients with moderate to high complex renal lesions was performed. Mean duration of surgery, WIT, and EBL were 132.5–250.8 min, 15.5–30 min, and 100-321 ml, respectively. Eighty-one patients (3.9%) had positive surgical margin and blood transfusion was required in 5.2% of the patients. Mean duration of LOS ranged between 1 and 7.8 days. According to Clavien-Dindo classification,[34] minor complications (Grade I and II) were seen in 19.3% of patients whereas major complications were seen in 6.3% of the patients [Table 3].
Table 3.
Baseline and perioperative outcomes in studies included in the review
| First author, years (n) | Age (years) | Sex (male/female) | BMI (kg/m2) | Duration (min), mean | WIT (min), mean | Blood loss (ml), mean | Blood transfusions, n (%) | Intra-operative complications, n (%) | Grade I and II complications, n (%) | Grade III, IV complications, n (%) | Hospital stay (days), mean | Margin positivity, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abedali, 2020 (148) | 60.3 | 87/61 | 31.2 | 226.5 | 19.7 | 156.5 | 2 (1.6) | - | - | 16 (10.8) | 3 | - |
| Beksac, 2019 (144) | 59 | 83/61 | 30.35 | 214.6 | 20.25 | 168.75 | 1 (0.69) | 12 (8.3) | 12 (8.3) | 3 (2.3) | 1 | 8 (5.56) |
| Buffi, 2019 (255) | 62 | 166/89 | 26 | 162 | 18.5 | 162.5 | - | - | 62 (24.3) | 13 (5.1) | 4 | 4 (1.9) |
| Bora, 2017 (53) | 49.4 | 29/24 | 23.6 | 169.5 | 27.4 | 252.8 | - | - | 22 (41.5) | 2 (3.9) | 5.8 | 1 (1.8) |
| Deng, 2019 (58) | - | - | - | 198.8 | 22.4 | 158.1 | - | 5 (8.6) | 9 (15.5) | 3 (5.2) | 7.5 | 2 (3.4) |
| Ellison, 2012 (62) | 58 | - | 30.2 | 230 | 30 | 250 | 4 (6.4) | - | 31 (50) | 9 (14.5) | 3.3 | 3 (4.8) |
| Garisto, 2018 (203) | 59.8 | 125/78 | 31 | 208 | 28 | 200 | 6 (2.9) | 15 (7.3) | 43 (21.1) | 14 (6.9) | 3 | 18/179 (10) |
| Hennessey, 2017 (31) | 58.7 | 20/10 | - | 155 | 23 | 200 | - | 0 | 2 (6.4) | 0 | 3.5 | 1 (3.2) |
| Jang, 2014 (89) | 49 | 59/30 | 24.3 | 144 | 24. | 197.6 | 4 (4.5) | 1 (1.1) | 17 (19.1) | 1 (1.1) | 7.1 | 0 |
| Kim, 2019 (85) | 51.2 | 55/30 | 25 | 150 | 24 | 200 | 8 (9.4) | 8 (9.4) | 8 (9.4) | 8 (9.4) | 5 | 0 |
| Long, 2012 (199) | 58.5 | 119/80 | 30.7 | 197 | 22.4 | 280.2 | 24 (12) | 6 (3.0) | 53 (26.6) | 11 (5.5) | 3.4 | 2 (1) |
| Png, 2013 (39) | - | - | - | 215 | 24 | 100 | - | 2 (5.1) | - | - | - | - |
| Raheem, 2016 (223) | 52.3 | 147/76 | 24.5 | 163.5 | 25.0 | 321 | 17 (7.6) | 18 (8.0) | 38 (17) | 14 (6.2) | 5.5 | 18 (8) |
| Schiavina, 2016 (165) | - | - | - | 167.3 | 21.1 | 233.2 | - | - | 18 (11) | 13 (7.8) | - | - |
| Simhan, 2012 (91) | - | - | - | 207.6 | 29.8 | 141.5 | - | - | 27 (29.6) | 10 (10.9) | 3.6 | 3 (3.2) |
| Tomaszewski, 2014 (210) | 57.5 | 133/77 | 30.6 | 199 | 29.4 | 159 | - | - | - | - | - | - |
| Ubrig, 2018 (212) | 57.2 | 147/65 | 34.3 | 193.7 | 15.5 | - | 3 (1.4) | 14 (6.6) | 35 (16.5) | 19 (8.9) | - | 13 (6.1) |
| Volpe, 2014 (44) | 63.75 | - | 26.7 | 132.5 | 18 | 381 | 2 (4.5) | 2 (4.5) | 6 (13.6) | 4 (9) | 7.7 | 2 (4.5) |
| Wang, 2015 (81) | 61.2 | 55/26 | 25.2 | 135.6 | 20.5 | 196.6 | 6 (7.4) | 4 (4.9) | 11 (13.5) | 3 (3.7) | 7.6 | 1 (1.2) |
| Wang, 2016 (190) | 61.8 | 139/51 | 25.4 | 141.7 | 21.3 | 196.8 | 12 (6.3) | 10 (5.2) | 25 (13.1) | 5 (2.6) | 7.8 | 3 (1.5) |
| White, 2011 (67) | 60.7 | 31/35 | 30.9 | 175.1 | 19.4 | 221.1 | - | - | 14 (20.8) | 1 (1.5) | 3 | 1 (1.5) |
| Zargar, 2014 (10) | 61.3 | 32/8 | 30 | 250.8 | 22.7 | - | 0 | 1 (10) | 0 | 4 (25) | - | 1 (10) |
| n=2659 | 49-63.75 (range) | - | 23.6-34.3 (range) | 132.5-250.8 (range) | 15.5-30 (range) | 100-321 (range) | 89/1690 (5.2) (weighted mean) | 98/1608 (6.1) (weighted mean) | 433/2262 (19.1) (weighted mean) | 153/2410 (6.3) (weighted mean) | 1-7.8 (range) | 81/2073 (3.9) (weighted mean) |
BMI=Body mass index, WIT=Warm ischemia time
High complexity renal masses
Subgroup analysis of patients with high complexity (RENAL and PADUA score ≥10) renal masses was also performed. In this subgroup, duration of surgery, WIT, EBL, and LOS were 132.5–214.6 min, 15.5–36.1 min, 200–456.2 ml, and 1–7.7 days, respectively. Minor and major complications were seen in 16.2% and 6.2% of the patients, respectively. Pooled margin positivity rate was 5.2% and need for blood transfusion was seen in 3.5% of patients.
Laparoscopic partial nephrectomy versus robot-assisted partial nephrectomy
Five nonrandomized studies including 969 patients compared robotic and LPN. There was no statistically significant difference in the two groups for any of the perioperative outcome, i.e., duration of surgery (mean difference [MD] ‒11.74 95% confidence interval [CI] [‒38.17,14.69], P = 0.38), EBL (MD ‒16.98 95% CI [‒52.03, 18.08], P = 0.34), need for blood transfusions (OR 0.77 95% CI [0.50, 1.19], P = 0.24), LOS (MD ‒0.36 95% CI [‒1.04, 0.32], P = 0.30), positive surgical margins (OR 0.89 95% CI [0.35, 2.27], P = 0.81), major (OR 1.14 95% CI [0.65, 1.99], P = 0.65) or minor (OR 0.98 95% CI [0.65, 1.46], P = 0.91) surgical complications, and WIT (MD ‒1.04 95% CI [‒2.49, 0.42], P = 0.16) [Table 2].
Open partial nephrectomy versus robot-assisted partial nephrectomy
Six nonrandomized studies including 1373 patients compared perioperative outcomes for OPN and RAPN. Study by Simhan et al.[26] contained separate data for moderately and highly complex lesion so they were grouped separately for the meta-analysis. Due to high heterogeneity, random effect model was used for comparison of duration of surgery, WIT, and EBL. There was no significant difference in the two groups for duration of surgery (MD 3.37 95% CI [‒2.87, 9.61], P = 0.29) and WIT (MD ‒1.78 95% CI [‒7.44, 3.88], P = 0.54) but EBL (MD ‒82.4 95% CI [‒133.8, ‒30.9], P = 0.002) and LOS (MD ‒1.71 95% CI [‒2.02, ‒1.40], P < 0.00001) were significantly lower for RAPN. Need for blood transfusion (OR 0.33 95% CI [0.14, 0.81], P = 0.02), complication rates (Major Complications OR 0.65, P = 0.02; minor complications OR 0.58 P = 0.0004) were significantly higher with OPN, whereas incidence of positive surgical margin (OR 0.65 95% CI [0.36,1.19], P = 0.16) was similar in the two groups [Table 2].
DISCUSSION
Robotic assistance has revolutionized the practice of PN to include highly complex renal masses, however, the data regarding the same are still not robust. In this study, we performed pooled analysis of various perioperative outcomes following RAPN in patients with complex renal masses and also compared RAPN to other surgical techniques, i.e., LPN and OPN. The studies included in this review are of good quality but all were nonrandomized case series which have inherent limitations.
In this study, the duration of surgery (132.5–250.8 min), WIT (15.5–30 min), and EBL (100–321 ml) showed a wide variation. In the subgroup analysis of high complexity renal masses (RENAL and PADUA score ≥10), similar results were noted with mean duration of surgery, WIT, blood loss, and hospital stay being 132.5–214.6 min, 15.5–36.1 min, 200–456.2 ml, respectively. In previously reported series of RAPN including mixed complexity data, mean WIT reported were 18.8 min, 15.7 min, and 20 min by Tanagho et al.,[35] Peyronnet et al.[36] and Laydner et al.,[37] respectively. On observing WIT data from our Tables 3 and 4, mean WIT for most of the studies appears to be higher than previously reported studies of RAPN with mixed complexity tumors. Thompson et al.[38] in their study of 362 solitary functioning kidneys who underwent OPN or LPN reported that a WIT of more than 25 min was associated with higher rates of acute renal failure (ARF), glomerular filtration rate (GFR) <15 ml/min/1.73 m2, and new onset stage IV chronic kidney disease. They also reported that every minute increase in WIT raised the odds of developing ARF and GFR <15 ml/min/1.73 m2 by 5% and 6%, respectively. Most of the studies in this review [16/22, Table 3] had WIT <25 min. Furthermore, in highly complex renal masses (RNS >10), the WIT was <25 min for 7 out of 12 studies [Table 4]. Thus, WIT, despite being on slightly higher side, still falls into the acceptable range of <25 min for most of the studies included in this review. Mean duration of surgery in previous RAPN series was 183.6 min and 153 min by Tanagho et al.[35] and Peyronnet et al.,[36] respectively, which appears lower than the duration of surgery reported from most of the studies in this review [Tables 3 and 4]. Mean EBL as reported by Laydner et al.[37] Tanagho et al.,[35] and Peyronnet et al.[36] were 182 ml, 191 ml, and 275 ml, respectively, whereas blood loss reported in the series by Gill et al.[39] in LPN and OPN were 300 ml and 376 ml, respectively, which compares well to our data from overall and highly complex renal masses [Tables 3 and 4]. From pooled analysis need for transfusion was 5.2% and 3.2% for overall and highly complex renal masses cohort which also compared well to the data from previous studies with renal masses of various complexities.[40]
Table 4.
Perioperative outcomes in studies included in the review with high complexity renal masses (radius, endophytic/ exophytic, nearness, anterior/posterior location or preoperative aspects of dimension used for anatomic classification score ≥10)
| Study, year (n) | Duration (min), mean | Blood loss (ml), mean | Blood transfusion, n (%) | WIT (min), mean | Grade I or II complication, n (%) | Grade III, IV complication, n (%) | Hospital stay (days), mean | Margin positivity, n (%) |
|---|---|---|---|---|---|---|---|---|
| Garisto, 2018 (203) | 208 | 200 | 6 (2.9) | 28 | 43 (21.1) | 14 (6.9) | 3 | 18/179 (10) |
| Hennessey, 2017 (31) | 155 | 200 | - | 23 | 2 (6.4) | 0 | 3.5 | 1 (3.2) |
| Kim, 2019 (85) | 150 | 200 | 8 (9.4) | 24 | 8 (9.4) | 10 (11.7) | 5 | 0 |
| Raheem, 2016 (121) | 164 | 360 | 10 (8.2) | 26 | 20 (16.5) | 6 (4.9) | 5.6 | 12 (9.9) |
| Schiavina, 2016 (79) | 169 | 243 | - | 24.7 | 2 (2.5) | 4 (5) | - | - |
| Simhan, 2012 (10) | 221 | 225 | - | 33.8 | - | - | 3.7 | 0 |
| Tomaszewski, 2014 (24) | 215 | 262 | - | 36.1 | - | - | - | - |
| Ubrig, 2018 (212) | 193.7 | - | 3 (1.4) | 15.5 | 35 (16.5) | 19 (8.9) | - | 13 (6.1) |
| Volpe, 2014 (44) | 132.5 | 381 | 2 (4.5) | 18 | 6 (13.6) | 4 (9) | 7.7 | 2 (4.5) |
| White, 2011 (11) | 201 | 456.25 | - | 27 | - | - | 4 | 1 (9) |
| Beksac, 2019 (144) | 214.6 | 168.75 | 1 (0.69) | 20.25 | 12 (8.3) | 3 (2.3) | 1 | 8 (5.5) |
| Buffi, 2019 (255) | 162 | 162.5 | - | 18.5 | 62 (24.3) | 13 (5.1) | 4 | 4 (1.9) |
| n=1246 | 132.5-214.6 (range) | 200-456.25 (range) | 30/836 (3.5) (weighted mean) | 15.5-36.1 (range) | 190/1170 (16.2) (weighted mean) | 73/1170 (6.2) (weighted mean) | 1-7.7 (range) | 59/1119 (5.2) (weighted mean) |
WIT=Warm ischemia time
In this review, we noted pooled minor complications rate to be 19.1% and 16.2% and the major complications rate to be 6.3% and 6.2% in overall and highly complex renal masses cohort, respectively [Tables 3 and 4]. In a multi-institutional study of RAPN by Spana et al.,[41] authors reported an overall complication rate of 15.8% of which 12% patients had minor and 3.7% patients had major complications. In a study by Tanagho et al.,[35] intraoperative and overall complication rates were 2.6% and 15.6%, respectively. They also stratified complication rates according to the RENAL score, where intraoperative complications were 2.3%, 2.7%, and 8.2% in low, moderate, and high complexity renal masses. Overall complications rates were 9%, 15.8%, and 18% in low, moderate, and high complexity masses. In a meta-analysis, comparing OPN and RAPN, RAPN group reported 19.2%, 14.3%, and 8.9% overall, minor, and major complication rates, respectively.[41] Thus, complication rates noted in our pooled analysis for complex renal masses are similar to RAPN cohort of mixed complexity reported previously.
Margin positivity reported in the present study is 3.9% and 5.2% in overall and high complexity group, respectively. A systematic review of 36 studies containing data on 45,786 patients reported 6.7%, 7%, 5%, and 4.3% positive surgical margins in overall, RAPN, LPN, and OPN, respectively. Authors also reported that positive surgical margins were associated with significant increases in risk of local recurrence, recurrence-free survival, and metastasis-free survival, however, had no effect on cancer-specific and overall survival.[42] Wu et al.[40] and Choi et al.[43] reported a margin positivity rate of 3.3% and 4.3% in their studies, respectively, for RAPN. Thus, data on margin positivity for complex renal masses following RAPN falls within acceptable ranges as noted in previous large studies.
Robot-assisted partial nephrectomy versus laparoscopic partial nephrectomy
In the present meta-analysis, comparison of perioperative variables revealed no significant difference in the two groups. These results are in contrary to previous meta-analysis by Choi et al.[43] comparing the two groups. They found robotic group had favorable change in WIT and LOS. Similarly, in an earlier meta-analysis, Aboumarzouk et al.[44] reported WIT to be favorable with robotic group compared to LPN. Theoretically, robotic platform with the added advantage of wristed instruments and 3D vision is expected to provide better WIT than laparoscopy. However, with complex renal tumors, this advantage gets blurred.
Robot-assisted partial nephrectomy versus open partial nephrectomy
In comparison of RAPN to OPN, we noted that RAPN had significantly lower EBL, complication rate (major and minor), need for blood transfusion, and LOS [Table 2]. Possibility of selection bias in these studies could not be ruled out with higher complexity lesions being preferentially dealt with open approach. In a previous meta-analysis, by Xia et al.[45] comparing the two groups included 19 studies irrespective of their nephrometery scores. Similar to our study, authors noted RAPN to be advantageous with lower complication rates, need for blood transfusion, EBL and LOS.
Limitations
There are multiple limitations of this study, firstly the studies included in this study are case series or nonrandomized comparative studies that amount to low quality of evidence. Furthermore, most of the studies included in this review were retrospective, further lowering the quality of evidence. We did pooled analysis of studies using both RENAL and PADUA score that differs slightly from each other, as describing them separately would not have produced meaningful results in our opinion. Furthermore, pooled analysis for moderately complex renal tumors could not be performed as the included studies lacked separate data for the same.
We did not include survival outcomes in the present meta-analysis as the duration of follow-up in the studies included in this meta-analysis were of short duration hence appropriate conclusions could not be drawn. The studies included in this review are from various parts of the world which have different national insurance policies which might affect LOS.
CONCLUSIONS
RAPN is a feasible option for moderate to high complexity renal masses with slightly longer but acceptable operative time and WIT. EBL, need for transfusion, margin positivity, and complications rate is consistent with previously reported studies of mixed complexity renal masses. LPN and RAPN were equal in terms of perioperative outcomes for complex masses whereas, OPN had significantly higher EBL, complications rate, need for blood transfusion, and LOS as compared to RAPN.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
- 1.Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Kim SP, Murad MH, Thompson RH, Boorjian SA, Weight CJ, Han LC, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol. 2012;188:51–7. doi: 10.1016/j.juro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: A systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71:606–17. doi: 10.1016/j.eururo.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 4.Janda G, Deal A, Yang H, Nielsen M, Smith A, Pruthi RS, et al. Single-institution experience with robotic partial nephrectomy for renal masses greater than 4cm. J Endourol. 2016;30:384–9. doi: 10.1089/end.2015.0254. [DOI] [PubMed] [Google Scholar]
- 5.Autorino R, Khalifeh A, Laydner H, Samarasekera D, Ouzaid I, Brandao LF, et al. Robotic partial nephrectomy for completely endophytic renal masses: A single institution experience. J Endourol. 2013;27:A398–9. [Google Scholar]
- 6.Boris R, Proano M, Linehan WM, Pinto PA, Bratslavsky G. Initial experience with robot assisted partial nephrectomy for multiple renal masses. J Urol. 2009;182:1280–6. doi: 10.1016/j.juro.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauza Quetglas JL, Sagalovich D, Bertolo R, Garisto J, Pieras E, Piza P, et al. Robotic partial nephrectomy for complex hilar tumors: Step by step. Urology. 2018;120:271–2. doi: 10.1016/j.urology.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–53. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–93. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abedali ZA, Monn MF, Huddleston P, Cleveland BE, Sulek J, Bahler CD, et al. Robotic and open partial nephrectomy for intermediate and high complexity tumors: A matched-pairs comparison of surgical outcomes at a single institution. Scand J Urol. 2020;54:313–7. doi: 10.1080/21681805.2020.1765017. [DOI] [PubMed] [Google Scholar]
- 13.Beksac AT, Okhawere KE, Elbakry AA, Dayal BD, Paulucci DJ, Rothberg MB, et al. Management of high complexity renal masses in partial nephrectomy: A multicenter analysis. Urol Oncol. 2019;37:437–44. doi: 10.1016/j.urolonc.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Buffi NM, Saita A, Lughezzani G, Porter J, Dell’Oglio P, Amparore D, et al. Robot-assisted partial nephrectomy for complex (PADUA Score≥10) tumors: Techniques and results from a multicenter experience at four high-volume centers. Eur Urol. 2020;77:95–100. doi: 10.1016/j.eururo.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Bora GS, Mavuduru RS, Sharma AP, Devana SK, Kakkar N, Lal A, et al. Initial experience of robotic nephron sparing surgery in cases of high renal nephrometry scores. Indian J Urol. 2017;33:230–5. doi: 10.4103/iju.IJU_331_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng W, Li J, Liu X, Chen L, Liu W, Zhou X, et al. Robot-assisted versus laparoscopic partial nephrectomy for anatomically complex T1b renal tumors with a RENAL nephrometry score≥7: A propensity score-based analysis. Cancer Med. 2020;9:586–94. doi: 10.1002/cam4.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison J, Montgomery J, Hafez K, Miller D, He C, Wolf S, et al. Effect of R.E.N.A.L nephrometry score on peri-operative outcomes in minimally invasive partial nephrectomy. J Urol. 2011;185:e276–7. [Google Scholar]
- 18.Garisto J, Bertolo R, Dagenais J, Sagalovich D, Fareed K, Fergany A, et al. Robotic versus open partial nephrectomy for highly complex renal masses: Comparison of perioperative, functional, and oncological outcomes. Urol Oncol. 2018;36:471–e1-9. doi: 10.1016/j.urolonc.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Hennessey DB, Kinnear N, Bolton DM, Moon D, Lawrentschuk N, Chan YK. Robotic partial nephrectomy for complex renal lesions: Strategies for success. A multi-institutional study. BJU Int. 2017;120:38. doi: 10.1111/bju.14059. [DOI] [PubMed] [Google Scholar]
- 20.Jang HJ, Song W, Suh YS, Jeong US, Jeon HG, Jeong BC, et al. Comparison of perioperative outcomes of robotic versus laparoscopic partial nephrectomy for complex renal tumors (RENAL nephrometry score of 7 or higher) Korean J Urol. 2014;55:808–13. doi: 10.4111/kju.2014.55.12.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JK, Lee H, Oh JJ, Lee S, Hong SK, Lee SE, et al. Comparison of robotic and open partial nephrectomy for highly complex renal tumors (RENAL nephrometry score≥10) PLoS One. 2019;14:e0210413. doi: 10.1371/journal.pone.0210413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long JA, Yakoubi R, Lee B, Guillotreau J, Autorino R, Laydner H, et al. Robotic versus laparoscopic partial nephrectomy for complex tumors: Comparison of perioperative outcomes. Eur Urol. 2012;61:1257–62. doi: 10.1016/j.eururo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Png KS, Bahler CD, Milgrom DP, Lucas SM, Sundaram CP. The role of R.E.N.A.L. nephrometry score in the era of robot-assisted partial nephrectomy. J Endourol. 2013;27:304–8. doi: 10.1089/end.2012.0182. [DOI] [PubMed] [Google Scholar]
- 24.Abdel Raheem A, Alatawi A, Kim DK, Sheikh A, Alabdulaali I, Han WK, et al. Outcomes of high-complexity renal tumours with a Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) score of≥10 after robot-assisted partial nephrectomy with a median 46.5-month follow-up: A tertiary centre experience. BJU Int. 2016;118:770–8. doi: 10.1111/bju.13501. [DOI] [PubMed] [Google Scholar]
- 25.Schiavina R, Novara G, Borghesi M, Ficarra V, Ahlawat R, Moon DA, et al. PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: Analysis of theVattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. 2017;119:456–63. doi: 10.1111/bju.13628. [DOI] [PubMed] [Google Scholar]
- 26.Simhan J, Smaldone MC, Tsai KJ, Li T, Reyes JM, Canter D, et al. Perioperative outcomes of robotic and open partial nephrectomy for moderately and highly complex renal lesions. J Urol. 2012;187:2000–4. doi: 10.1016/j.juro.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 27.Tomaszewski JJ, Smaldone MC, Mehrazin R, Kocher N, Ito T, Abbosh P, et al. Anatomic complexity quantitated by nephrometry score is associated with prolonged warm ischemia time during robotic partial nephrectomy. Urology. 2014;84:340–4. doi: 10.1016/j.urology.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ubrig B, Roosen A, Wagner C, Trabs G, Schiefelbein F, Witt JH, et al. Tumor complexity and the impact on MIC and trifecta in robot-assisted partial nephrectomy: A multi-center study of over 500 cases. World J Urol. 2018;36:783–8. doi: 10.1007/s00345-018-2191-0. [DOI] [PubMed] [Google Scholar]
- 29.Volpe A, Rogers C, Mottrie A, Ahlawat R, Rawal S, Moon D, et al. Robot-assisted partial nephrectomy for renal tumors with RENAL nephrometry score≥10: Perioperative outcomes from a large multicentre international dataset (Vattikuti global quality initiative on robotic urologic surgery) BJU Int. 2014;113:66–7. [Google Scholar]
- 30.Wang Y, Ma X, Huang Q, Du Q, Gong H, Shang J, et al. Comparison of robot-assisted and laparoscopic partial nephrectomy for complex renal tumours with a RENAL nephrometry score≥7: Peri-operative and oncological outcomes. BJU Int. 2016;117:126–30. doi: 10.1111/bju.13214. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Shao J, Ma X, Du Q, Gong H, Zhang X. Robotic and open partial nephrectomy for complex renal tumors: A matched-pair comparison with a long-term follow-up. World J Urol. 2017;35:73–80. doi: 10.1007/s00345-016-1849-8. [DOI] [PubMed] [Google Scholar]
- 32.White MA, Haber GP, Autorino R, Khanna R, Forest S, Altunrende F, et al. Outcomes of robotic partial nephrectomy for complex renal masses with a nephrometry score≥7. J Endourol. 2010;24:A237–8. doi: 10.1016/j.urology.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Zargar H, Bhayani S, Allaf ME, Stifelman M, Rogers C, Larson J, et al. Comparison of perioperative outcomes of robot-assisted partial nephrectomy and open partial nephrectomy in patients with a solitary kidney. J Endourol. 2014;28:1224–30. doi: 10.1089/end.2014.0297. [DOI] [PubMed] [Google Scholar]
- 34.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanagho YS, Kaouk JH, Allaf ME, Rogers CG, Stifelman MD, Kaczmarek BF, et al. Perioperative complications of robot-assisted partial nephrectomy: Analysis of 886 patients at 5 United States centers. Urology. 2013;81:573–9. doi: 10.1016/j.urology.2012.10.067. [DOI] [PubMed] [Google Scholar]
- 36.Peyronnet B, Seisen T, Oger E, Vaessen C, Grassano Y, Benoit T, et al. Comparison of 1800 robotic and open partial nephrectomies for renal tumors. Ann Surg Oncol. 2016;23:4277–83. doi: 10.1245/s10434-016-5411-0. [DOI] [PubMed] [Google Scholar]
- 37.Laydner H, Kassab A, Khalifeh A, Autorino R, Stein R, Haber GP, et al. 1097 robotic, laparoscopic and open partial nephrectomies: Comparison of surgical outcomes at a single institution. J Urol. 2013;189:e679–80. [Google Scholar]
- 38.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58:340–5. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–6. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Li M, Liu B, Cai C, Ye H, Lv C, et al. Robotic versus open partial nephrectomy: A systematic review and meta-analysis. PLoS One. 2014;9:e94878. doi: 10.1371/journal.pone.0094878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spana G, Haber GP, Dulabon LM, Petros F, Rogers CG, Bhayani SB, et al. Complications after robotic partial nephrectomy at centers of excellence: Multi-institutional analysis of 450 cases. J Urol. 2011;186:417–21. doi: 10.1016/j.juro.2011.03.127. [DOI] [PubMed] [Google Scholar]
- 42.Ficarraa V, Crestanib A, Inferreraa A, Novarac G, Rossanesea M, Subbaa E, et al. Positive surgical margins after partial nephrectomy: A systematic review and meta-analysis of comparative studies. Kidney Cancer. 2018;2:133–45. [Google Scholar]
- 43.Choi JE, You JH, Kim DK, Rha KH, Lee SH. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: A systematic review and meta-analysis. Eur Urol. 2015;67:891–901. doi: 10.1016/j.eururo.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 44.Aboumarzouk OM, Stein RJ, Eyraud R, Haber GP, Chlosta PL, Somani BK, et al. Robotic versus laparoscopic partial nephrectomy: A systematic review and meta-analysis. Eur Urol. 2012;62:1023–33. doi: 10.1016/j.eururo.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 45.Xia L, Zhang X, Wang X, Xu T, Qin L, Zhang X, et al. Transperitoneal versus retroperitoneal robot-assisted partial nephrectomy: A systematic review and meta-analysis. Int J Surg. 2016;30:109–15. doi: 10.1016/j.ijsu.2016.04.023. [DOI] [PubMed] [Google Scholar]


