ABSTRACT
Introduction:
Literature on the factors predicting functional and oncological outcomes following robot-assisted radical prostatectomy (RARP) is sparse for the Indian population. Hence, the primary objective of this study was to develop preoperative and postoperative nomograms predicting these outcomes in patients with prostate cancer undergoing RARP.
Methods:
This retrospective analysis identified the predictors of quadrifecta outcomes, i.e., the patients who did not have complications, were continent, had negative surgical margins, and were biochemical recurrence free with at least 1 year of follow-up following RARP. We excluded the return of sexual potency as the majority of the patients in our series were sexually inactive preoperatively. We used the backward stepwise logistic regression analysis method to identify the predictors of quadrifecta. Preoperative and postoperative nomograms using these predictors were developed and validated with bootstrapping, goodness of fit, calibration plot, decision curve analysis (DCA), and theits receiver operating characteristic (ROC) analysis.
Results:
Of the 688 patients who underwent RARP, 399 were included in this study, and 123 (30.8%) of these achieved the quadrifecta outcomes. Preoperative nomogram was developed using four variables, i.e., prostate-specific antigen (PSA), Charlson Comorbidity Index (CCI), biopsy Gleason score, and clinical stage. Postoperative nomogram included PSA, CCI, pathological tumor stage, tumor grade, and positive lymph node. Both the models were internally valid on bootstrapping, calibration plots, and goodness of fit. On the ROC analysis, preoperative and postoperative nomograms had an area under the curve of 0.71 and 0.79, respectively. On the DCA, at a threshold probability of 5%, both the models showed a net benefit.
Conclusions:
We developed and validated accurate nomograms for predicting quadrifecta outcomes following RARP for the Indian population.
INTRODUCTION
Prostate cancer is one of the common malignancies noted in the elderly males.[1] Radical prostatectomy (RP) has been the standard of care for decades with similar oncological and functional outcomes as compared to radiotherapy.[2,3] With the advent of the robotic platforms, robot-assisted RP (RARP) has rapidly disseminated worldwide and has essentially replaced open surgery at most of the centers.[4,5] Following the surgical management, functional and oncological outcomes remain an important area of concern for the surgeon as well as the patients. To gauge the quality of outcomes following RP, three essential variables, i.e., the erectile function, the biochemical recurrence-free survival (BCRFS), and the urinary incontinence, were combined to give the trifecta outcomes.[6] This was followed by the addition of the surgical margins and the complication rates to the trifecta to give the pentafecta outcomes.[7,8] There is no standard definition for the two functional outcomes included in the trifecta, i.e., the incontinence and erectile dysfunction.[6] Furthermore, there is a variability in the point of time at which these outcomes are reported in the different studies. This limitation has been previously realized and has essentially prevented the conduct of a pooled or summative analysis of the various observational studies.[6]
Multiple studies have been performed to identify the predictors of the trifecta and the pentafecta outcomes following RP.[7,9,10,11,12] Various factors such as the prostate-specific antigen (PSA), clinical stage, Gleason score, baseline erectile function, time from RP, and age at RP have been identified as the predictors of the trifecta or the pentafecta outcomes.[9,10,11,12,13] Most of these studies have been conducted at the centers of excellence in the Western countries. There is a dearth of good quality data on the factors predicting these outcomes for the Indian population. To the best of our knowledge, there is a lack of data on the predictors of trifecta or pentafecta outcomes following RARP in the Indian population. Hence, this study was aimed to identify the preoperative and postoperative factors predicting the quadrifecta outcomes (urinary incontinence, margin status, BCRFS, and complications) following RARP in patients with prostate cancer. We excluded the erectile function as one of the parameters as the majority of our patients were sexually inactive at the baseline. Another objective of this study was to develop and internally validate a nomogram from the prediction model thus obtained.
MATERIALS AND METHODS
In this retrospective study, we queried our prospectively maintained RARP database from October 2011 to July 2020. All the patients included in this study had undergone clinical, biochemical, and radiological investigations before the surgery. The clinical examination followed a standard pattern in all the patients, including a detailed history and physical examination. All the patients were required to fill out the International Prostate Symptom Score and the Sexual Health Inventory for Male[14] forms except those with language or understanding barriers. Biochemical investigations included complete blood count, kidney function test, liver function test, coagulation profile, and PSA. Radiological investigations were variable and included a combination of multiparametric magnetic resonance imaging (mpMRI) or contrast-enhanced computed tomogram of the abdomen and pelvis and the prostate-specific membrane antigen positron emission tomogram scan or the technetium-99m-methylene diphosphonate bone scan. Initial diagnosis in all the patients was obtained by the transrectal ultrasound-guided prostate biopsy or transurethral resection of the prostate (TURP). Due to the retrospective nature of this study, the need for off by the institutional ethics committee (IEC). The authors confirm the availability of and the access to all the original data reported in this study. The study protocol has been approved by the IEC (BHR/RS/MSSH/DDF/SKT-2/IEC/ONCO/21–35).
Baseline data
For every patient, the baseline data included age, height, body weight, body mass index (BMI), medical comorbidities, Charlson Comorbidity Index (CCI),[15] American Society of Anesthesiologists (ASA) grade, preoperative PSA, clinical stage, neoadjuvant hormonal therapy, previous TURP, and D’Amico risk staging.[16]
Radiological and biopsy data
Radiological data were also retrieved, and the local clinical stage was determined using the mpMRI findings in addition to the digital rectal examination (DRE) findings. Data from the biopsy included the Gleason score, the International Society of Urological Pathology (ISUP) grade,[17] and the percentage of the positive cores.
Operative technique and variables considered
Surgical procedures were performed by two experienced robotic surgeons (G. G and P. A) using the Si and the Xi da Vinci systems (Intuitive Surgical, Sunnyvale, CA, USA).[18,19,20] Data for the operative variables including the console time, the extent of the lymph node dissection, the extent of nerve sparing, and the estimated blood loss (EBL) were also extracted.
Postoperative follow-up
Complications were determined within a 30-day period as per the Clavien–Dindo classification.[21] After the discharge, the patients were followed up at 1 week for catheter removal and thereafter at 1 month with fresh serum PSA. Thereafter, the patients were followed with serum PSA as per the European Association of Urology guidelines.[3] From the final biopsy report, the data for Gleason score, ISUP grade, margin status, and the percentage of positive lymph nodes were retrieved.
Quadrifecta
Quadrifecta included four variables, i.e., no complications, negative margins, strict zero pad continence, and the absence of biochemical recurrence (BCR), at a minimum of 1-year follow-up. The BCR was defined as a PSA value of ≥0.2 ng/ml after the surgery.
Statistical analysis
Categorical data were presented as proportions or percentages and the continuous data as mean with standard deviation or median and range, as applicable. The normality of the data was checked using the Kolmogorov–Smirnov and the Shapiro's test. For the normally distributed data, the mean was compared using the Student's t-test for two groups. For the skewed data, nonparametric tests (Mann–Whitney test or Kruskal–Wallis test) were used, as applicable. Qualitative or categorical variables were described as frequencies and proportions. Categorical data were compared using the Chi-square test or the Fisher's exact test, whichever was appropriate. For identifying the predictors of the quadrifecta, we used the backward stepwise logistic regression analysis. Backward regression analysis begins with a full or saturated model. At each step, nonsignificant variables are excluded from the regression model to provide a final reduced best model. A prediction model based on the above mentioned criteria was used to develop a nomogram. Nomogram was internally validated using bootstrapping (5000 reps), a maximum area under the curve (AUC) from the receiver operating curve (ROC), and the goodness of fit, calculated using the Hosmer–Lemeshow test. Nomogram was generated using “nomolog” package on STATA (version 16; StataCorp, College Station, TX, USA).[22] Receiver operative characteristic (ROC) curves were used to predict the ability of the developed nomogram for quadrifecta. The calibration of the model was checked using the calibration plots. Calibration plots were constructed from the study by drawing 50% random samples and comparing the observed and expected probability of the quadrifecta. For generating calibration plots, “pmcal” package for STATA was used. We used decision curve analysis (DCA) to assess the clinical utility of the model. All statistical tests were two-sided and performed at a significance level of P < 0.05. The statistical analysis was performed using the Statistical Product and Service Solutions (SPSS Inc., Chicago, IL, version 23.0 for Windows) and STATA (version 16; StataCorp, College Station, TX, USA).[22]
RESULTS
From October 2011 to July 2020, 630 patients underwent RARP, of which 399 patients were included in the final analysis. We excluded 98 non-Indians and 133 Indian patients who lacked adequate follow-up data were also excluded. Of these 399 patients, only 134 men were sexually active at the baseline (33.5%). The median age of the patients included in this study was 66 years, ranging from 43 to 83 years. The median duration of the follow-up was 25 months, ranging from 12 to 108 months. A history of prior transurethral resection of the prostate (TURP) or neoadjuvant hormonal therapy was present in 21 (5.3%) and 31 (7.8%) of the patients, respectively. The mean CCI and BMI were 4.6 ± 1 and 22.4 ± 27.8, respectively. The median PSA was 14.7 ng/ml with a range of 0.5–310 ng/ml. On biopsy, ISUP Grades I, II, III, IV, and V were noted in 21.3%, 29.8%, 18.3%, 24.1%, and 6.5% of the patients, respectively. Clinically, patients were stratified into D’Amico risk categories as low risk (5%), intermediate risk (32.3%), and high risk (62.7%). The median console time and EBL were 180 min and 100 ml, respectively. Nerve sparing was performed bilaterally and unilaterally in 20.5% and 53.9%, respectively, whereas in 25.6%, bilateral wide resection of the neurovascular bundle was performed. The median length of stay was 2 days (range: 1–7 days). The median time to drain and catheter removal were 1 and 8 days, respectively. From the pathological analysis of the surgical specimen, ISUP Grades I, II, III, IV, and V were noted in 12.5%, 34.3%, 21.3%, 21.8%, and 9.8% of the patients, respectively. Lymph nodes were positive in 28.8% of the patients. Final pathology revealed T2 and T3 stages in 36.1% and 63.7% of the patients, respectively. Positive surgical margin was noted in 33.3% of the patients. The median duration of follow-up was 21 months. Clavien–Dindo classification Grade I, II, and III complications were recorded in 3.5%, 2.8%, and 3.5% of the patients, respectively. BCR and zero pad continence were noted in 45% and 81% of the patients, respectively. Quadrifecta outcomes were achieved in 123 (30.8%) of the included patients. Univariate analysis on comparison of the patients who achieved the quadrifecta outcomes to those who did not has been provided in Table 1.
Table 1.
Comparison of patients who did and did not achieve quadrifecta outcomes
| Variable | Quadrifecta achieved (123) | Quadrifecta not achieved (276) | P |
|---|---|---|---|
| Age | 64.0±7.2 | 65.9±6.5 | 0.009 |
| PSA (ng/ml) | 13.6±8.9 | 26.3±32.2 | 0.000 |
| BMI (kg/m2) | 26.7±4.0 | 26.8±4.5 | 0.730 |
| CCI | 4.2±1 | 4.6±1 | 0.001 |
| History of TURP (%) | 8 (6.5) | 13 (4.7) | 0.459 |
| Neoadjuvant hormonal therapy (%) | 8 (6.5) | 23 (8.3) | 0.528 |
| Clinical stage (using mpMRI and digital rectal examination) (%) | |||
| T1 | 54 (43.9) | 87 (31.5) | 0.032 |
| T2 | 57 (46.3) | 134 (48.5) | |
| T3a | 7 (5.7) | 23 (8.3) | |
| T3b | 5 (4.06) | 32 (11.6) | |
| Biopsy ISUP grade (%) | |||
| I | 38 (30.9) | 47 (17.02) | 0.002 |
| II-V | 85 (69.1) | 229 (83) | |
| D’Amico risk group (%) | |||
| Low | 15 (12.2) | 5 (1.8) | 0.000 |
| Intermediate | 50 (40.6) | 79 (28.6) | |
| High | 58 (47.1) | 192 (69.5) | |
| Nerve sparing (%) | |||
| Bilateral | 38 (30.8) | 44 (15.9) | 0.000 |
| Unilateral | 69 (56.1) | 146 (52.9) | |
| None | 16 (13) | 86 (31.1) | |
| EBL (ml) | 146.7±70.5 | 156.1±90.8 | 0.320 |
| Console time (min) | 173.0±39.2 | 178.8±42.6 | 0.202 |
| Time for drain removal (days) | 1.0±0.1 | 1.1±1.2 | 0.217 |
| Length of stay (days) | 2.0±0.5 | 2.1±0.6 | 0.030 |
| Time for catheter removal (days) | 9.0±1.9 | 9.4±2.1 | 0.079 |
| Radical prostatectomy specimen data ISUP grade (%) | |||
| I | 32 (26) | 18 (6.5) | 0.000 |
| II | 48 (39) | 89 (32.2) | |
| III | 27 (21.9) | 58 (21) | |
| IV | 13 (10.5) | 74 (26.8) | |
| V | 3 (2.4) | 36 (13) | |
| Stage (localized vs. locally advanced) (%) | 74 (60.1)/49 (39.8) | 71 (25.7)/205 (74.2) | 0.000 |
| Positive lymph nodes (%) | 10 (8.1) | 105 (38) | 0.000 |
PSA=Prostate-specific antigen, BMI=Body mass index, EBL=Estimated blood loss, ISUP=International Society of Urological Pathology, TURP=Transurethral resection of the prostate, CCI=Charlson Comorbidity Index, mpMRI=Multiparametric magnetic resonance imaging
Development of preoperative prediction model and nomogram
From Table 1, we noted that the following preoperative variables were statistically different between the two groups, i.e., age, PSA, CCI, clinical staging (using DRE and mpMRI prostate), biopsy tumor grade, and D’Amico risk stratification. Using the stepwise backward regression analysis, these six variables were analyzed for predicting the quadrifecta outcomes. Finally, a multivariate model including four variables was obtained [Table 2]. A preoperative nomogram including these four variables was then developed [Figure 1].
Table 2.
Multivariate analysis of final selected models for predicting quadrifecta outcomes
| Variables included | OR | 95% CI for OR | P | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
|
| ||||
| Preoperative model | ||||
| PSA | 0.95 | 0.93 | 0.97 | 0.000 |
| CCI | 0.70 | 0.56 | 0.89 | 0.003 |
| Clinical stage | ||||
| T1 | Reference | |||
| T2 | 0.77 | 0.47 | 1.26 | 0.311 |
| T3 | 0.45 | 0.21 | 0.96 | 0.039 |
| Biopsy Gleason score | ||||
| 3+3 (I) | 0.510 | 0.30 | 0.86 | 0.012 |
| >(3+3) (II-V) | Reference | |||
|
| ||||
| Postoperative model | ||||
|
| ||||
| Localized tumor (yes vs. no) | 2.012 | 1.193 | 3.395 | 0.009 |
| Positive lymph node (yes vs. no) | 0.344 | 0.161 | 0.737 | 0.006 |
| ISUP grade pathological (Grade 1) | Reference | 0.015 | ||
| ISUP grade pathological (Grade 2) | 0.452 | 0.221 | 0.923 | 0.029 |
| ISUP grade pathological (Grade 3) | 0.566 | 0.255 | 1.259 | 0.163 |
| ISUP grade pathological (Grade 4) | 0.266 | 0.108 | 0.653 | 0.004 |
| ISUP grade pathological (Grade 5) | 0.146 | 0.036 | 0.592 | 0.007 |
| Prebiopsy PSA (continuous) | 0.971 | 0.950 | 0.992 | 0.007 |
| CCI (continuous) | 0.721 | 0.561 | 0.926 | 0.011 |
PSA=Prostate-specific antigen, CCI=Charlson Comorbidity Index, ISUP=International Society of Urological Pathology, CI=Confidence interval, OR=Odds ratio
Figure 1.
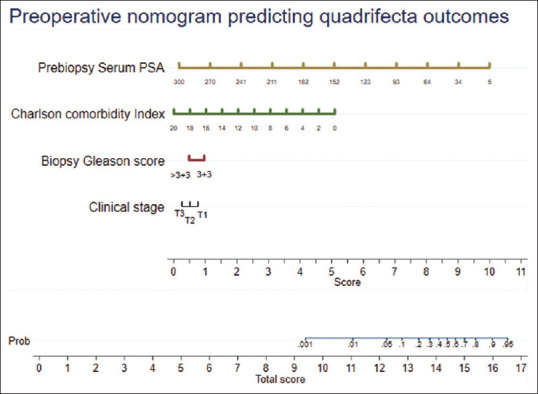
Preoperative nomogram predicting quadrifecta outcomes following robot-assisted radical prostatectomy
Development of postoperative prediction model and nomogram
Eleven variables were considered for the construction of the postoperative prediction model (age, PSA, CCI, previous TURP, clinical stage, biopsy ISUP grade, D’Amico risk classification, RP specimen ISUP grade and stage, positive lymph nodes, and nerve sparing). Finally, a model including five variables (prebiopsy serum PSA, CCI, pathological stage of tumor [RP specimen], ISUP grade of RP specimen, and positive lymph nodes on the final histopathology) was selected using the stepwise backward regression analysis. Multivariate logistic regression analysis, including these five variables, was run for developing the prediction model [Table 2]. A nomogram including these variables was developed for predicting the quadrifecta outcomes [Figure 2].
Figure 2.
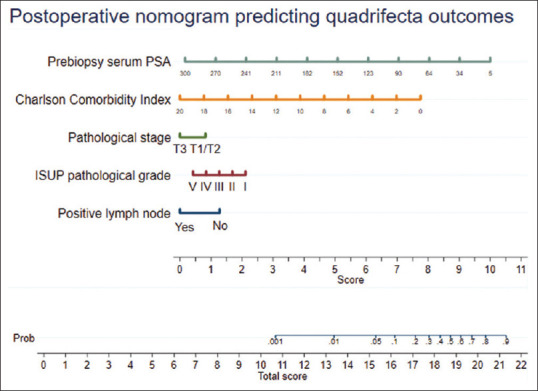
Postoperative nomogram predicting quadrifecta outcomes following robot-assisted radical prostatectomy
Validation of prediction model
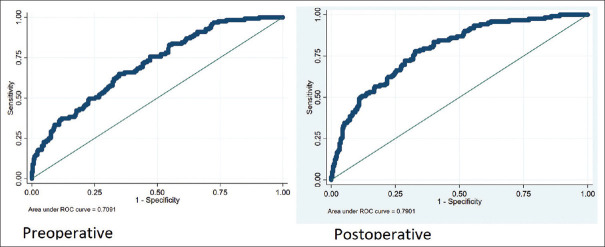
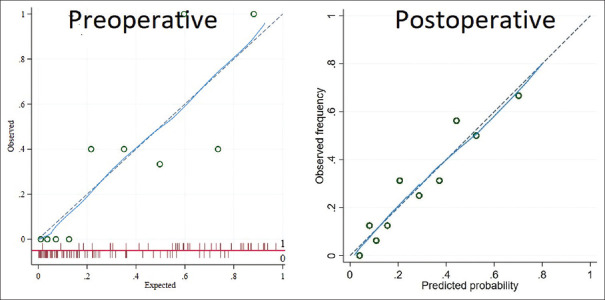
Preoperative and postoperative prediction models had an AUC of 0.71 and 0.79, respectively, in predicting the quadrifecta outcomes [Figure 3]. Bootstrapping of the models and Hosmer–Lemeshow goodness of fit showed that the models were valid. The calibration plot revealed good agreement between the predicted and the observed probabilities [Figure 4] for both the models. DCA for both the models revealed that the models were clinically useful at a threshold probability >0.05 [Figure 5].
Figure 3.
Receiver operating curve analysis depicts area under the curve for both the nomograms
Figure 4.
Calibration plot depicting agreement between the predicted and the observed probabilities for both the models
Figure 5.
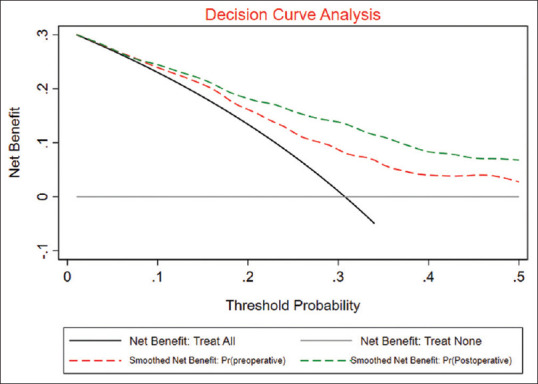
Decision curve analysis for preoperative and postoperative models depicting their clinical utility in predicting quadrifecta outcomes
DISCUSSION
RP is one of the standard treatments for the management of localized PC with up to 90% 5-year disease-free survival.[3] Similar oncological and functional outcomes have been noted with radiation therapy and surgical treatment. Hence, the choice of the therapy rests upon the patient in a given situation. Therefore, the ability to predict favorable outcomes following the surgical management for PC could guide the decision-making in a given case. Trifecta and pentafecta outcomes have been previously proposed as quality gauges for assessing the outcomes following RP. Various factors have been identified as predictors in different populations and different surgical modalities (open or robotic). In the present study, we developed clinically relevant nomograms for preoperative and postoperative prediction of the quadrifecta outcomes using various factors. Both the nomograms showed good fit statistics and the calibration plots showed good agreement between the predicted and the observed probabilities. Furthermore, both the study models had moderate diagnostic accuracy with an AUC of 0.71 and 0.79 in predicting the quadrifecta outcomes. Thus, both the models showed reasonable accuracy in predicting the quadrifecta outcomes following RARP. Furthermore, we noted that our models were clinically relevant as they showed net clinical benefit at a threshold of 0.05 on DCA.
Multiple studies have been published to identify the predictors of outcomes following RP in patients with PC. Eastham et al., developed a nomogram including six variables, i.e., PSA, clinical stage, Gleason score, erectile function, months from RP, and age at RP.[13] In a study by Jazayeri et al., the authors found that only the Gleason score was a predictor of the trifecta and the pentafecta outcomes on the multivariate analysis following RARP in patients with PC.[10] In a similar study by Patel et al., the authors noted that age alone was able to predict the pentafecta outcomes following RARP on the multivariate analysis.[8] In another study by Novara et al. in patients with PC, the authors noted that the preoperative International Index of Erectile Function (IIEF) score and the age were the predictors of the trifecta outcomes.[12] Similar to these previously published studies, we also found the serum PSA, pathological stage, grade, and the lymph node status to be the independent predictors of the quadrifecta outcomes. However, unlike these studies, age at RP is not a part of either of our two prediction models, as on the univariate analysis, there was no statistical difference between the two groups for age. Instead, we found CCI to be a predictor of the quadrifecta outcomes following RARP. CCI has been identified as a predictor of survival in various malignancies, including the prostate cancer.[23] However, for the trifecta or the pentafecta outcomes, very few studies have evaluated this variable.
In the present study, we noted that BCRFS was the least satisfied variable, with about 45% of the patients having BCR within a minimum of 1 year of follow-up. These rates of BCR are higher than those reported by other studies noted previously. However, it is to be pointed out that the patient cohort of our study differs remarkably from the Western literature. First, nearly two-third of the patients in our study belonged to the high-risk category, in contrast to other studies. Second, a substantial number of patients had locally advanced disease (63.7%) at the final pathological analysis. Third, the median initial PSA noted in the present study (median: 14.7 ng/ml) was higher than the previous studies (about 6 ng/ml). Hence, the higher BCR rates noted in our cohort could be due to the advanced disease status at the baseline. Similar argument could also be applied to the higher rates of margin positivity found in the present study (33.3%). Complication rates, both overall and major, as noted in the present study compare well with the other studies on RARP. Rates of continence found in the present study compare well with the other studies (81% with minimum 1-year follow-up) in the RARP literature. Various factors have been associated with poor continence-related outcomes such as advanced age, longer operative time, previous history of TURP, obesity, urethral wall thickness, and length.[24,25,26] Of the above mentioned variables, we have considered age, BMI, and previous history of TURP as the possible predictors of the quadrifecta outcomes.[15,27,28] On exploratory analysis, we noted that of all the four factors included in the quadrifecta, higher CCI was significantly associated with poor urinary continence outcomes. As previously mentioned, various factors have been identified as independent predictors of continence, including various comorbidity scales such as the CCI and the American Anesthesiologist Association (ASA grading).[27,28] CCI is a comprehensive tool used to define a patient's medical comorbidities.[15,28] However, which comorbidity, among the included comorbidities, is most relevant to the urinary continence is difficult to define.
Rates of trifecta and pentafecta outcomes following RP have varied between 57%–83% and 60.4%–70.4%, respectively, in the various studies. Compared to these studies, the quadrifecta outcomes noted in our study were much lower at 30.8% at a median follow-up of 21 months. Again, this could be attributed to the fact that the majority of the patients in our series were high risk and locally advanced as compared to the previously mentioned studies. Second, for urinary incontinence, we used a strict zero pad criteria. Finally, as noted earlier, most of the patients in our series were sexually inactive and the baseline erectile function has been reported to be a predictor of the trifecta outcomes. In our patient cohort, we noted that most of the patients were not sexually active at the baseline, therefore, we decided to exclude the potency outcomes from the originally proposed pentafecta outcomes. However, we understand that sexual function is an essential functional and the least satisfied parameter following RP. In a study by Inoue et al., the authors performed a longitudinal analysis of trifecta outcomes at 1, 3, 6, and 12 months following RARP in patients with PC.[9] The authors noted that potency was the least satisfied parameter of the trifecta items and even in the patients where nerve sparing was possible, potency rates remained low. This remains one of the significant limitations of our study and the nomograms developed herein.
Limitations
There are some limitations of this study worth mentioning. First, the nomogram developed here have been generated from a single-center study with a limited number of patients. Second, the nomograms developed by us are only internally validated and lacks external validation. Third, as previously mentioned, exclusion of the sexual outcomes from the quadrifecta remains one of the significant limitations. Another limitation of this study is that we decided to include patients who had received neoadjuvant hormonal therapy in the final analysis. Neoadjuvant hormonal therapy can influence many factors of the quadrifecta such as the BCR and the margin positivity rate. However, the use of neoadjuvant hormonal treatment is not our standard practice. All these patients had received neoadjuvant hormonal treatment prior to referral. We decided to include these patients for the final analysis as this may represent the current practice scenario for prostate cancer in our country. Due to the higher number of locally advanced prostate cancers, such patients may be given hormonal treatment by the local practitioners before referral to a higher center. Finally, for defining incontinence rates, we used a subjective method of the absence of use of pad rather than a validated scale for incontinence. Furthermore, the follow-up period in our study for BCR was relatively short (median 25 months).
CONCLUSIONS
With this study, we developed and internally validated preoperative and postoperative nomograms for predicting the quadrifecta outcomes following RARP in patients with prostate cancer. Both the models were fairly accurate in predicting the quadrifecta outcomes following RARP in patients with prostate cancer. On DCA, both the models showed net clinical benefit at a threshold probability of 0.05.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer.Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.van Poppel H, Everaerts W, Tosco L, Joniau S. Open and robotic radical prostatectomy. Asian J Urol. 2019;6:125–8. doi: 10.1016/j.ajur.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh I, Hemal AK. Robotic-assisted radical prostatectomy in 2010. Expert Rev Anticancer Ther. 2010;10:671–82. doi: 10.1586/era.10.35. [DOI] [PubMed] [Google Scholar]
- 6.Borregales LD, Berg WT, Tal O, Wambi C, Kaufman S, Gaya JM, et al. ’Trifecta’ after radical prostatectomy: Is there a standard definition? BJU Int. 2013;112:60–7. doi: 10.1111/bju.12002. [DOI] [PubMed] [Google Scholar]
- 7.Patel VR, Abdul-Muhsin HM, Schatloff O, Coelho RF, Valero R, Ko YH, et al. Critical review of ‘pentafecta’ outcomes after robot-assisted laparoscopic prostatectomy in high-volume centres. BJU Int. 2011;108:1007–17. doi: 10.1111/j.1464-410X.2011.10521.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel VR, Sivaraman A, Coelho RF, Chauhan S, Palmer KJ, Orvieto MA, et al. Pentafecta: A new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011;59:702–7. doi: 10.1016/j.eururo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, Hieda K, Hayashi T, Teishima J, Matsubara A. Longitudinal analysis of trifecta outcome in Japanese patients with prostate cancer following robot-assisted laparoscopic radical prostatectomy. World J Urol. 2020 doi: 10.1007/s00345-020-03515-2. [doi: 10.1007/s00345-020-03515-2] [DOI] [PubMed] [Google Scholar]
- 10.Jazayeri SB, Weissman B, Samadi DB. Outcomes following robotic-assisted laparoscopic prostatectomy: Pentafecta and Trifecta achievements. Minerva Urol Nefrol. 2018;70:66–73. doi: 10.23736/S0393-2249.17.02909-5. [DOI] [PubMed] [Google Scholar]
- 11.Ou YC, Yang CK, Kang HM, Chang KS, Wang J, Hung SW, et al. Pentafecta outcomes of 230 cases of robotic-assisted radical prostatectomy with bilateral neurovascular bundle preservation. Anticancer Res. 2015;35:5007–13. [PubMed] [Google Scholar]
- 12.Novara G, Ficarra V, D’Elia C, Secco S, Cavalleri S, Artibani W. Trifecta outcomes after robot-assisted laparoscopic radical prostatectomy. BJU Int. 2011;107:100–4. doi: 10.1111/j.1464-410X.2010.09505.x. [DOI] [PubMed] [Google Scholar]
- 13.Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: The trifecta nomogram. J Urol. 2008;179:2207–10. doi: 10.1016/j.juro.2008.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): A 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–19. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 17.van Leenders GJ, van der Kwast TH, Grignon DJ, Evans AJ, Kristiansen G, Kweldam CF, et al. The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020;44:e87–99. doi: 10.1097/PAS.0000000000001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamhankar AS, Patil SR, Ahluwalia P, Gautam G. Does continuation of low-dose aspirin during robot-assisted radical prostatectomy compromise surgical outcomes? J Endourol. 2018;32:852–8. doi: 10.1089/end.2018.0390. [DOI] [PubMed] [Google Scholar]
- 19.Batra V, Gautam G, Jaipuria J, Suryavanshi M, Khera R, Ahlawat R. Predictive factors for lymph node positivity in patients undergoing extended pelvic lymphadenectomy during robot assisted radical prostatectomy. Indian J Urol. 2015;31:217–22. doi: 10.4103/0970-1591.156918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbin DD, Tamhankar AS, Ahluwalia P, Gautam G. Robot-assisted radical prostatectomy in indian men of age 75 years and above: A propensity score-matched analysis. J Robot Surg. 2021 doi: 10.1007/s11701-021-01301-9. [doi: 10.1007/s11701-021-01301-9] [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp LLC. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. 2019 [Google Scholar]
- 23.Guzzo TJ, Dluzniewski P, Orosco R, Platz EA, Partin AW, Han M. Prediction of mortality after radical prostatectomy by Charlson comorbidity index. Urology. 2010;76:553–7. doi: 10.1016/j.urology.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallami S. Predictive factors of urinary incontinence after radical prostatectomy: Systematic review. Tunis Med. 2017;95:229–35. [PubMed] [Google Scholar]
- 25.Shao IH, Chang YH, Hou CM, Lin ZF, Wu CT. Predictors of short-term and long-term incontinence after robot-assisted radical prostatectomy. J Int Med Res. 2018;46:421–9. doi: 10.1177/0300060517715396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707–13. [PubMed] [Google Scholar]
- 27.Matsushita K, Kent MT, Vickers AJ, von Bodman C, Bernstein M, Touijer KA, et al. Preoperative predictive model of recovery of urinary continence after radical prostatectomy. BJU Int. 2015;116:577–83. doi: 10.1111/bju.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novara G, Ficarra V, D’elia C, Secco S, Cioffi A, Cavalleri S, et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J Urol. 2010;184:1028–33. doi: 10.1016/j.juro.2010.04.069. [DOI] [PubMed] [Google Scholar]