Abstract
Objectives
Inflammatory rheumatic and musculoskeletal diseases (iRMDs) are associated with increased systemic bone loss that is mediated by chronic inflammation, treatment with glucocorticoids (GCs) and other factors. Our objective was to analyse the impact of variables that influence osteoporosis (OP) in patients with iRMD treated with GC.
Methods
Rh-GIOP (acronyme) is a prospective observational cohort study investigating bone health in consecutive patients with iRMD and current or prior GC treatment. We present an analysis of the patients’ baseline data here. Bone mineral density (BMD) measured by dual X-ray absorptiometry was the primary outcome. Multivariable linear regression models were performed to identify variables associated with BMD.
Results
Data from 1066 patients with iRMD were analysed. GC doses of <5 mg prednisone equivalent per day, cumulative dose and duration of GC therapy were not associated with negative effects on BMD. Dosages of ≥5 mg/day lost their negative association with BMD after adjustment for confounders. When subanalysing patients with exactly 5 mg/day, no negative effect was seen. For patients with rheumatoid arthritis (RA), GC doses of >7.5 mg/day showed a negative association with BMD overall, but this effect seemed to be specific only to patients with moderate or high disease activity (Disease Activity Score 28–C reactive protein >3.2).
Conclusions
GCs of ≤5 mg/day did not seem to be associated with a reduction of BMD in patients with iRMD and current or prior exposure to GC. This is most likely due to the dampening of inflammation by GC, which exerts a mitigating effect on the risk of OP. In RA, current GC doses of >7.5 mg/day were negatively associated with BMD, but only in patients with moderate to high disease activity.
Trial registration number
Keywords: glucocorticoids; osteoporosis; outcome assessment, health care; arthritis, rheumatoid
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with inflammatory rheumatic and musculoskeletal diseases (iRMD) have an increased risk of osteoporosis and fragility fractures. The influence of glucocorticoid (GC) therapy on this risk has been controversial for years.
WHAT THIS STUDY ADDS
In this large cross-sectional study of patients previously or currently exposed to GCs, doses of ≤5 mg/day prednisone equivalent did not seem to be associated with negative effects on bone mineral density (BMD).
Higher daily GC dosages lost their negative association with BMD after adjustment for confounding factors.
In patients with rheumatoid arthritis, GC doses of >7.5 mg/day seemed to be negatively associated with BMD only in combination with moderate or high disease activity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
GCs should be used in an optimum dose, titrated with both benefit and harm in mind, in order to achieve remission and to support bone health in patients with iRMD.
Introduction
Glucocorticoids (GCs) exert powerful anti-inflammatory and immunosuppressive effects1 2 and are widely used to treat inflammatory rheumatic diseases (eg, rheumatoid arthritis (RA), vasculitis, lupus and inflammatory myopathies). In addition to their beneficial effects on reducing inflammation, GCs are also associated with many well-known adverse effects. Their use often elicits fierce debates on the benefit–risk profile.3 Among the most worrisome and unwanted effects of GC therapy is osteoporosis (OP).4 Despite the common use of these drugs, now employed for the treatment of inflammatory diseases for more than 70 years, there remain many unanswered questions about their use, such as the following: is there a safe (long-term) dose for bone? what is the dose-dependent effect size of GC therapy on bone health compared with other influencing factors?
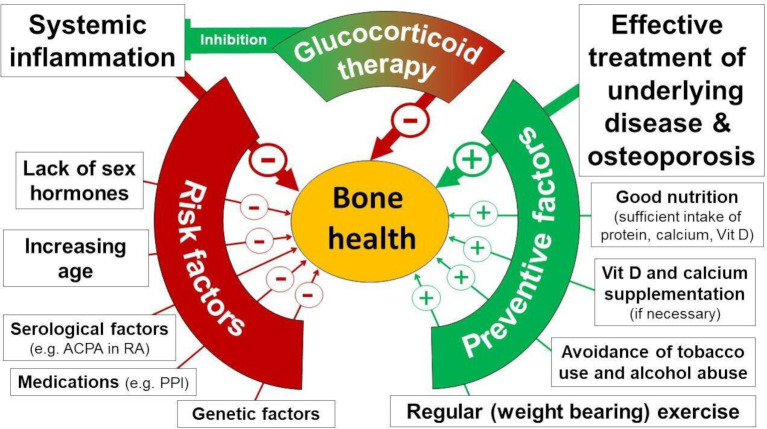
This study focuses on ‘glucocorticoid-induced’ osteoporosis (GIOP), the most common form of secondary OP.5 This condition affects up to one-third of GC-treated patients suffering from inflammatory rheumatic and musculoskeletal diseases (iRMDs).6 The deleterious bone effects of GC at dosages above 10 mg/day of prednisone equivalent for prolonged periods are undisputed.7 In recent years, however, it has been recognised that overall bone health on GC treatment is actually a result not only of the dose and duration of GC treatment, but also of several highly interactive factors that impact the potential for OP both positively and negatively (see also online supplemental box S1).8 9 For instance, inflammation also has deleterious effects and, in turn, is dampened by GC. In patients with iRMD, the net effect of GC treatment is modified by other factors such as inflammatory activity, age, regular exercise (which, in turn, is also determined by disease activity and pain), menopausal status, vitamin D levels and current therapy of both the underlying disease and OP (figure 1).
Figure 1.
Protective and risk factors for osteoporosis-related bone health. This figure illustrates selected factors influencing bone health according to current evidence but is not meant to be exhaustive. + indicates that the factors exert a protective effect on bone; − indicates a negative impact on bone health. Font size reflects presumed importance. ACPA, anticitrullinated protein antibody; PPI, proton pump inhibitor; RA, rheumatoid arthritis.
annrheumdis-2022-222339supp001.pdf (905.4KB, pdf)
This background was the driving force to initiate the Inflammatory Rheumatic and Musculoskeletal Diseases and Glucocorticoid-Induced Osteoorosis (Rh-GIOP) open cohort study in 2015. Its aim is to examine the effects of protective and risk factors contributing to bone health in iRMD in a comprehensive manner, including ‘old’ as well as ‘new’ antirheumatic and antiosteoporotic therapies. We present the results of the first analysis of baseline data including more than 1000 patients.
Methods
Study design and patient involvement
Rh-GIOP is an ongoing single-centre open cohort study designed to collect and analyse disease-related and bone-related data from patients with iRMD with prior or current exposure to GC. We partnered with a patient representative from the Deutsche Rheuma-Liga to centre our research outcomes and questionnaire on patients’ preferences. Patients receiving longitudinal care at the inpatient or outpatient clinic of the Department of Rheumatology and Clinical Immunology of Charité University Medicine are eligible. Data collection started in July 2015, and data are entered into an access database (programmed by Medikadat, Leverkusen, Germany). Five years of data extending through July 2020 are included.
Eligibility criteria
Inclusion criteria include (1) age at least 18 years, (2) clinical diagnosis of an iRMD, (3) current or previous treatment with GC, and (4) eligibility for OP diagnostics as recommended by the Dachverband Osteologie (http://dv-osteologie.org; see online supplemental box S2).
Key exclusion criteria: (1) pregnancy or lactation and (2) inability to provide informed consent for any reason (for full description, see online supplemental table S1).
Data collection
Data collected on each patient are summarised in table 1.
Table 1.
Data collected in each patient (by questionnaire and measurements)
| Demographics and general information | Age, sex, BMI, smoking status, alcohol consumption, type and frequency of physical exercise, exposure to direct sunlight, daily calcium intake, use of care services and socioeconomic status |
| Description of GC therapy | Current GC dose, mean daily GC dose, cumulative (lifetime) GC dose* and duration of GC therapy |
| Description of underlying disease | Onset of disease, current disease activity (DAS28–ESR, DAS28–CRP, CDAI, SDAI, SLEDAI, BASDAI, BASMI, BVAS, concomitant diseases and organ manifestation of iRMD (such as diabetes, hypertension, stroke, cancer, pericarditis in SLE, lung fibrosis in systemic sclerosis, etc), selected patient-reported outcomes (pain according to numerical rating scale, health assessment questionnaire, bath ankylosing spondylitis functional index), and past and current antirheumatic drugs |
| General bone-relevant parameters | Vitamin D and calcium supplementation, treatment with antiosteoporotic drugs, treatment with drugs having a known or potential impact on bone (eg, proton pump inhibitors) |
| Clinical bone-relevant parameters | Family history of osteoporosis/osteoporotic fractures, frailty assessment (timed-up-and-go test, chair-rising test and tandem stand), back pain, prior low-trauma vertebral and non-vertebral fractures,† date of fracture, management of fractures, fracture sequalae, weight loss, loss of height, past falls, risk assessment of falls, back pain, menarche/menopause/pregnancies/lactation/past use of hormone-based contraceptives |
| Technical bone-relevant parameters | Routine laboratory parameters such as calcium, phosphate, vitamin D levels (1, 25 and 25), iPTH, bone alkaline phosphatase, crosslinks and other, BMD/T-score measured by DXA and TBS |
Parameters in italic were retrieved through measurements. All other parameters were assessed through a questionnaire. When patients were not able to provide full or detailed information, patient charts were used to complement the investigated parameters.
*Cumulative GC dose was calculated meticulously from patients’ self-reported dose and duration of GC therapy with the help of supplemental data retrieved from patient charts.
†History of fractures was self-reported and verified from patient charts, if available. Fractures were adjudicated under osteoporotic fractures when having occurred due to inadequate trauma or fall from standing height.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMD, bone mineral density; BMI, body mass index; BVAS, Birmingham Vasculitis Activity Score; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; DAS28, Disease Activity Score 28; DXA, dual-energy X-ray absorptiometry; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; HAQ, Health Assessment Questionnaire; iPTH, intact parathyroid hormone; iRMD, inflammatory rheumatic and musculoskeletal disease; SDAI, Simplified Disease Activity Index; SLEDAI, Systemic Lupus Erythematodes Disease Activity Index; TBS, trabecular bone score.
Bone densitometry
Bone mineral density (BMD) was measured at the lumbar spine and bilateral proximal femur by dual X-ray absorptiometry (DXA). All participants were scanned on Lunar Prodigy bone densitometers (GE Medical Systems Lunar Corporation, Madison, Wisconsin, USA) per manufacturer recommendations and analysed with enCORE Software. The results are presented as T-scores. Scores <−1.0 to >−2.5 were classified as osteopenic (‘low bone mass’) and ≤−2.5 osteoporotic.
Statistical analysis
The primary outcome was BMD expressed by the T-score, more specifically, the lowest (minimum) T-score measured at either the lumbar spine (L1–L4), the left and right femoral neck or total hip. Secondary outcomes were the lowest T-score of the individual lumbar vertebrae (L1–L4) and the lower T-score obtained at the left and right femoral neck. To identify variables associated with the T-score, multivariable linear regression models were formed that included a full set of factors preselected according to published evidence, medical and clinical expertise and subcategorised into factors known to have an impact on bone health (online supplemental table S2). Our aim was data mining but not a specific regression model, to identify variables strongly associated with the respective T-scores out of a large pool of potential factors competing in one model. The full model, including non-significant variables, is reported. In addition, we performed a sensitivity analysis that excluded patients treated with anti-OP drugs (bisphosphonates, denosumab and teriparatide). Collinearity between explanatory variables was ignored. Multiple imputation with 10 replications were used to address missing data. Variables categorised a priori as having weak effects on BMD were excluded if values were missing in more than 30% of the patients. Exceptions were made for alanine transaminase (31% missing), alkaline phosphatase (32%) and urinary deoxypyridinoline (34%) due to their known relevance as laboratory markers for the assessment of bone health.
To explore the impact of current GC dose on the T-score, three commonly discussed GC threshold doses were tested as binary variables in separate multivariable models: <5, ≤5 and ≤7.5 mg/day prednisone equivalent. Subdivisions at doses below 5 mg/day were not feasible due to low case numbers. The models with the lowest cut-off with significant impact for any of the considered T-scores are reported. The following categorical variables were analysed in three models: (1) in crude models without any adjustment; (2) in models adjusted for age, sex, menopause, body mass index (BMI), disease duration, alkaline phosphatase, and the use of denosumab and bisphosphonates; and (3) in models specifically adjusted for those variables retained from the data mining processes after backward selection. The results are displayed in forest plots for comparison. Because the majority of patients received 5 mg/day, another categorisation of current GC dose was analysed in the process described previously with ‘no GC’, ‘>0 mg/day to <5 mg/day’, ‘5 mg/day’ and ‘>5 mg/day’, with a specific focus on 5 mg/day.
Currently, no generic clinical composite score of iRMDs is available to assess the influence of disease activity on BMD. For patients with RA—the largest patient subgroup—the DAS28–CRP as a specific composite score of disease activity was available in 93% of individuals. The interplay of GC dose and disease activity on the lowest T-score was explored with a combination variable of dose and activity in a separate model including all variables that were significant in the prior model selection for patients with RA. Apart from RA, the number of scored patients in individual disease groups was too small to perform a subgroup analysis for associations between composite score and BMD.
In order to investigate the impact of anticitrullinated protein antibodies (ACPAs)/rheumatoid factor (RF), four variants of possible singlets/combinations were considered in separate multivariable linear regression models as described previously (online supplemental table S3): (1) positive ACPA status, (2) positive RF status, defined as either IgA or IgM positivity; (3) double positive, defined as both positive ACPA and RF status; and (4) double negative.
Values are reported as mean/SD for normally distributed data and median/inner quartiles for non-normally distributed continuous variables. Subgroup comparisons were performed by non-parametric tests for continuous variables and χ2 tests for categorical variables. P values lower than 0.05 were considered significant. Given the explorative nature of this study, no adjustment for multiple testing was done. IBM SPSS Statistics V.27.0 was used for analysis.
Results
A total of 1246 patients were enrolled, comprising >95% of eligible patients (ie, those who met inclusion/exclusion criteria after screening). Approximately 60% of the patients enrolled were from the outpatient clinic, and the remainder were from day or in-hospital care. Patients with the following iRMD were included in the current analysis: RA (n=434), connective tissue diseases (CTDs) (n=281), vasculitides (n=173) and spondyloarthritides, including psoriatic arthritis (n=178). Patients in other disease groups, totaling 174, were excluded because of low numbers in any individual disease group. Six patients were excluded because of clinically manifest hyperparathyroidism, leaving 1066 patients for the final analysis. The patients’ age was 62 (±13) years, and 76% were women, of whom 89% were postmenopausal (table 2). Further baseline characteristics are summarised in tables 2–4, and details on disease, treatment and bone health are listed.
Table 2.
Demographics, GC therapy and bone status*†
| All | RA‡ | CTD§ | Vasculitides¶ | Spondyloarthritides** | |
| N=1066 | N=434 | N=281 | N=173 | N=178 | |
| Demographics | |||||
| Age (years) | 62.2 (±13) | 64.2 (±12) | 57.5 (±15) | 67.6 (±12) | 59.4 (±12) |
| Female patients | 806 (76) | 348 (80) | 240 (85) | 115 (67) | 103 (58) |
| Menopause | 706 (89) | 314 (91) | 193 (81) | 111 (97) | 88 (87) |
| BMI (kg/m²) | 27.1 (±5.4) | 27.7 (±5.6) | 25.3 (±5.2) | 26.6 (±4.4) | 28.7 (±5.7) |
| GC therapy | |||||
| Patients with current GC†† therapy | 705 (66) | 311 (72) | 201 (72) | 150 (87) | 43 (24) |
| Current GC dose (mg/day), median (IQ) | 5.0 (5–10) | 5.0 (4–8) | 5.0 (5–10) | 8.2 (5–30) | 10.0 (5–40) |
| ≤2.5 (% of total current GC) | 85 (12) | 48 (15) | 17 (9) | 16 (11) | 4 (9) |
| >2.5–4.9 | 75 (11) | 40 (13) | 20 (10) | 11 (7) | 4 (9) |
| 5.0–7.4 | 285 (40) | 143 (46) | 92 (46) | 41 (27) | 9 (21) |
| 7.5–10.0 | 108 (15) | 41 (13) | 38 (19) | 20 (13) | 9 (21) |
| >10.0 | 152 (22) | 39 (13) | 34 (17) | 62 (41) | 17 (40) |
| Cumulative GC dose (g)‡‡ | 18.2 (±24.7) | 18.0 (±23.8) | 23.4 (±26.3) | 13.9 (±22.6) | 12.9 (±26.0) |
| Duration of GC therapy (years) | 8.2 (±8.8) | 8.7 (±9.1) | 10.5 (±9.1) | 5.0 (±6.2) | 6.1 (±8.2) |
| Bone status (T-score§§) | |||||
| Spine | –0.7 (±1.5) | –0.8 (±1.5) | –1.0 (±1.3) | –0.6 (±1.5) | –0.6 (±1.5) |
| Normal | 512 (51) | 205 (51) | 116 (44) | 91 (56) | 100 (61) |
| Osteopenia | 374 (38) | 157 (39) | 109 (41) | 60 (37) | 48 (29) |
| OP | 107 (11) | 40 (10) | 39 (15) | 11 (7) | 17 (10) |
| Left femoral neck | –1.1 (±1.1) | –1.1 (±1.0) | –1.2 (±1.2) | –1.2 (±1.0) | –0.9 (±1.1) |
| Normal | 395 (41) | 155 (39) | 97 (38) | 53 (34) | 90 (54) |
| Osteopenia | 486 (50) | 196 (50) | 135 (52) | 89 (57) | 66 (39) |
| OP | 94 (9) | 42 (11) | 26 (10) | 14 (9) | 12 (7) |
| Right femoral neck | –1.1 (±1.1) | –1.1 (±1.1) | –1.2 (±1.1) | –1.2 (±1.0) | –0.9 (±1.2) |
| Normal | 395 (41) | 156 (40) | 96 (37) | 58 (37) | 85 (51) |
| Osteopenia | 475 (49) | 193 (40) | 133 (51) | 85 (54) | 64 (38) |
| OP | 101 (11) | 38 (10) | 31 (12) | 14 (9) | 18 (11) |
| Osteoporotic fractures¶¶ | |||||
| Vertebral | 67 (6) | 34 (8) | 12 (4) | 14 (8) | 7 (4) |
| Non-vertebral | 290 (27) | 124 (29) | 70 (25) | 41 (24) | 55 (31) |
*Categorical variables are presented as number and per cent of valid observations (%) unless otherwise noted.
†Continuous variables are presented as mean values with SD unless otherwise noted.
‡RA comprises patients with seropositive and seronegative RA as well as late-onset RA.
§CTDs include patients with systemic lupus erythematosus, progressive systemic sclerosis, limited cutaneous systemic sclerosis, mixed CTD, polymyositis, undifferentiated CTD, antisynthetase syndrome, eosinophilic fasciitis, inclusion body myositis, dermatomyositis, scleroderma with overlap RA and Sjogren’s syndrome.
¶Vasculitides include polymyalgia rheumatica, giant cell arteritis, panarteritis nodosa, microscopic polyangiitis, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, Cogan’s syndrome, anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis and undifferentiated vasculitis.
**Spondyloarthritides include psoriatic arthritis and ankylosing spondylitis.
††GCs include both oral and intravenous application forms of prednis(ol)one, methylprednisolone and modified-release prednisone. All doses are given in prednisone equivalent.
‡‡Cumulative GC dose is an estimate calculated from information provided by the patient with the help of patient charts for the entire duration of GC therapy.
§§BMD and T-score are measured with GE Healthcare Lunar Prodigy DF+15629 dual X-ray absorptiometry scanner. Normal, ≥−1.0; osteopenia, <–1.0; and >–2.5; OP, ≤–2.5.
¶¶History of fractures was self-reported and/or verified from patient charts, if available. In case of clinical suspicion of a vertebral fracture, a conventional X-ray examination was performed. Fractures were adjudicated under osteoporotic fractures when having occurred due to inadequate trauma or fall from standing height.
BMD, bone mineral density; BMI, body mass index; CTD, connective tissue disease; GC, glucocorticoid; IQ, inner quartile; OP, osteoporosis; RA, rheumatoid arthritis.
Table 3.
Risk factors for OP*†
| All | RA | CTD | Vasculitides | Spondyloarthritides | |
| N=1066 | N=434 | N=281 | N=173 | N=178 | |
| Disease activity | |||||
| HAQ score | 0.8 (±0.8) | 0.9 (±0.8) | 0.9 (±0.9) | 0.6 (±0.8) | 0.8 (±0.7) |
| S-CRP mg/L (<5), median (IQR) | 2.3 (0.8–6.6) | 2.4 (0.8–6.7) | 1.6 (0.7–4.9) | 4.8 (1.3–10.9) | 2.0 (0.8–4.9) |
| RA–DAS28–CRP score | 2.7 (±1.3) | ||||
| Disease duration (years) | 11.9 (±10) | 11.9 (±10) | 12.8 (±9) | 5.4 (±6) | 17.2 (±13) |
| Use of care services‡ | 473 (51) | 189 (49) | 147 (59) | 53 (36) | 84 (57) |
| Age (years) | |||||
| Age group | |||||
| <50 | 164 (15) | 43 (10) | 80 (9) | 12 (7) | 29 (16) |
| 50–64 | 427 (40) | 180 (42) | 103 (27) | 50 (29) | 94 (53) |
| 65–84 | 458 (43) | 203 (47) | 97 (25) | 107 (62) | 51 (29) |
| ≥85 | 17 (2) | 8 (2) | 1 (<1) | 4 (2) | 4 (2) |
| Underweight (BMI <18.5 kg/m²) | 28 (3) | 8 (2) | 18 (6) | 2 (1) | 0 |
| Family history | |||||
| OP | 212 (27) | 100 (30) | 54 (25) | 22 (19) | 36 (33) |
| Osteoporotic fractures | 101 (13) | 44 (14) | 28 (14) | 13 (11) | 16 (14) |
| Comedication | |||||
| Proton pump inhibitors | 468 (44) | 175 (40) | 138 (49) | 98 (57) | 57 (32) |
| NSAIDs | 249 (23) | 117 (27) | 45 (16) | 14 (8) | 73 (41) |
| Antidepressants | 75 (7) | 18 (4) | 38 (14) | 6 (4) | 13 (7) |
| Oral antidiabetics | 61 (6) | 26 (6) | 4 (1) | 11 (6) | 20 (11) |
| Insulin | 49 (4) | 19 (4) | 9 (3) | 9 (5) | 12 (7) |
| Antihyperuricaemic drugs | 42 (4) | 18 (4) | 9 (3) | 8 (5) | 7 (4) |
| Oestrogens (female patients only) | 17 (2) | 6 (1) | 7 (3) | 0 | 5 (5) |
| Concomitant diseases§ | |||||
| Osteoarthritis | 153 (14) | 79 (18) | 38 (14) | 10 (6) | 26 (15) |
| Diabetes | 130 (12) | 56 (13) | 16 (6) | 26 (15) | 32 (18) |
| Dyslipidaemia | 119 (11) | 43 (10) | 32 (11) | 25 (15) | 19 (11) |
| Depression | 94 (9) | 39 (9) | 27 (10) | 11 (6) | 17 (10) |
| Renal insufficiency | 76 (7) | 21 (5) | 22 (8) | 25 (15) | 8 (5) |
| Hyperuricaemia/gout | 53 (5) | 23 (5) | 12 (4) | 8 (5) | 10 (6) |
*Categorical variables are presented as number and per cent of valid observations (%) unless otherwise noted.
†Continuous variables are presented as mean values with SD unless otherwise noted.
‡Use of care services comprises any level of care received, including low-level support. The latter applied for most patients.
§Concomitant diseases: shown are diseases or medications that are either particularly common and/or variables considered to have a ‘weakly expected’ impact on the T-score. To avoid overfitting, diseases or medications were not considered in our model when case numbers were low (such as history of transplantation, chronic obstructive pulmonary disease, antiepileptic therapy, heart failure, aromatase inhibitors and hypogonadism).
BMI, body mass index; CTD, connective tissue diseases; HAQ, Health Assessment Questionnaire; NSAID, non-steroidal anti-inflammatory drug; OP, osteoporosis; RA, rheumatoid arthritis; S-CRP, serum C reactive protein.
Table 4.
Factors with a confirmed or potential anti-OP effect and bone turnover markers*†
| All | RA | CTD | Vasculitides | Spondyloarthritides | |
| N=1066 | N=434 | N=281 | N=173 | N=178 | |
| Treatment of underlying disease | |||||
| csDMARDs‡ | 637 (60) | 288 (66) | 210 (75) | 81 (47) | 58 (33) |
| Biologics | 313 (29) | 154 (36) | 38 (14) | 24 (14) | 97 (55) |
| TNF-alpha antagonists§ (n, % of total biologics) | 134 (43) | 76 (49) | 1 (3) | 0 | 57 (59) |
| IL-6R antagonists¶ | 47 (15) | 33 (21) | 4 (11) | 10 (42) | 0 |
| Rituximab | 57 (18) | 25 (16) | 18 (47) | 14 (58) | 0 |
| Abatacept | 23 (7) | 20 (13) | 1 (3) | 0 | 2 (2) |
| IL-17 and IL-12/23 antagonists** | 38 (12) | 0 | 0 | 0 | 38 (39) |
| Belimumab | 15 (5) | 0 | 15 (40) | 0 | 0 |
| tsDMARDs†† | 26 (2) | 18 (4) | 1 (<1) | 0 | 7 (4) |
| Antiosteoporotic therapy | |||||
| Vitamin D supplementation | 865 (81) | 365 (84) | 250 (89) | 144 (83) | 87 (49) |
| Calcium supplementation | 51 (5) | 24 (6) | 18 (6) | 6 (4) | 3 (2) |
| Bisphosphonates‡‡ | 124 (12) | 60 (14) | 31 (11) | 29 (17) | 4 (2) |
| Denosumab | 32 (3) | 13 (3) | 10 (4) | 6 (4) | 3 (2) |
| Teriparatide | 2 (<1) | 2 (1) | 0 | 0 | 0 |
| Strontium ranelate | 1 (<1) | 0 | 1 (<1) | 0 | 0 |
| Behavioural | |||||
| Sun exposure (>30 min/day) | 490 (47) | 218 (51) | 111 (40) | 82 (49) | 79 (44) |
| Non-smoker (never) | 540 (51) | 214 (50) | 171 (61) | 85 (50) | 70 (39) |
| Former smoker | 347 (33) | 138 (32) | 71 (25) | 72 (42) | 66 (37) |
| Active smoker§§ | 171 (16) | 78 (18) | 38 (14) | 13 (7) | 42 (24) |
| No Alcohol consumption | 487 (46) | 216 (51) | 141 (50) | 72 (43) | 58 (33) |
| Regular physical exercise | 658 (63) | 257 (61) | 173 (63) | 113 (67) | 115 (67) |
| Laboratory tests | |||||
| S-25-hydroxy vitamin D (nmol/L) (50–150), median (IQR) | 80.0 (61–97) | 78.2 (62–96) | 85.8 (67.7–103) | 86.4 (71.0–97.6) | 67.7 (49.8–6.8) |
| Vitamin D deficiency¶¶ | 123 (14) | 50 (11) | 29 (13) | 11 (8) | 43 (25) |
| S-osteocalcin (ng/mL) (11.0–46.0) | 12.3 (8–18) | 12.6 (9–17) | 11.8 (8–17) | 9.9 (7–16) | 14.6 (11–21) |
| S-BAP (µg/L) (5.5–38.0) | 16.9 (13–21) | 17.2 (14–22) | 15.3 (12–20) | 15.0 (11–19) | 19.3 (16–25) |
| S-AP (U/L) (35–130) | 66 (66–81) | 67 (56–82) | 61 (50–75) | 64 (54–84) | 70 (60–86) |
| Gamma-GT (U/L) (5–61) | 24 (17–39) | 23 (16–36) | 23 (15–35) | 29 (19–48) | 24 (17–44) |
| Urinary deoxypyridinoline (nmol/L) (<64) | 43 (23–76) | 48 (25–81) | 35 (17–76) | 39 (18–59) | 47 (27–82) |
* Continuous variables are presented as mean values with SD unless otherwise noted.
† Categorical variables are presented as number and per cent of valid observations (%) unless otherwise noted.
‡csDMARDs include azathioprine, chloroquine, ciclosporin, cyclophosphamide, hydroxychloroquine, leflunomide, methotrexate, mycophenolate mofetil and sulfasalazine.
§TNF-alpha antagonists include adalimumab, certolizumab, etanercept, infliximab and golimumab, both originator products as well as biosimilars.
¶IL-6R antagonists include tocilizumab and sarilumab.
**IL-17 and IL-12/23 antagonists include secukinumab, ixekizumab, guselkumab, brodalumab and ustekinumab.
††tsDMARDs include tofacitinib, baricitinib and apremilast.
‡‡Bisphosphonates include alendronate, ibandronate, risedronate, pamidronic acid and zoledronate.
§§Active smoking is a known risk factor for OP and is only listed in this table for completeness of information.
¶¶Vitamin D deficiency is defined as serum 25-hydroxy vitamin D level below the lower range of normal <50 nmol/L.
csDMARD, conventional synthetic disease-modifying antirheumatic drug; CTD, connective tissue disease; Gamma-GT, gamma-glutamyltransferase; IL, interleukin; OP, osteoporosis; RA, rheumatoid arthritis; S-AP, serum alkaline phosphatase; S-BAP, serum bone alkaline phosphatase; S-CRP, serum C reactive protein; TNF, tumour necrosis factor; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
Two-thirds were taking GC at baseline; the median daily dose was 5 mg. OP, as indicated by the lowest measured T-score, was present in 22% of patients and osteopenia in 49%; 31% had fragility fractures. Of note, 24% of the latter group had normal T-scores; 44% were osteopenic; and 32% osteoporotic. Overall, 43% had OP (12% OP by DXA, 21% by fragility fracture and 10% by both).
Prevalence of OP risk modifiers
Most patients had low CRP levels (median 2.3 mg/L, normal <5), suggesting low systemic inflammatory activity. In patients with RA, mean DAS28–CRP score was 2.7 (±1.3). Disease duration in the entire cohort was 12 (±10) years, with mild to moderate disability (mean HAQ score 0.8, table 3). Fifty-one per cent required (mostly low-level) support from care services. Some patients were on antirheumatic or antiosteoporotic drugs at baseline; others were not (table 4).
Factors associated with BMD
Age, male sex, menopause and HAQ were negatively associated with T-scores. For laboratory parameters, only alkaline phosphatase (negative) and gamma-GT levels (positive) were associated with T-scores (all patients, online supplemental table S4A; patients with RA, online supplemental table S4B; sensitivity analysis excluding patients on anti-OP drugs, online supplemental table S4C).
Treatment with antiosteoporotic medication was strongly associated with low T-scores at any site, with regression coefficients of −0.42 for denosumab and −0.45 for bisphosphonates. Prior vertebral (−0.39) and non-vertebral fractures were associated with low BMD, with the latter, however, only at the femoral neck (−0.53). Non-steroidal anti-inflammatory drugs were positively associated with T-scores (+0.10) at the femoral neck, while proton pump inhibitors (PPIs) were negatively associated with the lowest T-score (−0.19) at all sites measured (online supplemental table S4A).
All variables emerging in the three models were confirmed in regression analyses with backward selection except for diabetes and calcium supplementation for lumbar T-score (online supplemental table S5).
Of note, disease-modifying antirheumatic drug (DMARD) use, including biological, conventional synthetic and targeted synthetic agents, was not associated with an impact on BMD.
Impact of GCs on BMD
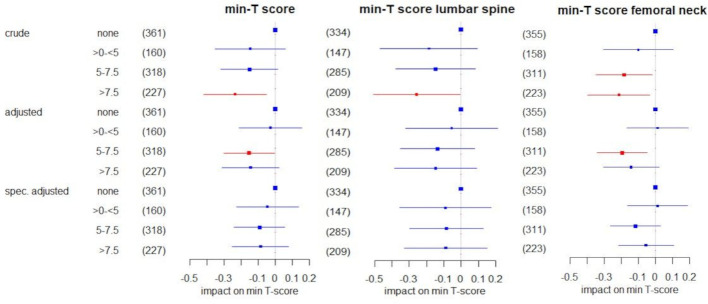
In the crude analysis of the effect of the current GC dose categorised as no GC, >0 mg/day to <5 mg/day, ‘5.0–7.5 mg/day’ and ‘>7.5 mg/day’, we found no differential effects on T-scores between patients on current GC at doses below five and patients not on GC. There were negative effects, however, in patients on dosages >7.5 mg/day (figure 2). At the femoral neck, this effect was already noticeable at dosages of 5.0–7.5 mg/day. However, after adjustment for age, sex, menopause, BMI, disease duration, alkaline phosphatase, and the use of denosumab and bisphosphonates, this effect was seen only for dosages of 5.0–7.5 mg/day (for min- and min-/T-score femoral neck). When further optimising adjustment to include only significant variables for the respective score (online supplemental table S5), the effect estimates shifted even closer to 0, suggesting no meaningful impact of any GC dose (figure 2).
Figure 2.
Impact of the current GC dose on the lowest (min) T-score in all patients in linear regression using (1) a crude model only including GC categories; (2) a multivariable model adjusted for age, sex, menopause, body mass index, alkaline phosphatase, disease duration, bisphosphonates and denosumab; and (3) a multivariable model specifically adjusted for the variables that emerged in the data mining process and were confirmed with backward selection for the respective T score (compare online supplemental table S5). The regression coefficient β and respective 95% CIs are shown. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For ‘no GC’ as the reference group, no coefficient was estimated. GC, glucocorticoid.
Similar results were obtained with the GC dose categorisation no GC, >0 mg/day to <5 mg/day, 5 mg/day and >5 mg/day. In the crude model the difference in T-score between 5 mg/day compared with no GC use was significant only at the femoral neck, persisting after predefined adjustment but disappearing after specified adjustment.
As described in table 1, we also quantified the GC therapy by estimating the cumulative dose and duration of GC therapy. These did not show strong associations with T-scores.
Since approximately 15% of patients in our cohort received anti-OP drugs at baseline (mostly bisphosphonates), we performed a sensitivity analysis that excluded patients with anti-OP drugs. This did not change our findings and conclusions (online supplemental table S4C).
Impact of GCs, ACPA or RF, and the use of DMARDs on BMD in patients with RA
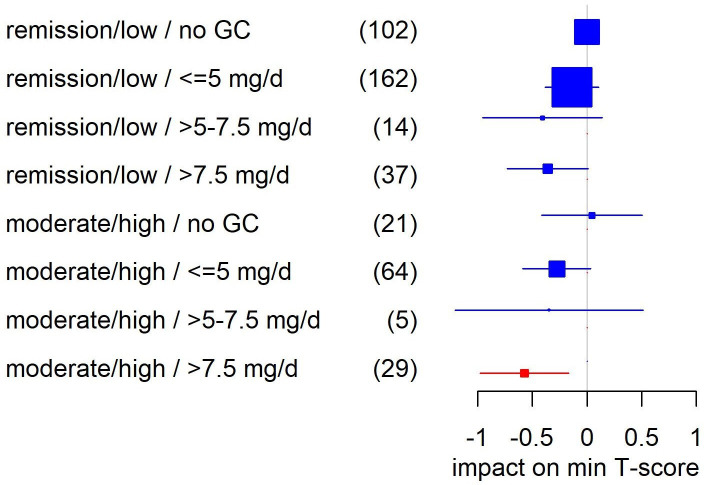
In patients with RA, current GC doses of >5 mg per day had a significant negative association with the lowest overall (−0.49) and lumbar spine T-score (−0.77), together with age, menopause, BMI, alkaline phosphatase, bisphosphonates, disease duration, denosumab and male sex (compare online supplemental table S4B). These results were confirmed at the higher cut-off of >7.5 mg/day (−0.60 for the lowest T-score and −0.90 at the lumbar spine). However, in the interaction analysis of GC with disease activity, T-scores seemed to be only negatively affected in patients with moderate to high disease activity and current GC doses of >7.5 mg/day (figure 3). In other words, doses of 5 mg/day or above did not seem to be associated with lower T-scores in patients either in remission or with low disease activity. In a specific examination of the 5 mg/day group—the largest subgroup—no negative effects were seen at any disease activity level (remission/low −0.12, p=0.38, n=93; moderate/high −0.35, p=0.051, n=45).
Figure 3.
Impact of the interaction of disease activity and current GC dose on the lowest (min) T-Score in patients with rheumatoid arthritis in multivariable linear regression. Adjusted for age, menopause, body mass index, alkaline phosphatase, bisphosphonates, disease duratio, denosumab and male sex (compare online supplemental table S4B). Shown are regression coefficients β and respective 95% CIs. Significant coefficients are highlighted in red. Yhe size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For ‘remission/low/no GC’ as the reference group, no coefficient was estimated. GC, glucocorticoid; min, minimum.
No impact of seropositivity for ACPA or RF on BMD was found for any of the combinations explored in patients with RA (online supplemental table S2).
Discussion
In this study of patients with rheumatic disease with prior GC exposure, current GC doses of ≤5 mg/day prednisone equivalent did not seem to be associated with deleterious effects on BMD. For higher GC doses, crude models showed negative associations with lower BMD measured as lowest (minimum) T-score either at the lumbar spine and/or femoral neck and/or total hip. However, after adjusting for age, sex, menopause, BMI, disease duration, alkaline phosphatase, and the use of denosumab and bisphosphonates, these associations disappeared.
GC usage is seen as the main culprit for OP in iRMD.10 Indeed, a multitude of observational studies have found correlations between current GC use and both low BMD and fracture incidence.11–13 One report, however, suggested that prednisone was beneficial for femoral neck BMD in patients with RA with concomitant adalimumab therapy.14 However, observational research in GC, including our research here, is highly susceptible to confounding/bias by indication.15 16 In other words, we know that active inflammation itself deteriorates bone health, and patients with the most active disease are precisely the patients most likely to be treated with high doses of GCs.9 17–19 Thus, a patient’s inherent inflammatory state may confound an accurate determination of the specific effects of GC. Even if disease activity is recorded and adjusted for, confounding may persist because one patient may need a higher dose of GC to remain at a certain disease activity level than another, and the motivation for a certain dose is usually not recorded. In our study, we attempted to address this problem by broad and accurate data collection and by adjusting for as many relevant influencing factors as possible.
In RA, bone loss occurs even before clinical onset,20 and the risk of hip and vertebral fracture is doubled merely by the presence of RA (without GC).21 Previous work demonstrated that small elevations of C reactive protein significantly increased the risk of non-traumatic fractures.22 Therefore, in observational research, disentanglement of the effects of disease activity and GC (dose) is challenging if not impossible, despite adjustment for these variables in statistical models. Pragmatic trials can solve the confounding problem but cannot be continued long enough to provide full information on long-term effects. Observational studies complement clinical trials and may approach the truth if they feature prospective, high-quality data collection and analyses. Ideally, such observational studies include detailed information on dosing over time and documentation of the motivation for a certain dose and dose changes. The hurdles to achieving such standards in observational studies are substantial.
In our patients with RA, daily intake of higher GC doses seemed to be associated with lower T-scores only in the presence of moderate or high disease activity. Furthermore, our data suggest that in the presence of remission or low disease activity, there is no association between GC dose and low T-scores. These results are consistent with findings from previous studies that also failed to identify links between current or cumulative GC dose and bone loss, vertebral fractures or trabecular bone scores in chronic inflammatory disease.23–26 In contrast, Tong et al recently reported the cumulative GC dose to be associated with vertebral osteoporotic fractures in patients with RA27 but did not properly adjust for factors with (potential) influence on bone health. Other studies confirmed the association between BMD loss and disease activity.28 29 Trials offer unconfounded observations. In these, low-dose prednisone has clearly been shown to provide a safe and more sustainable disease control in conjunction with biological DMARDs compared with biological DMARD regimens that do not include simultaneous, continuous GC treatment.30 Low-dose prednisone also prevents or slows radiographic progression in patients with RA.31–33 In the recent Glucocorticoid Low-dose Outcome in Rheumatoid Arthritis Study (GLORIA), in which patients with RA aged 65+ were treated with prednisolone 5 mg/day or placebo for 2 years, these beneficial effects were confirmed without relevant effects on bone health (Boers et al, Ann Rheum Dis, in press). Current recommendations in rheumatology agree that the treatment goal in iRMD should be remission. GCs continue to play an important role, but their dosing must be optimised, that is, titrated with a view toward both benefit and harm. In other words, the dose of GCs should be as high as necessary but as low as possible, and the dose must be re-evaluated frequently with an eye toward optimisation. This approach achieves the dual goals of tempering inflammation towards remission while supporting bone health. Thus, at these low dosages, the anti-inflammatory effects of GC can potentially counter their negative effects on BMD.29
There is no optimal parameter for homogeneous measurement of systemic inflammatory activity across all iRMDs, but measurement of CRP allows a generic and feasible estimate. It is, however, not perfect because (1) disease activity is not always reflected as CRP elevation; and (2) CRP elevation may have other causes. Nevertheless, associations between elevated CRP levels with inflammation-related complications such as cardiovascular disease and OP are well documented. Gulyás et al recently reported in RA and ankylosing spondylitis an inverse correlation of baseline CRP levels with BMD values both at baseline measurement and after 12 months of treatment, suggesting that baseline high-grade inflammation was associated with lower BMD.34
Although RA is included as an independent risk factor in fracture risk calculators such as the FRAX, the complexity of inter-relating factors is often not adequately appreciated or addressed analytically. For instance, bone loss is also a feature of many other iRMDs.35 Moreover, analyses that focus on binary representations of exposure to GC (eg, ≥5 mg/day, yes or no?) oversimplify the question because the dosage categorisation is too rough, and the impact of disease activity is excluded.36 Recent efforts, however, have addressed this situation more definitively. For example, the updated Japanese Society for Bone and Mineral Research guidelines on GC-induced OP evaluate and indeed weight fracture risk and treatment indication according to GC dose categories of <5 mg/day, ≥5 mg/day to <7.5 mg/day and ≥7.5 mg/day.37 The current German Osteoporosis Guidelines also specifically adjust fracture risk assessment in GC users when RA is present (http://dv-osteologie.org).
As another result, we observed in our patients with RA with long-standing disease that seropositivity for ACPA or RF was not associated with negative effects on BMD, confirming recent evidence38 39 and suggesting that ACPA positivity is associated with low BMD in early RA only.39
Our study offers a more thorough understanding of non-GC factors determining bone fragility in patients with iRMD. We found a relatively high prevalence of reduced bone mass and fragility fractures in our cohort, confirming previous epidemiological studies.6 Our study also confirms that older age, menopause, prior vertebral and non-vertebral fractures, high AP levels, and intake of PPIs are important risk factors for OP. Moreover, our targeted data mining approach revealed some new findings. First, in contrast to the well-known risks in postmenopausal women,40 41 we found that men with iRMD in particular had low BMD. As male OP in general remains underdiagnosed and undertreated, our findings suggest we should pay more attention to the bone health of men with iRMD.42 Second, we found that in patients with iRMD, a higher BMI is associated with higher T-scores.
In our cohort, 81% received vitamin D supplementation. Only 14% had vitamin D deficiency, which is lower than would be expected in a random adult German population (about 30%).43 This might have been a relevant factor pertaining to the results of our analysis about risk factors for OP in our cohort.
We did not find an association of DMARD use with an impact on BMD. It should be noted, however, that we included in our multivariable model several protective and potential harmful factors whose strength of influence may be greater than that of DMARD therapy. Second, our data are still limited with regard to subanalyses of patients treated with, for example, anti-TNF or IL-6R blocking agents.
Strengths of this study include a large sample size of patients with a variety of iRMD and prospective state-of-the art collection of a very broad spectrum of data, increasing the level of detail in the analysis. Our study also has some limitations. First, we cannot derive causal relationships from our cross-sectional study. The level of evidence will be improved by longitudinal observations. Second, BMD alone may not fully account for the elevated fracture risk in patients treated with GC as suggested by Van Staa et al.44 BMD is at best a surrogate for fragility fractures. In our study, history of fractures was either self-reported and/or verified from patient charts, if available. In case of clinical suspicion of a vertebral fracture, a conventional X-ray examination was performed. This approach holds the possibility that radiographic morphometric vertebral fractures were missed, which is why we did not consider fractures as a primary outcome parameter in this cross-sectional analysis. Concerning Trabecular Bone Score (TBS), a meta-analysis demonstrated the combination of BMD and TBS to provide a better estimation of fracture risk than BMD (or BMD+FRAX) alone.45 We started TBS measurement in our cohort in July 2019. Consequently, the amount of available data is still too small to allow for meaningful analysis. Third, we conducted a pooled analysis of a variety of iRMDs. While this increased statistical power, the actual benefits and risks of certain factors may vary between diseases. A subgroup analysis of patients with RA was performed and yielded similar results compared with the overall cohort. Subgroup analyses of other diseases were not yet performed due to the rather low numbers of patients.
We conclude that in patients with iRMD, (1) both optimal disease control, optimum GC doses and sufficient OP treatment measures (such as normal vitamin D levels and appropriate use of anti-OP drugs) are essential for bone protection, and (2) low GC dosages (≤5 mg/day), aimed at achieving sustained remission or low disease activity, are likely to be safe in terms of bone health. A final conclusion is that a better term for GIOP might be ‘GC-associated’ OP.
Acknowledgments
We thank Gabriele May and Manuela Jakstadt for their excellent data collection and entering, Kim Nicola Zeiner for clinical support in recruiting and screening patients, Ulrike Liessmann for technical assistance and Peter Böhm for his help as a patient representative.
Footnotes
Handling editor: Josef S Smolen
Contributors: We declare that all authors included on this paper fulfil the criteria of authorship. Concept and planning: FB, EW, DH and CD; supervision: FB; conduct/data collection: EW, DS, SH, TB, RB, GRB, YP and FB; data analyses: FB, EW, DH, CD, MB, G-RRB and JHS; visualisation: FB, EW, DH and CD; writing (draft): FB, EW, DH, AP, CD, MB, GRB and JHS; writing and approving (final manuscript): all authors. Guarantor: FB
Funding: Rh-GIOP (acronyme) was supported by a joint funding of Amgen, Biogen, BMS, Chugai, Generic Assays, GSK, Hexal, Horizon Therapeutics, Lilly, Medac, Mundipharma, Novartis, Pfizer, Roche and Sanofi-Genzyme.
Competing interests: EW reported consultancy fees, honoraria and travel expenses from Medac and Novartis. DH reported receiving travel expenses from Shire. TB received consultancy fees and honoraria from Roche, Novartis, Sanofi and GSK. RB reported receiving consultancy fees, honoraria and travel expenses from Novartis. GRB reported receiving consultancy fees, honoraria and travel expenses from Roche and Sanofi and grant support from Medac. MB received consulting fees from Novartis. JS reported receiving consulting fees from AbbVie, ChemoCentryx, Sanofi, Spruce, Zenas, Bristol-Myers Squib, Sana, Q32Bio, Novartis, Kyverna, Horizon, Steritas and Argenx. CD reported receiving consultancy fees and honoraria from MSD, Pfizer, UCB, AbbVie, Roche, Novartis, Lilly, Sanofi and Galapagos. FB reported receiving consultancy fees, honoraria and travel expenses from Abbvie, Horizon Therapeutics, Pfizer and Roche, and grant support from Horizon Therapeutics, Roche and Abbvie.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethical committee of the Charité Universitätsmedizin Berlin (EA1/367/14). The participants gave informed consent to participate in the study before taking part.
References
- 1. Palmowski Y, Buttgereit T, Buttgereit F. The 70th anniversary of glucocorticoids in rheumatic diseases: the second youth of an old Friend. Rheumatology 2019;58:580–7. 10.1093/rheumatology/key169 [DOI] [PubMed] [Google Scholar]
- 2. Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol 2008;4:525–33. 10.1038/ncprheum0898 [DOI] [PubMed] [Google Scholar]
- 3. Buttgereit F, Bijlsma JW. Glucocorticoids in rheumatoid arthritis: the picture is shaping up. Ann Rheum Dis 2017;76:1785–7. 10.1136/annrheumdis-2017-211187 [DOI] [PubMed] [Google Scholar]
- 4. van der Goes MC, Jacobs JWG, Boers M, et al. Patient and rheumatologist perspectives on glucocorticoids: an exercise to improve the implementation of the European League against rheumatism (EULAR) recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 2010;69:1015–21. 10.1136/ard.2009.114579 [DOI] [PubMed] [Google Scholar]
- 5. Mirza F, Canalis E. Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 2015;173:R131–51. 10.1530/EJE-15-0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossini M, Viapiana O, Vitiello M, et al. Prevalence and incidence of osteoporotic fractures in patients on long-term glucocorticoid treatment for rheumatic diseases: the glucocorticoid induced osteoporosis tool (GIOTTO) study. Reumatismo 2017;69:30–9. 10.4081/reumatismo.2017.922 [DOI] [PubMed] [Google Scholar]
- 7. Strehl C, Bijlsma JWJ, de Wit M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR Task force. Ann Rheum Dis 2016;75:952–7. 10.1136/annrheumdis-2015-208916 [DOI] [PubMed] [Google Scholar]
- 8. Briot K, Geusens P, Em Bultink I, et al. Inflammatory diseases and bone fragility. Osteoporos Int 2017;28:3301–14. 10.1007/s00198-017-4189-7 [DOI] [PubMed] [Google Scholar]
- 9. Güler-Yüksel M, Hoes JN, Bultink IEM, et al. Glucocorticoids, inflammation and bone. Calcif Tissue Int 2018;102:592–606. 10.1007/s00223-017-0335-7 [DOI] [PubMed] [Google Scholar]
- 10. Leipe J, Holle JU, Weseloh C, et al. [German Society of Rheumatology Recommendations for the management of glucocorticoid-induced Osteoporosis. German version]. Z Rheumatol 2021;80:670–87. 10.1007/s00393-021-01028-w [DOI] [PubMed] [Google Scholar]
- 11. Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993–1000. 10.1359/jbmr.2000.15.6.993 [DOI] [PubMed] [Google Scholar]
- 12. Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int 2004;15:323–8. 10.1007/s00198-003-1548-3 [DOI] [PubMed] [Google Scholar]
- 13. van Staa TP, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002;13:777–87. 10.1007/s001980200108 [DOI] [PubMed] [Google Scholar]
- 14. Wijbrandts CA, Klaasen R, Dijkgraaf MGW, et al. Bone mineral density in rheumatoid arthritis patients 1 year after adalimumab therapy: arrest of bone loss. Ann Rheum Dis 2009;68:373–6. 10.1136/ard.2008.091611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boers M. Observational studies on glucocorticoids are harmful! Lupus Sci Med 2017;4:e000219. 10.1136/lupus-2017-000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buttgereit F. Views on glucocorticoid therapy in rheumatology: the age of convergence. Nat Rev Rheumatol 2020;16:239–46. 10.1038/s41584-020-0370-z [DOI] [PubMed] [Google Scholar]
- 17. Chiodini I, Falchetti A, Merlotti D, et al. Updates in epidemiology, pathophysiology and management strategies of glucocorticoid-induced osteoporosis. Expert Rev Endocrinol Metab 2020;15:283–98. 10.1080/17446651.2020.1772051 [DOI] [PubMed] [Google Scholar]
- 18. Dougados M. Comorbidities in rheumatoid arthritis. Curr Opin Rheumatol 2016;28:282–8. 10.1097/BOR.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 19. Briot K, Geusens P, Em Bultink I, et al. Inflammatory diseases and bone fragility. Osteoporos Int 2017;28:3301–14. 10.1007/s00198-017-4189-7 [DOI] [PubMed] [Google Scholar]
- 20. Kleyer A, Finzel S, Rech J, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis 2014;73:854–60. 10.1136/annrheumdis-2012-202958 [DOI] [PubMed] [Google Scholar]
- 21. Cooper C, Coupland C, Mitchell M. Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann Rheum Dis 1995;54:49–52. 10.1136/ard.54.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schett G, Kiechl S, Weger S, et al. High-Sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med 2006;166:2495–501. 10.1001/archinte.166.22.2495 [DOI] [PubMed] [Google Scholar]
- 23. Lems WF, Baak MME, van Tuyl LHD, et al. One-Year effects of glucocorticoids on bone density: a meta-analysis in cohorts on high and low-dose therapy. RMD Open 2016;2:e000313. 10.1136/rmdopen-2016-000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okano T, Inui K, Tada M, et al. High frequency of vertebral fracture and low bone quality in patients with rheumatoid arthritis-Results from tomorrow study. Mod Rheumatol 2017;27:398–404. 10.1080/14397595.2016.1213943 [DOI] [PubMed] [Google Scholar]
- 25. Florez H, Hernández-Rodríguez J, Muxi A, et al. Trabecular bone score improves fracture risk assessment in glucocorticoid-induced osteoporosis. Rheumatology 2020;59:1574–80. 10.1093/rheumatology/kez464 [DOI] [PubMed] [Google Scholar]
- 26. Nowakowska-Płaza A, Wroński J, Sudoł-Szopińska I, et al. Clinical utility of trabecular bone score (tbs) in fracture risk assessment of patients with rheumatic diseases treated with glucocorticoids. Horm Metab Res 2021;53:499–503. 10.1055/a-1528-7261 [DOI] [PubMed] [Google Scholar]
- 27. Tong J-J, Xu S-Q, Zong H-X, et al. Prevalence and risk factors associated with vertebral osteoporotic fractures in patients with rheumatoid arthritis. Clin Rheumatol 2020;39:357–64. 10.1007/s10067-019-04787-9 [DOI] [PubMed] [Google Scholar]
- 28. Book C, Karlsson M, Akesson K, et al. Disease activity and disability but probably not glucocorticoid treatment predicts loss in bone mineral density in women with early rheumatoid arthritis. Scand J Rheumatol 2008;37:248–54. 10.1080/03009740801998747 [DOI] [PubMed] [Google Scholar]
- 29. Kim J-W, Jung J-Y, Kim H-A, et al. Anti-Inflammatory effects of low-dose glucocorticoids compensate for their detrimental effects on bone mineral density in patients with rheumatoid arthritis. J Clin Med 2021;10:2944. 10.3390/jcm10132944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burmester GR, Buttgereit F, Bernasconi C, et al. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet 2020;396:267–76. 10.1016/S0140-6736(20)30636-X [DOI] [PubMed] [Google Scholar]
- 31. Jacobs JWG, van Everdingen AA, Verstappen SMM, et al. Followup radiographic data on patients with rheumatoid arthritis who participated in a two-year trial of prednisone therapy or placebo. Arthritis Rheum 2006;54:1422–8. 10.1002/art.21809 [DOI] [PubMed] [Google Scholar]
- 32. Wassenberg S, Rau R, Steinfeld P, et al. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:3371–80. 10.1002/art.21421 [DOI] [PubMed] [Google Scholar]
- 33. Svensson B, Boonen A, Albertsson K, et al. Low-Dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum 2005;52:3360–70. 10.1002/art.21298 [DOI] [PubMed] [Google Scholar]
- 34. Gulyás K, Horváth Ágnes, Végh E, et al. Effects of 1-year anti-TNF-α therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol 2020;39:167–75. 10.1007/s10067-019-04771-3 [DOI] [PubMed] [Google Scholar]
- 35. Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 2011;365:62–70. 10.1056/NEJMcp1012926 [DOI] [PubMed] [Google Scholar]
- 36. Kanis JA, Johansson H, Oden A, et al. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int 2011;22:809–16. 10.1007/s00198-010-1524-7 [DOI] [PubMed] [Google Scholar]
- 37. Suzuki Y, Nawata H, Soen S, et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for bone and mineral research: 2014 update. J Bone Miner Metab 2014;32:337–50. 10.1007/s00774-014-0586-6 [DOI] [PubMed] [Google Scholar]
- 38. Amkreutz JAMP, de Moel EC, Theander L, et al. Association between bone mineral density and autoantibodies in patients with rheumatoid arthritis. Arthritis Rheumatol 2021;73:921–30. 10.1002/art.41623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wysham KD, Baker JF, Shoback DM. Osteoporosis and fractures in rheumatoid arthritis. Curr Opin Rheumatol 2021;33:270–6. 10.1097/BOR.0000000000000789 [DOI] [PubMed] [Google Scholar]
- 40. Jones G, Nguyen T, Sambrook PN, et al. Symptomatic fracture incidence in elderly men and women: the Dubbo osteoporosis epidemiology study (does). Osteoporos Int 1994;4:277–82. 10.1007/BF01623352 [DOI] [PubMed] [Google Scholar]
- 41. Kanis JA, Johnell O, Oden A, et al. Long-Term risk of osteoporotic fracture in Malmö. Osteoporos Int 2000;11:669–74. 10.1007/s001980070064 [DOI] [PubMed] [Google Scholar]
- 42. Kaufman J-M. Management of osteoporosis in older men. Aging Clin Exp Res 2021;33:1439–52. 10.1007/s40520-021-01845-8 [DOI] [PubMed] [Google Scholar]
- 43. Rabenberg M, Scheidt-Nave C, Busch MA, et al. Vitamin D status among adults in Germany--results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015;15:641. 10.1186/s12889-015-2016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Staa TP, Laan RF, Barton IP, et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003;48:3224–9. 10.1002/art.11283 [DOI] [PubMed] [Google Scholar]
- 45. McCloskey EV, Odén A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 2016;31:940–8. 10.1002/jbmr.2734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2022-222339supp001.pdf (905.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request.