Abstract
Deletion of the citC gene, coding for isocitrate dehydrogenase, arrests sporulation of Bacillus subtilis at stage I after bipolar localization of the cell division protein FtsZ but before formation of the asymmetric septum. A spontaneous extragenic suppressor mutation that overcame the stage I block was found to map within the spoVG gene. The suppressing mutation and other spoVG loss-of-function mutations enabled citC mutant cells to form asymmetric septa and to activate the forespore-specific sigma factor ςF. However, little induction of mother cell-specific, ςE-dependent sporulation genes was observed in a citC spoVG double mutant, indicating that there is an additional defect(s) in compartmentalized gene expression in the citC mutant. These other defects could be partially overcome by reducing the synthesis of citrate, by buffering the medium, or by adding excess MnCl2. Overexpression of the spoVG gene in wild-type cells significantly delayed ςF activation. Increased expression and stability of SpoVG in citC mutant cells may contribute to the citC mutant phenotype. Inactivation of the spoVG gene caused a population of otherwise wild-type cells to produce a small number of minicells during growth and caused sporulating cells to complete asymmetric septation more rapidly than normal. Unlike the case for inactivation of the cell division inhibitor gene minD, many of these minicells contained DNA and appeared only when the primary sporulation signal transduction pathway, the Spo0A phosphorelay, was active. These results suggest that SpoVG interferes with or is a negative regulator of the pathway leading to asymmetric septation.
Spore formation in Bacillus subtilis is a developmental pathway in which sequential, compartmentalized gene expression is achieved by interlocking cascades of regulatory factors and morphological cues (67). The primary environmental signal for initiation of sporulation is nutrient limitation (63), but this same condition also induces other adaptive responses (e.g., genetic competence, degradative enzyme synthesis, chemotaxis and motility, and antibiotic production) characteristic of slowly growing or stationary-phase cells.
The earliest morphological change that distinguishes a sporulating from a nonsporulating stationary-phase cell is the formation of an asymmetrically disposed division septum (48). During exponential growth, B. subtilis cells, like those of most other rod-shaped bacteria, divide exclusively at mid-cell. Mid-cell division requires the assembly at the septation site of a protein complex that includes FtsZ (4, 6), a tubulin-like GTPase (11, 50). Formation of a ring of FtsZ at the site of future septation is a prerequisite for association of other proteins with this site (25) and for septation itself. When a B. subtilis cell initiates sporulation, rings of FtsZ protein form at the two poles of the cell rather than at mid-cell (36). One of these rings becomes the site of asymmetric (polar) septation; the other ring dissociates. Bipolar localization of FtsZ is thought to be mediated by the product of a gene that depends on the Spo0A transcription factor for its expression, since in a spo0A mutant strain, polar rings of FtsZ do not form in stationary phase (36). Another factor required for polar septation is thought to be the product of a gene transcribed by the ςH form of RNA polymerase, since a spo0H (ςH) mutant does not form polar septa even though FtsZ rings assemble at polar sites (36).
Asymmetric septation permits forespore-specific activation of ςF (1, 16, 42), the first step in a cascade of gene expression determined by sequentially active ς factors (67). Soon after ςF becomes active, its activity leads to signals that activate ςE in the mother cell (27, 32, 37), followed by activation of ςG in the forespore (46, 68) and ςK in the mother cell (9, 10, 39).
The nutritional signal that initiates sporulation is unknown but is assumed to be created intracellularly by normal metabolism (63). It has long been known that the enzymes of the Krebs citric acid cycle are induced as cells leave exponential growth phase; activities of the enzymes are required for successful sporulation (18, 21, 54, 72). To investigate the specific roles of Krebs cycle enzymes in spore formation, we have analyzed the stages of sporulation blockage in mutants deficient in various steps of the cycle (8, 29, 31). We found that the absence of the third enzyme, isocitrate dehydrogenase (ICDH), causes a specific block at stage I (31); mutant cells enter stationary phase, organize their chromosomes in an axial filament (as in wild-type cells), and assemble apparently normal rings of FtsZ protein at both poles (31). No polar septation occurs, however. In the accompanying paper (41), we show that abnormally high accumulation of citrate is responsible, at least in part, for this phenotype.
To understand the basis for this stage I block and to identify proteins that may participate in or regulate asymmetric septation, we sought suppressor mutations that would restore polar septation to an ICDH (citC) mutant strain. To do so, we searched for spontaneous pseudorevertants of a citC null mutant in which expression of the ςF-dependent gene spoIIQ (38) was restored. One such mutation proved to be in the spoVG gene (53, 56, 58), whose product was not previously known to affect the pathway leading to asymmetric septation.
MATERIALS AND METHODS
Strains and growth conditions.
The B. subtilis strains and plasmids used in this study are listed in Table 1. Cells were grown in nutrient broth sporulation (DS) medium (19) supplemented with antibiotics (5), if necessary. Sporulation was induced either by nutrient exhaustion in DS broth or by a resuspension method (65). LacZ indicator plates contained 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) at 80 μg/ml. Escherichia coli JM107 (71) was used for construction of plasmids.
TABLE 1.
Bacterial strains and B. subtilis plasmids used in this study
| Strain or plasmid | Genotype or description | Source, reference, or construction |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM107 | endA1 gyrA96 thi hsdR17 (rK− mK+) supE44 relA1 λ− Δ(lac-proAB) e14−/F′ traD36 proAB lacIqlacZΔM15 | Laboratory stock |
| B. subtilis | ||
| JH642 | trpC2 pheA1 | J. A. Hoch |
| FJS107 | trpC2 SPβs | 62 |
| AG918 | trpC2 pheA1 sof-1 cat | A. D. Grossman |
| DZR143 | Δspo0A::erm trpC2 pheA1 | 28 |
| KS265 | spoVG::Tn917ΩHU265 | 56 |
| MB284 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(spoVG′-lacZ) cat] | 43 |
| MO1657 | ΔamyE::[Φ(sspE′-lacZ) cat] trpC2 pheA1 | 20 |
| MO1778 | ΔspoVG::tet ΔspoIIB::kan trpC2 pheA1 | 66 |
| PL33 | minD::pPL6 (cat) | 35 |
| SJB78 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(spoVG′-lacZ) cat] trpC2 pheA1 | MB284 DNA→JH642a |
| SJB219 | ΔcitC::spc trpC2 pheA1 | 31 |
| SJB225 | Φ(spoIID′-lacZ) cat trpC2 pheA1 | 31 |
| SJB229 | Φ(spoIID′-lacZ) cat ΔcitC::spc trpC2 pheA1 | 31 |
| SJB231 | ΔcitZC::spc trpC2 pheA1 | 41 |
| SJB294 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] trpC2 pheA1 | 31 |
| SJB295 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitC::spc trpC2 pheA1 | 31 |
| SC432 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] | S. Cutting |
| SR10 | Φ(spoIID′-lacZ) cat | 52 |
| AS5 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitC::spc ΔspoVG::tet sof-1 cat trpC2 pheA1 | SJB219 DNA→KMB433 |
| AS9 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitC::spc ΔspoVG::tet spo0A+ cat trpC2 pheA1 | SJB219 DNA→KMB434 |
| KMB97 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitC::spc spoVG2 trpC2 pheA1 (mutant of SJB295) | |
| KMB136 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitC::spc spoVG::Tn917ΩHU265 trpC2 pheA1 | KS265 DNA→SJB295 |
| KMB158 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔthrC::(spoVG+ erm) ΔcitC::spc spoVG2 trpC2 pheA1 | pKM56→KMB97 |
| KMB162 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(spoVG′-lacZ) cat] ΔcitC::spc trpC2 pheA1 | MB284 DNA→SJB219 |
| KMB197 | ΔcitC::spc ΔspoVG::tet trpC2 pheA1 | MO1778 DNA→SJB219 |
| KMB198 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitC::spc ΔspoVG::tet trpC2 pheA1 | MO1778 DNA→SJB295 |
| KMB199 | ΔspoVG::tet trpC2 pheA1 | MO1778 DNA→JH642 |
| KMB200 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔspoVG::tet trpC2 pheA1 | KMB199 DNA→SJB294 |
| KMB207 | ΔcitZC::spc ΔspoVG::tet trpC2 pheA1 | MO1778 DNA→SJB231 |
| KMB208 | Φ(spoIID′-lacZ) cat ΔcitC::spc ΔspoVG::tet trpC2 pheA1 | SR10 DNA→KMB197 |
| KMB210 | ΔamyE::[Φ(sspE′-lacZ) cat] ΔcitC::spc ΔspoVG::tet trpC2 pheA1 | MO1657 DNA→KMB197 |
| KMB212 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitC::spc ΔspoVG::tet trpC2 pheA1 | SC432→KMB197 |
| KMB217 | ΔamyE::[Φ(spoIIQ′-lacZ) cat] ΔcitZC::spc ΔspoVG::tet trpC2 pheA1 | SJB294 DNA→KMB207 |
| KMB218 | Φ(spoIID′-lacZ) cat ΔcitZC::spc ΔspoVG::tet trpC2 pheA1 | SR10 DNA→KMB207 |
| KMB219 | ΔamyE::[Φ(sspE′-lacZ) cat] ΔcitZC::spc ΔspoVG::tet trpC2 pheA1 | MO1657 DNA→KMB207 |
| KMB220 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitZC::spc ΔspoVG::tet trpC2 pheA1 | SC432→KMB207 |
| KMB271 | minD::pPL6 (cat) ΔspoVG::tet trpC2 pheA1 | PL33 DNA→KMB199 |
| KMB272 | minD::pPL6 (cat) trpC2 pheA1 | PL33 DNA→JH642 |
| KMB352 | Δspo0A::erm ΔspoVG::tet trpC2 pheA1 | DZR143 DNA→KMB199 |
| KMB353 | Δspo0A::erm minD::pPL6 (cat) trpC2 pheA1 | DZR143 DNA→KMB272 |
| KMB367 | ΔcitZC::spc ΔspoVG::tet spo0A cat trpC2 pheA1 | AG918 DNA→KMB207 |
| KMB377 | ΔcitZC::spc ΔspoVG::tet sof-1 cat trpC2 pheA1 | AG918 DNA→KMB207 |
| KMB398 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitZC::spc ΔspoVG::tet spo0A+ cat trpC2 pheA1 | SC432 DNA→KMB367 |
| KMB399 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔcitZC::spc ΔspoVG::tet sof-1 cat trpC2 pheA1 | SC432 DNA→KMB377 |
| KMB422 | ΔspoVG::tet sof-1 cat trpC2 pheA1 | MO1778 DNA→KMB415 |
| KMB423 | ΔspoVG::tet spo0A+ cat trpC2 pheA1 | MO1778 DNA→KMB416 |
| KMB433 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔspoVG::tet sof-1 cat trpC2 pheA1 | SC432 DNA→KMB422 |
| KMB434 | SPβc2 Δ2::Tn917::pSK10Δ6::[Φ(cotA′-lacZ) cat] ΔspoVG::tet spo0A+ cat trpC2 pheA1 | SC432 DNA→KMB423 |
| Plasmids | ||
| pHP13 | Vector | |
| pJL52 | Helper plasmidb | |
| pKM98 | spoVG+ | |
| pKM157 | spoVG2 |
MB284 DNA was introduced into strain JH642.
Plasmid pJL52 was used to increase the stability of pHP13-derived plasmids in B. subtilis by the method of LeDeaux and Grossman (33).
DNA manipulation and transformation.
Plasmid DNA was isolated from E. coli cells by a modified version of the method of He et al. (26). Other DNA manipulations were done by standard protocols (55). Preparation of electroporation-competent cells of E. coli and transformation with a Bio-Rad GenePulser apparatus (Bio-Rad Laboratories) were performed by the method of Dower et al. (14). Chromosomal DNA from B. subtilis was prepared as described previously (19). Transformation of B. subtilis cells with chromosomal DNA or plasmids was done by the procedure of Dubnau and Davidoff-Abelson (15).
Construction of an integrative genomic library of B. subtilis.
A B. subtilis integrative plasmid, pPS34, and a B. subtilis genomic library were constructed by Pascale Serror (59). pPS34 was created by ligating the 2.8-kb SspI fragment of pBluescript SK(−) (pSK−) (Stratagene, Inc.) to a 1.35-kb erythromycin resistance gene (erm) excised from pJPM9 (43) as a blunt-ended EcoRI-HindIII fragment. The erythromycin cassette was inserted in the same orientation as the lacZ gene of pSK−. For construction of a B. subtilis genomic library, chromosomal DNA from strain FJS107 (Table 1) was digested separately with AluI, HinpI, and HpaII. After HinpI and HpaI fragments were blunt ended, they and the AluI fragments were inserted in separate ligations in the EcoRV site of pPS34. The cloned DNAs were pooled and, in a subsequent transformation, integrated into the chromosome of JH642 by homologous recombination. DNA from the pooled Ermr transformants was prepared for use as a random insertion library.
Transfer of a suppressor mutation.
Competent cells of strain SJB219 (ΔcitC::spc) (Table 1) were transformed with the chromosomal DNA from a spontaneous pseudorevertant, KMB77S3 (ΔcitC::spc ΔamyE::[spoIIQ′-lacZ cat] spoVG2), and chloramphenicol-resistant (Camr) transformants were selected. Most were slightly blue on DS plates containing X-Gal, but some colonies were as dark blue as KMB77S3, indicating that the unlinked spoVG2 mutation and the spoIIQ′-lacZ fusion were cotransferred by congression. One of the congressants, KMB97, was used for subsequent mapping experiments (see Results).
Cloning of the spoVG2 mutant allele.
The spoVG locus of KMB97, located within a 680-bp HindIII fragment, was amplified by PCR with the following primers: 5′-AAGTGATTCTGGGAGAGCCGGGATC-3′ (which anneals about 170 bp upstream of the spoVG initiation codon) and 5′-AGGCTTACCGCAAACTGGATGAAGG-3′ (which anneals about 280 bp downstream of the spoVG termination codon). The amplified DNA fragment (770 bp) was cloned directly into the modified EcoRV site of pT7Blue(R) (Novagen, Inc.). The resulting plasmid (pKM73) was sequenced to confirm that there were no additional mutations other than spoVG2 in the spoVG open reading frame. In order to eliminate possible PCR-derived mutations in the spoVG promoter, the BstXI-SacI fragment containing the spoVG2 mutation was excised and fused to the 5′ end of wild-type spoVG in pKM70 which had been digested with the same enzymes, creating pKM89. To create pKM70, the spoVG-containing 680-bp HindIII fragment from pLS5 (a pBR322 derivative) (64) was inserted at the HindIII site of a version of pSK− with a disrupted BstXI site in its multicloning site.
Overexpression of spoVG and preparation of anti-SpoVG antibodies.
A strain producing a maltose-binding protein (MBP)-SpoVG fusion protein was constructed as follows. A BamHI site was introduced by PCR just upstream of the initiation codon of spoVG, and the amplified fragment was cloned in pT7Blue(R). A 300-bp BamHI fragment from the resulting plasmid was inserted in the proper orientation at the BamHI site of pMAL-c2 (New England Biolabs), a plasmid for construction of MBP fusions, to create pKM102. E. coli JM107 harboring pKM102 was used for overexpression of MBP-SpoVG.
Cells were grown to mid-exponential phase (optical density at 600 nm of 0.5) in 500 ml of L broth supplemented with 0.2% glucose and 100 μg of ampicillin per ml; 1 mM isopropyl-thio-β-d-galactosidase (IPTG) was added to the culture, and incubation was continued for 2.5 h. Cells were harvested, washed once with column buffer (20 mM Tris-HCl buffer [pH 7.4] containing 200 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol, and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]), and resuspended in 10 ml of the same buffer. Cells were disrupted by sonication and centrifuged to remove cell debris. Supernatant fluid was applied to an amylose column (≈5 ml) that had been equilibrated with column buffer. After extensive washing of the column, MBP-SpoVG was eluted with column buffer containing 10 mM maltose. Fractions containing MBP-SpoVG were collected and dialyzed against 20 mM Tris-HCl buffer (pH 8) containing 100 mM NaCl and 2 mM CaCl2. About 30 μg of factor Xa (Boehringer Mannheim) was incubated overnight at 4°C with 9 mg of MBP-SpoVG. The reaction mixture was dialyzed against 20 mM Tris-HCl buffer (pH 8) containing 25 mM NaCl and 0.5 mM PMSF and then centrifuged. The supernatant fluid was subjected to DEAE-Sephacel chromatography to remove factor Xa. SpoVG and MBP-SpoVG coeluted from the DEAE column in a gradient of NaCl (25 to 500 mM). Fractions containing SpoVG and MBP-SpoVG were pooled and subsequently applied to an amylose column to separate SpoVG from residual MBP-SpoVG fusion protein. The flowthrough fraction from each preparation typically contained about 0.75 mg of SpoVG polypeptide.
SpoVG polypeptide (1 mg) was mixed with adjuvant and injected into a female New Zealand White rabbit. Injections and bleedings were done with the assistance of Sada Yaser, Tufts University Animal Care Facility. To remove nonspecific antibodies, the antisera against SpoVG were mixed with a crude extract of a spoVG null mutant and kept on ice for 1 h. After centrifugation, antibodies in the supernatant fluid were partially purified by differential ammonium sulfate precipitation (35 to 50% of saturation).
Immunoblot analysis.
Cells were harvested from a 50-ml culture in DS medium, washed once with 50 mM HEPES buffer (pH 8) containing 1 mM EDTA, 150 mM NaCl, 5 mM MgCl2, 10% glycerol, and 0.5 mM PMSF, and resuspended in 2 ml of the same buffer. Cells were disrupted by sonication followed by centrifugation to remove cell debris. Supernatant fluid (crude extract; 10 μg protein) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrotransferred to an Immobilon-P (Millipore Corp.) membrane. The membrane was exposed to anti-SpoVG antibodies, and the immune complexes were visualized by using color-forming substrates of an alkaline phosphatase-linked secondary antibody (30).
Microscopic analysis.
Fixation of cells and DNA staining with 4,6-diamidino-2-phenylindole (DAPI) were done essentially by the method of Wu et al. (70). Cells were harvested before and after induction of sporulation by the resuspension method (65) and fixed in 70% ethanol. Cell suspensions were kept at 4°C overnight, and subsequently subjected to nucleoid staining (1 μg of DAPI per ml). Samples were observed by using an Olympus BX60 microscope equipped with a C4742-95 digital camera (Hamamatsu). Images were processed with Image Pro Plus version 3.0 (Media Cybernetics) and Adobe Photoshop.
Samples for thin-section electron microscopic analysis were prepared as described previously (31) and visualized by A. Brown-Cormier, Electron Microscopy Unit, Department of Anatomy and Cellular Biology, Tufts University.
Construction of other plasmids.
pKM56 was constructed by inserting the 680-bp HindIII fragment of pLS5 (see above) in the HindIII site of pDG1664, a B. subtilis integration vector for the thrC locus (22).
Plasmid pKM82, a derivative of pSK−, contains the spoVG gene within a HindIII fragment excised from pLS5 (see above). The orientation of the spoVG gene in pKM82 is such that transcription is in the direction opposite that of lacZ. pKM98 was constructed by ligating a PstI-SalI fragment of pKM82 to pHP13 (23), which had been digested with the same enzymes. The spoVG2 allele was removed from pKM89 as a BamHI-SalI fragment and inserted at the corresponding sites of pHP13, creating pKM157.
Other methods.
DNA sequencing was done by using a Sequenase reagent kit (U.S. Biochemical Corp.) or by M. Berne, Tufts University Protein and Nucleic Acid Analysis Facility, using an ABI Automated Sequencer. Direct sequencing of PCR-amplified fragments was performed after treatment of the fragments with shrimp alkaline phosphatase and exonuclease I (U.S. Biochemical Corp.) according to the manufacturer’s instructions. DNA sequences were analyzed with the DNA Strider and BLAST programs (2). β-Galactosidase activity was measured and expressed as Miller units as described previously (61). All oligonucleotides used in this study were synthesized by M. Berne.
RESULTS
Isolation of spontaneous suppressor mutations that restore expression of spoIIQ-lacZ in a ΔcitC mutant.
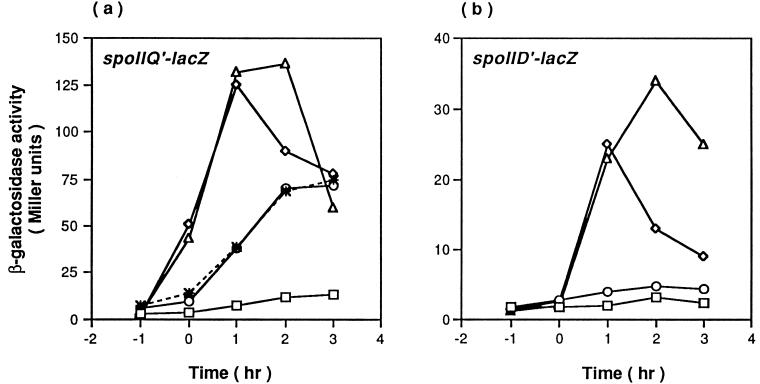
Sporulation of an ICDH (ΔcitC::spc) null mutant is blocked at stage I after expression of Spo0A-phosphate-dependent genes and bipolar localization of FtsZ rings early in stationary phase but before the formation of an asymmetric septum at one of the cell poles (31). Because of the failure of asymmetric septum formation, neither forespore-specific, ςF-dependent genes nor mother cell-specific, ςE-dependent genes are turned on in the ΔcitC mutant (31). To investigate the basis of the stage I block, extragenic suppressor mutations were sought by looking for spontaneous variants of the ΔcitC mutant in which expression of ςF-dependent genes was restored. For this purpose, we used a ΔcitC mutant strain (SJB295) carrying a fusion of the E. coli lacZ gene to the ςF-dependent promoter of the spoIIQ gene (38). In independent trials, two spontaneous mutants were isolated as dark blue colonies on plates of DS medium containing X-Gal. These colonies were as translucent as those of the ΔcitC mutant, indicating that they were still defective in sporulation. In transformation crosses, the suppressing mutations proved to be unlinked to citC or to the spoIIQ′-lacZ fusion integrated at the amyE locus. The spoIIQ′-lacZ fusion and the suppressing mutation of one of the strains were simultaneously moved by congression into strain SJB219 (ΔcitC::spc), creating strain KMB97 (see Materials and Methods). As shown in Fig. 1a, spoIIQ′-lacZ expression in strain KMB97 was increased in stationary phase to almost 50% of that of the wild-type strain.
FIG. 1.
Expression of spoIIQ′-lacZ (a) and spoIID′-lacZ (b) fusions in citC spoVG double mutants. Cells were grown in DS broth and harvested at the indicated times for measurement of β-galactosidase activity. Symbols: ▵, SJB294 (a) and SJB225 (b) (citC+); □, SJB295 (a) and SJB229 (b) (ΔcitC);  , KMB97 (ΔcitC spoVG2); ○, KMB198 (a) and KMB208 (b) (ΔcitC ΔspoVG); ◊, KMB200 (a) and KMB209 (b) (ΔspoVG). Time zero is defined as the onset of stationary phase.
, KMB97 (ΔcitC spoVG2); ○, KMB198 (a) and KMB208 (b) (ΔcitC ΔspoVG); ◊, KMB200 (a) and KMB209 (b) (ΔspoVG). Time zero is defined as the onset of stationary phase.
Identification of the spoVG2 mutation.
To map the suppressing mutation in KMB97, we initially transformed strain KMB97 with a chromosomal library of random integrations of pPS34 (Ermr; see Materials and Methods). DNA from the pooled transformants was then used to transform strain SJB295. Ermr transformants that formed blue colonies on plates of DS medium containing X-Gal were tested individually for linkage of the suppressing mutation to erm. The location of an integrated plasmid showing about 50% linkage to the suppressing mutation was determined by cleavage of chromosomal DNA with EcoRI, ligation at a low DNA concentration, transformation of E. coli, extraction of plasmid, and sequencing of the cloned insert. The site of plasmid insertion was in the 5′ end of the mfd gene (44), located at 7° on the chromosomal map. Further genetic crosses demonstrated that the mutation is 30% linked to abrB (47), located about 15 kb upstream of the mfd gene, and 70% linked to each of two insertion mutations previously shown to flank the spoVG gene (24). The spoVG locus of KMB97 was amplified by PCR and direct sequence analysis of the PCR-amplified fragments showed a point mutation (CCT to CTT), causing a single amino acid substitution from proline to leucine at residue 63. This mutation was designated spoVG2. Subsequently, the spoVG2 mutant allele was cloned and the presence of the mutation was confirmed by sequence analysis. The second, independently isolated, suppressor mutant was found to have the same mutation, perhaps because this mutation causes the same phenotype as a null mutation (see below).
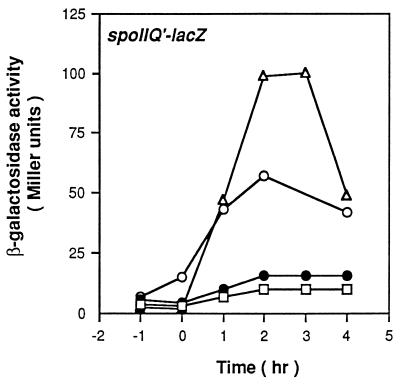
Proof that spoVG2 is the suppressing mutation and is recessive.
When the wild-type spoVG gene was introduced at the thrC locus in KMB97, the suppressor mutant phenotype disappeared (Fig. 2), indicating that spoVG2 is required for suppression and that the spoVG2 mutation is recessive to the wild-type spoVG+. The recessiveness of spoVG2 suggests that it is a loss-of-function mutation. To confirm this point, we tested the ability of null alleles of spoVG to suppress the ΔcitC mutation. When spoVG::Tn917ΩHU265 (56) or ΔspoVG::tet (66) was introduced into the ΔcitC mutant (creating strain KMB136 or KMB198, respectively), the expression of the spoIIQ gene in each of the citC spoVG mutants was indistinguishable from that in KMB97 (Fig. 1a and data not shown), implying that the spoVG2 mutation abolishes a negative effect of SpoVG on ςF activation in the ΔcitC mutant.
FIG. 2.
spoVG2 is a recessive mutation. Cells carrying a spoIIQ′-lacZ fusion were grown in DS broth and harvested at the indicated times for measurement of β-galactosidase activity. Symbols: ▵, SJB294 (citC+); □, SJB295 (ΔcitC); ○, KMB97 (ΔcitC spoVG2); ●, KMB158 (ΔcitC spoVG2 ΔthrC::spoVG+). Time zero is defined as the onset of stationary phase.
Expression of spoVG in a citC mutant.
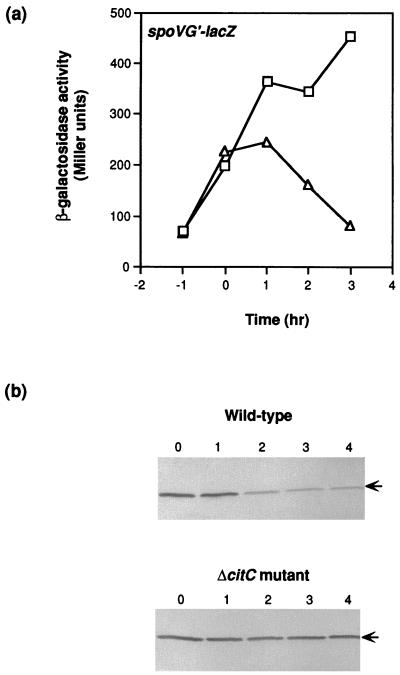
Although ΔcitC mutant cells were known to express the spoVG gene and have no defect in ςH activity (31), we reexamined spoVG gene expression in detail. As shown in Fig. 3a, spoVG expression was induced at the normal time in the mutant at the onset of sporulation but, unlike the case for the wild-type strain, failed to shut off after T1 (1 h after the onset of stationary phase), reaching a maximum level approximately twofold higher than that seen in the wild-type strain.
FIG. 3.
Expression of a spoVG′-lacZ fusion in a ΔcitC mutant. (a) Wild-type (SJB78; ▵) and ΔcitC mutant (KMB162; □) strains were grown in DS broth and harvested at the indicated times for measurement of β-galactosidase activity. (b) Samples of wild-type (SJB294) and ΔcitC mutant (SJB295) cultures were harvested at the 1-h intervals after the onset of stationary phase indicated over the gels and disrupted by sonication. Proteins (10 μg) in the crude extract were separated in sodium dodecyl sulfate-polyacrylamide gels and subjected to immunoblot analysis with anti-SpoVG antibodies.
Consistent with the results of spoVG′-lacZ expression, the level of SpoVG protein in the wild-type strain was high in early stationary phase, at least until T1, but decreased significantly thereafter (Fig. 3b). In the ΔcitC mutant, the level of SpoVG was constant throughout stationary phase, remaining at nearly the maximal level seen in the wild-type strain (Fig. 3b), raising the possibility that the phenotype of a ΔcitC mutant is due, at least in part, to prolonged expression or stability of SpoVG.
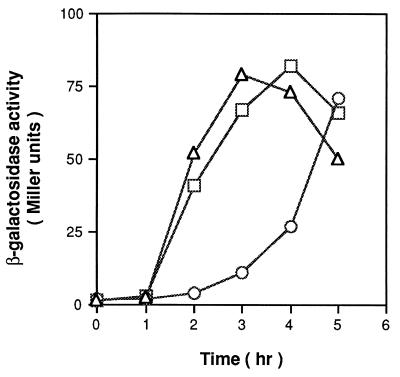
Overexpression of spoVG in B. subtilis.
To assess further the potential negative effect of SpoVG on asymmetric septation, we measured the effect on wild-type cells of overexpression of spoVG. We used a low-copy-number plasmid, pHP13, for this purpose, since it is known that high dosage of the ςH-dependent spoVG promoter results in severe inhibition of sporulation at an early stage (3, 73). Figure 4 shows that, in wild-type cells harboring spoVG+ on pHP13 (pKM98), ςF-dependent spoIIQ expression was significantly delayed. This inhibitory effect was not caused by titration by the spoVG promoter of any regulatory factor, since no detectable difference in spoIIQ expression was found in cells carrying the plasmid-borne spoVG2 allele compared to cells carrying the vector only.
FIG. 4.
Expression of spoIIQ′-lacZ in strains harboring the spoVG gene and the spoVG2 allele on a multicopy plasmid. Cells having pHP13 (vector only; ▵), pKM98 (spoVG+; ○), or pKM157 (spoVG2; □) were grown in DS broth and harvested at the indicated times for measurement of β-galactosidase activity. Time zero is defined as the onset of stationary phase.
Sporulation gene expression in a citC spoVG double mutant.
Since colonies of a ΔcitC::spc ΔspoVG::tet double mutant were as translucent as those of the original ΔcitC mutant and the number of heat-resistant spores, measured at about T20, was nearly identical to that of the ΔcitC mutant (Table 2), we examined later stages of the sporulation pathway to identify the defect. The expression of the mother cell-specific, ςE-dependent spoIID gene (52) is normally induced following activation of ςF in the forespore. However, in the ΔcitC ΔspoVG double mutant, there was relatively little induction of spoIID′-lacZ (Fig. 1b). As expected from the low level of ςE activity in the mother cell, there was little or no expression of the later sporulation genes sspE (17) and cotA (57), which are transcribed by RNA polymerases containing ςG and ςK, respectively (data not shown). These defects were not due to the spoVG mutation, since a spoVG single mutant had no detectable deficit in spo gene expression (data not shown) and sporulated at close to the wild-type frequency (reference 53 and Table 2).
TABLE 2.
Sporulation of citC spoVG double mutanta
| Strain (relevant genotype) | Viable cells (no./ml) | Spores (no./ml) |
|---|---|---|
| JH642 (wild type) | 6.3 × 108 | 5.0 × 108 |
| KMB199 (ΔspoVG) | 1.7 × 108 | 1.2 × 108 |
| SJB219 (ΔcitC) | 2.6 × 107 | 3.3 × 103 |
| KMB197 (ΔcitC ΔspoVG) | 1.2 × 107 | 2.4 × 103 |
Cells were grown in DS broth. At T20, viable-cell titers were determined by plating and spore titers were determined by plating after heating at 80°C for 10 min.
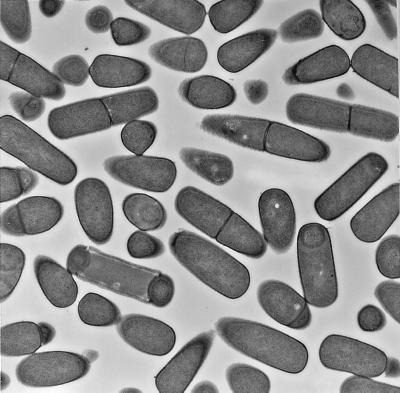
Thin-section electron microscopy of the ΔcitC ΔspoVG mutant revealed that approximately 20% of the population of cells formed the sporulation-specific asymmetric septum, i.e., overcame the stage I block in sporulation characteristic of the ΔcitC single mutant, and proceeded further in the developmental pathway (Fig. 5). At T6, engulfment of the forespore (normally seen at T3 to T4) may have been completed in a few cells, but no further development was seen. It seems that post-stage I defects remain unsuppressed in the citC spoVG double mutant.
FIG. 5.
Thin-section electron microscopic analysis. A sample of a culture of ΔcitC ΔspoVG mutant cells (KMB197) was harvested at T6 and prepared for transmission electron microscopy as described in Materials and Methods.
In the accompanying paper, we show that the stage I block in a ΔcitC mutant can be partially overcome by a reduction in citrate accumulation (41). To see whether the citrate and spoVG effects are additive, we created a triple mutant strain from which citZ (the major citrate synthase gene), citC, and spoVG had been deleted. In this strain, expression of spoIIQ′-lacZ was nearly as high as in wild-type cells, and expression of both spoIID′-lacZ and sspE′-lacZ was increased (Table 3). The inhibitory effect of excess citrate is due to a decrease in pH and chelation of divalent cations (41). Thus, the effect of the citZ mutation on a citC spoVG double mutant could be mimicked by supplementing the medium with HEPES buffer (pH 8) or excess MnCl2 (Table 3). Simultaneous addition of HEPES and excess Mn2+ to citC spoVG double-mutant cells allowed significant restoration of expression of all spo genes tested (Table 3), yet the cells still did not form heat-resistant spores efficiently (7.2 × 106 per ml). This result presumably indicates that the absence of ICDH activity causes additional physiological changes that we have yet to identify.
TABLE 3.
Expression of sporulation genes in tricarboxylic acid cycle mutants
| Relevant genotype | Supplement to mediumb | Relative expression of sporulation genes (%)a
|
|||
|---|---|---|---|---|---|
| spoIIQ | spoIID | sspE | cotA | ||
| ΔcitC | None | <10 | <10 | <10 | <10 |
| ΔcitC ΔspoVG | None | 43 | 19 | <10 | <10 |
| HEPES | 81 | 20 | 29 | <10 | |
| MnCl2 | 62 | 36 | 27 | <10 | |
| HEPES + MnCl2 | 170 | 43 | 30 | 37 | |
| ΔcitC ΔspoVG sof-1 | None | <10 | |||
| ΔcitZC | None | 26 | 13 | <10 | <10 |
| ΔcitZC ΔspoVG | None | 76 | 36 | 29 | <10 |
| ΔcitZC ΔspoVG sof-1 | None | 110 | |||
B. subtilis strains carrying a promoter of a sporulation gene (ςF-dependent spoIIQ, ςE-dependent spoIID, ςG-dependent sspE, or ςK-dependent cotA) fused to the E. coli lacZ gene were grown in DS broth and harvested every hour after onset of stationary phase for measurements of β-galactosidase activity. The levels of expression of the lacZ fusions in mutants were estimated relative to the maximum level of expression of the same fusions in the wild-type strain. The average maximal β-galactosidase activities (in Miller units) of the various fusions in the wild-type strains were: spoIIQ-lacZ, 120; spoIID-lacZ, 27; sspE-lacZ, 250; and cotA-lacZ, 100.
In some experiments, 50 mM HEPES (pH 8) or 0.75 mM MnCl2 or both were added to DS medium.
The only combination of conditions known to suppress the sporulation defect of a citC mutant nearly completely is to reduce citrate production (as in a citZ mutant) and modify the spo0A gene by the sof-1 mutation (41). When the sof-1 allele of spo0A was introduced into citZC spoVG mutant cells, late spo gene expression and spore formation were restored to near-normal levels (Table 3 and data not shown).
Minicell formation in spoVG null mutant.
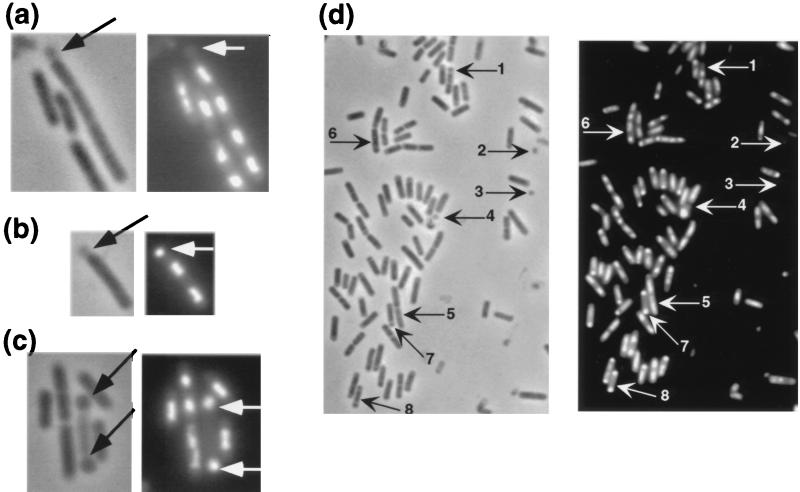
Since the phenotype of the spoVG mutant suggests that the wild-type protein interferes with asymmetric septation, we tested the effects on cell division and sporulation of a spoVG mutation alone or in combination with a mutation in minD, a gene whose product inhibits polar septation during exponential growth (34, 35, 69). We confirmed that single null mutations in either spoVG or minD had relatively small effects on sporulation frequency (34, 35, 53). In our hands, the numbers of viable cells and spores obtained with either strain were decreased to about 50% of those of the wild-type strain after growth overnight in DS broth (Table 2 and data not shown). The minD spoVG double mutant suffered further losses in viability and spore titer to 30 and 10%, respectively (data not shown). The impaired ability of the double mutant to maintain viability and produce spores was reflected in the production of minicells by single and double mutants. O. Resnekov (51) had noted previously that a spoVG null mutant produces minicells. We estimated the minicell fraction at 2 to 3% of total spoVG mutant cells during vegetative growth (Fig. 6 and Table 4). A minD mutant produced about 13% minicells.
FIG. 6.
Minicell formation in a ΔspoVG mutant. Cells of strain KMB200 (ΔspoVG) were grown to mid-exponential phase and induced to sporulate by the resuspension method of Sterlini and Mandelstam (65). Ethanol-fixed cells were observed under phase-contrast microscopy (left-hand panels), and nucleoids were visualized by DAPI staining (right-hand panels). (a to c) Exponential-phase cells, showing an anucleate minicell (a), a minicell containing a condensed nucleoid (b), and a disporic cell (c). (d) Cell population of the mutant at 1 h after resuspension. Arrows 1 to 4 indicate anucleate minicells, arrow 5 indicates a sporulating cell at stage 0-I, arrow 6 indicates a sporulating cell at stage II, and arrows 7 and 8 indicate disporic cells.
TABLE 4.
Minicell formation in ΔspoVG mutant
| Type of cella | No. of cellsb at:
|
|||||
|---|---|---|---|---|---|---|
| Mid-exponential phase
|
1 hc
|
2 hc
|
||||
| ΔspoVG | WT | ΔspoVG | WT | ΔspoVG | WT | |
| Vegetative cell or stage 0-I cell | 1,223 | 1,442 | 1,003 | 1,009 | 741 | 652 |
| Anucleate minicell | 40 | 1 | 15 | 1 | 13 | 1 |
| Minicell containing a nucleoid | 2 | 0 | 9 | 0 | 2 | 0 |
| Sporulating cell at stage II | 2 | 1 | 55 | 16 | 122 | 126 |
| Disporic cell | 3 | 0 | 3 | 0 | 9 | 0 |
Examples of vegetative cells, anucleate minicells, minicells containing a nucleoid, disporic cells, and sporulating cells at stages 0-I and II are shown in Fig. 6.
Cells of wild-type (WT) and ΔspoVG mutant strains were grown in Casamino Acids-containing rich medium of Sterlini and Mandelstam (65) to mid-log phase and induced to sporulate by resuspension into poor medium. Cells were fixed with 70% ethanol to visualize septa, and nucleoid distribution was analyzed by DAPI staining (see Materials and Methods for details). Numbers represent total counts obtained from four individual experiments.
After induction of sporulation.
We examined minicell formation in a mid-exponential-phase culture of the ΔspoVG mutant in more detail by visualization of septa and nucleoids. Surprisingly, in addition to typical anucleate minicells, the ΔspoVG mutant culture also included cells having unusual distributions of nucleoids, such as minicells containing a condensed nucleoid or cells with a disporic phenotype (60), both occurring at a very low but significantly higher frequency than in the wild-type strain (Fig. 6 and Table 4). When mid-exponential-phase cells of the spoVG mutant were induced to sporulate by the resuspension method to synchronize the initiation of sporulation, significant numbers of anucleate minicells, minicells containing nucleoids, and disporic cells were found in the mutant culture at T1, and stage II cells appeared earlier in the mutant culture than in the wild-type strain culture (Fig. 6 and Table 4).
Since polar septation concomitant with condensation of the nucleoid in the forespore compartment is the cell division event unique to initiation of sporulation in B. subtilis, we tested the contribution of the Spo0A phosphorelay (7), the essential signal transduction system for initiation of sporulation, to the formation of these kinds of minicells. When spo0F and spo0B mutations were introduced into the ΔspoVG mutant, few minicells of any type were observed in a vegetative cell culture of the triple mutant. Similarly, a spo0A deletion greatly decreased the frequency of minicells in the ΔspoVG mutant culture (<0.13%). In contrast, formation of anucleate minicells by a minD mutant was not affected by a spo0A mutation.
To see whether the effects of a citC mutation would be suppressed by a minD mutation, we tested spoIIQ′-lacZ expression and sporulation in a citC minD double mutant. A minD mutation had no effect on sporulation frequency or expression of spoIIQ in a citC mutant (data not shown).
DISCUSSION
Null mutations in the spoVG gene partially relieve the stage I block of a ΔcitC mutant, allowing a significant fraction of the ΔcitC mutant cell population to form an asymmetric septum and activate expression of ςF-dependent genes in the forespore compartment. This evidence suggests that a normal function of ICDH overcomes the activity of SpoVG, which is itself an inhibitor or a negative regulator of the ςH-dependent pathway to the asymmetric septum. In other work, we have shown that it is the enzymatic activity of ICDH that is critical for completing stage I (31, 41).
spoVG was the first developmentally regulated gene cloned from B. subtilis (58). Close homologs are now known to exist in Bacillus megaterium, Clostridium acetobutylicum, Borrelia burgdorferi, and Archaeoglobus fulgidus, organisms which collectively represent gram-positive and -negative eubacteria and the archaea. Proline-63, the residue mutated in our suppressor strains, is conserved in all five SpoVG proteins.
The absence of SpoVG in sporulating cells of B. subtilis is known to cause aberrations in the germ cell wall and the cortex, defects seen at T5 to T6 (stage V) (53), and to make the stage II defect of a spoIIB mutant more severe (40). However, extensive gene expression studies have shown that the spoVG gene is a very early stationary phase gene, expressed at a significant level even during exponential growth phase and further induced at the end of exponential phase (45, 74). This transcription depends on the ςH form of RNA polymerase and is partially repressed during early exponential phase by a global negative regulator, AbrB (74). The concentration of SpoVG protein reaches a maximum level at the onset of stationary phase and decreases substantially after T2, indicating that SpoVG most likely has its primary function early in stationary phase. In the ΔcitC mutant, spoVG expression is induced at the normal time but continues to increase even after several hours in stationary phase. Moreover, the level of SpoVG protein remains high at a time when it is greatly diminished in wild-type cells. Thus, the dependence of asymmetric septation on ICDH activity might be attributed to an effect on SpoVG synthesis or stability. Asymmetric septation, however, is an early event in stationary phase; it is uncertain whether prolonged expression of SpoVG (seen after T1) is in fact responsible for the primary effect of the citC mutation. On the other hand, overexpression of spoVG in wild-type cells interferes with asymmetric septation.
The MinCD complex is a cell division inhibitor in exponential-phase cells of B. subtilis (34, 35, 69), as well as in E. coli (12, 13). Cells lacking these proteins form septa at potential division sites near cell poles and produce anucleate minicells during vegetative growth (13). The Min proteins appear to mask the polar cell division sites in normal growing cells, restricting FtsZ ring formation to medial sites (49). (Under some growth conditions, other proteins can carry out this function in B. subtilis [36a].) The masked sites, however, must become accessible to FtsZ when B. subtilis cells enter the spore formation program, implying that polar septation inhibitors (e.g., MinCD) lose activity in stationary phase. Our finding that deletion of the spoVG gene increases the rate and extent of asymmetric septation in both wild-type and citC mutant cells suggests that a normal role of SpoVG in early stationary phase is to temporarily prevent asymmetric septation after the Min proteins cease to function. Perhaps the subtle defects observed in late sporulating cells of a spoVG mutant (40, 53) are due to the loss of precise timing of asymmetric septum formation.
Although the primary function of SpoVG seems to be to regulate asymmetric septation in stationary-phase cells, the spoVG gene is also expressed, albeit at a lower level, in a population of exponential-phase cells. The fact that an exponential-phase culture of the spoVG mutant contains some minicells with condensed nucleoids and cells with a disporic phenotype is consistent with a heterogeneous population in which some cells initiate sporulation while others grow. This supposition is consistent with our finding that appearance of the aberrant cells is dependent on the Spo0A phosphorelay.
While inactivation of SpoVG partially overcomes the stage I block of a ΔcitC mutant, the cells do not complete the sporulation process or even proceed beyond stage III. Moreover, only a subpopulation (<30%) of the citC spoVG double mutant cells form asymmetric septa and the level of ςF-dependent gene expression reaches only 30 to 50% of the level in wild-type cells. Further suppression of the stage I block in a citC mutant requires reducing citrate synthesis or raising the pH of the medium and supplementation with MnCl2 to compensate for overaccumulation of citrate (41).
The mechanism by which SpoVG regulates the extent and timing of asymmetric septation is unknown. SpoVG may interact directly with the septation apparatus or may act indirectly by regulating the synthesis or stability of proteins required for septation. Future experiments will be directed toward identifying the mechanism by which SpoVG regulates asymmetric septation and the means by which ICDH activity intervenes in this process. A working model has the following elements. As cells make the transition from exponential growth to stationary phase, citC and spoVG are simultaneously induced. At the same time, polar sites for assembly of FtsZ-containing cell division complexes become unmasked, but polar septation is still prevented in an unknown way by newly accumulated SpoVG. As Krebs cycle enzymes, including ICDH, begin to function, acidic metabolites of the glycolytic pathway are assimilated, raising extracellular pH, inactivating SpoVG, and inducing asymmetric septation, a process that requires one or more Mn2+-dependent proteins. Our data, however, do not rule out the possibility that low pH, Mn2+ limitation, and SpoVG affect polar septation by independent mechanisms.
ACKNOWLEDGMENTS
We thank O. Resnekov, R. Losick, P. Levin, A. Grossman, and P. Stragier for providing strains, for helpful discussions, and for sharing unpublished results; B. Belitsky, P. Fawcett, A. Grossman, R. Losick, N. King, and D. RayChaudhuri for helpful criticism of the manuscript; S. Jin for providing strains; A. Serio for constructing strains and assaying β-galactosidase activity; P. Serror for constructing pPS28, pPS34, and genomic libraries; A. Brown-Cormier for assistance with electron microscopy; and S. Yaser for preparation of anti-SpoVG antisera.
This work was supported in part by a research grant (GM42219) from the U.S. Public Health Service to A.L.S.
REFERENCES
- 1.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Banner C D, Moran C P, Jr, Losick R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J Mol Biol. 1983;168:351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- 4.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 5.Belitsky B R, Gustafsson M C U, Sonenshein A L, Wachenfeldt C V. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J Bacteriol. 1997;179:5448–5457. doi: 10.1128/jb.179.17.5448-5457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature (London) 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 7.Burbulys D, Trach K A, Hoch J A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 8.Craig J E, Ford M J, Blaydon D C, Sonenshein A L. A null mutation in the Bacillus subtilis aconitase gene causes a block in Spo0A-phosphate-dependent gene expression. J Bacteriol. 1997;179:7351–7359. doi: 10.1128/jb.179.23.7351-7359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutting S, Driks A, Schmidt R, Kunkel B, Losick R. Forespore-specific transcription of a gene in the signal transduction pathway that governs pro-ςK processing in Bacillus subtilis. Genes Dev. 1991;5:456–466. doi: 10.1101/gad.5.3.456. [DOI] [PubMed] [Google Scholar]
- 10.Cutting S, Roels S, Losick R. Sporulation operon spoIVF and the characterization of mutations that uncouple mother-cell from forespore gene expression in Bacillus subtilis. J Mol Biol. 1991;221:1237–1256. doi: 10.1016/0022-2836(91)90931-u. [DOI] [PubMed] [Google Scholar]
- 11.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature (London) 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 12.de Boer P A J, Crossley R E, Rothfield L I. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J Bacteriol. 1988;170:2106–2112. doi: 10.1128/jb.170.5.2106-2112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer P A J, Crossley R E, Rothfield L I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 14.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. J Mol Biol. 1971;56:209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- 16.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 17.Fajardo-Cavazos P, Tovar-Rojo F, Setlow P. Effect of promoter mutations and upstream deletions on the expression of genes coding for small, acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1991;173:2011–2016. doi: 10.1128/jb.173.6.2011-2016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortnagel P, Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968;95:1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouet A, Sonenshein A L. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990;172:835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frandsen N, Stragier P. Identification and characterization of the Bacillus subtilis spoIIP locus. J Bacteriol. 1995;177:716–722. doi: 10.1128/jb.177.3.716-722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freese E B, Marks C L. Developmental block in citric acid mutants of Bacillus subtilis. J Bacteriol. 1973;116:1466–1468. doi: 10.1128/jb.116.3.1466-1468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guérout-Fleury A-M, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- 23.Haima P, Bron S, Venema G. The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol Gen Genet. 1987;209:335–342. doi: 10.1007/BF00329663. [DOI] [PubMed] [Google Scholar]
- 24.Haldenwang W G, Banner C D B, Ollington J F, Losick R, Hoch J A, O’Connor M B, Sonenshein A L. Mapping a cloned gene under sporulation control by insertion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980;142:90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hale C A, de Boer P A J. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- 26.He M, Kaderdhai M A, Adcock I, Austen B M. An improved and rapid procedure for isolating RNA-free Escherichia coli plasmid DNA. Genet Anal Tech Appl. 1991;8:107–110. doi: 10.1016/1050-3862(91)90045-s. [DOI] [PubMed] [Google Scholar]
- 27.Hofmeister A E M, Londoño-Vallejo A, Harry E, Stragier P, Losick R. Extracellular signal protein triggering the proteolytic activation of a developmental transcription factor in B. subtilis. Cell. 1995;83:219–226. doi: 10.1016/0092-8674(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 28.Ireton K, Rudner D Z, Siranosian K J, Grossman A D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 29.Ireton K, Jin S, Grossman A D, Sonenshein A L. Krebs cycle function is required for activation of the Spo0A transcription factor in Bacillus subtilis. Proc Natl Acad Sci USA. 1995;92:2845–2849. doi: 10.1073/pnas.92.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin S, Sonenshein A L. Characterization of the major citrate synthase of Bacillus subtilis. J Bacteriol. 1996;178:3658–3660. doi: 10.1128/jb.178.12.3658-3660.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin S, Levin P A, Matsuno K, Grossman A D, Sonenshein A L. Deletion of the Bacillus subtilis isocitrate dehydrogenase gene causes a block at stage I of sporulation. J Bacteriol. 1997;179:4725–4732. doi: 10.1128/jb.179.15.4725-4732.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karow M L, Glaser P, Piggot P J. Identification of a gene, spoIIR, that links the activation of ςE to the transcriptional activity of ςF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 1995;92:2012–2016. doi: 10.1073/pnas.92.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeDeaux J R, Grossman A D. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J Bacteriol. 1995;177:166–175. doi: 10.1128/jb.177.1.166-175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Price C W. The minCD locus of Bacillus subtilis lacks the minE determinant that provides topological specificity to cell division. Mol Microbiol. 1993;7:601–610. doi: 10.1111/j.1365-2958.1993.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 35.Levin P A, Margolis P, Setlow P, Losick R, Sun D. Identification of Bacillus subtilis genes for septum placement and shape determination. J Bacteriol. 1992;174:6717–6728. doi: 10.1128/jb.174.21.6717-6728.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 36a.Levin P A, Shim J J, Grossman A D. Effect of minCD on FtsZ ring position and polar septation in Bacillus subtilis. J Bacteriol. 1998;180:6048–6051. doi: 10.1128/jb.180.22.6048-6051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Londoño-Vallejo J-A, Stragier P. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 1995;9:503–508. doi: 10.1101/gad.9.4.503. [DOI] [PubMed] [Google Scholar]
- 38.Londoño-Vallejo J-A, Fréhel C, Stragier P. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- 39.Lu S, Halberg R, Kroos L. Processing of the mother-cell ς factor, ςK, may depend on events occurring in the forespore during Bacillus subtilis development. Proc Natl Acad Sci USA. 1990;87:9722–9726. doi: 10.1073/pnas.87.24.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margolis P S, Driks A, Losick R. Sporulation gene spoIIB from Bacillus subtilis. J Bacteriol. 1993;175:528–540. doi: 10.1128/jb.175.2.528-540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuno K, Blais T, Serio A W, Conway T, Henkin T M, Sonenshein A L. Metabolic imbalance and sporulation in an isocitrate dehydrogenase mutant of Bacillus subtilis. J Bacteriol. 1999;181:3382–3391. doi: 10.1128/jb.181.11.3382-3391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-ς factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 43.Mueller J P, Bukusoglu G, Sonenshein A L. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992;174:4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogasawara N, Nakai N, Yoshikawa H. Systematic sequencing of the 180 kilobase region of the Bacillus subtilis chromosome containing the replication origin. DNA Res. 1994;1:1–14. doi: 10.1093/dnares/1.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Ollington J F, Haldenwang W G, Huynh T V, Losick R. Developmentally regulated transcription in a cloned segment of the Bacillus subtilis chromosome. J Bacteriol. 1981;147:432–442. doi: 10.1128/jb.147.2.432-442.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Partridge S R, Foulger D, Errington J. The role of ςF in prespore-specific transcription in Bacillus subtilis. Mol Microbiol. 1991;5:757–767. doi: 10.1111/j.1365-2958.1991.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 47.Perego M, Spiegelman G B, Hoch J A. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol. 1988;2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 48.Piggot P J, Coote J G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raskin D M, de Boer P A J. The MinE ring: an FtsZ-independent cell structure required for selection of the correct division site in E. coli. Cell. 1997;91:685–694. doi: 10.1016/s0092-8674(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 50.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature (London) 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 51.Resnekov, O. Personal communication.
- 52.Rong S, Rosenkrantz M S, Sonenshein A L. Transcriptional control of the Bacillus subtilis spoIID gene. J Bacteriol. 1986;165:771–779. doi: 10.1128/jb.165.3.771-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbluh A, Banner C D B, Losick R, Fitz-James P C. Identification of a new developmental locus in Bacillus subtilis by construction of a deletion mutation in a cloned gene under sporulation control. J Bacteriol. 1981;148:341–351. doi: 10.1128/jb.148.1.341-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutberg B, Hoch J A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970;104:826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 56.Sandman K, Losick R, Youngman P. Genetic analysis of Bacillus subtilis spo mutations generated by Tn917-mediated insertional mutagenesis. Genetics. 1987;117:603–617. doi: 10.1093/genetics/117.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandman K, Kroos L, Cutting S, Youngman P, Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988;200:461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- 58.Segall J, Losick R. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell. 1977;11:751–761. doi: 10.1016/0092-8674(77)90289-6. [DOI] [PubMed] [Google Scholar]
- 59.Serror, P. Personal communication.
- 60.Setlow B, Magill N, Febbroriello P, Nakhimovsky L, Koppel D E, Setlow P. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J Bacteriol. 1991;173:6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slack F J, Mueller J P, Strauch M A, Mathiopoulos C, Sonenshein A L. Transcriptional regulation of a Bacillus subtilis dipeptide transport operon. Mol Microbiol. 1991;5:1915–1925. doi: 10.1111/j.1365-2958.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 62.Slack F J, Mueller J P, Sonenshein A L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sonenshein A L. Metabolic regulation of sporulation and other stationary-phase phenomena. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C: American Society for Microbiology; 1989. pp. 109–130. [Google Scholar]
- 64.Sonenshein, A. L. Unpublished data.
- 65.Sterlini J M, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stragier, P. Personal communication.
- 67.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 68.Sun D, Cabrera-Martinez R M, Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor ςG. J Bacteriol. 1991;173:2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Varley A W, Stewart G C. The divIVB region of the Bacillus subtilis chromosome encodes homologues of Escherichia coli septum placement (MinCD) and cell shape (MreBCD) determinants. J Bacteriol. 1992;174:6729–6742. doi: 10.1128/jb.174.21.6729-6742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu L J, Lewis P J, Allmansberger R, Hauser P M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- 71.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 72.Yousten A A, Hanson R S. Sporulation of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1972;109:886–894. doi: 10.1128/jb.109.2.886-894.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zuber P, Healy J M, Losick R. Effects of plasmid propagation of a sporulation promoter on promoter utilization and sporulation in Bacillus subtilis. J Bacteriol. 1987;169:461–469. doi: 10.1128/jb.169.2.461-469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuber P, Marahiel M, Robertson J. Influence of abrB on the transcription of the sporulation-associated genes spoVG and spo0H in Bacillus subtilis. In: Ganesan A T, Hoch J A, editors. Genetics and biotechnology of bacilli. Vol. 2. San Diego, Calif: Academic Press, Inc.; 1988. pp. 123–127. [Google Scholar]