Abstract
Objective
The objective of the current study was to analyse the association between treatment with tumour necrosis factor inhibitors (TNFi) and radiographic spinal progression in patients with axial spondyloarthritis (axSpA) from a long-term inception cohort.
Methods
A total of 243 patients with axSpA from the German Spondyloarthritis Inception Cohort with at least two sets of spinal radiographs obtained at least 2 years apart during a 10-year follow-up were included. Spinal radiographs were evaluated by three trained and calibrated readers according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). The association between the current TNFi, previous TNFi and radiographic spinal progression defined as the absolute mSASSS change score over 2 years was analysed using longitudinal generalised estimating equations analysis.
Results
TNFi treatment in the current 2-year interval was not associated with retardation of radiographic spinal progression (β=−0.02 (95% CI −0.37 to 0.34) and −0.17 (95% CI −0.54 to 0.20) for any and ≥12 months treatment duration, respectively, adjusted for sex, the Ankylosing Spondylitis Disease Activity Score, smoking, presence of definite radiographic sacroiliitis, mSASSS at baseline and non-steroidal anti-inflammatory drug intake). TNFi treatment in the previous 2-year interval, was, however, significantly associated with reduction of mSASSS progression, which was especially evident in patients who received TNFi in the previous and in the current intervals: β=−0.58 (95% CI −1.02 to –0.13), adjusted for the same variables.
Conclusion
TNFi treatment was associated with a time-shifted effect on radiographic spinal progression in axSpA that became evident between years 2 and 4 after treatment initiation.
Keywords: Tumor Necrosis Factor Inhibitors; Spondylitis, Ankylosing; Epidemiology; Biological Therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Observational studies demonstrated that tumour necrosis factor inhibitors (TNFi) may retard radiographic progression in patients with ankylosing spondylitis.
The minimal duration of TNFi treatment that is needed to observe reduction of radiographic progression and the question if such an effect can also be observed in patients at an earlier disease stage remained uncertain.
WHAT THIS STUDY ADDS
In this long-term (10 years) inception cohort of patients with axial spondyloarthritis, treatment TNFi was significantly associated with a time-shifted retardation of radiographic spinal progression, which became evident between year 2 and 4 after treatment initiation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
This study suggests that continuous treatment with an effective anti-inflammatory drug such as TNFi has disease-modifying properties in axial spondyloarthritis.
Introduction
Radiographic spinal progression in axial spondyloarthritis (axSpA) is largely attributable to the process of new bone formation with development of so-called syndesmophytes, which build bridges between vertebral bodies resulting into spinal ankylosis.1 Also other spinal structures (facet joints, costrovertebral and costotranversal joints) might become damaged and ankylosed in axSpA,2 but syndesmophyte formation is usually considered as the main proxy in the assessment of structural damage that is referred to as radiographic spinal progression in axSpA if evaluated on conventional radiographs. Disease activity and structural damage in the spine are the two major determinants of spinal mobility and function in axSpA3 4; at the advanced disease stage, the contribution of structural damage to the functional impairment might become leading. New bone formation in axSpA is assumed to be preceded by bony inflammation, which induces repair mechanisms with subchondral granulation tissue formation and subsequent stimulation of osteogenesis.5–7 After first studies had shown that effective anti-inflammatory treatment with tumour necrosis factor inhibitors (TNFi) over 2 years does not inhibit radiographic progression in patients with advanced axSpA (radiographic axSpA - r-axSpA also termed ankylosing spondylitis—AS) as compared with historical cohorts8–10 it became evident that a long-term suppression of inflammation might be necessary to see the effect of anti-inflammatory treatment on structural damage development.11–18 The minimal duration of TNFi treatment that is needed to observe reduction of radiographic progression and the question if such an effect can also be observed in patients at an earlier disease stage remained uncertain.
The objective of the current study was to analyse the association between the TNFi exposure and radiographic spinal progression in patients with axSpA in a long-term inception cohort.
Methods
Cohort description and patient selection
The German Spondyloarthritis Inception Cohort (GESPIC) is an ongoing longitudinal study focussing on clinical and radiographic outcomes of patients with SpA. The study design and the inclusion criteria have been reported in detail elsewhere.19 Briefly, the cohort was initiated in 2000 as a national multicentre study within the German Competence Network Rheumatology programme and comprised four university clinics, five community hospitals and four private practices. The last patient was enrolled in the cohort in 2009. Patients with axSpA were included if they had r-axSpA (AS) fulfilling the modified New York criteria and symptom duration of up to 10 years or non-radiographic axSpA(nr-axSpA) fulfilling the slightly modified European Spondyloarthropathy Study Group criteria and symptom duration of up to 5 years.19 Classification as r-axSpA or nr-axSpA was performed based on central evaluation of sacroiliac X-rays as described elsewhere20; in the absence of central reading results, the local rheumatologist’s assessment was used for the classification. There were no restrictions in terms of treatment, but the majority of patients were recruited before introduction of TNFi in daily clinical practice. Patients were investigated at baseline, every 6 months during the first 2 years and annually thereafter up to year 10. Disease activity was assessed by the Bath Ankylosing Spondylitis Disease Activity Index, C reactive protein (CRP) and the patient global assessment of disease activity. Furthermore, the Ankylosing Spondylitis Disease Activity Score (ASDAS) was calculated. Function was evaluated by the Bath Ankylosing Spondylitis Functional Index, spinal mobility—by the Bath Ankylosing Spondylitis Metrology Index (BASMI). Information on treatment was collected at every visit. If TNFi intake was recorded on two consecutive visits, it was assumed that TNFi was taken during the period between those visits. For non-steroidal anti-inflammatory drugs (NSAIDs), the Assessment of Spondyloarthritis International Society NSAIDs intake score21 was calculated as previously described.22 Cervical and lumbar radiographs were obtained at baseline and every 2 years thereafter. Radiographs had to be performed in a ±6 months window around the date of the clinical visit. For the purpose of the present analysis, we selected patients who had at least two sets of spinal radiographs (cervical and lumbar spine, lateral views) during the 10-year follow-up period. The mean and median interval lengths between radiographs were 25.2 and 24 (IQR: 22 to 28) months, respectively. A total of 243 patients (130 with nr-axSpA and 113 with r-axSpA) were finally included in the current study; the flowchart of patient selection is presented in online supplemental figure S1.
annrheumdis-2022-222324supp001.pdf (259.4KB, pdf)
Patients and the public involvement
Patients and the public were not involved in the design, conduct, reporting or dissemination plans of the current research.
Reading of radiographs
Three trained and calibrated readers (AD, VRR, MT) scored spinal radiographs (up to six time points per patient: baseline, year 2, year 4, year 6, year 8 and year 10) according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) system. The readers were blinded for all clinical information but knew the chronology of the images.
Statistical analyses
A total mSASSS ranging from 0 to 72 was calculated for each reader. The final mSASSS was calculated as a mean of three reader score for per patient and time point. We allowed for up to six missing scores of single vertebral corners per time point and up to three for the anatomical region (cervical or lumbar spine). Missing value of a single vertebral corner was replaced by the values of the same vertebral corner obtained at the next available time point (previous for the last one) or by 0 if all were missing. Furthermore, we imputed missing time points if the previous and the next available time points had the same mSASSS. No further imputations were performed.
The reliability of assessment was evaluated by the intraclass correlation coefficient (ICC) for the mSASSS status and change scores between the readers.
The primary outcome used in this analysis was the absolute change in mSASSS in a 2-year interval. The secondary outcomes included progression in the mSASSS by ≥2 points over 2 years and formation of ≥1 new syndesmophyte (as recorded by at least two out of three readers) over 2 years.
The TNFi exposure was defined as follows:
any TNFi use in the current 2-year interval.
TNFi for ≥12 months in the current 2-year interval,
Any TNFi use in the previous 2-year interval.
TNFi for ≥12 months in the previous 2-year interval.
TNFi for ≥12 months in the previous and ≥12 months in the current 2-year interval.
The longitudinal association between TNFi treatment and radiographic spinal progression was evaluated using linear and binomial generalised estimating equations (GEE). All relevant interactions between mSASSS, TNFi exposure and other covariates were tested and revealed no significant interaction (p>0.15). An autoregressive correlation structure was used for the models. In choosing the best correlation structure, we followed the guide suggested by Hardin and Hilbe.23 Briefly, since our data contained missings and were collected over time, we chose the autoregressive structure. This provides a better understanding of the true longitudinal relationship because the cross-sectional effects (within-participant) are removed and the value of the outcome at a given time point is predicted by the outcome variable at the previous time point (‘autoregression’). In addition, we tested the correlation structures based on Quasilikelihood under the Independence model Criterion. Univariable and multivariable GEE analyses were performed for primary and secondary outcomes. All multivariable GEE models with different TNFi definitions were adjusted for the following variables: mSASSS at the beginning of the 2-year interval, classification status (radiographic or non-radiographic), sex (male vs female), symptom duration, current smoking status (yes vs no), time-averaged ASDAS and the NSAID intake score. In addition, we evaluated direct and indirect (mediated by disease activity—ASDAS) effects of TNFi on mSASSS progression as described by Hayes,24 and described in online supplemental figure S2. This graph depicts the conceptional causal framework between TNFi exposure (X), mediator (ASDAS (M)), and outcome (progression in mSASSS (Y)). While ‘a*b’ represents the indirect effect via the mediator, c' represents the direct effect of TNFi exposure on progression. Parameter estimates (β/OR—OR, where appropriate) with 95% CIs were calculated.
Results
The baseline characteristics of 243 included patients are shown in table 1. In comparison to patients excluded (n=282) due to missing radiographs which precluded the assessment of radiographic progression, included patients were less frequently male (49.4% vs 58.9%), had more frequently a family history of SpA (35.1% vs 26.2%) and more often a history of psoriasis (11.5% vs 8.9%). Included patients had similar ASDAS (2.5 vs 2.6) and BASMI (1.6 vs 1.4), and received less frequently systemic steroids (6.2% vs 11.7%). In addition, baseline mSASSS was slightly lower (2.6 vs 2.7) in the included patients, but there was no difference in the proportion of patients with baseline syndesmophytes. Among included patients, only eight (3.3%) patients were under TNFi treatment at baseline, while 70 (28.8%) patients received TNFi during follow-up. The included patients contributed a total of 531 2-year radiographic intervals, with 1, 2, 3, 4 and 5 intervals obtained from 114, 41, 44, 17 and 27 patients, respectively. Of these, 103 (19.4%) and 78 (14.7%) intervals were covered by TNFi treatment of any duration and TNFi treatment of at least 12 months, respectively. The distributions of the intervals and number of patients with respect to each definition of the TNFi exposure are shown in online supplemental figure S3.
Table 1.
Baseline characteristics of the patients with axial spondyloarthritis in GESPIC who included and excluded from the present study
| Parameter | All patients in GESPIC (n=525) |
Included patients (n=243) |
Excluded patients (n=282) |
| Age, years, mean±SD | 35.7±10.3 | 36.2±10.2 | 35.2±10.4 |
| Male sex, n (%) | 286 (54.5) | 120 (49.4) | 166 (58.9) |
| Symptom duration, years, mean±SD | 3.9±2.7 | 4.0±2.5 | 3.9±2.8 |
| Smoking current, n (%) | 132 (25.1) | 67 (27.6) | 65 (23.1) |
| HLA-B27 positivity, n (%) | 406 (77.9) | 191 (79.3) | 215 (76.8) |
| Positive family history for SpA, n (%) | 159 (30.3) | 85 (35.1) | 74 (26.2) |
| Peripheral arthritis, current, n (%) | 77 (14.7) | 28 (11.5) | 49 (17.4) |
| Enthesitis, current, n (%) | 105 (20.0) | 46 (18.9) | 59 (20.9) |
| Dactylitis, current, n (%) | 27 (5.1) | 13 (5.4) | 14 (5.0) |
| Uveitis ever, n (%) | 86 (16.4) | 45 (18.5) | 41 (14.5) |
| Psoriasis ever, n (%) | 53 (10.1) | 28 (11.5) | 25 (8.9) |
| IBD ever, n (%) | 14 (2.7) | 7 (2.9) | 7 (2.5) |
| CRP, mg/litre, mean±SD | 11.1±17.5 | 11.4±18.8 | 10.9±16.3 |
| ASDAS-CRP, mean±SD | 2.6±1.0 | 2.5±1.0 | 2.6±0.9 |
| BASDAI (0–10 points NRS), mean±SD | 3.9±2.1 | 3.7±2.1 | 4.1±2.1 |
| BASFI (0–10 points NRS), mean±SD | 2.8±2.4 | 2.7±2.3 | 2.8±2.4 |
| BASMI (0–10 points NRS), mean±SD | 1.5±1.6 | 1.6±1.6 | 1.4±1.7 |
| Treatment with NSAIDs, n (%) | 352 (67.1) | 163 (67.1) | 189 (67.0) |
| Treatment with csDMARDs, n (%) | 121 (23.1) | 58 (23.9) | 63 (22.3) |
| Treatment with TNFi, n (%) | 13 (2.5) | 8 (3.3) | 5 (1.8) |
| Treatment with systemic steroids, n (%) | 48 (9.1) | 15 (6.2) | 33 (11.7) |
| Patients with r-axSpA, n (%) | 249 (47.4) | 113 (46.5) | 136 (48.2) |
| mSASSS points, mean±SD | 2.6±5.9 N=378 |
2.6±6.6 N=225 |
2.7±4.9 N=153 |
| ≥1 syndesmophyte, n (%) | 66 (17.5) N=378 |
40 (17.8) N=225 |
26 (17.0) N=153 |
ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C reactive protein; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; HLA-B27, human leucocyte antigen B27; IBD, inflammatory bowel disease; mSASSS, modified Stroke Ankylosing Spondylitis Spine Score; NRS, numeric rating scale; NSAIDs, non-steroidal anti-inflammatory drugs; SpA, spondyloarthritis; TNFi, tumour necrosis factor alpha inhibitor.
The interobserver reliability between the three readers with regards to the mSASSS status score was good to excellent at all time points with ICC ranging from 0.84 to 0.97 (online supplemental table S1). The reliability of the mSASSS change score was poor to good with ICC ranging from 0.31 to 0.84 (online supplemental table S2).
Longitudinal association between TNFi exposure and mSASSS change
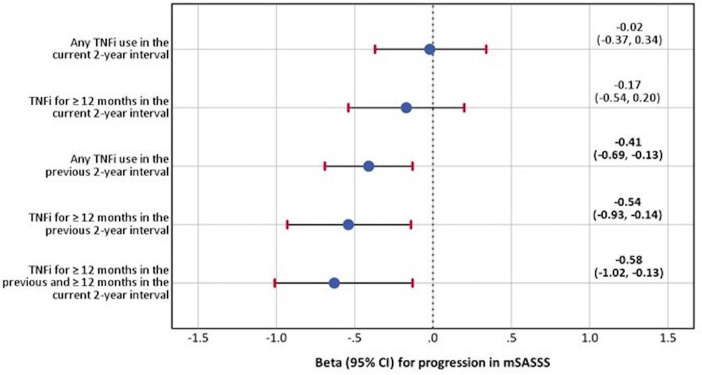
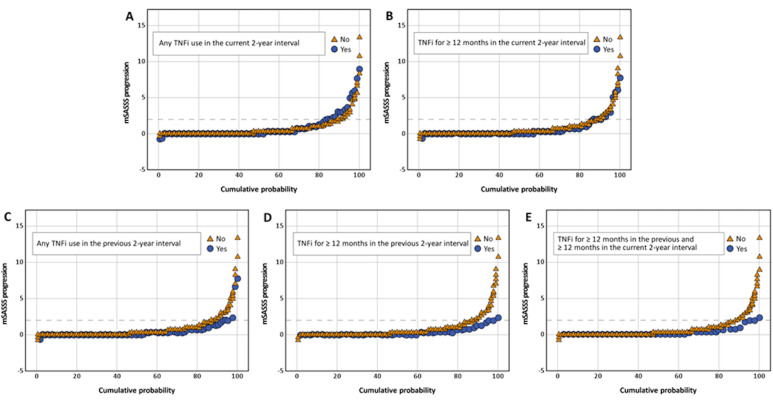
In the univariable analyses, TNFi exposure for ≥12 months in the previous 2-year interval was significantly associated with lower mSASSS progression (table 2). This was confirmed in the multivariable analyses: any TNFi exposure in the previous 2-year interval, exposure ≥12 months in the previous 2-year interval and same exposure that continued in the current interval were associated with reduction of the mSASSS progression by 0.41, 0.54, 0.58 mSASSS points, respectively, having TNFi unexposed patients as a reference (table 2 and figure 1). Of note, exposure to TNFi in the current 2-year interval was not associated with a reduction of the mSASSS progression. Cumulative probability plots (figure 2A–E) reflect the mSASSS progression in TNFi exposed and unexposed patients according to the different definitions across all available 2-year intervals. Interestingly, NSAIDs intake in the current 2-year interval was consistently associated with reduction of radiographic progression in the same interval in all multivariable models (table 2).
Table 2.
The association between progression of the mSASSS over 2 years and TNFi exposure in patients with axial spondyloarthritis from in the longitudinal generalised estimating equation analysis
| Variable | Univariable analysis β (95% CI) |
Multivariable model 1 β (95% CI) |
Multivariable model 2 β (95% CI) |
Multivariable model 3 β (95% CI) |
Multivariable model 4 β (95% CI) |
Multivariable model 5 β (95% CI) |
| Any TNFi use in the current 2-year interval | 0.26 (−0.14 to 0.65) | −0.02 (−0.37 to 0.34) | – | – | – | – |
| TNFi for ≥12 months in the current 2-year interval | −0.03 (−0.40 to 0.34) | – | −0.17 (−0.54 to 0.20) | – | – | – |
| Any TNFi use in the previous 2-year interval | −0.31 (−0.61 to 0.00) | – | – | −0.41 (−0.69 to −0.13) | – | – |
| TNFi for ≥12 months in the previous 2-year interval | −0.41 (−0.69 to −0.13) | – | – | – | −0.54 (−0.93 to −0.14) | – |
| TNFi for ≥12 months in the previous and ≥12 months in the current 2-year interval | −0.43 (−0.73 to −0.14) | – | – | – | – | −0.58 (−1.02 to −0.13) |
| Male sex | 0.52 (0.19 to 0.84) | 0.27 (0.01 to 0.53) | 0.27 (0.01 to 0.53) | 0.27 (0.01 to 0.53) | 0.26 (0.00 to 0.52) | 0.27 (0.01 to 0.53) |
| Symptom duration, years | 0.04 (0.00 to 0.08) | 0.00 (−0.03 to 0.03) | 0.01 (−0.03 to 0.04) | 0.01 (−0.02 to 0.04) | 0.01 (−0.02 to 0.05) | 0.01 (−0.02 to 0.05) |
| Time-averaged ASDAS, points | 0.24 (0.00 to 0.47) | 0.20 (−0.03 to 0.42) | 0.19 (−0.03 to 0.41) | 0.18 (−0.04 to 0.40) | 0.18 (−0.04 to 0.39) | 0.18 (−0.03 to 0.40) |
| Current smoking | 0.52 (0.15 to 0.89) | 0.37 (0.08 to 0.65) | 0.36 (0.08 to 0.64) | 0.37 (0.09 to 0.65) | 0.38 (0.10 to 0.66) | 0.38 (0.10 to 0.66) |
| Classification as r-axSpA | 0.61 (0.27 to 0.94) | 0.24 (−0.02 to 0.50) | 0.25 (−0.01 to 0.51) | 0.24 (−0.02 to 0.50) | 0.23 (−0.03 to 0.49) | 0.23 (−0.03 to 0.49) |
| mSASSS, points | 0.10 (0.07 to 0.13) | 0.09 (0.06 to 0.12) | 0.09 (0.06 to 0.12) | 0.09 (0.06 to 0.12) | 0.09 (0.06 to 0.12) | 0.09 (0.06 to 0.12) |
| NSAID score, per 10 points | 0.02 (−0.03 to 0.06) | −0.04 (−0.09 to −0.00) | −0.04 (−0.09 to −0.00) | −0.05 (−0.09 to −0.00) | −0.05 (−0.09 to −0.00) | −0.05 (−0.09 to −0.00) |
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; mSASSS, modified Stroke Ankylosing Spondylitis Spine Score; NSAID, non-steroidal anti-inflammatory drugs; r-axSpA, radiographic axSpA; TNFi, tumour necrosis factor alpha inhibitor.
Figure 1.
The multivariable longitudinal generalised estimating equation analysis* for the association between progression in the mSASSS over 2 years and TNFi use in patients with axial spondyloarthritis. *Parameter estimates from the multivariable models adjusted for sex, symptom duration at the beginning of the current 2-year interval, time-averaged ASDAS in the current 2-year interval, smoking in the current 2-year interval, classification as radiographic axSpA, mSASSS at the beginning of the current 2-year interval and NSAID intake score. ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; mSASSS, modified Stroke Anlylosing Spondylitis Spine Score; NSAID, non-steroidal anti-inflammatory drug; TNFi, tumour necrosis factor alpha inhibitor.
Figure 2.
Cumulative probability plot of the 2-year mSASSS change scores stratified by TNFi exposure status. (A) Any TNFi in the current interval, (B) at least 12 months TNFi in the current interval, (C) any TNFi in the previous interval, (D) at least 12 months TNFi in the previous interval and (E) TNFi for ≥12 months in the previous and ≥12 months in the current 2-year intervals. mSASSS, the modified Stoke Ankylosing Spondylitis Spinal Score; TNFi, tumour necrosis factor alpha inhibitor.
The analysis of the direct and indirect (mediated by reduction in disease activity as reflected by ASDAS) effects of TNFi on radiographic spinal progression is presented in table 3 and described in online supplemental figure S2. In the current 2-year interval, neither indirect (via reduction in ASDAS) nor direct effect of TNFi on mSASSS change was significant. However, in the models that included TNFi exposure in the previous 2-year radiographic interval, we observed significant direct effects of TNFi on radiographic progression in the current 2-year interval (β values were −0.40, –0.55, and −0.57, for any TNFi use in the previous 2-year interval, TNFi for ≥12 months in the previous 2-year interval and TNFi for ≥12 months in the previous and ≥12 months in the current 2-year interval, respectively—table 3).
Table 3.
The effects of TNFi on spinal radiographic progression in mediation analyses
| β | SE | 95% CI | |
| Any TNFi use in the current 2-year interval* | |||
| TNF → ASDAS | −0.20 | 0.09 | −0.38 to −0.02 |
| ASDAS → mSASSS progression | 0.18 | 0.08 | 0.02 to 0.34 |
| Indirect effect | −0.04 | 0.03 | −0.11 to 0.01 |
| Direct effect (TNF → mSASSS progression) | −0.05 | 0.16 | −0.37 to 0.28 |
| Total (direct and indirect) effect | −0.08 | 0.16 | −0.40 to 0.24 |
| TNFi for ≥12 months in the current 2-year interval* | |||
| TNF → ASDAS | −0.36 | 0.10 | −0.57 to −0.16 |
| ASDAS → mSASSS progression | 0.17 | 0.08 | 0.01 to 0.33 |
| Indirect effect | −0.06 | 0.05 | −0.18 to 0.01 |
| Direct effect (TNF → mSASSS progression) | −0.19 | 0.19 | −0.56 to 0.18 |
| Total (direct and indirect) effect | −0.25 | 0.19 | −0.62 to 0.11 |
| Any TNFi use in the previous 2-year interval* | |||
| TNF → ASDAS | −0.25 | 0.11 | −0.46 to −0.04 |
| ASDAS → mSASSS progression | 0.16 | 0.08 | 0.00 to 0.33 |
| Indirect effect | −0.04 | 0.04 | −0.14 to 0.01 |
| Direct effect (TNF → mSASSS progression) | −0.40 | 0.19 | −0.77 to −0.04 |
| Total (direct and indirect) effect | −0.45 | 0.19 | −0.81 to −0.08 |
| TNFi for ≥12 months in the previous 2-year interval* | |||
| TNF → ASDAS | −0.34 | 0.13 | −0.59 to −0.08 |
| ASDAS → mSASSS progression | 0.16 | 0.08 | 0.00 to 0.32 |
| Indirect effect | −0.05 | 0.05 | −0.18 to 0.02 |
| Direct effect (TNF → mSASSS progression) | −0.55 | 0.23 | −0.99 to −0.10 |
| Total (direct and indirect) effect | −0.60 | 0.23 | −1.05 to −0.15 |
| TNFi for ≥12 months in the previous and ≥12 months in the current 2-year interval* |
|||
| TNF → ASDAS | −0.27 | 0.14 | −0.55 to 0.01 |
| ASDAS → mSASSS progression | 0.17 | 0.08 | 0.01 to 0.33 |
| Indirect effect | −0.04 | 0.05 | −0.17 to 0.02 |
| Direct effect (TNF → mSASSS progression) | −0.57 | 0.25 | −1.17 to −0.08 |
| Total (direct and indirect) effect | −0.62 | 0.25 | −1.11 to −0.13 |
The table represents the direct, indirect and total effects of TNFi on mSASSS progression in patients with axSpA. These effects are described in the diagram in online supplemental figure S2 in detail.
*Parameter estimates from all multivariable models with different TNFi exposure definitions were adjusted for sex, symptom duration at the beginning of the current 2-year interval, smoking in the current 2-year interval, classification as radiographic axSpA, mSASSS at the beginning of the current 2-year interval, and NSAID intake score.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; mSASSS, modified Stroke Anlylosing Spondylitis Spine Score; NSAID, non-steroidal anti-inflammatory drugs; SE, standard error; TNFi, tumour necrosis factor alpha inhibitor.
Online supplemental table 3 presents the changes in mSASSS in the whole axSpA group and in the subgroups according to ASDAS-CRP categories (derived from the time-averaged ASDAS in the current 2 year interval) based on different definitions of TNFi exposure. The progression rate was highest in patients with very high disease activity who received no TNFi. Overall, the effect of TNFi on radiographic spinal progression was largely consistent across all subgroups.
Longitudinal association between TNFi exposure and binary outcomes
The results for the binary definitions of progression (progression ≥2 mSASSS points over 2 years, formation of ≥1 new syndesmophyte over 2 years) were in line with the analyses that used the continuous mSASSS change score as an outcome, although the precision of the effect estimation was lower as reflected by large 95% CIs (table 4). In general, TNFi exposure in the previous 2-year interval was associated with lower odds for progression in the current one; for example, any TNFi exposure in the previous 2-year interval was associated with a 69% reduction of the odds of formation of new syndesmophytes in the current interval, OR 0.31, 95% CI 0.10 to 0.95 (table 4).
Table 4.
The association between progression ≥2 mSASSS points and formation of ≥1 new syndesmophyte over 2 years and TNFi exposure in patients with axial spondyloarthritis in a binomial generalised estimating equation analysis
| Model* | TNFi exposure definition | Reference | Progression ≥2 mSASSS points OR (95% CI) |
Formation of ≥1 new syndesmophyte OR (95% CI) |
| 1 | Any TNFi use in the current 2-year interval | No TNFi use in the current 2-ear interval | 1.39 (0.64 to 3.01) | 1.18 (0.50 to 2.79) |
| 2 | TNFi for ≥12 months in the current 2-year interval | No TNFi for ≥12 months in the current 2-year interval | 0.96 (0.35 to 2.66) | 0.75 (0.25 to 2.28) |
| 3 | Any TNFi use in the previous 2-year interval | No TNFi use in the previous 2-year interval | 0.30 (0.08 to 1.20) | 0.31 (0.10 to 0.95) |
| 4 | TNFi for ≥12 months in the previous 2-year interval | No TNFi for ≥12 months in the previous 2-year interval | 0.21 (0.02 to 3.08) | 0.36 (0.12 to 1.07) |
| 5 | TNFi for ≥12 months in the previous and ≥12 months in the current 2-year interval | No TNFi for ≥12 months in the previous and ≥12 months in the current 2-year interval | 0.29 (0.02 to 4.86) | 0.43 (0.12 to 1.55) |
*Parameter estimates from the multivariable models adjusted for sex, symptom duration at the beginning of the current 2-year interval, time-averaged ASDAS in the current 2-year interval, smoking in the current 2-year interval, classification as radiographic axSpA, mSASSS at the beginning of the current 2-year interval and NSAID intake score.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial Spondyloarthritis; mSASSS, modified Stroke Anlylosing Spondylitis Spine Score; NSAID, non-steroidal anti-inflammatory drugs; TNFi, tumour necrosis factor alpha inhibitor.
Discussion
In the present study, we could demonstrate that TNFi treatment is associated with reduction of radiographic spinal progression in patients with axSpA. Importantly, the effect could not be observed immediately after treatment initiation (in the first 2 years) but became evident after 4 years of observation. The effect was clinically relevant if considered in the context of natural radiographic progression in axSpA with a mean of 1–2 mSASSS points per 2 years25 26: TNFi treatment was associated with reduction of radiographic progression by 0.5–0.6 mSASSS points in 2 years as compared with patients not treated with TNFi.
We hope that our data will contribute to current knowledge in the field that evolved after publication of first long-term extension studies with TNFi in AS. These studies showed that TNFi treatment over 2 years was not associated with retardation of radiographic spinal progression as compared with historical controls.8–10 Subsequent works indicated, however, that such a retardation might be possible, especially if treatment is applied long-term, which is in line with our results, although there are some differences in terms of the study design, patient characteristics and definition of TNFi exposure in the intervals.11–18 Indeed, it seems that the effect of anti-inflammatory treatment with TNFi cannot be observed immediately after treatment initiation—at least not with radiographs as the method of structural damage assessment. This is related to the fact that inflammation in the vertebral body is followed by the process of repair characterised by the replacement of the inflammatory-affected bone marrow by fibrous repair tissue that gives raise to new bone formation (syndesmophytes) later on.5 27 This sequel has been confirmed by recent data correlating both, MRI and histological data7 28 and MRI and radiographic data.6 29 It can be, therefore, expected that in the first 2 years after TNFi initiation, we observe the process of new bone formation that has started already before or just after (‘TNF-brake’) release30 treatment initiation that slows down radiographic progression between year 2 and year 4. This means that effective and continuous (and ideally early) control of inflammation is necessary to modify the natural course of structural damage progression in axSpA, which is also in line with our analysis, where we demonstrated a similar effect of TNFi on radiographic sacroiliitis progression.20 The observed direct effect of TNFi exposure—especially when used in the previous interval—on radiographic progression, suggests that either the inflammatory burden is not fully captured by ASDAS or CRP (we think this is the most likely explanation since we have not captured the presence and extent of local inflammation in the spine) or that TNFi might also have additional effects on new bone formation independent of anti-inflammatory properties.31 32 Indeed, earlier studies indicated that TNF might stimulate new bone formation (especially at the early stages of endochondral ossification) via upregulation of osteogenic mediators such as bone morphogenic proteins.33 34
It is important to mention that the natural course of structural progression is very heterogeneous and that not all patients with axSpA develop clinically relevant (in terms of irreversible reduction of function and spinal mobility) damage in the spine.25 Therefore, ‘early’ is a term that is not well defined in the context of axSpA since duration of symptoms correlates only to some extent with the presence of the structural damage in the spine. The same imprecision holds also true for the so-called ‘window of opportunity’—in some patients, the window is rather small (high-risk patients with early syndemophytes, high inflammatory activity as reflected by elevated CRP and spinal inflammation on MRI), but in other cases, the window remains open many years, sometimes life long. In any case, a treatment strategy focussing on symptom and inflammation control seems to be beneficial for all patients; patients with a high risk of structural damage development might need, however, special attention with a tight-control strategy.
Is there a possibility to stop structural damage in axSpA immediately on treatment initiation? This question remains unsolved until now. Such a treatment modality would need to have a direct inhibitory effect on osteoblasts participating in the process of new bone formation in the spine. NSAIDs showed some promising results in an earlier study (with patients treated mainly with a selective cyclooxygenase (COX)−2 drug celecoxib),35 while a subsequent study (with non-selective COX-inhibitor diclofenac as an investigational drug) could not demonstrate an inhibitory effect of a continuous versus on-demand intake on radiographic spinal progression in axSpA.36 Interestingly, in our present analysis, higher NSAID intake was associated with reduction of radiographic spinal progression in the current 2-year interval. Similar effect of NSAIDs was observed in the previous work that analysed 2-year data from GESPIC.22 An ongoing prospective controlled study comparing a TNFi monotherapy with a combination of TNFi plus celecoxib should clarify the question about the potential role of NSAIDs in reducing structural damage progression in axSpA.37
There is an ongoing discussion, whether IL-17 blockade is able to retard structural damage progression in axSpA not only through inhibition of inflammation but also through a direct inhibition of osteoblastic activity as suggested by some preclinical data.38 A currently ongoing head-to-head comparison of an IL-17 inhibitor secukinumab with the TNFi adalimumab focussing on radiographic spinal progression39 should demonstrate if there is a clinically relevant difference between these drug classes. In GESPIC, the 10-year follow-up visit was completed for most patients before IL-17 inhibitors’ approval; therefore, we could not investigate the effect of this drug class on radiographic spinal progression.
There are some other limitations of the current study we need to acknowledge. First, with conventional spinal radiographs, we capture only a relatively small part of the structural damage occurring in the spine of patients with axSpA. CT might be able to substitute radiographs in the future for a comprehensive assessment of structural damage and a better sensitivity to change.40 41 Second, MRI scans of the spine were not performed in GESPIC, thus, the presence and extent of spinal inflammation could only be captured indirectly by CRP. Third, unblinded reading of radiographs might lead to an overestimation of progression. Nevertheless, this method was chosen due to its higher sensitivity to change and potential reduction of ‘background noise’ not related to true structural changes especially in a setting with multiple time points.42 Although the interobserver reliability of the mSASSS was good to excellent for the status scores across all time points, the poor to good ICCs concerning the change scores may be considered as a further limitation. Finally, we had to exclude a substantial number of patients who had no complete sets of spinal radiographs. Although the included and the excluded groups were largely comparable, the risk of attrition bias cannot be completely excluded.
In conclusion, in the present study, we could demonstrate retardation of radiographic spinal progression associated with TNFi treatment in patients with axSpA. This effect was time shifted and observed between 2 and 4 years after treatment initiation.
Acknowledgments
We thank Prof. M. Leirisalo-Repo (Finland), Prof. D. van der Heijde (The Netherlands) and Prof. M. Dougados (France) for scientific advice on the design of the cohort. We are grateful to Beate Buss, Petra Tietz, and Annegret Langdon for monitoring the cohort, Johanna Callhoff, Anja Weiss, Joachim Listing and Martina Niewerth for the data management support and statistical advice, Torsten Karge for the development of the image scoring interface, and to all patients who voluntarily participated in this cohort. Further, we would like to thank the following rheumatologists for inclusion of their patients: J. Brandt, H. Brandt, G.-R. Burmester, H. Deister, E. Edelmann, J. Emmerich, M. Enderlein, A. Gauliard, E. Gromnica-Ihle, F. Heldmann, S. Hermann, U. von Hinüber, Ü. Hübner, K. Karberg, H. Nüßlein, R. Pelle-Lohfink, D. Pick, G. Reichmuth, E. Riechers, M. Rihl, R. Schmidt, S. Schnarr, U. Schneider, I.-H. Song, I. Spiller, U. Syrbe, V. Walz, S. Wassenberg, H. M. Wisseler, S. Zinke.
Footnotes
Handling editor: Josef S Smolen
Twitter: @MProtopopov
Contributors: All authors have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; finally approved the version to be published; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DP is responsible for the overall content as the guarantor and accepts full responsibility for the work, had access to the data and controlled the decision to publish.
Funding: GESPIC has been financially supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung-BMBF). As funding by BMBF was reduced according to schedule in 2005 and stopped in 2007, complementary financial support has been obtained also from Abbott/Abbvie, Amgen, Centocor, Schering-Plough, and Wyeth. Since 2010 GESPIC was supported by Abbvie, additional support has been obtained also from ANCYLOSS (grant number FKZ 01EC1002D), ArthroMark (grant numbers FKZ 01EC1009A and FKZ 01EC1401A), and METARTHROS (grant number FKZ 01EC1407A) projects funded by BMBF.
Competing interests: VRR: reports grants and personal fees from Falk e.V., consulting fees from AbbVie. FP: reports grants and personal fees from Novartis and Lilly, and personal fees from AbbVie, AMGEN, BMS, Celgene, MSD, Pfizer, Roche, UCB, and Janssen. MP: reports personal fees from Novartis and UCB. JR: reports personal fees from Novartis and UCB. HH: reports consulting fees from Boehringer, Janssen, MSD, Novartis, Sobi; personal fees from MSD, Janssen, Roche, Pfizer, Novartis, AbbVie, and Sobi. JS: reports consulting fees from: AbbVie, Lilly, Merck, Novartis, UCB; consulting fees from: Abbvie, Lilly, Merck, Novartis, UCB; speaker fees from: Abbvie, Janssen, Lilly, Merck, Novartis, UCB; personal fees from: AbbVie. MR: reports personal fees from: Abbvie, Janssen, Eli Lilly, Novartis, Galapagos, Pfizer, and UCB; speaker fees from: Abbvie, Boehringer Ingelheim, Celgene, Chugai, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB. DP: reports research support from: AbbVie, Eli Lilly, MSD, Novartis, Pfizer; consulting fees from: AbbVie, Biocad, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, Samsung Bioepis, and UCB; speaker fees from: AbbVie, Bristol-Myers Squibb, Eli Lilly, MSD, Novartis, Pfizer, and UCB.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The original study data can be made available upon reasonable request that should be directed to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the ethics committee of the coordinating centre (Charité—Universitätsmedizin Berlin, Germany/ethics approval number 188-19) and by local ethical committees of participating centres. Participants gave informed consent to participate in the study before taking part.
References
- 1. Sieper J, Poddubnyy D. Axial spondyloarthritis. The Lancet 2017;390:73–84. 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 2. Stal R, van Gaalen F, Sepriano A, et al. Facet joint ankylosis in r-axSpA: detection and 2-year progression on whole spine low-dose CT and comparison with syndesmophyte progression. Rheumatology 2020;59:3776–83. 10.1093/rheumatology/keaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Machado P, Landewé R, Braun J, et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465–70. 10.1136/ard.2009.124206 [DOI] [PubMed] [Google Scholar]
- 4. Poddubnyy D, Listing J, Haibel H, et al. Functional relevance of radiographic spinal progression in axial spondyloarthritis: results from the German spondyloarthritis inception cohort. Rheumatology 2018;57:703–11. 10.1093/rheumatology/kex475 [DOI] [PubMed] [Google Scholar]
- 5. Poddubnyy D, Sieper J. Mechanism of new bone formation in axial spondyloarthritis. Curr Rheumatol Rep 2017;199:55. 10.1007/s11926-017-0681-5 [DOI] [PubMed] [Google Scholar]
- 6. Machado PM, Baraliakos X, van der Heijde D, et al. Mri vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016;75:1486–93. 10.1136/annrheumdis-2015-208011 [DOI] [PubMed] [Google Scholar]
- 7. Bleil J, Maier R, Hempfing A, et al. Granulation tissue Eroding the Subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheumatol 2016;68:2456–65. 10.1002/art.39715 [DOI] [PubMed] [Google Scholar]
- 8. van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. 10.1186/ar2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Heijde D, Landewé R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31. 10.1002/art.23471 [DOI] [PubMed] [Google Scholar]
- 10. van der Heijde D, Landewé R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. 10.1002/art.23901 [DOI] [PubMed] [Google Scholar]
- 11. Haroon N, Inman RD, Learch TJ. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;6510:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baraliakos X, Haibel H, Listing J, et al. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:710–5. 10.1136/annrheumdis-2012-202698 [DOI] [PubMed] [Google Scholar]
- 13. Sepriano A, Ramiro S, Wichuk S, et al. Tumor necrosis factor inhibitors reduce spinal radiographic progression in patients with radiographic axial spondyloarthritis: a longitudinal analysis from the Alberta prospective cohort. Arthritis Rheumatol 2021;73:1211–9. 10.1002/art.41667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maas F, Arends S, Wink FR, et al. Ankylosing spondylitis patients at risk of poor radiographic outcome show diminishing spinal radiographic progression during long-term treatment with TNF-α inhibitors. PLoS One 2017;12:e0177231. 10.1371/journal.pone.0177231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koo BS, Oh JS, Park SY, et al. Tumour necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18-year real-world evidence. Ann Rheum Dis 2020;79:1327–32. 10.1136/annrheumdis-2019-216741 [DOI] [PubMed] [Google Scholar]
- 16. Park JW, Kim MJ, Lee JS, et al. Impact of tumor necrosis factor inhibitor versus nonsteroidal antiinflammatory drug treatment on radiographic progression in early ankylosing spondylitis: its relationship to inflammation control during treatment. Arthritis Rheumatol 2019;71:82–90. 10.1002/art.40661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molnar C, Scherer A, Baraliakos X, et al. Tnf blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss clinical quality management cohort. Ann Rheum Dis 2018;77:63–9. 10.1136/annrheumdis-2017-211544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hebeisen M, Micheroli R, Scherer A, et al. Spinal radiographic progression in axial spondyloarthritis and the impact of classification as nonradiographic versus radiographic disease: data from the Swiss clinical quality management cohort. PLoS One 2020;15:e0230268. 10.1371/journal.pone.0230268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum 2009;60:717–27. 10.1002/art.24483 [DOI] [PubMed] [Google Scholar]
- 20. Torgutalp M, Rios Rodriguez V, Proft F, et al. Treatment with Tumour Necrosis Factor Inhibitors is Associated with a Time‐Shifted Retardation of Radiographic Sacroiliitis Progression in Patients with Axial Spondyloarthritis: 10‐year Results from the German Spondyloarthritis Inception Cohort. Arthritis & Rheumatology 2022. 10.1002/art.42144 [DOI] [PubMed] [Google Scholar]
- 21. Dougados M, Paternotte S, Braun J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. 10.1136/ard.2010.133488 [DOI] [PubMed] [Google Scholar]
- 22. Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German spondyloarthritis inception cohort. Ann Rheum Dis 2012;71:1616–22. 10.1136/annrheumdis-2011-201252 [DOI] [PubMed] [Google Scholar]
- 23. Hardin J, Hilbe J. Generalized estimating equations. 2 ed. CRC Press. Taylor & Francis Group. Chapman and Hall book, 2012. [Google Scholar]
- 24. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. 2 ed. The Guilford Press, 2017. [Google Scholar]
- 25. Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015;74:52–9. 10.1136/annrheumdis-2013-204055 [DOI] [PubMed] [Google Scholar]
- 26. Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012;64:1388–98. 10.1002/art.33465 [DOI] [PubMed] [Google Scholar]
- 27. Sieper J, Appel H, Braun J, et al. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum 2008;58:649–56. 10.1002/art.23260 [DOI] [PubMed] [Google Scholar]
- 28. Baraliakos X, Boehm H, Bahrami R, et al. What constitutes the fat signal detected by MRI in the spine of patients with ankylosing spondylitis? A prospective study based on biopsies obtained during planned spinal osteotomy to correct hyperkyphosis or spinal stenosis. Ann Rheum Dis 2019;78:1220–5. 10.1136/annrheumdis-2018-214983 [DOI] [PubMed] [Google Scholar]
- 29. Baraliakos X, Heldmann F, Callhoff J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? a long-term observational study using MRI and conventional radiography. Ann Rheum Dis 2014;73:1819–25. 10.1136/annrheumdis-2013-203425 [DOI] [PubMed] [Google Scholar]
- 30. Pedersen SJ, Chiowchanwisawakit P, Lambert RGW, et al. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J Rheumatol 2011;38:1349–54. 10.3899/jrheum.100925 [DOI] [PubMed] [Google Scholar]
- 31. Sedger LM, McDermott MF,. Tnf and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev 2014;25:453–72. 10.1016/j.cytogfr.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 32. Kalliolias GD, Ivashkiv LB. Tnf biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016;12:49–62. 10.1038/nrrheum.2015.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lories RJ, Haroon N. Bone formation in axial spondyloarthritis. Best Pract Res Clin Rheumatol 2014;28:765–77. 10.1016/j.berh.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 34. Carter S, Braem K, Lories RJ. The role of bone morphogenetic proteins in ankylosing spondylitis. Ther Adv Musculoskelet Dis 2012;4:293–9. 10.1177/1759720X12444175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wanders A, Heijde Dvander, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005;52:1756–65. 10.1002/art.21054 [DOI] [PubMed] [Google Scholar]
- 36. Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43. 10.1136/annrheumdis-2015-207897 [DOI] [PubMed] [Google Scholar]
- 37. Proft F, Muche B, Listing J, et al. Study protocol: comparison of the effect of treatment with nonsteroidal anti-inflammatory drugs added to anti-tumour necrosis factor a therapy versus anti-tumour necrosis factor a therapy alone on progression of structural damage in the spine over two years in patients with ankyLosing spondylitis (CONSUL) – an open-label randomized controlled multicenter trial. BMJ Open 2017;7:e014591. 10.1136/bmjopen-2016-014591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Tok MN, van Duivenvoorde LM, Kramer I, et al. Interleukin-17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis Rheumatol 2019;71:612–25. 10.1002/art.40770 [DOI] [PubMed] [Google Scholar]
- 39. Baraliakos X, Østergaard M, Gensler LS, et al. Comparison of the effects of Secukinumab and adalimumab Biosimilar on radiographic progression in patients with ankylosing spondylitis: design of a randomized, phase IIIB study (surpass). Clin Drug Investig 2020;40:269–78. 10.1007/s40261-020-00886-7 [DOI] [PubMed] [Google Scholar]
- 40. Ward MM, Tan S. Better quantification of Syndesmophyte growth in axial spondyloarthritis. Curr Rheumatol Rep 2018;20:46. 10.1007/s11926-018-0759-8 [DOI] [PubMed] [Google Scholar]
- 41. de Bruin F, de Koning A, van den Berg R, et al. Development of the CT Syndesmophyte score (CTSS) in patients with ankylosing spondylitis: data from the SIAS cohort. Ann Rheum Dis 2018;77:371–7. 10.1136/annrheumdis-2017-212553 [DOI] [PubMed] [Google Scholar]
- 42. Wanders A, et al. Scoring of radiographic progression in randomised clinical trials in ankylosing spondylitis: a preference for paired reading order. Ann Rheum Dis 2004;63:1601–4. 10.1136/ard.2004.022038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2022-222324supp001.pdf (259.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The original study data can be made available upon reasonable request that should be directed to the corresponding author.