ABSTRACT
Modern medicine is threatened by the rising tide of antimicrobial resistance, especially among Gram-negative bacteria, where resistance to β-lactams is most often mediated by β-lactamases. The penicillin and cephalosporin ascendancies were, in their turn, ended by the proliferation of TEM penicillinases and CTX-M extended-spectrum β-lactamases. These class A β-lactamases have long been considered the most important. For carbapenems, however, the threat is increasingly from the insidious rise of a class D carbapenemase, OXA-48, and its close relatives. Over the past 20 years, OXA-48 and “OXA-48-like” enzymes have proliferated to become the most prevalent enterobacterial carbapenemases across much of Europe, Northern Africa, and the Middle East. OXA-48-like enzymes are notoriously difficult to detect because they often cause only low-level in vitro resistance to carbapenems, meaning that the true burden is likely underestimated. Despite this, they are associated with carbapenem treatment failures. A highly conserved incompatibility complex IncL plasmid scaffold often carries blaOXA-48 and may carry other antimicrobial resistance genes, leaving limited treatment options. High conjugation efficiency means that this plasmid is sometimes carried by multiple Enterobacterales in a single patient. Producers evade most β-lactam–β-lactamase inhibitor combinations, though promising agents have recently been licensed, notably ceftazidime-avibactam and cefiderocol. The molecular machinery enabling global spread, current treatment options, and the development pipeline of potential new therapies for Enterobacterales that produce OXA-48-like β-lactamases form the focus of this review.
KEYWORDS: OXA-48 β-lactamase, treatment, pharmacology, drug development, OXA-48, beta-lactamases, epidemiology
INTRODUCTION
An estimated 4.95 million deaths associated with bacterial antimicrobial resistance (AMR) in 2019 make it a leading cause of mortality worldwide and a serious threat to modern medicine (1). The rise in resistance to carbapenems—until recently the last-resort β-lactams—is a particular concern. OXA-48-like β-lactamases, including OXA-48 itself, are important because they hydrolyze carbapenems, readily transmit among a wide range of Enterobacterales (2), frequently associate with other resistances, and have recently (though rarely) been identified among Pseudomonas aeruginosa and Acinetobacter baumannii (3, 4).
OXA-48-like β-lactamases are notoriously difficult to detect in the clinical laboratory, impeding implementation of infection control and facilitating their stealthy rise. Since around 2010, nosocomial spread and outbreaks have increased worldwide (5–12). This has merged into endemicity among a range of Enterobacterales across much of Eurasia and Africa (13).
Therapeutic options remain limited (14), though promising agents have been recently licensed (ceftazidime-avibactam and cefiderocol) or are progressing through development (various β-lactam inhibitor combinations and monobactams). The utility of older therapies remains debatable.
Here, we review the structure and function of OXA-48-like β-lactamases, how their genetic support allowed proliferation engendering a serious threat to health care systems, their current epidemiology and, lastly, current and emerging treatment options.
CLASSIFICATION
OXA-48-like enzymes are serine β-lactamases (SBLs) (15, 16) belonging to molecular class D. This family is the most diverse of the four β-lactamase classes defined by Ambler et al., who divide these enzymes based on their amino acid sequences (17, 18).
SBLs hydrolyze their substrates by forming an acyl intermediate through the active-site serine, and class D β-lactamases (DBLs) or “OXA types” have oxacillin as their preferred substrate in terms of kcat/Km, which measures catalytic efficiency. DBLs vary greatly in both sequence and substrate spectrum. Some (e.g., OXA-1 and -2) are primarily penicillinases, others (e.g., OXA-11 and -14) have an extended spectrum (extended-spectrum β-lactamases [ESBLs]), and a few, including OXA-48 and most OXA-48-like enzymes, are carbapenemases. Each has a unique number assigned based on the chronological order of first description.
Bush and Jacoby’s functional β-lactamase classification (18) places OXA-48 β-lactamases, along with the carbapenemases of Acinetobacter spp. (OXA-23 and similar) in group 2 (subgroup 2df), where the defining characteristics are the ability to hydrolyze cloxacillin, oxacillin, and carbapenems, with no inhibition by EDTA. Alternatively, in 2010, Poirel et al. (19) proposed a division of DBLs into four groups: (i) acquired narrow-spectrum β-lactamases, (ii) acquired expanded-spectrum β-lactamases, (iii) acquired carbapenem-hydrolyzing β-lactamases, and (iv) naturally occurring (chromosomal) types (Table 1). A complexity is that the “acquired” types are escaped chromosomal types from other species, blurring that distinction. For example, OXA-23 originated from Acinetobacter radioresistens (20) and OXA-48 from Shewanella spp. (see below). A second complexity is diversity among acquired class D carbapenemases comprising group 3: OXA-48 types attack penicillins but mostly not extended-spectrum cephalosporins, whereas the Acinetobacter OXA types attack both penicillins and cephalosporins (19).
TABLE 1.
Proposed division of class D β-lactamases by Poirel et al. (19)
| Group | Examples | Host |
|---|---|---|
| Acquired narrow-spectrum β-lactamases | OXA-1, OXA-2, and OXA-10 subgroups; OXA-20, OXA-21, OXA-22, OXA-29, and OXA-30 | Mainly identified from Enterobacterales and P. aeruginosa |
| Acquired expanded-spectrum β-lactamases | OXA-11, OXA-14, OXA-15, OXA-16, OXA-17, OXA-18, OXA-19, OXA-28, OXA-31, OXA-32, OXA-35, OXA-45, and OXA-53 | Mainly identified from P. aeruginosa |
| Acquired carbapenem-hydrolyzing β-lactamases | OXA-23, OXA-40, OXA-58, and OXA-97 | Mainly identified from Acinetobacter spp. |
| OXA-48 and OXA-48-like | Mainly identified from Enterobacterales | |
| Naturally occurring types | OXA-10 | Acinetobacter baumannii |
| OXA-12 | Aeromonas jandaei | |
| OXA-23 | Acinetobacter radioresistans | |
| OXA-61 | Campylobacter jejuni | |
| OXA-42 and OXA-43 | Burkholderia pseudomallei | |
| OXA-50 | Pseudomonas aeruginosa | |
| OXA-51 | Acinetobacter baumannii | |
| OXA-54 | Shewanella oneidensis | |
| OXA-57 | Burkholderia pseudomallei |
Since OXA-48 enzyme was first described, several variants have been observed, collectively forming the “OXA-48-like” subfamily. Variants differ from OXA-48 by one to five amino acid substitutions and/or deletions (21). More-prevalent members include OXA-181 (four substitutions at Thr104Ala, Asn110Asp, Glu168Gln, and Ser171Ala) (22), OXA-232 (single substitution at Arg214Ser) (23), OXA-204 (two substitutions at Gln98His and Thr99Arg) (24), OXA-162 (single substitution at Thr213Ala) (25), OXA-244 (single substitution at Arg214Gly), OXA-163 (four deletions at Arg214, Ile215, Glu216, and Pro217 and a single substitution at Ser220Asp) (26) OXA-245 (single substitution at Glu125Tyr) (27), OXA-370 (single substitution at Gly220Glu) (28), and OXA-405 (four deletions at Thr213 to Glu216) (29). Almost all are carbapenemases unable to hydrolyze cephalosporins, but some, including OXA-163, OXA-405 (29), and OXA 247 (30), show the converse pattern.
ENZYME STRUCTURE AND FUNCTION
OXA-48 has a dimeric structure, similar to OXA-10 (31), OXA-13 (32), and OXA-46 (33), comprising two identical subunits. The active site is in a narrow crevice presenting three motifs typical of DBLs in addition to the carbamylated side chain of Lys73 (34). These elements (notably Arg214) are critical for substrate recognition and for catalysis (15, 35). OXA-48-like enzymes have a unique β-5–β-6 loop conformation, which extends to the outer portion of the active-site crevice, modifying the charge distribution and narrowing the active site compared with more remote DBLs, such as OXA-10 (31, 36). This loop is considered important for carbapenem hydrolysis, although there is a different conformation in A. baumannii OXA enzymes (i.e., OXA-23, -24, and -58) (37–39). OXA-48 preferentially hydrolyzes imipenem (kcat, 5 s−1) compared with the 1-β-methyl-carbapenems, meropenem and ertapenem, whose kcat values are ≤1 s−1 (34, 40); the kcat for oxacillin, for comparison, is 25 s−1 (41). Nonetheless, ertapenem MICs are raised more than for other carbapenems, including imipenem.
Efficient hydrolysis relies on rotation of the carbapenem’s alpha-hydroxyethyl group, as promoted by the conformation of residues located in or close to the β-5–β-6 loop, which subsequently allows for the deacylating water molecule to access the acylated serine residue (34). Sequence modifications in OXA-163 (see above) correspond to positions belonging to, or close to, the β-5 strand (34), explaining spectrum changes compared with other OXA-48-like enzymes. Specifically, the lack of Arg214 in OXA-163 disturbs the active-site conformation and compromises interactions with carbapenems (26), whereas cephalosporin breakdown is efficient due to control of active-site solvation requiring orientation of Leu158 in the Ω loops (42).
Of significant concern is the recent prediction that a single mutation in the OXA-48 β-5–β-6 loop (Arg214Ser) would increase the efficiency of ceftazidime deacylation relative to that of OXA-163 (42), though the authors do not speculate on how this might affect carbapenemase activity.
MOLECULAR MACHINERY: MOBILIZATION AND GLOBAL SPREAD
The original reservoir of blaOXA-48 was demonstrated to be the waterborne Gram-negative saprophyte Shewanella oneidensis, which has a chromosomal β-lactamase gene, blaOXA-54. blaOXA-54 has genetic components upstream and downstream similar to those of blaOXA-48, and its product has 92% amino acid identity to plasmid-associated OXA-48 (41). Other Shewanella species have been sequenced, and their role as progenitors of blaOXA-48-like genes has been confirmed (43), although the relative importance of new escapes versus postescape mutations is uncertain.
Plasmids are the primary vehicle for the transmission and propagation of blaOXA-48-like genes. Several plasmid types have been observed to host blaOXA-48-like genes, including IncL, IncA/C, IncF, ColKP3, ColE2, IncX3, IncN1, and IncT plasmids (13). The most frequent hosts for blaOXA-48 itself are self-conjugative 60- to 70-kb plasmids with an IncL scaffold (13, 44, 45) also encoding the replicon protein RepP (46). IncA/C plasmids often carry blaOXA-204 (47), whereas blaOXA-181/232 are predominantly associated with IncX3 and ColKP3 plasmids (44, 48, 49).
In contrast to many other oxacillinase determinants, blaOXA-48-like genes are not associated with class 1 integrons (50, 51). Rather, the “typical” IncL plasmid harbors blaOXA-48 via acquisition of transposon Tn1999 with an upstream, and often a downstream, IS1999 insertion sequence (52). Expression is driven by the outward-directed promoter Pout, located in IS1999 (53). When blaOXA-48 was originally described from Klebsiella pneumoniae in Turkey in 2001, IS1999 was immediately upstream of the enzyme’s gene (50) and the transposon had integrated within the tir gene, which is recognized as key for high conjugation and transfer frequency (54, 55). This Tn1999 transposon structure has several variants, designated Tn1999.2, Tn1999.3, Tn1999.4, and Tn1999.5 (46, 56, 57). IncL plasmids with embedded Tn1999.2 were critical to the dissemination of blaOXA-48 across Libya, Turkey, and the Netherlands (58).
OXA-181 has been identified among diverse sequence types (STs) but with nearly identical IncX3 plasmids responsible for spread in different countries, including Jordan, Egypt, Turkey, South Korea, Thailand, South Africa, and Kuwait (59). Furthermore, although superclone K. pneumoniae ST307 was implicated as an OXA-181 producer in a large outbreak across hospitals in South Africa, the IncX3 plasmid was universally identified and found to be identical to others reported with blaOXA-181 from China and Angola (48). Plasmid-borne OXA-48-like carbapenemases have been observed in high-risk international clones, including K. pneumoniae ST147 (12), ST307 (48), ST15 (13), ST14 (51), ST23 (60), and ST405 (61) and Escherichia coli ST38 and ST410 (13), but these are not the major factor behind the enzymes’ global spread (46). Thus, E. coli, K. pneumoniae, and Enterobacter cloacae were the predominant blaOXA-48-like hosts in the United Kingdom; these were polyclonal, and the common denominator was that many harbored a disseminated IncL plasmid carrying the carbapenemase gene (62).
Inevitably, exceptions to these generalizations occur. Clones with OXA-48-like enzymes do, of course, cause local and regional outbreaks; examples are given (see above and below). Furthermore, while plasmids are the principal mechanism responsible for global dissemination (13, 55), a chromosome-integrated blaOXA-48-like gene has been observed in emerging clones such as E. coli ST38 with OXA-244 β-lactamase, which has spread across several European countries (63). Last, there is a growing problem, internationally, with K. pneumoniae, commonly of ST14 (an anyway frequent lineage) carrying OXA-48-like (often OXA-232) enzymes together with NDM (New Delhi metallo-β-lactamase) types (64–66). These points should not, however, detract from the great importance of plasmids for the dissemination of blaOXA-48-like and the overall diversity of producers. This contrasts with KPC carbapenemases, where the K. pneumoniae ST258 has been a considerable vector of global dissemination.
CHALLENGES IN THE CLINICAL MICROBIOLOGICAL LABORATORY
A wide range of Enterobacterales can carry blaOXA-48 and its variants, with a few reports also for P. aeruginosa (3). In principle, biochemical or genetic detection can be achieved by immunochromatography, multiplex PCR, or matrix-assisted laser desorption ionization–time of flight (MALDI-TOF), among other assays. However, the practical difficulties for OXA-48-like β-lactamases are greater than for other carbapenemases. These challenges have recently been reviewed (67, 68) and are not discussed in detail here. Briefly, they include the following: (i) that carbapenem resistance is often low level, meaning that a high level of suspicion is required to ensure that all likely producers are recognized and examined further; (ii) that unlike for KPC carbapenemases and metallo-β-lactamases (MBLs), there are no simple synergy tests to aid detection; (iii) that owing to weak expression, biochemical tests (e.g., Carba-NP) have a greater risk of false-negative results, and finally (iv) that some variants (including OXA-163, OXA-405, and OXA-247) with increased expanded-spectrum cephalosporin hydrolysis may be incorrectly identified as class A, although these are rare. These challenges mean that OXA-48-like enzymes often pass undetected, impacting our understanding of their global epidemiology, burden of associated infection, and current treatment outcomes.
Figure 1 shows a meropenem susceptibility distribution for 906 carbapenemase-producing Enterobacterales (CPEs) submitted to Public Health England (PHE), illustrating how MICs for OXA-48 producers are mainly ≤2 mg/L and lower than those for Enterobacterales with other carbapenemases (69, 70). Consequently, many producers count as “susceptible” based on current EUCAST (≤2 mg/L) and CLSI (≤1 mg/L) clinical breakpoints (71, 72).
FIG 1.
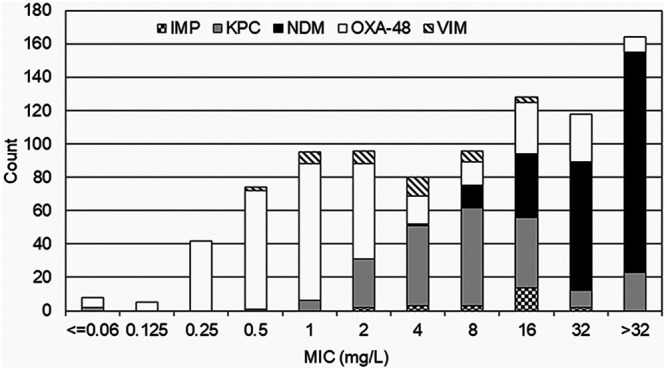
MIC distribution of meropenem for Enterobacterales. MIC distributions of meropenem for Enterobacterales that were submitted to United Kingdom Health Security Agency (previously Public Health England) Antimicrobial Resistance and Healthcare Associated Infection Reference Unit from July 2015 to July 2016 are shown. (Reproduced from reference 69 with permission of the Infectious Diseases Society of America.)
Two useful indicators in a clinical laboratory workflow are that producers of OXA-48-like enzymes reliably display high-level resistance to piperacillin-tazobactam (MIC, >64 mg/L) and temocillin (MIC, >128 mg/L), though the latter drug has limited availability and so is not widely tested (62). Ertapenem resistance (MIC, >0.5 mg/L) provides a useful clue, even when imipenem and meropenem still appear active, but is also seen in isolates with combinations of ESBL or AmpC activity and impermeability. Most producers should be identified when screening breakpoints for meropenem (MIC, >0.12 mg/L) trigger subsequent biochemical or genotypic testing (73). Rare producers are susceptible to meropenem at ≤0.12 mg/L and/or temocillin at ≤32 mg/L (69).
A diagnostic trap is that most OXA-48-like carbapenemases do not hydrolyze third- and fourth- generation cephalosporins, meaning that producers lacking ESBLs remain susceptible to these agents (67). The unwary easily dismiss carbapenem-borderline-resistant, cephalosporin-susceptible isolates as test failures, when the phenotype should prompt suspicion of an OXA-48-like β-lactamase.
EPIDEMIOLOGY
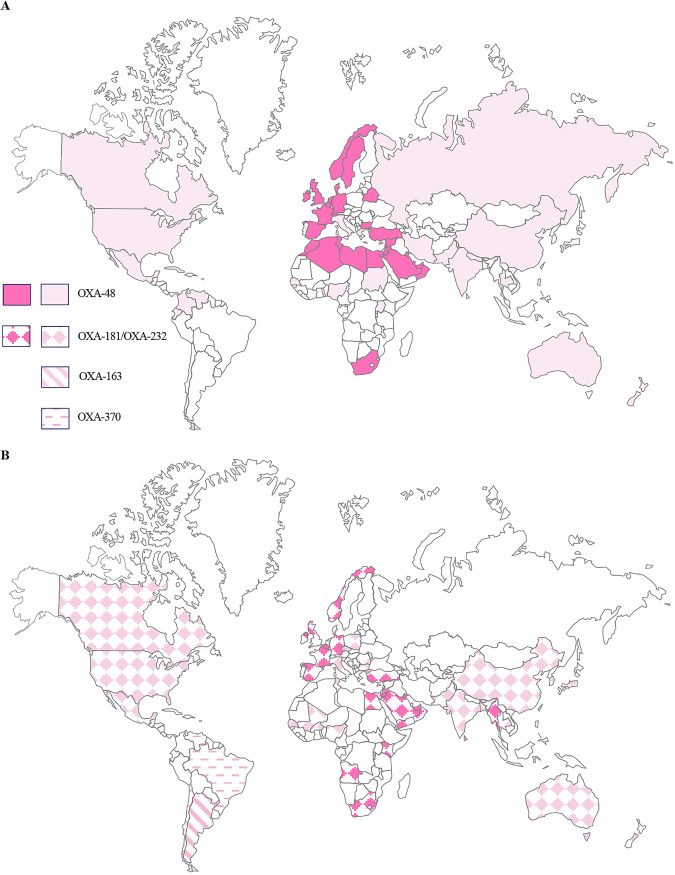
OXA-48 itself was initially identified from a carbapenem-resistant K. pneumoniae isolate from a patient in Istanbul, Turkey, in 2001 (50). OXA-48-like β-lactamases have subsequently disseminated to every inhabited continent (Fig. 2) (59, 74). Turkey remains an important reservoir for OXA-48-like producers, with OXA-48, OXA-181, OXA-232, and increasing reports of OXA-48-like/NDM coproducers linked with different hosts and various STs (59, 64, 75, 76).
FIG 2.
Global distribution of acquired OXA-48-like β-lactamases among Enterobacterales. (A) OXA-48; (B) prevalent OXA-48-like variants. Full-tone color is used to indicate where OXA-48-like enzymes are reported as the most prevalent carbapenemases in the country. Lighter-tone color is used to indicate countries where OXA-48-like enzymes are reported in outbreaks but where other carbapenemases (KPC or metalloenzymes) are more prevalent or the most prevalent carbapenemase is unclear.
Aside from human clinical samples, OXA-48-like enzymes have been widely detected among different bacterial species and in different countries globally from the environment, particularly from water (Table 2). The potential role of monitoring hospital sewage as an early warning system for clinical outbreaks (77) is of interest, but false positives from environmental Shewanella spp. may ensue if PCR is simply performed on sewage or hospital wastewater. Different niches have been reviewed (21) and updated here. The dissemination of OXA-48-like carbapenemases (particularly OXA-181) among nonhuman settings in Switzerland (78), including in companion animal clinics (Table 3) (79, 80) and in the food chain (Table 4), is a concern. These new data enhance our understanding of important links between humans and other reservoirs for OXA-48-like carbapenemases, which remains poorly understood, with uncertainty on whether the predominant direction of spread is from animals to humans or vice versa.
TABLE 2.
Examples of environmental reservoirs of OXA-48-like enzymes globally
| Yr isolated | OXA-48-like enzyme | Host | Reservoir | Country | Reference |
|---|---|---|---|---|---|
| 2011 | OXA-48 | E. coli and Serratia marcescens | Puddles in and around Marrakech | Morocco | 231 |
| 2011–2012 | OXA-48 | E. coli | Domestic sewage and hospital wastewater treatment plant | Austria | 232 |
| 2015 | OXA-48 | Enterobacterales (mainly E. coli) | River water | Algeria | 233 |
| 2015–2016 | OXA-48-likea | Host species not definedb | Hospital wastewater effluent | Tunisia | 234 |
| 2015–2017 | OXA-48 | E. coli and other coliforms | Hospital sewage | Sweden | 77 |
| 2015–2021 | OXA-48 | E. cloacae (mainly ST66) | Hospital (shower drains) | Germany | 235 |
| 2016 | OXA-48-likea | Bacteriophages and unspecified bacterial hostsb | Wastewater, river water, and irrigation water | USA | 236 |
| 2016–2017 | OXA-48 | S. marcescens | Hospital wastewater (sink trap) | Israel | 237 |
| 2016–2019 | OXA-48 | Citrobacter freundii | Hospital wastewater (toilets) | France | 238 |
| 2017 | OXA-48 | ST131 E. coli and ST101 K. pneumoniae | Seawater bathing site | Ireland | 239 |
| 2018 | OXA-48-likea | Enterobacterales (mainly Klebsiella spp.) | River water and farm soil | South Africa | 240 |
| 2019 | OXA-48 | K. pneumoniae and Raoultella ornithinolytica | Hospital wastewater treatment plant | United Kingdom | 241 |
| 2019–2020 | OXA-48 | Enterobacterales | Municipal wastewater treatment plant | Romania | 242 |
| 2020 | OXA-48 | Enterobacterales (mainly E. coli) | Municipal wastewater treatment plant | Croatia | 243 |
| 2020 | OXA-48 | K. pneumoniae | Hospital wastewater treatment plant | Mexico | 244 |
| Sampling date not specified | OXA-48-like (including OXA-48, OXA-181, OXA-199, and OXA-204, among others) | Host species not definedb | Estuarine water with flow from domestic, agricultural, and domestic origin | Portugal | 245 |
Specific variant not specified.
Where host species are not defined among water reservoirs, caution must be used to interpret data due to the naturally occurring chromosomally encoded OXA-54 in Shewanella spp. (as described in the text).
TABLE 3.
Dissemination of OXA-48-like enzymes among animals globally
| Yr isolated | OXA-48-like enzyme | Host | Reservoir | Country | Reference |
|---|---|---|---|---|---|
| 2009–2011 | OXA-48 | Enterobacterales (mainly E. coli and K. pneumoniae) | Companion animals (dogs, cats, horses) | Germany | 246 |
| 2009–2013 | OXA-48 | E. coli | Companion animals (dogs and cats) | U.S.A. | 247 |
| 2009–2016 | OXA-48-likea | Enterobacterales (mainly K. pneumoniae) | Companion animals (dogs, cats, guinea pig, rat, mouse, rabbit) | Germany | 248 |
| 2013 | OXA-48 | E. coli and K. pneumoniae | Companion animals (dog) | Germany | 249 |
| 2014–2015 | OXA-48 | E. coli | Companion animals (dogs and cats) | Algeria | 250 |
| 2015 | OXA-48 | E. cloacae | German cockroaches | Algeria | 251 |
| 2015 | OXA-48 | E. coli | Companion animals (dog) | France | 252 |
| 2015–2016 | OXA-48 | Enterobacterales (mainly E. cloacae ST527) | Companion animals (dogs, cats, horses, pet birds) | Algeria | 253 |
| 2016 | OXA-48-like (OXA-181, OXA-232) | E. coli, K. pneumoniae, E. cloacae | Cockroaches, ants, moths, spiders, flies in human hospital setting | Pakistan | 254 |
| 2016–2019 | OXA-48-like (OXA-181) | E. coli ST410 | Companion animals postdischarge from veterinary hospital (dog) | Portugal | 49 |
| 2018 | OXA-48-like (OXA-181) | E. coli | Companion animals postdischarge from veterinary hospital (dogs, cats) | Switzerland | 80 |
Specific variant not specified.
TABLE 4.
Dissemination of OXA-48-like enzymes in the food chain
| Yr isolated | OXA-48-like enzyme | Host | Reservoir | Country | Reference |
|---|---|---|---|---|---|
| 2013 | OXA-48 | E. coli ST38 | Fowl | Lebanon | 255 |
| 2015 | OXA-48-like (OXA-181) | Klebsiella variicola | Coriander herb | Import from Thailand, Vietnam, and India to Switzerland | 256 |
| 2018 | OXA-48 | K. pneumoniae | Retail pork meat in farmers' market | China | 257 |
| 2018 | OXA-48 | E. cloacae | Retail pork meat in supermarket chain | China | 257 |
| 2018–2019 | OXA-48 | E. coli | Poultry in foodchain | Nepal | 131 |
| 2019 | OXA-48 | E. coli | Fattening pig | Germany | 258 |
International travel is well known to facilitate the spread of carbapenemases. This is better documented for blaNDM than for blaOXA-48, but nonetheless, some data are available. Among early (2007 to 2014) United Kingdom patients colonized or infected with bacteria producing OXA-48-like enzymes, a travel history was available for 24%, of whom 42% had documented travel to 17 different countries, several of which had previously reported outbreaks involving bacteria with OXA-48-like enzymes (62). Casualties from the Libyan conflict were a source of export of OXA-48-like producers to Germany (81), Denmark (82), United Kingdom, the Netherlands, and Malta (83, 84). Notably, a polytrauma patient from Libya was the source of OXA-48-producing Salmonella enterica serovar Kentucky ST198 into Switzerland in 2011 (85). The recent COVID pandemic has greatly reduced travel and, no doubt, reduced the international spread of resistance. Nevertheless, case reports continue to appear that highlight the importation of OXA-48-like carbapenemases by way of repatriation of patients; one such example was the transfer of a patient colonized with an E. coli carrying blaOXA-484 from India to Switzerland (86).
Europe and Russia.
OXA-48-like enzymes are the most prevalent carbapenemases among Enterobacterales in much of Western Europe (87). They are most frequently detected in K. pneumoniae and E. coli but may be associated with other Enterobacterales.
One of the first cases outside Turkey was reported from Belgium in 2007. Notably, this imipenem-susceptible, ertapenem-resistant K. pneumoniae isolate was cultured from a patient who had no connection to Turkey (88). France reported its first OXA-48 outbreak in 2010, involving a K. pneumoniae strain (89). As in Belgium, those affected had not travelled to anywhere the enzyme had previously been recorded, suggesting that blaOXA-48 was already endemic in these regions (89). OXA-48-like enzymes have become the most prevalent carbapenemase group in France (90) and Spain (55, 91, 92) although KPC types remain more prevalent in Italy and Greece. Colistin resistance among OXA-48-producers was first reported from France in 2014, likely mediated by mgrB gene alterations (93), and cocarriage with plasmid-mediated mcr-1 has also been reported (see below). Enterobacterales producing OXA-48-like carbapenemases appear to have been introduced to Israel—a country where KPC otherwise dominates among carbapenemases—first by medical tourism in 2007 (6) and then by wounded Syrian nationals, transferred for medical care, in 2013 (94).
Elsewhere in Europe, away from the Mediterranean, OXA-48 types are now the most prevalent carbapenemases in Germany (45, 95), the Netherlands (7), Switzerland (96), and the United Kingdom (97, 98). E. coli ST38 with blaCTX-M-27/14b together with blaOXA-48/244 has spread across Germany and Switzerland (99, 100), precluding the use of unprotected third- or fourth- generation cephalosporins. The case is similar in the United Kingdom and Spain, where clonally diverse isolates with OXA-48-like carbapenemases often also produce CTX-M-15 enzyme, narrowing the therapeutic options (27).
In 2018, there was a remarkable increase in K. pneumoniae producing OXA-48 or OXA-244 enzymes across Russia (75). In Moscow, reports describe an outbreak of infection in a neurosurgical intensive care unit (ICU) caused by hypermucoviscous virulent K. pneumoniae ST23 isolates harboring both blaOXA-48 and blaCTX-M-15 (101, 102). This is worrisome owing to the clone’s propensity to cause severe metastatic infection, and this concern is redoubled by subsequent detections of the lineage in Sweden (2019), Ireland and France (2020) (103), and then Switzerland (104).
Surveillance across 31 countries from 2016 to 2018 revealed 40/354 isolates with second carbapenemases in addition to their OXA-48-like-enzymes. Most of these were from Europe (75). Twenty carried NDM-1 plus OXA-232 (United Kingdom), 16 had NDM-1 plus OXA-48 (Germany, Belarus, Greece, Turkey), two had NDM-5 plus OXA-232 (United Kingdom), and one had NDM-1 plus OXA-181 (Turkey) (75). The Middle East, where double carbapenemase producers may be particularly prevalent (105), was not represented.
Africa.
Tunisia was another country with early reports of OXA-48-like enzymes (87, 106). Subsequently, OXA-48-like carbapenemases proliferated in neighboring North African countries, including Egypt (107), Algeria (106, 108), and Morocco and Libya (46, 109, 110).
A lack of health care infrastructure, limited molecular diagnostics, and a paucity of skilled scientists mean that the epidemiology of blaOXA-48-like is poorly understood across much of Africa. Nonetheless, a study conducted from 2015 to 2016 examined 117 clinical K. pneumoniae isolates from Sudan; 44/117 produced a carbapenemase, and most were NDM types but seven had OXA-48-like enzymes (111). On the other side of the continent, in Senegal, OXA-48 was reported among hospital and community Enterobacterales as early as 2011 (112). More recently, a systematic review reported OXA-48-like carbapenemases in human samples from Algeria, Nigeria, Libya, São Tomé and Principe, Tunisia, South Africa, and Uganda, though not in environmental or animal samples (113).
OXA-48-like enzymes are dominant among carbapenemases in South Africa (114), with at least one early case having been imported from Egypt (115). Colistin resistance emerged from a K. pneumoniae strain producing OXA-181 during selective digestive decontamination (11), while K. pneumoniae ST307 with OXA-181 enzymes have spread across several private hospitals in South Africa, concurrent with a period of high carbapenem use (116).
Middle East, Gulf region, and the Levant.
Enterobacterales producing OXA-48-like β-lactamases have swiftly become endemic in the Gulf and non-Mediterranean Middle East, with startling high prevalence rates reported in several studies (117–120). Surveillance of ICU patients in Bahrain, Kuwait, Saudi Arabia, Oman, and the United Arab Emirates in 2019 reported blaOXA-48 to be the most prevalent carbapenemase gene in isolates from rectal swab samples (representing colonization), being present in 15% of all specimens screened and up to 51% of those in Saudi Arabia (121). Risk factors for colonization included being on antibiotics at admission to ICU, age of >65 years, and prolonged stay. Travel was not associated with increased risk (121). In Saudi Arabia, 81.5% of carbapenem-resistant K. pneumoniae isolates collected across two large hospitals produced OXA-48. The authors attributed this prevalence to large numbers of migrant workers from Turkey, India, and Pakistan (122), though the latter two countries are more associated with NDM carbapenemases, which were much less prevalent. Of particular concern is the emergence of Enterobacterales that harbor both blaOXA-48-like and blaNDM in Iran (123), Saudi Arabia (124), and Oman (125) from as early as 2010. OXA-48 types dominate in the Levant, including Lebanon (126), Jordan (127, 128), and Syria, as well Turkey (see above).
Indian subcontinent, East Asia, and Australasia.
In India, OXA-48-like enzymes are the second-most-prevalent carbapenemases among Enterobacterales after New Delhi metallo-β-lactamases (NDM) and are often represented by OXA-181 (87, 129) and OXA-232 (130). Surveillance and reports show increasing numbers of Enterobacterales that coproduce both OXA-48-like and NDM enzymes in hospital settings across the Indian subcontinent and in Thailand (51, 75, 129). Cocarriage of blaOXA-48 and plasmid-mediated mcr-1, encoding colistin resistance, has been identified in E. coli from clinical and poultry isolates in Nepal (131) and in K. pneumoniae from pediatric patients in Vietnam (132). These patients had no epidemiological link to each other, further indicating that reservoirs are poorly understood.
Enterobacterales producing OXA-48-like enzymes remain sporadic in China (12, 133) and are (or, pre-COVID, were) occasionally linked to isolates from Western European travelers (134). K. pneumoniae ST15 isolates producing OXA-232 have caused several hospital outbreaks in the country (135–137), as have, less frequently, K. pneumoniae ST11 strains coproducing KPC-2 and OXA-48 enzymes (133). In Singapore, OXA-181 producers appear increasingly important (138). In Japan, producers of OXA-48-like enzymes are still uncommon and most often from patients transferred for medical care from elsewhere, including India (139) and Kuwait (140).
In Australia and New Zealand, reports are few and sporadic (14). From 2009 to 2017, 9% of cases with OXA-48-like producers in New Zealand had no history of travel, suggesting local transmission (8).
North America.
The first reports of OXA-48-like carbapenemases in North America were published in 2013 and included retrospectively identified isolates from 2009 (141). From 2010 to 2015, the Centers for Disease Control and Prevention (CDC) received reports of 52 Enterobacterales that produced OXA-48-like enzymes; international travel was the predominant risk factor (66% of cases, frequently India) in addition to hospitalization abroad (55% of cases) (142). U.S. surveillance of OXA-48-like producers from 2010 to 2014 across 12 states showed several variants represented (OXA-181, 43%; OXA-232, 33%; OXA-48, 23%) (143). From 2016 to 2018, Enterobacterales expressing OXA-232 alone or together with NDM-1 were the second-largest group of carbapenemase producers (75), although they remain considerably rarer than those with KPC types.
OXA-48-like (OXA-181, 52.2%; OXA-48, 31.3%) enzymes are reported to be emerging in Canada, with recent international travel reported for 40% of cases identified between 2011 and 2014 and IncL plasmids implicated (44), although this trend has likely been disrupted by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced travel restrictions.
Latin America.
Reports of Enterobacterales producing OXA-48-like β-lactamases remain relatively scarce in Latin America, where KPC enzymes dominate (144). Nevertheless, a case series from Mexico in 2014 reported the first finding of Enterobacterales producing OXA-232 (145), followed by a 2016 study reporting classical OXA-48 (146). In Argentina, OXA-163 has spread extensively among Enterobacterales (147, 148) and is exceptional among OXA-48-like enzymes (see above) in not attacking carbapenems. Other variants, including OXA-247, have also emerged here (147). The OXA-370 variant has been recorded in Brazil (28, 149), spreading also into Chile (150) and conferring small rises in carbapenem MICs. This variant coproduced with NDM-1 by K. pneumoniae was first isolated from a patient transferred from Brazil to Chile (150), although there is no current evidence of ongoing spread in Chile within the published literature. There is little evidence of wide dissemination of OXA-48-like enzymes in human samples in Latin America, although there remain questions on the reliability of detection and on environmental, animal, or food chain reservoirs.
TREATMENT OPTIONS
Much of the available data relate to infections involving strains with classical OXA-48 enzymes. This is assumed to extend to other prevalent variants, including OXA-181 and OXA-232, which are associated with similar phenotypes. Data on noncarbapenemase variants such as OXA-163 remain scant.
Combination therapy.
Until recently, the treatment of infections due to carbapenemase producers was mostly with polymyxins, tigecycline, aminoglycosides, and fosfomycin, often in combination and often together with carbapenems.
A systematic review in 2014 concluded that combination therapy reduced mortality for infections involving carbapenemase-producing Enterobacterales (CPE), with fewer treatment failures than with monotherapy (151). However, only one study represented patients infected by OXA-48 producers. This was a cohort of 34 elderly patients with bloodstream infection (BSI) among whom the 30-day mortalities were 52.4% (11/21) for those treated with ≥2 active drugs (mostly including colistin) but not including a carbapenem, 33.3% (2/6) in patients receiving ≥2 “active” drugs including a carbapenem, and 33.3% (1/3) in patients receiving amikacin monotherapy (152). While this study was prospective, the numbers were tiny, the patient population heterogenous, and the infection sources diverse. Moreover, numerous antimicrobial combinations were reported, and tigecycline susceptibility was judged by FDA interpretive criteria, with a breakpoint 4-fold higher than that of EUCAST criteria, meaning that definitive conclusions cannot be drawn.
Following this, a prospective multicenter randomized controlled trial in 2017 (INCREMENT) showed that patients who received “appropriate” combination therapy (based on in vitro susceptibilities) for infections caused by CPE had reduced mortality compared with those receiving appropriate monotherapy only when their pretreatment mortality hazard score was high (153). Colistin monotherapy was concluded to be adequate for those with a low-mortality risk. A caveat is that KPC carbapenemases were heavily represented and OXA-48-like carbapenemases were underrepresented.
In an observational study of bacteremia involving OXA-48-producing Enterobacterales, a triple combination (colistin-aminoglycoside-ceftazidime/cefepime) was associated with slightly better 28-day survival than that with colistin-based dual combinations (P = 0.075), although the authors failed to comment on the incidence of renal toxicity with aminoglycoside-colistin combinations (154).
Based on these data, it remains unclear whether and when there is a benefit to colistin-based combination therapy versus monotherapy for patients with infections due to bacteria with OXA-48-like enzymes. Increasingly, though, this debate is being overtaken by the availability of ceftazidime-avibactam and cefiderocol (discussed below). Scenarios that may still necessitate consideration of a colistin-based combination include (i) patients showing an anaphylaxis risk with cephalosporins, or (ii) patients already receiving a high-dose central nervous system (CNS)-penetrating β-lactam, e.g., for concurrent pneumococcal meningitis, precluding further cephalosporin coadministration. In such cases, the neurotoxicity of colistin-based regimens should also be considered.
Ceftazidime, cefepime, and aztreonam.
Ceftazidime, cefepime, and aztreonam evade hydrolysis by OXA-48-like enzymes (with the exceptions noted above). Ceftazidime activity was demonstrated in a K. pneumoniae murine peritonitis model when the challenge strain produced OXA-48 without accompanying ESBL or AmpC enzymes (155). However, these drugs lack efficacy against strains that coexpress ESBLs and, for ceftazidime and aztreonam, also those with derepressed AmpC. Since as many as 80% of OXA-48-positive isolates (rates vary by country) coproduce ESBLs (7, 61), sometimes with blaCTX-M-15 contained within a mosaic Tn1999.4 transposon (156, 157), neither unprotected ceftazidime, cefepime, nor aztreonam is a reliable option for monotherapy.
Ceftazidime-avibactam.
The ceftazidime-avibactam combination protects ceftazidime with a novel diazabicyclooctane β-lactamase inhibitor, avibactam, which binds covalently and reversibly to serine β-lactamases, including ESBLs and AmpC enzymes. Inhibition of OXA-48 enzyme is weak, but this matters little given ceftazidime’s stability to this carbapenemase.
Nonrandomized controlled trials and registry-based analyses suggest that ceftazidime-avibactam is an effective therapy for a range of infections due to pathogens with OXA-48-mediated resistance to carbapenems (158). It displays in vitro activity against most Enterobacterales producing OXA-48-like enzymes at the EUCAST and FDA susceptibility breakpoints (8 + 4 mg/L) (159–163). Case series suggest better clinical outcomes than when colistin- or carbapenem-based regimens are used against OXA-48 producers (164–167), though further in vivo data and prospective outcome studies are required.
Emerging resistance to ceftazidime-avibactam was documented in one patient infected with a K. pneumoniae strain producing OXA-48 enzyme. However, this individual was first treated with unprotected ceftazidime, and resistance emerged via Pro170Ser and Thr264Ile substitutions to a coproduced CTX-M-14 enzyme, with OXA-48 remaining unaltered (70).
An in vitro exploration revealed that Pro68Ala and Pro68Ala,Tyr211Ser amino acid substitutions in OXA-48 resulted in an increased ability to hydrolyze ceftazidime and a decreased ability to withstand inhibition by avibactam, respectively (168). Although this has not been reported in clinical practice, and unlike for KPC carbapenemases, emerging mutational resistance appears rare (169).
Further pharmacokinetics/pharmacodynamics (PK/PD) and clinical outcome data, for specific OXA-48-like carbapenemase variants, would be welcomed; nonetheless, ceftazidime-avibactam appears widely effective for infections involving OXA-48-like-β-lactamase-producing Enterobacterales and should be seen as a therapy of choice.
Cefepime-tazobactam and ceftolozane-tazobactam.
A cefepime-tazobactam (1 g + 0.125 g) combination is available in India and China (170). While positive case series have been described (171), the low tazobactam dose is contentious and appears to be predicated upon the 8:1 ratio of piperacillin to tazobactam [4 g to 0.5 g] rather than robust PK/PD data. There is concern that the low tazobactam concentrations achieved are insufficiently reliable to protect cefepime from hydrolysis by ESBL enzymes. A higher dose combination (2 g + 2 g) is currently in development (172). Tazobactam is a weaker inhibitor of ESBLs than enmetazobactam (below), but the higher dose combination may render this difference insignificant.
Around two-thirds (90/136) of ceftazidime-resistant (i.e., ESBL-producing) OXA-48-producing Enterobacterales were inhibited by cefepime-tazobactam at 8 + 8 mg/L along with almost all (113/114) ceftazidime-susceptible OXA-48-producing Enterobacterales (173).
A phase 3 clinical trial to compare cefepime-tazobactam to meropenem for complicated urinary tract infections (cUTI) is planned (174).
Ceftolozane, like cefepime and ceftazidime, must approach stability to OXA-48-like enzymes, since it has low MICs for producers that lack ESBLs. Wide activity against OXA-48-like producers would therefore be anticipated for ceftolozane-tazobactam, given tazobactam’s ability to inhibit coproduced ESBLs. Nonetheless, MICs of tazobactam-protected ceftolozane are consistently high for ceftazidime-resistant OXA-48 producers, indicating that ESBLs are being poorly inhibited in practice (175). A simple explanation—that OXA-48 might hydrolyze tazobactam—is refuted by the observation that cefepime-tazobactam MICs are much lower than those of ceftolozane-tazobactam (173). Unlike cefepime-tazobactam, ceftolozane-tazobactam should not be seen as a potential option against OXA-48 β-lactamase producers.
Cefiderocol.
Cefiderocol is a siderophore cephalosporin with a catechol moiety on the 3 position of the R2 side chain (176). It exploits the iron uptake pathway to permeate Gram-negative bacteria and binds mainly to PBP3; moreover, it is stable to most β-lactamases, including ESBLs and OXA-48-like enzymes (177, 178), with low induction of chromosomal AmpC (179).
Despite this potential, preclinical animal models of infection assessing efficacy against Enterobacterales with OXA-48 enzymes are limited. Moreover, although cefiderocol demonstrated efficacy and safety in a phase 3 clinical trial for infections due to carbapenem-resistant pathogens, (CREDIBLE CR), it is unclear how many patients had OXA-48-like producers (180). Its role against producers of more globally prevalent variants, including OXA-181 and OXA-232, requires further investigation. Clarification of these aspects is needed, along with a fuller understanding of the hazard of selecting mutants with reduced CirA-mediated uptake (181); nonetheless, it is likely that cefiderocol will find a role in treating infections due to producers as an alternative to ceftazidime-avibactam.
Aminoglycosides.
Aminoglycosides may be used for the treatment of infections due to Enterobacterales that produce OXA-48-like enzymes, provided that in vitro susceptibility is confirmed. Except in UTI, they would not ordinarily be considered for monotherapy.
Historically, most resistance has been mediated by aminoglycoside-modifying enzymes (182), with amikacin evading more of these than gentamicin and tobramycin. Cocarriage of methyltransferases, which alter rRNA and prevent binding of 3-ring aminoglycosides, is an emerging issue (183). These confer resistance to plazomicin as well as to long-established analogues such as amikacin, gentamicin, and tobramycin. In Greece, most isolates with both NDM and OXA-48-like carbapenemases also had these methyltransferases (183). Notably, armA was observed together with OXA-48 and CTX-M in the absence of NDM enzymes in both Greece (184) and Oman (125). In the United Kingdom, pan-aminoglycoside resistance (to amikacin, tobramycin, and gentamicin), as is typical of methyltransferase production, was seen in 14.8% to 15.9% of Enterobacterales with OXA-48-like carbapenemases sent to the reference laboratory in 2018 and 2021, respectively (S.E.B., personal correspondence with United Kingdom Health Security Agency). Rates vary globally although with much higher resistance due to associated aac(6’)-Ib (185, 186) and/or rmtB and armA (187) genes having been observed in Egypt.
Polymyxin B and colistin.
Although most Enterobacterales with OXA-48-like carbapenemases are susceptible to polymyxins, susceptibility is not universal, with resistance reported in K. pneumoniae, E. coli, and Enterobacter spp. (93, 188). Much of this resistance is likely mediated by mgrB gene alterations (93) but cocarriage of blaOXA-48-like with plasmid-mediated mcr-1 has also been reported (see above).
A combination of azithromycin and colistin has recently been proposed to treat infections caused by K. pneumoniae producing OXA-48-like enzymes, based on in vitro synergism but without supportive in vivo or clinical data (189). However, the approach is potentially undermined by genes increasing resistance to macrolides [e.g., mph(A), erm(B)], which are commonly carried by the plasmids of Gram-negative bacteria. These genes were identified alongside blaOXA-181 in K. pneumoniae isolates from septic neonates in India (51) and alongside blaOXA-48 among different Enterobacterales associated with outbreaks in China (133).
Tigecycline and eravacycline.
Tigecycline is a glycylcycline with in vitro activity against Enterobacterales except Proteeae (190, 191) but not against Pseudomonas spp. It is generally used in combination, often with colistin, but was successfully used as monotherapy against OXA-48-β-lactamase-producing K. pneumoniae in two patients with bacteremia secondary to intra-abdominal or skin and soft tissue sources (152).
Caution is advocated in sepsis because of bacteriostatic activity, the low plasma levels of the currently recommended regimen, and disagreement concerning the appropriate breakpoint (EUCAST susceptibility breakpoint, ≤0.5 mg/L for E. coli only; FDA, ≤2 mg/L for all Enterobacterales; CLSI, no values). Mixed trial outcomes and excess mortality contributed to an FDA black box warning in 2013 (192). Time-kill curve assays support its use with ceftazidime-avibactam as synergistic against OXA-48 producers, but clinical data are lacking (193).
Eravacycline is a fluorocycline which, like classical tetracyclines, inhibits protein synthesis by binding to the 30S ribosomal subunit. Like tigecycline, it evades acquired efflux pumps (194); potential advantages over tigecycline are that it has slightly lower MICs for Enterobacterales (195), achieves higher serum levels (196), and induces less nausea and vomiting (197). Consequently, it may be a preferable combination agent to tigecycline, but clinical data are scant. In 2019, the FDA approved eravacycline for use in complicated intra-abdominal infections based on data from the IGNITE4 clinical trial (198), with subsequent United Kingdom and European Union (EU) licenses. Eravacycline was not approved for cUTI, where it failed to achieve noninferiority to fluoroquinolone and carbapenem comparators (199). Clinical outcome data against OXA-48-like producers remain unavailable, but evidently, the carbapenemase itself will not compromise activity.
Fosfomycin.
Fosfomycin inhibits cell wall synthesis by a unique irreversible mechanism, inactivating UDP N-acetylglucosamine-GlcNAc enolpyruvyl transferase (MurA) (200). Despite having been available for over 50 years, its intravenous (i.v.) formulation has garnered interest as a treatment for severe infections only in the past decade; it is available in the United Kingdom, much of Europe, and Japan, but approval remains pending in the United States.
Fosfomycin retains in vitro activity against many multidrug-resistant (MDR) Enterobacterales, including most with OXA-48-like enzymes (201). Synergy, defined by a fractional inhibitory concentration index (FICI) of ≤0.5, has been reported in vitro for combinations with imipenem, meropenem, and tigecycline, whereas antagonism (FICI, >0.5 to 4) has been observed with colistin (188).
A multicenter, noninterventional, prospective clinical registry (FORTRESS) across Europe is in progress to evaluate the clinical outcomes of severely infected patients treated with i.v. fosfomycin and is expected to complete recruitment in 2023 (202), although how well OXA-48-like producers will be represented is unclear.
An oral fosfomycin formulation is widely available, including in the United States, but its utility is limited to uncomplicated lower UTI, with EUCAST and CLSI breakpoints only for E. coli among Gram-negative bacteria. It may be useful in E. coli cystitis involving carbapenemase producers, including those with OXA-48-like enzymes.
Carbapenems.
Carbapenems are often considered in combination regimens against infections due to “carbapenem-resistant Enterobacterales” (CRE) (203), particularly if the MIC is low (69) or if ceftazidime-avibactam and cefiderocol are unavailable. However, clinical studies investigating treatments for CRE infections often include pathogens with mixed mechanisms of resistance (180, 204), and a growing body of evidence from animal models, small trials, and case series suggests that the type of carbapenemase is as important as the MIC (69).
Uncertainty surrounds the utility of carbapenems for infections involving Enterobacterales with OXA-48-like enzymes: clinical cure and survival rates ranged from 0 to 66% with meropenem or imipenem monotherapy (61, 89, 154, 205). True figures are likely to be lower, as some “successes” involved source control by line removal (14). Clinical outcomes were poor when carbapenems were used to treat patients infected with Enterobacterales producing both OXA-48 and CTX-M-15 enzymes, despite low imipenem and meropenem MICs (89).
Crucially, and despite these poor outcomes, many OXA-48-like-positive isolates are categorized as susceptible according to EUCAST and CLSI breakpoints (69, 70). It may be that under challenge, the bacteria swiftly accumulate secondary mechanisms precluding carbapenem efficacy. When meropenem was administered to recapitulate humanized PK in a hollow fiber model of infection with a “EUCAST and CLSI susceptible” challenge strain (E. cloacae; meropenem MIC, 1 mg/L), the total bacterial population expanded and a drug-resistant population emerged. The drug-resistant population had reduced permeability via inactivation of OmpC rather than increased expression of OXA-48 (206). Likewise, (i) doripenem, administered to simulate a humanized regimen, failed to achieve efficacy in a murine thigh infection model where the strain produced OXA-48 β-lactamase despite a doripenem MIC of only 0.38 mg/L (versus EUCAST and CLSI susceptible breakpoints of ≤1 mg/L) (207), and (ii) imipenem-cilastatin had little or no impact on lethality in a K. pneumoniae murine peritonitis model where the challenge strain, with an imipenem MIC of 0.5 mg/L (versus the CLSI susceptibility breakpoint of <1 mg/L and the EUCAST susceptibility breakpoint of ≤2 mg/L) produced OXA-48 enzyme with no associated ESBL or AmpC β-lactamase (155). Synergy has been reported in vitro with a dual-carbapenem combination for OXA-48 producers (208, 209), but this is not an approach supported by current clinical safety or outcome data. In particular, it may be precluded by central nervous system toxicity.
Carbapenems combined with β-lactamase inhibitors.
The β-lactamase inhibitors vaborbactam and relebactam do not inhibit OXA-48-like enzymes and do not potentiate partner carbapenems against producers (67); rather, they are specific inhibitors of KPC and other class A carbapenemases. Consequently, meropenem-vaborbactam and imipenem-relebactam-cilastatin are not options against infections with OXA-48-like producers.
Interpretive pitfalls arise owing to the different breakpoints for meropenem and meropenem-vaborbactam. EUCAST defines Enterobacterales as susceptible to meropenem when the MIC is ≤2 mg/L, based upon 1 g every 8 h (q8h) dosing, “susceptible, increased exposure” when the MICs are 4 to 8 mg/L with 2 g q8h dosing, and resistant when MICs are >8 mg/L. For meropenem-vaborbactam, the breakpoints are for susceptible, ≤8 plus 8 mg/L, and for resistant, >8 plus 8 mg/L, based on the sole licensed regimen of 2 g + 2 g q8h administered over a 3-h infusion (71). CLSI criteria for meropenem are for susceptible, ≤1 mg/L, for intermediate, 2 mg/L, and for resistant, ≥4 mg/L; those for meropenem-vaborbactam are for susceptible, ≤4 + 8 mg/L, for intermediate, 8 + 8 mg/L, and for resistant, ≥16 + 8 mg/L (72).
Consequently, an isolate with MICs of 4 mg/L meropenem and 4 + 4 mg/L meropenem-vaborbactam would count as meropenem resistant based on CLSI criteria, “susceptible–increased exposure” based on EUCAST criteria, and fully susceptible to meropenem-vaborbactam based on both sets of criteria, despite vaborbactam not inhibiting OXA-48-like enzymes. As already highlighted, no data exist to show that an increased dosage of meropenem (alone or combined as in meropenem-vaborbactam) will overcome reduced susceptibility associated with OXA-48 β-lactamases in clinical settings.
Flomoxef and other 7-α-methoxy (oxa)cephems.
Flomoxef is an off-patent 7-α-methoxy oxacephem β-lactam drug, developed in the 1980s. It is stable to ESBLs, though not AmpC enzymes (210). It may retain activity against isolates with DBLs, including OXA-48-like enzymes, but further research is required. It is licensed in China, South Korea, Taiwan, and Japan, where OXA-48-like producers are sporadic, perhaps explaining the lack of data thus far. Other 7-α-methoxy (oxa)cephems, moxalactam and cefotetan, also deserve fuller investigation; published data on their interactions are scant, and the drugs themselves have largely fallen into disuse.
Cefoxitin MICs for producers of OXA-48-like enzymes are widely scattered, from 4 to >64 mg/L, with low values for some that coproduce ESBLs (D.M.L., data on file), but there is a general acceptance that activity is marginal against MDR Enterobacterales, and mutational resistance readily arises via porin loss.
DEVELOPMENT PIPELINE
The development pipeline includes several cefepime-based β-lactamase inhibitor combinations. Given cefepime’s stability to prevalent OXA-48-like enzymes (see above), the key issue is whether the inhibitor inactivates coproduced ESBLs (also NDM types, coproduced in around 5 to 10% of United Kingdom isolates with OXA-48-like enzymes) (62).
Cefepime-taniborbactam.
Taniborbactam is a boronic acid β-lactamase inhibitor able to inactivate many class A (ESBL, KPC), class B (VIM, NDM, SPM-1, GIM-1), class C (AmpC), and class D (OXA-48-like) β-lactamases (211); IMP metallo-carbapenemases are not inhibited. The inhibition of serine β-lactamases involves covalent binding to the active-site serine. Activity at likely breakpoints (4 or 8 mg/L) includes Enterobacterales with OXA-48 carbapenemases (212).
A randomized, double-blind, phase 3 noninferiority trial (CERTAIN-1) has completed recruitment, comparing cefepime-taniborbactam with meropenem for the treatment of complicated urinary tract infection (cUTI) in adults (213). Given the comparator, it is unlikely that this trial will inform on efficacy against carbapenemase producers.
Cefepime-zidebactam.
Cefepime-zidebactam is widely active against Enterobacterales that produce class A (ESBL, KPC), class B (IMP, VIM, NDM), class C (AmpC), and class D (OXA-48-like) β-lactamases and retains activity against P. aeruginosa with multiple modes of resistance (214). Direct inhibition of OXA-48-like enzymes by zidebactam is limited; rather, activity depends substantially (i) on the stability of cefepime (see above), (ii) on the ability of zidebactam to inhibit coproduced ESBLs, and (iii) on zidebactam’s direct antibacterial activity and ability to potentiate PBP3-targeted β-lactams by binding PBP2 (215).
A phase 3, randomized, double-blind, multicenter noninferiority clinical trial evaluating the efficacy, safety, and tolerability of cefepime-zidebactam versus meropenem in the treatment of hospitalized adults with cUTI is planned (216). As with cefepime-taniborbactam, the design and comparator mean that few conclusions will be drawn for the treatment of infections caused by carbapenemase producers.
Cefepime-enmetazobactam.
Enmetazobactam is a methylated analog of tazobactam with potent activity against class A (ESBL) enzymes (217–219). Eight of 10 Enterobacterales producing OXA-48 carbapenemases were inhibited at 8 + 8 mg/L cefepime-enmetazobactam compared to 4/10 with cefepime-tazobactam at 8 + 4 mg/L (220); this suggests an advantage, but further data are needed (219).
The ALLIUM phase 3 clinical trial recently demonstrated cefepime-enmetazobactam’s superiority over piperacillin-tazobactam for cUTI at the primary efficacy endpoint (221), but given the comparator, this trial is unlikely to contain any data relevant to efficacy against OXA-48-like producers. At a dose of 2 g + 0.5 g q8h infused over 2 h, a probability of target attainment is achieved for >90% Enterobacterales with MICs of ≤8 mg/L (222).
Aztreonam-avibactam.
Aztreonam-avibactam is active, at a prospective breakpoint of 8 + 4 mg/L, against Enterobacterales with class A (ESBL, KPC), class B (MBLs), class C (AmpC), and class D (OXA-48-like) enzymes but not against P. aeruginosa (223). This combination is attractive, especially given the growing numbers of isolates that coproduce NDM and OXA-48-like carbapenemases reported across India, the United Kingdom, Thailand, Turkey, Germany, the United States, and Belarus (75).
Nonetheless, the emergence of aztreonam-avibactam resistance via PBP3 modifications in E. coli is a concern. This is documented mostly for strains with NDM carbapenemases alone and is particularly prevalent in India (224) but was also seen for isolates producing both OXA-48-like and NDM-type carbapenemases in Germany (225). These modifications also compromise cefiderocol and ceftazidime-avibactam, though not cefepime-zidebactam (226, 227).
Other therapies.
Ancremonam (formerly LYS228 or BOS228) is a monobactam antibiotic currently in phase 2 development (Boston Pharmaceuticals). It is stable to class A (ESBL, KPC), B (IMP, VIM, NDM), C (AmpC), and D (OXA-48-like) enzymes but is not active against P. aeruginosa.
QPX7728 is a novel cyclic boronic acid inhibitor of class A (ESBLs, KPC), B (NDM, VIM, IMP), C (AmpC), and D enzymes, including OXA-48-like enzymes from Enterobacterales and OXA types from A. baumannii (228). Its future partner β-lactam remains uncertain (229), and it may instead be developed as a “stand-alone” inhibitor (230). Direct comparison with taniborbactam remains to be published.
CONCLUSIONS
The spread and threat of OXA-48-like producers have been underappreciated for the last 2 decades. Patchy data on epidemiology undoubtedly reflect diagnostic difficulties. Consequently, it is likely that the published literature reflects only the tip of the iceberg. Several treatment options (ceftazidime-avibactam, cefiderocol, tigecycline, eravacycline) have been licensed since 2001, when these enzymes first began to spread. Unfortunately, data on their merits and limitations remain scantier than those for KPC producers, despite OXA-48-like β-lactamases being more prevalent globally. A limited appetite to generate specific PK/PD or clinical outcome data for newer agents, let alone for repurposed older drugs, means it is difficult to definitively guide treatment practice.
Despite these limitations, it seems reasonable to now consider ceftazidime-avibactam, followed (simply because there are fewer data) by cefiderocol, as first-choice options if in vitro susceptibility allows. While “OXA-48-like” enzymes currently fall under the same umbrella, we are continuously developing our understanding of how broad and unique these enzymes are. A concerted effort is required to ensure that new epidemiological, PK/PD, and clinical outcome data are deeply understood, so that differences in the therapeutics for specific OXA-48-like variants can be explored. The development pipeline includes promising new agents, notably cefepime combined with taniborbactam and zidebactam, but it is unlikely that licensing trials will provide extensive information on activity against pathogens with OXA-48-like enzymes. Assuming that these combinations proceed to licensure, an urgent need will arise to evaluate efficacy against cefiderocol and ceftazidime-avibactam and to monitor the impact of evolution within the OXA-48 family, the incidence of coproduction with metallo-carbapenemases, and the spread of reduced susceptibility owing to PBP3 inserts.
ACKNOWLEDGMENTS
S.E.B. held research funding through an MRC RCUK/UKRI Innovation Fellowship (MR/R016895/1) and the North West MRC Scheme in Clinical Pharmacology (MR/N025989/1). A.H. is supported by the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with the UK Health Security Agency (previously Public Health England [PHE]), in collaboration with Imperial Healthcare Partners, University of Cambridge, and University of Warwick. W.H. holds awards from the Medical Research Council, National Institute of Health Research, FDA, and the European Commission.
S.E.B. received research support from Roche Pharma. S.E.B. has consulted for/received speaker fees from Sumitovant and Shionogi. A.H. cosupervises a Ph.D. program partly funded by Shionogi in an industrial partnership doctoral training program and has received consultation fees from bioMérieux. R.P. is an employee of F. Hoffmann La Roche and holds stock and options amounting to <10% of total portfolio value. He is also a consultant to InsightRX. D.M.L. serves on advisory boards or provides ad hoc consultancy to Accelerate, Antabio, Centauri, GenPax, Meiji, Menarini, Mutabilis, Nordic, Paion, ParaGraf, ParaPharm, Pfizer, QPEX, Shionogi, Sumitovant, Summit, T.A.Z., Venatorx, Wockhardt, and Zambon. He has delivered lectures paid for by bioMérieux, GSK, Hikma, Merck/MSD, Menarini, Nordic, Pfizer, and Shionogi. He has relevant shareholdings with Dechra, GSK, Merck, and PerkinElmer amounting to <10% of portfolio value. He has share options with T.A.Z. and GenPax. He also has nominated holdings in Arecor, Avacta, Diaceutics, Creo Medical, Destiny Pharma Evgen, Genedrive, Poolbeg, Renalytix AI, and Trellus (all with research/products pertinent to medicines or diagnostics) through Enterprise Investment Schemes but has no authority to trade these shares directly. W.H. holds or has recently held research grants with F2G, Astellas Pharma, Spero Therapeutics, Antabio, Allecra, Bugworks, and NAEJA-RGM. W.H. has received personal fees in his capacity as a consultant for F2G, Amplyx, Auspherix, Spero Therapeutics, VenatoRx, Pfizer, and BLC/TAZ.
Footnotes
[This article was published on 20 July 2022 with errors in the article text. The text was corrected in the current version, posted on 26 July 2022.]
REFERENCES
- 1.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, McManigal B, Agarwal R, Akech S, Albertson S, Amuasi J, Andrews J, Aravkin A, Ashley E, Bailey F, Baker S, Basnyat B, Bekker A, Bender R, Bethou A, Bielicki J, Boonkasidecha S, Bukosia J, Carvalheiro C, Castañeda-Orjuela C, Chansamouth V, Chaurasia S, Chiurchiù S, Chowdhury F, Cook AJ, Cooper B, Cressey TR, Criollo-Mora E, Cunningham M, Darboe S, Day NPJ, De Luca M, Dokova K, et al. 2022. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399:629–655. 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera‐Izquierdo M, Láinez‐Ramos‐Bossini AJ, Rivera‐Izquierdo C, López‐Gómez J, Fernández‐Martínez NF, Redruello‐Guerrero P, Martín‐Delosreyes LM, Martínez‐Ruiz V, Moreno‐Roldán E, Jiménez‐Mejías E. 2021. OXA-48 carbapenemase-producing Enterobacterales in Spanish hospitals: an updated comprehensive review on a rising antimicrobial resistance. Antibiotics 10:89. 10.3390/antibiotics10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vatansever C, Menekse S, Dogan O, Gucer LS, Ozer B, Ergonul O, Can F. 2020. Co-existence of OXA-48 and NDM-1 in colistin resistant Pseudomonas aeruginosa ST235. Emerg Microbes Infect 9:152–154. 10.1080/22221751.2020.1713025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarafdar F, Jafari B, Azimi T. 2020. Evaluating the antimicrobial resistance patterns and molecular frequency of blaoxa-48 and blaGES-2 genes in Pseudomonas aeruginosa and Acinetobacter baumannii strains isolated from burn wound infection in Tehran, Iran. New Microbes New Infect 37:100686. 10.1016/j.nmni.2020.100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzon G, Naas T, Lesenne A, Benhamou M, Nordmann P. 2010. Plasmid-mediated carbapenem-hydrolysing OXA-48 β-lactamase in Klebsiella pneumoniae from Tunisia. Int J Antimicrob Agents 36:91–93. 10.1016/j.ijantimicag.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Adler A, Shklyar M, Schwaber MJ, Navon-Venezia S, Dhaher Y, Edgar R, Solter E, Benenson S, Masarwa S, Carmeli Y. 2011. Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. J Antimicrob Chemother 66:2763–2766. 10.1093/jac/dkr382. [DOI] [PubMed] [Google Scholar]
- 7.Dautzenberg MJ, Ossewaarde JM, de Kraker ME, van der Zee A, van Burgh S, de Greeff SC, Bijlmer HA, Grundmann H, Cohen Stuart JW, Fluit AC, Troelstra A, Bonten MJ. 2014. Successful control of a hospital-wide outbreak of OXA-48 producing Enterobacteriaceae in the Netherlands, 2009 to 2011. Euro Surveill 19:20723. 10.2807/1560-7917.ES2014.19.9.20723. [DOI] [PubMed] [Google Scholar]
- 8.Howard JC, Anderson T, Creighton J, Freeman JT. 2018. Geographical and temporal clustering of OXA-48-producing Escherichia coli ST410 causing community-onset urinary tract infection in Christchurch, New Zealand. J Antimicrob Chemother 73:2900–2901. 10.1093/jac/dky269. [DOI] [PubMed] [Google Scholar]
- 9.Espedido BA, Steen JA, Ziochos H, Grimmond SM, Cooper MA, Gosbell IB, van Hal SJ, Jensen SO. 2013. Whole genome sequence analysis of the first Australian OXA-48-producing outbreak-associated Klebsiella pneumoniae isolates: the resistome and in vivo evolution. PLoS One 8:e59920. 10.1371/journal.pone.0059920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharbi M, Moore LSP, Gilchrist M, Thomas CP, Bamford K, Brannigan ET, Holmes AH. 2015. Forecasting carbapenem resistance from antimicrobial consumption surveillance: lessons learnt from an OXA-48-producing Klebsiella pneumoniae outbreak in a West London renal unit. Int J Antimicrob Agents 46:150–156. 10.1016/j.ijantimicag.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brink AJ, Coetzee J, Corcoran C, Clay CG, Hari-Makkan D, Jacobson RK, Richards GA, Feldman C, Nutt L, Van Greune J, Deetlefs JD, Swart K, Devenish L, Poirel L, Nordmann P. 2013. Emergence of OXA-48 and OXA-181 carbapenemases among Enterobacteriaceae in South Africa and evidence of in vivo selection of colistin resistance as a consequence of selective decontamination of the gastrointestinal tract. J Clin Microbiol 51:369–372. 10.1128/JCM.02234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo L, An J, Ma Y, Ye L, Luo Y, Tao C, Yang J. 2016. Nosocomial outbreak of OXA-48-producing Klebsiella pneumoniae in a Chinese hospital: clonal transmission of ST147 and ST383. PLoS One 11:e0160754. 10.1371/journal.pone.0160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitout JDD, Peirano G, Kock MM, Strydom KA, Matsumura Y. 2019. The global ascendency of OXA-48-type carbapenemases. Clin Microbiol Rev 33:e00102-19. 10.1128/CMR.00102-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart A, Harris P, Henderson A, Paterson D. 2018. Treatment of infections by OXA-48-producing Enterobacteriaceae. Antimicrob Agents Chemother 62:e01195-18. 10.1128/AAC.01195-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walther-Rasmussen J, Høiby N. 2006. OXA-type carbapenemases. J Antimicrob Chemother 57:373–383. 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 16.Ambler RP. 1980. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci 289:321–331. 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 17.Naas T, Nordmann P. 1999. OXA-type β-lactamases. Curr Pharm Des 5:865–879. [PubMed] [Google Scholar]
- 18.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel L, Naas T, Nordmann P. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother 54:24–38. 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel L, Figueiredo S, Cattoir V, Carattoli A, Nordmann P. 2008. Acinetobacter radioresistens as a silent source of carbapenem resistance for Acinetobacter spp. Antimicrob Agents Chemother 52:1252–1256. 10.1128/AAC.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mairi A, Pantel A, Sotto A, Lavigne JP, Touati A. 2018. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur J Clin Microbiol Infect Dis 37:587–604. 10.1007/s10096-017-3112-7. [DOI] [PubMed] [Google Scholar]
- 22.Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, Poirel L. 2011. Characterization of OXA-181, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 55:4896–4899. 10.1128/AAC.00481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. 2013. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents 41:325–329. 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Potron A, Nordmann P, Poirel L. 2013. Characterization of OXA-204, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 57:633–636. 10.1128/AAC.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasap M, Torol S, Kolayli F, Dundar D, Vahaboglu H. 2013. OXA-162, a novel variant of OXA-48 displays extended hydrolytic activity towards imipenem, meropenem and doripenem. J Enzyme Inhib Med Chem 28:990–996. 10.3109/14756366.2012.702343. [DOI] [PubMed] [Google Scholar]
- 26.Poirel L, Castanheira M, Carrër A, Rodriguez CP, Jones RN, Smayevsky J, Nordmann P. 2011. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob Agents Chemother 55:2546–2551. 10.1128/AAC.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oteo J, Hernández JM, Espasa M, Fleites A, Sáez D, Bautista V, Pérez-Vázquez M, Fernández-García MD, Delgado-Iribarren A, Sánchez-Romero I, García-Picazo L, Miguel MD, Solís S, Aznar E, Trujillo G, Mediavilla C, Fontanals D, Rojo S, Vindel A, Campos J. 2013. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J Antimicrob Chemother 68:317–321. 10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 28.Sampaio JLM, Ribeiro VB, Campos JC, Rozales FP, Magagnin CM, Falci DR, Silva RCFD, Dalarosa MG, Luz DI, Vieira FJ, Antochevis LC, Barth AL, Zavascki AP. 2014. Detection of OXA-370, an OXA-48-related class D β-lactamase, in Enterobacter hormaechei from Brazil. Antimicrob Agents Chemother 58:3566–3567. 10.1128/AAC.02510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dortet L, Oueslati S, Jeannot K, Tandé D, Naas T, Nordmann P. 2015. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum β-lactamase without significant carbapenemase activity. Antimicrob Agents Chemother 59:3823–3828. 10.1128/AAC.05058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oueslati S, Retailleau P, Marchini L, Dortet L, Bonnin RA, Iorga BI, Naas T. 2019. Biochemical and structural characterization of OXA-405, an OXA-48 variant with extended-spectrum β-lactamase activity. Microorganisms 8:24. 10.3390/microorganisms8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strynadka NCJ, Paetzel M, Danel F, de Castro L, Mosimann SC, Page MGP. 2000. Crystal structure of the class D β-lactamase OXA-10. Nat Struct Biol 7:918–925. 10.1038/79688. [DOI] [PubMed] [Google Scholar]
- 32.Pernot L, Frénois F, Rybkine T, L'Hermite G, Petrella S, Delettré J, Jarlier V, Collatz E, Sougakoff W. 2001. Crystal structures of the class D β-lactamase OXA-13 in the native form and in complex with meropenem. J Mol Biol 310:859–874. 10.1006/jmbi.2001.4805. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani F, Docquier JD, Riccio ML, Pagani L, Rossolini GM. 2005. OXA-46, a new class D β-lactamase of narrow substrate specificity encoded by a blaVIM-1-containing integron from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother 49:1973–1980. 10.1128/AAC.49.5.1973-1980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Docquier J-D, Calderone V, De Luca F, Benvenuti M, Giuliani F, Bellucci L, Tafi A, Nordmann P, Botta M, Rossolini GM, Mangani S. 2009. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem Biol 16:540–547. 10.1016/j.chembiol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Couture F, Lachapelle J, Levesque RC. 1992. Phylogeny of LCR-1 and OXA-5 with class A and class D β-lactamases. Mol Microbiol 6:1693–1705. 10.1111/j.1365-2958.1992.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 36.Maveyraud L, Golemi D, Kotra LP, Tranier S, Vakulenko S, Mobashery S, Samama JP. 2000. Insights into class D β-lactamases are revealed by the crystal structure of the OXA10 enzyme from Pseudomonas aeruginosa. Structure 8:1289–1298. 10.1016/S0969-2126(00)00534-7. [DOI] [PubMed] [Google Scholar]
- 37.Smith CA, Antunes NT, Stewart NK, Toth M, Kumarasiri M, Chang M, Mobashery S, Vakulenko SB. 2013. Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii. Chem Biol 20:1107–1115. 10.1016/j.chembiol.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santillana E, Beceiro A, Bou G, Romero A. 2007. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc Natl Acad Sci USA 104:5354–5359. 10.1073/pnas.0607557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith CA, Antunes NT, Toth M, Vakulenko SB. 2014. Crystal structure of carbapenemase OXA-58 from Acinetobacter baumannii. Antimicrob Agents Chemother 58:2135–2143. 10.1128/AAC.01983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oueslati S, Nordmann P, Poirel L. 2015. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother 70:1059–1063. 10.1093/jac/dku524. [DOI] [PubMed] [Google Scholar]
- 41.Poirel L, Héritier C, Nordmann P. 2004. Chromosome-encoded Ambler class D β-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob Agents Chemother 48:348–351. 10.1128/AAC.48.1.348-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirvonen VHA, Mulholland AJ, Spencer J, Van Der Kamp MW. 2020. Small changes in hydration determine cephalosporinase activity of OXA-48 β-lactamases. ACS Catal 10:6188–6196. 10.1021/acscatal.0c00596. [DOI] [Google Scholar]
- 43.Tacão M, Araújo S, Vendas M, Alves A, Henriques I. 2018. Shewanella species as the origin of blaOXA-48 genes: insights into gene diversity, associated phenotypes and possible transfer mechanisms. Int J Antimicrob Agents 51:340–348. 10.1016/j.ijantimicag.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Mataseje LF, Boyd DA, Fuller J, Haldane D, Hoang L, Lefebvre B, Melano RG, Poutanen S, Van Caeseele P, Mulvey MR. 2018. Characterization of OXA-48-like carbapenemase producers in Canada, 2011–14. J Antimicrob Chemother 73:626–633. 10.1093/jac/dkx462. [DOI] [PubMed] [Google Scholar]
- 45.Morita Y, Pecora N, Göttig S, Hamprecht A, Sommer J, Willmann M, Brender C, Stelzer Y, Krause FF, Tsvetkov T, Wild F, Riedel-Christ S, Kutschenreuter J, Imirzalioglu C, Gonzaga A, Nübel U. 2019. Pathogenicity of clinical OXA-48 isolates and impact of the OXA-48 IncL plasmid on virulence and bacterial fitness. Front Microbiol 10:2509. 10.3389/fmicb.2019.02509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrër A, Poirel L, Yilmaz M, Akan ÖA, Feriha C, Cuzon G, Matar G, Honderlick P, Nordmann P. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob Agents Chemother 54:1369–1373. 10.1128/AAC.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potron A, Bernabeu S, Cuzon G, Pontiès V, Blanchard H, Seringe E, Naas T, Nordmann P, Dortet L. 2017. Analysis of OXA-204 carbapenemase-producing Enterobacteriaceae reveals possible endoscopy-associated transmission, France, 2012 to 2014. Euro Surveill 22:17-00048. 10.2807/1560-7917.ES.2017.22.49.17-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strydom KA, Chen L, Kock MM, Stoltz AC, Peirano G, Nobrega DB, Lowe M, Ehlers MM, Mbelle NM, Kreiswirth BN, Pitout JDD. 2020. Klebsiella pneumoniae ST307 with OXA-181: threat of a high-risk clone and promiscuous plasmid in a resource-constrained healthcare setting. J Antimicrob Chemother 75:896–902. 10.1093/jac/dkz550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brilhante M, Menezes J, Belas A, Feudi C, Schwarz S, Pomba C, Perreten V. 2020. OXA-181-producing extraintestinal pathogenic Escherichia coli sequence type 410 isolated from a dog in Portugal. Antimicrob Agents Chemother 64:e02298-19. 10.1128/AAC.02298-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:15–22. 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naha S, Sands K, Mukherjee S, Saha B, Dutta S, Basu S. 2021. OXA-181-like carbapenemases in Klebsiella pneumoniae ST14, ST15, ST23, ST48, and ST231 from septicemic neonates: coexistence with NDM-5, resistome, transmissibility, and genome diversity. mSphere 6:e01156-20. 10.1128/mSphere.01156-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrër A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. 2008. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother 52:2950–2954. 10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aubert D, Naas T, Héritier C, Poirel L, Nordmann P. 2006. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J Bacteriol 188:6506–6514. 10.1128/JB.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potron A, Poirel L, Nordmann P. 2014. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother 58:467–471. 10.1128/AAC.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poirel L, Bonnin RA, Nordmann P. 2012. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 56:559–562. 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giani T, Conte V, Di Pilato V, Aschbacher R, Weber C, Larcher C, Rossolini GM. 2012. Escherichia coli from Italy producing OXA-48 carbapenemase encoded by a novel Tn1999 transposon derivative. Antimicrob Agents Chemother 56:2211–2213. 10.1128/AAC.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skalova A, Chudejova K, Rotova V, Medvecky M, Studentova V, Chudackova E, Lavicka P, Bergerova T, Jakubu V, Zemlickova H, Papagiannitsis CC, Hrabak J. 2017. Molecular characterization of OXA-48-like-producing Enterobacteriaceae in the Czech Republic and evidence for horizontal transfer of pOXA-48-like plasmids. Antimicrob Agents Chemother 61:e01889-16. 10.1128/AAC.01889-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hidalgo L, de Been M, Rogers MRC, Schürch AC, Scharringa J, van der Zee A, Bonten MJM, Fluit AC. 2019. Sequence-based epidemiology of an OXA-48 plasmid during a hospital outbreak. Antimicrob Agents Chemother 63:e01204-19. 10.1128/AAC.01204-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peirano G, Chen L, Nobrega D, Finn TJ, Kreiswirth BN, DeVinney R, Pitout JDD. 2022. Genomic epidemiology of global carbapenemase-producing Escherichia coli, 2015–2017. Emerg Infect Dis 28:924–931. 10.3201/eid2805.212535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaidullina E, Shelenkov A, Yanushevich Y, Mikhaylova Y, Shagin D, Alexandrova I, Ershova O, Akimkin V, Kozlov R, Edelstein M. 2020. Antimicrobial resistance and genomic characterization of OXA-48-and CTX-M-15-co-producing hypervirulent Klebsiella pneumoniae ST23 recovered from nosocomial outbreak. Antibiotics 9:862. 10.3390/antibiotics9120862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pano-Pardo JR, Ruiz-Carrascoso G, Navarro-San Francisco C, Gomez-Gil R, Mora-Rillo M, Romero-Gomez MP, Fernandez-Romero N, Garcia-Rodriguez J, Perez-Blanco V, Moreno-Ramos F, Mingorance J. 2013. Infections caused by OXA-48-producing Klebsiella pneumoniae in a tertiary hospital in Spain in the setting of a prolonged, hospital-wide outbreak. J Antimicrob Chemother 68:89–96. 10.1093/jac/dks364. [DOI] [PubMed] [Google Scholar]
- 62.Findlay J, Hopkins KL, Loy R, Doumith M, Meunier D, Hill R, Pike R, Mustafa N, Livermore DM, Woodford N. 2017. OXA-48-like carbapenemases in the UK: an analysis of isolates and cases from 2007 to 2014. J Antimicrob Chemother 72:1340–1349. 10.1093/jac/dkx012. [DOI] [PubMed] [Google Scholar]
- 63.European Centre for Disease Prevention and Control. 2021. Rapid risk assessment: increase in OXA-244 -producing Escherichia coli in the European Union/European Economic Area and the UK since 2013, first update. https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-increase-oxa-244-producing-escherichia-coli-eu-eea. Accessed 3 June 2022.
- 64.Isler B, Özer B, Çınar G, Aslan AT, Vatansever C, Falconer C, Dolapçı İ, Şimşek F, Tülek N, Demirkaya H, Menekşe Ş, Akalin H, Balkan İİ, Aydın M, Tigen ET, Demir SK, Kapmaz M, Keske Ş, Doğan Ö, Arabacı Ç, Yağcı S, Hazırolan G, Bakır VO, Gönen M, Chatfield MD, Forde B, Saltoğlu N, Azap A, Azap Ö, Akova M, Paterson DL, Can F, Ergönül Ö. 2022. Characteristics and outcomes of carbapenemase harbouring carbapenem-resistant Klebsiella spp. bloodstream infections: a multicentre prospective cohort study in an OXA-48 endemic setting. Eur J Clin Microbiol Infect Dis 41:841–847. 10.1007/s10096-022-04425-4. [DOI] [PubMed] [Google Scholar]
- 65.Moubareck CA, Mouftah SF, Pál T, Ghazawi A, Halat DH, Nabi A, AlSharhan MA, AlDeesi ZO, Peters CC, Celiloglu H, Sannegowda M, Sarkis DK, Sonnevend Á. 2018. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int J Antimicrob Agents 52:90–95. 10.1016/j.ijantimicag.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Mushtaq S, Garello P, Vickers A, Woodford N, Livermore DM. 2021. Activity of cefepime/zidebactam (WCK 5222) against “problem” antibiotic-resistant Gram-negative bacteria sent to a national reference laboratory. J Antimicrob Chemother 76:1511–1522. 10.1093/jac/dkab067. [DOI] [PubMed] [Google Scholar]
- 67.Kidd JM, Livermore DM, Nicolau DP. 2020. The difficulties of identifying and treating Enterobacterales with OXA-48-like carbapenemases. Clin Microbiol Infect 26:401–403. 10.1016/j.cmi.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Dabos L, Oueslati S, Bernabeu S, Bonnin RA, Dortet L, Naas T. 2022. To be or not to be an OXA-48 carbapenemase. Microorganisms 10:258. 10.3390/microorganisms10020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Livermore DM, Nicolau DP, Hopkins KL, Meunier D. 2020. Carbapenem-resistant Enterobacterales, carbapenem resistant organisms, carbapenemase-producing Enterobacterales, and carbapenemase-producing organisms: terminology past its “sell-by date” in an era of new antibiotics and regional carbapenemase epidemiology. Clin Infect Dis 71:1776–1782. 10.1093/cid/ciaa122. [DOI] [PubMed] [Google Scholar]
- 70.Livermore DM, Meunier D, Hopkins KL, Doumith M, Hill R, Pike R, Staves P, Woodford N. 2018. Activity of ceftazidime/avibactam against problem Enterobacteriaceae and Pseudomonas aeruginosa in the UK, 2015-16. J Antimicrob Chemother 73:648–657. 10.1093/jac/dkx438. [DOI] [PubMed] [Google Scholar]
- 71.EUCAST. 2022. Clinical breakpoints and dosing of antibiotics v.12.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf. Accessed 3 May 2022.
- 72.Clinical and Laboratory Standards Institute. 2021. CLSI performance standards for antimicrobial susceptibility testing, 31st ed. M100-ED31. http://em100.edaptivedocs.net/Login.aspx?_ga=2.33444157.2034438934.1644156008-100107473.1644156008. Accessed 6 February 2022.
- 73.Fattouh R, Tijet N, McGeer A, Poutanen SM, Melano RG, Patel SN. 2015. What is the appropriate meropenem MIC for screening of carbapenemase-producing Enterobacteriaceae in low-prevalence settings? Antimicrob Agents Chemother 60:1556–1559. 10.1128/AAC.02304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Seifert H, Woodford N, Nordmann P, Poirel L, Bogaerts P, Navon-Venezia S, Cornaglia G, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 75.Castanheira M, Doyle TB, Collingsworth TD, Sader HS, Mendes RE. 2021. Increasing frequency of OXA-48-producing Enterobacterales worldwide and activity of ceftazidime/avibactam, meropenem/vaborbactam and comparators against these isolates. J Antimicrob Chemother 76:3125–3134. 10.1093/jac/dkab306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otlu B, Yakupoğullari Y, Gürsoy NC, Duman Y, Bayindir Y, Tekerekoğlu MS, Ersoy Y. 2018. Co-production of OXA-48 and NDM-1 carbapenemases in Providencia rettgeri: the first report. Mikrobiyol Bul 52:300–307. 10.5578/mb.67153. (In Turkish.) [DOI] [PubMed] [Google Scholar]
- 77.Flach CF, Hutinel M, Razavi M, Åhrén C, Larsson DGJ. 2021. Monitoring of hospital sewage shows both promise and limitations as an early-warning system for carbapenemase-producing Enterobacterales in a low-prevalence setting. Water Res 200:117261. 10.1016/j.watres.2021.117261. [DOI] [PubMed] [Google Scholar]
- 78.Campos-Madueno EI, Moser AI, Jost G, Maffioli C, Bodmer T, Perreten V, Endimiani A. 2022. Carbapenemase-producing Klebsiella pneumoniae strains in Switzerland: human and non-human settings may share high-risk clones. J Glob Antimicrob Resist 28:206–215. 10.1016/j.jgar.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Schmitt K, Biggel M, Stephan R, Willi B. 2022. Massive spread of OXA-48 carbapenemase-producing Enterobacteriaceae in the environment of a Swiss companion animal clinic. Antibiotics 11:213. 10.3390/antibiotics11020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nigg A, Brilhante M, Dazio V, Clément M, Collaud A, Brawand SG, Willi B, Endimiani A, Schuller S, Perreten V. 2019. Shedding of OXA-181 carbapenemase-producing Escherichia coli from companion animals after hospitalisation in Switzerland: an outbreak in 2018. Euro Surveill 24:1900071. 10.2807/1560-7917.ES.2019.24.39.1900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lohr B, Pfeifer Y, Heudorf U, Rangger C, Norris DE, Hunfeld KP. 2018. High prevalence of multidrug-resistant bacteria in Libyan war casualties admitted to a tertiary care hospital, Germany. Microb Drug Resist 24:578–584. 10.1089/mdr.2017.0141. [DOI] [PubMed] [Google Scholar]
- 82.Zorgani A, Ziglam H. 2013. Injured Libyan combatant patients: both vectors and victims of multiresistance bacteria? Libyan J Med 8:20325. 10.3402/ljm.v8i0.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koole K, Ellerbroek PM, Lagendijk R, Leenen LPH, Ekkelenkamp MB. 2013. Colonization of Libyan civil war casualties with multidrug-resistant bacteria. Clin Microbiol Infect 19:E285–E287. 10.1111/1469-0691.12135. [DOI] [PubMed] [Google Scholar]
- 84.Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL, The European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. 2015. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 20:30062. 10.2807/1560-7917.ES.2015.20.45.30062. [DOI] [PubMed] [Google Scholar]
- 85.Seiffert SN, Perreten V, Johannes S, Droz S, Bodmer T, Endimiani A. 2014. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob Agents Chemother 58:2446–2449. 10.1128/AAC.02417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moser AI, Campos-Madueno EI, Sendi P, Perreten V, Keller PM, Ramette A, Endimiani A. 2021. Repatriation of a patient with COVID-19 contributed to the importation of an emerging carbapenemase producer. J Glob Antimicrob Resist 27:267–272. 10.1016/j.jgar.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuzon G, Naas T, Bogaerts P, Glupczynski Y, Huang TD, Nordmann P. 2008. Plasmid-encoded carbapenem-hydrolyzing β-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob Agents Chemother 52:3463–3464. 10.1128/AAC.00543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. 2011. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob Agents Chemother 55:2420–2423. 10.1128/AAC.01452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gauthier L, Dortet L, Cotellon G, Creton E, Cuzon G, Ponties V, Bonnin RA, Naas T. 2018. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012-2013. Antimicrob Agents Chemother 62:e00266-18. 10.1128/AAC.00266-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lázaro-Perona F, Dahdouh E, Sotillo A, Pérez-Blanco V, Villa J, Viedma E, Ruiz-Carrascoso G, Mingorance J. 2022. Dissemination of a single ST11 clone of OXA-48-producing Klebsiella pneumoniae within a large polyclonal hospital outbreak determined by genomic sequencing. Microb Genom 8:808. 10.1099/mgen.0.000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miro E, Rossen JWA, Chlebowicz MA, Harmsen D, Brisse S, Passet V, Navarro F, Friedrich AW, García-Cobos S. 2020. Core/whole genome multilocus sequence typing and core genome SNP-based typing of OXA-48-producing Klebsiella pneumoniae clinical isolates from Spain Front Microbiol 10:2961. 10.3389/fmicb.2019.02961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayol A, Poirel L, Dortet L, Nordmann P. 2016. National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant OXA-48-producing Klebsiella pneumoniae, France, 2014. Euro Surveill 21:30339. 10.2807/1560-7917.ES.2016.21.37.30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lerner A, Solter E, Rachi E, Adler A, Rechnitzer H, Miron D, Krupnick L, Sela S, Aga E, Ziv Y, Peretz A, Labay K, Rahav G, Geffen Y, Hussein K, Eluk O, Carmeli Y, Schwaber MJ. 2016. Detection and characterization of carbapenemase-producing Enterobacteriaceae in wounded Syrian patients admitted to hospitals in northern Israel. Eur J Clin Microbiol Infect Dis 35:149–154. 10.1007/s10096-015-2520-9. [DOI] [PubMed] [Google Scholar]
- 95.Kola A, Piening B, Pape UF, Veltzke-Schlieker W, Kaase M, Geffers C, Wiedenmann B, Gastmeier P. 2015. An outbreak of carbapenem-resistant OXA-48-producing Klebsiella pneumoniae associated to duodenoscopy. Antimicrob Resist Infect Control 4:8. 10.1186/s13756-015-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zurfluh K, Nüesch-Inderbinen MT, Poirel L, Nordmann P, Hächler H, Stephan R. 2015. Emergence of Escherichia coli producing OXA-48 β-lactamase in the community in Switzerland. Antimicrob Resist Infect Control 4:9. 10.1186/s13756-015-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nordmann P, Poirel L. 2014. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 20:821–830. 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 98.UK Health Security Agency. 2021. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) 2020–2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1033851/espaur-report-2020-to-2021-16-Nov.pdf. Accessed 6 February 2022.
- 99.Falgenhauer L, Nordmann P, Imirzalioglu C, Yao Y, Falgenhauer J, Hauri AM, Heinmüller P, Chakraborty T. 2020. Cross-border emergence of clonal lineages of ST38 Escherichia coli producing the OXA-48-like carbapenemase OXA-244 in Germany and Switzerland. Int J Antimicrob Agents 56:106157. 10.1016/j.ijantimicag.2020.106157. [DOI] [PubMed] [Google Scholar]
- 100.Masseron A, Poirel L, Falgenhauer L, Imirzalioglu C, Kessler J, Chakraborty T, Nordmann P. 2020. Ongoing dissemination of OXA-244 carbapenemase-producing Escherichia coli in Switzerland and their detection. Diagn Microbiol Infect Dis 97:115059. 10.1016/j.diagmicrobio.2020.115059. [DOI] [PubMed] [Google Scholar]
- 101.Lev AI, Astashkin EI, Kislichkina AA, Solovieva EV, Kombarova TI, Korobova OV, Ershova ON, Alexandrova IA, Malikov VE, Bogun AG, Borzilov AI, Volozhantsev NV, Svetoch EA, Fursova NK. 2018. Comparative analysis of Klebsiella pneumoniae strains isolated in 2012–2016 that differ by antibiotic resistance genes and virulence genes profiles. Pathog Glob Health 112:142–151. 10.1080/20477724.2018.1460949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Volozhantsev NV, Kislichkina AA, Mukhina TN, Fursova NK. 2020. Draft genome sequences of clinical K1-type Klebsiella pneumoniae strains isolated in Russia. Microbiol Resour Announc 9:e01250-19. 10.1128/MRA.01250-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.European Centre for Disease Prevention and Control. 2021. Emergence of hypervirulent Klebsiella pneumoniae ST23 carrying carbapenemase genes in EU/EEA countries. https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-hypervirulent-Klebsiella-pneumoniae-ST23-carrying-carbapenemase-genes.pdf. Accessed 6 February 2022.
- 104.Blanc DS, Poirel L, Van Singer M, Greub G, Nordmann P. 2021. Hypervirulent Klebsiella pneumoniae ST23 producing OXA-48 in Switzerland. Int J Antimicrob Agents 58:106457. 10.1016/j.ijantimicag.2021.106457. [DOI] [PubMed] [Google Scholar]
- 105.Al-Baloushi AE, Pál T, Ghazawi A, Sonnevend A. 2018. Genetic support of carbapenemases in double carbapenemase producer Klebsiella pneumoniae isolated in the Arabian Peninsula. Acta Microbiol Immunol Hung 65:135–150. 10.1556/030.65.2018.005. [DOI] [PubMed] [Google Scholar]
- 106.Saïdani M, Hammami S, Kammoun A, Slim A, Boutiba-Ben Boubaker I. 2012. Emergence of carbapenem-resistant OXA-48 carbapenemase-producing Enterobacteriaceae in Tunisia. J Med Microbiol 61:1746–1749. 10.1099/jmm.0.045229-0. [DOI] [PubMed] [Google Scholar]
- 107.Sherif M, Palmieri M, Mirande C, El-Mahallawy H, Rashed HG, Abd-El-Reheem F, El-Manakhly AR, Abdel-Latif RAR, Aboulela AG, Saeed LY, Abdel-Rahman S, Elsayed E, van Belkum A, El-Kholy A. 2021. Whole-genome sequencing of Egyptian multidrug-resistant Klebsiella pneumoniae isolates: a multi-center pilot study. Eur J Clin Microbiol Infect Dis 40:1451–1460. 10.1007/s10096-021-04177-7. [DOI] [PubMed] [Google Scholar]
- 108.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 109.Lahlaoui H, Poirel L, Barguellil F, Moussa MB, Nordmann P. 2012. Carbapenem-hydrolyzing class D β-lactamase OXA-48 in Klebsiella pneumoniae isolates from Tunisia. Eur J Clin Microbiol Infect Dis 31:937–939. 10.1007/s10096-011-1389-5. [DOI] [PubMed] [Google Scholar]
- 110.Alp E, Perçin D, Colakoğlu S, Durmaz S, Kürkcü CA, Ekincioğlu P, Güneş T. 2013. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary university hospital in Turkey. J Hosp Infect 84:178–180. 10.1016/j.jhin.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 111.Osman EA, El-Amin NI, Al-Hassan LL, Mukhtar M. 2021. Multi-clonal spread of Klebsiella pneumoniae across hospitals in Khartoum, Sudan. J Glob Antimicrob Resist 24:241–245. 10.1016/j.jgar.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 112.Moquet O, Bouchiat C, Kinana A, Seck A, Arouna O, Bercion R, Breurec S, Garin B. 2011. Class D OXA-48 carbapenemase in multidrug-resistant Enterobacteria, Senegal. Emerg Infect Dis 17:143–144. 10.3201/eid1701.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Osei Sekyere J, Reta MA. 2020. Genomic and resistance epidemiology of Gram-negative bacteria in Africa: a systematic review and phylogenomic analyses from a One Health perspective. mSystems 5:e00897-20. 10.1128/mSystems.00897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manenzhe RI, Zar HJ, Nicol MP, Kaba M. 2015. The spread of carbapenemase-producing bacteria in Africa: a systematic review. J Antimicrob Chemother 70:23–40. 10.1093/jac/dku356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perovic O, Britz E, Chetty V, Singh-Moodley A. 2016. Molecular detection of carbapenemase-producing genes in referral Enterobacteriaceae in South Africa: a short report. S Afr Med J 106:975–977. 10.7196/SAMJ.2016.v106i10.11300. [DOI] [PubMed] [Google Scholar]
- 116.Lowe M, Kock MM, Coetzee J, Hoosien E, Peirano G, Strydom KA, Ehlers MM, Mbelle NM, Shashkina E, Haslam DB, Dhawan P, Donnelly RJ, Chen L, Kreiswirth BN, Pitout JDD. 2019. Klebsiella pneumoniae ST307 with blaOXA-181, South Africa, 2014–2016. Emerg Infect Dis 25:739–747. 10.3201/eid2504.181482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Touati A, Mairi A. 2020. Epidemiology of carbapenemase-producing Enterobacterales in the Middle East: a systematic review. Expert Rev Anti Infect Ther 18:241–250. 10.1080/14787210.2020.1729126. [DOI] [PubMed] [Google Scholar]
- 118.Solgi H, Giske CG, Badmasti F, Aghamohammad S, Havaei SA, Sabeti S, Mostafavizadeh K, Shahcheraghi F. 2017. Emergence of carbapenem resistant Escherichia coli isolates producing bla NDM and bla OXA-48-like carried on IncA/C and IncL/M plasmids at two Iranian university hospitals. Infect Genet Evol 55:318–323. 10.1016/j.meegid.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 119.Pérez-López A, Sundararaju S, Tsui KM, Al-Mana H, Hasan MR, Suleiman M, Al Maslamani E, Imam O, Roscoe D, Tang P. 2021. Fecal carriage and molecular characterization of carbapenemase-producing Enterobacterales in the pediatric population in Qatar. Microbiol Spectr 9:e0112221. 10.1128/Spectrum.01122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharahi JY, Hashemi A, Ardebili A, Davoudabadi S. 2021. Molecular characteristics of antibiotic-resistant Escherichia coli and Klebsiella pneumoniae strains isolated from hospitalized patients in Tehran, Iran. Ann Clin Microbiol Antimicrob 20:32. 10.1186/s12941-021-00437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alqahtani M, Tickler IA, Al Deesi Z, AlFouzan W, Al Jabri A, Al Jindan R, Al Johani S, Alkahtani SA, Al Kharusi A, Mokaddas E, Nabi A, Saeed N, Madian A, Whitmore J, Tenover FC. 2021. Molecular detection of carbapenem resistance genes in rectal swabs from patients in Gulf Cooperation Council hospitals. J Hosp Infect 112:96–103. 10.1016/j.jhin.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 122.Al-Zahrani IA, Alsiri BA. 2018. The emergence of carbapenem-resistant Klebsiella pneumoniae isolates producing OXA-48 and NDM in the Southern (Asir) province, Saudi Arabia. Saudi Med J 39:23–30. 10.15537/smj.2018.1.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hosseinzadeh Z, Sedigh Ebrahim-Saraie H, Sarvari J, Mardaneh J, Dehghani B, Rokni-Hosseini SMH, Motamedifar M. 2018. Emerge of bla NDM-1 and bla OXA-48-like harboring carbapenem-resistant Klebsiella pneumoniae isolates from hospitalized patients in southwestern Iran. J Chin Med Assoc 81:536–540. 10.1016/j.jcma.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 124.Shibl A, Al-Agamy M, Memish Z, Senok A, Khader SA, Assiri A. 2013. The emergence of OXA-48- and NDM-1-positive Klebsiella pneumoniae in Riyadh, Saudi Arabia. Int J Infect Dis 17:e1130–e1133. 10.1016/j.ijid.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 125.Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. 2012. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect 18:E144–E148. 10.1111/j.1469-0691.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 126.Matar GM, Cuzon G, Araj GF, Naas T, Corkill J, Kattar MM, Nordmann P. 2008. Oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Lebanon. Clin Microbiol Infect 14:887–888. 10.1111/j.1469-0691.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 127.Hajj A, Adaimé A, Hayajneh W, Abdallah A, Itani T, Hakimé N, Mallah M, Alsamarneh R, Badal R, Sarkis DK. 2018. Post Syrian war impact on susceptibility rates and trends in molecular characterization of Enterobacteriaceae. Future Microbiol 13:1419–1430. 10.2217/fmb-2018-0109. [DOI] [PubMed] [Google Scholar]
- 128.Aqel AA, Findlay J, Al-Maayteh M, Al-Kaabneh A, Hopkins KL, Alzoubi H, Masalha I, Turton J, Woodford N, Ellington MJ. 2018. Characterization of carbapenemase-producing Enterobacteriaceae from patients in Amman, Jordan. Microb Drug Resist 24:1121–1127. 10.1089/mdr.2017.0238. [DOI] [PubMed] [Google Scholar]
- 129.Gupta V, Singh M, Datta P, Goel A, Singh S, Prasad K, Chander J. 2020. Detection of various beta-lactamases in Escherichia coli and Klebsiella sp.: a study from tertiary care centre of North India. Indian J Med Microbiol 38:390–396. 10.4103/ijmm.IJMM_20_253. [DOI] [PubMed] [Google Scholar]
- 130.Shankar C, Mathur P, Venkatesan M, Pragasam AK, Anandan S, Khurana S, Veeraraghavan B. 2019. Rapidly disseminating bla OXA-232 carrying Klebsiella pneumoniae belonging to ST231 in India: multiple and varied mobile genetic elements. BMC Microbiol 19:137. 10.1186/s12866-019-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muktan B, Thapa Shrestha U, Dhungel B, Mishra BC, Shrestha N, Adhikari N, Banjara MR, Adhikari B, Rijal KR, Ghimire P. 2020. Plasmid mediated colistin resistant mcr-1 and co-existence of OXA-48 among Escherichia coli from clinical and poultry isolates: first report from Nepal. Gut Pathog 12:44. 10.1186/s13099-020-00382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berglund B, Hoang NTB, Tarnberg M, Le NK, Welander J, Nilsson M, Khu DTK, Nilsson LE, Olson L, Le HT, Larsson M, Hanberger H. 2018. Colistin- and carbapenem-resistant Klebsiella pneumoniae carrying mcr-1 and blaOXA-48 isolated at a paediatric hospital in Vietnam. J Antimicrob Chemother 73:1100–1102. 10.1093/jac/dkx491. [DOI] [PubMed] [Google Scholar]
- 133.Wang L, Guo L, Ye K, Yang J. 2021. Genetic characteristics of OXA-48-producing Enterobacterales from China. J Glob Antimicrob Resist 26:285–291. 10.1016/j.jgar.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 134.Yu F, Wang S, Lv J, Qi X, Guo Y, Tang YW, Kreiswirth BN, Wang L, Chen L. 2017. Coexistence of OXA-48-producing Klebsiella pneumoniae and Escherichia coli in a hospitalized patient who returned from Europe to China. Antimicrob Agents Chemother 61:e02580-16. 10.1128/AAC.02580-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu Z, Huang H, Xu Y, Wang M, Lv J, Xu L, Shi C, Xu Y, Yang R, Chen L, Du H. 2021. Emergence and genomics of OXA-232-producing Klebsiella pneumoniae in a hospital in Yancheng, China. J Glob Antimicrob Resist 26:194–198. 10.1016/j.jgar.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 136.Li X, Ma W, Qin Q, Liu S, Ye L, Yang J, Li B. 2019. Nosocomial spread of OXA-232-producing Klebsiella pneumoniae ST15 in a teaching hospital, Shanghai, China. BMC Microbiol 19:235. 10.1186/s12866-019-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jia H, Zhang Y, Ye J, Xu W, Xu Y, Zeng W, Liao W, Chen T, Cao J, Wu Q, Zhou T. 2021. Outbreak of multidrug-resistant OXA-232-producing ST15 Klebsiella pneumoniae in a teaching hospital in Wenzhou, China. Infect Drug Resist 14:4395–4407. 10.2147/IDR.S329563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balm MND, Ngan G, Jureen R, Lin RTP, Teo JWP. 2013. OXA-181-producing Klebsiella pneumoniae establishing in Singapore. BMC Infect Dis 13:58–55. 10.1186/1471-2334-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nagano N, Endoh Y, Nagano Y, Toyama M, Matsui M, Shibayama K, Arakawa Y. 2013. First report of OXA-48 carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in Japan from a patient returned from Southeast Asia. Jpn J Infect Dis 66:79–81. 10.7883/yoken.66.79. [DOI] [PubMed] [Google Scholar]
- 140.Nishida S, Asahara M, Nemoto K, Ishigaki S, Furukawa T, Sano K, Ono Y. 2019. Emergence of Escherichia coli producing OXA-48-like carbapenemase in a patient with percutaneous transhepatic biliary drainage. Infect Prev Pract 1:100008. 10.1016/j.infpip.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. 2015. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 59:6605–6607. 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lyman M, Walters M, Lonsway D, Rasheed K, Limbago B, Kallen A. 2015. Notes from the field: carbapenem-resistant Enterobacteriaceae producing OXA-48-like carbapenemases—United States, 2010–2015. MMWR Morb Mortal Wkly Rep 64:1315–1316. 10.15585/mmwr.mm6447a3. [DOI] [PubMed] [Google Scholar]
- 143.Lutgring JD, Zhu W, De Man TJB, Avillan JJ, Anderson KF, Lonsway DR, Rowe LA, Batra D, Rasheed JK, Limbago BM. 2018. Phenotypic and genotypic characterization of Enterobacteriaceae producing oxacillinase-48-like carbapenemases, United States. Emerg Infect Dis 24:700–709. 10.3201/eid2404.171377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boyd SE, Livermore DM, Hooper DC, Hope WW. 2020. Metallo-β-lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob Agents Chemother 64:e00397-20. 10.1128/AAC.00397-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Torres-Gonzalez P, Cervera-Hernandez ME, Niembro-Ortega MD, Leal-Vega F, Cruz-Hervert LP, García-García L, Galindo-Fraga A, Martinez-Gamboa A, Bobadilla-Del Valle M, Sifuentes-Osornio J, Ponce-De-Leon A. 2015. Factors associated to prevalence and incidence of carbapenem-resistant Enterobacteriaceae fecal carriage: a cohort study in a Mexican tertiary care hospital. PLoS One 10:e0139883. 10.1371/journal.pone.0139883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aquino-Andrade A, Merida-Vieyra J, Arias De La Garza E, Arzate-Barbosa P, De Colsa Ranero A. 2018. Carbapenemase-producing Enterobacteriaceae in Mexico: report of seven non-clonal cases in a pediatric hospital. BMC Microbiol 18:38. 10.1186/s12866-018-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Belder D, Ghiglione B, Pasteran F, De Mendieta JM, Corso A, Curto L, Di Bella A, Gutkind G, Gomez SA, Power P. 2020. Comparative kinetic analysis of OXA-438 with related OXA-48-type carbapenem-hydrolyzing class D β-lactamases. ACS Infect Dis 6:3026–3033. 10.1021/acsinfecdis.0c00537. [DOI] [PubMed] [Google Scholar]
- 148.Pasteran F, Denorme L, Ote I, Gomez S, De Belder D, Glupczynski Y, Bogaerts P, Ghiglione B, Power P, Mertens P, Corso A. 2016. Rapid identification of OXA-48 and OXA-163 subfamilies in carbapenem-resistant Gram-negative bacilli with a novel immunochromatographic lateral flow assay. J Clin Microbiol 54:2832–2836. 10.1128/JCM.01175-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Magagnin CM, Rozales FP, Antochevis L, Nunes LS, Martins AS, Barth AL, Sampaio JM, Zavascki AP. 2017. Dissemination of blaOXA-370 gene among several Enterobacteriaceae species in Brazil. Eur J Clin Microbiol Infect Dis 36:1907–1910. 10.1007/s10096-017-3012-x. [DOI] [PubMed] [Google Scholar]
- 150.Carrasco-Anabalón S, Conceição Neto CO, D'Alincourt Carvalho-Assef AP, Lima CA, Cifuentes M, Silva F, Barrera B, Domínguez M, González-Rocha G, Bello-Toledo H. 2019. Introduction of NDM-1 and OXA-370 from Brazil into Chile in strains of Klebsiella pneumoniae isolated from a single patient. Int J Infect Dis 81:28–30. 10.1016/j.ijid.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 151.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58:654–663. 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gómez-Gil R, Arribas-López JR, Mingorance J, Paño-Pardo JR. 2013. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 19:E72–E79. 10.1111/1469-0691.12091. [DOI] [PubMed] [Google Scholar]
- 153.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh P-R, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J, del Toro MD, Gálvez J, Falcone M, Russo A, Giamarellou H, Trecarichi EM, Losito AR, García-Vázquez E, Hernández A, Gómez J, Bou G, Iosifidis E, Prim N, Navarro F, Mirelis B, Skiada A, Origüen J, Juan RS, Fernández-Ruiz M, et al. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 154.Balkan II, Aygün G, Aydın S, Mutcalı SI, Kara Z, Kuşkucu M, Midilli K, Şemen V, Aras Ş, Yemişen M, Mete B, Özaras R, Saltoğlu N, Tabak F, Öztürk R. 2014. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis 26:51–56. 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 155.Mimoz O, Grégoire N, Poirel L, Marliat M, Couet W, Nordmann P. 2012. Broad-spectrum β-lactam antibiotics for treating experimental peritonitis in mice due to Klebsiella pneumoniae producing the carbapenemase OXA-48. Antimicrob Agents Chemother 56:2759–2760. 10.1128/AAC.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Potron A, Nordmann P, Rondinaud E, Jaureguy F, Poirel L. 2013. A mosaic transposon encoding OXA-48 and CTX-M-15: towards pan-resistance. J Antimicrob Chemother 68:476–477. 10.1093/jac/dks397. [DOI] [PubMed] [Google Scholar]
- 157.Potron A, Poirel L, Rondinaud E, Nordmann P. 2013. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill 18:20549. 10.2807/1560-7917.es2013.18.31.20549. [DOI] [PubMed] [Google Scholar]
- 158.Hughes S, Gilchrist M, Heard K, Hamilton R, Sneddon J. 2020. Treating infections caused by carbapenemase-producing Enterobacterales (CPE): a pragmatic approach to antimicrobial stewardship on behalf of the UKCPA Pharmacy Infection Network (PIN). JAC Antimicrob Resist 2:dlaa075. 10.1093/jacamr/dlaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Livermore DM, Mushtaq S, Warner M, Miossec C, Woodford N. 2008. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum β-lactamases and carbapenemases. J Antimicrob Chemother 62:1053–1056. 10.1093/jac/dkn320. [DOI] [PubMed] [Google Scholar]
- 160.Stachyra T, Levasseur P, Péchereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Endimiani A, Choudhary Y, Bonomo RA. 2009. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother 53:3599–3601. 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sousa A, Pérez-Rodríguez MT, Soto A, Rodríguez L, Pérez-Landeiro A, Martínez-Lamas L, Nodar A, Crespo M. 2018. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:3170–3175. 10.1093/jac/dky295. [DOI] [PubMed] [Google Scholar]
- 165.Castón JJ, Lacort-Peralta I, Martín-Dávila P, Loeches B, Tabares S, Temkin L, Torre-Cisneros J, Paño-Pardo JR. 2017. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis 59:118–123. 10.1016/j.ijid.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 166.Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. 2019. Efficacy of ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. BMC Infect Dis 19:772. 10.1186/s12879-019-4409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, MacHuca I, Jiménez-Martín MJ, Martínez JA, Mora-Rillo M, Navas E, Osthoff M, Pozo JC, Ramos JCR, Rodriguez M, Sánchez-García M, Viale P, Wolff M, Carmeli Y. 2017. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 61:e01964-16. 10.1128/AAC.01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fröhlich C, Sørum V, Thomassen AM, Johnsen PJ, Leiros H-KS, Samuelsen Ø. 2019. OXA-48-mediated ceftazidime-avibactam resistance is associated with evolutionary trade-offs. mSphere 4:e00024-19. 10.1128/mSphere.00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shapiro AB, Moussa SH, Carter NM, Gao N, Miller AA. 2021. Ceftazidime-avibactam resistance mutations V240G, D179Y, and D179Y/T243M in KPC-3 β-lactamase do not alter cefpodoxime-ETX1317 susceptibility. ACS Infect Dis 7:79–87. 10.1021/acsinfecdis.0c00575. [DOI] [PubMed] [Google Scholar]
- 170.Palwe S, Veeraraghavan B, Periasamy H, Khobragade K, Kharat AS. 2020. Unorthodox parenteral β-lactam and β-lactamase inhibitor combinations: flouting antimicrobial stewardship and compromising patient care. Antimicrob Agents Chemother 64:e00168-20. 10.1128/AAC.00168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ghafur A, Tayade A, Kannaian P. 2012. Clinical profile of patients treated with cefepime/tazobactam: a new ß-lactam/ß-lactamase inhibitor combination. J Microbiol Infect Dis 2:79–86. 10.5799/ahinjs.02.2012.03.0049. [DOI] [Google Scholar]
- 172.Gill CM, Abdelraouf K, Nicolau DP. 2021. In vivo activity of WCK 4282 (high-dose cefepime/tazobactam) against serine β-lactamase-producing Enterobacterales and Pseudomonas aeruginosa in the neutropenic murine thigh infection model. J Antimicrob Chemother 76:993–1000. 10.1093/jac/dkaa551. [DOI] [PubMed] [Google Scholar]
- 173.Mushtaq S, Garello P, Vickers A, Woodford N, Livermore DM. 2021. Cefepime/tazobactam compared with other tazobactam combinations against problem Gram-negative bacteria. Int J Antimicrob Agents 57:106318. 10.1016/j.ijantimicag.2021.106318. [DOI] [PubMed] [Google Scholar]
- 174.U.S. National Library of Medicine. 2018. Study of cefepime-tazobactam (FEP-TAZ) in complicated urinary tract infection (cUTI) or acute pyelonephritis (AP). https://clinicaltrials.gov/ct2/show/NCT03630081. Accessed 6 February 2022.
- 175.Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, Adkin R, Chaudhry A, Pike R, Staves P, Woodford N, Allen M, Brown DFG, Livermore DM, Longshaw C, MacGowan APG, BSAC Resistance Surveillance Standing Committee. 2017. Activity of ceftolozane/tazobactam against surveillance and “problem” Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 72:2278–2289. 10.1093/jac/dkx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, Rhomberg PR, Jones RN, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother 71:670–677. 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 177.Jacobs MR, Abdelhamed AM, Good CE, Rhoads DD, Hujer KM, Hujer AM, Domitrovic TN, Rudin SD, Richter SS, van Duin D, Kreiswirth BN, Greco C, Fouts DE, Bonomo RA. 2019. ARGONAUT-I: activity of cefiderocol (s-649266), a siderophore cephalosporin, against Gram-negative bacteria, including carbapenem-resistant nonfermenters and Enterobacteriaceae with defined extended-spectrum β-lactamases and carbapenemases. Antimicrob Agents Chemother 63:e01801-18. 10.1128/AAC.01801-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Poirel L, Kieffer N, Nordmann P. 2018. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int J Antimicrob Agents 52:866–867. 10.1016/j.ijantimicag.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 179.Wu JY, Srinivas P, Pogue JM. 2020. Cefiderocol: a novel agent for the management of multidrug-resistant Gram-negative organisms. Infect Dis Ther 9:17–40. 10.1007/s40121-020-00286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, Portsmouth S, Torre-Cisneros J, Toyoizumi K, Wunderink RG, Nagata TD. 2021. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 21:226–240. 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 181.Klein S, Boutin S, Kocer K, Fiedler MO, Störzinger D, Weigand MA, Tan B, Richter D, Rupp C, Mieth M, Mehrabi A, Hackert T, Zimmermann S, Heeg K, Nurjadi D. 2021. Rapid development of cefiderocol resistance in carbapenem-resistant Enterobacter cloacae during therapy is associated with heterogeneous mutations in the catecholate siderophore receptor cirA. Clin Infect Dis 74:905–908. 10.1093/cid/ciab511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aishwarya KVL, Geetha PV, Eswaran S, Mariappan S, Sekar U. 2020. Spectrum of aminoglycoside modifying enzymes in Gram-negative bacteria causing human infections. J Lab Physicians 12:27–31. 10.1055/s-0040-1713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Galani I, Nafplioti K, Adamou P, Karaiskos I, Giamarellou H, Souli M, Study Collaborators. 2019. Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regard to plazomicin and aminoglycoside resistance. BMC Infect Dis 19:167. 10.1186/s12879-019-3801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Galani I, Anagnostoulis G, Chatzikonstantinou M, Petrikkos G, Souli M. 2016. Emergence of Klebsiella pneumoniae co-producing OXA-48, CTX-M-15, and ArmA in Greece. Clin Microbiol Infect 22:898–899. 10.1016/j.cmi.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 185.Elshamy AA, Saleh SE, Alshahrani MY, Aboshanab KM, Aboulwafa MM, Hassouna NA. 2021. OXA-48 carbapenemase-encoding transferable plasmids of Klebsiella pneumoniae recovered from Egyptian patients suffering from complicated urinary tract infections. Biology 10:889. 10.3390/biology10090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abdelaziz SM, Aboshanab KM, Yahia IS, Yassien MA, Hassouna NA. 2021. Correlation between the antibiotic resistance genes and susceptibility to antibiotics among the carbapenem-resistant Gram-negative pathogens. Antibiotics 10:255–215. 10.3390/antibiotics10030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.ElBaradei A, Shawky SM. 2022. Co-existence of blaOXA-48, rmtB and armA among Klebsiella pneumoniae isolates causing respiratory tract infections in Alexandria, Egypt. J Med Microbiol 31:23–28. 10.21608/ejmm.2022.228619. [DOI] [Google Scholar]
- 188.Evren E, Azap OK, Çolakoğlu Ş, Arslan H. 2013. In vitro activity of fosfomycin in combination with imipenem, meropenem, colistin and tigecycline against OXA 48-positive Klebsiella pneumoniae strains. Diagn Microbiol Infect Dis 76:335–338. 10.1016/j.diagmicrobio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 189.Singkham-In U, Muhummudaree N, Chatsuwan T. 2021. In vitro synergism of azithromycin combination with antibiotics against OXA-48-producing Klebsiella pneumoniae clinical isolates. Antibiotics 10:1551. 10.3390/antibiotics10121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kazmierczak KM, Bradford PA, Stone GG, De Jonge BLM, Sahm DF. 2018. In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance program from 2012 to 2015. Antimicrob Agents Chemother 62:e00592-18. 10.1128/AAC.00592-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ozbek B, Mataraci-Kara E, Er S, Ozdamar M, Yilmaz M. 2015. In vitro activities of colistin, tigecycline and tobramycin, alone or in combination, against carbapenem-resistant Enterobacteriaceae strains. J Glob Antimicrob Resist 3:278–282. 10.1016/j.jgar.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 192.Dixit D, Madduri RP, SharMA R. 2014. The role of tigecycline in the treatment of infections in light of the new black box warning. Expert Rev Anti Infect Ther 12:397–400. 10.1586/14787210.2014.894882. [DOI] [PubMed] [Google Scholar]
- 193.Mataraci Kara E, Yilmaz M, Istanbullu Tosun A, Özbek Çelik B. 2020. Evaluation of the synergy of ceftazidime/avibactam in combination with colistin, doripenem, levofloxacin, tigecycline, and tobramycin against OXA-48 producing Enterobacterales. J Chemother 32:171–178. 10.1080/1120009X.2020.1761172. [DOI] [PubMed] [Google Scholar]
- 194.Grossman TH, Starosta AL, Fyfe C, O'Brien W, Rothstein DM, Mikolajka A, Wilson DN, Sutcliffe JA. 2012. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother 56:2559–2564. 10.1128/AAC.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Morrissey I, Olesky M, Hawser S, Lob SH, Karlowsky JA, Corey GR, Bassetti M, Fyfe C. 2020. In vitro activity of eravacycline against Gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob Agents Chemother 64:e01699. 10.1128/AAC.01699-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhanel GG, Cheung D, Adam H, Zelenitsky S, Golden A, Schweizer F, Gorityala B, Lagacé-Wiens PRS, Walkty A, Gin AS, Hoban DJ, Karlowsky JA. 2016. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 76:567–588. 10.1007/s40265-016-0545-8. [DOI] [PubMed] [Google Scholar]
- 197.Hobbs ALV, Gelfand MS, Cleveland KO, Saddler K, Sierra-Hoffman MA. 2021. A retrospective, multicentre evaluation of eravacycline utilisation in community and academic hospitals. J Glob Antimicrob 29:430–433. 10.1016/j.jgar.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 198.Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, Tsai L. 2019. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis 69:921–929. 10.1093/cid/ciy1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Doi Y. 2019. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis 69:S565–S575. 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim DH, Lees WJ, Kempsell KE, Lane WS, Duncan K, Walsh CT. 1996. Characterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycin. Biochemistry 35:4923–4928. 10.1021/bi952937w. [DOI] [PubMed] [Google Scholar]
- 201.Berleur M, Guérin F, Massias L, Chau F, Poujade J, Cattoir V, Fantin B, de Lastours V. 2018. Activity of fosfomycin alone or combined with temocillin in vitro and in a murine model of peritonitis due to KPC-3- or OXA-48-producing Escherichia coli. J Antimicrob Chemother 73:3074–3080. 10.1093/jac/dky283. [DOI] [PubMed] [Google Scholar]
- 202.U.S. National Library of Medicine. 2016. Fosfomycin i.v. for treatment of severely infected patients (FORTRESS). https://clinicaltrials.gov/ct2/show/NCT02979951. Accessed 6 February 2022.
- 203.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 204.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. 2018. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 31:e00079-17. 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ahn C, Butt AA, Rivera JI, Yaqoob M, Hag S, Khalil A, Pitout M, Doi Y. 2015. OXA-48-producing Enterobacteriaceae causing bacteremia, United Arab Emirates. Int J Infect Dis 30:e36–e37. 10.1016/j.ijid.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Boyd S, Johnson A, McEntee L, Farrington N, Harper N, Unsworth J, Jimenez-Valverde A, Barrera-Briceno L, Healy F, Xuan L, Peck R, Hope W. 2022. Pharmacodynamics of meropenem against Enterobacterales producing OXA-48 with low meropenem minimum inhibitory concentrations, poster 4027, p 1239. 32nd Eur Congr Clin Microbiol Infect Dis, Lisbon, Portugal.
- 207.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. Efficacy of humanized carbapenem and ceftazidime regimens against Enterobacteriaceae producing OXA-48 carbapenemase in a murine infection model. Antimicrob Agents Chemother 58:1678–1683. 10.1128/AAC.01947-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Erdem F, Abulaila A, Aktas Z, Oncul O. 2020. In vitro evaluation of double carbapenem and colistin combinations against OXA-48, NDM carbapenemase-producing colistin-resistant Klebsiella pneumoniae strains. Antimicrob Resist Infect Control 9:70. 10.1186/s13756-020-00727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Poirel L, Kieffer N, Nordmann P. 2016. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 71:156–161. 10.1093/jac/dkv294. [DOI] [PubMed] [Google Scholar]
- 210.Darlow CA, Hope W. 2022. Flomoxef for neonates: extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales. J Antimicrob Chemother 77:711–718. 10.1093/jac/dkab468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hamrick JC, Docquier JD, Uehara T, Myers CL, Six DA, Chatwin CL, John KJ, Vernacchio SF, Cusick SM, Trout REL, Pozzi C, De Luca F, Benvenuti M, Mangani S, Liu B, Jackson RW, Moeck G, Xerri L, Burns CJ, Pevear DC, Daigle DM. 2020. VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:e01963-19. 10.1128/AAC.01963-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mushtaq S, Vickers A, Doumith M, Ellington MJ, Woodford N, Livermore DM. 2021. Activity of β-lactam/taniborbactam (VNRX-5133) combinations against carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 76:160–170. 10.1093/jac/dkaa391. [DOI] [PubMed] [Google Scholar]
- 213.U.S. National Library of Medicine. 2019. Safety and efficacy study of cefepime/VNRX-5133 in patients with complicated urinary tract infections (CERTAIN-1). https://clinicaltrials.gov/ct2/show/NCT03840148. Accessed 6 February 2022.
- 214.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 215.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 216.U.S. National Library of Medicine. 2021. Study of cefepime-zidebactam (FEP-ZID) in complicated urinary tract infection (cUTI) or acute pyelonephritis (AP). https://clinicaltrials.gov/ct2/show/NCT04979806. Accessed 6 February 2022.
- 217.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.D'Angelo RG, Johnson JK, Bork JT, Heil EL. 2016. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin Pharmacother 17:953–967. 10.1517/14656566.2016.1154538. [DOI] [PubMed] [Google Scholar]
- 219.Morrissey I, Magnet S, Hawser S, Shapiro S, Knechtle P. 2019. In vitro activity of cefepime-enmetazobactam against Gram-negative isolates collected from U.S. and European hospitals during 2014–2015. Antimicrob Agents Chemother 63:e00514-19. 10.1128/AAC.00514-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Carmeli Y, Adler A, Rashi E, Pypstra R, Shapiro S. 2018. In vitro activity of cefepime-AAI101 vs. drug-resistant Klebsiella pneumoniae clinical isolates, poster. Eur Congr Clin Microbiol Infect Dis, Madrid, Spain.
- 221.Kaye K, Belley A, Lahlou O, Motta P, Kashap S, Knechtle P, Velicitat P. 2020. Outcomes of the novel β-lactam/β-lactamase inhibitor combination of cefepime-enmetazobactam versus piperacillin-tazobactam in adult patients with complicated urinary tract infections—the ALLIUM phase 3 trial. 30th Eur Congr Clin Microbiol Infect Dis.
- 222.Das S, Fitzgerald R, Ullah A, Bula M, Collins AM, Mitsi E, Reine J, Hill H, Rylance J, Ferreira DM, Tripp K, Bertasini A, Franzoni S, Massimiliano M, Lahlou O, Motta P, Barth P, Velicitat P, Knechtle P, Hope W. 2020. Intrapulmonary pharmacokinetics of cefepime and enmetazobactam in healthy volunteers: towards new treatments for nosocomial pneumonia. Antimicrob Agents Chemother 65:e01468-20. 10.1128/AAC.01468-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sader HS, Carvalhaes CG, Arends SJR, Castanheira M, Mendes RE. 2021. Aztreonam/avibactam activity against clinical isolates of Enterobacterales collected in Europe, Asia and Latin America in 2019. J Antimicrob Chemother 76:659–666. 10.1093/jac/dkaa504. [DOI] [PubMed] [Google Scholar]
- 224.Periasamy H, Joshi P, Palwe S, Shrivastava R, Bhagwat S, Patel M. 2020. High prevalence of Escherichia coli clinical isolates in India harbouring four amino acid inserts in PBP3 adversely impacting activity of aztreonam/avibactam. J Antimicrob Chemother 75:1650–1651. 10.1093/jac/dkaa021. [DOI] [PubMed] [Google Scholar]
- 225.Nordmann P, Yao Y, Falgenhauer L, Sadek M, Imirzalioglu C, Chakraborty T. 2021. Recent emergence of aztreonam-avibactam resistance in NDM and OXA-48 carbapenemase-producing Escherichia coli in Germany. Antimicrob Agents Chemother 65:e0109021. 10.1128/AAC.01090-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bhagwat SS, Hariharan P, Joshi PR, Palwe SR, Shrivastava R, Patel MV, Devanga Ragupathi NK, Bakthavatchalam YD, Ramesh MS, Soman R, Veeraraghavan B. 2020. Activity of cefepime/zidebactam against MDR Escherichia coli isolates harbouring a novel mechanism of resistance based on four-amino-acid inserts in PBP3. J Antimicrob Chemother 75:3563–3567. 10.1093/jac/dkaa353. [DOI] [PubMed] [Google Scholar]
- 227.Wang Q, Jin L, Sun S, Yin Y, Wang R, Chen F, Wang X, Zhang Y, Hou J, Zhang Y, Zhang Z, Luo L, Guo Z, Li Z, Lin X, Bi L, Wang H. 2022. Occurrence of high levels of cefiderocol resistance in carbapenem-resistant Escherichia coli before its approval in China: a report from China CRE-Network. Microbiol Spectr 10:e0267021. 10.1128/spectrum.02670-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tsivkovski R, Totrov M, Lomovskaya O. 2020. Biochemical characterization of QPX7728, a new ultrabroad-spectrum beta-lactamase inhibitor of serine and metallo-beta-lactamases. Antimicrob Agents Chemother 64:e00130-20. 10.1128/AAC.00130-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lomovskaya O, Rubio-Aparicio D, Nelson K, Sun D, Tsivkovski R, Castanheira M, Lindley J, Loutit J, Dudley M. 2021. In vitro activity of the ultrabroad-spectrum beta-lactamase inhibitor QPX7728 in combination with multiple beta-lactam antibiotics against Pseudomonas aeruginosa. Antimicrob Agents Chemother 65:e00210-21. 10.1128/AAC.00210-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yahav D, Giske CG, Gramatniece A, Abodakpi H, Tam VH, Leibovici L. 2021. New β-lactam–β-lactamase inhibitor combinations. Clin Microbiol Rev 34:e00015-20. 10.1128/CMR.00115-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Potron A, Poirel L, Bussy F, Nordmann P. 2011. Occurrence of the carbapenem-hydrolyzing β-lactamase gene blaOXA-48 in the environment in Morocco. Antimicrob Agents Chemother 55:5413–5414. 10.1128/AAC.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Galler H, Feierl G, Petternel C, Reinthaler FF, Haas D, Grisold AJ, Luxner J, Zarfel G. 2014. KPC-2 and OXA-48 carbapenemase-harbouring Enterobacteriaceae detected in an Austrian wastewater treatment plant. Clin Microbiol Infect 20:O132–O134. 10.1111/1469-0691.12336. [DOI] [PubMed] [Google Scholar]
- 233.Tafoukt R, Touati A, Leangapichart T, Bakour S, Rolain JM. 2017. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res 120:185–189. 10.1016/j.watres.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 234.Nasri E, Subirats J, Sànchez-Melsió A, Mansour H, Ben Borrego CM, Balcázar JL. 2017. Abundance of carbapenemase genes (bla KPC, bla NDM and bla OXA-48) in wastewater effluents from Tunisian hospitals. Environ Pollut 229:371–374. 10.1016/j.envpol.2017.05.095. [DOI] [PubMed] [Google Scholar]
- 235.Nurjadi D, Scherrer M, Frank U, Mutters NT, Heininger A, Späth I, Eichel VM, Jabs J, Probst K, Müller-Tidow C, Brandt J, Heeg K, Boutin S. 2021. Genomic investigation and successful containment of an intermittent common source outbreak of OXA-48-producing Enterobacter cloacae related to hospital shower drains. Microbiol Spectr 9:e01380-21. 10.1128/Spectrum.01380-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang A, Call DR, Besser TE, Liu J, Jones L, Wang H, Davis MA. 2019. β-Lactam resistance genes in bacteriophage and bacterial DNA from wastewater, river water, and irrigation water in Washington State. Water Res 161:335–340. 10.1016/j.watres.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 237.Regev-Yochay G, Smollan G, Tal I, Pinas Zade N, Haviv Y, Nudelman V, Gal-Mor O, Jaber H, Zimlichman E, Keller N, Rahav G. 2018. Sink traps as the source of transmission of OXA-48-producing Serratia marcescens in an intensive care unit. Infect Control Hosp Epidemiol 39:1307–1315. 10.1017/ice.2018.235. [DOI] [PubMed] [Google Scholar]
- 238.Jolivet S, Couturier J, Vuillemin X, Gouot C, Nesa D, Adam M, Brissot E, Mohty M, Bonnin RA, Dortet L, Barbut F. 2021. Outbreak of OXA-48-producing Enterobacterales in a haematological ward associated with an uncommon environmental reservoir, France, 2016–2019. Euro Surveill 26:2000118. 10.2807/1560-7917.ES.2021.26.21.2000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mahon BM, Brehony C, Cahill N, McGrath E, O'Connor L, Varley A, Cormican M, Ryan S, Hickey P, Keane S, Mulligan M, Ruane B, Jolley KA, Maiden MC, Brisse S, Morris D. 2019. Detection of OXA-48-like-producing Enterobacterales in Irish recreational water. Sci Total Environ 690:1–6. 10.1016/j.scitotenv.2019.06.480. [DOI] [PubMed] [Google Scholar]
- 240.Ebomah KE, Okoh AI. 2020. Detection of carbapenem-resistance genes in Klebsiella species recovered from selected environmental niches in the Eastern Cape Province, South Africa. Antibiotics 9:425–412. 10.3390/antibiotics9070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gibbon MJ, Couto N, David S, Barden R, Standerwick R, Jagadeesan K, Birkwood H, Dulyayangkul P, Avison MB, Kannan A, Kibbey D, Craft T, Habib S, Thorpe HA, Corander J, Kasprzyk-Hordern B, Feil EJ. 2021. A high prevalence of bla OXA-48 in Klebsiella (Raoultella) ornithinolytica and related species in hospital wastewater in South West England. Microb Genom 7:mgen000509. 10.1099/mgen.0.000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Teban-Man A, Szekeres E, Fang P, Klümper U, Hegedus A, Baricz A, Berendonk TU, Pârvu M, Coman C. 2022. Municipal wastewaters carry important carbapenemase genes independent of hospital input and can mirror clinical resistance patterns. Microbiol Spectr 10:e02711-21. 10.1128/spectrum.02711-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Puljko A, Milaković M, Križanović S, Kosić-Vukšić J, Babić I, Petrić I, Maravić A, Jelić M, Udiković-Kolić N. 2022. Prevalence of enteric opportunistic pathogens and extended-spectrum cephalosporin- and carbapenem-resistant coliforms and genes in wastewater from municipal wastewater treatment plants in Croatia. J Hazard Mater 427:128155. 10.1016/j.jhazmat.2021.128155. [DOI] [PubMed] [Google Scholar]
- 244.Galarde-López M, Velazquez-Meza ME, Bobadilla-Del-Valle M, Carrillo-Quiroz BA, Cornejo-Juárez P, Ponce-De-León A, Sassoé-González A, Alpuche-Aranda CM. 2022. Surveillance of antimicrobial resistance in hospital wastewater: identification of carbapenemase-producing Klebsiella spp. Antibiotics 11:288. 10.3390/antibiotics11030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tacão M, Silva I, Henriques I. 2017. Culture-independent methods reveal high diversity of OXA-48-like genes in water environments. J Water Health 15:519–525. 10.2166/wh.2017.260. [DOI] [PubMed] [Google Scholar]
- 246.Schmiedel J, Falgenhauer L, Domann E, Bauerfeind R, Prenger-Berninghoff E, Imirzalioglu C, Chakraborty T. 2014. Multiresistant extended-spectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol 14:187. 10.1186/1471-2180-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu X, Thungrat K, Boothe DM. 2016. Occurrence of OXA-48 carbapenemase and other β-lactamase genes in ESBL-producing multidrug resistant Escherichia coli from dogs and cats in the United States, 2009–2013. Front Microbiol 7:1057. 10.3389/fmicb.2016.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pulss S, Stolle I, Stamm I, Leidner U, Heydel C, Semmler T, Prenger-Berninghoff E, Ewers C. 2018. Multispecies and clonal dissemination of OXA-48 carbapenemase in Enterobacteriaceae from companion animals in Germany, 2009–2016. Front Microbiol 9:1265. 10.3389/fmicb.2018.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stolle I, Prenger-Berninghoff E, Stamm I, Scheufen S, Hassdenteufel E, Guenther S, Bethe A, Pfeifer Y, Ewers C. 2013. Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J Antimicrob Chemother 68:2802–2808. 10.1093/jac/dkt259. [DOI] [PubMed] [Google Scholar]
- 250.Yousfi M, Touati A, Mairi A, Brasme L, Gharout-Sait A, Guillard T, De Champs C. 2016. Emergence of carbapenemase-producing Escherichia coli isolated from companion animals in Algeria. Microb Drug Resist 22:342–346. 10.1089/mdr.2015.0196. [DOI] [PubMed] [Google Scholar]
- 251.Loucif L, Gacemi-Kirane D, Cherak Z, Chamlal N, Grainat N, Rolain JM. 2016. First report of German cockroaches (Blattella germanica) as reservoirs of CTX-M-15 extended-spectrum-β-lactamase- and OXA-48 carbapenemase-producing Enterobacteriaceae in Batna University Hospital, Algeria. Antimicrob Agents Chemother 60:6377–6380. 10.1128/AAC.00871-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Melo LC, Boisson MNG, Saras E, Médaille C, Boulouis HJ, Madec JY, Haenni M. 2017. OXA-48-producing ST372 Escherichia coli in a French dog. J Antimicrob Chemother 72:1256–1258. 10.1093/jac/dkw531. [DOI] [PubMed] [Google Scholar]
- 253.Yousfi M, Touati A, Muggeo A, Mira B, Asma B, Brasme L, Guillard T, de Champs C. 2018. Clonal dissemination of OXA-48-producing Enterobacter cloacae isolates from companion animals in Algeria. J Glob Antimicrob Resist 12:187–191. 10.1016/j.jgar.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 254.Hassan B, Ijaz M, Khan A, Sands K, Serfas GI, Clayfield L, El-Bouseary MM, Lai G, Portal E, Khan A, Watkins WJ, Parkhill J, Walsh TR. 2021. A role for arthropods as vectors of multidrug-resistant Enterobacterales in surgical site infections from South Asia. Nat Microbiol 6:1259–1270. 10.1038/s41564-021-00965-1. [DOI] [PubMed] [Google Scholar]
- 255.Al Bayssari C, Olaitan AO, Dabboussi F, Hamze M, Rolain JM. 2015. Emergence of OXA-48-producing Escherichia coli clone ST38 in fowl. Antimicrob Agents Chemother 59:745–746. 10.1128/AAC.03552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zurfluh K, Poirel L, Nordmann P, Klumpp J, Stephan R. 2015. First detection of Klebsiella variicola producing OXA-181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrob Resist Infect Control 4:38. 10.1186/s13756-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhuang Z, Lv L, Lu J, Lin J, Liu JH. 2019. Emergence of Klebsiella pneumoniae and Enterobacter cloacae producing OXA-48 carbapenemases from retail meats in China, 2018. J Antimicrob Chemother 74:3632–3634. 10.1093/jac/dkz394. [DOI] [PubMed] [Google Scholar]
- 258.Irrgang A, Pauly N, Tenhagen BA, Grobbel M, Kaesbohrer A, Hammerl JA. 2020. Spill-over from public health? First detection of an OXA-48-producing Escherichia coli in a German pig farm. Microorganisms 8:855. 10.3390/microorganisms8060855. [DOI] [PMC free article] [PubMed] [Google Scholar]