ABSTRACT
Morbidity and mortality related to ventriculitis in neurocritical care patients remain high. Antibiotic dose optimization may improve therapeutic outcomes. In this study, a population pharmacokinetic model of meropenem in infected critically ill patients was developed. We applied the final model to determine optimal meropenem dosing regimens required to achieve targeted cerebrospinal fluid exposures. Neurocritical care patients receiving meropenem and with a diagnosis of ventriculitis or extracranial infection were recruited from two centers to this study. Serial plasma and cerebrospinal fluid samples were collected and assayed. Population pharmacokinetic modeling and Monte Carlo dosing simulations were performed using Pmetrics. We sought to determine optimized dosing regimens that achieved meropenem cerebrospinal fluid concentrations above pathogen MICs for 40% of the dosing interval, or a higher target ratio of meropenem cerebrospinal fluid trough concentrations to pathogen MIC of ≥1. In total, 53 plasma and 34 cerebrospinal fluid samples were obtained from eight patients. Meropenem pharmacokinetics were appropriately described using a three-compartment model with linear plasma clearance scaled for creatinine clearance and cerebrospinal fluid penetration scaled for patient age. Considerable interindividual pharmacokinetic variability was apparent, particularly in the cerebrospinal fluid. Percent coefficients of variation for meropenem clearance from plasma and cerebrospinal fluid were 41.7% and 89.6%, respectively; for meropenem, the volume of distribution in plasma and cerebrospinal fluid values were 63.4% and 58.3%, respectively. High doses (up to 8 to 10 g/day) improved attainment of meropenem cerebrospinal fluid target exposures, particularly for less susceptible organisms (MICs, ≥0.25 mg/L). Standard meropenem doses of 2 g every 8 h may not achieve effective concentrations in cerebrospinal fluid in all critically ill patients. Higher doses, or alternative dosing methods (e.g., loading dose followed by continuous infusion) may be required to optimize cerebrospinal fluid exposures. Doses of up to 8 to 10 g/day either as intermittent boluses or continuous infusion would be suitable for patients with augmented renal clearance; lower doses may be considered for patients with impaired renal function as empirical suggestions. Ongoing dosing should be tailored to the individual patient circumstances. Notably, the study population was small and dosing recommendations may not be generalizable to all critically ill patients.
KEYWORDS: CSF, carbapenem, dosing, pharmacodynamics, pharmacokinetics, sepsis
INTRODUCTION
Morbidity and mortality related to nosocomial infections in the intensive care unit (ICU) remain high. Ventriculitis, or ventriculostomy-associated infection (VAI), is the most common nosocomial central nervous system (CNS) infection in neurocritical care patients and is a serious complication following the surgical insertion of external ventricular drains (EVD) (1). Optimizing antibiotic dosing to maximize antibiotic exposures at the infection site (cerebrospinal fluid [CSF]) is an important strategy that may improve therapeutic outcomes in critically ill patients (2, 3).
Meropenem is a broad-spectrum carbapenem antibiotic used in the ICU for the treatment of severe sepsis, including infections with a CNS focus (4). Meropenem bacterial killing best correlates with the percentage of time the free drug concentration remains above pathogen MIC during the dosing interval (%f T>MIC) (5). A f T>MIC of 40 to 70% in plasma has been associated with ≥1-log10 CFU/mL of bacterial killing over 24 h in preclinical studies, the minimum target established by licensing authorities (6–9). Although no clinical data for adult critically ill patients with CNS infections exist, a plasma f T>MIC of 100% has been associated with improved outcomes in other infection syndromes (7, 10, 11). Importantly, it remains unclear what meropenem exposures in CSF are necessary for optimal benefit in adults (1).
Dosing of antibiotics, such as meropenem, in critically ill patients with ventriculitis can be particularly challenging. Augmented renal clearance (ARC; creatinine clearance of ≥130 mL/min/1.73 m2), including in patients with head trauma, increases clearance of renally cleared drugs (12). In addition, the variable (and possibly low) meningeal inflammation in this patient population further increases the risk of subtherapeutic exposures at the infection site (13). This variability has dose selection implications. Previous in silico simulation studies have demonstrated that standard meropenem doses of 1 to 2 g every 8 h may not achieve an adequate exposure in the CSF against “susceptible” pathogens for which the MIC is ≥0.25 mg/L (14, 15). Therefore, increased doses or altered dosing methods may need to be considered.
The aims of this study were to (i) describe the plasma and CSF population pharmacokinetics (PK) of meropenem in infected critically ill patients with an EVD in situ and (ii) apply the final PK model for dosing simulations to determine optimal meropenem dosing regimens required to achieve targeted CSF exposures.
RESULTS
Study population.
Eight patients met inclusion criteria and were included in the study. The sampling sequence was plasma to be drawn 0.5, 1, 2, 4, 6, and 8 h after infusion commencement and CSF drawn 0.5, 2, 4, and 6 h after start of infusion. In total, 53 plasma and 34 CSF samples were obtained. Demographic and clinical details are presented in Table 1. Seven patients had an admission diagnosis of subarachnoid hemorrhage and one was admitted for meningitis initially. Six patients were receiving meropenem for treatment of ventriculitis, one for pneumonia, and one for a urinary tract infection. Patients received 3 to 8 g/day as divided doses given every 6 to 12 h, with one patient receiving a continuous infusion. Fifty percent of patients displayed ARC, and younger patients were more likely to have greater creatinine clearance. Measured interleukin-1 (IL-1) concentrations ranged from undetectable to 7.79 pg/mL and were highly variable in both plasma and CSF. Interleukin-1 was undetectable in 9 of 13 samples.
TABLE 1.
Patient demographic details
| Demographica | Value(s) for our sample (N = 8) |
|---|---|
| Age, yrs, median (range) | 63 (46–75) |
| Female sex, n (%) | 5 (62.5) |
| Wt, kg, median (range) | 75 (46–78) |
| Albumin, g/L, median (range) | 25 (15–59) |
| Creatinine clearance, mL/min, median (range) | 124 (36–240) |
| Augmented renal clearance,b n (%) | 4 (50) |
| CSF protein, g/L, median (range) | 1.2 (0.45–2.9) |
| CSF pleocytosis, ×106, median (range) | 235 (10–7,600) |
| CSF protein:serum albumin, median (range) | 0.036 (0.012–0.133) |
| CSF vol drained from EVD, mL, median (range) | 40 (10–113) |
| SOFA score, median (range) | 7 (4–9) |
| APACHE scores, median (range) | 13 (12–27) |
| Infection source, n (%) | |
| Ventriculitis | 6 (75) |
| Pneumonia | 1 (12.5) |
| Urinary tract | 1 (12.5) |
| Isolate identified, nc | |
| Acinetobacter spp. | 3 |
| Coagulase-negative Staphylococcus spp. | 2 |
| Escherichia coli | 1 |
| No organism identified | 1 |
| Pantoea agglomerans | 1 |
| Unspeciated Gram-negative bacilli | 1 |
| Meropenem dose administered, n (%) | |
| 1 g every 8 h | 2 (25) |
| 2 g every 8 h | 3 (37.5) |
| 2 g every 12 h | 1 (12.5) |
| 2 g every 8 h for 4 doses then 250 mg/h continuous infusion for 24 h | 1 (12.5) |
| 2 g every 6 h | 1 (12.5) |
CSF, cerebrospinal fluid; EVD, external ventricular drain; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation.
Defined as creatinine clearance of ≥130 mL/min.
Two patients had two organisms isolated.
Pharmacokinetic model.
Cerebrospinal fluid and plasma concentrations of meropenem were adequately described by a three-compartment model incorporating a linear entry into CSF, linear intercompartmental distribution, linear clearance, and nonlinear elimination from the CSF compartment (incorporating volume of CSF drained from EVD over the sampling interval) and linear clearance from the plasma compartment (Fig. 1). Including mean creatinine clearance (calculated using the Cockcroft-Gault formula) as a covariate on meropenem clearance and mean age on distribution rate constant from plasma to CSF compartment as covariates improved the goodness-of-fit plots and the Akaike information criterion (AIC), Bayesian information criterion (BIC), and log-likelihood ratio (LLR) for the final population model (see Table S1 in the supplemental material).
FIG 1.

Pharmacokinetic model. K12, transfer rate constant from central to CSF compartment; K21, transfer rate constant from CSF to central compartment; Kcp, transfer rate constant from central to peripheral compartment; Kpc, transfer rate constant from peripheral to central compartment; CL, clearance from central compartment; CL2, clearance from CSF compartment.
The final covariate model relied on the following equations: TVCL = (CL × creatinine clearance/130) and TVK12 = (K12 × age/63), where TVCL is the typical value of meropenem clearance, K12 is the transfer rate constant from plasma to the CSF, and TVK12 is the typical meropenem distribution rate constant from plasma into the CSF.
The final model performed adequately, as evidenced by the plasma and CSF population and Bayesian posterior observed-predicted plots, respectively (Fig. 2A and B), and the plasma and CSF visual predictive check (Fig. 3). Meropenem PK parameters displayed considerable interpatient variability, particularly in CSF. See Tables 2 and 3 for the parameter estimates and the percent coefficient of variation (%CV) predicted by the final model.
FIG 2.
Comparison between observed antibiotic concentrations versus concentrations predicted by pharmacokinetic model in plasma and cerebrospinal fluid. (A) Plasma model for population and individual; (B) cerebrospinal fluid model for population and individual.
FIG 3.
Observed meropenem concentration-time data and visual predictive check of the final model. (A) Plasma model, dots represent observed meropenem concentrations in plasma; (B) cerebrospinal fluid model, dots represent observed meropenem concentrations in CSF.
TABLE 2.
Meropenem dosing, patient renal function, and plasma and CSF meropenem concentrations achieved
| Patient | Creatinine clearance (mL/min) | Meropenem dosinga | Minimum and maximum plasma concn (mg/L) | Minimum and maximum CSF concn (mg/L) |
|---|---|---|---|---|
| RB01 | 245 | 2 g every 6 h | 0.2–26.1 | 0.3–0.5 |
| RB02 | 133 | 2 g every 6 h | 0.6–79.1 | 0.5–1.1 |
| HK01 | 41 | 1 g every 8 h | 2.9–31.8 | 1.3–2.4 |
| HK04 | 127 | 2 g every 8 h | 0.2–43.6 | 1.3–4.3 |
| RB06 | 161 | 2 g every 6 h | 2.5–96.6 | 0.3–3.3 |
| RB07 | 170 | 2 g every 8 h | 0.4–83.3 | 0.8–1.9 |
| RB14 | 52 | 2 g every 8–12 h | 16.7–61.7 | 0.8 |
| RB18 | 65 | 2 g every 8 h for 3 doses then continuous infusion of 250 mg/h | 19.1–22.4 | 4.4–4.5 |
All bolus doses were given over 30 min.
TABLE 3.
Meropenem pharmacokinetic model parameter estimates
| Parametera | Mean (SD) | Median | %CV |
|---|---|---|---|
| CL (L/h) | 14.87 (6.20) | 14.00 | 41.72 |
| Vc (L) | 7.11 (4.51) | 5.46 | 63.42 |
| K12 (h−1) | 0.61 (0.45) | 0.35 | 73.66 |
| K21 (h−1) | 0.06 (0.06) | 0.08 | 90.00 |
| Vcsf (L) | 108.94 (9.20) | 84.49 | 58.27 |
| Kcp (h−1) | 24.69 (4.79) | 22.10 | 37.23 |
| Kpc (h−1) | 7.09 (4.32) | 6.57 | 61.01 |
| CL2 (L/h) | 0.25 (0.22) | 0.09 | 89.63 |
SD, standard deviation; CV, covariance; CL, clearance from central compartment; Vc, volume of distribution of the central compartment; K12, transfer rate constant from plasma to cerebrospinal fluid compartment; K21, transfer rate constant from cerebrospinal fluid to plasma compartment; Vcsf, volume of distribution of cerebrospinal fluid compartment; Kcp, transfer rate constant from central to peripheral compartment; Kpc, transfer rate constant from peripheral to central compartment; CL2, linear clearance from CSF compartment.
Penetration into CSF was variable but low; the median (range) CSF/plasma area under the time-concentration curve (AUC) ratio was 18% (2 to 33%). Meropenem mean and 95% confidence interval (CI) plasma concentrations at 3.2 and 8 h postdosing (reflecting the 40% fT>MIC and 100% fT>MIC) were 13.02 mg/L (95% CI, 2.07 to 38.85) and 4.05 mg/L (95% CI, 0.19 to 14.04), respectively. Conversely, the mean (95% CI) meropenem CSF concentrations at 3.2 and 8 h postdosing were 1.55 mg/L (95% CI, 0.49 to 4.04) and 1.00 mg/L (95% CI, 0.28 to 2.92), respectively.
Probability of target attainment and fractional target attainment.
The probability of target attainment (PTA) and fractional target attainment (FTA) for both targets were appreciably higher in plasma than in CSF samples. Both the PTA and FTA (fT>MIC 40%) were >95% in plasma for all isolates (including in young patients with ARC) with a standard dosing regimen of 2 g every 8 h. Simulated high-dose continuous infusions achieved a PTA and FTA (for which the ratio of the trough concentration [Cmin] and the MIC was ≥1) of up to 70% more than intermittent bolus dosing in plasma. Conversely, intermittent bolus doses of 2 g every 6 h attained PTA and FTA (Cmin/MIC of ≥1) up to 28% greater than a continuous infusion in patients with ARC; however, in patients without ARC, the PTA and FTA were comparable between equivalent daily meropenem doses administered as a continuous infusion or with intermittent dosing. The PTAs in CSF are presented in Fig. 4 and 5. The FTAs in CSF are presented in Tables 4 and 5.
FIG 4.
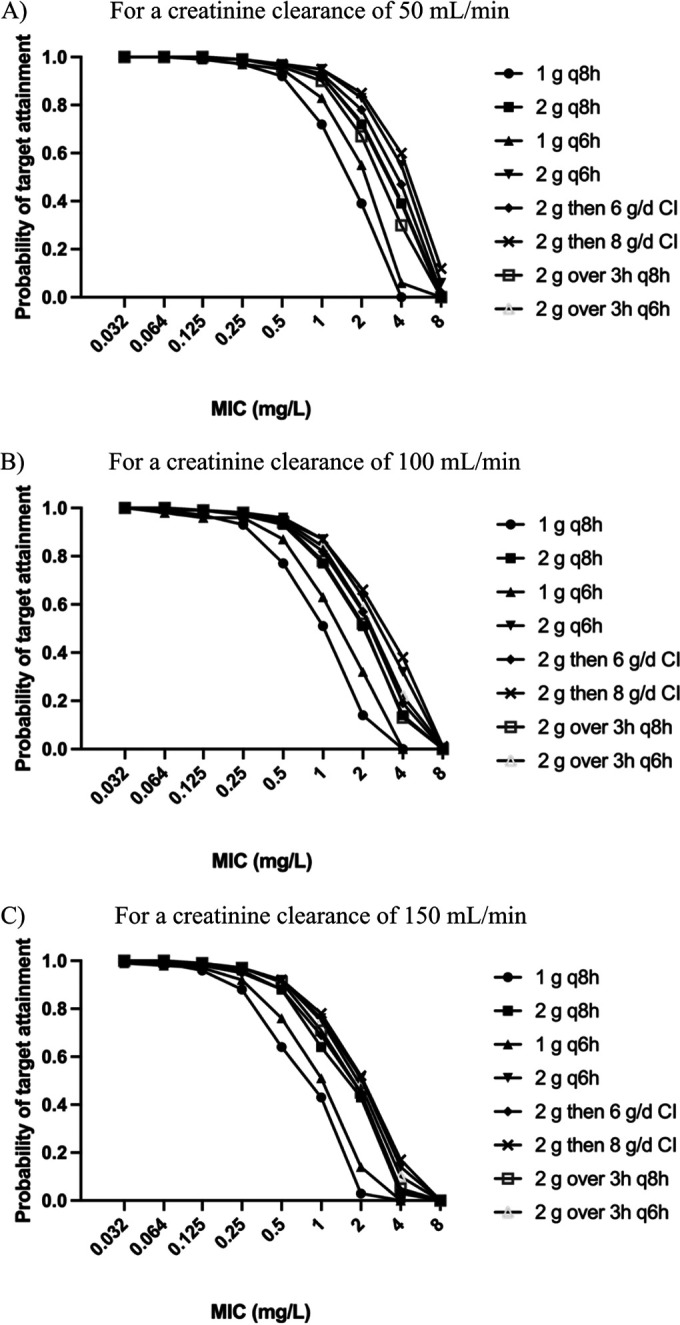
Probabilities of target attainment in CSF for a 40% fT>MIC with simulated patient age of 60 years for a creatinine clearance of 50 mL/min (A), 100 mL/min (B), or 150 mL/min (C). q8h, administered every 8 h; q6h, administered every 6 h; CI, continuous infusion; g/d, grams per day.
FIG 5.

Probabilities of target attainment in CSF for a Cmin/MIC of ≥1 with a simulated patient age of 60 years and a creatinine clearance of 50 mL/min (A), 100 mL/min (B), or 150 mL/min (C). q8h, administered every 8 h; q6h, administered every 6 h; CI, continuous infusion; g/d, grams per day.
TABLE 4.
Fractional target attainment in CSF for 40% fT>MIC in simulated patient, age 60 years
| Dosing regimena | CrCL (mL/min) | FTA in CSF |
|||||
|---|---|---|---|---|---|---|---|
| E. coli | E. cloacae | K. pneumoniae | S. epidermidis | S. aureus | P. aeruginosa | ||
| 1 g every 8 h | 50 | 1 | 0.99 | 0.99 | 0.70 | 0.97 | 0.73 |
| 100 | 1 | 0.97 | 0.98 | 0.63 | 0.95 | 0.63 | |
| 150 | 0.99 | 0.96 | 0.97 | 0.58 | 0.93 | 0.56 | |
| 2 g every 8 h | 50 | 1 | 0.99 | 0.99 | 0.81 | 0.99 | 0.84 |
| 100 | 1 | 0.99 | 0.99 | 0.74 | 0.98 | 0.77 | |
| 150 | 1 | 0.98 | 0.99 | 0.70 | 0.97 | 0.71 | |
| 1 g every 6 h | 50 | 1 | 0.99 | 0.99 | 0.74 | 0.97 | 0.78 |
| 100 | 0.99 | 0.98 | 0.98 | 0.67 | 0.96 | 0.69 | |
| 150 | 0.99 | 0.97 | 0.97 | 0.62 | 0.94 | 0.62 | |
| 2 g every 6 h | 50 | 1 | 0.99 | 0.99 | 0.85 | 0.99 | 0.87 |
| 100 | 1 | 0.99 | 0.99 | 0.78 | 0.98 | 0.81 | |
| 150 | 1 | 0.98 | 0.99 | 0.73 | 0.97 | 0.76 | |
| 2-g load, 6 g/day CI | 50 | 1 | 0.99 | 0.99 | 0.83 | 0.98 | 0.86 |
| 100 | 1 | 0.99 | 0.99 | 0.76 | 0.97 | 0.79 | |
| 150 | 1 | 0.98 | 0.98 | 0.70 | 0.96 | 0.72 | |
| 2-g load, 8 g/day CI | 50 | 1 | 0.99 | 1 | 0.86 | 0.99 | 0.88 |
| 100 | 1 | 0.99 | 0.99 | 0.79 | 0.98 | 0.82 | |
| 150 | 1 | 0.98 | 0.99 | 0.74 | 0.97 | 0.77 | |
| 2 g over 3 h every 8 h | 50 | 1 | 0.99 | 0.99 | 0.79 | 0.98 | 0.83 |
| 100 | 1 | 0.99 | 0.99 | 0.77 | 0.98 | 0.77 | |
| 150 | 1 | 0.99 | 0.99 | 0.71 | 0.97 | 0.74 | |
| 2 g over 3 h every 6 h | 50 | 1 | 0.99 | 0.99 | 0.82 | 0.99 | 0.85 |
| 100 | 1 | 0.99 | 0.99 | 0.87 | 0.98 | 0.80 | |
| 150 | 1 | 0.99 | 0.99 | 0.73 | 0.97 | 0.76 | |
CrCL, creatinine clearance; CI, continuous infusion.
TABLE 5.
Fractional target attainment in CSF for Cmin/MIC ≥ 1 for simulated patient age of 60 years
| Dosing regimena | CrCL (mL/min) | FTA in CSF |
|||||
|---|---|---|---|---|---|---|---|
| E. coli | E. cloacae | K. pneumoniae | S. epidermidis | S. aureus | P. aeruginosa | ||
| 1 g every 8 h | 50 | 0.98 | 0.93 | 0.96 | 0.51 | 0.87 | 0.43 |
| 100 | 0.98 | 0.91 | 0.94 | 0.46 | 0.84 | 0.36 | |
| 150 | 0.96 | 0.88 | 0.91 | 0.41 | 0.78 | 0.28 | |
| 2 g every 8 h | 50 | 0.99 | 0.97 | 0.98 | 0.62 | 0.94 | 0.60 |
| 100 | 0.99 | 0.96 | 0.97 | 0.57 | 0.92 | 0.54 | |
| 150 | 0.99 | 0.94 | 0.95 | 0.52 | 0.89 | 0.46 | |
| 1 g every 6 h | 50 | 0.98 | 0.93 | 0.95 | 0.52 | 0.87 | 0.46 |
| 100 | 0.97 | 0.92 | 0.94 | 0.50 | 0.86 | 0.43 | |
| 150 | 0.97 | 0.91 | 0.93 | 0.47 | 0.83 | 0.38 | |
| 2 g every 6 h | 50 | 0.99 | 0.97 | 0.97 | 0.64 | 0.94 | 0.62 |
| 100 | 0.99 | 0.96 | 0.97 | 0.61 | 0.93 | 0.59 | |
| 150 | 0.99 | 0.95 | 0.96 | 0.57 | 0.92 | 0.55 | |
| 2 g load, 6 g/d CI | 50 | 0.99 | 0.95 | 0.96 | 0.57 | 0.90 | 0.53 |
| 100 | 0.99 | 0.95 | 0.96 | 0.56 | 0.90 | 0.52 | |
| 150 | 0.98 | 0.94 | 0.96 | 0.56 | 0.88 | 0.51 | |
| 2 g load, 8 g/d CI | 50 | 0.99 | 0.95 | 0.96 | 0.57 | 0.90 | 0.53 |
| 100 | 0.99 | 0.95 | 0.96 | 0.56 | 0.90 | 0.52 | |
| 150 | 0.98 | 0.94 | 0.96 | 0.56 | 0.89 | 0.51 | |
| 2 g over 3 h every 8 h | 50 | 0.98 | 0.92 | 0.94 | 0.50 | 0.85 | 0.43 |
| 100 | 0.98 | 0.92 | 0.94 | 0.50 | 0.85 | 0.43 | |
| 150 | 0.98 | 0.92 | 0.94 | 0.50 | 0.85 | 0.43 | |
| 2 g over 3 h every 6 h | 50 | 0.98 | 0.92 | 0.94 | 0.51 | 0.85 | 0.44 |
| 100 | 0.98 | 0.92 | 0.94 | 0.51 | 0.85 | 0.44 | |
| 150 | 0.98 | 0.92 | 0.94 | 0.51 | 0.85 | 0.44 | |
CrCL, creatinine clearance; g/d, grams per day; CI, continuous infusion.
Increasing patient age was associated with an increased PTA and FTA in CSF, while increased creatinine clearance reduced the PTA and FTA in both plasma and CSF. The plasma PTA for both targets of the 40% fT>MIC and the Cmin/MIC of ≥1 was >90% for standard doses of 1 g every 8 h with a 0.5-h infusion even in patients with ARC. At higher MICs up to 8 mg/L, the PTA in plasma decreased substantially, even in patients with normal renal function and with higher doses and continuous infusions. As expected, achieving >95% FTA was more difficult with a target Cmin/MIC of ≥1 compared with the 40% fT>MIC in both plasma and CSF. A 2-g bolus followed by a continuous infusion of 8 g over 24 h resulted in the highest PTA and FTA. Prolonged infusions (over 3 h) did not improve the PTA or FTA compared with bolus dosing regimens for both targets in patients without ARC; however, the PTA was increased by up to 34% for prolonged infusions compared with bolus dosing regimens in patients with ARC.
DISCUSSION
This study highlights the considerable interindividual PK variability of critically ill patients, particularly for CSF, and the difficulty attaining targeted exposures of meropenem in CSF against less susceptible organisms (MICs of ≥0.25 mg/L). Younger patients with ARC were found to be more likely to have subtherapeutic meropenem exposures in CSF.
High PK variability was a key feature of meropenem in these patients. The volume of distribution (V) and clearance (CL) of meropenem in plasma predicted by the PK model in this present study was comparably variable as the values obtained in previous studies involving critically ill patients (16–19). For example, Jaruratanasirikul et al. predicted a %CV of 35% and 64% for meropenem CL and V, respectively, in patients with septic shock (16). Likewise, Mattioli and colleagues described %CV values of 47.7% and 55.6% for meropenem CL and V, respectively, in critically ill patients with sepsis (18). This contrasts with the reduced variability of meropenem PK parameters described for healthy volunteers (20–22). For example, Bax et al. demonstrated a %CV of 4.1% and 4.7% for CL and V, respectively, of meropenem in healthy adults (20). Similarly, Nilsson-Ehle and colleagues predicted a %CV of 16.5% and 12%, respectively, for meropenem CL and V in healthy male volunteers (21). Penetration into CSF in our study also varied significantly but was consistent with findings from previous studies in patients that had variable meningeal inflammation (23).
Meropenem concentrations achieved in CSF were significantly lower than plasma concentrations and display marked inter- and intraindividual variability over the dosing interval. Blassmann et al. found an increased meropenem clearance from CSF compared with its rate of CSF penetration, thus contributing to low CSF meropenem concentrations (19). This can be explained by the dual routes of antibiotic clearance from CSF: diffusion through the blood-CSF barrier and bulk transfer via the filtration of CSF into venous blood (13). This may be exacerbated by high CSF output through the EVD, which differed among study patients, as well as the baseline permeability of the blood-CSF barrier. Increasing age has previously been shown to correlate with blood-CSF barrier permeability, supporting the inclusion of age in our model (24). Further contributing to the low CSF concentrations is the presence of variable (and often low) meningeal inflammation in the setting of VAI. During meningitis, there is increased endothelial permeability of the blood-CSF barrier and decreased CSF outflow (13, 25), changes that may be present to varying degrees in the context of ventriculitis. Interestingly, incorporating the CSF protein/plasma albumin ratio as a marker of meningeal inflammation did not improve our model fit; nor was there an association between CSF protein and achieved concentrations. Increased CSF protein concentration has previously been shown to correlate with increasing meropenem and vancomycin CSF penetration (26, 27). This discrepancy between studies could be explained by multiple reasons: differing population sizes, variable CSF drainage through the EVD, heterogenous sampling protocols, difficulty in diagnosing ventriculitis, and CSF sampling occurred on different infection timelines. Konig et al. also fixed the volume of CSF, making a physiological assumption (27).
Higher intravenous doses of meropenem may be required to achieve targeted exposures in the CSF. In the absence of ARC, patients receiving standard doses for CNS infections, such as 2 g every 8 hour, will likely only attain targeted CSF exposures against highly susceptible organisms (MICs of ≤0.125 mg/L). Moreover, to uniformly achieve this target in younger patients, especially those at risk of ARC, higher doses of 2 g every 6 h or a 2-g loading dose followed by an 8-g continuous infusion over 24 h may be necessary. A previous meropenem PK study identified similar dosing recommendations for isolates with a low MIC (≤0.25 mg/L); however, doses as high as 4 g every 6 hours may be required for isolates currently deemed susceptible (MIC, ≤2 mg/L) (19). Considering the FTA for specific bacterial pathogens, even high doses of meropenem (total of 8 to 10 g/day) either via intermittent bolus dosing or a bolus followed by continuous infusion may not result in sufficiently favorable targeted exposures for susceptible Staphylococcus epidermidis and Pseudomonas aeruginosa. Continuous infusion of meropenem has previously been considered problematic due to limited stability in aqueous solutions and the associated challenges of line availability and compatibility (28). However, the ongoing large multicenter BLING trial program has demonstrated the feasibility of continuous meropenem infusion (29–31). Tiede et al. used continuous meropenem and vancomycin infusion with therapeutic drug monitoring-guided dose optimization for successful treatment of ventriculitis in a retrospective cohort study (32). Continuous infusion may have varying impacts upon CSF penetration. Higher mean concentrations may lead to increased CSF penetration; alternatively, higher peak concentrations associated with bolus dosing may increase antibiotic distribution into CSF. Data remain inconsistent. Alternative antibiotics or the intraventricular route, which has the advantage of bypassing the blood-CSF barrier, may need consideration. In one retrospective study, intrathecal meropenem and vancomycin for treatment of postoperative intracranial infections was associated with greater clinical efficacy, shorter cure time, and reduced costs compared to an intravenous regimen (33). In a population PK simulation study of combined intrathecal and intravenous meropenem for treatment of postoperative intracranial infections, Li et al. found CSF concentrations inversely correlated with CSF drainage volume (34). However, in line with the Infectious Disease Society of America Guidelines, the risk of serious neurotoxicity and seizures from carbapenems precludes their routine intrathecal administration (35). Intrathecal carbapenem administration, although pharmacokinetically sensible, is still in the experimental space.
This study has some limitations. First, the small patient sample size may not be sufficient to capture the true PK variability inherent in the critically ill population, although our patients were heterogenous with respect to age, gender, renal function, infection source, meropenem dosing regimen, underlying diagnosis, and serum and CSF biochemistry. Additionally, it should be noted that two patients were being treated for non-CNS infections. However, ventriculitis may be associated with low meningeal inflammation (particularly compared to meningitis), during which the blood-brain barrier may be less affected (36). No appreciable difference in CSF protein:plasma albumin ratios was found between patients with and without ventriculitis, although the sample size precludes robust statistical analysis. The MIC distribution for specific isolates was obtained from the EUCAST database and so may not necessarily reflect local susceptibility and resistance patterns. Furthermore, the PK/PD targets used for modeling simulations used have not been validated in CSF and have been extrapolated from plasma targets. Importantly, a precise calculation of creatinine clearance, such as urinary creatinine clearance, was not available for patients. Finally, the impact of such dosing regimens was not assessed against patient-centered outcomes, such as clinical cure, ICU length of stay, or mortality.
Conclusions.
The present study has illustrated high interindividual PK variability, especially in CSF, of meropenem in critically ill patients. It has also highlighted the influence of plasma concentrations, ARC, and age on meropenem CSF concentrations. A standardized approach to meropenem dosing is likely inappropriate, particularly for young patients with higher creatinine clearances, such as ARC. An intermittent bolus dosing regimen of 2 g every 6 h, or a 2-g loading dose followed by 8 g over 24 h as continuous infusion, has the highest probability of attaining targeted CSF exposures. Even higher doses may be required for VAI caused by P. aeruginosa or S. epidermidis; alternative antibiotics or the intraventricular route may be a consideration in these circumstances. Future studies should seek to explore the relationship between dosing regimen, probability of achieving therapeutic exposures in CSF, and patient outcomes.
MATERIALS AND METHODS
Setting.
This was a binational prospective observational PK study conducted at two university-associated tertiary level ICUs: Royal Brisbane and Women’s Hospital, University of Queensland, and the Prince of Wales Hospital, Chinese University of Hong Kong. Ethical approval was obtained from both the Royal Brisbane and Women’s Hospital Research Ethics Committee (HREC/16/QRBW/157) and the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CREC 2016.030).
Patient population.
Inclusion criteria for this study were the following: (i) age 18 to 85 years; (ii) EVD in situ; (iii) receiving meropenem treatment; and (iv) a diagnosis of ventriculitis or extracranial infection. Definitions for ventriculitis vary, with some studies using positive CSF culture, others using positive CSF culture along with clinical or microbiological criteria, and others using either positive culture or clinical or microbiological criteria to diagnose VAI (37). In this study, VAI was diagnosed by the treating intensivist. Exclusion criteria were the following: (i) small ventricles as determined by computed tomography with intracranial pressure of >20 mmHg; (ii) preexisting renal impairment, defined as the need for replacement therapy or a serum creatinine concentration of >200 µmol/L on the day of participant screening; (iii) preexisting hepatic dysfunction, defined as a gamma glutamyl transferase level of >200 IU/L; (iv) hypersensitivity to beta-lactams; (v) pregnancy; and (vi) patients in whom death was deemed inevitable within 24 h. Clinical and anthropometric data were prospectively recorded and included patient age (years), weight (kilograms), height (centimeters), body mass index, suspected infection source, clinical morality prediction scores (including sequential organ failure assessment [SOFA] scores and acute physiology and chronic health evaluation [APACHE] scores), microbiological results (if available), serum albumin (grams per liter), CSF parameters such as pleocytosis, glycorrhachia (millimoles per liter), red blood cell count, and protein (grams per liter), serum albumin (grams per liter), serum creatinine (micromoles per liter), creatinine clearance (CrCL; milliliters per minute, calculated per the Cockcroft-Gault equation), and presence of ARC (defined as a CrCL of ≥130 mL/min [12]).
Sample handing, storage, and measurement.
Blood samples were immediately stored in an ice bath and centrifuged within 6 h. Between 1.5 and 1.8 mL of the ensuing plasma was transferred to a labeled cryovial, which was immediately stored at −20°C. Cerebrospinal fluid samples were immediately placed on ice and centrifuged within 60 min. All samples were stored at −80°C within 2 days of collection. Samples were subsequently transferred on dry ice to the University of Queensland Centre for Clinical Research, Herston, Brisbane.
Meropenem was measured in plasma and CSF by a validated ultrahigh-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) method on a Nexera UHPLC apparatus connected to an 8030+ triple-quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). Test samples were assayed in batches alongside calibrators, and quality controls and results were subject to batch acceptance criteria (U.S. FDA Guidance for Industry: Bioanalytical Method Validation, May 2018 [38]).
Cerebrospinal fluid samples were processed in the same way as plasma samples. Plasma and CSF are biological liquids with similar potential interferences in terms of salts and proteins, and a stable isotope-labeled meropenem internal standard was used that is resilient against ion suppression. Samples (10 µL of plasma and CSF) were spiked with deuterium-labeled meropenem internal standard, and acetonitrile (60 µL) was added to precipitate any proteins. An aliquot of the solution was injected onto the UHPLC-MS/MS apparatus. Chromatographic separation was achieved using a SeQuant analytical guard column as the stationary phase, 0.1% formic acid in water as mobile phase A, and 100% acetonitrile with 0.1% formic acid as mobile phase B. Meropenem was monitored by positive-mode electrospray optimized with multiple reaction monitoring at fragmentation ions of 383.3 → 68.0 (sample meropenem) and 390.15 → 147.00 (internal standard). Mean intrabatch accuracy and precision values were 9% and 5.5%, respectively, with a range of linearity between 0.2 and 100 mg/L.
Additionally, interleukin-1 (IL-1) concentrations were measured in plasma and CSF samples using the Abcam IL-1 human enzyme-linked immunosorbent assay kit (Abcam, Cambridge, UK) over a calibration range of 0.412 to 100 pg/mL. Cerebrospinal fluid samples were processed as plasma samples. Samples were centrifuged and diluted with Abcam proprietary sample diluent NS; 50 µL of this solution was then added to a microplate well, to which an antibody cocktail was added, the well was washed, and a colorimetric detection method was applied.
Population pharmacokinetic modeling.
Meropenem concentrations in plasma and CSF were modeled using the Pmetrics version 1.5.2 software package for R (39). Two- and three-compartment models were developed that incorporated linear clearance from the central compartment and combined linear clearance and nonlinear elimination from the CSF compartment. A linear intercompartmental distribution process was also modeled for both two- and three-compartment models. For each model, the additive and multiplicative error models incorporating a linear residual error estimate within Pmetrics were assessed and optimized. Diagnostic plots, including the observed versus predicted concentrations, residuals versus predicted concentrations, concentrations versus time, and the visual predictive plots, as well as the AIC, BIC, and LLR, were considered to compare different models.
Clinical and demographic patient variables such as age, sex, weight, height, body mass index, APACHE and SOFA scores, serum albumin, creatinine clearance (calculated using the Cockcroft-Gault equation using total body weight and expressed in milliliters per minute), serum alanine aminotransferase levels, volume of CSF drained over sampling interval, CSF protein concentration, and CSF protein:serum albumin ratio were considered for inclusion as covariates in the modeling process. Covariates were included in the final model if they improved the goodness-of-fit plots and/or reduced the AIC, BIC, and LLR. Cerebrospinal fluid penetration was estimated using the posterior estimates of CSF/plasma AUC ratios during the first 24 h of therapy.
Probability of target attainment and fractional target attainment.
Monte Carlo simulations (n = 1,000) using Pmetrics were performed using the final population model with simulated doses of 1 or 2 g administered every 6 or 8 h, a 2-g loading dose followed by a 6-g continuous infusion over 24 h, and a 2-g loading dose followed by an 8-g continuous infusion over 24 h. Simulated patient covariates included creatinine clearance, age, and daily CSF volume output. The probability of such dosing regimens achieving a primary target, f T>MIC, of 40% and an alternative higher target, a Cmin/MIC of ≥1 in CSF and plasma, was determined for MICs ranging from 0.032 to 8 mg/L (7, 40, 41). Traditionally, a 40% f T>MIC is extrapolated from in vitro studies, with some literature suggesting the higher Cmin/MIC of ≥1 exposure is likely necessary (7). Moreover, it is likely inappropriate to consider a target of 40% f T>MIC in patients receiving a continuous infusion, given that the exposure will be either 0% f T>MIC or 100% f T>MIC. In this population, the minimum target exposure should therefore be a Cmin/MIC of ≥1 (7).
The FTA describes the probability of simulated patients attaining the meropenem PK/PD targets in CSF (f T>MIC of 40% and Cmin/MIC of ≥1) against a MIC distribution for specific bacterial species. For our analyses, we tested up to a maximum MIC of 8 mg/L. The FTAs were calculated for methicillin-susceptible Staphylococcus aureus, Staphylococcus epidermidis, Enterobacter cloacae, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli isolates by using the MIC distribution obtained from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) database (available from www.eucast.org). Staphylococcus spp. and Enterobacterales are commonly implicated pathogens causing ventriculitis in critically ill patients (36).
Dosing regimens were considered successful if the PTA was ≥90% of the simulated patient population and if the FTA was ≥95%.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kumta N, Roberts JA, Lipman J, Wong WT, Joynt GM, Cotta MO. 2020. A systematic review of studies reporting antibiotic pharmacokinetic data in the cerebrospinal fluid of critically ill patients with uninflamed meninges. Antimicrob Agents Chemother 65:e01998-20. 10.1128/AAC.01998-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases . 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonçalves-Pereira J, Póvoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta-lactams. Crit Care 15:R206. 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin CM, Lyseng-Williamson KA, Keam SJ. 2008. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs 68:803–838. 10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Mouton J. 2016. General concepts of pharmacodynamics for anti-infective agents. In Rotschafer J, Andes D, Rodvold K (ed), Antibiotic pharmacodynamics. Springer, New York, NY. [Google Scholar]
- 6.Ong CT, Tessier PR, Li C, Nightingale CH, Nicolau DP. 2007. Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diagn Microbiol Infect Dis 57:153–161. 10.1016/j.diagmicrobio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva JA, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA, Infection Section of European Society of Intensive Care Medicine, Pharmacokinetic/Pharmacodynamic, Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy . 2020. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 46:1127–1153. 10.1007/s00134-020-06050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 47:S32–S40. 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 9.Agency EM. 2017. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products. European Medicines Agency, London, UK. [Google Scholar]
- 10.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Roberts JA, Lipman J, Starr T, Wallis SC, Paul SK, Margarit Ribas A, De Waele JJ, De Crop L, Spapen H, Wauters J, Dugernier T, Jorens P, Dapper I, De Backer D, Taccone FS, Rello J, Ruano L, Afonso E, Alvarez-Lerma F, Gracia-Arnillas MP, Fernandez F, Feijoo N, Bardolet N, Rovira A, Garro P, Colon D, Castillo C, Fernado J, Lopez MJ, Fernandez JL, Arribas AM, Teja JL, Ots E, Carlos Montejo J, Catalan M, DALI Study . 2014. DALI: defining antibiotic levels in intensive care unit patients. Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 12.Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, Deans R, Roberts JA, Senthuran S, Boots R, Bisht K, Bulmer AC, Lipman J. 2017. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma 34:137–144. 10.1089/neu.2015.4328. [DOI] [PubMed] [Google Scholar]
- 13.Nau R, Sorgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodise TP, Nau R, Kinzig M, Drusano GL, Jones RN, Sörgel F. 2007. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother 60:1038–1044. 10.1093/jac/dkm325. [DOI] [PubMed] [Google Scholar]
- 15.Nau R, Lassek C, Kinzig-Schippers M, Thiel A, Prange HW, Sörgel F. 1998. Disposition and elimination of meropenem in cerebrospinal fluid of hydrocephalic patients with external ventriculostomy. Antimicrob Agents Chemother 42:2012–2016. 10.1128/AAC.42.8.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaruratanasirikul S, Thengyai S, Wongpoowarak W, Wattanavijitkul T, Tangkitwanitjaroen K, Sukarnjanaset W, Jullangkoon M, Samaeng M. 2015. Population pharmacokinetics and Monte Carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob Agents Chemother 59:2995–3001. 10.1128/AAC.04166-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adnan S, Li JX, Wallis SC, Rudd M, Jarrett P, Paterson DL, Lipman J, Udy AA, Roberts JA. 2013. Pharmacokinetics of meropenem and piperacillin in critically ill patients with indwelling surgical drains. Int J Antimicrob Agents 42:90–93. 10.1016/j.ijantimicag.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Mattioli F, Fucile C, Del Bono V, Marini V, Parisini A, Molin A, Zuccoli ML, Milano G, Danesi R, Marchese A, Polillo M, Viscoli C, Pelosi P, Martelli A, Di Paolo A. 2016. Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur J Clin Pharmacol 72:839–848. 10.1007/s00228-016-2053-x. [DOI] [PubMed] [Google Scholar]
- 19.Blassmann U, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Hope W, Briegel J, Huge V. 2016. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care 20:343. 10.1186/s13054-016-1523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bax RP, Bastain W, Featherstone A, Wilkinson DM, Hutchinson M, Haworth SJ. 1989. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother 24:311–320. 10.1093/jac/24.suppl_A.311. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson-Ehle I, Hutchison M, Haworth SJ, Norrby SR. 1991. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur J Clin Microbiol Infect Dis 10:85–88. 10.1007/BF01964413. [DOI] [PubMed] [Google Scholar]
- 22.Krueger WA, Bulitta J, Kinzig-Schippers M, Landersdorfer C, Holzgrabe U, Naber KG, Drusano GL, Sorgel F. 2005. Evaluation by Monte Carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob Agents Chemother 49:1881–1889. 10.1128/AAC.49.5.1881-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heffernan AJ, Roberts JA. 2021. Dose optimisation of antibiotics used for meningitis. Curr Opin Infect Dis 34:581–590. 10.1097/QCO.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 24.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. 2015. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85:296–302. 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutsar I, McCracken G, Friedland I. 1998. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis 27:1117–1127. 10.1086/515003. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Wu Y, Sun S, Zhao Z, Wang Q. 2016. Population pharmacokinetics of vancomycin in postoperative neurosurgical patients and the application in dosing recommendation. J Pharm Sci 105:3425–3431. 10.1016/j.xphs.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Konig C, Grensemann J, Czorlich P, Schlemm E, Kluge S, Wicha SG. 2022. A dosing nomograph for cerebrospinal fluid penetration of meropenem applied by continuous infusion in patients with nosocomial ventriculitis. Clin Microbiol Infect 28:1022.e9–1022.e16. 10.1016/j.cmi.2022.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Roberts JA, Ulldemolins M, Lipman J. 2010. Meropenem: focus on its use in serious bacterial infections. Clin Med Rev Ther 2:S4598. 10.4137/CMRT.S4598. [DOI] [Google Scholar]
- 29.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Starr T, Paul SK, Lipman J, BLING II Investigators for the ANZICS Clinical Trials Group . 2015. A multicenter randomized trial of continuous versus intermittent beta-lactam infusion in severe sepsis. Am J Respir Crit Care Med 192:1298–1305. 10.1164/rccm.201505-0857OC. [DOI] [PubMed] [Google Scholar]
- 30.Lipman J, Brett SJ, De Waele JJ, Cotta MO, Davis JS, Finfer S, Glass P, Knowles S, McGuinness S, Myburgh J, Paterson DL, Peake S, Rajbhandari D, Rhodes A, Roberts JA, Shirwadkar C, Starr T, Taylor C, Billot L, Dulhunty JM. 2019. A protocol for a phase 3 multicentre randomised controlled trial of continuous versus intermittent beta-lactam antibiotic infusion in critically ill patients with sepsis: the BLING III trial. Crit Care Resusc 21:63–68. [PubMed] [Google Scholar]
- 31.Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Lipman J. 2013. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicentre double-blind, randomised controlled trial. Clin Infect Dis 56:236–244. 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 32.Tiede C, Chiriac U, Dubinski D, Raimann FJ, Frey OR, Rohr AC, Wieduwilt A, Eibach M, Filmann N, Senft C, Zacharowski K, Seifert V, Mersmann J. 2021. Cerebrospinal fluid concentrations of meropenem and vancomycin in ventriculitis patients obtained by TDM-guided continuous infusion. Antibiotics (Basel) 10:1421. 10.3390/antibiotics10111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Chen H, Zhu C, Chen F, Sun S, Liang N, Zheng W. 2019. Efficacy and safety of intrathecal meropenem and vancomycin in the treatment of postoperative intracranial infection in patients with severe traumatic brain injury. Exp Ther Med 17:4605–4609. 10.3892/etm.2019.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Wang X, Wu Y, Sun S, Chen K, Lu Y, Wang Q, Zhao Z. 2019. Plasma and cerebrospinal fluid population pharmacokinetic modeling and simulation of meropenem after intravenous and intrathecal administration in postoperative neurosurgical patients. Diagn Microbiol Infect Dis 93:386–392. 10.1016/j.diagmicrobio.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL, Zunt JR. 2017. 2017 Infectious Diseases Society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis 64:e34–e65. 10.1093/cid/ciw861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumta N, Roberts JA, Lipman J, Cotta MO. 2018. Antibiotic distribution into cerebrospinal fluid: can dosing safely account for drug and disease factors in the treatment of ventriculostomy-associated infections? Clin Pharmacokinet 57:439–454. 10.1007/s40262-017-0588-3. [DOI] [PubMed] [Google Scholar]
- 37.Ramanan M, Lipman J, Shorr A, Shankar A. 2015. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis 15:3. 10.1186/s12879-014-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.FDA. 2001. Guidance for industry: bioanalytical method validation. FDA, Rockville, MD. [Google Scholar]
- 39.Neely MN, Van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleibinger M, Steinbach CL, Topper C, Kratzer A, Liebchen U, Kees F, Salzberger B, Kees MG. 2015. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol 80:525–533. 10.1111/bcp.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download aac.00142-22-s0001.pdf, PDF file, 0.1 MB (110.5KB, pdf)




