Abstract
Exochelin is the primary extracellular siderophore of Mycobacterium smegmatis, and the iron-regulated fxbA gene encodes a putative formyltransferase, an essential enzyme in the exochelin biosynthetic pathway (E. H. Fiss, Y. Yu, and W. R. Jacobs, Jr., Mol. Microbiol. 14:557–569, 1994). We investigated the regulation of fxbA by the mycobacterial IdeR, a homolog of the Corynebacterium diphtheriae iron regulator DtxR (M. P. Schmitt, M. Predich, L. Doukhan, I. Smith, and R. K. Holmes, Infect. Immun. 63:4284–4289, 1995). Gel mobility shift experiments showed that IdeR binds to the fxbA regulatory region in the presence of divalent metals. DNase I footprinting assays indicated that IdeR binding protects a 28-bp region containing a palindromic sequence of the fxbA promoter that was identified in primer extension assays. fxbA regulation was measured in M. smegmatis wild-type and ideR mutant strains containing fxbA promoter-lacZ fusions. These experiments confirmed that fxbA expression is negatively regulated by iron and showed that inactivation of ideR results in iron-independent expression of fxbA. However, the levels of its expression in the ideR mutant were approximately 50% lower than those in the wild-type strain under iron limitation, indicating an undefined positive role of IdeR in the regulation of fxbA.
Iron is an essential nutrient for almost all living microorganisms, including pathogenic bacteria. In an aerobic environment, iron is oxidized to its ferric state, which forms insoluble ferric oxides at physiological pH; thus, the concentration of free iron is approximately 10−18 M (1). In mammalian hosts, iron is chelated by molecules such as transferrin, lactoferrin, heme, and ferritin, and the concentration of free iron in human serum is also extremely low, 10−15 M or less (3). Thus, regardless of the external environment, the levels of free iron are far below the nutritional requirements of any bacterium. To acquire this essential metal, many microorganisms secrete low-molecular-weight iron chelators termed siderophores that solubilize iron its ferric form, which is then transported into the cell by membrane-bound receptors (20). The Fe2+ produced by intracellular reduction of the newly imported Fe3+ is then available for its essential functions.
Mycobacterial iron assimilation relies upon synthesis of two structurally unrelated types of siderophores, i.e., secreted carboxymycobactins in slow-growing pathogenic members of this genus as well as nonpathogens and exochelins that are found only in fast-growing mycobacteria (44). In addition, most mycobacteria possess mycobactins, which are highly lipophilic cell wall-associated molecules thought to facilitate the transport of iron across the cell wall and to store iron (35, 44). Mycobacterium tuberculosis carboxymycobactin can remove iron from the host’s transferrin and lactoferrin and transport it to mycobactins (10). The structures of mycobactins and exochelins from several mycobacterial species have been elucidated (11, 16, 33–35, 45); however, until recently, only a single gene, M. smegmatis fxbA (9), had been demonstrated to be involved in siderophore biosynthesis. fxbA encodes a putative formyltransferase necessary for exochelin biosynthesis. More recently, a group of closely linked genes also involved in M. smegmatis exochelin biosynthesis (46, 47) and a cluster of M. tuberculosis genes that encode the mycobactin biosynthetic enzymes (25) have been described. It has long been known that iron represses the production of mycobactins, exochelins, and iron-regulated envelope proteins that could be involved in iron uptake (15, 36, 37). However, at that time, the molecular basis of siderophore regulation in mycobacteria was unknown. Since these early reports, IdeR, a functional homolog of Corynebacterium diphtheriae DtxR (32), has been described (5). DtxR is an iron-dependent repressor that controls siderophore biosynthesis and transcription of the tox gene, which encodes the C. diphtheriae diphtheria toxin. DtxR, when activated by Fe2+, binds to operator sequences known as iron boxes, repressing transcription of iron-regulated genes (31, 40). More recently, IdeR has been shown to negatively regulate siderophore biosynthesis in M. smegmatis (6).
To provide more insight into the role of IdeR in mycobacterial iron regulation, we initiated a study of its interaction with mycobacterial siderophore biosynthetic genes. When this project was started, fxbA was the only known iron-regulated mycobacterial gene, and its promoter region had been found to contain sequences that resembled a DtxR binding site (9). We have now investigated the regulation of fxbA by IdeR in cell-free experiments and in whole-cell physiological studies. The work reported here demonstrates that IdeR binds to the fxbA promoter region in the presence of divalent metals and specifically protects a 28-bp region which encompasses a palindromic sequence of the fxbA promoter region that contains the postulated iron box. The transcription start point (TSP) of fxbA has been mapped by primer extension, and fxbA regulation has been studied in strains containing fxbA-lacZ transcriptional fusions. Inactivation of ideR in M. smegmatis results in constitutive expression of fxbA, independent of the iron levels. However, the levels of fxbA expression in the ideR mutant are 50% lower than those in the wild-type strain in nonrepressing (low-iron) conditions, indicating an undetermined positive role of IdeR in fxbA regulation.
MATERIALS AND METHODS
Media and growth conditions.
Mycobacterial strains were grown on Middlebrook 7H10 solid medium (Difco) and in Middlebrook 7H9 liquid medium (Difco) at 37°C. Both media were routinely supplemented with 0.2% glycerol and 0.05% Tween 80. Mycobacteria were also grown on solid LB (Difco) medium and LB broth, both supplemented with 0.05% Tween 80. The antibiotics kanamycin and streptomycin, when required, were each added at a concentration of 20 μg/ml. For growth under low-iron conditions, 7H9L (7H9 that had been treated with a Chelex resin [Chelex 100; Bio-Rad]) was used. The concentration of iron in this medium was determined by atomic absorption spectroscopy to be less than 1 μM. For primer extension experiments and β-galactosidase assays with bacteria grown under low-iron conditions, mycobacteria were grown in 7H9L for approximately nine generations by periodically diluting cultures that were growing exponentially. LB broth cultures in the exponential phase of growth were starved for iron by adding the iron chelator 2,2′-dipyridyl (DP) to a final concentration of 200 μM, and the cultures were grown overnight. These cultures were used to inoculate fresh LB broth containing DP prior to harvesting in the exponential growth phase. For primer extension and β-galactosidase assays with bacteria grown under high-iron conditions, mycobacteria were grown in 7H9L with 50 μM ferric chloride (7H9H) or in LB broth not treated with DP. The levels of iron normally found in LB broth, approximately 30 μM (43), are high enough to repress DtxR- and IdeR-regulated genes (32). Plasticware was systematically used in experiments that required low-iron media to avoid leaching of iron from glass surfaces. Escherichia coli was routinely grown in LB medium at 37°C.
DNA techniques.
DNA manipulations were performed by standard procedures as described elsewhere (27). Restriction and modifying enzymes were obtained from Promega. DNA fragments used in the cloning procedures and PCR products were isolated from agarose gels with a Qiaex or Qiaquick gel extraction kit (Qiagen Inc.) according to the manufacturer’s instructions.
Labeling of DNA fragments.
T4 polynucleotide kinase and [γ-32P]ATP (NEN) were used to end label the oligonucleotides FXB5′2 (5′-GTGGTGGTCTTCCCCCTGGC-3′), FXB5′7 (5′-AACCGGCATGCTATCAAAGG-3′), and FXB3′7 (5′-TGGCAGGTTCGGGGGCGG-3′). A DNA fragment corresponding to the first 23 codons of the fxbA gene and its regulatory region was subcloned into pBluescript KS (Stratagene), creating pKSfxbA. 32P-labeled FXB5′2 and unlabeled FXB3′7 were used to amplify by PCR a 126-bp product from pKSfxbA. The 126-bp PCR product was isolated by electrophoresis on a 2% agarose gel, purified by using a Qiaquick gel extraction kit, and used in the gel shift assay. 32P-labeled FXB5′7 and unlabeled FXB3′7 were used to amplify by PCR a 67-bp fragment from pKSfxbA. The 67-bp PCR product was isolated and purified as described above and used in the DNase I protection experiments of the first strand. For analysis of the binding of IdeR to the complementary strand, unlabeled FXB5′7 and 32P-labeled FXB3′7 were used to amplify by PCR the same 67-bp fragment from pKSfxbA, and the PCR product was purified as described above.
Gel mobility shift assay.
The IdeR protein was purified by nickel affinity chromatography as previously described (32). Binding reactions were carried out in a final volume of 20 μl in a buffer composed of 20 mM Tris-HCl (pH 8), 50 mM KCl, 5 mM MgCl2, 50 μg of poly(dI-dC) per ml, 50 μg of bovine serum albumin per ml, and 10% glycerol. The reaction mixtures contained approximately 10 fmol of 32P-labeled DNA fragment, 200 μM divalent metal salts, and purified IdeR as indicated in Results, and incubation was carried for 30 min at room temperature. When Fe2+ salts were used in the assays, 2 mM dithiothreitol (DTT) was added to decrease its oxidation to Fe3+. A 15-μl aliquot of each reaction mixture was loaded without dye onto a 5.5% polyacrylamide gel containing 40 mM Tris-acetate (pH 8). Gels were run at 110 V at room temperature and dried, and radioactivity was visualized by autoradiography.
DNase I footprinting.
Binding reaction mixtures contained approximately 10 fmol of 32P-labeled DNA fragment and 18 pmol of purified IdeR in 50 μl of reaction buffer (20 mM Na2HPO4 [pH 7.0]), 50 mM NaCl, 5 mM MgCl2, 2 mM DTT, 100 μg of bovine serum albumin per ml, 10 μg of sonicated salmon sperm DNA per ml, 10% glycerol). Freshly prepared nickel sulfate was added to a final concentration of 500 μM. Reaction mixtures were incubated for 10 min at 37°C. After incubation, 50 μl of a solution containing 5 mM CaCl2 and 10 mM MgCl2 was added to the reaction mixtures at room temperature. Then 0.15 U of DNase I (Promega) was added, and the mixtures were incubated for 3 min at room temperature. The digestion reactions were terminated by addition of 90 μl of stop solution (200 mM NaCl, 30 mM EDTA, 1% sodium dodecyl sulfate, 100 mg of yeast RNA per ml) and extracted with phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]). The nucleic acids were ethanol precipitated, and the dried pellets were resuspended in formamide loading dye. After electrophoresis on an 8% denaturing polyacrylamide sequencing gel, the gel was dried and the footprinting patterns were analyzed by autoradiography. To locate the protected sequence on each strand, Maxam and Gilbert G+A reactions were performed on both 32P-labeled 67-bp DNA fragments (27).
RNA isolation and TSP mapping.
RNA from mycobacteria grown in 7H9L or 7H9H was prepared by mechanical disruption with glass beads in the presence of phenol and lithium chloride as described previously (4). The fxbA TSP was determined by primer extension analysis, using previously described methods with minor modifications (4). Briefly, oligonucleotide fxbA23 (5′-CATGACCACGCGCACAGGAAACACCC-3′), complementary to the sequence between nucleotides (nt) 44 to 69 relative to the first nucleotide in the fxbA start codon, was 5′-end labeled with [γ-32P]ATP (NEN). The labeled fxbA23 primer was annealed to 30 μg of RNA (1 min at 100°C, 2 min at 60°C, 10 min on ice) and then extended with avian myeloblastosis virus reverse transcriptase (15 min at 48°C). To obtain the size of the extended product, plasmid pKSfxbA and primer fxbA23 were used to generate a sequencing ladder by the dideoxy-chain termination method with Sequenase T7 DNA polymerase (Sequenase 2.0; Amersham). Primer extension products were loaded onto a 6% polyacrylamide sequencing gel along with the sequencing ladder and run 2 h at 1,800 V. To estimate the relative amount of the fxbA transcript in M. smegmatis wild-type mc2155 (46) and the ideR mutant SM3 (6) grown in 7H9L or 7H9H, autoradiographs were scanned with a Hewlett-Packard Scanjet IICX/T scanner and quantitated with ScanAnalysis version 2.56 (BioSoft).
Construction of an integrative promoter-probe vector for mycobacteria.
To construct an integrative promoter-probe vector for mycobacteria, the replicative plasmid pJEM15 (41) was modified. pJEM15 contains a cII-lacZ fusion preceded by a synthetic ribosome binding site (RBS), multiple cloning sites, and the transcription terminator of the T4 bacteriophage (T4t). T4t had been shown to be an efficient transcription terminator in mycobacteria, reducing readthrough transcription from vector sequences (42). A DNA fragment of approximately 1.3 kb containing the T4t, multiple cloning site, and synthetic RBS and part of the cII-lacZ fusion was obtained by digesting pJEM15 with PstI and EcoRI. This fragment was then treated with the Klenow fragment of DNA polymerase I and cloned between the DraI and EcoRV sites of the integrative vector pMV361-lacZ (38). The resulting vector was then linearized with ScaI and ligated to the omega cassette harboring a streptomycin/spectinomycin resistance gene (22), resulting in plasmid pSM128 (a map of this vector is available on request).
Construction of fxbA-lacZ and tox-lacZ fusions.
Transcriptional fusions to lacZ were obtained by cloning PCR-amplified fragments of the fxbA and C. diphtheriae tox regulatory regions into the ScaI site of pSM128. A PCR fragment of fxbA that contained two predicted IdeR binding sites and comprised the first 23 codons of the gene and its upstream regulatory region up to −187 bp, relative to the fxbA TSP, was obtained by using as primers fxbAUP (5′-AGATTTTCGGCCACCGTAATAC-3′) and fxbA3-1 (5′-AGCTTCATGACCACGCGCACAGG-3′). The pSM128 derivative harboring this fragment was named pSM371. A shorter fxbA-lacZ fusion, pSM353, contained the same 3′ extremity as in pSM371 and the upstream regulatory region up to −108 bp, relative to the TSP. This fragment did not contain the putative distal iron box. It was obtained by using as primers in the PCR amplification fxbAS (5′-CCCTCGTCGTTGACCAGG-3′) and fxbA3-1. Finally, the third fxbA-lacZ fusion, pSM353, contained a DNA fragment with the coordinates −187 to +6 bp, relative to the TSP. This fragment, containing the predicted distal iron box and a truncated proximal iron box, was produced by using as primers in the PCR amplification fxbAS and fxbAWOIB (5′-ACCTTTGATAGCATGCCGGTTG-3′). In the case of tox, the PCR fragment was obtained by using as primers tox 5-2 (5′-TTGCTAGTGAAGCTTAGCTAGT-3′) and tox 3-2 (5′-GTTTTCTGCTCACAACGTATCCC-3′) and comprised the first five codons of the gene and approximately 100 bp of its upstream regulatory region that contains a DtxR binding site. The plasmid containing this tox-lacZ fusion was named pSM223. The structures of all lacZ transcriptional fusions were verified by restriction analysis and DNA sequencing.
β-Galactosidase assays.
Cultures of M. smegmatis strains harboring the different lacZ fusions were grown in either 7H9 or LB that contained low or high iron, collected at an optical density at 600 nm of 0.6 to 1, washed, and resuspended in Z buffer (19). Suspensions were lysed in a Mini Bead-beater (Biospec Products) for 30 s three times. Protein concentration of the extracts was estimated by using the Bio-Rad protein assay, with bovine serum albumin as the standard. β-Galactosidase activity of the extracts was determined as described by Miller (19), and units of activity are expressed as nanomoles of nitrophenol produced per minute per milligram of protein. Comparable β-galactosidase levels were observed in cells growing in either LB or 7H9 medium.
RESULTS
Interaction of IdeR with the regulatory region of the fxbA gene.
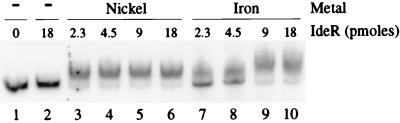
Previous studies have identified and characterized fxbA, an iron-regulated gene in M. smegmatis that encodes an enzyme essential for exochelin biosynthesis (9). A potential binding site (iron box) for a DtxR-like regulator was also observed in the region of fxbA immediately upstream from the putative translational start codon. To determine whether IdeR interacts directly with the regulatory region of fxbA, we carried out gel mobility shift assays using a 126-bp fragment containing the putative iron box (Fig. 1). A retarded DNA band was observed in presence of IdeR and divalent iron or nickel. No retarded complexes were observed when divalent metals were absent from the reaction. IdeR binding to the 126-bp fragment was also activated in the presence of Mn2+, Co2+, Zn2+, and Cd2+, and the protein-DNA complexes showed similar shifts (data not shown). Cu2+ did not induce binding of IdeR to the fragment. These results are similar to those previously reported for the metal-dependent binding of both DtxR and IdeR to the C. diphtheriae tox iron box (32). As a control for the specificity of the observed binding of IdeR to fxbA, several DNA fragments of various sizes, lacking iron boxes, including the promoter regions of the M. tuberculosis sodA, katG, and sigA genes, were used in similar assays, and none of these were retarded in the presence of divalent nickel or iron (data not shown).
FIG. 1.
Gel mobility shift assay of the fxbA-IdeR interaction. The ability of IdeR to bind to the fxbA regulatory region was measured by acrylamide gel electrophoresis of protein-DNA complexes. Approximately 10 fmol of a 126-bp 32P-labeled fragment containing the regulatory region of fxbA was incubated with no metal or with 200 μM Ni2+ or Fe2+ and increasing amounts of IdeR from 0 to 18 pmol, as indicated above the lanes. Lane 1 contained the DNA alone, and lane 2 had the DNA and 18 pmol of IdeR with no metal. Binding conditions and gel electrophoresis are described in Materials and Methods.
In general, we preferred to use Ni2+ salts for activation of the DNA binding property of IdeR since this transition state metal is more resistant to oxidation than Fe2+ (31). This could explain the observation that more IdeR was needed to shift fxbA when binding reactions were carried out in the presence of Fe2+ compared to those performed with Ni2+ (Fig. 1; compare lanes 3 to 7 and 4 to 8). It was essential to add DTT to the IdeR-fxbA binding reactions with iron salts to observe any gel shift (data not shown).
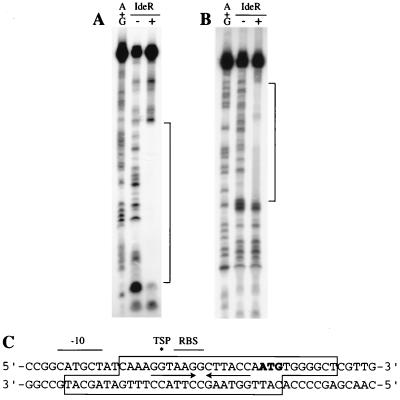
To determine the exact location of the IdeR binding site in the fxbA regulatory region, DNase I footprinting experiments were conducted with a 67-bp DNA fragment containing the putative iron box. A single protected region of 28 bp was obtained when IdeR was allowed to bind to this DNA in presence of Ni2+ (Fig. 2). This sequence contained the predicted iron box and encompassed the putative translation initiation codon and RBS. The iron box contained a 13-bp sequence that formed a perfect palindrome around a central G. IdeR protected slightly different regions of the fxbA promoter/operator on the coding and noncoding strands. The protection was common to both strands over the first 21 bp, containing the palindromic sequences, and extended only on one strand over the last 7 bp symmetrically to the dyad axis. The IdeR binding sequence identified by DNase footprinting was compared with known DtxR binding sites and DtxR-like operator sequences and was shown to have 74% identity with the 19-bp consensus DtxR operator (Fig. 3).
FIG. 2.
DNase I footprinting of the fxbA-IdeR complex. The specific fxbA sequences to which IdeR binds were determined by treating protein-DNA complexes with DNase I and analyzing the products on DNA sequencing gels; 10 fmol of a 67-bp fragment containing the regulatory region of fxbA, labeled on the coding (A) or the noncoding (B) strand, was incubated with 500 μM Ni2+, with (+) or without (−) 18 picomoles of IdeR. Binding and DNase I digestion conditions are described in Materials and Methods. Maxam and Gilbert A+G sequencing reactions were performed on both strands, and gel electrophoresis was performed as described in Materials and Methods. Brackets indicate the sequences protected by IdeR from DNase I digestion. (C) DNA sequence of the fxbA regulatory region. The boxed region indicates the IdeR box, i.e., sequences protected from DNase I digestion by IdeR. Inverted arrows indicate sequences that form a palindrome. The asterisk indicates the TSP observed in the primer extension illustrated in Fig. 4. The translation initiation codon is indicated by boldface, and the putative RBS and −10 region are indicated by lines over the corresponding sequences.
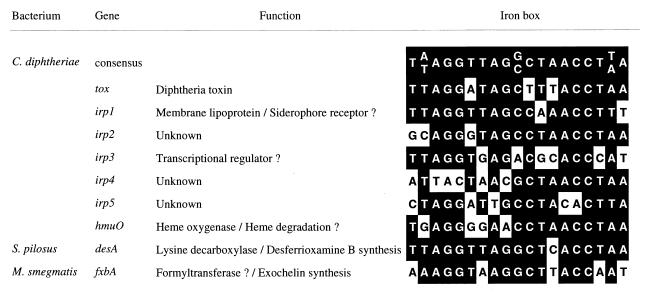
FIG. 3.
Comparison of DtxR and IdeR binding sites. The sequences shown, except for the Streptomyces pilosus desA sequence, were identified by their ability to bind IdeR or DtxR, as measured by gel mobility and/or DNase footprinting assays. desA is repressed by iron (13), and since Streptomyces species have a DtxR homolog (14), it is assumed that the sequence shown here is an operator site for this protein. Other relevant references are cited in the text.
Mapping of the fxbA TSP.
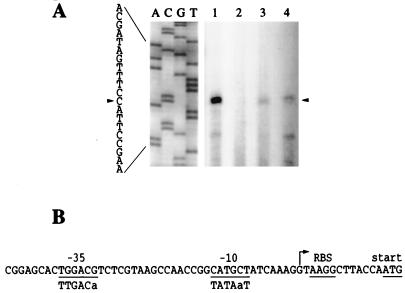
The fxbA TSP was determined by primer extension using RNA extracted from strains mc2155 and SM3 grown in 7H9L (low-iron) or 7H9H (high-iron) media. The results indicated demonstrated a TSP located 13 bp upstream of the fxbA start codon. Transcription of fxbA in mc2155 was detected only when the cells had been growing in iron-depleted medium. From the data obtained from this and other primer extension experiments, it was observed that the quantity of fxbA transcript in strain SM3 was essentially the same in both 7H9L and 7H9H and was four times lower than the quantity of fxbA transcript in strain mc2155 grown in 7H9L. This latter result is discussed below.
Sequences similar to the E. coli ς70 consensus for −35 and −10 boxes, TgGACg and cATgcT, respectively (where uppercase denotes identity with the consensus), were identified upstream of the fxbA TSP. The −10 hexamer was also similar to the consensus for constitutively expressed M. smegmatis promoters, TATAaT (2). The spacer between the putative −10 and −35 sequences was 17 nt, and the distance between the TSP and the −10 box was 7 nt (Fig. 4B).
FIG. 4.
Identification of the fxbA promoter. (A) The 5′ terminus of the fxbA transcript was determined by primer extension using oligonucleotide fxbA23 and RNA prepared from cultures of strains mc2155 and SM3 growing in 7H9L or 7H9H. Lanes: 1, mc2155 in 7H9L; 2, mc2155 in 7H9H; 3, SM3 in 7H9L; 4, SM3 in 7H9H. The nucleotide sequencing ladder shown at the left was obtained with oligonucleotide fxbA23 as the primer and plasmid pKSfxbA as the template. (B). The putative −10 and −35 boxes of the fxbA promoter are underlined, as are a possible RBS and the start codon. The TSP is indicated with an arrow. The consensus sequence for E. coli ς70 promoters is shown for comparison. Uppercase denotes more than 50% conservation.
Regulation of fxbA by using transcriptional lacZ fusions.
To study the regulation of the M. smegmatis fxbA gene by using lacZ gene fusions, it was first necessary to construct a new promoter-probe vector. Most mycobacterial reporter vectors contain a kanamycin resistance gene and cannot be selected for in the M. smegmatis ideR mutant strain SM3 that harbors the same antibiotic marker in the chromosome (6). Moreover, in preliminary experiments, we had noticed that the cells containing the fxbA-lacZ fusion previously used to study fxbA regulation (9) showed a relatively high enzyme level in high-iron medium (data not shown), possibly due to readthrough from vector sequences that acted as promoters. To solve these problems, we constructed a new E. coli-mycobacteria shuttle vector designated pSM128. This plasmid contains the integrative cassette from mycobacteriophage L5 (18), a spectinomycin/streptomycin resistance gene, a promoterless lacZ, which is preceded by a T4t element and a unique ScaI cloning site. M. smegmatis transformed with this vector exhibited extremely low β-galactosidase levels (legend to Fig. 5).
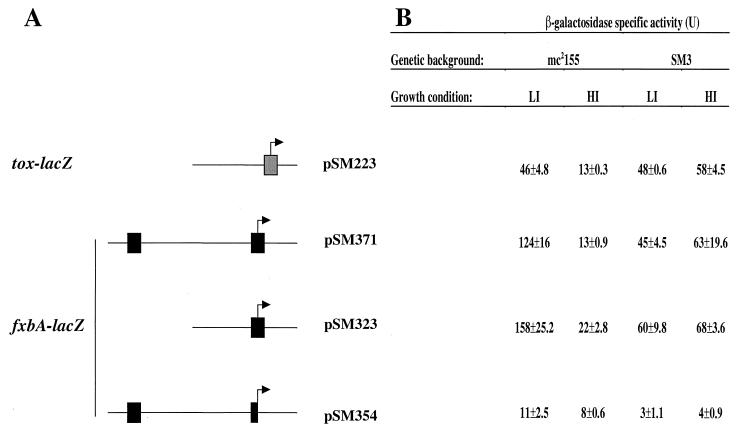
FIG. 5.
fxbA expression in wild-type and ideR mutant strains. The upstream regulatory region of the C. diphtheriae tox gene and different segments of the fxbA promoter region were cloned into the integrative lacZ reporter vector pSM128. These constructs and the vector were transformed into the wild-type M. smegmatis strain mc2155 and its isogenic ideR mutant derivative SM3. Individual colonies were purified and then grown in LB broth (HI [high iron]) that has repressing levels of iron (30 μM) and also in LB that had been treated with the iron chelator DP to remove Fe (LI [low iron]). Cells were harvested, and β-galactosidase specific activities were determined as described in Materials and Methods. (A) Plasmids containing the promoter −lacZ constructs and schema of their structure. IdeR binding sites are indicated by boxes. Coordinates of the DNA fragments used for the constructions are given in Materials and Methods. Arrows indicate the approximate site of the TSP and direction of transcription. The proximal IdeR box is truncated in pSM354, as indicated in the diagram. (B) Averages of experiments done in triplicate. The β-galactosidase activities of strains containing the promoterless vector pSM 128, less than 3 U in all conditions, were subtracted to give the values presented.
We first wanted to study the role of the newly identified IdeR binding site (Fig. 2) in the regulation of fxbA and also to examine the possible involvement of another potential IdeR box that was observed 140 bp upstream of the fxbA start codon (9). Using pSM128, we constructed transcriptional fusions to lacZ of various parts of the fxbA upstream regulatory region as described in Materials and Methods and depicted in Fig. 5. As a positive control, we constructed a transcriptional fusion with the C. diphtheriae tox gene that can be regulated by IdeR (32). These plasmid constructs and the promoterless vector pSM128 were transformed into M. smegmatis wild-type mc2155 and ideR mutant SM3. Cells carrying these fusions were then grown in high- or low-iron medium, and β-galactosidase assays were performed. As shown in Fig. 5, fxbA-lacZ fusions containing the downstream, proximal IdeR box were repressed by iron in mc2155, as the β-galactosidase levels obtained in low iron were 7- to 10-fold higher than those obtained in high iron. Similarly, the expression of the tox promoter was approximately fourfold higher when mc2155 containing pSM223 was grown in low iron. Expression of the tox- and fxbA-lacZ transcriptional fusions was independent of iron in the ideR mutant strain SM3. However, the levels of fxbA-lacZ expression in the mutant were approximately 50% lower than those observed during growth of the wild-type strain in low iron. These observations are consistent with the results obtained by primer extension presented above that also indicated that IdeR is required for full expression of fxbA under nonrepressive (low-iron) conditions. This was not true for tox expression, since the tox-lacZ fusion β-galactosidase levels in SM3 grown in both low- and high-iron media were comparable to those observed in mc2155 grown in low-iron medium.
The presence or absence of the putative distal iron box had little or no effect on the regulation of fxbA, as the β-galactosidase values obtained in cells containing the pSM371 construct that has both iron boxes are very similar to those observed in the strain carrying pSM323 that contains only the proximal one (Fig. 5). Gel retardation experiments have also shown that DNA fragments containing the putative upstream IdeR binding site do not bind IdeR (data not shown), indicating that this sequence does not play a role in fxbA expression. We also tried to modify the fxbA promoter by disrupting the proximal IdeR binding site while allowing full expression of this gene, as this would conclusively prove that this site was essential for repression. However, this first attempt was unsuccessful, as all fxbA-lacZ activity was lost when we removed the downstream arm of the IdeR binding site palindrome, situated 6 bp downstream from the TSP in the fusion construct pSM354. Similar low levels of activity were observed when a lacZ fusion construct was made with an DNA fragment from the fxbA promoter region missing both potential iron boxes (data not shown). This finding suggests that the 5′ terminus of the fxbA mRNA, removed by the cloning, may be necessary for RNA stability. However, further experiments that make other modifications of the promoter region will be necessary to understand the actual mechanism of IdeR repression of fxbA.
DISCUSSION
We had previously shown that the M. smegmatis IdeR represses mycobacterial siderophore biosynthesis in the presence of iron (6) but at that time had not identified any mycobacterial genes that were directly repressed by this protein. IdeR can replace DtxR, the C. diphtheriae iron-dependent regulator of toxin and siderophore biosynthesis, as it can bind to DtxR target genes in cell-free assays, and ideR, its structural gene, can complement C. diphtheriae dtxR mutants (32). It was also shown that IdeR and DtxR are 80% identical in the first 140 amino acids (5), where the helix-turn-helix DNA binding motif, metal binding sites, and multimerization domains are found in DtxR (23, 24, 28). This postulated structural similarity of IdeR and DtxR has been confirmed, as the crystal structure of the M. tuberculosis IdeR, recently determined, shows that IdeR and DtxR have almost identical structures in the first two domains (21). The above genetic, physiological, and structural observations strongly suggested that IdeR represses its target genes in a manner identical to that of DtxR. This mechanism would include the formation of a Fe2+-stabilized IdeR dimer that enables it to bind to operator sequences in the promoters of mycobacterial iron acquisition genes, inhibiting their transcription.
To provide evidence for this hypothesis, we have now analyzed the interaction of IdeR with the M. smegmatis fxbA, the first siderophore biosynthetic gene described for this genus (9). The binding of IdeR to the fxbA operator was characterized by gel mobility shift assays. IdeR required activation by divalent metals, e.g., Fe2+, Ni2+, Co2+, Zn2+, Mn2+, or Cd2+, in order to bind to the fxbA operator, as has been previously shown for DtxR and Fur binding to their operator sites (1, 30) and also for IdeR binding to the C. diphtheriae tox operator (32). After metal binding, IdeR protected a 28-bp sequence of the fxbA regulatory region from DNase I digestion. The IdeR binding box was 74% identical to the 19-bp consensus DtxR binding site selected in vitro and shown to be the minimal essential target sequence (39). The protected region observed in our experiments, i.e., the IdeR iron box, was found to encompass the translational start codon, the putative RBS, the TSP, the 13-bp palindromic sequence, and most of the −10 region. This is similar to DtxR and DtxR-like regulated promoters in which the operator sequence overlaps the −10 and downstream regions (13, 17, 30, 40). Binding of a protein to this region would be expected to interfere with the binding of the RNA polymerase and transcription initiation, as would the binding of Fur to its target promoters. In these latter genes, mainly studied in enteric bacteria, Fur binding sites are generally located between the −40 and +1 bp relative to the TSP (12).
Our results show that fxbA expression is negatively regulated by iron only when IdeR is present. This indicates that the repression must be mediated by the ferration of IdeR and the subsequent binding of the activated protein to the fxbA promoter, as discussed above. However, the levels of fxbA expression in the ideR mutant were approximately 50% lower than those observed in the wild-type strain in nonrepressive conditions. A similar effect was observed when levels of fxbA mRNA were measured by primer extension analyses. These results are similar to those previously observed when total siderophore production was assayed in M. smegmatis ideR mutants (6). In these experiments, exochelin and mycobactin synthesis were partially independent of iron levels in ideR mutant strains, but their levels were approximately 50% of those observed when the wild-type strain was grown in low iron. It is possible that IdeR positively controls fxbA transcription and siderophore synthesis as a directly acting positive regulator or as an activator of a second activator for these genes. It could also act as a repressor of a repressor. A similar cascade mechanism has been hypothesized for the heme-dependent induction of hmuO, a DtxR- and iron-regulated gene encoding a heme oxygenase in C. diphtheriae (29).
The possibility that IdeR acts directly as a positive regulator of fxbA led us to examine the function of a potential IdeR binding site observed approximately 140 bp upstream of the TSP of fxbA (9). However, this sequence did not bind IdeR, and its removal had no effect on fxbA expression, indicating it had no function in fxbA regulation. The inactivation of ideR caused reduced transcription of fxbA but not of tox or several other iron/IdeR-regulated genes that have recently been characterized from M. tuberculosis (11a, 26). On the other hand, inactivation of ideR caused pleiotropic effects such as reduced expression of the antioxidant enzymes KatG and SodA in M. smegmatis and increased sensitivity to isoniazid (6–8). Thus, it is also possible that altered metabolic pathways in the ideR mutant indirectly affect the transcription of some promoters. Experiments are now in progress to determine the molecular mechanism of IdeR’s positive function in mycobacterial siderophore biosynthesis, oxidative stress response, and isoniazid resistance.
ACKNOWLEDGMENTS
Olivier Dussurget and Juliano Timm contributed equally to this work.
We thank Michael Heller for atomic absorption spectroscopy and Jeanie Dubnau, Marcela Rodriguez and Riccardo Manganelli for valuable discussions.
This work was supported by grants AI-26170 (to W.R.J.), AI-14107 (to R.K.H.), and GM 32651 and AI-46655 (both to I.S.) from the National Institutes of Health and by a fellowship from the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche to O.D.
Footnotes
Publication no. 64 from the TB Center, Public Health Research Institute.
REFERENCES
- 1.Bagg A, Neilands J B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashyam M D, Kaushal D, Dasgupta S K, Tyagi A K. A study of mycobacterial transcriptional apparatus: identification of novel features in promoter elements. J Bacteriol. 1996;178:4847–4853. doi: 10.1128/jb.178.16.4847-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullen J J, Rogers H J, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 4.Cutting S, Roels R, Losick R. Sporulation operon spoIVF and the characterization of mutations that uncouple mother-cell from ferespore gene expression in Bacillus subtilis. J Mol Biol. 1991;221:1237–1256. doi: 10.1016/0022-2836(91)90931-u. [DOI] [PubMed] [Google Scholar]
- 5.Doukhan L, Predich M, Nair G, Dussurget O, Mandic-Mulec I, Cole S T, Smith D R, Smith I. Genomic organization of the mycobacterial sigma gene cluster. Gene. 1995;165:67–70. doi: 10.1016/0378-1119(95)00427-8. [DOI] [PubMed] [Google Scholar]
- 6.Dussurget O, Rodriguez G M, Smith I. An ideR mutant of Mycobacterium smegmatis has a derepressed siderophore production and an altered oxidative-stress response. Mol Microbiol. 1996;22:535–544. doi: 10.1046/j.1365-2958.1996.1461511.x. [DOI] [PubMed] [Google Scholar]
- 7.Dussurget O, Rodriguez G M, Smith I. Protective role of the mycobacterial IdeR against reactive oxygen species and isoniazid toxicity. Tuberc Lung Dis. 1998;79:99–106. doi: 10.1054/tuld.1998.0011. [DOI] [PubMed] [Google Scholar]
- 8.Dussurget O, Smith I. Interdependence of mycobacterial iron regulation, oxidative stress and INH resistance. Trends Microbiol. 1998;6:354–358. doi: 10.1016/s0966-842x(98)01307-9. [DOI] [PubMed] [Google Scholar]
- 9.Fiss E H, Yu S, Jacobs W R., Jr Identification of genes involved in the sequestration of iron in mycobacteria: the ferric exochelin biosynthetic and uptake pathways. Mol Microbiol. 1994;14:557–569. doi: 10.1111/j.1365-2958.1994.tb02189.x. [DOI] [PubMed] [Google Scholar]
- 10.Gobin J, Horwitz M. Exochelins of Mycobacterium tuberculosis remove iron from human iron-binding proteins and donate iron to mycobactins in the M. tuberculosis cell wall. J Exp Med. 1996;183:1527–1532. doi: 10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobin J, Moore C H, Reeve J J R, Wong D K, Gibson B W, Horwitz M A. Iron acquisition by Mycobacterium tuberculosis: isolation and characterization of a family of iron-binding exochelins. Proc Natl Acad Sci USA. 1995;92:5189–5193. doi: 10.1073/pnas.92.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Gold, B., M. Rodriguez, and I. Smith. Unpublished data.
- 12.Gralla J D, Collado-Vides J. Organization and function of transcription regulatory elements. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1232–1245. [Google Scholar]
- 13.Gunter K, Toupet C, Schupp T. Characterization of an iron-regulated promoter involved in desferrioxamine B synthesis in Streptomyces pilosus: repressor-binding site and homology to the diphtheria toxin gene promoter. J Bacteriol. 1993;175:3295–3302. doi: 10.1128/jb.175.11.3295-3302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunter-Seeboth K, Schupp T. Cloning and sequence analysis of the Corynebacterium diphtheriae dtxR homologue from Streptomyces lividans and Streptomyces pilosus encoding a putative iron repressor protein. Gene. 1995;166:117–119. doi: 10.1016/0378-1119(95)00628-7. [DOI] [PubMed] [Google Scholar]
- 15.Hall R M, Sritharan M, Messenger A J M, Ratledge C. Iron transport in Mycobacterium smegmatis: occurrence of iron-regulated envelope proteins as potential receptors for iron uptake. J Gen Microbiol. 1987;133:2107–2114. doi: 10.1099/00221287-133-8-2107. [DOI] [PubMed] [Google Scholar]
- 16.Lane S J, Marshall P S, Upton R J, Ratledge C, Ewing M. Novel extracellular mycobactins, the carboxymycobactins from Mycobacterium avium. Tetrahedron Lett. 1995;36:4129–4132. [Google Scholar]
- 17.Lee J H, Wang T, Ault K, Liu J, Schmitt M P, Holmes R. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect Immun. 1997;65:4273–4280. doi: 10.1128/iai.65.10.4273-4280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M H, Pascopella L, Jacobs W R, Jr, Hatfull G F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guérin. Proc Natl Acad Sci USA. 1991;88:3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 20.Neilands J B. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 21.Pohl E, Holmes R K, Hol W G M. Crystal structure of the iron-dependent regulator (IdeR) from Mycobacterium tuberculosis shows both metal binding sites to be fully occupied. J Mol Biol. 1999;285:1145–1186. doi: 10.1006/jmbi.1998.2339. [DOI] [PubMed] [Google Scholar]
- 22.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 23.Qiu X, Pohl E, Holmes R K, Hol W G J. High-resolution structure of the diphtheria toxin repressor complexed with cobalt and manganese reveals an SH3-like third domain and suggests a possible role of phosphate as a co-repressor. Biochemistry. 1996;35:12292–12302. doi: 10.1021/bi960861d. [DOI] [PubMed] [Google Scholar]
- 24.Qiu X, Verlinde C L M J, Zhang L, Schmitt M P, Holmes R K, Hol W G J. Three-dimensional structure of the diphtheria toxin repressor in complex with divalent cation co-repressors. Structure. 1995;3:87–100. doi: 10.1016/s0969-2126(01)00137-x. [DOI] [PubMed] [Google Scholar]
- 25.Quadri L E, Keating J S A, Weinreb P H, Walsh C T. Identification of a Mycobacterium tuberculosis gene cluster encoding the biosynthetic enzymes for assembly of the virulence-conferring siderophore mycobactin. Chem Biol. 1998;5:631–645. doi: 10.1016/s1074-5521(98)90291-5. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez, G. M., B. Gold, M. Gomez, O. Dussurget, and I. Smith. Identification and characterization of two divergently transcribed iron regulated genes in Mycobacterium tuberculosis. Tuberc. Lung Dis., in press. [DOI] [PubMed]
- 27.Sambrook J, Fritsch E K, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Schiering N, Tao X, Zeng H, Murphy J R, Petsko G A. Structures of the apo- and the metal ion-activated forms of the diphtheria tox repressor from Corynebacterium diphtheriae. Proc Natl Acad Sci USA. 1995;92:9843–9850. doi: 10.1073/pnas.92.21.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt M P. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J Bacteriol. 1997;179:838–845. doi: 10.1128/jb.179.3.838-845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt M P, Holmes R K. Analysis of diphtheria toxin repressor-operator interactions and characterization of a mutant repressor with decreased binding activity for divalent metals. Mol Microbiol. 1993;9:173–181. doi: 10.1111/j.1365-2958.1993.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt M P, Holmes R K. Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J Bacteriol. 1994;176:1141–1149. doi: 10.1128/jb.176.4.1141-1149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt M P, Predich M, Doukhan L, Smith I, Holmes R K. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect Immun. 1995;63:4284–4289. doi: 10.1128/iai.63.11.4284-4289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharman G J, Williams D N, Ewing D F, Ratledge C. Determination of the structure of exochelin MN, the extracellular siderophore of Mycobacterium neoaurum. Chem Biol. 1995;2:553–561. doi: 10.1016/1074-5521(95)90189-2. [DOI] [PubMed] [Google Scholar]
- 34.Sharman G J, Williams D H, Ewing D F, Ratledge C. Isolation, purification and structure of exochelin MS, the extracellular siderophore from Mycobacterium smegmatis. Biochem J. 1995;305:187–196. doi: 10.1042/bj3050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow G A. Mycobactins: iron chelating growth factors from mycobacteria. Bacteriol Rev. 1970;34:99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sritharan M, Ratledge C. Co-ordinated expression of the components of iron transport (mycobactin, exochelin and envelope proteins) FEMS Microbiol Lett. 1989;60:183–186. doi: 10.1016/0378-1097(89)90505-3. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson M C, Ratledge C. Iron transport in Mycobacterium smegmatis: uptake of iron from ferric exochelin. J Gen Microbiol. 1979;110:193–202. doi: 10.1099/00221287-110-1-193. [DOI] [PubMed] [Google Scholar]
- 38.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Jr, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 39.Tao X, Murphy J R. Determination of the minimal essential nucleotide sequence for diphtheria tox repressor binding by in vitro affinity selection. Proc Natl Acad Sci USA. 1994;91:9646–9650. doi: 10.1073/pnas.91.20.9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao X, Schiering N, Zeng H Y, Ringe D, Murphy J R. Iron, DtxR, and the regulation of diphtheria toxin expression. Mol Microbiol. 1994;14:191–197. doi: 10.1111/j.1365-2958.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 41.Timm J, Lim E M, Gicquel B. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J Bacteriol. 1994;176:6749–6753. doi: 10.1128/jb.176.21.6749-6753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timm J, Perilli M G, Duez C, Trias J, Orefici G, Fattorini L, Amicosante G, Oratore A, Joris B, Frere J M, et al. Transcription and expression analysis, using lacZ and phoA gene fusions, of Mycobacterium fortuitum β-lactamase genes cloned from a natural isolate and a high-level β-lactamase producer. Mol Microbiol. 1994;12:491–504. doi: 10.1111/j.1365-2958.1994.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Schmitt M P, Holmes R K. Characterization of mutations that inactivate the diphtheria toxin repressor gene (dtxR) Infect Immun. 1994;62:1600–1608. doi: 10.1128/iai.62.5.1600-1608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler P R, Ratledge C. Metabolism of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 353–385. [Google Scholar]
- 45.Wong D K, Gobin J, Horwitz M A, Gibson B W. Characterization of exochelins of Mycobacterium avium: evidence for saturated and unsaturated and for acid and ester forms. J Bacteriol. 1996;178:6394–6398. doi: 10.1128/jb.178.21.6394-6398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu S, Fiss E, Jacobs W R., Jr Analysis of the exochelin locus in Mycobacterium smegmatis: biosynthesis genes have homology with genes of the peptide synthetase family. J Bacteriol. 1998;180:4676–4685. doi: 10.1128/jb.180.17.4676-4685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu W, Arceneaux J E L, Beggs M L, Byers R B, Eisenach K D, Lundrigan M D. Exochelin genes in Mycobacterium smegmatis: identification of an ABC transporter and two non-ribosomal peptide synthetase genes. Mol Microbiol. 1998;29:629–639. doi: 10.1046/j.1365-2958.1998.00961.x. [DOI] [PubMed] [Google Scholar]