Abstract
We have determined that the genes encoding the secreted proteins EspA, EspD, and EspB of enterohemorrhagic Escherichia coli (EHEC) are organized in a single operon. The esp operon is controlled by a promoter located 94 bp upstream from the ATG start codon of the espA gene. The promoter is activated in the early logarithmic growth phase, upon bacterial contact with eukaryotic cells and in response to Ca2+, Mn2+, and HEPES. Transcription of the esp operon seems to be switched off in tightly attached bacteria. The activation process is regulated by osmolarity (induction at high osmolarities), modulated by temperature, and influenced by the degree of DNA supercoiling. Transcription is ςS dependent, and the H-NS protein contributes to its fine tuning. Identification of the factors involved in activation of the esp operon and the signals responsible for modulation may facilitate understanding of the underlying molecular events leading to sequential expression of virulence factors during natural infections caused by EHEC.
Enterohemorrhagic Escherichia coli (EHEC) is the most common cause of hemorrhagic colitis, a bloody diarrhea which can lead to the life-threatening hemolitic-uremic syndrome (4). This pathogen can cause large food-borne epidemic outbreaks and belongs to the group of Shiga toxin-producing E. coli (STEC) (34). Infections caused by EHEC and the closely related enteropathogenic E. coli (EPEC) are associated with histopathological changes called attaching and effacing (A/E) lesions (33, 42). These changes consist of effacement of the intestinal microvilli followed by intimate association of bacteria with host cells and reorganization of cytoskeletal components beneath adherent bacteria (8). Most of the factors required to produce A/E lesions are encoded by a large chromosomal locus called LEE (for “locus of enterocyte effacement”) (31). LEE codes for a type III secretion system (30); an outer membrane protein called intimin (EaeA), which is required for intimate attachment to host cells (22, 46); the secreted proteins EspA, EspD, and EspB, which are required in EPEC for signal transduction events leading to formation of A/E lesions; the Tir (EPEC), or EspE (STEC), protein, which, after translocation within the host cell, phosphorylation, and surface display, constitutes the intimin receptor (6, 26); and the Pas (EHEC), or EscD (EPEC), protein, which seems to be involved in the secretion process (28). Other genes that appear to be involved in the pathogenesis process are located on plasmids (14, 22, 24).
The EspA protein plays a key role during the infection processes of both EHEC and EPEC (11, 25). It has recently been shown that EspA is involved in the formation of a novel type of pilus-like structure, which is essential for early bacterial attachment to epithelial cells and seems to be involved in EspB translocation within host cells (11, 27). Formation of these surface structures is transient, disappearing once the attachment is strengthened (11, 27). Thus, major synthesis and secretion of the EspA and EspB proteins presumably occur during early infection and are enhanced when bacteria are grown at 37°C in tissue culture medium and by the presence of micronutrients or signals produced by eukaryotic cells (10, 21). However, neither the transcriptional regulation of Esp proteins nor the real signals required for gene activation are known.
Coordinated regulation of gene activation according to environmental stimuli is a common feature among microorganisms to optimize performance, avoiding the energetic cost of synthesizing unnecessary products. This becomes a more compelling requirement for those infectious agents that during their biological cycle transit across different niches. In fact, untimely expression of virulence factors may have a devastating effect on pathogenic bacteria (1). Thus, genes encoding proteins involved in the pathogenesis process are expressed only when required in response to environmental regulatory signals. The control process is usually very complex and orchestrated by a cascade of regulatory factors (12, 32). However, the underlying mechanism and true nature of the signals involved in triggering and fine tuning this response remain elusive. In enteropathogenic bacteria, expression of virulence genes is mainly required within the intestinal tract. Therefore, control circuits which respond to a range of local signals have evolved. In Salmonella spp., different regulators and stimulating factors have been identified (9, 13); however, there is very limited information concerning EHEC. Thus, we have investigated the regulation of expression of the esp genes, which are essential during the first steps of the infection process.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Strains were grown in Luria-Bertani medium, M9 minimal medium supplemented with 0.2% glucose as a carbon source (39), or Dulbecco modified Eagle medium (DMEM) (GIBCO, Karlsruhe, Germany). Where required, media were supplemented with ampicillin (100 μg/ml), nalidixic acid (20 μg/ml), or novobiocin (5, 20, or 50 μg/ml). For β-galactosidase assays, bacteria were grown until they reached the exponential phase, and cultures were reinoculated to an optical density at 600 nm (OD600) of 0.1 into the appropriate medium. To test the influence of oligoelements on gene expression, bacteria were grown in M9-glucose medium supplemented with either MgSO4 (1, 7, or 30 mM), MnSO4 (0.0033, 0.33, or 3.3 mM), CaCl2 (0.01, 0.1, or 1 mM), FeSO4 (0.25, 25, or 250 μM), or Fe(NO3)3. NH4Cl was added to nitrogen-free M9 medium at a concentration of 0.5, 2, or 10 mM. Osmotic regulation was tested in M9-glucose minimal medium by the addition of NaCl or sucrose to a final concentration ranging from 10 to 600 mM.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli EDL933 | Prototypic O157:H7 EHEC strain | 35 |
| E. coli MC4100 | F− Δ(argF-lac)U169 araD139 rpsL150 ptsF25 flbB5301 rbsR deoC relA1 | 29 |
| E. coli RH90 | MC4100 rpoS359::Tn10 | 29 |
| E. coli GM37 | Wild type | 19 |
| E. coli GM230 | GM37 hns | 19 |
| Plasmids | ||
| pCR2.1 | Apr Kmr; high-copy-number vector for cloning of PCR products | Invitrogen |
| pMAK700oriT | Cmr; mobilizable suicide vector bearing the oriT region of pJFF350 | 43 |
| pANK1 | Cmr Kmr; pMAK700oriT derivative containing additional cloning sites and the Kmr gene (aphA3) inserted in the HindIII restriction site | This work |
| pANK111 | Cmr Kmr; pANK1 derivative containing the 548-bp PCR fragment encompassing the espA gene containing an in-frame deletion, generated with primers 9188 and 9185 | This work |
| pUJ9TT | Apr; multicopy promoter probe vector to generate fusions with the lacZ gene | 23 |
| pUJ3 | Apr; pUJ9TT derivative containing a 653-bp BamHI PCR fragment generated with primers EspA-lac1 and EspA-lac2 | This work |
| pUJ3-285 | Apr; pUJ9TT derivative containing a 371-bp EcoRV/BamHI fragment from pUJ3 inserted into the SmaI/BamHI sites | This work |
| pUJ3-56 | Apr; pUJ9TT derivative containing a 580-bp BamHI PCR fragment generated with primers EspA-lac1 and FAB 56 | This work |
Tissue culture and cell infections.
HeLa cells (ATCC CCL2) were cultured in six-well Nunclon Delta tissue culture plates (Inter Med Nunc, Roskilde, Denmark) in DMEM supplemented with 10% fetal calf serum and glutamine (2 mM) at 37°C. Semiconfluent monolayers were infected for 4 h at 37°C with a bacterium/cell ratio of 100:1. For immunofluorescence studies, cells were seeded onto 12-mm-diameter glass coverslips in 24-well tissue culture plates (Inter Med Nunc), infected with overnight-grown bacteria resuspended in DMEM for 3 h, fixed with 3.7% paraformaldehyde in phosphate-buffered saline (PBS), and permeabilized with 0.2% Triton X-100 in PBS. Bacteria were stained with a rabbit polyclonal antiserum against O157 K− (Behring, Marburg, Germany) as primary antibody and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit as secondary antibody (Dianova, Hamburg, Germany), whereas F-actin was stained with fluorescein isothiocyanate (FITC)-labelled phalloidin (Sigma, Deisenhofen, Germany). Then, coverslips were washed and mounted, and cells were examined by epifluorescence with a Zeiss axiophot microscope (Carl Zeiss, Jena, Germany).
Recombinant DNA techniques.
All DNA manipulations were performed by standard methods (39). Amplification by PCR of the chromosomal region encompassing the espA, espD, and espB genes from strain EDL933 was performed as previously described (28); all reported DNA positions refer to the EMBL database (accession no. Y13068) (28). Plasmid DNA was isolated with the QIAprep Spin Miniprep kit (Qiagen, Chatsworth, Calif.) and sequenced with a Taq dyedeoxy terminator cycle sequencing kit and an automatic DNA sequencer, model 373A (Applied Biosystems, Foster City, Calif.), according to the manufacturer’s instructions.
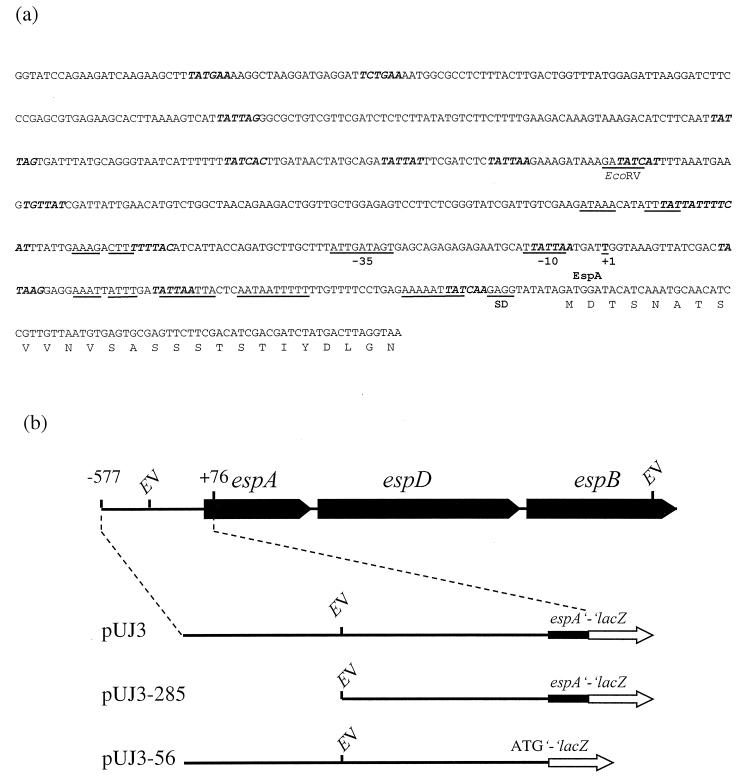
esp promoter fragments were generated by PCR (see Fig. 3) with primers which incorporated restriction sites (underlined) to facilitate construction of translational fusions with the lacZ gene present in the promoter probe vector pUJ9TT (23). Plasmid pUJ3 contains a 653-bp BamHI fragment generated with the oligonucleotides EspA-lac1 (2164 5′-CCGGATCCGGTATCCAGAAGATCAAGAAGC-3′ 2185) and EspA-lac2 (2817 5′-GCGGATCCTTACCTAAGTCATAGATCGTCGAT-3′ 2794). Plasmid pUJ3-285 was constructed by subcloning the 371-bp EcoRV/BamHI fragment from plasmid pUJ3 into the SmaI/BamHI-digested pUJ9TT. Finally, pUJ3-56 contains a BamHI fragment generated with the primers EspA-lac1 (see above) and FAB56 (2742 5′-GGGGATCCATCTATATACCTCTTGATAATTTTTC-3′ 2728).
FIG. 3.
(a) Sequence of the esp promoter region. The start of transcription (+1), the putative −10 and −35 consensus sequences (underlined), the Shine-Dalgarno sequence (SD), the consensus binding sequence for H-NS (5′-TNTNAN-3′ [in boldface italic type]), and several inverted and direct repeats (underlined) are indicated. (b) Schematic representation of the constructs employed to study transcriptional regulation of the esp operon. Abbreviations: EV, EcoRV; lacZ, β-galactosidase-encoding gene.
The espA, espD, espB, and sepL (region upstream of espA) probes used for Northern blot analysis were generated by PCR with the primer pairs A293 (2611 5′-GATAGTGAGCAGAGAGAATGC-3′ 2633) and EspAP1 (3161 5′-CCGCCTTCACTGTTTGCAGATC-3′ 3139), 9189 (3618 5′-GCTATCCCTATCTCTCTCAGGT-3′ 3640) and 9530 (4113 5′-CCAATTTTGTTAGCAACATTAC-3′ 4091), 6556 (4477 5′-ATGAATACTATTGATAATACTC-3′ 4499) and 7191 (4739 5′-GCTTTATTCTGGCTCTCAAAAA-3′ 4717), and A291 (1616 5′-GTGAGTTTCCAATGGCTAATGG-3′ 1638) and A292 (1880 5′-AGCAGCTTCTCGATTGTCGAGC-3′ 1858), respectively. [α-32P]dATP (Amersham Life Science, Braunschweig, Germany) was incorporated into the probes with the Random Primed DNA labelling kit (Boehringer, Mannheim, Germany), according to the manufacturer’s instructions.
Generation of a nonpolar mutation of the espA gene.
Overlap extension PCR (18) was used to generate an in-frame deletion of the espA gene. Two PCR fragments were generated with the primer pair 9188 (2524 5′-CGGGTATCGATTGTCGAAG-3′ 2542) and 9187 (2803 5′-GATCGTCGATGTCGAAGAACTCG-3′ 2780) and the primer pair 9186 (5′-CTTCGACATCGACGATC-3254-AGTGCACGTTCTGATGTGCAATC-3′ 3277) and 9185 (3523 5′-CGTCACTAATGAGTGACCTGCC-3′ 3501). The resulting products contained the first 63 bp and the last 66 bp of the espA open reading frame (ORF), respectively. A 17-bp overlap in their sequences (underlined) permitted amplification of a 548-bp fragment during a second PCR performed with primers 9188 and 9185. The resulting product was cloned into plasmid pCR2.1 (Invitrogen), digested with KpnI and XbaI, and subcloned into the pMAK700oriT (43) derivative pANK1, thereby generating plasmid pANK111. Transfer of the suicide vector by conjugation, cointegration, and excision was performed as previously described (28). The in-frame deletion was confirmed by PCR with the primers ANK25 (2164 5′-GGTATCCAGAAGATCAAGAAGC-3′ 2185) and A289 (3549 5′-CAACCCGGGCTAAGGACATCCTCAGCAGC-3′ 3578), which hybridize with adjacent external sequences.
Northern blot and primer extension analyses.
Bacterial strains were grown on DMEM-HEPES (pH 7) to an OD600 of 0.8, and total RNA was extracted with the RNeasy Midi Kit (Qiagen), according to the supplier’s instructions. Aliquots of 10 μg of RNA were denatured at 100°C in the presence of formaldehyde (2 M) and 50% formamide, separated on a 1% agarose–10% formaldehyde gel, blotted on a Byodine B transfer membrane (0.45 μm) (Pall, Dreieich, Germany), and then hybridized as described by Sambrook et al. (39) at 50°C with the probes described above. For primer extension analysis, strains were grown to an OD600 of 0.8 on M9 minimal medium with or without NaCl (430 mM), and total RNA was extracted as described above. Primer FAB56 (2743 5′-CATCTATATACCTCTTGATAATTT-3′ 2720) was end labelled with [γ-32P]dATP at 37°C for 40 min. The labelled primer was hybridized with 25 μg of RNA at 50°C for 20 min and extended with 1 U of avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) at 42°C for 40 min. Sequencing ladders were generated by using the same primer with the Deaza G/A T7Sequencing Mixes kit (Pharmacia Biotech, Piscataway, N.J.), according to the supplier’s instructions. Primer extension products were analyzed on a sequencing gel with the sequence ladder as a reference.
Detection of secreted proteins.
Bacteria were grown in DMEM-HEPES (pH 7) until they reached an OD600 of 0.6. Then, the proteins present in the supernatant fluids were precipitated by the addition of 10% (vol/vol) trichloroacetic acid, overnight incubation at 4°C, and subsequent centrifugation at 4,000 × g for 30 min. The dry pellet was resuspended in 1.5 M Tris (pH 8), and proteins (20 μg/lane) were fractionated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (39) with a 12.5% separating gel. They were then transferred to a positively charged Biodyne B nylon membrane (Pall) with a semidry device (Bio-Rad Laboratories, Richmond, Calif.), and proteins were detected with monoclonal antibodies against EspA, EspB, and EspD (10, 11) and horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G and immunoglobulin M as second antibodies (Bio-Rad Laboratories). Antigen-antibody complexes were visualized by chemiluminescence with the ECL system (Amersham Life Science).
β-Galactosidase assays.
Samples were taken at different time intervals, the OD600 was determined, and aliquots were removed and centrifuged at 8,000 × g to recover bacterial pellets, which were immediately processed to determine β-galactosidase activity or were stored at −80°C. To study activation of the esp promoter during bacterial infection of HeLa cells, monolayers were infected; at different time intervals, supernatants fluids were removed and unattached bacteria were collected by centrifugation. Then, the monolayers were gently washed and lysed with 1% Triton X-100 in PBS to collect attached bacteria. These samples were processed to determine the number of viable microorganisms and β-galactosidase activity. The β-galactosidase assay was performed with the β-GAL Reporter Gene Assay Chemiluminescent Kit (Boehringer) according to the supplier’s instructions, except that lysis was performed by resuspending bacteria in 500 μl of the lysis solution from the kit supplemented with chloroform (20 μl) and 0.1% SDS (20 μl) for 30 min at room temperature. The samples were measured with a Victor 1420 Multilabel Counter fluorometer (EG&G Wallac, Turku, Finland), and the results were normalized for the number of bacterial cells.
RESULTS
A functional espA gene is necessary for production of A/E lesions after infection with the EHEC strain EDL933.
Although the relevance of the EspA protein has been established for EPEC, limited information is available for EHEC. Therefore, we analyzed the role played by EspA in the initial interaction between the prototypic EHEC strain EDL933 (O157:H7) and eukaryotic cells. To assess whether the product encoded by the espA gene was also necessary for formation of the A/E lesion in EDL933, a mutant which contains an in-frame deletion in the espA gene was generated (see Materials and Methods). Immunofluorescence studies revealed a marked reduction in the numbers of attaching bacteria and actin accumulation when EDL933 ΔespA was compared with the parental strain (Fig. 1). To confirm that the observed effect was due to production of a truncated (i.e., nonfunctional) EspA protein and not to an affected transcription or translation of genes located downstream, production of the EspA, EspB, and EspD proteins was analyzed by Western blotting. As expected, EspA was not present in concentrated culture supernatants, whereas bands reacting with EspD- and EspB-specific antibodies were detected (not shown). This demonstrated that the EspA protein plays similar roles in the interactions between eukaryotic cells and EPEC, STEC, or EHEC.
FIG. 1.
Infection of HeLa cells with the EHEC strain EDL933. Cells were infected with EDL933 (a and c) or its ΔespA derivative (b and d) for 3 h. Then, monolayers were fixed, bacteria were labelled with TRITC-conjugated antibodies (a and b) and F-actin was stained with FITC-labelled phalloidin (c and d), and coverslips were examined by immunofluorescence microscopy. While the wild-type strain forms microcolonies with consistent actin accumulation (a and c), the ΔespA mutant has lost the ability to attach to HeLa cells (b) and to induce actin accumulation (d). Scales are in micrometers.
The espADB genes of EHEC are transcribed as a single operon.
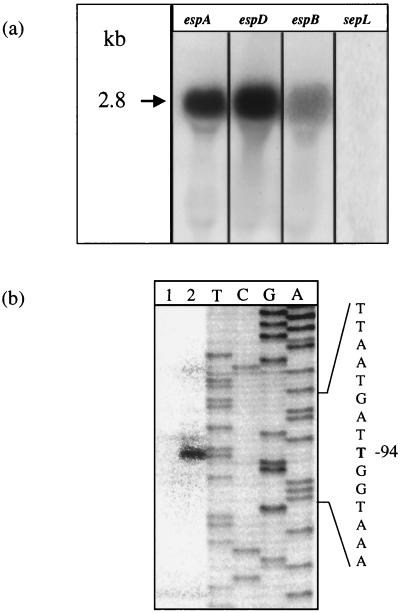
sepL and the genes encoding the secreted proteins EspA, EspD, and EspB are positioned in tandem on LEE, suggesting that they are cotranscribed as a polycistronic mRNA. The available information about secreted proteins in EPEC and STEC indicates that both the temperature and the composition of the culture medium are critical factors for expression (10, 21, 26). Therefore, to identify the transcript of the esp genes, Northern blot analysis was performed with RNA extracted from bacteria grown in DMEM supplemented with HEPES (100 mM) and PCR-generated fragments encompassing internal sequences from the three esp ORFs as probes. All probes hybridized with a unique band of approximately 2.8 kb. The length of the transcript corresponds to that of the espA, espD, and espB genes, suggesting that the promoter is located immediately upstream of espA. Probes specific for sepL, which is located upstream of espA, did not give any signal, ruling out the possibility that the observed band resulted from 5′ processing of a major transcript. This suggests that the esp genes, but not sepL, are transcribed as a single operon (subsequently designated the esp operon) (Fig. 2a).
FIG. 2.
(a) Northern blot analysis of the mRNA transcript encompassing the espA, espD, and espB genes. Total RNA extracted from EDL933 grown on DMEM-HEPES was fractionated on a 1% agarose gel, transferred to Byodine B membranes, and hybridized with probes specific for espA, espD, and espB. As a control, a probe that hybridizes within regions located upstream of espA (sepL) was used. The main RNA transcript is indicated by an arrow (approximately 2.8 kb). (b) Identification of the transcriptional start site from the esp operon by primer extension analysis. Total RNA was extracted from EDL933(pUJ3) grown exponentially at 37°C in medium supplemented with either 10 (lane 1) or 430 (lane 2) mM NaCl. A 24-bp oligonucleotide (FAB56), which hybridizes with positions +3 to −21 of the espA region, was used to perform primer extension and to generate a sequence ladder. The position of the first base in the main RNA message relative to the adenosine (base +1) of the ATG start codon is indicated.
Primer extension analysis was performed to identify the start of transcription of the esp promoter. RNA was extracted from cells grown on M9-glucose medium supplemented with either 10 or 430 mM NaCl. The major start site was mapped to 94 bp upstream from the ATG start codon of the espA gene (position 2646 of the published sequence) (Fig. 2b). The intensity of the signal was increased when bacteria were grown at high osmolarity, confirming the data obtained in studies on esp promoter regulation (see below). Analysis of the region upstream from the start of transcription led to identification of putative −10 and −35 sequences (Fig. 3). The −10 sequence exhibits a high degree of homology both to the −10 sequences of the bfpA gene of EPEC (37), which seems to be ς70 dependent, and to the osmE promoter, which is ςS dependent (5).
Transcription of the esp operon is activated upon contact with HeLa cells.
To study regulation of the esp operon, a DNA fragment spanning nucleotides −577 to +76 (with respect to the espA ATG start codon) was amplified by PCR and used to generate a translational fusion with the lacZ gene present in plasmid pUJ9TT, thereby generating plasmid pUJ3 (Fig. 3). This fragment was considered sufficiently long both to include the promoter and upstream regions containing potential binding sites for regulatory factors and to retain intact the translation initiation region to avoid potential artifacts resulting from altered translational efficiency (40).
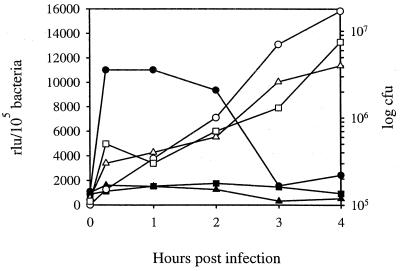
EspA seems to be produced in the early phase of infection, and it disappears when bacteria are stably attached to eukaryotic cells (11, 27). However, EspA was also detected in supernatant fluids and attached to bacterial surfaces, and it is unclear whether its transcription or translation results from interaction with eukaryotic cells. To elucidate this point, HeLa cells were infected with EDL933 harboring pUJ3 and the kinetics of espA activation was analyzed by determining the level of β-galactosidase produced by bacteria present in supernatant fluids or attached to HeLa cells. As shown in Fig. 4, rapid transcriptional activation was observed when bacteria came in contact with the eukaryotic cells, whereas almost no increment in β-galactosidase activity over the basal level was observed in bacteria present in supernatant fluids. Thus, the esp promoter appears to be induced upon contact with HeLa cells. The detected enzymatic activity began to decrease 1 to 2 h after infection, suggesting that a repression of the esp promoter takes place after the initial attachment. During the course of infection, the ratio between tightly and loosely attached bacteria increases; thus, transcription of the esp operon is probably switched off in tightly attached bacteria. These results demonstrate that transcription of the esp operon is induced by direct bacterial contact with HeLa cells rather than by components present in tissue culture medium or by soluble factors released by eukaryotic cells.
FIG. 4.
Expression of β-galactosidase by EDL933(pUJ3) after infection of HeLa cells. At different time intervals after infection, enzymatic activity was determined in bacteria present in supernatants (■) or attached to HeLa cells (●) and compared to that produced by EDL933(pUJ3) grown in DMEM (▴). The basal values of β-galactosidase obtained from EDL933 containing the promoterless plasmid under matching conditions were at least 10-fold lower than the basal levels of the tested clones and were subtracted from each sample. β-Galactosidase activities are expressed as relative light units (rlu) per 105 bacteria and are means of three independent experiments; standard deviations were lower than 5%. Open symbols indicate numbers of CFU at each time point.
esp operon induction by growth in different media.
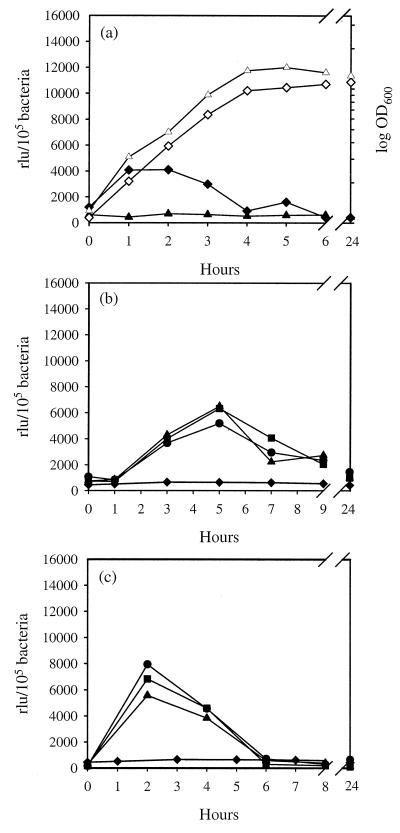
Since growth in tissue culture medium is known to stimulate secretion of EHEC proteins involved in the infection process (21), activation of the esp promoter in DMEM was analyzed. Strain EDL933(pUJ3) was grown in DMEM and expression of β-galactosidase was determined at different time intervals. An increment in β-galactosidase activity was observed in the exponential phase; however, this activation was blocked when bacteria were grown in DMEM without HEPES (Fig. 5a).
FIG. 5.
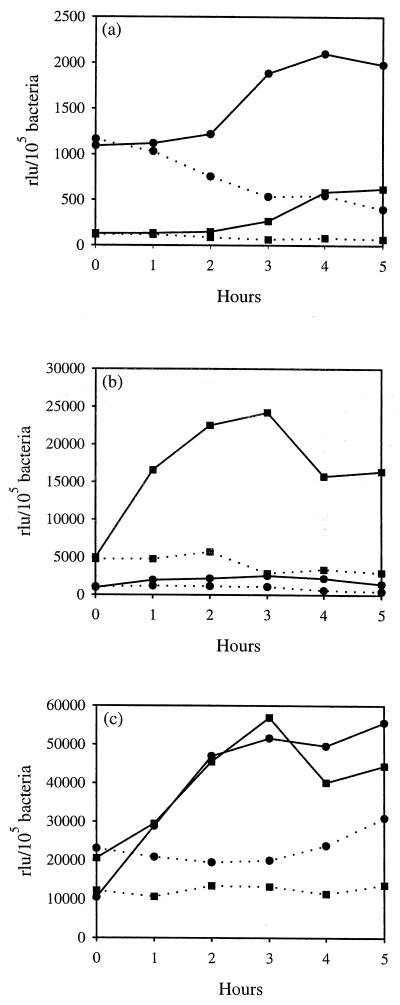
β-Galactosidase induction in response to different media and micronutrients. EDL933(pUJ3) was grown in DMEM (▴) or DMEM supplemented with 100 mM HEPES (pH 7) (⧫) (a) or in M9-glucose medium supplemented with either CaCl2 (⧫, 0 mM; ●, 0.01 mM; ■, 0.1 mM; ▴, 1 mM) (b) or MnSO4 (⧫, 0 mM; ●, 0.0033 mM; ■, 0.33 mM; ▴, 3.3 mM) (c), and β-galactosidase activities were determined at different time intervals. Growth rate is indicated by open symbols (OD600). Results are expressed as relative light units (rlu) per 105 bacteria and are means of three independent experiments; standard deviations were lower than 5%. The background values for EDL933 containing the promoterless plasmid under matching conditions were at least 10-fold lower than the basal values at the tested conditions and were subtracted from each sample.
Many micronutrients are known to induce expression of virulence genes in a wide range of pathogenic microorganisms (reviewed in reference 32). The influence of some micronutrients on protein secretion by EPEC and STEC has been analyzed previously but only in connection with other inducing conditions (e.g., HEPES and tissue culture medium). Therefore, their individual contributions to activation of the esp promoter were analyzed in the present study. The virulence of EPEC appears to be inhibited by the consistent amount of NH4+ present in the colon (26, 32). Supplementation of nitrogen-deficient M9 medium with different concentrations of NH4Cl did not affect the basal activity of the esp promoter, indicating that promoter activation is independent of the presence of either NH4+ or chloride (data not shown). Kenny et al. (26) reported that addition of calcium and iron to the culture medium resulted in improved export of secreted proteins in EPEC. Supplementation of M9-glucose medium with CaCl2 resulted in increased β-galactosidase activity from the early to middle exponential growth phases (Fig. 5b). In contrast, no significant changes in transcription were observed when the minimal medium was supplemented with FeSO4 or Fe(NO3)3, suggesting that iron, nitrate, and sulfate contribute very little, if at all, to activation of the esp operon (data not shown). Interestingly, the addition of MnSO4 resulted in an increased transcription, similar to that observed with CaCl2 (Fig. 5b and c). However, activity of the esp promoter was not affected in the presence of Mg2+, indicating that divalent ions per se were not responsible for the observed effect.
Effects of temperature, pH, and osmolarity on activation of the esp promoter.
The first sudden change that enteropathogenic bacteria face when they infect their hosts is the increment in temperature. Previous studies have suggested that secreted proteins are upregulated at 37°C (10, 26); however, the individual contribution of temperature was buried among other potential stimuli (e.g., pH and culture medium, etc.) due to the poor sensitivity of the reading system. Therefore, the effects of changes in growth temperature on induction of the esp promoter were analyzed. Interestingly, no significant differences were observed in the activity of the promoter when strain EDL933(pUJ3) was incubated in standard M9 medium (10 mM NaCl) at 25, 37, or 42°C (Fig. 6). EHEC is also confronted during the first phase of infection with a very acidic environment in the stomach. It then transits across the duodenum, which receives the alkaline biliary content. Finally, it reaches the ileum, cecum, and colon, which constitute its primary targets and in which the pH is neutral or slightly alkaline. However, no significant differences in promoter activity were observed when bacteria were grown at pH 6, 7, or 8 (data not shown).
FIG. 6.
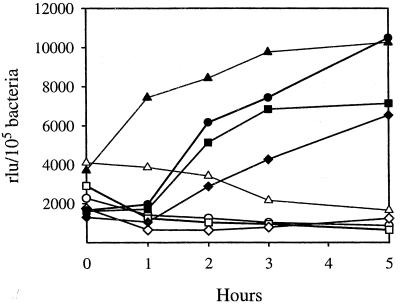
Activation of the esp promoter in response to changes in temperature and osmolarity. EDL933 containing either pUJ3 (●) or its deletion derivative, pUJ3-285 (■), was grown in minimal medium (pH 7) supplemented with 10 mM (dotted lines) or 430 mM (solid lines) NaCl at 25°C (a), 37°C (b), or 42°C (c), and β-galactosidase activities were determined at different time intervals. Results are expressed as relative light units (rlu) per 105 bacteria and are means of three independent experiments; standard deviations were lower than 5%. The background values for EDL933 containing the promoterless plasmid were at least 10-fold lower than the values at the tested conditions and were subtracted from each sample.
The external niches in which E. coli is present are in general characterized by low osmolarity. Therefore, it might be expected that the high osmolarity of the gut lumen can be exploited by EHEC as an expression signal. In fact, osmolarity plays an important role in activation of virulence genes from other enteropathogenic microorganisms (26, 32, 36). It is known that, depending on the specific infection site, maximal gene expression occurs at different osmolarities (32). Therefore, experiments were performed to establish the effect of osmolarity on activation of the esp promoter. Preliminary studies demonstrated (data not shown) that the maximal effect was observed at 1,120 mosmol/kg (430 mM NaCl). Concentrations below or above this value resulted in a suboptimal level of induction. To avoid interference with potential osmoprotectants present in Luria-Bertani medium, strain EDL933(pUJ3) was grown in M9-glucose medium in the presence of 430 mM NaCl or an equimolar concentration of sucrose.
As shown in Fig. 6b and c, when bacteria were grown at 37 and 42°C, high osmolarity resulted in an 8- to 10-fold increased β-galactosidase activity during exponential and stationary phases. A similar activation pattern was observed when NaCl was replaced by sucrose (data not shown). This demonstrates that induction of the esp promoter depends on osmolarity rather than on an indirect stress effect due to elevated concentrations of NaCl. It has been frequently observed that promoters sensitive to osmolarity are also induced in the stationary phase. However, the activation of the esp promoter was independent of bacterial entrance into the stationary phase and was triggered immediately following inoculation in high-osmolarity medium. Furthermore, when bacteria were grown on low-osmolarity medium (10 mM NaCl), no increment in enzymatic activity was observed in the stationary phase (Fig. 6). Temperature and osmolarity are thought to play key roles in the expression of virulence genes in many enteropathogenic bacteria (32). Since EHEC can also face any of these individual conditions outside the host, we analyzed whether at suboptimal (nonphysiologic) temperatures the promoter was activated at high osmolarities. Despite bacteria being grown at optimal osmolarity, the esp promoter was not induced at 25°C (Fig. 6), whereas minimal differences in activation were observed at between 37 and 42°C. These results suggest that the promoter is optimally activated by a combination of temperature and osmolarity.
To assess the contribution of the regions located upstream from the start of transcription, the pUJ3 derivative pUJ3-285, which contains a 285-bp deletion, was generated (Fig. 3). An increment in the enzymatic activity of EDL933(pUJ3-285) with respect to EDL933(pUJ3) was observed when strains were grown at 37°C at either low or high osmolarity (Fig. 6b). This suggests the presence of a binding site for a negative regulator in the deleted region. The differences were less evident at the suboptimal temperatures of 25 and 42°C.
We then generated a hybrid plasmid (pUJ3-56) in which the fragment located downstream from the ATG start codon was deleted (Fig. 3). The resulting construct was transformed into EDL933 to determine β-galactosidase activity under different conditions. The obtained results showed 60 to 80% reductions in enzymatic activity when bacteria were grown in high- and low-salt medium (data not shown). This suggests that the initial part of the espA ORF is essential for allowing optimal translation efficiency, as has been previously reported for other genes of E. coli (40).
Transcription of the esp operon is dependent on the presence of a functional ςS factor.
It has been shown that ςS controls a regulon of more than 30 genes expressed in response to starvation or during the transition to stationary phase and influences the response to osmotic stress (15). Interestingly, motifs located upstream from the start of transcription of the esp promoter exhibit similarity with ςS-dependent promoters (see above). Therefore, to assess whether transcription of the esp operon is dependent on the ςS factor, the pUJ3 plasmid was introduced into E. coli MC4100 and its ςS-deficient derivative, RH90. As shown in Fig. 7a, the expression levels of the reporter gene were dramatically reduced in the mutant strain (10-fold) under both inducing (430 mM NaCl) and noninducing (10 mM NaCl) conditions. The differences were more striking at high osmolarity and in the early stationary phase. Interestingly, when the wild-type strain MC4100 was tested, the β-galactosidase activities under both growth conditions were approximately two- to fourfold lower than that observed in EDL933(pUJ3), suggesting that additional factors are required to trigger full activation of the esp promoter in EDL933.
FIG. 7.
Transcription of the esp operon is dependent on ςS and H-NS. (a) Plasmid pUJ3 was transformed into E. coli MC4100 (●) and its ςS-deficient derivative, RH90 (■), and β-galactosidase activities were determined under inducing (430 mM NaCl [solid lines]) and noninducing (10 mM NaCl [dotted lines]) conditions. (b and c) To investigate the role of the H-NS protein, plasmids pUJ3 (●) and pUJ3-285 (■) were transformed into E. coli GM37 (b) and its hns derivative GM230 (c). Strains were grown in M9-glucose medium supplemented with 10 mM (dotted lines) or 430 mM (solid lines) NaCl, and production of β-galactosidase was determined after different time intervals. Results are expressed as relative light units (rlu) per 105 bacteria and are means of three independent experiments; standard deviations were lower than 5%. The background values for strains containing the promoterless plasmid were at least 10-fold lower than the basal values at the tested conditions and were subtracted from each sample. No differences in growth were observed.
Transcription of the esp operon is dependent on the presence of a functional H-NS protein.
The global negative regulator H-NS is also involved in osmoregulation and can act either indirectly, through the maintenance of low ςS levels in exponentially growing (nonstressed) bacteria, or directly, in a ςS-independent manner (reviewed in reference 2). To analyze whether the H-NS protein was also involved in regulation of the esp promoter, plasmids pUJ3 and pUJ3-285 were introduced into E. coli GM37 and its hns derivative, GM230. When the production of β-galactosidase of strains GM37(pUJ3) and GM230(pUJ3) were compared, a 10- to 20-fold increment was observed in the hns mutant grown in the presence of either low or high levels of NaCl (Fig. 7b and c). The strong increase in transcription can be explained by an overexpression of ςS or an indirect H-NS-mediated effect in the plasmid copy and linking numbers (17).
H-NS has the strongest effect under conditions in which expression of the target gene is not induced by positive regulators, whereas under inducing conditions H-NS-mediated repression is almost eliminated. Therefore, by comparing the osmotic induction ratios (induction at high/low osmolarity) in the hns+ and hns strains, a direct effect of H-NS can be demonstrated (the greater the ratio, the more complete the repression). Ratios of 2.5 and 4 were observed for GM230(pUJ3) and GM37(pUJ3) after 4 h of incubation, suggesting that the observed activation was directly dependent on H-NS. This hypothesis was further supported by the results obtained with plasmid pUJ3-285. When strains harboring this plasmid were tested, both basal and induced levels of β-galactosidase were increased up to 10-fold in GM37(pUJ3-285) with respect to GM37(pUJ3), whereas in the hns mutant the basal and induced levels of the strain harboring pUJ3-285 were only slightly affected in comparison to those of GM230(pUJ3) (Fig. 7b and c). Abolition of H-NS-mediated repression in pUJ3-285 suggests that the deleted region encompasses binding motifs for this protein. This hypothesis is further supported by the presence of several stretches containing the H-NS binding consensus sequence (5′-TNTNAN-3′) (38) upstream and downstream from the EcoRV site present in the esp promoter region (Fig. 3).
Influence of DNA supercoiling in transcription of the esp operon.
Osmoinduction of several promoters is determined by changes in the degree of DNA supercoiling (32). Since EHEC should face an anaerobic environment in the intestinal niche, and anaerobicity can also affect DNA supercoiling (45), we investigated whether the degree of supercoiling influences activation of the esp promoter. Novobiocin was used to inhibit the DNA gyrase, which facilitates initiation of transcription by introducing negative supercoils. EDL933(pUJ3) was grown in the presence of subinhibitory concentrations of novobiocin, and β-galactosidase activity was measured. The obtained results showed that novobiocin reduced the levels of β-galactosidase in a dose-dependent manner when EDL933 was grown in the presence of 430 mM NaCl (Fig. 8), suggesting that the degree of supercoiling is critical in regulation of the esp promoter.
FIG. 8.
Influence of the degree of supercoiling on transcription of the esp promoter. EDL933(pUJ3) was grown in M9-glucose medium supplemented with 10 mM (open symbols) or 430 mM (solid symbols) NaCl in the presence of 0 (triangles), 5 (circles), 20 (squares), or 50 (diamonds) μg of the gyrase inhibitor novobiocin per ml, and β-galactosidase activities were determined at different time intervals. Results are expressed as relative light units (rlu) per 105 bacteria and are means of three independent experiments; standard deviations were lower than 5%. The background values for EDL933 containing the promoterless plasmid were at least 10-fold lower than the basal values at the tested conditions and were subtracted from each sample. No differences in growth were observed at the novobiocin concentrations tested (not shown).
DISCUSSION
The products encoded by LEE confer upon EHEC and EPEC their distinctive virulence property, namely, the ability to produce A/E lesions. We studied transcriptional regulation of the genes encoding the secreted proteins EspA, EspD, and EspB, which play a key role in A/E lesion formation. Recent results from our group and others have demonstrated that the EspA protein from STEC and EPEC is involved in the formation of filamentous surface appendages that appear during early infection and seem to be critical for bacterial adherence (11, 27). Although these studies strongly suggested that EspA is involved in the first steps of infection, they provided no definitive proof about the kinetics of appearance of EspA and the potential induction mechanism. Northern blot and primer extension analyses showed that espA is cotranscribed with espD and espB and permitted identification of a promoter located 94 bp upstream of the espA gene. A 5- to 10-fold induction of the esp promoter was observed upon bacterial attachment to HeLa cells. The fact that the esp promoter was switched off later during infection is consistent with the lack of EspA production by bacteria forming microcolonies (11, 27).
The esp promoter appears to be subjected to different environmental stimuli, similar to those faced by EHEC in the intestine. Previous reports showed that expression of the secreted proteins occurred when bacteria were grown in tissue culture medium (10, 26). No induction of the esp promoter was observed when bacteria were grown in DMEM, whereas the addition of 100 mM HEPES resulted in four- to fivefold-increased transcription. Therefore, the previously reported effect on protein secretion seems to be due to the presence of HEPES rather than to specific components of the tissue culture medium. The presence of Ca2+ also resulted in strong activation of the esp promoter over the broad range of concentrations tested. Therefore, calcium seems to play an important role not only in the signal transduction events leading to the rearrangement of cytoskeletal proteins (20) but also in the early interactions of EHEC with enterocytes via induction of the esp promoter. This is in agreement with the general role played by Ca2+ in regulation of virulence genes from several pathogenic microorganisms (26, 32, 37). Similar activation levels were observed when media were supplemented with Mn2+. Interestingly, Mn2+ is involved in regulation of expression of metal transporter systems in Streptococcus spp. and Yersinia spp. (3, 7). Although the molecular mechanism by which Mn2+ exerts its effect on the esp promoter is unclear, surface proteins are affected in EHEC, Streptococcus pneumoniae, and Yersinia pestis, suggesting common underlying processes in unrelated pathogens. Temperature has a weak effect on induction of the esp promoter; however, increased levels of activation are achieved when it acts together with osmolarity. Although EHEC can be confronted with any of these stimuli outside the host, the combination of 37°C and high osmotic pressure represents an excellent indicator that bacteria have reached their target within the host intestine.
It is known that the presence of the 60-MDa plasmid pMAR2 is required in EPEC to achieve full virulence; plasmidless bacteria exhibit a reduced ability to infect HeLa cells (14). Although a 90-kb plasmid (pO157) has been identified in EHEC, its role in the infection process is still unclear (24). When the STEC strain 413.89-1 (44) and its plasmidless derivative (413.89-1/6) were transformed with pUJ3, a significant impairment (sixfold) in production of β-galactosidase was observed under inducing conditions when the megaplasmid was absent (not shown). This suggests that activation of the esp promoter is also fine tuned by a product(s) encoded by the megaplasmid.
Results obtained with an rpoS mutant and the homology between putative consensus sequences and the promoter of osmE (5) suggested that transcription from the esp promoter is ςS dependent. Interestingly, activation of the esp promoter preferentially occurs during the exponential phase of growth, whereas during the stationary phase it slightly decreases. However, the role of ςS is more complex than that of other alternative ς factors, as it plays a role under various conditions of slow growth, such as those observed during the stationary phase and under osmotic shock (15, 16). Although basal expression of the reporter was strongly reduced in the rpoS mutant, osmoinduction was preserved (Fig. 7). Tanaka et al. (41) showed that several promoters can be recognized by either the Eς70 or EςS RNA polymerase holoenzymes. Therefore, ςS-independent transcription of the esp promoter may be directly dependent on Eς70. The esp promoter also exhibits homology with the bfpA promoter (36). Although it has been suggested, without experimental evidence, that this promoter is ς70 dependent, it is intriguing that promoters driving expression of proteins involved in the synthesis of surface appendages required for initial attachment have common motifs. The apparent decreased activity of the esp promoter in the late stationary phase might reflect a mechanism evolved by EHEC to avoid the extra energetic cost required to synthesize products which are required only in the initial phases of infection.
The H-NS protein is involved in regulation of many genes activated by environmental signals (2). We have demonstrated that the levels of transcription of the esp promoter are significantly increased in an hns mutant. The presence of this regulator usually results in 2- to 20-fold repression, which is stronger when H-NS both acts at the promoter level and affects the expression of positive regulators (2). Therefore, the observed influence of H-NS in activation of the esp promoter can be explained by (i) hyperexpression of the ςS factor which is repressed by H-NS (2) and (ii) a direct effect on the promoter itself, since putative H-NS-binding regions have been identified.
No vaccines able to prevent infections caused by EHEC are presently commercially available, and antibiotics are not useful for therapy since they can worsen symptoms by enhancing the release of bacterial toxins. Study of the interactions between bacteria and host cells may permit identification of novel molecular targets for therapeutic interventions. It might be possible to modulate the expression of virulence factors to make bacteria more susceptible to chemotherapeutics or host clearance mechanisms. Thus, an understanding of fine regulatory mechanisms may be the first step towards development of new tools to fight EHEC infections.
The data emerging from this work show that overall regulation of the esp promoter is an extremely complex process. During their transit across different niches, EHEC organisms must integrate different signals to optimize and fine tune the expression of virulence factors. The activation process is in part modulated by factors which are also needed for regulation of housekeeping genes from nonpathogenic E. coli. This has been demonstrated as well for other pathogens (12) and suggests that virulence genes, which were inherited later during bacterial evolution, exploit previously established regulatory networks.
ACKNOWLEDGMENTS
We are grateful to F. Sasse for insight into the performance of fluorometry experiments, F. Ebel for providing antibodies and strain 413.89-1/6, and K. N. Timmis for generous support and encouragement.
Part of this work was supported by a grant from the Lower Saxony-Israel Cooperation Programme, founded by the Volkswagen Foundation (21.45-75/2).
REFERENCES
- 1.Akerley J F, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 3.Bearden S W, Staggs T M, Perry R D. An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J Bacteriol. 1998;180:1135–1147. doi: 10.1128/jb.180.5.1135-1147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce T G, Swerdlow D L, Griffin P M. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 5.Conter A, Menchon C, Gutierrez C. Role of DNA supercoiling and RpoS sigma factor in the osmotic and growth phase-dependent induction of the gene osmE of Escherichia coli K12. J Mol Biol. 1997;273:75–83. doi: 10.1006/jmbi.1997.1308. [DOI] [PubMed] [Google Scholar]
- 6.Deibel C, Kramer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 7.Dintilhac A, Alloing G, Granadel C, Claverys J. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Kaper J B, Finlay B B. Interaction between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 9.Dorman C J. DNA supercoiling and environmental regulation of gene expression in pathogenic bacteria. Infect Immun. 1991;59:745–749. doi: 10.1128/iai.59.3.745-749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebel F, Deibel C, Kresse A U, Guzmán C A, Chakraborty T. Temperature- and medium-dependent secretion of proteins by shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebel F, Podzadel T, Rohde M, Kresse A U, Kramer S, Deibel C, Guzmán C A, Chakraborty T. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol Microbiol. 1998;30:147–161. doi: 10.1046/j.1365-2958.1998.01046.x. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster J W, Spector M P. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Duarte O, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengge-Aronis R, Lange R, Henneberg N, Fisher D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengge-Aronis R. Back to the log phase: ςS as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 17.Hinton J C D, Santos D S, Seirafi A, Hulton C S J, Pavitt G D, Higgins F C. Expression and mutational analysis of the nucleoid-associated protein H-NS of Salmonella typhimurium. Mol Microbiol. 1992;6:2327–2337. doi: 10.1111/j.1365-2958.1992.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 18.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 19.Hulton C S J, Seirafi A, Hinton J C D, Sidebotham J M, Wadel L, Pavit G D, Owen-Hughes T, Spassky A, Buc H, Higgins C F. Histone like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990;63:631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- 20.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jungnitz H, West N P, Walker M J, Chhatwal G S, Guzmán C A. A second two-component regulatory system of Bordetella bronchiseptica required for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence. Infect Immun. 1998;66:4640–4650. doi: 10.1128/iai.66.10.4640-4650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaper J B. Enterohemorrhagic Escherichia coli. Curr Opin Microbiol. 1998;1:103–108. doi: 10.1016/s1369-5274(98)80149-5. [DOI] [PubMed] [Google Scholar]
- 25.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 26.Kenny B, De Vinney R, Stein M, Reinsheid D J, Frey E A, Finlay B B. Enteropathogenic Escherichia coli (EPEC) transfers its receptor for intimate adherence to mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 27.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kresse A U, Schulze K, Deibel C, Ebel F, Rohde M, Chakraborty T, Guzmán C A. Pas, a novel protein required for protein secretion and attaching and effacing activities of enterohemorrhagic Escherichia coli. J Bacteriol. 1998;180:4370–4379. doi: 10.1128/jb.180.17.4370-4379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange R, Hengge-Aronis R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor ςS. J Bacteriol. 1991;173:4474–4481. doi: 10.1128/jb.173.14.4474-4481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel T K, Jarvis K G, Donnenberg K S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon H V, Whipp S C, Argenzio R A, Levine M M, Ginnella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien A D, Lively T A, Chen M E, Rothman S W, Formal S B. Escherichia coli O157-H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (Shiga) like cytotoxin. Lancet. 1983;i:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 36.Porter M E, Dorman C J. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J Bacteriol. 1994;176:4187–4191. doi: 10.1128/jb.176.13.4187-4191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puente J L, Bieber D, Ramer S W, Murray W, Schoolnik G K. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol Microbiol. 1996;20:87–100. doi: 10.1111/j.1365-2958.1996.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 38.Rimsky S, Spassky A. Sequence determinants for H1 binding on Escherichia coli lac and gal promoters. Biochemistry. 1990;29:3765–3771. doi: 10.1021/bi00467a024. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Schauder B, McCarthy J E G. The role of basis upstream of the Shine-Dalgarno region and in the coding sequence in the control of gene expression in Escherichia coli: translation and stability of mRNAs in vivo. Gene. 1989;78:59–72. doi: 10.1016/0378-1119(89)90314-4. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal ς factor in Escherichia coli: the rpoS gene product ς38 is a secondary principal ς factor of RNA polymerase in stationary-phase. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzipori S, Wachsmuth I K, Chapman C, Birner R, Brittingham J, Jackson C, Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 43.Viret J F, Cryz S J, Favre D. Expression of Shigella sonnei lipopolysaccharide in Vibrio cholerae. Mol Microbiol. 1996;19:949–963. doi: 10.1046/j.1365-2958.1996.435967.x. [DOI] [PubMed] [Google Scholar]
- 44.Wieler L H, Vieler E, Erpenstein C, Schlapp T, Steinruck H, Bauerfeind R, Byomi A, Baljer G. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J Clin Microbiol. 1996;34:2980–2984. doi: 10.1128/jcm.34.12.2980-2984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto N, Droffner M L. Mechanisms determining aerobic or anaerobic growth in the facultative anaerobe Salmonella typhimurium. Proc Natl Acad Sci USA. 1985;82:2077–2081. doi: 10.1073/pnas.82.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]