Abstract
8-Nitro-1,3-benzothiazin-4-ones (BTZs) are known as potent antitubercular agents. BTZ043 as one of the most advanced compounds has reached clinical trials. The putative oxidation products of BTZ043, namely, the corresponding BTZ sulfoxide and sulfone, were reported in this journal (Tiwari et al. ACS Med. Chem Lett.2015, 6, 128–133). The molecular structures were later revised to the constitutionally isomeric benzisothiazolone and its 1-oxide, respectively. Here, we report two BTZ043-derived benzisothiazolinones (BITs) with in vitro activity against mycobacteria. The constitutionally isomeric O-acyl benzisothiazol-3-ols, in contrast, show little or no antimycobacterial activity in vitro. The structures of the four compounds were investigated by X-ray crystallography and NMR spectroscopy. Molecular covalent docking of the new compounds to Mycobacerium tuberculosis decaprenylphosphoryl-β-d-ribose 2′-epimerase (DprE1) suggests that the active BITs exert antimycobacterial activity through inhibition of DprE1 like BTZs.
Keywords: benzisothiazolinone, BTZ043, crystal structure, antimycobacterial activity, DprE1, tuberculosis
Tuberculosis (TB), the disease usually caused by Mycobacterium tuberculosis, globally continues to be the leading cause of death from a bacterial infectious agent.1 According to the World Health Organization, a total of 1.5 million people died from and an estimated 9.9 million people fell ill with TB in 2020.2 In particular, the increasing emergence of drug-resistant TB is a threat to public health.3,4 Whereas about 85% of patients with drug-susceptible active TB can be successfully treated with a six-month regime of four first-line drugs,2 treatment of drug-resistant TB in general takes longer, requires more expensive drugs that result in more side effects, and has lower success rates.5,6 Drug discovery efforts are thus vital to combat drug-resistance and to reduce the duration, cost, and side effects of TB therapy through maintaining a copious anti-TB drug supply.7−9
8-Nitro-1,3-benzothiazin-4-ones (BTZs) have been identified as potent antitubercular agents.10 The two most advanced compounds of this class, BTZ043 (Scheme 1) and macozinone (PBTZ169),11 have progressed to clinical studies.7 BTZs and several other electron-deficient nitroaromatic agents target the mycobacterial enzyme decaprenylphosphoryl-β-d-ribose 2′-epimerase (DprE1),12−14 which is crucial for the biosynthesis of arabinogalactan, a major structural component of the mycobacterial cell wall. Upon binding to the active site of DprE1, the BTZ nitro group is reduced to a reactive nitroso group, which then forms a covalent semimercaptal with the thiol group of cysteine 387.15 BTZs are thus mechanism-based inhibitors, which irreversibly inactivate the enzyme. For this mechanism of action, an electron-deficient warhead is essential. Besides the nitro group it was recently shown that 8-cyano BTZs also have antimycobacterial activity.16 Efforts to modify the substituent at position 6 and the side chain at position 2 of the BTZ scaffold to improve physicochemical properties have been made.17−20 Alteration of the BTZ core by means of scaffold simplification21 and metabolically relevant chemical transformations of the scaffold have also been reported.22,23
Scheme 1. Reaction of BTZs 1a and 1b (BTZ043) with m-Chloroperoxybenzoic Acid (mCPBA): Putative Chemical Structures “BTZ-SO” and “BTZ-SO2” Assigned by Tiwari et al.24 and Corresponding Revised Chemical Structures Reported by Eckhardt et al.25.
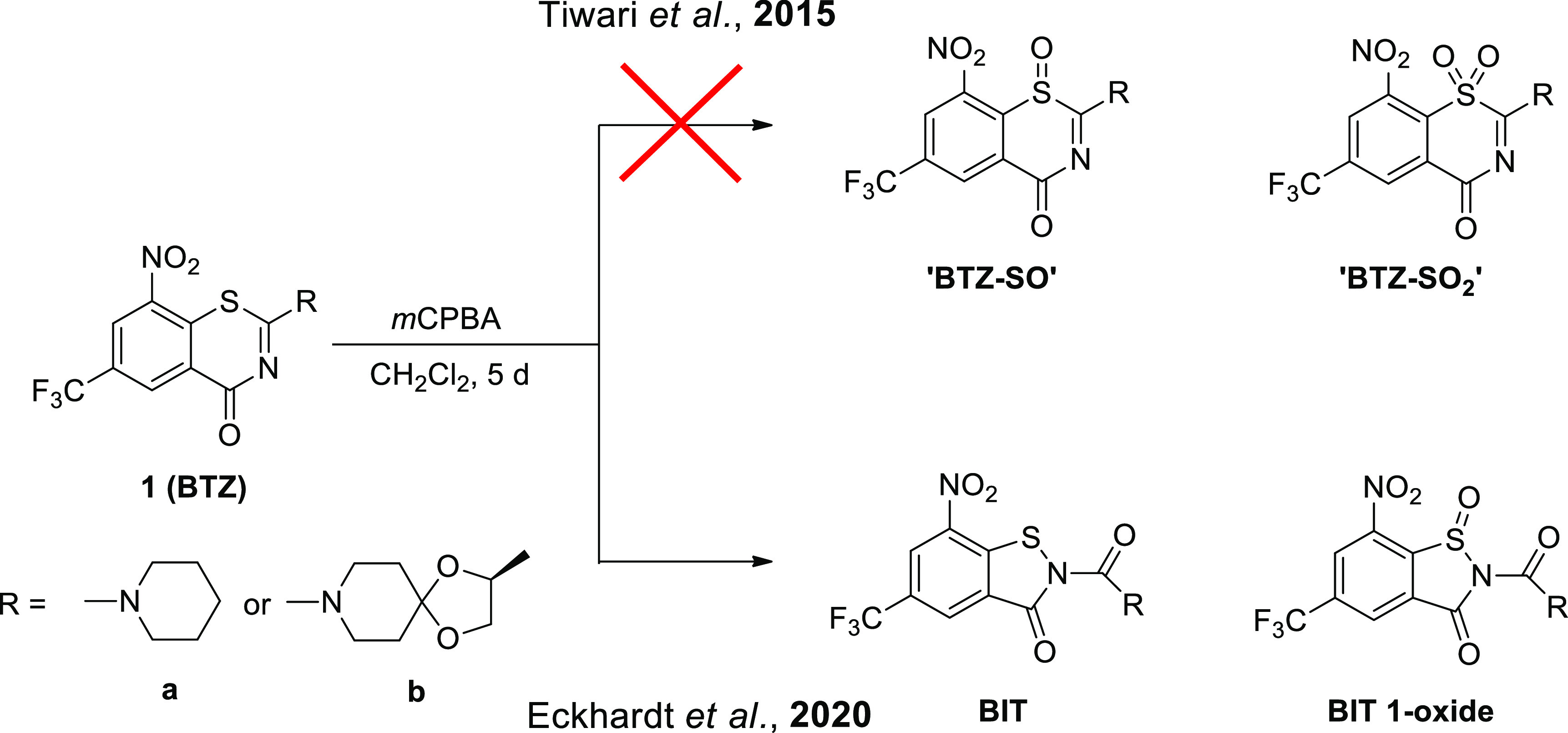
In 2015, Tiwari et al. described the purported sulfoxide (“BTZ-SO”) and sulfone (“BTZ-SO2”) of BTZ043 in this journal,24 putatively obtained in very low yields by reaction of BTZ043 with the m-chloroperoxybenzoic acid (Scheme 1). The chemical structures of “BTZ-SO” and “BTZ-SO2”, as depicted in Scheme 1, were assigned on the basis of 1H NMR spectroscopy and ESI mass spectrometry.
Unexpected upfield shifts of the two aromatic 1H NMR signals, as compared with the parent BTZ043, were attributed to the influence of the lone pair at the sulfur atom and loss of aromaticity due to puckering of the thiazinone ring, as observed in AM1 computed molecular structures of the anticipated “BTZ-SO” and “BTZ-SO2”. Recently, X-ray crystallography revealed that the supposed BTZ sulfones resulting from treatment of BTZs 1a and 1b with m-chloroperoxybenzoic acid are in fact the corresponding benzisothiazolinone (BIT) 1-oxides (Scheme 1), which represent constitutional isomers.25 Crystallographic evidence for the unintended BIT 1-oxide formation, retrosynthesis of a BIT and revisiting the 1H NMR data eventually led to the revision of the chemical structures as depicted in Scheme 1 by Eckhardt et al. in 2020. In vitro activity data included in the previous reports revealed that the BITs exhibit comparable or better in vitro activity against M. tuberculosis and Mycobacterium aurum than the corresponding BTZs 1a and 1b.25 This prompted us to further investigate these compounds. In this contribution, we report on the synthesis, structural characterization, and in vitro and in silico antimycobacterial evaluation of two BITs and their O-acyl benzisothiazol-3-ol constitutional isomers. The chemistry used to prepare the compounds studied is outlined in Scheme 2. N-Acyl BITs 4 and O-acyl benzisothiazolols 5 were synthesized in two steps from 2-chloro-5-trifluoromethyl-3-nitrobenzamide26 (2). In the first step, BIT 3 was synthesized from 2 by a copper-mediated sulfur–nitrogen coupling reaction.27 In the second step, 3 was reacted with piperidine-1-carbonyl chloride (for 4a and 5a) or (S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]decane-8-carbonyl chloride23,28 (for 4b and 5b)29,30 to obtain 4 and 5. Using pyridine as a base instead of triethylamine significantly improved the yield in the latter step. Compounds 4a and 5a as well as 4b and 5b represent constitutional isomers, which were separated by flash chromatography in both cases. The benzisothiazol-3-ol system in 5a and 5b represents a tautomeric form of the BIT system in 4a and 4b. Concomitant O-acylation benzisothiazol-3-ol of the tautomer, which is expected to be less stable than the BIT form, affords isolable O-acyl derivatives 5. The ratio of 4 to 5 was around 1:2 in both cases. Higher electron density and better steric accessibility of the oxygen atom of the isothiazol-3-ol form compared with the isothiazol-3-one nitrogen atom likely explain the observed regioselectivity.
Scheme 2. Syntheses of N-Acyl BITs 4a and 4b and O-Acyl Benzisothiazolols 5a and 5b.
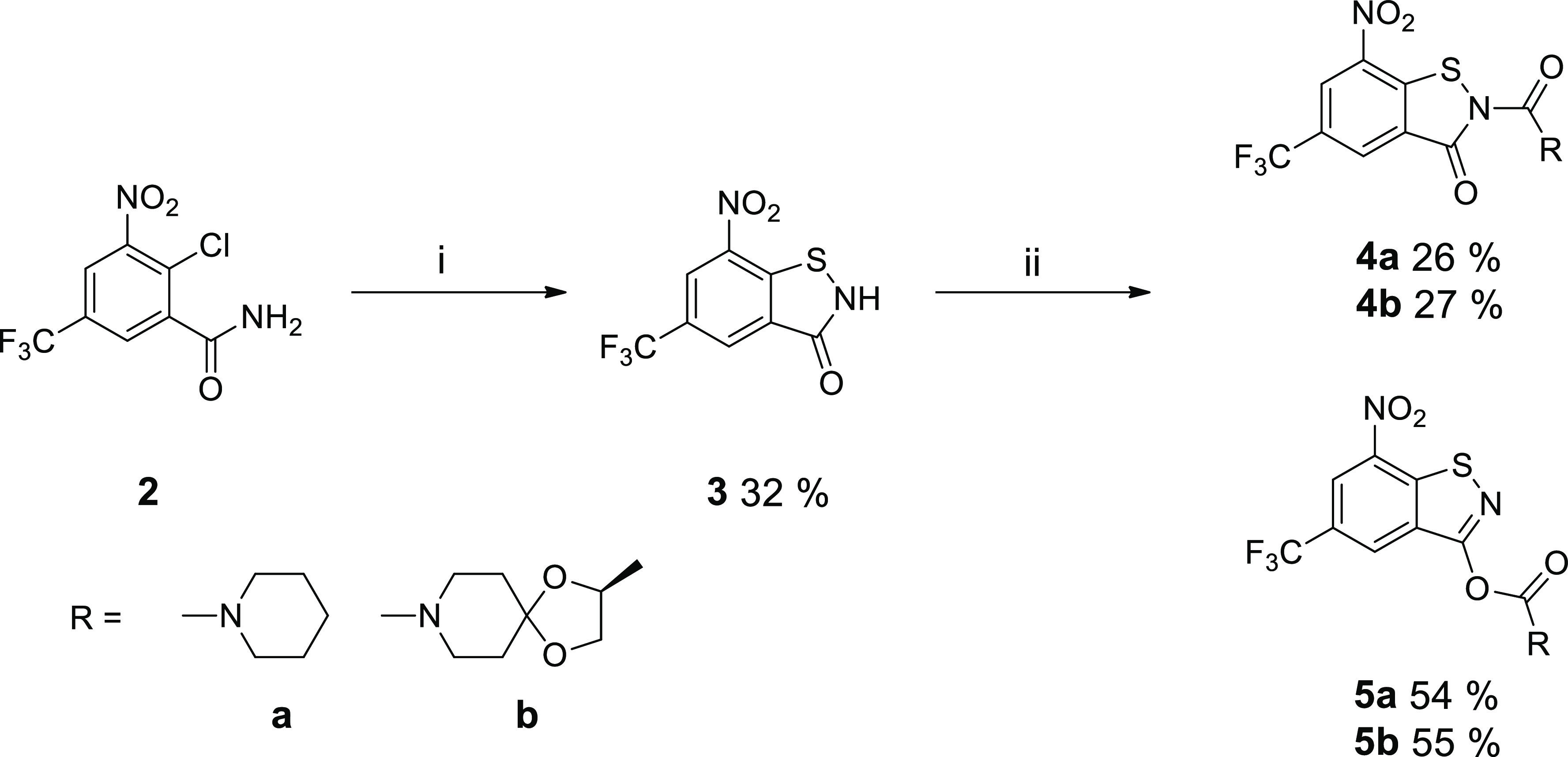
Reagents and conditions: (i) CuI, 1,10-phenanthroline, sulfur, K2CO3, DMF, 110 °C, overnight; (ii) piperidine-1-carbonyl chloride (a) or (S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]decane-8-carbonyl chloride (b), pyridine, CH2Cl2, rt, 24 h.
The structures of the reaction products 4a and 4b as well as isomeric 5a and 5b were unambiguously assigned by X-ray crystallography. Compound 4a crystallized as a 0.25 hydrate with three crystallographically unique molecules.31 As shown in Figure 1a, the three distinct molecules all adopt a Z conformation about the Cacyl–N2BIT bond. The appended piperidine ring adopts a chair conformation in each case (Figure 1b).
Figure 1.
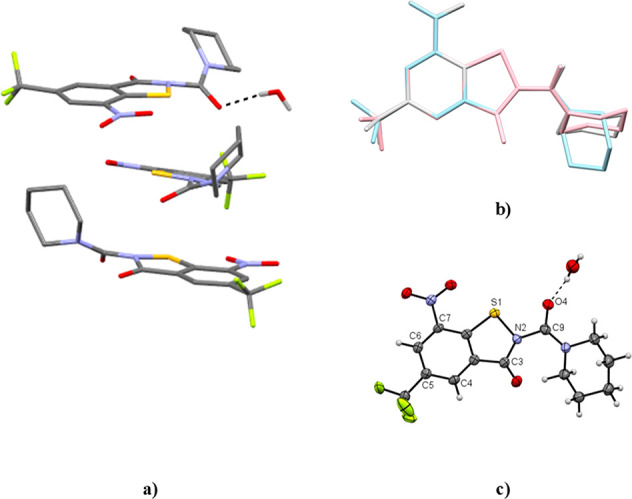
Crystal structure of 4a·0.25 H2O. (a) Asymmetric unit. Carbon-bound hydrogen atoms are omitted for clarity. (b) Structure overlay (benzene rings) of the three crystallographically unique molecules (pink, blue, gray). (c) Displacement ellipsoid plot (50% probability) of molecule 1. Rotational disorder of the trifluoromethyl group and positional disorder of a water hydrogen atom are not shown for clarity. Color scheme: C, gray; H, white; N, blue; O, red; S, yellow.
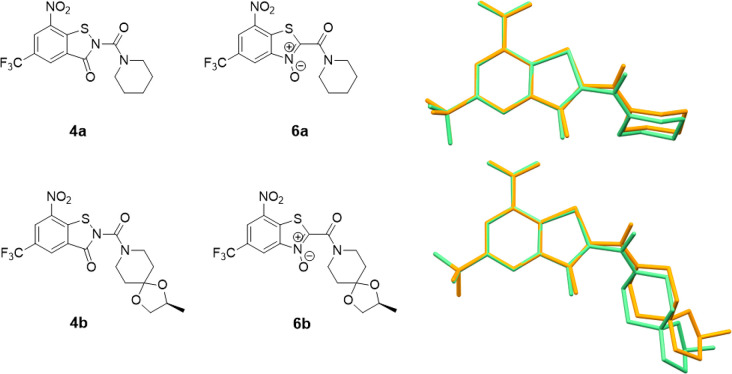
The solvate water forms a hydrogen bond to the piperidinoyl oxygen atom of molecule 1 (Figure 1c). Figure 2 depicts overlay diagrams of DFT-calculated molecular structures of 4a and 4b and the corresponding constitutionally isomeric benzothiazole N-oxides (BTOs, 6), potent antimycobacterial agents described previously in the literature (vide infra),32,33 which reveals that the molecules are isosteric.
Figure 2.
Chemical diagrams and structure overlay (benzene rings) of DFT-optimized molecular structures of 4a and 4b (orange) and the BTO constitutional isomers 6a(32) and 6b (green).33 Hydrogen atoms are omitted for clarity.
Compound 4b crystallizes with two molecules in the asymmetric unit (Figure 3).34 Like in 4a, the conformation about the Cacyl–N2BIT bond is Z in both unique molecules. The two crystallographically distinct molecules are thus diastereomeric conformers. Such was also encountered in the crystal structure of 1b (BTZ043).35
Figure 3.
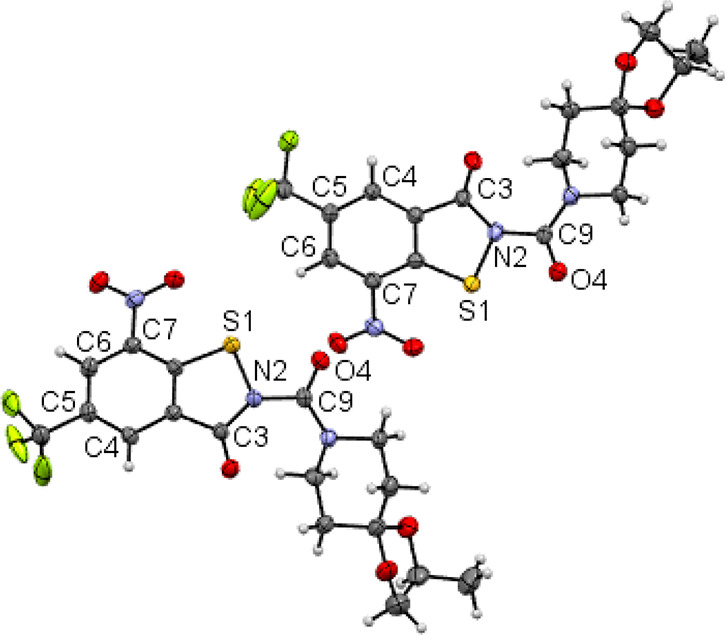
Asymmetric unit of the crystal structure of 4b. Displacement ellipsoid plots are drawn at the 50% probability level. Rotational disorder of the trifluoromethyl groups is omitted for clarity. Color scheme: C, gray; H, white; N, blue; O, red; S, yellow.
Figures 4 and 5 depict the molecular structures of 5a and 5b, respectively, in the crystal.36,37 The C3–N2 bond length of the benzothiazole heterocyclic system of 1.298(1) Å is significantly shorter by ca. 0.4 Å than that in 4a and 4b, consistent with C=N double bond character in 5a and 5b. The opposite enantiomeric conformer is also present in the centrosymmetric crystal structure. As for 1b(35) and 4b, the crystal structure of 5b comprises two crystallographically unique diastereomeric conformers.
Figure 4.
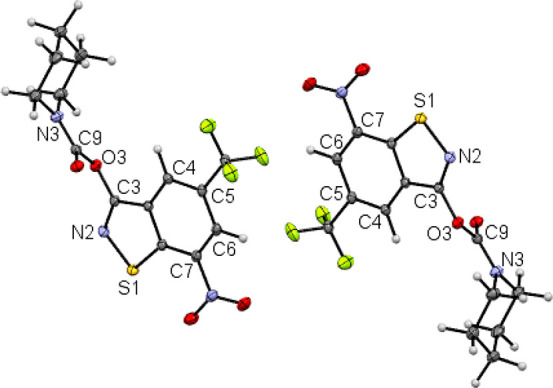
Section of the crystal structure of 5a, showing two molecules related by a crystallographic inversion center. Displacement ellipsoids are drawn at the 50% probability level. The crystal structure was refined with aspherical scattering factors using NoSpherA2.38 Color scheme: C, gray; H, white; N, blue; O, red; S, yellow.
Figure 5.
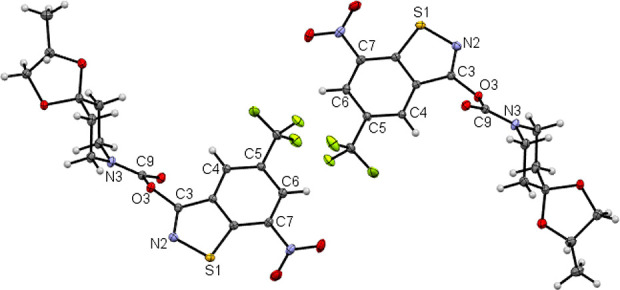
Asymmetric unit of the crystal structure of 5b. Displacement ellipsoids are drawn at the 50% probability level. Color scheme: C, gray; H, white; N, blue; O, red; S, yellow.
Compounds 4 and 5 were also structurally characterized by 1H and 13C NMR spectroscopy in solution at room temperature (see the Supporting Information). Figure 6 shows the aliphatic regions of the 13C NMR spectra in CDCl3, which exhibit the most marked differences for the compounds studied. The resonance signals in the aliphatic regions were assigned based on chemical shifts. With three signals, 4a shows the simplest spectrum in this region. The signals are assigned to the piperidine methylene groups. The occurrence of one set of signals for the α- and β-methylene groups indicates that rotation about the urea Ccarbonyl–Npiperidine bond is fast. The same was observed for BTZ 1a.20 In contrast, two signals are encountered for each of the α- and β-methylene groups in the spectrum of 5a, which can the explained by restricted rotation of the piperidine ring around the Ccarbonyl–Npiperidine bond. For 4b and 5b, the most upfield shifted signal is assigned to the methyl group of the methyldioxolan moiety. For 4b, two signals appear for the piperidine β-methylene groups, whereas the α-methylene carbon atoms are virtually isochronous, suggesting that there is no restricted rotation of the side chain around the Ccarbonyl–Npiperidine bond. For BTZ 1b, variable-temperature 13C NMR spectroscopy revealed a coalescence temperature Tc of 301 ± 1 K and a Gibbs free energy ΔG⧧ of 14.6 ± 1 kcal mol–1 for this process.35 The 13C NMR spectrum of 5b displays four discrete signals for each of the α- and β-piperidine methylene groups, which can be ascribed to diastereomeric conformers, as revealed by X-ray crystallography (Figure 5), resulting from restricted rotation of the side chain around both the carbamate Ccarbonyl–Npiperidine and CBIT–OBIT bonds. Restricted rotation around the CBIT–OBIT bond in 5a due to steric clashes between the carbamate group and benzisothiazolol moiety should result in stable enantiomeric conformers, which cannot be distinguished in the NMR spectrum. As shown in Figure 4, both enantiomeric conformers of 5a are present in the solid state.
Figure 6.
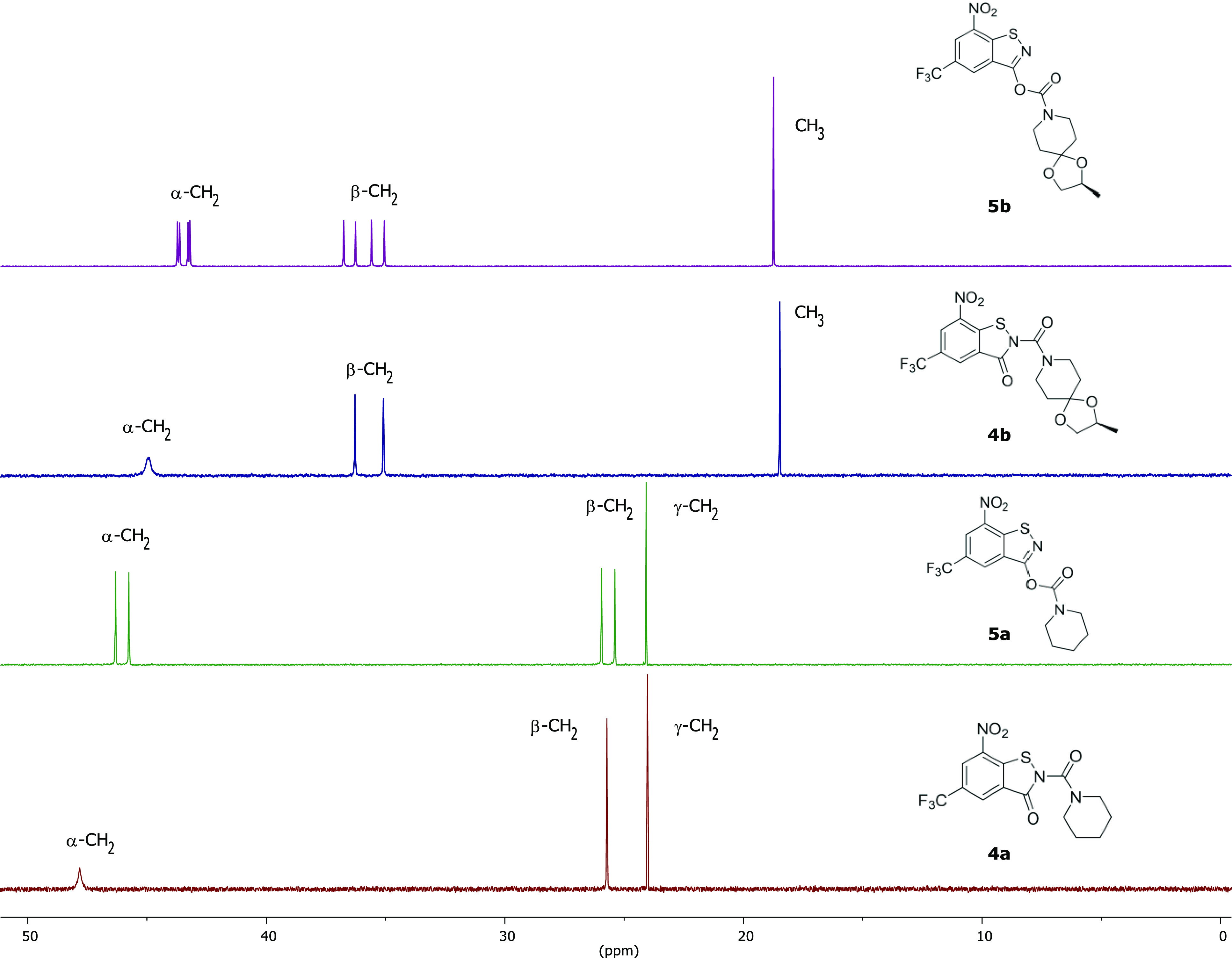
Aliphatic region of the 13C NMR spectra of 4a, 5a, 4b, and 5b in CDCl3 at room temperature (the full spectra are shown in the Supporting Information).
Antimycobacterial activities of 4a, 4b, 5a, and 5b and BTZs 1a and 1b as reference compounds were determined in vitro against Mycobacterium smegmatis, M. aurum, and M. tuberculosis (Table 1). M. smegmatis and M. tuberculosis are susceptible to irreversible inhibition of DprE1 through BTZs,10,39 because of the presence of a cysteine residue in the active site (vide supra). M. smegmatis is a fast-growing mycobacterium, generally considered nonpathogenic, and a useful model organism for M. tuberculosis.40 We found that the N-acyl BITs 4a and 4b with the side chain appended to the BIT nitrogen atom exert activity against the three mycobacterial strains. Interestingly, 4a is more active than BTZ 1a bearing the same piperidine side chain. A similar trend, however, is not observed for 4b and the corresponding BTZ 1b. The in vitro activity of 4b bearing the BTZ043-like side chain does not reach that of the 1b but is similar to that of 4a. The MIC values against the three mycobacterial strains are still some orders of magnitude higher for 4b than for 1b. The isomeric O-acyl benzisothiazol-3-ol derivatives 5a and 5b do not inhibit mycobacterial growth up to the concentrations tested. We propose that the carbamate group attached to the BIT oxygen atom (O3) and resulting altered hydrogen bond preferences account for the inactivity of 5a and 5b (vide infra). The BIT precursor 3, lacking a side chain, was tested in vitro against M. smegmatis and found to be inactive (MIC90 > 100 μM) as expected. The in vitro test results for 3, 5a, and 5b confirm that not only an electron-deficient nitroaromatic warhead but also the nature and spatial orientation of the side chain are critical for efficient mechanism-based mycobacterial growth inhibition. Kinetic solubilities of the compounds studied in PBS are essentially comparable with each other and with the two reference BTZs, and the c log P values are lower for the compounds bearing the BTZ043-like spiro ketal side chain than for those with an appended piperidine ring (Table 2). The c log P values of the active compounds 4a and 4b are higher than those of the corresponding inactive constitutional isomers 5a and 5b but do not exceed a value of 5, which is desirable for drug-like compounds.41
Table 1. In Vitro Activity (MIC90 in μM) of 4a, 4b, 5a, and 5b and the Two Reference BTZs 1a and 1b against M. smegmatis mc2 155, M. aurum DSM 43999 and M. tuberculosis H37Rv.
| M. smegmatis mc2 155a | M. aurum DSM 43999b | M. tuberculosis H37Rvb | |
|---|---|---|---|
| 1a | 6.25 | 25–12.5 | >4.0 |
| 1b (BTZ043) | 0.025 | 0.008 | <0.06 |
| 4a | 0.20 | 0.10 | 1.0 |
| 4b | 0.32 | 0.25–0.13 | 0.5 |
| 5a | >100 | >100 | >32 |
| 5b | >100 | >100 | >32 |
7H9 medium supplemented with ADS.
7H9 medium supplemented with OADC.
Table 2. Physicochemical Properties of 4a, 4b, 5a, and 5b, the Two Reference BTZs 1a and 1b, and the precursor3.
| solubility (μM)a | c log Pb | |
|---|---|---|
| 1a | 85 | 3.39 |
| 1b (BTZ043) | 74 | 2.45 |
| 3 | 444 | 1.23 |
| 4a | 92 | 4.14 |
| 4b | 103 | 3.47 |
| 5a | 60 | 3.75 |
| 5b | 39 | 2.81 |
Determined by nephelometry in PBS (pH 7.4) with 2% (v/v) DMSO.44
Calculated with Chem3D Ultra version 20.1.1.125.
These results raise the question of how the antimycobacterial properties of 4a and 4b compare with those of the structurally most related compounds. As reported previously, 4a and 4b exhibited higher in vitro activity against M. tuberculosis and M. aurum than the corresponding BIT 1-oxides.25 For the corresponding isosteric BTO constitutional isomers (vide supra), in vitro activity data against M. tuberculosis H37Rv can be found in the literature: 0.25 μg mL–1 (0.67 μM) for 6a(32) (medium unknown) and 0.24 μM (7H12 medium) and 0.23 μM (GAS medium) for 6b.33 Thus, 4a/4b and 6a/6b exhibit comparable in vitro activities against M. tuberculosis. Comparing the molecular electrostatic potential (MEP) mapped onto the van der Waals surface for 4a/4b versus 6a/6b reveals a striking similarity of the isosteric molecules (see the Supporting Information). The comparable antimycobacterial properties are consistent with these findings. The authors of 6a also reported the crystal structure of a related BTO (bearing a different side chain) in complex with M. tuberculosis DprE1 (PDB ID: 4PFA),32 which revealed a semimercaptal adduct formed between the nitroso form of the ligand and the Cys387 residue like for BTZs (vide supra). The authors of 6b proved by metabolic labeling of M. tuberculosis DprE1 and in vitro testing against M. aurum that the target for this BTO compound is DprE1, based on the assumption that M. aurum lacks a cysteine residue in the DprE1 active site42 [U. Möllmann, M. Miller, personal communication: In ref (33), the M. aurum strain SB66 from the collection of the Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute – (HKI, Jena, Germany) was used.].33
As shown in Table 2 below, the active BIT derivatives 4a and 4b have a similar low solubility as lead compound BTZ043 (≤100 μM), whereas the c log P values are slightly higher.
In the present work, we observed that BITs 4a/4b and BTZs 1a/1b inhibit growth of M. aurum DSM 43999, which is consistent with our previously reported results for 1a and 4a.25 In their report on the purported “BTZ-SO”,24 whose structure we revised to 4b (vide supra), Tiwari et al. described growth inhibition of M. aurum SB66 by “BTZ-SO”, but not by 1b (BTZ043). This also could be due to DprE1 sequence differences between the two M. aurum strains. We should also note that BITs are known to have various molecular targets in microorganisms.43
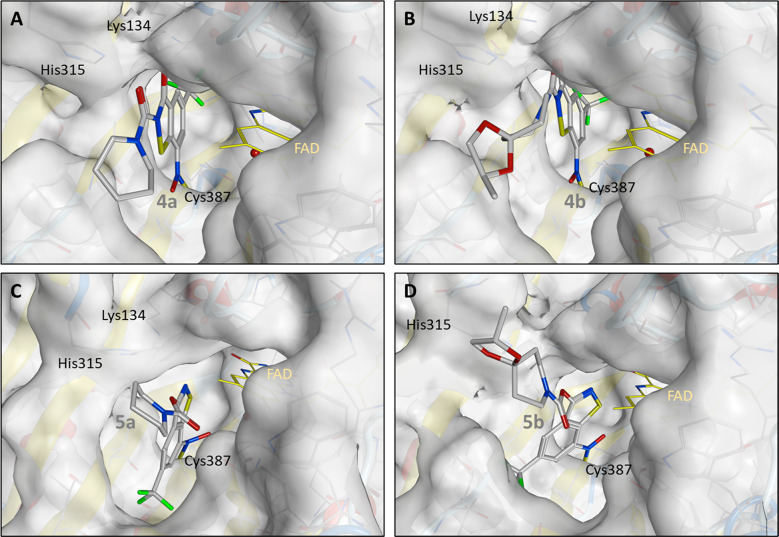
Molecular covalent docking of the newly synthesized compounds 4a, 4b, 5a, and 5b to M. tuberculosis DprE1 was performed to gain insight into possible binding modes. The docking pose of 1b corresponds to the orientation encountered in the crystal structure in complex with the M. tuberculosis DprE1 (PDB ID: 6HEZ)15 (see the Supporting Information). The binding mode of the active compounds 4a and 4b shows a comparable orientation in the active site for all highly evaluated docking poses. Therefore, it is reasonable to assume that the secondary interactions can be formed similarly to 1b. In comparison to 4b, the position of 4a changes slightly. This can be attributed to the fact that the bridged hydrogen bond, formed to FAD, which is apparent in the crystal structure of 1b in complex with DprE1 (chain B), is observed in docked solutions of 4b but not 4a. Stabilizing effects are, however, noticeable via hydrogen bonds to Lys134 and His315 for this compound. In particular, the possible formation of a hydrogen bond between the amino group of the Lys134 side chain and O3 of 4a is striking (Figure 7).
Figure 7.
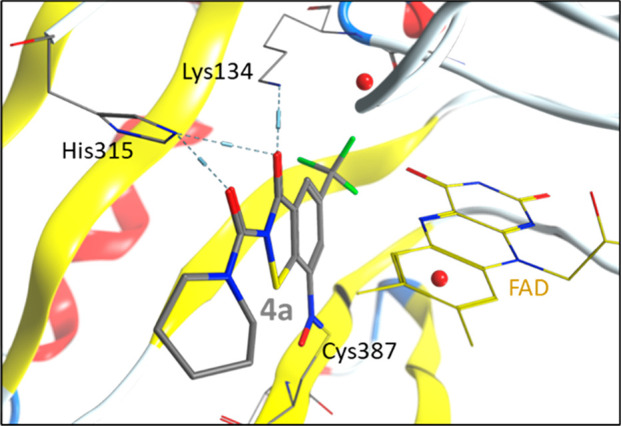
Highest ranked molecular covalent docking solution of 4a to M. tuberculosis DprE1.
The results for the inactive 5a and 5b are quite different. For these molecules, none of the in silico methods could provide a placement similar to that described above. All docking solutions for 5a and 5b resulted in a pose of the molecules rotated by nearly 180°. This is likely due to the fundamental change in the molecular structure with regard to the linkage of the piperidinoyl side chain to the molecular scaffold via the benzothiazole oxygen atom O3, which subsequently leads to steric hindrance in the DprE1 active site. In addition, the hydrogen bond observed in the crystal structure of 1b in complex with DprE1 can no longer be formed. All these indications rationalize the inactivity of 5a and 5b, which is confirmed by the total fitness scores of active 4a and 4b and inactive 5a and 5b (Table S1). The highest ranked solutions are shown in Figure 8.
Figure 8.
Highest ranked molecular docking solutions for (A) 4a, (B) 4b, (C) 5a, and (D) 5b (docked to 6HEZ.B; flexible side chains for Lys134, Trp230, Val365, Leu363, Lys418; toggled water molecules; solution number as in Table S2).
In summary, the N-acyl BITs 4 and O-acyl benzisothiazolols 5 were synthesized and their structures investigated by X-ray crystallography and NMR spectroscopy. The N-acyl BITs 4 exhibited only slightly poorer activity against M. smegmatis mc2 155a, M. aurum DSM 43999b and M. tuberculosis H37Rv than 1b (BTZ043) and significantly better activity than 1a, whereas 5 showed little or no antimycobacterial activity. Notably, 4a is more active by an order of magnitude than BTZ 1a bearing the same piperidine side chain. The in vitro test results for the BIT precursor 3 lacking a side chain, 5a and 5b confirm that not only an electron-deficient nitroaromatic warhead but also the nature and spatial orientation of the side group are critical for efficient mechanism-based mycobacterial growth inhibition. The inability of 5 to inhibit M. tuberculosis DprE1 was rationalized using in silico docking experiments on 4 and 5, which attributed this to the linkage of the piperidinoyl side chain to the molecular scaffold via the benzothiazole oxygen atom O3.
Acknowledgments
Professor Christian W. Lehmann is gratefully acknowledged for providing access to the X-ray diffraction facility. We would to thank Heike Schucht and Elke Dreher for technical assistance with the X-ray intensity data collections, Dr. Christian Ihling and Antje Herbrich-Peters for recording the HRMS spectra and Julia Seiser for the nephelometric measurements. Thanks are due to Dr. Jens-Ulrich Rahfeld and Dr. Nadine Taudte for providing and maintaining the biosafety level 2 facility. We also thank Lisa Niwinski (Borstel) for her expert technical assistance with the M. tuberculosis growth analyses under biosafety level 3 conditions.
Glossary
Abbreviations
- BTZ
8-nitro-1,3-benzothiazin-4-one
- BIT
benzisothiazolinone
- BTO
benzothiazole N-oxides
- DprE1
decaprenylphosphoryl-β-d-ribose 2′-epimerase
- mCPBA
m-chloroperoxybenzoic acid
- MEP
molecular electrostatic potential
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00215.
Experimental and computational details along with characterizations of the compounds synthesized (PDF)
DFT-calculated structure of 4a (XYZ)
DFT-calculated structure of 4b (XYZ)
DFT-calculated structure of 5a (XYZ)
DFT-calculated structure of 5b (XYZ)
DFT-calculated structure of 6a (XYZ)
DFT-calculated structure of 6b (XYZ)
Author Contributions
The manuscript was written through contributions of all authors.
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 432291016.
The authors declare no competing financial interest.
Dedication
Dedicated to Professor Bodo Dobner on the occasion of his 70th birthday.
Supplementary Material
References
- Dartois V. A.; Rubin E. J., Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nature Reviews Microbiology 2022, 10.1038/s41579-022-00731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2021.
- Koch A.; Cox H.; Mizrahi V. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Current Opinion in Pharmacology 2018, 42, 7–15. 10.1016/j.coph.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah I.; Poojari V.; Meshram H. Multi-Drug Resistant and Extensively-Drug Resistant Tuberculosis. The. Indian Journal of Pediatrics 2020, 87 (10), 833–839. 10.1007/s12098-020-03230-1. [DOI] [PubMed] [Google Scholar]
- Lange C.; Chesov D.; Heyckendorf J.; Leung C. C.; Udwadia Z.; Dheda K. Drug-resistant tuberculosis: An update on disease burden, diagnosis and treatment. Respirology 2018, 23 (7), 656–673. 10.1111/resp.13304. [DOI] [PubMed] [Google Scholar]
- Lange C.; Dheda K.; Chesov D.; Mandalakas A. M.; Udwadia Z.; Horsburgh C. R. Management of drug-resistant tuberculosis. Lancet 2019, 394 (10202), 953–966. 10.1016/S0140-6736(19)31882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetye G. S.; Franzblau S. G.; Cho S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Translational Research 2020, 220, 68–97. 10.1016/j.trsl.2020.03.007. [DOI] [PubMed] [Google Scholar]
- Chauhan A.; Kumar M.; Kumar A.; Kanchan K. Comprehensive review on mechanism of action, resistance and evolution of antimycobacterial drugs. Life Sciences 2021, 274, 119301. 10.1016/j.lfs.2021.119301. [DOI] [PubMed] [Google Scholar]
- Sharma A.; De Rosa M.; Singla N.; Singh G.; Barnwal R. P.; Pandey A. Tuberculosis: An Overview of the Immunogenic Response, Disease Progression, and Medicinal Chemistry Efforts in the Last Decade toward the Development of Potential Drugs for Extensively Drug-Resistant Tuberculosis Strains. J. Med. Chem. 2021, 64 (8), 4359–4395. 10.1021/acs.jmedchem.0c01833. [DOI] [PubMed] [Google Scholar]
- Makarov V.; Manina G.; Mikusova K.; Möllmann U.; Ryabova O.; Saint-Joanis B.; Dhar N.; Pasca M. R.; Buroni S.; Lucarelli A. P.; Milano A.; De Rossi E.; Belanova M.; Bobovska A.; Dianiskova P.; Kordulakova J.; Sala C.; Fullam E.; Schneider P.; McKinney J. D.; Brodin P.; Christophe T.; Waddell S.; Butcher P.; Albrethsen J.; Rosenkrands I.; Brosch R.; Nandi V.; Bharath S.; Gaonkar S.; Shandil R. K.; Balasubramanian V.; Balganesh T.; Tyagi S.; Grosset J.; Riccardi G.; Cole S. T. Benzothiazinones Kill Mycobacterium tuberculosis by Blocking Arabinan Synthesis. Science 2009, 324 (5928), 801–804. 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V.; Mikušová K. Development of Macozinone for TB treatment: An Update. Applied Sciences 2020, 10 (7), 2269. 10.3390/app10072269. [DOI] [Google Scholar]
- Gawad J.; Bonde C. Decaprenyl-phosphoryl-ribose 2′-epimerase (DprE1): challenging target for antitubercular drug discovery. Chemistry Central Journal 2018, 12 (1), 72. 10.1186/s13065-018-0441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale R. V.; Barmade M. A.; Murumkar P. R.; Yadav M. R. Overview of the Development of DprE1 Inhibitors for Combating the Menace of Tuberculosis. J. Med. Chem. 2018, 61 (19), 8563–8593. 10.1021/acs.jmedchem.8b00281. [DOI] [PubMed] [Google Scholar]
- Imran M..; A.S. A.; Thabet H. K..; Abida; Afroz Bakht M.. Synthetic molecules as DprE1 inhibitors: A patent review. Expert Opinion on Therapeutic Patents 2021, 31 (8), 759–772. 10.1080/13543776.2021.1902990. [DOI] [PubMed] [Google Scholar]
- Richter A.; Rudolph I.; Möllmann U.; Voigt K.; Chung C.-w.; Singh O. M. P.; Rees M.; Mendoza-Losana A.; Bates R.; Ballell L.; et al. Novel insight into the reaction of nitro, nitroso and hydroxylamino benzothiazinones and of benzoxacinones with Mycobacterium tuberculosis DprE1. Sci. Rep. 2018, 8 (1), 13473. 10.1038/s41598-018-31316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Sheng L.; Hegde P.; Li Y.; Aldrich C. C. 8-cyanobenzothiazinone analogs with potent antitubercular activity. Med. Chem. Res. 2021, 30, 449–458. 10.1007/s00044-020-02676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D.; Wang B.; Stelitano G.; Savková K.; Shi R.; Huszár S.; Han Q.; Mikušová K.; Chiarelli L. R.; Lu Y.; Qiao C. Structural and Activity Relationships of 6-Sulfonyl-8-Nitrobenzothiazinones as Antitubercular Agents. J. Med. Chem. 2021, 64 (19), 14526–14539. 10.1021/acs.jmedchem.1c01049. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Howe M.; Aldrich C. C. Spirocyclic and Bicyclic 8-Nitrobenzothiazinones for Tuberculosis with Improved Physicochemical and Pharmacokinetic Properties. ACS Med. Chem. Lett. 2019, 10 (3), 348–351. 10.1021/acsmedchemlett.8b00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C.; Ye T.-H.; Wang N.-Y.; Zeng X.-X.; Zhang L.-D.; Xiong Y.; You X.-Y.; Xia Y.; Xu Y.; Peng C.-T.; Zuo W.-Q.; Wei Y.; Yu L.-T. Synthesis and structure–activity relationships evaluation of benzothiazinone derivatives as potential anti-tubercular agents. Bioorg. Med. Chem. Lett. 2013, 23 (17), 4919–4922. 10.1016/j.bmcl.2013.06.069. [DOI] [PubMed] [Google Scholar]
- Richter A.; Narula G.; Rudolph I.; Seidel R. W.; Wagner C.; Av-Gay Y.; Imming P. Efficient Synthesis of Benzothiazinone Analogues with Activity against Intracellular Mycobacterium tuberculosis. ChemMedChem. 2022, 17, e202100733. 10.1002/cmdc.202100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R.; Möllmann U.; Cho S.; Franzblau S. G.; Miller P. A.; Miller M. J. Design and Syntheses of Anti-Tuberculosis Agents Inspired by BTZ043 Using a Scaffold Simplification Strategy. ACS Med. Chem. Lett. 2014, 5 (5), 587–591. 10.1021/ml500039g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss F.; Krchnak V.; Krchnakova A.; Schieferdecker S.; Dreisbach J.; Krone V.; Möllmann U.; Hoelscher M.; Miller M. J. In Vivo Dearomatization of the Potent Antituberculosis Agent BTZ043 via Meisenheimer Complex Formation. Angew. Chem., Int. Ed. 2017, 56 (8), 2187–2191. 10.1002/anie.201609737. [DOI] [PubMed] [Google Scholar]
- Tiwari R.; Moraski G. C.; Krchňák V.; Miller P. A.; Colon-Martinez M.; Herrero E.; Oliver A. G.; Miller M. J. Thiolates Chemically Induce Redox Activation of BTZ043 and Related Potent Nitroaromatic Anti-Tuberculosis Agents. J. Am. Chem. Soc. 2013, 135 (9), 3539–3549. 10.1021/ja311058q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R.; Miller P. A.; Cho S.; Franzblau S. G.; Miller M. J. Syntheses and Antituberculosis Activity of 1,3-Benzothiazinone Sulfoxide and Sulfone Derived from BTZ043. ACS Med. Chem. Lett. 2015, 6 (2), 128–133. 10.1021/ml5003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T.; Goddard R.; Lehmann C.; Richter A.; Sahile H. A.; Liu R.; Tiwari R.; Oliver A. G.; Miller M. J.; Seidel R. W.; Imming P. Crystallographic evidence for unintended benzisothiazolinone 1-oxide formation from benzothiazinones through oxidation. Acta Crystallographica Section C 2020, 76 (9), 907–913. 10.1107/S2053229620010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A.; Goddard R.; Schlegel T.; Imming P.; Seidel R. W. 2-Chloro-3-nitro-5-(trifluoromethyl)benzoic acid and -benzamide: structural characterization of two precursors for antitubercular benzothiazinones. Acta Crystallographica Section E 2021, 77 (2), 142–147. 10.1107/S2056989021000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakuni B. S.; Balkrishna S. J.; Kumar A.; Kumar S. An efficient copper mediated synthetic methodology for benzo[d]isothiazol-3(2H)-ones and related sulfur–nitrogen heterocycles. Tetrahedron Lett. 2012, 53 (11), 1354–1357. 10.1016/j.tetlet.2012.01.003. [DOI] [Google Scholar]
- Nishizawa A.; Takahira T.; Yasui K.; Fujimoto H.; Iwai T.; Sawamura M.; Chatani N.; Tobisu M. Nickel-Catalyzed Decarboxylation of Aryl Carbamates for Converting Phenols into Aromatic Amines. J. Am. Chem. Soc. 2019, 141 (18), 7261–7265. 10.1021/jacs.9b02751. [DOI] [PubMed] [Google Scholar]
- Liu D.; Tian Z.; Yan Z.; Wu L.; Ma Y.; Wang Q.; Liu W.; Zhou H.; Yang C. Design, synthesis and evaluation of 1,2-benzisothiazol-3-one derivatives as potent caspase-3 inhibitors. Biorg. Med. Chem. 2013, 21 (11), 2960–2967. 10.1016/j.bmc.2013.03.075. [DOI] [PubMed] [Google Scholar]
- Wu L.; Lu M.; Yan Z.; Tang X.; Sun B.; Liu W.; Zhou H.; Yang C. 1,2-Benzisothiazol-3-one derivatives as a novel class of small-molecule caspase-3 inhibitors. Biorg. Med. Chem. 2014, 22 (8), 2416–2426. 10.1016/j.bmc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Crystal data for 4a: C14H12.50F3N3O4.25S, Mr = 379.83, T = 100(2) K, λ = 1.54178 Å, triclinic, space group P1̅, a = 10.0915(4), b = 14.5439(5), c = 17.3813(6) Å, β = 78.721(2), γ = 80.711(2)°, V = 2377.82(15) Å3, Z = 6, ρcalc = 1.592 mg m–3, μ = 2.408 mm–1, F(000) = 1167, crystal size 0.133 × 0.073 × 0.043 mm3, θ range = 2.69–72.00°, reflections collected/unique = 88503/8735 (Rint = 0.0834), 750 parameters, 237 restraints, S = 1.073, R1 [I > 2σ(I)] = 0.0420, wR2 = 0.1499, Δρmax/Δρmin = 0.43/–0.89 e Å–3.
- Landge S.; Mullick A. B.; Nagalapur K.; Neres J.; Subbulakshmi V.; Murugan K.; Ghosh A.; Sadler C.; Fellows M. D.; Humnabadkar V.; Mahadevaswamy J.; Vachaspati P.; Sharma S.; Kaur P.; Mallya M.; Rudrapatna S.; Awasthy D.; Sambandamurthy V. K.; Pojer F.; Cole S. T.; Balganesh T. S.; Ugarkar B. G.; Balasubramanian V.; Bandodkar B. S.; Panda M.; Ramachandran V. Discovery of benzothiazoles as antimycobacterial agents: Synthesis, structure–activity relationships and binding studies with Mycobacterium tuberculosis decaprenylphosphoryl-β-d-ribose 2′-oxidase. Bioorg. Med. Chem. 2015, 23 (24), 7694–7710. 10.1016/j.bmc.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Liu R.; Markley L.; Miller P. A.; Franzblau S.; Shetye G.; Ma R.; Savková K.; Mikušová K.; Lee B. S.; Pethe K.; Moraski G. C.; Miller M. J. Hydride-induced Meisenheimer complex formation reflects activity of nitro aromatic anti-tuberculosis compounds. RSC Medicinal Chemistry 2021, 12 (1), 62–72. 10.1039/D0MD00390E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal data for 4b: C17H16F3N3O6S, Mr = 447.39, T = 100(2) K, λ = 1.54178 Å, monoclinic, space group P21, a = 13.4977(4), b = 9.0737(3), c = 15.2780(4) Å, β = 90.489(2)°, V = 1871.09(10) Å3, Z = 4, ρcalc = 1.588 mg m–3, μ = 2.212 mm–1, F(000) = 920, crystal size 0.161 × 0.062 × 0.051 mm3, θ range = 2.89–71.99°, reflections collected/unique = 58657/7075 (Rint = 0.0634), 600 parameters, 235 restraints, S = 1.099, R1 [I > 2σ(I)] = 0.0485, wR2 = 0.1309, Δρmax/Δρmin = 0.37/–0.29 e Å–3.
- Richter A.; Patzer M.; Goddard R.; Lingnau J. B.; Imming P.; Seidel R. W. Structural elucidation of the antitubercular benzothiazinone BTZ043: A combined X-ray, variable temperature NMR and DFT study. J. Mol. Struct. 2022, 1248, 131419. 10.1016/j.molstruc.2021.131419. [DOI] [Google Scholar]
- Crystal data for 5a: C14H12F3N3O4S, Mr = 375.33, T = 100(2) K, λ = 0.71073 Å, orthorhombic, space group Pbca, a = 16.8517(10), b = 7.6877(5), c = 23.4052(14) Å, V = 3032.2(3) Å3, Z = 8, ρcalc = 1.644 mg m–3, μ = 0.275 mm–1, F(000) = 1538.080, crystal size 0.094 × 0.034 × 0.031 mm3, θ range = 1.74–31.75°, reflections collected/unique = 96114/5114 (Rint = 0.0535), 274 parameters, S = 1.0508, R1 [I > 2σ(I)] = 0.0241, wR2 = 0.0466, Δρmax/Δρmin = 0.46/–0.44 e Å–3.
- Crystal data for 5b: C17H16F3N3O6S, Mr = 447.39, T = 100(2) K, λ = 0.71073 Å, monoclinic, space group P21, a = 7.2884(7), b = 17.5581(16), c = 14.3508(13) Å, β = 98.454(4)°, V = 1816.5(3) Å3, Z = 4, ρcalc = 1.636 mg m–3, μ = 0.252 mm–1, F(000) = 920, crystal size 0.130 × 0.051 × 0.041 mm3, θ range = 1.44–48.67°, reflections collected/unique = 434272/34730 (Rint = 0.0734), 543 parameters, 1 restraint, S = 1.008, R1 [I > 2σ(I)] = 0.0473, wR2 = 0.1191, Δρmax/Δρmin = 0.57/–0.69 e Å–3.
- Kleemiss F.; Dolomanov O. V.; Bodensteiner M.; Peyerimhoff N.; Midgley L.; Bourhis L. J.; Genoni A.; Malaspina L. A.; Jayatilaka D.; Spencer J. L.; White F.; Grundkötter-Stock B.; Steinhauer S.; Lentz D.; Puschmann H.; Grabowsky S. Accurate crystal structures and chemical properties from NoSpherA2. Chemical Science 2021, 12 (5), 1675–1692. 10.1039/D0SC05526C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neres J.; Pojer F.; Molteni E.; Chiarelli L. R.; Dhar N.; Boy-Röttger S.; Buroni S.; Fullam E.; Degiacomi G.; Lucarelli A. P.; et al. Structural Basis for Benzothiazinone-Mediated Killing of Mycobacterium tuberculosis. Science Translational Medicine 2012, 4 (150), 150ra121. 10.1126/scitranslmed.3004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T J. A. S.; J R.; Rajan A.; Shankar V. Features of the biochemistry of Mycobacterium smegmatis, as a possible model for Mycobacterium tuberculosis. Journal of Infection and Public Health 2020, 13 (9), 1255–1264. 10.1016/j.jiph.2020.06.023. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1PII of original article: S0169–409X(96)00423–1. The article was originally published in Advanced Drug Delivery Reviews 23 (1997) 3–25.1. Adv. Drug Delivery Rev. 2001, 46 (1), 3–26. 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Riccardi G.; Manina G.; Pasca M. R.. An effective new drug target for the treatment of tuberculosis. WO2009100731, 2009.
- Gopinath P.; Yadav R. K.; Shukla P. K.; Srivastava K.; Puri S. K.; Muraleedharan K. M. Broad spectrum anti-infective properties of benzisothiazolones and the parallels in their anti-bacterial and anti-fungal effects. Bioorg. Med. Chem. Lett. 2017, 27 (5), 1291–1295. 10.1016/j.bmcl.2017.01.027. [DOI] [PubMed] [Google Scholar]
- Hoelke B.; Gieringer S.; Arlt M.; Saal C. Comparison of Nephelometric, UV-Spectroscopic, and HPLC Methods for High-Throughput Determination of Aqueous Drug Solubility in Microtiter Plates. Anal. Chem. 2009, 81 (8), 3165–3172. 10.1021/ac9000089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.