Abstract
Low levels of vitamin C have been observed in patients with schizophrenia and psychosis, and vitamin C may affect the dopaminergic system. Likewise, antipsychotic medication modulates striatal dopamine D2 receptors. We measured vitamin C levels in 52 patients with first-episode psychoses (24 females, age 23.1 ± 5.2 years) and 57 matched HCs (20 females, age 22.7 ± 4.3 years) before and after 6 weeks where patients received aripiprazole monotherapy (mean dose 10.4 mg ± 4.8 mg). At baseline, patients displayed lower levels of vitamin C (57.4 ± 25.9 µM) than controls (72.7 ± 21.4 µM) (t = 3.4, P = .001). Baseline symptoms and vitamin C levels were not correlated. Higher baseline vitamin C levels were associated with more improvement in negative symptoms (n = 39, R2 = 0.20, F = 8.2, P = .007), but not with age, sex, or p-aripiprazole. Because negative symptoms are generally considered challenging to alleviate, a potential adjunctive effect of vitamin C on treatment response should be tested in future randomized clinical trials.
Keywords: Vitamin C, negative symptoms, first-episode psychoses
Introduction
Psychotic symptoms often lead to changes in lifestyle and eating habits, such as increased consumption of fat and refined carbohydrates (Saugo et al., 2020), which in turn may result in lower intake of minerals and vitamins (Aucoin et al., 2020). Psychosis is associated with increased dopamine turnover in striatum, where dopamine D2 receptors are mostly expressed and hypofunction of the dopaminergic system in other areas in the brain such as the prefrontal cortex (PFC) (Howes and Kapur, 2009). All marketed antipsychotics target the D2 receptor.
Vitamin C is a hydrophilic carbohydrate abundantly present in fruits and vegetables. Humans are unable to synthesize vitamin C and rely solely on dietary intake. In vivo, vitamin C mainly exists in the reduced form, ascorbic acid, and the oxidized form, dehydroascorbic acid. With a daily intake of 400 mg/d or more, a homeostatic state is reached with maximal steady-state concentrations of approximately 70 to 80 µmol/L (Lykkesfeldt et al., 2014). Vitamin C is an electron donor, and high concentrations of vitamin C in the CNS are required for the synthesis of monoamine neurotransmitters. Specifically, vitamin C is a co-factor in the synthesis of serotonin (Englard and Seifter, 1986) and in the transformation of dopamine to norepinephrine (Harrison and May, 2009). Finally, vitamin C has an antagonistic effect on the dopamine D1 and D2 receptors (Tolbert et al., 1992).
Accumulating evidence suggests an association between a poor diet and several psychiatric conditions (Adan et al., 2019), and patients with first-episode psychoses display several nutritional deficiencies, including vitamin C (Firth et al., 2018). A few studies have reported lower vitamin C levels in medicated patients with schizophrenia (Dadheech et al., 2006; Suprapaneni, 2007). In a preclinical study, the effect of haloperidol, risperidone, and clozapine on apomorphine-induced stereotypical movements in mice was enhanced by vitamin C in a dose-dependent manner (Deshpande et al., 2006). A small double-blind intervention study in schizophrenia patients showed that co-administration of vitamin C resulted in an improved response to antipsychotic medication (Dakhale et al., 2005). Whether vitamin C also potentiates the effect of the newer partial dopamine D2 receptor agonists such as aripiprazole, brexipiprazole, and cariprazine has not been investigated.
In the present longitudinal study, vitamin C levels were assessed in antipsychotic-naïve patients with first-episode psychosis (FEP) and a group of matched healthy controls (HCs). We hypothesized that patients at baseline would exhibit lower concentrations of vitamin C in plasma, which would be associated with increased symptoms. Furthermore, we expected that lower baseline vitamin C status in patients would be associated with a poorer effect of 6 weeks of treatment with a partial dopamine agonist, aripiprazole.
METHODS
Participants
The present data were collected between 2014 and 2019 as a part of the PECANS II project, a large multimodal study carried out in accordance with the Declaration of Helsinki and approved by the Committee on Biomedical Research Ethics (H-3-2013-149). All participants provided informed consent. The study design was a prospective 6-week follow-up cohort study, which included a total of 66 antipsychotic-naïve patients with FEP and 58 HC, matched on age, sex, and parental socioeconomic status. Patients were recruited from hospitals and outpatient mental health centers in the capital region in Denmark and were between 18 and 45 years of age; were legally competent; and fulfilled the diagnostic criteria of schizophrenia, persistent delusional disorder, acute and transient psychotic disorders, schizoaffective disorder, or other or unspecified non-organic psychotic disorders according to ICD-10 (or schizophrenia schizophreniform- or schizoaffective disorder according to DSM-IVR). The diagnosis was confirmed by a diagnostic interview (Schedules for Clinical Assessment in Neuropsychiatry) (Wing et al., 1990).
Exclusion criteria for patients were any prior exposure to antipsychotics, daily substance abuse or fulfilling the criteria of ongoing substance dependency according to ICD-10/DSM-IVR, recent treatment with antidepressant medication, patients involuntarily admitted or treated, pregnancy, or severe physical illness. Exclusion criteria for controls were first-degree relatives with psychotic symptoms, substance abuse or positive screening of drugs in urine sample, pregnancy, or severe physical illness.
HCs were recruited using internet advertisement (https://www.forsoegsperson.dk/) and matched on age (±2 years of age), gender, and parental socioeconomic status.
Assessments
Blood samples were acquired in the morning in fasting state at baseline and at 6 weeks follow-up. Blood tubes were kept on ice and immediately centrifuged for 2 minutes, after which the plasma was acidified by adding 10% meta-phosphoric acid containing 2 mM disodium ethylenediaminetetraacetic acid. After another short centrifugation, the samples were stored at −80°C. Time from phlebotomy to storage in the freezer was registered. Vitamin C and its oxidation ratio have been shown to be stable for at least 5 years under these conditions (Lykkesfeldt, 2012). After study completion, all samples were analyzed by means of high-performance liquid chromatography as described in detail elsewhere (Lykkesfeldt, 2000). Analyses were performed at the Department of Veterinary and Animal Sciences, Faculty of Health and Medical Science, University of Copenhagen, Denmark.
Information about use of cannabis, alcohol, and tobacco during the last month was obtained. Height and weight were measured, and body mass index (BMI) was calculated. After baseline examinations, patients received monotherapy with the antipsychotic compound aripiprazole in individual doses balancing clinical effect and side effects.
Psychopathology in patients was evaluated by trained raters using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Percentual change in PANSS was calculated (Obermeier et al., 2011). Depressive symptoms were assessed with the Calgary Depression Scale for Schizophrenia (Addington et al., 1993). Level of function was assessed for all participants using the Personal and Social Performance Scale (Morosini et al., 2000).
Statistics
Statistical analyses were performed using Statistical Package for the Social Sciences for Windows version 25 (SPSS Inc., Chicago, IL, USA). Normal distribution of the data was tested with Shapiro–Wilks test. Group comparisons were performed with independent sample t test or chi-squared test.
Multiple stepwise regression with total vitamin C as the dependent variable and group, age, gender, regular tobacco use (yes/no), BMI, fruit/vegetable consumption (multiple times per day/daily/less than daily), and vitamin C supplements (yes/no) as independent variables was performed. Repeated-measures ANOVA was used to examine the effect of time and group on vitamin C levels, and post hoc analyses included independent and paired t tests, which were also used for change in psychopathology and level of function. Relation between baseline vitamin C levels and psychopathology, and relation between p-aripiprazole and change in symptoms were examined with Spearman’s correlation coefficient. Multiple regressions were performed with percentual change in PANSS scores corrected for minimal score as the dependent variable and vitamin C, gender, age, and p-aripiprazole as independent variables.
RESULTS
Baseline
Vitamin C was obtained from 52 patients and 57 HCs. The groups were well matched, but patients had fewer years of education and more tobacco use (Table 1).
Table 1.
Demographic and clinical data
| FEP n = 52 |
HC n = 57 |
Statistics | |
|---|---|---|---|
| Age, y, mean (SD) | 23.0 (±5.2) | 22.7(±4.3) | t = 0.5, P = .655 |
| Gender (f/m) | 24/28 | 20/37 | χ² = 1.4, P = .240 |
| BMI | 24.9 (±6.3) | 23.1 (±2.3) | T = 1,8, P = .73 |
| Education, y | 11.5 (±2.0) | 13.7 (±2.0) | t = −4,0, P < .001 |
| Regular tobacco use (yes/no) | 22/30 | 12/45 | χ² = 5.7, P = .017 |
| Regular alcohol use (yes/no) | 38/14 | 49/8 | χ² = 2.8, P = .094 |
| Regular cannabis use (yes/no) | 6/46 | 2/55 | χ² = 2.6, P = .108 |
| Fruit and vegetable intakea | 14/18/18 | 37/12/8 | χ² = 14.6, P = .001 |
| Vitamin C supplementsb (yes/no) | 7/45 | 10/47 | χ² = 0.3 P = .557 |
| Total vitamin C level, μmol/L | 57.4 (±25.9) | 72,7 (±21.4) | t = 3.4, P = .001 |
| Vitamin C below/above medianc | 32/20 | 23/34 | χ² = 4.8, P = .027 |
| Dehydroscorbic acid, μmol/L | 1.27 (±1.2) | 0.95 (±1.6) | t = 1.0, P = .301 |
| Blood processing time, min | 6.9(±3.2) | 7.4(±3.2) | t = −1.4, P = .162 |
Bold values indicates significant group difference, P < .05.
Abbreviation: BMI, body mass index.
a Consumptions of fruit and vegetables: (multiple times pr. day/daily/less than daily).
b Counting multivitamin and/or vitamin C.
c Median for the whole sample was 69.5 μM.
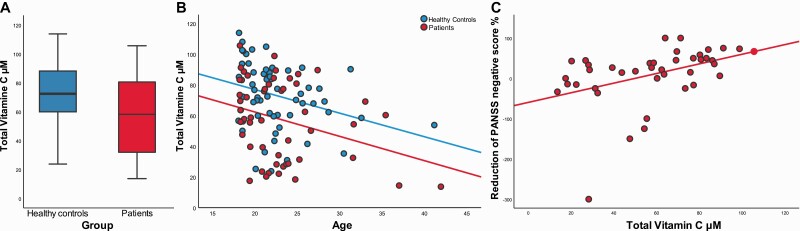
At baseline, patients had lower levels of vitamin C levels (t = 3.4, P = .001) (Figure 1A). Median vitamin C in the whole sample was 69.5 µM. A higher proportion of patients had vitamin C levels below the median split (62% vs 40% χ² = 4.8, P = .027). Multiple stepwise regression with total vitamin C as the dependent variable showed that total vitamin C was associated with group and age (R2 = 0.20, F = 12.5, P < .001). Group was the main predictor for vitamin C (R2 = 0.11, F = 13.2, P < .001) (Figure 1B). Variables removed (criteria P > .10) were gender (β = .144, t = 1.56, P = .12), tobacco use (β = .005, t = 0.55, P = .957), BMI (β = .063, t = .69, P = .49), and vitamin C supplements (β = .110, t = 1.23, P = .22), whereas variable not entered (criteria P < .05) was fruit/vegetable consumption (β = .17, t = 1.83, P = .07).
Figure 1.
(A) Boxplot of baseline vitamin C divided by group. (B) The relation between age and levels of vitamin C at baseline. (C) Correlation between baseline levels of vitamin C and percentage reduction in negative symptoms corrected for minimum score (baseline − 7) − (follow-up − 7) * 100/(baseline − 7).
Because of group difference in several independent variables (smoking, vitamin C supply, and fruit and vegetable intake), we additionally performed the regression without including group as an independent variable. In this model, vitamin C was associated with age and fruit and vegetable intake (R2 = 0.17, F = 10.5, P < .001), with age being the main predictor for vitamin C (R2 = 0.10, F = 11.6, P = .001) when group was excluded. Variables removed (criteria P > .10) were gender (β = .145, t = 1.54, P = .13), tobacco use (β = .024, t = .26, P = .79), BMI (β = .105, t = 1.15, P = .25), and vitamin C supplements (β = .09, t = .97, P = .33).
Because group was the main predictor, groupwise analyses were performed post hoc and showed that vitamin C levels in HCs were associated with gender and regular tobacco use (R2 = 0.20, F = 6.3, P = .003), but only associated with age in patients (R2 = 0.10, F = 4.9, P = .031). There was no group difference in processing time of the blood samples or in dehydroascorbic acid.
Follow-up
After 6 weeks, data on 39 patients and 55 HCs were obtained. Drop-out analyses showed no differences between the patients, who completed follow-up examinations and patients, who left the study (all P > .1). The patients who remained in the study received a mean dose of 10.3 (range: 2.5–25) mg aripiprazole and had a significant reduction on PANSS-total, -positive, -negative, and -general symptoms. Symptom improvement was not associated with aripiprazole dose or plasma aripiprazole levels. The treatment also resulted in a significant improvement in level of functioning (Table 2).
Table 2.
Data on patients at baseline and follow up
| Baseline n = 52 | 6-Week follow-up n = 39 | Statistics baseline follow-up |
|
|---|---|---|---|
| Total vitamin C μmol/L, mean (SD) | 57.4 (±25.9) | 58.2 (±22.7) | t = 0.7, P = .472 |
| PANSS total | 75.6 (±14.4) | 59.9 (±13.5) | t = 8.1, P < .001 |
| Positive | 18.6 (±4,4) | 13.8 (±4.4) | t = 9.4, P < .001 |
| Negative | 19.4 (±5,3) | 16.9 (±16.7) | t = 3.5, P = .001 |
| General | 37.6 (±7.5) | 29.3 (±7.1,) | t = 7.7, P < .001 |
| PSP | 48.3a (±14.2) | 56.9b (±12.5) | t = 4,5, P < .001 |
| CDSS | 8.3 (±3.9) | 4.6 (±4.4) | t = 4.1, P < .001 |
| BMI | 24.9 (±6.6) | 24.2(±6.0) | T = 0.8, P = .408 |
| Aripiprazole dose (mg/d) | 10.3c (2.5-25) | ||
| P-aripiprazole (ng/mL) | 303.3 (±235.9) | ||
| P-aripiprazole and dehydroaripiprazole (ng/mL) | 450.1(±128.8) |
Bold values indicates significant group difference, P < .05.
Abbreviations: BMI, body mass index; CDSS, Calgary Depression Scale for Schizophrenia; PANSS, Positive and Negative Symptom Scale; PSP, Personal and Social Performance scale.
a Consumptions of fruit and vegetables: (multiple times pr. day/daily/less than daily).
b Counting multivitamin and/or vitamin C.
c Median for the whole sample was 69.5 µM.
a: n = 51 b: n = 37 c: n = 34
Vitamin C levels were stable over 6 weeks in both patients and controls as a repeated-measure ANOVA showed a main effect of group (F = 13.9, P < .001), no effect of time (F = 0.821, P = .367), and no group × time interaction (F = 0.081, P = .777).
Vitamin C and Psychopathology
At baseline, PANSS and Calgary Depression Scale for Schizophrenia did not correlate with vitamin C levels (all P > .1). Multiple regression showed that improvement in negative symptoms was associated with higher baseline vitamin C (R2 = 0.17, F = 8.2, P = .007) but not with age, sex, or p-aripiprazole (illustrated as a correlation in Figure 1C). Explorative analyses showed that higher vitamin C was correlated with reductions in emotional withdrawal (N2), poor report (N3), passive-apathetic thinking (N4), and difficulty in abstract thinking (N5) (Spearman’s correlations, all P < .01). Analyses using PANSS positive, -general, and -total scores as independent variables were not significant (P > .05). Within patients, vitamin C levels did not correlate with aripiprazole dose or p-aripiprazole (P > .5).
Discussion
We found markedly lower vitamin C levels in this sample of antipsychotic-naive patients with FEP, but vitamin C levels did not correlate with severity of symptoms. Levels of vitamin C remained stable during 6 weeks of antipsychotic monotherapy, but symptoms improved during treatment and there was an association between higher level of plasma vitamin C and improvement in negative symptoms.
As hypothesized, our patients exhibited lower vitamin C levels than HCs also after adjusting for dietary habits and substance use. This group difference was present before antipsychotic treatment and was unaffected by aripiprazole, thereby corroborating previous findings (Dadheech et al., 2006; Suprapaneni, 2007). Patients reported less consumption of fruit and vegetables, which was found to be associated with lower baseline vitamin C levels. This indicates that inexpedient dietary habits and poorer nutritional status are present early in the disease and support that metabolic abnormalities are not solely related to antipsychotic treatment (Williamson et al., 2015; Saugo et al., 2020).
Different predictors for vitamin C were observed between the groups; among patients, age was the main explanatory factor, whereas gender and regular tobacco use were explanatory factors in the HC group. The observation that regular tobacco use is associated with lower vitamin C levels is well documented in a normal population (Lykkesfeldt et al., 2000). Because this was not the case in our patients, there may be other disease-related factors contributing to the vitamin C level in patients with psychosis. Nevertheless, we did not, as expected, find any association between vitamin C levels and symptom severity.
In our data, higher vitamin C levels were associated with improvement in negative symptoms. The levels of vitamin C remained stable over time in both groups, which reflected that participants did not undergo any dietary intervention. Primary negative symptoms and cognitive deficits observed in patients with schizophrenia have been linked to dopaminergic hypofunction in the PFC (Collo et al., 2020). Aripiprazole is a partial D2-receptor agonist hypothesized to exert an agonistic effect in PFC, where the endogen dopamine levels are reduced (Tuplin and Holahan, 2017), which may in turn improve negative symptoms. The current data could suggest that vitamin C enhances the agonistic effect on D2-receptors in PFC. Because aripiprazole also has affinity to other receptor systems, including 5-HT2A receptors (Tuplin and Holahan, 2017), further studies are needed to clarify the possible potentiating effect of vitamin C on negative symptoms.
Finally, it could be speculated that our findings represent the presence of a subgroup of patients with deficit schizophrenia characterized by primary and persistent negative symptoms (Carpenter et al., 1988). Thus, longstanding negative symptoms may underlie inexpedient dietary habits (Kirkpatrick et al., 2006) and in turn partly explain the poorer treatment response on negative symptoms often reported in these patients. Because our sample consisted of acutely ill and newly diagnosed psychotic patients, we were not able to identify a subgroup of patients with deficit syndrome according to the original definition (Carpenter et al., 1988). Future studies on stable patients with and without a high level of primary negative symptoms may be able to explore this.
In conclusion, antipsychotic-naïve patients with FEP exhibited lower vitamin C level than HCs. Although this was not associated with patients’ symptom severity, lower vitamin C levels were associated with more persistent negative symptoms after short-term treatment with a partial D2 agonist. Whether vitamin C potentiates the antipsychotic effect and supplementary vitamin C as adjuvant treatment may improve treatment effect should be further examined in future studies.
Acknowledgments
We thank Gitte Saltoft Andersen and Helle Schæbel for collecting blood samples and performing clinical assessments and Ricki Thanning for technical assistance with vitamin C measurements.
This work was supported by an independent grant from the Lundbeck Foundation (R155-2013-16337) to the Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS) to B.G.; support from the Mental Health Services, Capital Region of Denmark to B.G.; a grant from the Gangsted Foundation to B.G.; and Scholarship grant from the Lundbeck Foundation to A.M. The funding sources played no further role in the study design, collection, analysis, or interpretation of data, writing of the report, or the decision to submit the paper for publication.
Contributor Information
Anders N Myken, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark; Faculty of health and Medical Science, Department of Clinical Medicine, University of Copenhagen, Denmark.
Bjørn H Ebdrup, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark; Faculty of health and Medical Science, Department of Clinical Medicine, University of Copenhagen, Denmark.
Mikkel E Sørensen, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark.
Brian V Broberg, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark.
Martin W Skjerbæk, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark.
Birte Y Glenthøj, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark; Faculty of health and Medical Science, Department of Clinical Medicine, University of Copenhagen, Denmark.
Jens Lykkesfeldt, Faculty of Health and Medical Sciences, Department of Veterinary and Animal Sciences, University of Copenhagen, Denmark (Dr Lykkesfeldt).
Mette Ø Nielsen, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Denmark; Faculty of health and Medical Science, Department of Clinical Medicine, University of Copenhagen, Denmark.
Interest Statement
B.H.E. has received lecture fees and/or is part of the advisory boards of Bristol-Myers Squibb, Eli Lilly and Company, Janssen-Cilag, Otsuka Pharma Scandinavia AB, Takeda Pharmaceutical Company, Boehringer Ingelheim, and Lundbeck Pharma A/S. B.Y.G. is the leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), which is partially financed by an independent grant from the Lundbeck Foundation based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administrated by them. She has no other conflicts to disclose. J.L. is Director of the LifePharm Centre for In Vivo Pharmacology, which is partly funded by Novo Nordisk, and has received lecture fees and travel reimbursement from DSM. B.V.B. was employed at CNSR during study design and data collection but is now employed at Novo Nordisk. A.M. received a scholarship from the Lundbeck foundation. M.E.S., M.S., and M.Ø.N. declare no conflicts of interest.
References
- Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, Schellekens H, Dickson SL (2019) Nutritional psychiatry: towards improving mental health by what you eat. Eur Neuropsychopharmacol 29:1321–1332. [DOI] [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E (1993) Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry 22:39–44. [PubMed] [Google Scholar]
- Aucoin M, LaChance L, Cooley K, Kidd S (2020) Diet and psychosis: a scoping review. Neuropsychobiology 79:20–42. [DOI] [PubMed] [Google Scholar]
- Carpenter WT Jr, Heinrichs DW, Wagman AM (1988) Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 145:578–583. [DOI] [PubMed] [Google Scholar]
- Collo G, Mucci A, Giordano GM, Merlo Pich E, Galderisi S (2020) Negative symptoms of schizophrenia and dopaminergic transmission: translational models and perspectives opened by iPSC techniques. Front Neurosci 14:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadheech G, Mishra S, Gautam S, Sharma P (2006) Oxidative stress, alpha-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem 21:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakhale GN, Khanzode SD, Khanzode SS, Saoji A (2005) Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology 182:494–498. [DOI] [PubMed] [Google Scholar]
- Deshpande C, Dhir A, Kulkarni SK (2006) Antagonistic activity of ascorbic acid (vitamin C) on dopaminergic modulation: apomorphine-induced stereotypic behavior in mice. Pharmacology 77:38–45. [DOI] [PubMed] [Google Scholar]
- Englard S, Seifter S (1986) The biochemical functions of ascorbic acid. Annu Rev Nutr 6:365–406. [DOI] [PubMed] [Google Scholar]
- Firth J, Carney R, Stubbs B, Teasdale SB, Vancampfort D, Ward PB, Berk M, Sarris J (2018) Nutritional deficiencies and clinical correlates in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull 44:1275–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, May JM (2009) Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 46:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR (2006) The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkesfeldt J (2000) Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: reliable reduction with tris[2-carboxyethyl]phosphine hydrochloride. Anal Biochem 282:89–93. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J (2012) Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: validity of clinical data depends on vacutainer system used. Nutr Res 32:66–69. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN (2000) Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr 71:530–536. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Michels AJ, Frei B (2014) Vitamin C. Adv Nutr 5:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R (2000) Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 101:323–329. [PubMed] [Google Scholar]
- Obermeier M, Schennach-Wolff R, Meyer S, Möller HJ, Riedel M, Krause D, Seemüller F (2011) Is the PANSS used correctly? A systematic review. BMC Psychiatry 11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugo E, Lasalvia A, Bonetto C, Cristofalo D, Poli S, Bissoli S, Bertani M, Lazzarotto L, Gardellin F, Ceccato E, Pavanati M, Tosato S, Ruggeri M; GET UP Group (2020) Dietary habits and physical activity in first-episode psychosis patients treated in community services. Effect on early anthropometric and cardio-metabolic alterations. Schizophr Res 216:374–381. [DOI] [PubMed] [Google Scholar]
- Suprapaneni K (2007) Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in schizophrenia patients. J Clin Diagnostic Res 1:39–44. [PubMed] [Google Scholar]
- Tolbert LC, Morris PE Jr, Spollen JJ, Ashe SC (1992) Stereospecific effects of ascorbic acid and analogues on D1 and D2 agonist binding. Life Sci 51:921–930. [DOI] [PubMed] [Google Scholar]
- Tuplin EW, Holahan MR (2017) Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol 15:1192–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson K, Kilner K, Clibbens N (2015) A comparison of the nutrient intake of a community-dwelling first-episode psychosis cohort, aged 19-64 years, with data from the UK population. J Nutr Sci 4:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N (1990) SCAN. schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry 47:589–593. [DOI] [PubMed] [Google Scholar]