Abstract
Mechanical variables such as stiffness, stress, strain, and fluid shear stress are central to tissue functions, thus, must be maintained within the proper range. Mechanics are especially important in the cardiovascular system and lung, the functions of which are essentially mechanical. Mechanical homeostasis is characterized by negative feedback in which deviations from the optimal value or set point activates mechanisms to return the system to the correct range. In chronic diseases, homeostatic mechanisms are generally overcome or replaced with positive feedback loops that promote disease progression. Recent work has shown that microRNAs (miRNAs) are essential to mechanical homeostasis in a number of biological systems and that perturbations to miRNA biogenesis play key roles in cardiovascular and pulmonary diseases. In this review, we integrate current knowledge of miRNAs in mechanical homeostasis and how these mechanisms are altered in disease.
MECHANICAL HOMEOSTASIS
Homeostasis, a concept introduced in the early 1900s by Walter Cannon (Cannon 1926), posits that many biological variables such as blood pressure, body temperature, and plasma pH are maintained within a defined range or set point. This occurs through an active process in which the variable is first sensed, and deviations from the set point activate mechanisms to return the variable toward normal values (Modell et al. 2015). For example, elevated body temperature triggers dilation of surface blood vessels and sweating to mediate cooling, whereas low body temperature triggers contraction of surface blood vessels to conserve heat and, at lower temperatures, shivering to generate heat. Homeostasis is thus characterized by negative feedback in which a deviation sets in motion mechanisms to move the system back toward the initial state (Humphrey and Schwartz 2021).
Mechanical properties are important for the proper function of all tissues (Orr et al. 2006; Vogel and Sheetz 2006). This is especially important in the pulmonary and cardiovascular systems, whose main functions, pumping air or pumping blood, are fundamentally mechanical. Large arteries undergo elastic expansion during systole, which stores energy that is released during diastole; this mechanism dampens pulse pressure and maximizes blood delivery to the tissues (Humphrey and Schwartz 2021). In the lung, elasticity enables efficient expansion and contraction of alveoli during breathing (Tschumperlin et al. 2010). Increased stiffness and decreased elasticity in large arteries are both an early indicator and a contributing factor in progressive decline of cardiovascular function during aging or disease (Humphrey and Tellides 2019). Cardiac elasticity also aids pumping, and compromised elasticity in fibrosis contributes to heart failure. In the lung, decreased elasticity is strongly linked to declining function in fibrosis and emphysema (Tschumperlin et al. 2010). In fact, proper control of stiffness and elasticity is critical for normal function of most tissues, with changes in mechanics seen in prominent diseases of muscle, skin, and bone, among others (Orr et al. 2006).
Developing embryos show viscoelastic behaviors, with force-induced flow of the cells within tissues and solid-to-liquid phase transitions during morphogenesis (Petridou et al. 2019); however, the vast majority of adult tissues behave largely as solids, characterized by stiffness and elasticity (Humphrey et al. 2014). Stiffness is usually expressed as Young's modulus, or stress/strain, a measure of how far the material deforms under force (commonly expressed as pascals [Pa; 1 N/m2]).
Extracellular matrix (ECM) is the main determinant of tissue mechanics, at least in stiffer tissues. Very soft tissues such as brain or fat (<1 kPa) are largely cellular with relatively low fractions of ECM. Muscle, skin, and blood vessels are stiffer (on the order of 10 kPa) due to higher content of fibrillar collagens, elastin, and associated components. Scar tissue or cartilage, comprised mainly of ECM with a small fraction of cells, can be ∼100 kPa, and bone, consisting mainly of calcified collagen fibrils with a small fraction of cells, is on the order of megapascals (Humphrey et al. 2014).
It is now generally accepted that tissue mechanics, principally stiffness, is under homeostatic control. This concept is supported by the observation that, with a few exceptions, constituents of the cells and the ECM turn over many times during a human life, yet tissue stiffness remains relatively constant (Humphrey et al. 2014). Perturbations in mechanical stresses, at least over a modest range, trigger responses that restore tissue stresses. For example, increases or decreases in blood pressure trigger remodeling of artery walls to increase or decrease wall thickness and return stress (force/unit area) toward initial values (Humphrey and Schwartz 2021). Small injuries typically trigger a healing response in which excess collagen is initially deposited to serve as a scaffold for subsequent remodeling, transiently stiffening the tissue, but as healing proceeds, the collagen is cleared and normal tissue composition and organization are restored (Martin 1997). Liver can undergo substantial fibrosis secondary to chemical or viral injury, but if the injury is terminated, normal tissue architecture and stiffness are frequently restored, consistent with the impressive regenerative capacity of this tissue (Cordero-Espinoza and Huch 2018).
WHEN HOMEOSTASIS FAILS
More severe, chronic, or repetitive injury frequently leads not to recovery of normal tissue organization and stiffness but to scar formation, commonly termed fibrosis (Weiskirchen et al. 2019). Fibrotic tissue consists mainly of dense, aligned bundled collagen fibrils, sparsely populated by cells, that is substantially stiffer than the original tissue. Scarring is thought to be beneficial in that the damage is rapidly repaired to withstand infection or new insults, but the immediate benefit can be associated with deleterious effects in the long term.
This dichotomy is exemplified by events following myocardial infarction (MI) (Prabhu and Frangogiannis 2016; Lafuse et al. 2020). Blockage of a coronary artery leads to severe hypoxia and death of cardiac myocytes in the affected region. If the patient survives the immediate decline in heart function, a healing response is set in motion. Damage signals from the myocytes as well as hypoxia itself activates both cardiac fibroblasts and cells of the innate immune system (neutrophils, monocytes, and macrophages). Together, these cells degrade and phagocytose the dead cells and damaged ECM, and secrete a variety of inflammatory factors, fibrillar collagens, and other ECM components. Cytokines secreted by the immune cells, the most important of which is TGF-β, induce fibroblasts to differentiate into myofibroblasts that express smooth muscle actin and myosin and become strongly contractile. These cells both secrete abundant fibrillar collagens and mediate force-dependent reorganization of collagen fibrils into denser, stiffer networks. Rapid post-MI fibrosis is beneficial because it strengthens the tissue against the mechanical stresses of the beating heart. If it fails, fatal rupture of the heart wall is the result, usually 1- to 2-wk post-MI. Thus, efficient fibrosis is essential for survival. However, the stiffer, nonbeating fibrotic tissue impairs cardiac function. In severe cases, this stresses the remaining healthy heart tissue and leads to heart failure. In some less severe cases, abnormal strains within the boundary zone between the soft, beating tissue and the stiff, nonbeating scar result in fibrosis of this otherwise normal tissue (Rouillard and Holmes 2014). As that tissue stiffens, it creates a new boundary zone, which can lead to progressive expansion of the fibrotic zone and eventually heart failure.
Chronic, unresolved inflammation or injury of any tissue can result in fibrosis, which further impairs normal function and in severe cases results in organ failure (Weiskirchen et al. 2019). In most tissues (kidney, liver, heart, skin, etc.), scarring is secondary to tissue injury, infection, or inflammation. In such instances, fibrosis exacerbates organ dysfunction but does not initiate the problem. Idiopathic pulmonary fibrosis (IPF) is an exception in that fibrosis is the primary cause of tissue dysfunction (Wolters et al. 2014). IPF typically begins at the lung periphery and then invades centrally into the normal alveoli, eventually taking over most of the lung and leading to asphyxiation.
Blood vessels also become fibrotic under conditions of chronic inflammation. In atherosclerosis (which means stiffening of arteries), the increased stiffness itself has adverse consequences. Stiffness of the subendothelial ECM shifts endothelial gene expression profiles toward a more inflammatory phenotype (VanderBurgh and Reinhart-King 2018) and amplifies inflammatory responses of monocyte/macrophage cell types (Li et al. 2020). Increased stiffness of the vessel wall also leads to decreased energy storage during systole, which amplifies pulse pressure and increases damage to small vessels downstream (Humphrey and Tellides 2019). This effect also interferes with cardiac circulation. Arterial stiffness is thus one of several positive feedback loops that contribute to disease progression.
INFLAMMATION TIPS THE BALANCE BETWEEN HOMEOSTASIS AND FIBROSIS
The duration or intensity of inflammation is, in most cases, the variable that distinguishes normal healing from fibrosis (Martin 1997; Cordero-Espinoza and Huch 2018; Lafuse et al. 2020). In all cases, tissue cells sense damage or infection and express cytokines and cell-adhesion molecules that recruit and retain a variety of leukocytes. Early arriving neutrophils fight infection and clean up debris. Later arriving monocytes or resident tissue macrophages are activated to phagocytose debris and dead cells, release factors that recruit and activate fibroblasts, and begin building and strengthening the tissue. In the case of small wounds or minor damage, this process is relatively rapid and self-limiting. Macrophages switch from inflammation to repair, anti-inflammatory factors like IL-10 and TGF-β are produced, and inflammatory status decreases (i.e., is resolved). Fibroblasts differentiate to myofibroblasts under the influence of secreted inflammatory factors, hypoxia and TGF-β (Hinz 2016), and further condense and strengthen the wound by producing and contracting fibrillar collagens. As the tissue damage is repaired and normal tissue homeostasis is restored, immune cells and fibroblasts depart, deactivate, or die, leading to restoration of normal tissue architecture.
When the injury is more severe, infection arises and healing is delayed, and/or when underlying inflammatory conditions are present, scar rather than normal tissue is formed. The evolutionary logic is that rapid healing to prevent further injury or infection is more important than perfect restoration of tissue organization. Immune functions that deal with acute, potentially fatal threats are thus given priority (Kotas and Medzhitov 2015). Severe, chronic, or repeated insults that give rise to more severe inflammation thus generally lead to fibrosis and loss of homeostasis (Humphrey and Schwartz 2021).
REVERSIBLE VERSUS NONREVERSIBLE CHANGES IN TISSUE ARCHITECTURE
The distinction between normal healing and fibrosis is remarkably age- and species-dependent. In mice, amputation of a small portion of the left ventricular apex leads to regeneration in late embryos or early neonates, but this capacity is lost over the first week or so after birth; past this age, fibrosis irreversibly replaces the normal tissue (Uygur and Lee 2016; Vujic et al. 2020). By contrast, zebrafish and amphibians retain the ability to regenerate heart tissue during adulthood. Mechanistic explanations for these differences vary. One study reported that tissue stiffening in fibrosis is the key factor via activation of Notch signaling, which impedes regeneration (Notari et al. 2018). Inability of terminally differentiated cardiac myocytes to reenter the cell cycle is likely an important mechanism (Vujic et al. 2020). As discussed above, inhibiting inflammation blocks both regeneration and scarring, underscoring its dual role (Uygur and Lee 2016).
In contrast to heart, liver shows substantial ability to reverse at least moderate fibrosis and recover normal tissue architecture and function (Cordero-Espinoza and Huch 2018). Instances of this are seen clinically where fibrosis secondary to viral infection substantially reverses following eradication of the virus. This correlates with the greater proliferative capacity of hepatocytes, although the distinctly softer, nonbeating mechanical environment of the liver versus the heart is a potential contributor.
MicroRNAs IN MECHANICAL HOMEOSTASIS
We have established that tissues exhibit mechanical homeostasis in which deviations in mechanical properties from the set point activate pathways to return to the initial state. Evidence to date identifies microRNAs (miRNAs) as the critical mediators of this process. MiRNAs are small regulatory RNAs, usually 22 nucleotides long, that when bound in an active RNA-induced silencing complex (RISC), induce translational repression or degradation of target mRNAs (Ha and Kim 2014). Given that miRNAs are rapidly produced and among the longest-lived transcripts (Reichholf et al. 2019) and that a single miRNA can modulate hundreds of mRNA transcripts (Baek et al. 2008), they are well positioned to mediate the complex web of functions involved in tissue homeostasis.
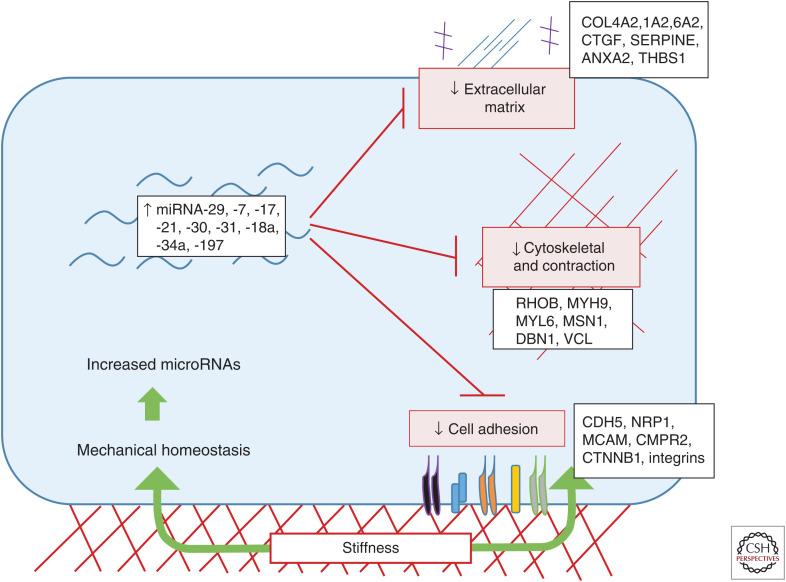
Support for this concept comes primarily from a study that investigated the role of miRNAs in endothelial cells cultured on tissue culture plastic, a substrate of pathological stiffness (Moro et al. 2019). They found that elevated substrate stiffness dramatically up-regulated >100 RISC-associated miRNAs whose collective function was to dampen expression or translation of cytoskeletal, contractile, adhesion, and matrix (CAM) genes (Moro et al. 2019). Remarkably, this set of miRNAs comprised nearly 60% of the total RISC-bound miRNA pool. Prominent miRNAs included miRNA-29, -7, -30, and the 17–92 miR cluster family members that play major roles in suppressing expression of fibrillar collagens, other ECM proteins, and critical pathways (e.g., proliferation and TGF-β) that contribute to fibrosis (Fig. 1). As these are the principal genes involved in tissue stiffening, the modulation by miRNAs had the characteristics of a negative feedback circuit that would oppose tissue stiffness. To test this notion in vivo, the authors showed that blocking miRNA processing by loss of Argonaute 2 (Ago2) or mutation of the miRNA target sequences in the 3′ untranslated regions (UTRs) of key target genes caused tissue stiffening, elevated contractility, and ECM deposition in zebrafish embryos. Another study demonstrating that tissue stiffness caused a general increase of miRNAs whose targets include focal adhesion proteins and the mechanosensitive Hippo pathway agree with these findings (Song et al. 2019). Together, these data lead to the conclusion that mechanical homeostasis is significantly mediated by miRNA regulatory networks.
Figure 1.
Mechanical homeostasis via microRNAs. Cellular sensing of matrix stiffness induces a network of microRNAs that dampen cytoskeletal, contractile, adhesion, and matrix (CAM) gene expression to restore physiological conditions. This negative feedback mechanism is central to mechanical homeostasis.
HOMEOSTATIC REGULATION OF FLUID SHEAR STRESS
A second form of mechanical homeostasis that implicate miRNAs involves endothelial cells and fluid shear stress, the frictional force from blood flow. Endothelial cells activate a wide range of pathways in response to shear stress (for reviews, see Zhou et al. 2014; Baeyens et al. 2016). One important consequence of shear stress sensing is adjustment of artery diameters to accommodate the required blood flow to the downstream tissues. Decreased vascular resistance in a tissue (due to growth or increased metabolic demand and vasodilation) increases flow through the feeding arteries, which then remodel to increase lumen diameter and bring shear stress back toward the initial range (for review, see Humphrey and Schwartz 2021). Endothelial cells have also been shown to encode a shear stress set point, at which specific pathways are maximally activated at different values (Baeyens et al. 2015). For example, the Smad1/5/8 pathway shows a peak of activation at physiological shear stress and induces genes that confer vascular stability (Baeyens et al. 2015). Notch, which also supports vascular stability, also peaks in the physiological range (Fang et al. 2017). By contrast, NF-κB, which induces inflammatory genes in endothelial cells, exhibits a maximum at both low and high shear stress values with a minimum at physiological shear stress, as befits a process that is central to remodeling (Baeyens et al. 2015). Smad2/3 signaling is activated with a sharp peak at low shear stress values and promotes artery inward remodeling, consistent with mechanical homeostasis (Deng et al. 2021).
FAILED HOMEOSTASIS
Failure of homeostasis is a hallmark of disease in general. Failure of mechanical homeostasis is a theme in a multitude of pathologies, most prominently in the cardiovascular system and the lung. Both hypertrophic and dilated cardiomyopathy are strongly, if not universally, associated with fibrosis (Dobaczewski et al. 2011). Just as negative feedback is the hallmark of homeostasis, positive feedback is a key aspect of disease, especially chronic, progressive disease. For example, stiffening of the heart walls impedes pumping, which further stresses cardiac myocytes, which release factors that stimulate fibroblasts, thus accelerating heart failure (Kong et al. 2014).
Positive feedback loops are highly evident at the cellular level. Stiff environments, both 2D and 3D, increase fibrosis via multiple mechanisms, including increased activation of TGF-β due to increased cellular contractility (for review, see Hinz 2015), increased cell responsiveness to TGF-β expression (Chambers et al. 2018), and assembly of ECMs that facilitate the transition of fibroblasts to myofibroblasts (Klingberg et al. 2018). An interesting feature of these effects includes slow time courses that have been characterized as mechanical memory. Prolonged exposure and growth of fibroblasts on very stiff tissue culture plastic or glass substrates not only promotes the transition to the myofibroblast phenotype (increased contractile state) but induces a long-lived fibrotic state that is not readily reversed upon transfer to a soft substratum (Balestrini et al. 2012). Durable profibrotic states have also been observed with fibroblasts isolated from stiff IPF tissue (IPF fibroblasts). IPF fibroblasts (as compared to fibroblasts isolated from soft elastic normal lung) show increased ECM expression and resistance to apoptosis even after multiple passages in culture, and, when implanted into mice, they also form fibrotic lesions in vivo (Ramos et al. 2001; Nho and Hergert 2014; Xia et al. 2014).
Long-lived miRNAs are well positioned to mediate mechanical memory. A study of mesenchymal stromal cells (MSCs) found that miRNA-21 was elevated on a pathologically stiff substratum and remained high after transfer to a softer substrate, which contributed to a persistent fibrotic state (Li et al. 2017). Elevated levels of miRNA-21 have been observed in fibrotic tissues from multiple sources including IPF specimens (for review, see Huang et al. 2015). Target genes for miRNA-21 include the tumor-suppressor phosphatase and tensin homolog (PTEN), which hydrolyzes PI3 lipids to inhibit Akt and other pathways, and SMAD7, which inhibits TGF-β signaling. MiRNA-21 suppression of PTEN is consistent with fibrosis progression, as this will lead to increased cell growth, migration, and invasion (Meng et al. 2007). In addition, miRNA-21-mediated suppression of SMAD7 will increase ECM production (McClelland et al. 2015).
MicroRNAs IN DISEASE
Changes in miRNAs in disease states have been extensively cataloged, including fibrotic disease of arteries, lungs, and elsewhere (Xin et al. 2014; Bagnato et al. 2017; Wojciechowska et al. 2017), although interpretations are less clear. The mechanical homeostasis concept predicts that miRNA regulation may be physiological but inadequate, meaning that miRNAs change in the right direction to oppose disease processes but are ineffective, or they may lead to pathology, meaning that homeostatic regulation is lost and miRNA changes contribute to disease processes. Reexamination of the literature provides instances of both.
Stiffness and Abnormal miRNA Regulation
Mechanical homeostasis dictates that elevated tissue stiffness will drive increased expression of homeostatic miRNA networks that limit expression of adhesion, contractile, and ECM genes. Consistent with this framework, miRNAs are generally up-regulated in scleroderma (Zhu et al. 2012), cardiac fibrosis (Thum et al. 2007; van Rooij et al. 2008), and liver fibrosis (Chen et al. 2017); in all these cases, miRNA-21 levels are increased. These effects can be interpreted as regulation that is physiological but presumably insufficient to prevent or reverse disease. By contrast, reported changes in miRNA levels in kidney disease are variable (Assmann et al. 2018). Changes in miR-29 levels also vary, decreasing in scleroderma, cardiac fibrosis, and liver-induced injury models but either increasing or decreasing in liver fibrosis (Bandyopadhyay et al. 2011; Hyun et al. 2014). These more complex changes may reflect the multiple disease processes involved, that is, fibrosis and stiffening is only one aspect of tissue dysregulation in kidney disease, or it could indicate that homeostatic miRNA mechanisms are disrupted.
Normal lung has a Young's modulus in the range of 1–2 kPa, which increases ∼10× in IPF lung (Booth et al. 2012; Sicard et al. 2018). Several studies reported that, in IPF tissue, ∼10% of miRNAs were deregulated and generally decreased, suggesting that homeostatic mechanisms were no longer operating (Pandit et al. 2010; Oak et al. 2011). For instance, miRNA-7, -17, -18a, -20a, -29, -30a, -106b, and -186 were increased with high stiffness in the mechanical homeostasis pathway described by Moro et al. (2019) but suppressed in IPF tissues. Similarly, miRNA-214 and -509 were suppressed on soft substrates in normal cells but elevated in IPF. Interestingly, miRNA-128, -31, and -324 were up-regulated on stiff substrates in normal cells and elevated in IPF, indicating that not all miRNAs are deregulated.
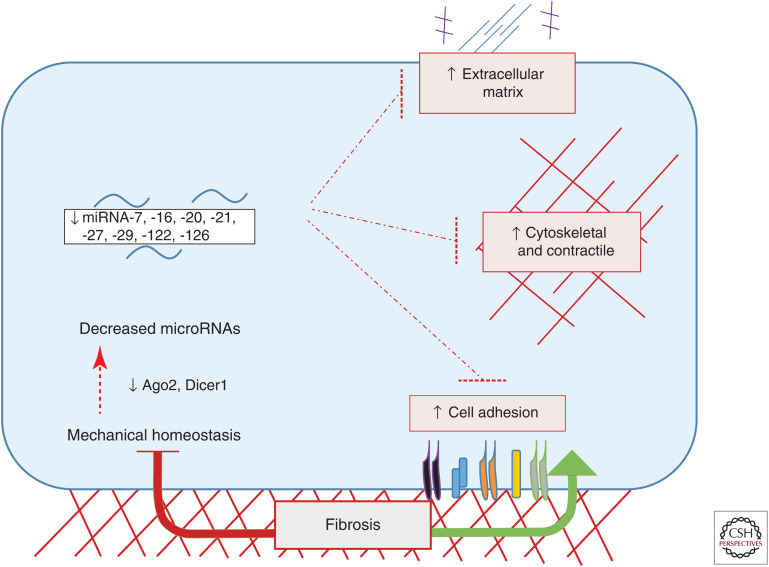
Ex vivo studies using decellularized human lung slices have provided important insights into these processes. These scaffolds maintain their initial mechanical properties (Booth et al. 2012; Herrera et al. 2018), consistent with the notion that the ECM is the main determinant of tissue mechanics (Humphrey et al. 2014). Culturing normal lung fibroblasts on decellularized IPF lung tissue (IPF-ECM) compared to normal lung ECM induced translation of ECM proteins typical of IPF tissue (Parker et al. 2014). This finding suggests the existence of positive feedback in which abnormal cells deposit ECM that further promote abnormal behavior. Interestingly, miRNA-29, which is among the genes most strongly induced by stiff substrates in other settings (Herrera et al. 2018; Moro et al. 2019), decreased on IPF-ECM (Parker et al. 2014; Herrera et al. 2018). This miRNA is a major suppressor of ECM synthesis (Peng et al. 2012), and thus is likely to be functionally important in this setting. These results imply that the miRNA-driven mechanism that mediates mechanical homeostasis in normal tissue does not operate in IPF and, further, that its blockade is encoded in the matrix. This study also shows that IPF-ECM reduced miRNA-29 via an miRNA-processing defect caused by suppressing expression of Dicer1, the enzyme that cleaves miRNA precursors to generate the mature species. They also confirmed reduced Dicer1 mRNA and protein levels in fibrotic lesions in IPF and demonstrate that Dicer1 depletion was sufficient to induce a profibrotic phenotype in fibroblasts cultured on normal decellularized lung (Herrera et al. 2018). Induction of lung fibrosis via global suppression of miRNA processing by Dicer1 depletion is consistent with the previously discussed results in zebrafish where global suppression of miRNA processing via depletion or mutation of Argonaute 2 induced tissue stiffening and hypercontractility (Moro et al. 2019). Overcoming mechanical homeostasis by blocking miRNA biogenesis pathways can thus contribute to fibrosis (Fig. 2).
Figure 2.
Loss of mechanical homeostasis in fibrosis due to defects in microRNA (miRNA) processing. Cellular responses to fibrotic (stiff) tissues fail to increase miRNAs that target cytoskeletal, contractile, adhesion, and matrix (CAM) genes, often due to the loss of key miRNA processing machinery such as Argonaute 2 (Ago2) or Dicer1. Loss of miRNA processing leads to decreased miRNA expression alleviating its repression on CAM genes to promote fibrosis progression.
Cell migration/invasion and survival are also important aspects of fibrotic disease. Fibroblasts and immune cells generally migrate into inflamed or injured tissue (Eming et al. 2014). Apoptosis of myofibroblasts is a component of the resolution phase that restores normal tissue architecture, and thus is a key feature of healthy healing, whereas their persistence is linked to fibrosis in multiple tissues (Hinz and Lagares 2020). The PI3 kinase (PI3K) pathway is a central regulator of all of these processes (Hers et al. 2011). ECM stiffness can promote PI3K signaling and stimulate all of these functions, which again involves miRNAs. In hepatocellular carcinoma cells, stiffness increases expression of miR-17-5p, which directly targets PTEN, the phosphatase that hydrolyzes PI3 lipids to terminate PI3K and Akt signaling (Gao et al. 2020). Reducing PTEN expression activates PI3K and Akt signaling to promote cell migration, invasion, and survival (Gao et al. 2020). Stiff ECM was also reported to increase miR-18a, another PTEN-targeting miRNA, to activate Akt signaling (Mouw et al. 2014). Decreased PTEN/elevated Akt signaling are an established hallmark of IPF pathogenesis (Xia et al. 2008; Mercer et al. 2016). Thus, loss of mechanical homeostasis has the capacity to drive disease in an miRNA-dependent manner.
IPF is spatially heterogenous, with stiff fibrotic tissue immediately next to regions of intact, soft alveoli, and the fibroblastic focus (FF; the hallmark lesion of IPF) situated at this interface (Raghu et al. 2018). The FF is a signature lesion of IPF compared to other instances of fibrosis, and is where activated fibroblasts invade the normal tissue to deposit ECM and expand the fibrotic region (Herrera et al. 2019). Efforts to model this active fibrotic front has led to the theory that tissue stiffness drives myofibroblast activation and ECM production in an organized and directional manner. However, atomic force microscopy micro-indentation has recently shown that the FF is nearly as soft as normal lung (Young's modulus of ∼2 kPa) (Fiore et al. 2018). Failure of mechanical homeostasis at the FF must therefore involve other factors. We speculate that a combination of other mechanical properties, such as strain, may be a contributing factor, as the spatial outside-in fibrotic remodeling characteristic of IPF was shown to be driven by elevated mechanical strain (Wu et al. 2020). Strain may also be under homeostatic control, although this concept is largely unexplored.
Shear Stress and miRNA Deregulation
Atherosclerosis is the principal disease of large- to medium-sized arteries, leading to formation of fibro-fatty lesions that in late stages restricts blood flow to affected tissues (Libby et al. 2019). In extreme cases, complete blockade of blood flow induces severe ischemia and tissue damage, which can be fatal in the heart (MI) or brain (stroke) or can lead to amputation of affected limbs (peripheral arterial disease). Plaques form at regions of arteries that experience low and disturbed fluid shear stress, that is, shear stress with changes in direction during the cardiac cycle (Zhou et al. 2014; Baeyens et al. 2016). These shear stress patterns render these regions susceptible to the systemic metabolic and inflammatory stimuli that drive disease, while artery regions that are under high unidirectional shear are protected.
Shear stress regulates a large number of miRNAs, including miRNA-29, -7, 10a, 19a, 21, -30a, and 34a, many of which are potentially involved in disease (Qin et al. 2010; Weber et al. 2010; Lee et al. 2017). Consistent with mechanical homeostasis, miRNA-10a, which targets the leukocyte adhesion receptor VCAM-1 and the critical proinflammatory transcription factor NF-κB, is up-regulated by atheroprotective, high laminar shear stress but decreased by disturbed shear stress in vitro (Lee et al. 2017) and in vivo (Fang et al. 2010). Disturbed shear stress was also shown to promote inflammation by enhancing maturation of miRNA-93 and miRNA-484, which target KLF2, a potent anti-inflammatory transcription factor (Gongol et al. 2019). Disturbed shear stress regulates these miRNAs via dephosphorylation of nucleolin, which facilitates processing of miRNA-93 and miRNA-484 precursors into their mature species. This process is reversed by physiological flow, implicating miRNA processing in mechanical homeostasis.
An underappreciated aspect of atherosclerosis is that shear stress homeostasis is compromised only at late stages. The initial intrusion of plaques into artery lumens is compensated by outward (so-called Glagov) remodeling that maintains lumen diameter; however, this mechanism fails in severe disease for reasons that are not well understood (Korshunov et al. 2007). However, the recent identification of Smad2/3 as key mediators of inward remodeling provides a likely answer (Deng et al. 2021). Smad2/3 in endothelial cells is activated downstream of inflammatory cytokines or inflammatory disturbed flow; by contrast, in healthy arteries, Smad2/3 is suppressed by Let-7 family miRNAs (Chen et al. 2012, 2015a). Let-7 members are also increased by physiological laminar shear stress (Qin et al. 2010). However, in diseased arteries, Let-7 miRNAs decrease and Smad2/3 activation increases, which strongly correlates with disease severity in human coronary arteries. These results suggest a mechanism by which inflammation activates the pathway that under normal conditions mediates artery inward remodeling, which correlates with failure of compensatory (Glagov) remodeling.
Maintenance or expansion of artery lumen diameter is strongly dependent on expression and activation of NOS3 (endothelial NOS or eNOS)-dependent production of nitric oxide (NO) (Luque Contreras et al. 2006). Physiological shear stress induces eNOS expression in part via changes in the levels of miRNAs that target eNOS directly or indirectly via its upstream inducer Klf2 (for review, see Boon et al. 2012). Conversely, regions of arteries under low/disturbed shear stress show decreased Klf2 and eNOS expression; this occurs in part via changes in levels of miR-93 and miR-484 (Gongol et al. 2019) and in part via induction of miRNA-92a, which targets KLF2 and KLF4 (Wu et al. 2015; Loyer et al. 2014; Chen et al. 2015b). In the partial carotid artery ligation model of disturbed shear stress in vivo, increased miRNA-712 promotes endothelial inflammation and barrier dysfunction by down-regulation of the metalloproteinase inhibitor TIMP3, which would increase the activity of matrix-degrading proteases (Son et al. 2013); the human counterpart of miRNA-712, miRNA-663, is also up-regulated by disturbed shear stress in vitro (Ni et al. 2011).
Under low flow, endothelial inflammatory activation and leukocyte recruitment is an essential component of normal vessel remodeling to recover normal shear stress and terminate the activated state; by contrast, disturbed flow fails to induce remodeling, instead inducing a permanently activated state (for review, see Baeyens et al. 2016; Chen et al. 2020). This state is by itself relatively benign, but in the presence of other risk factors such as high low-density lipoprotein (LDL) cholesterol and triglycerides, diabetes, oxidative stress, or elevated inflammatory status, these regions are susceptible to development of atherosclerotic plaque.
SUMMARY AND PERSPECTIVES
Mechanics is central to the function of the heart, arteries, and lung, and altered mechanics play a central role in diseases of these organs. The identification of an miRNA network that maintains tissue stiffness permits reinterpretation of extensive data from disease studies, leading to a model in which disruption of miRNA-mediated mechanical homeostasis by pathological factors is an essential step in disease. MiRNA dysregulation most often leads to fibrosis, which accompanies unresolved injury or inflammation in most tissues but is especially deleterious in the heart, arteries, and lung. Inflammation is a major contributor to failed homeostasis but other mechanisms have been observed, such as loss of Dicer1 expression in IPF. An interesting implication is that global inhibition of miRNA processing is profibrotic and thus occurs in disease states. Indeed, Dicer1 deletion in mice promotes fibrosis and dysfunction of kidney, heart, and other organs (da Costa Martins et al. 2008; Hajarnis et al. 2018). These results imply that methods to restore miRNA regulatory networks is a promising direction for treatment of fibrosis. Delivery methods for RNA therapeutics, in many cases targeting specific tissues, are developing rapidly and are of intense interest to the pharmaceutical/biotechnology industry (Jiang et al. 2017). Convergence of these two new research directions offers great opportunities for treating a class of currently intractable diseases.
ACKNOWLEDGMENTS
This was work was supported by NIH Grant R01 HL135582 to M.A.S. and by Wellcome Centre for Cell-Matrix Research (WCCMR; 203128/Z/16/Z) to J.A.H.
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.cshperspectives.org
REFERENCES
- Assmann TS, Recamonde-Mendoza M, de Souza BM, Bauer AC, Crispim D. 2018. MicroRNAs and diabetic kidney disease: systematic review and bioinformatic analysis. Mol Cell Endocrinol 477: 90–102. 10.1016/j.mce.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. 2008. The impact of microRNAs on protein output. Nature 455: 64–71. 10.1038/nature07242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL, et al. 2015. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife 4: e04645. 10.7554/eLife.04645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. 2016. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 126: 821–828. 10.1172/JCI83083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato G, Roberts WN, Roman J, Gangemi S. 2017. A systematic review of overlapping microRNA patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur Respir Rev 26: 160125. 10.1183/16000617.0125-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini JL, Chaudhry A, Sarrazy V, Koehler A, Hinz B. 2012. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 4: 410–421. 10.1039/c2ib00149g [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Friedman RC, Marquez RT, Keck K, Kong B, Icardi MS, Brown KE, Burge CB, Schmidt WN, Wang Y, et al. 2011. Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J Infect Dis 203: 1753–1762. 10.1093/infdis/jir186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon RA, Hergenreider E, Dimmeler S. 2012. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb Haemost 108: 616–620. 10.1160/TH12-07-0491 [DOI] [PubMed] [Google Scholar]
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthew SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. 2012. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876. 10.1164/rccm.201204-0754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. 1926. Physiological regulation of normal states: some tentative postulates concerning biological homeostatics, p. 91. Les Éditions Médicales, Paris. [Google Scholar]
- Chambers DM, Moretti L, Zhang JJ, Cooper SW, Chambers DM, Santangelo PJ, Barker TH. 2018. LEM domain-containing protein 3 antagonizes TGFβ-SMAD2/3 signaling in a stiffness-dependent manner in both the nucleus and cytosol. J Biol Chem 293: 15867–15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, et al. 2012. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep 2: 1684–1696. 10.1016/j.celrep.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. 2015a. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125: 4514–4528. 10.1172/JCI82719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wen L, Martin M, Hsu CY, Fang L, Lin FM, Lin TY, Geary MJ, Geary GG, Zhao Y, et al. 2015b. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation 131: 805–814. 10.1161/CIRCULATIONAHA.114.013675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhao W, Yang A, Xu A, Wang H, Cong M, Liu T, Wang P, You H. 2017. Integrated analysis of microRNA and gene expression profiles reveals a functional regulatory module associated with liver fibrosis. Gene 636: 87–95. [DOI] [PubMed] [Google Scholar]
- Chen PY, Schwartz MA, Simons M. 2020. Endothelial-to-mesenchymal transition, vascular inflammation, and atherosclerosis. Front Cardiovasc Med 7: 53. 10.3389/fcvm.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Espinoza L, Huch M. 2018. The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest 128: 85–96. 10.1172/JCI93562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, Molkentin JD, De Windt LJ. 2008. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 118: 1567–1576. 10.1161/CIRCULATIONAHA.108.769984 [DOI] [PubMed] [Google Scholar]
- Deng H, Min E, Baeyens N, Coon BG, Hu R, Zhuang ZW, Chen M, Huang B, Afolabi T, Zarkada G, et al. 2021. Activation of Smad 2/3 signaling by low fluid shear stress mediates artery inward remodeling. Proc Natl Acad Sci 118: e2105339118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Chen W, Frangogiannis NG. 2011. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 51: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6: 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Shi C, Manduchi E. Civelek M, Davies PF. 2010. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci 107: 13450–13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, Chittenden TW, Burt JM, Schwartz MA, Hirschi KK. 2017. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun 8: 2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore VF, Wong SS, Tran C, Tan C, Xu W, Sulchek T, White ES, Hagood JS, Barker TH. 2018. αvβ3 integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 3: e97597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Qiao X, Xing X, Huang J, Qian J, Wang Y, Zhang Y, Zhang X, Li M, Cui J, et al. 2020. Matrix stiffness-upregulated microRNA-17-5p attenuates the intervention effects of metformin on HCC invasion and metastasis by targeting the PTEN/PI3K/Akt pathway. Front Oncol 10: 1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongol B, Marin T, Zhang J, Wang SC, Sun W, He M, Chen S, Chen L, Li J, Liu JH, et al. 2019. Shear stress regulation of miR-93 and miR-484 maturation through nucleolin. Proc Natl Acad Sci 116: 12974–12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Kim VN. 2014. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524. [DOI] [PubMed] [Google Scholar]
- Hajarnis S, Yheskel M, Williams D, Brefort T, Glaudemans B, Debaix H, Baum M, Devuyst O, Patel V. 2018. Suppression of microRNA activity in kidney collecting ducts induces partial loss of epithelial phenotype and renal fibrosis. J Am Soc Nephrol 29: 518–531. 10.1681/ASN.2017030334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J, Beisang DJ, Peterson M, Forster C, Gilbertsen A, Benyumov A, Smith K, Korenczuk CE, Borocas VH, Guenther K, et al. 2018. Dicer1 deficiency in the idiopathic pulmonary fibrosis fibroblastic focus promotes fibrosis by suppressing microRNA biogenesis. Am J Respir Crit Care Med 198: 486–496. 10.1164/rccm.201709-1823OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J, Forster C, Pengo T, Montero A, Swift J, Schwartz MA, Henke CA, Bitterman PB. 2019. Registration of the extracellular matrix components constituting the fibroblastic focus in idiopathic pulmonary fibrosis. JCI Insight 4: e125185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. 2011. Akt signalling in health and disease. Cell Signal 23: 1515–1527. [DOI] [PubMed] [Google Scholar]
- Hinz B. 2015. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 47: 54–65. [DOI] [PubMed] [Google Scholar]
- Hinz B. 2016. Myofibroblasts. Exp Eye Res 142: 56–70. [DOI] [PubMed] [Google Scholar]
- Hinz B, Lagares D. 2020. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat Rev Rheumatol 16: 11–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, He Y, Li J. 2015. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr Pharm Des 21: 2236–2242. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Schwartz MA. 2021. Vascular mechanobiology: homeostasis, adaptation, and disease. Ann Rev Biomed Eng 23: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Tellides G. 2019. Central artery stiffness and thoracic aortopathy. Am J Physiol Heart Circ Physiol 316: H169–H182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER, Schwartz MA. 2014. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J, Choi SS, Diehl AM, Jung Y. 2014. Potential role of Hedgehog signaling and microRNA-29 in liver fibrosis of IKKβ-deficient mouse. J Mol Histol 45: 103–112. 10.1007/s10735-013-9532-5 [DOI] [PubMed] [Google Scholar]
- Jiang L, Vader P, Schiffelers RM. 2017. Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Ther 24: 157–166. [DOI] [PubMed] [Google Scholar]
- Klingberg F, Chau G, Walraven M, Boo S, Koehler A, Chow ML, Olson AL, Im M, Lodyga M, Wells RG, et al. 2018. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J Cell Sci 131: jcs201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong P, Christia P, Frangogiannis NG. 2014. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71: 549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA, Schwartz SM, Berk BC. 2007. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol 27: 1722–1728. [DOI] [PubMed] [Google Scholar]
- Kotas ME, Medzhitov R. 2015. Homeostasis, inflammation, and disease susceptibility. Cell 160: 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuse WP, Wozniak DJ, Rajaram MVS. 2020. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Lin TE, Lee CI, Zhou J, Huang YH, Lee PL, Shih YT, Chien S, Chiu JJ. 2017. MicroRNA-10a is crucial for endothelial response to different flow patterns via interaction of retinoid acid receptors and histone deacetylases. Proc Natl Acad Sci 114: 2072–2077. 10.1073/pnas.1621425114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz B. 2017. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16: 379–389. [DOI] [PubMed] [Google Scholar]
- Li J, Wang S, Li Y, Zhang N, Gribskov M, Zhang X, Lin M, Shao D, Zhang C, Dai L, et al. 2020. miRNA-mediated macrophage behaviors responding to matrix stiffness and ox-LDL. J Cell Physiol 235: 6139–6153. [DOI] [PubMed] [Google Scholar]
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Sommer Bittencourt M, Tokgozoglu L, Lewis EF. 2019. Atherosclerosis. Nat Rev Dis Primers 5: 56. [DOI] [PubMed] [Google Scholar]
- Loyer X, Potteaux S, Vion AC, Guérin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, et al. 2014. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res 114: 434–443. 10.1161/CIRCRESAHA.114.302213 [DOI] [PubMed] [Google Scholar]
- Luque Contreras D, Vargas Robles H, Romo E, Rios A, Escalante B. 2006. The role of nitric oxide in the post-ischemic revascularization process. Pharmacol Ther 112: 553–563. [DOI] [PubMed] [Google Scholar]
- Martin P. 1997. Wound healing—aiming for perfect skin regeneration. Science 276: 75–81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. 2015. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 129: 1237–1249. [DOI] [PubMed] [Google Scholar]
- Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. 2007. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, Franklin L, Nathakuman CB, Man Y, Genovese F, et al. 2016. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 71: 701–711. 10.1136/thoraxjnl-2015-207429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell H, Cliff W, Michael J, McFarland J, Wenderoth MP, Wright A. 2015. A physiologist's view of homeostasis. Adv Physiol Educ 39: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro A, Discroll T, Boraas LC, Armero W, Kasper DM, Baeyens N, Jouy C, Mallikarjun V, Swift J, Ahn SJ, et al. 2019. MicroRNA-dependent regulation of biomechanical genes establishes tissue stiffness homeostasis. Nat Cell Biol 21: 348–358. 10.1038/s41556-019-0272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, et al. 2014. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med 20: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nho RS, Hergert P. 2014. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS ONE 9: e94616. 10.1371/journal.pone.0094616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni CW, Qiu H, Jo H. 2011. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol 300: H1762–H1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari M, Ventura-Rubio A, Bedford-Guaus SJ, Jorba I, Mulero L, Navajas D, Marti M, Raya A. 2018. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci Adv 4: eaao5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Towes GB, Knight D, et al. 2011. A microRNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS ONE 6: e21253. 10.1371/journal.pone.0021253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. 2006. Mechanisms of mechanotransduction. Dev Cell 10: 11–20. 10.1016/j.devcel.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, et al. 2010. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182: 220–229. 10.1164/rccm.200911-1698OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. 2014. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635. 10.1172/JCI71386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng WJ, Tao JH, Mei B, Chen B, Li BZ, Yang GJ, Zhang Q, Yao H, Wang BX, He Q, et al. 2012. MicroRNA-29: a potential therapeutic target for systemic sclerosis. Expert Opin Ther Targets 16: 875–879. [DOI] [PubMed] [Google Scholar]
- Petridou NI, Grigolon S, Salbreux G, Hannezo E, Heisenberg CP. 2019. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat Cell Biol 21: 169–178. [DOI] [PubMed] [Google Scholar]
- Prabhu SD, Frangogiannis NG. 2016. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112. 10.1161/CIRCRESAHA.116.303577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YSH, Chien S, Wang N. 2010. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci 107: 3240–3244. 10.1073/pnas.0914882107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, et al. 2018. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- Ramos C, Montaño M, Garcı´a-Alavarez J, Ruiz V, Uhal BD, Selman M, Pardo A. 2001. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24: 591–598. 10.1165/ajrcmb.24.5.4333 [DOI] [PubMed] [Google Scholar]
- Reichholf B, Herzog VA, Fasching N, Manenreither RA, Sowemimo I, Ameres SL. 2019. Time-resolved small RNA sequencing unravels the molecular principles of microRNA homeostasis. Mol Cell 75: 756–768.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillard AD, Holmes JW. 2014. Coupled agent-based and finite-element models for predicting scar structure following myocardial infarction. Prog Biophys Mol Biol 115: 235–243. [DOI] [PubMed] [Google Scholar]
- Sicard D, Haak AJ, Choi KM, Craig AR, Fredenburgh LE, Tschumperlin DJ. 2018. Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol 314: L946–L955. 10.1152/ajplung.00415.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Kang SW, et al. 2013. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun 4: 3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Sun Z, Chen G, Shang P, You G, Zhao J, Liu S, Han D, Zhou H. 2019. Matrix stiffening induces endothelial dysfunction via the TRPV4/microRNA-6740/endothelin-1 mechanotransduction pathway. Acta Biomater 100: 52–60. [DOI] [PubMed] [Google Scholar]
- Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, et al. 2007. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116: 258–267. 10.1161/CIRCULATIONAHA.107.687947 [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Boudreault F, Liu F. 2010. Recent advances and new opportunities in lung mechanobiology. J Biomech 43: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygur A, Lee RT. 2016. Mechanisms of cardiac regeneration. Dev Cell 36: 362–374. 10.1016/j.devcel.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderBurgh JA, Reinhart-King CA. 2018. The role of age-related intimal remodeling and stiffening in atherosclerosis. Adv Pharmacol 81: 365–391. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. 2008. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci 105: 13027–13032. 10.1073/pnas.0805038105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. 2006. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7: 265–275. 10.1038/nrm1890 [DOI] [PubMed] [Google Scholar]
- Vujic A, Natarajan N, Lee RT. 2020. Molecular mechanisms of heart regeneration. Semin Cell Dev Biol 100: 20–28. 10.1016/j.semcdb.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Baker MB, Moore JP, Searles CD. 2010. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun 393: 643–648. 10.1016/j.bbrc.2010.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowska A, Braniewska A, Kozar-Kamińska K. 2017. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med 26: 865–874. 10.17219/acem/62915 [DOI] [PubMed] [Google Scholar]
- Wolters PJ, Collard HR, Jones KD. 2014. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Huang RT, Kuo CH, Kuman S, Kim CW, Lin YC, Chen YJ, Birukova A, Birukov KG, Dulin NO, et al. 2015. Mechanosensitive PPAP2B regulates endothelial responses to atherorelevant hemodynamic forces. Circ Res 117: e41–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, Shi M, Zhao X, Yuan J, Li J, et al. 2020. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 180: 107–121.e17. [DOI] [PubMed] [Google Scholar]
- Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, et al. 2008. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 205: 1659–1672. 10.1084/jem.20080001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Bodempudi V, Benyumov A, Hergert P, Tank D, Herrera J, Braziunas J, Larsson O, Parker M, Rossi D, et al. 2014. Identification of a cell-of-origin for fibroblasts comprising the fibrotic reticulum in idiopathic pulmonary fibrosis. Am J Pathol 184: 1369–1383. 10.1016/j.ajpath.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Zhang Y, Liu X, Xin H, Cao Y, Geng M. 2014. MicroRNA in hepatic fibrosis and cirrhosis. Front Biosci (Landmark Ed) 19: 1418–1424. 10.2741/4292 [DOI] [PubMed] [Google Scholar]
- Zhou J, Li YS, Chien S. 2014. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34: 2191–2198. 10.1161/ATVBAHA.114.303422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Li Y, Qu S, Loo H, Zhou Y, Wang Y, Zhao H, You Y, Xiao X, Zuo X. 2012. MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol 32: 514–522. [DOI] [PubMed] [Google Scholar]