Abstract
The European Association for the Study of the Liver has recently published updated guidelines on the use of non-invasive tests to identify and stratify chronic liver disease. Here, we provide a summary of the key recommendations from the guideline.
Keywords: non-alcoholic steatohepatitis, screening, hepatic fibrosis, alcoholic liver disease, liver function test
Key points.
Two-step risk stratification with non-invasive tests (NITs) of patients with risk factors for liver disease in the general population setting has high diagnostic accuracy for detecting fibrosis.
Fibrosis-4<1.3 has good negative predictive value to rule out advanced fibrosis (F3/4) and requires measurement of aspartate transaminase and platelets.
Established simple transient elastography cut-offs for alcohol related liver disease (ARLD) and non-alcoholic fatty liver disease (NAFLD): liver stiffness measurement (LSM)<8 kPa to rule out compensated advanced chronic liver disease (cACLD), LSM≥12–15 kPa to rule in cACLD and LSM of ≥20–25 kPa can be used to diagnose clinically significant portal hypertension.
In cACLD due to alcohol, NAFLD, viral hepatitis, HIV−hepatitis C, primary biliary cholangitis and primary sclerosing cholangitis, the Baveno VI criteria (LSM<20 kPa and platelet count>150x109/L) should be used to exclude high-risk varices.
Overview
The Lancet Commission on Liver Disease called for screening and risk stratification for liver disease in high-risk groups to curb rising morbidity and mortality.1 Developments outlined in the 2021 European Association for the Study of the Liver (EASL) clinical practice guidelines (CPG) on non-invasive tests (NITs) for evaluation of liver disease severity and prognosis, updated from 2015, detail the evidence base, advantages and limitations of existing strategies.2 3 Liver biopsy has historically been gold standard for diagnosing and stratifying disease severity in most aetiologies. However, it is an imperfect gold standard, while cost and risk profile mandate pragmatic use of NITs.
Aetiology influences diagnostic performance of NITs so the CPG provides recommendations on this basis alongside discussing use in compensated advanced chronic liver disease (cACLD).
NITs in the general population
Use of NITs in an unselected general population is discouraged, as evidence supporting their use is derived from populations with high liver disease prevalence.
In the primary care setting, NITs are best used to exclude advanced fibrosis in at-risk groups, primarily those with type 2 diabetes and obesity and those who use alcohol. Previous guidelines suggested NITs should be performed by specialists. However, given the rising burden of liver disease, use of NITs in primary care is strongly advocated. Fibrosis-4 (FIB-4) is promoted as the index NIT of choice given its acceptable negative predictive value in low prevalence settings; however, its calculation requires an aspartate transaminase (AST), which is not included in routine liver function tests in many UK laboratories.
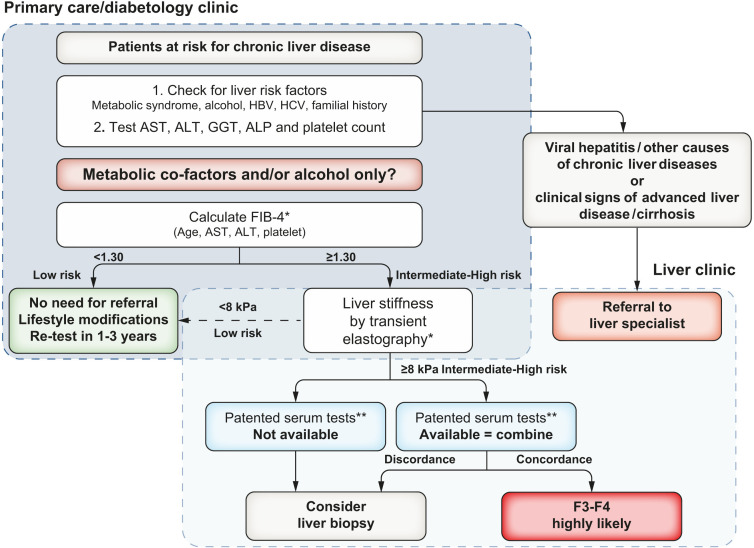
The guidelines support a risk stratification process of at least two tiers, providing the example of FIB-4 followed by transient elastography (TE) to identify patients most likely to have significant (≥F2) or advanced fibrosis (F3/4) who require further evaluation. This two-step risk stratification process is cost-effective in low prevalence settings and can safely rule out liver fibrosis. An example algorithm (see figure 1) mirrors pathways published by Srivastava et al and Chalmers et al. 4 5 Recent evidence has demonstrated that non-alcoholic fatty liver disease (NAFLD) fibrosis—not just cirrhosis—is associated with liver-related morbidity and mortality.6 Thus, early detection of liver fibrosis to facilitate behavioural change and pharmacological interventions could improve outcomes. Reflecting this, the term cACLD was introduced following the 2015 Baveno VI workshop7 referring to the continuum from severe fibrosis (≥F3) to cirrhosis in asymptomatic patients.
Figure 1.
As presented in the CPG: proposed use of non-invasive tests in patients observed in primary care or outside the liver clinic. As shown, FIB-4 can be used in patients with metabolic cofactors and/or alcoholic liver disease to identify patients requiring referral to the specialist liver clinic. Transient elastography or FIB-4 may be performed before or after referral to liver specialist according to local availability and pathways. Cut-offs to use: ELF<9.8 (NAFLD/ALD), FibroMeter<0.45 (NAFLD) and FibroTest<0.48 (NAFLD). ALD, alcohol-related liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPG, clinical practice guidelines; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4; GGT, gamma-glutamyl transferase; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease.
NITs in the alcohol-related liver disease (ALD)
ALD is responsible for the greatest liver-related morbidity and mortality worldwide. TE is the NIT with the strongest evidence in ALD. EASL recommend a liver stiffness measurement (LSM) of <8 kPa to rule out advanced fibrosis. If TE is unavailable, enhanced liver fibrosis (ELF)<9.8, FibroMeter<0.45, FibroTest<0.48 or FIB-4<1.3 can be used to rule out advanced fibrosis. LSM of ≥12–15 kPa provides high specificity for ruling in advanced fibrosis in ALD. Ongoing alcohol use is associated with false-positive LSMs, secondary to alcohol-related steatohepatitis which may correlate with deranged liver blood tests. In patients with elevated LSMs plus AST or GGT>2× upper limits of normal (ULN), clinicians are advised to repeat TE 1 week after reducing alcohol intake. How practical this is in the real-world setting remains to be seen; clinicians may continue to seek clarity through histological assessment in this cohort. There is no international consensus on cut-off values to rule in advanced fibrosis for patented biomarkers. At this stage, there is no strong evidence in support of NITs in prognosticating for compensated ALD or diagnosing alcoholic hepatitis.
NITs in the NASH and NAFLD
Conventional ultrasound remains the diagnostic modality of choice for detecting steatosis in patients with metabolic risk factors, being accessible, cheap and non-invasive. MRI proton density fat fraction (MRI-PDFF) has superior diagnostic performance to ultrasound; however, it is impractical on a population scale and expensive. Echosens developed controlled attenuation parameter (CAP), a proprietary algorithm to detect and quantify steatosis using a FibroScan device. While it has good sensitivity for detection of steatosis (CAP>275 dB/m), its role in quantifying steatosis has been challenged following a recent meta-analysis examining CAP performance with the FibroScan XL probe.8 Given steatosis alone is not associated with increased liver-related events and mortality, it is arguable whether quantification of steatosis is valuable in the clinical setting.9
Since the 2015 CPG, robust biomarkers for non-alcoholic steatohepatitis (NASH) diagnosis are yet to materialise and liver biopsy remains the only definitive diagnostic tool. LSM<8 kPa, ELF score<9.8, FibroMeter<0.45, FibroTest<0.48, FIB-4<1.3 or NAFLD fibrosis score (NFS)<−1.455 can exclude advanced NAFLD fibrosis. Given their ability to predict liver-related events and overall mortality, NITs are recommended to be repeated 3-yearly in patients with early-stage disease (ie, steatosis) and yearly in those with advanced NAFLD (ie, fibrosis).
NITs for assessment of treatment response in NAFLD are particularly desirable given the barriers to trial recruitment that repeated liver biopsy entails. MRI-PDFF is the most promising available tool in assessing treatment response; however, more data are needed to support its use as well as a consensus on the degree of change that would reflect clinical significance.
NITs in the hepatitis C (HCV) infection post sustained virological response (SVR)
With the global success of directly acting antivirals in HCV, the value of NITs to demonstrate regression of fibrosis post HCV SVR will become increasingly important, as liver biopsy is rarely used to confirm regression. EASL do not recommend using TE to confirm fibrosis regression, as reduced LSM could result from inflammation improvement opposed to fibrosis regression.10 Cut-offs for FIB-4, AST to platelet ratio index (APRI) and ELF need validation in large prospective studies at lower values post SVR to assess fibrosis regression. While hepatic venous pressure gradient (HVPG) remains the strongest prognostic determinant of outcomes in HCV-related cACLD, it is not widely available. TE has good accuracy for detecting clinically significant portal hypertension (CSPH) post SVR and should be performed annually.
NITs in the cholestatic and autoimmune liver disease
Despite small numbers of cholestatic liver disease studies of NITs, there is evidence to support use of TE to stratify disease severity. In primary biliary cholangitis (PBC), LSM≥10 kPa is diagnostic of advanced fibrosis. In primary sclerosing cholangitis (PSC), a TE≥9.5 kPa in combination with normal bilirubin and absence of high-grade stenosis is indicative of advanced fibrosis. Biomarker or non-invasive scores are not advocated to identify PBC fibrosis or differentiate between histological stages. However, they can risk stratify disease at baseline and during follow-up in PBC and PSC in conjunction with TE, which is advised annually in advanced disease.
LSM with TE is evidenced to play a role (in conjunction with IgG and transaminases) in monitoring autoimmune hepatitis (AIH) following initiation of treatment and staging fibrosis 6 months post initiation. Non-invasive scores such as APRI and FIB-4 perform poorly at predicting fibrosis in AIH.
NITs in the cACLD, portal hypertension and varices
Assessing cACLD is important to identify patients at risk of CSPH. TE remains the most validated modality for assessing cACLD and can exclude cACLD (due to NAFLD or ALD) with LSM of <8–10 kPa and rule in with LSM of >12–15 kPa. As detailed in the 2015 CPG, FibroTest, FibroMeter and ELF can be used to exclude cACLD.
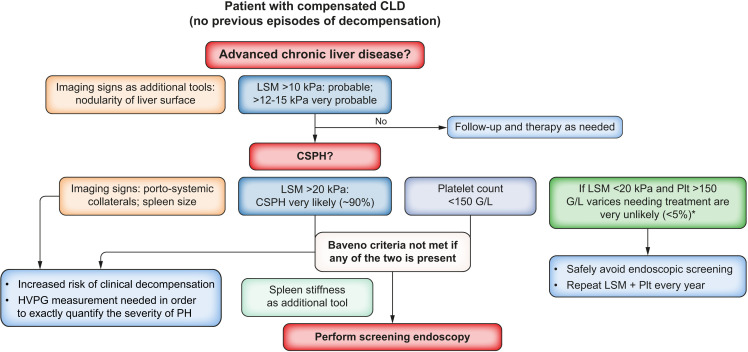
In cACLD, LSM of >20–25 kPa is indicative of CSPH, however there is no role for serum markers currently. Within CSPH, HVPG remains the only validated tool to assess severity. NITs including platelet count, spleen size and spleen stiffness can risk stratify for clinical outcomes. In cACLD due to alcohol, NAFLD, viral hepatitis, HIV−HCV coinfection, PBC, PSC and the Baveno VI criteria (LSM<20 kPa and platelet count>150x109/L) can exclude high-risk varices without need for annual endoscopic screening (see figure 2). Adding spleen stiffness measurement may even expand this to a larger group.
Figure 2.
As presented in the CPG: proposed use of NITs for risk stratification in patients with compensated chronic liver disease (CLD). CPG, clinical practice guidelines; CSPH, clinically significant portal hypertension; HVPG, hepatic venous pressure gradient; LSM, liver stiffness measurement; NITs, non-invasive tests; PH, portal hypertension; Plt, platelet count.
Conclusion
NITs are now established in ruling out advanced fibrosis in the low prevalence setting and risk stratifying fibrosis, ideally through a two-step process. Currently, TE is the most validated non-invasive measure for assessment of cACLD and CSPH. Readers are encouraged to review the original clinical practice guideline for a definitive overview.
Footnotes
Twitter: @Annie Archer, @KushAbey
Contributors: AJA reviewed the guideline and drafted the manuscript. KB reviewed the literature and provided critical revision of the manuscript. FHG and JGO provided critical revision of the manuscript. KWMA reviewed the literature and provided critical revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: KWMA has received lecture honoraria from Intercept Pharma. AJA is an NIHR academic clinical fellow.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Williams R, Alessi C, Alexander G, et al. New dimensions for hospital services and early detection of disease: a review from the Lancet Commission into liver disease in the UK. Lancet 2021;397:1770–80. 10.1016/S0140-6736(20)32396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado . EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–64. 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 3. Easl clinical practice guidelines (CpGs) on non-invasive tests for evaluation of liver disease severity and Prognosis- 2021 update. J Hepatol 2021;75. 10.1016/j.jhep.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71:371–8. 10.1016/j.jhep.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 5. Chalmers J, Wilkes E, Harris R, et al. The development and implementation of a commissioned pathway for the identification and stratification of liver disease in the community. Frontline Gastroenterol 2020;11:86–92. 10.1136/flgastro-2019-101177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanyal AJ, Van Natta ML, Clark J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559–69. 10.1056/NEJMoa2029349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Franchis R, Baveno VIF, Baveno VI Faculty . Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. 10.1016/j.jhep.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 8. Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol 2021;6:185–98. 10.1016/S2468-1253(20)30357-5 [DOI] [PubMed] [Google Scholar]
- 9. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557–65. 10.1002/hep.29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh S, Facciorusso A, Loomba R, et al. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:27–38. 10.1016/j.cgh.2017.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]