Abstract
Vedolizumab is a gut-selective monoclonal antibody approved for the management of Crohn’s disease and ulcerative colitis. The available data demonstrate a favourable response to dose escalation in patients with primary non-response or secondary loss of response to vedolizumab. While therapeutic drug monitoring has a proven clinical utility for tumour necrosis factor antagonists, the available guidance for therapeutic drug monitoring and dose escalation of vedolizumab is rather limited. The present review proposes a practical algorithm to use vedolizumab trough levels in the management of treatment failure. Therapeutic drug monitoring can differentiate underexposed patients from those with mechanistic failure. Underdosed patients can respond to dose escalation instead of unnecessarily switching to other treatment modalities. We also review the safety and potential cost-effectiveness of vedolizumab dose escalation, the role of antidrug antibodies and the possible applicability of this strategy to subcutaneous vedolizumab.
Keywords: chronic ulcerative colitis, crohn's colitis, crohn's disease, IBD, IBD clinical
Key points.
Vedolizumab demonstrates a clear dose–response relationship; dose escalation may induce or restore the clinical response.
Early vedolizumab trough levels correlate with the short and long-term therapeutic outcomes.
Dose escalation of vedolizumab could off-set the effect of poor prognostic factors in Crohn’s disease.
Antidrug antibodies against vedolizumab are infrequent, transient and probably not the driving force of treatment failure.
Optimum vedolizumab trough levels vary by the utilised assay, therapeutic targets and phase of treatment.
The frequency of vedolizumab adverse events does not correlate with the dosing regimen.
Introduction
Vedolizumab is a humanised IgG1 monoclonal antibody targeting the α4β7 integrin, which modulates lymphocyte trafficking in the gut without inducing systemic immunosuppression. In May 2014, the Medicines and Healthcare products Regulatory Agency (MHRA) authorised the use of vedolizumab for the treatment of moderate to severe ulcerative colitis (UC) or Crohn’s disease (CD). The recommended dose regimen of intravenous vedolizumab is 300 mg at 0, 2 and 6 weeks and every 8 weeks thereafter.1 The pivotal GEMINI trials reported primary response rates of 47.1% and 43.5% in patients with UC and CD on the standard dose regimen, respectively.2 3 A systematic review and meta-analysis reported the pooled incidence rates for loss of response (LOR) to vedolizumab as 47.9 per 100 person-years among patients with CD and 39.8 per 100 person-years among patients with UC.4 These studies highlight the prevalence of vedolizumab non-response and LOR and hence the need for an effective management strategy for vedolizumab treatment failure.
Standard guidance for tumour necrosis factor (TNFα) antagonists therapeutic drug monitoring (TDM) was published by the National Institute for Health and Care Excellence (NICE),5 while there are no standard recommendations for vedolizumab TDM. In order to develop a practical approach, we first present a brief review of the relevant literature regarding vedolizumab exposure–response relationship and the efficacy of vedolizumab dose escalation. We also discuss the suggested target trough levels and the role of antidrug antibodies (ADA) in vedolizumab treatment failure. Then, we attempt to evaluate the cost-effectiveness and safety of dose escalation, based on the available evidence. Finally, we examine the potential applicability of the suggested pathway to subcutaneous vedolizumab.
Pharmacokinetics and pharmacodynamics
Vedolizumab is eliminated through a process of cellular uptake and proteolytic degradation. The half-life of vedolizumab is 25.5 days during the linear elimination phase and it is similar in patients with either UC or CD.6 Several factors have a clinically relevant impact on vedolizumab clearance; low albumin concentrations, high body weight and increased inflammatory load all increase its clearance, while a concurrent immunosuppressive therapy with methotrexate or thiopurines has no clinically relevant pharmacokinetic effect.7
Vedolizumab achieves almost complete saturation of the α4β7 integrin receptors of the peripheral blood CD4+ T lymphocytes with drug concentrations as low as 1 μg/mL.6 Nevertheless, a clear exposure–response relationship has been demonstrated since its earliest phase 2 and 3 trials.8 9 As the vedolizumab-α4β7 receptor complex is internalised by the CD4+T lymphocytes and the α4β7 receptors are re-expressed after vedolizumab withdrawal, higher drug concentrations could help to maintain persistent blocking of lymphocyte trafficking.10 Furthermore, vedolizumab probably has additional modes of action; it was shown to exert effects on macrophage populations and expression of molecules involved in microbial sensing, chemoattraction and regulation of the innate immunity.11
In the Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis (GEMINI-1) study, vedolizumab trough concentrations at week 6 in the lowest quartile (<17 μg/mL) were associated with a clinical remission rate similar to placebo (6%), whereas the highest concentrations (>35.7 μg/mL) resulted in a remission rate of 37%. In addition, 62.9% of patients in the highest quartile achieved mucosal healing, compared with only 20.1% of patients in the lowest quartile.2 Similar data was reported by the GEMINI-2 study in patients with CD.3 Several studies emphasised the correlation between early vedolizumab trough levels at week 6 and the long-term clinical and endoscopic outcomes.12 13 Multiple systematic reviews, incorporating data from both clinical trials and real-world cohorts, reported a similar correlation.14–17 Vedolizumab trough levels have also been correlated with histological healing.18
In a cohort of 40 patients with inflammatory bowel disease (IBD) who discontinued vedolizumab, a trend was observed towards lower vedolizumab concentrations at week 6 in primary non-responders compared with patients with secondary LOR (20.3 vs 30.7 μg/mL, p=0.057).19 This further emphasises the importance of early vedolizumab concentrations in attaining the initial response in the induction phase. Indeed, a proportion of primary non-responders could simply be ‘underdosed’. TDM of vedolizumab may identify these ‘false’ primary non-responders, and treatment can be optimised instead of unnecessarily switching to alternative treatment.
Efficacy of dose escalation
The favourable response to vedolizumab dose escalation is well described, both for primary non-response and secondary LOR. In a prospective study of 47 patients with IBD, non-responders at week 6 were switched to a 4 weekly regimen. Although the dose escalation was based on clinical activity scores, vedolizumab trough levels<18.5 μg/mL at week 6 were associated with the need for dose escalation, with a 100% positive predictive value. Furthermore, all patients who required dose escalation achieved clinical response 4 weeks later.20 The mean change in vedolizumab trough levels after dose escalation was higher in responders.21These findings suggest that vedolizumab TDM, notably early trough levels at week 6, could differentiate between two groups of vedolizumab primary non-responders, those who are simply underexposed and will benefit from dose escalation, from patients with a primary mechanistic failure. Week 6 was identified as the earliest time at which vedolizumab concentrations were consistently associated with clinical remission at weeks 14 and 52.22 23
In a multicentre retrospective study of 58 patients with IBD with secondary LOR to vedolizumab, 62% of the study group responded to reactive dose escalation, and those with lower trough levels were more likely to respond.24 Similar response rate was reported in real-life settings.25 In the GEMINI LTS trials, vedolizumab dose escalation restored and maintained a clinical response in patients who had withdrawn early from the GEMINI-1 and GEMINI-2 trials due to LOR, and durable benefits on the Health-Related Quality of Life scale were also observed.26 27
The Clinical Decision Support Tool (CDST), which was developed using a combination of clinical and laboratory factors, aims to classify CD patients as low, intermediate or high probability of response to vedolizumab.28 None of the CDST high-probability patients in the Groupe d’Étude Thérapeutique des Affections Inflammatoires du Tube Digestif (GETAID) cohort required dose escalation for lack of response. In the Vedolizumab For Health Outcomes In Inflammatory Bowel Diseases (VICTORY) consortium, a clinical response was seen in 46% of the CDST low-probability group, 39% of the intermediate-probability group and none of the high-probability group (p=0.038) after vedolizumab dose escalation. These findings suggest that dose escalation of vedolizumab could off-set the effect of the poor prognostic factors in CD.29
Antidrug antibodies
The true incidence of ADA against vedolizumab is difficult to estimate, partly due to the wide variety of used assays. In the pivotal GEMINI-1 and GEMINI-2 trials, 3.7% and 4.1% of patients had samples that were positive for ADA, and only 1% and 0.4% were persistently positive to ADA, respectively. After testing the same cohorts with drug-tolerant affinity capture elution assay, only 4% of the GEMINI cohorts changed their ADA status.30 In a real-world cohort, all ADA-positive patients continued standard vedolizumab therapy at least up to 1 year and there was no correlation between vedolizumab trough levels and ADA status.31 Few studies reported a higher incidence of vedolizumab ADA. Ungar et al reported ADA in 17% of their study cohort during the induction phase. Nevertheless, ADAs were a transient phenomenon detected in only 3% of patients in the maintenance phase and the ADA status did not correlate with the clinical outcomes.32 ADAs are probably not the driving force of vedolizumab treatment failure.
Target trough levels
In a published consensus panel statement, vedolizumab TDM was suggested in two clinical scenarios, namely for primary non-responders and for secondary LOR. The consensus panel also acknowledged the current lack of sufficient data to guide specific induction or maintenance drug concentrations.33 The differences across studies could be partly secondary to disagreement of the utilised assays.17 Additionally, target trough levels vary by treatment target (eg, clinical vs endoscopic remission) and phase of therapy (induction vs maintenance).33 Table 1 demonstrates the inconsistency of the suggested target vedolizumab trough levels, in the context of variable sampling time points and target therapeutic outcomes in different studies.
Table 1.
Suggested vedolizumab trough levels33
| Sampling time point | Trough level | Target therapeutic outcome |
| Crohn’s disease | ||
| Induction (w2) | >35.2 | Biological remission (at week 6)43 |
| Induction (w2) | ≥24.5 | No need for dose escalation (at week 24)20 |
| Induction (w6) | ≥18.5 | No need for dose escalation20 |
| Induction (w6) | >27.5 | Sustained clinical response20 |
| Induction (w6) | >18 | Mucosal healing (at week 54)13 |
| Maintenance (w22) | >13.6 | Mucosal healing (at week 22)43 |
| Maintenance (w22) | >12 | Biological remission (at week 22)43 |
| Ulcerative colitis | ||
| Induction (w2) | >28.9 | Clinical response (at week 14)43 |
| Induction (w2) | >23.7 | Mucosal healing (at week 14)43 |
| Induction (w2) | ≥24.5 | No need for dose escalation (at week 24)20 |
| Induction (w6) | >20.8 | Clinical response (at week 14)43 |
| Induction (w6) | ≥18.5 | No need for dose escalation20 |
| Induction (w6) | >27.5 | Sustained clinical response20 |
| Induction (w6) | >18 | Mucosal healing (at week 54)13 |
| Postinduction (w14) | >12.6 | Clinical response (at week 14)43 |
| Postinduction (w14) | >17 | Mucosal healing (at week 14)43 |
Safety and cost-effectiveness
The GEMINI trials reported a similar incidence of adverse events (AEs) regardless of vedolizumab dosing regimen. Furthermore, the difference between the incidence of AE in the vedolizumab group and the placebo group was not statistically significant.2 3 These results suggest that vedolizumab dose escalation should not be associated with safety concerns.
Dose escalation to a 4 weekly regimen could add approximately £13 000 to the annual cost of treatment per patient, excluding the additional hospital visits and infusion costs.34 The proposed cost for vedolizumab trough level test is only £29.5.35 Performing a full cost–benefit analysis is beyond the scope of this article. Nevertheless, considering the aim of the proposed strategy is to identify IBD patients who may benefit from dose intensification instead of switching to another therapeutic modality, therefore, estimation of the cost-effectiveness should take in consideration the cost of alternative therapies, notably surgery. The NICE guidance describes several models which consider the costs and health benefits over a time horizon of 10 years. In one model, patients could progress to have surgery for primary non-response or secondary LOR. It was assumed that 40% would have a proctocolectomy with end ileostomy and 60% would have a subtotal proctocolectomy with pouch formation, with or without a loop ileostomy. After surgery, some patients had complications and needed additional surgeries. In this model, 8 weekly vedolizumab was more cost-effective than surgery with an incremental cost-effectiveness ratio of £33297 per quality-adjusted life-year gained.36 Nevertheless, cost-effectiveness could be significantly affected by dose escalation, depending on the proportion of patients receiving 4 weekly dosing. Additionally surgery may not be the main relevant comparator.37
Subcutaneous vedolizumab
In a population pharmacokinetic model which included data from four clinical trials, namely VISIBLE-1 and VISIBLE open-label extension (for subcutaneous vedolizumab), together with GEMINI-1 and GEMINI-2 (for intravenous vedolizumab), subcutaneous (SC) vedolizumab (108 mg) administered fortnightly produced average serum concentrations similar to those for intravenous vedolizumab (300 mg) 8 weekly infusions, and lower than those for intravenous vedolizumab 4 weekly infusions.38 The VISIBLE-1 study also reported a positive exposure-response correlation for SC vedolizumab. Additionally, ADAs were detected in only 6% of the subcutaneous arm and were generally a transient phenomenon.39
The present pathway may prove useful for SC vedolizumab, considering the comparable pharmacokinetics, pharmacodynamics and immunogenicity data. In an ongoing study of the long-term effects of subcutaneous vedolizumab, participants with secondary LOR are switched to weekly subcutaneous injections. This should theoretically be equivalent to escalating patients on intravenous vedolizumab to a 4 weekly regimen.40
Proposed pathway
Several studies highlighted the importance of early assessment of clinical response and vedolizumab trough levels notably at week 6, in the management of primary non-response and for the long-term therapeutic outcomes.20–23 Therefore, we propose early assessment of clinical response together with proactive TDM at week 6 for all patients commenced on vedolizumab. Patients with primary non-response and induction trough levels below 18 μg/mL should be considered for dose escalation, while we suggest early switching to alternative treatment modality for patients with higher trough levels and hence probably a mechanistic failure. For assessment of the clinical response, we recommend adopting the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) criteria, namely a decrease of at least 50% in patient-reported outcomes, the abdominal pain and stool frequency in patients with CD, and rectal bleeding and stool frequency in patients with UC. The STRIDE-II recommendations suggested these criteria as the immediate (ie, earliest) treatment targets, and advised to consider changing treatment if they have not been achieved.41 Considering the requirement for early assessment at week 6, objective measures notably faecal calprotectin (FCP) were not considered in the pathway (figure 1). Indeed, a post hoc analysis of the GEMINI-1 data reported that only a minority of patients achieved a significant reduction in FCP at week 6, regardless of their long-term endoscopic outcomes.42 We also suggest considering CD patients with low or intermediate CDST scores for early dose escalation. This is based on the data suggesting that dose escalation of vedolizumab could off-set the effect of the poor prognostic factors in CD.29
Figure 1.
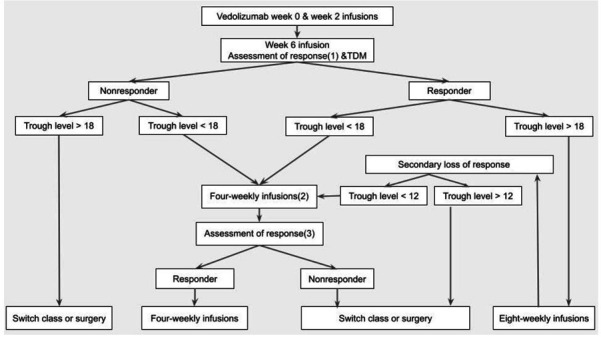
Proposed pathway for vedolizumab therapeutic drug monitoring (TDM) and dose optimisation. (1) using the STRIDE-II criteria.41 (2) also consider dose escalation for patients Clinical Decision Support Tool (CD): low or intermediate probability (<19).28 UC/CD: body weight >120 kg, albumin <3.2 and/or high FCP9 (3) at least 4 weeks after dose escalation.20 23 CD, Crohn’s disease; FCP, faecal calprotectin; STRIDE-II, Selecting Therapeutic Targets in Inflammatory Bowel Disease; UC, ulcerative colitis.
Considering the low incidence and transient nature of ADA, together with their insignificant impact on vedolizumab trough levels and clinical outcomes, the detection of ADA was omitted from the pathway.30–32 The pathway applies the lowest suggested trough levels in the induction and maintenance phases (18 and 12 μg/mL, respectively), in order to maximise the ability of TDM to distinguish underexposed patients from those with mechanistic failure.13 43 The same trough levels we used for CD and UC, considering the comparable pharmacokinetics6 and reported therapeutic trough levels13 20 of both IBD phenotypes. In the maintenance phase, we suggest reactive TDM for patients with secondary LOR, considering the lack of evidence for more frequent proactive monitoring after the first TDM check.44 Patients with secondary LOR and maintenance trough level above 12 μg/mL should be switched to alternative treatment modality, in order to avoid prolonging a probably futile therapy.
Although the aforementioned trials reported dose escalation depending on clinical assessment, the pathway depends on a combination of clinical assessment and TDM. The use of TDM to guide dose escalation compared with clinical decision making alone was associated with higher clinical response and endoscopic remission rates.17 Empiric dose escalation risks the potential complications of prolonging a futile therapy while delaying more effective alternative treatments. Furthermore, several studies reported TDM-guided strategies were consistently cost-saving or cost-effective for patients with IBD on TNFα antagonists.45 46
Conclusion
We propose a practical pathway for the management of vedolizumab primary non-response and secondary LOR, using a combination of clinical assessment and TDM. We acknowledge the limitations of the current evidence for vedolizumab TDM, notably regarding the target serum levels, sampling time points and optimum TDM strategy, that is, proactive versus reactive monitoring. The clinical utility of the present pathway should be validated by prospective studies and real-world cohorts.
Footnotes
Contributors: Conceptualisation: ION and NS. Methodology: ION. Data Acquisition: ION. Visualisations: ION. Writing the Manuscript: ION. Critical Review: JC, MNQ and NS. Supervision: NS. All the authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Electronic Medicines Compendium (emc) . Entyvio 300 mg powder for concentrate for solution for infusion, summary of product characteristics (SmPC) [online]. Available: https://www.medicines.org.uk/emc/product/5442/smpc#gref [Accessed 01 Dec 2021].
- 2. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Danese S, Argollo M, et al. Loss of response to Vedolizumab and ability of dose intensification to restore response in patients with Crohn's disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:838–46. 10.1016/j.cgh.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 5. The National Institute for Health and Care Excellence . Therapeutic monitoring of TNF-alpha inhibitors in Crohn’s disease (LISA-TRACKER ELISA kits, IDKmonitor ELISA kits, and Promonitor ELISA kits) Diagnostics guidance [DG22]. Available: https://www.nice.org.uk/guidance/dg22/chapter/9-Review [Accessed 01 Dec 2021].
- 6. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease. Aliment Pharmacol Ther 2015;42:188–202. 10.1111/apt.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of Vedolizumab. Clin Pharmacokinet 2017;56:1287–301. 10.1007/s40262-017-0546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis 2012;18:1470–9. 10.1002/ibd.21896 [DOI] [PubMed] [Google Scholar]
- 9. Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for Vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J Crohns Colitis 2017;11:921–9. 10.1093/ecco-jcc/jjx021 [DOI] [PubMed] [Google Scholar]
- 10. Wyant T, Yang L, Fedyk E. In vitro assessment of the effects of vedolizumab binding on peripheral blood lymphocytes. MAbs 2013;5:842–50. 10.4161/mabs.26392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68:25–39. 10.1136/gutjnl-2018-316023 [DOI] [PubMed] [Google Scholar]
- 12. Yarur AJ, Bruss A, Naik S, et al. Vedolizumab concentrations are associated with long-term endoscopic remission in patients with inflammatory bowel diseases. Dig Dis Sci 2019;64:1651–9. 10.1007/s10620-019-05570-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab Trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. 10.1111/apt.14548 [DOI] [PubMed] [Google Scholar]
- 14. Singh S, Dulai PS, Vande Casteele N, et al. Systematic review with meta-analysis: association between vedolizumab Trough concentration and clinical outcomes in patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2019;50:848–57. 10.1111/apt.15484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ward MG, Sparrow MP, Roblin X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Therap Adv Gastroenterol 2018;11:1756284818772786. 10.1177/1756284818772786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pugliese D, Privitera G, Pizzolante F, et al. Therapeutic drug monitoring with vedolizumab in inflammatory bowel disease. Minerva Gastroenterol Dietol 2019;65:280–90. 10.23736/S1121-421X.19.02625-4 [DOI] [PubMed] [Google Scholar]
- 17. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab Trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med 2019;17:89. 10.1186/s12916-019-1323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pouillon L, Rousseau H, Busby-Venner H, et al. Vedolizumab Trough levels and histological healing during maintenance therapy in ulcerative colitis. J Crohns Colitis 2019;13:970–5. 10.1093/ecco-jcc/jjz029 [DOI] [PubMed] [Google Scholar]
- 19. Van den Berghe N, Verstockt B, Tops S, et al. Immunogenicity is not the driving force of treatment failure in vedolizumab-treated inflammatory bowel disease patients. J Gastroenterol Hepatol 2019;34:1175–81. 10.1111/jgh.14584 [DOI] [PubMed] [Google Scholar]
- 20. Williet N, Boschetti G, Fovet M, et al. Association between low Trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–7. 10.1016/j.cgh.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 21. Gouynou C, Pouillon L, Rousseau H, et al. Early changes in the pharmacokinetic profile of vedolizumab-treated patients with IBD may predict response after dose optimisation. Gut 2019;68:178–9. 10.1136/gutjnl-2017-315766 [DOI] [PubMed] [Google Scholar]
- 22. Guidi L, Pugliese D, Panici Tonucci T, et al. Early vedolizumab Trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol J 2019;7:1189–97. 10.1177/2050640619873784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther 2019;49:408–18. 10.1111/apt.15113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaughn BP, Yarur AJ, Graziano E, et al. Vedolizumab serum Trough concentrations and response to dose escalation in inflammatory bowel disease. J Clin Med 2020;9:3142. 10.3390/jcm9103142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birdi S, Sierra Morales M, Samaan M. Vedolizumab dose escalation as a way of Recapturing response in patients with inflammatory bowel disease. Gut 2018;67:A69–70. [Google Scholar]
- 26. Loftus EV, Colombel J-F, Feagan BG, et al. Long-Term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017;11:400–11. 10.1093/ecco-jcc/jjw177 [DOI] [PubMed] [Google Scholar]
- 27. Vermeire S, Loftus EV, Colombel J-F, et al. Long-Term efficacy of Vedolizumab for Crohn's disease. J Crohns Colitis 2017;11:412–24. 10.1093/ecco-jcc/jjw176 [DOI] [PubMed] [Google Scholar]
- 28. Dulai PS, Boland BS, Singh S, et al. Development and Validation of a Scoring System to Predict Outcomes of Vedolizumab Treatment in Patients With Crohn's Disease. Gastroenterology 2018;155:687–95. 10.1053/j.gastro.2018.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dulai PS, Amiot A, Peyrin-Biroulet L, et al. A clinical decision support tool may help to optimise vedolizumab therapy in Crohn's disease. Aliment Pharmacol Ther 2020;51:553–64. 10.1111/apt.15609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyant T, Yang L, Rosario M. Comparison of the ELISA and ECL assay for vedolizumab anti-drug antibodies: assessing the impact on pharmacokinetics and safety outcomes of the phase 3 gemini trials. Aaps J 2020;23:3. 10.1208/s12248-020-00518-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bian S, Dreesen E, Tang HT, et al. Antibodies toward vedolizumab appear from the first infusion onward and disappear over time. Inflamm Bowel Dis 2017;23:2202–8. 10.1097/MIB.0000000000001255 [DOI] [PubMed] [Google Scholar]
- 32. Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705. 10.1016/j.cgh.2017.11.050 [DOI] [PubMed] [Google Scholar]
- 33. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019;17:1655–68. 10.1016/j.cgh.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joint Formulary Committee. British National Formulary London: BMJ Group and Pharmaceutical Press . Vedolizumab. Available: https://bnf.nice.org.uk/drug/vedolizumab.html [Accessed 01 Dec 2021].
- 35. Exeter Clinical Laboratory International . Vedolizumab drug levels. Available: https://www.exeterlaboratory.com/test/vedolizumab-drug-levels/ [Accessed 10 Dec 2021].
- 36. National Institute for Health and Care Excellence . Vedolizumab for treating moderately to severely active ulcerative colitis, technology appraisal guidance [TA342], 2015. Available: https://www.nice.org.uk/guidance/ta342
- 37. Scottish Medicines Consortium . Vedolizumab 300mg powder for concentrate for solution for infusion (Entyvio®) SMC No. 1064/15. Available: https://www.scottishmedicines.org.uk/media/2472/vedolizumab_entyvio_final_june_2015_for_website.pdf [Accessed 13 Jul 2015].
- 38. Rosario M, Polhamus D, Chen C, et al. P490 a vedolizumab population pharmacokinetic model including intravenous and subcutaneous formulations for patients with ulcerative colitis. J Crohns Colitis 2019;13:S357. 10.1093/ecco-jcc/jjy222.614 [DOI] [Google Scholar]
- 39. Sandborn W, Baert F, Danese S. Efficacy and safety of vedolizumab subcutaneous formulation for ulcerative colitis: results of the visible trial. Gut 2019;68:A60. [Google Scholar]
- 40. ClinicalTrials.gov . A study of long-term effects of vedolizumab subcutaneous in adults with ulcerative colitis and Crohn’s disease. Available: https://clinicaltrials.gov/ct2/show/record/NCT02620046
- 41. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International organization for the study of IBD (IOIBD): determining therapeutic goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 42. Reinisch W, Bressler B, Curtis R, et al. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: a post hoc analysis of gemini 1. Inflamm Bowel Dis 2019;25:803–10. 10.1093/ibd/izy304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab Trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–46. 10.1016/j.cgh.2018.04.040 [DOI] [PubMed] [Google Scholar]
- 44. Al Yassin S, Spataro J, Kang L, et al. S0715 Longer Duration of Response With Proactive Therapeutic Drug Monitoring for Infliximab but Not for Vedolizumab Treatment in Patients With Inflammatory Bowel Disease. Am J Gastroenterol 2020;115:S358–9. 10.14309/01.ajg.0000704908.05410.59 [DOI] [Google Scholar]
- 45. Yao J, Jiang X, You JHS. A systematic review on cost-effectiveness analyses of therapeutic drug monitoring for patients with inflammatory bowel disease: from immunosuppressive to anti-TNF therapy. Inflamm Bowel Dis 2021;27:275–82. 10.1093/ibd/izaa073 [DOI] [PubMed] [Google Scholar]
- 46. Guidi L, Pugliese D, Panici Tonucci T, et al. Therapeutic drug monitoring is more cost-effective than a clinically based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multicentre study. J Crohns Colitis 2018;12:1079–88. 10.1093/ecco-jcc/jjy076 [DOI] [PubMed] [Google Scholar]


