Abstract
Maternal psychosocial stress increases the risk of adverse birth and postnatal outcomes for the mother and child, but the role of maternal exposure to childhood traumatic events (CTE) and multi-domain psychosocial stressors for the level and rise of placental Corticotrophin-Releasing Hormone (pCRH) across pregnancy has been understudied. In a sociodemographically and racially diverse sample of 1303 women (64% Black, 36% White/others) with low-medical risk pregnancies at enrollment from Shelby County, Tennessee, USA, blood samples were drawn twice, corresponding roughly to second and third trimester, and extracted prior to conducting radioimmune assays for pCRH. Mothers reported CTE (physical abuse, sexual abuse, or family violence, in childhood), adulthood traumatic events, and interpersonal violence during pregnancy. Neighborhood crime/deprivation was derived using geospatially-linked objective databases. General linear and mixed models tested associations between stress exposure variables and pCRH levels and rate of rise, adjusting for obstetric/clinical/health related factors. Maternal CTE did not predict pCRH levels at time 1, but positively predicted levels at time 2, and the rate of rise in pCRH across pregnancy. Race did not moderate this association. No additional maternal stress exposures across adulthood or during pregnancy predicted pCRH outcomes. Findings indicate that childhood violence or abuse exposure can become biologically embedded in a manner predicting later prenatal physiology relevant for maternal and offspring health, and that such embedding may be specific to childhood, but not adulthood, stress. Findings also highlight the placental-fetal unit as a mechanistic pathway through which intergenerational transmission of the adverse effects of childhood adversities may occur.
Keywords: Childhood Traumatic Events, maternal psychosocial stress, placental corticotrophin releasing hormone, stress during pregnancy
Introduction
A large empirical literature suggests that maternal exposure to psychosocial stress during pregnancy increases the risk of multiple adverse birth-related outcomes as well as maternal psychiatric and child developmental outcomes, including spontaneous abortions, preterm birth, low birth weight, postpartum depression, infant growth retardation, postnatal health, augmented stress reactivity, and, in older children, reduced cognitive abilities and behavioral- and socioemotional problems (Bergman et al., 2007; Buffa et al., 2018; Class et al., 2011; Davis et al., 2011; Entringer et al., 2009; Hobel et al., 2008; Loomans et al., 2012; Madigan et al., 2018; Qobadi et al., 2016; M. Robinson et al., 2008). While such associations have been found across a wide range of psychosocial stressor types, the biological pathways linking prenatal maternal stress to these outcomes are only starting to become illuminated.
Since the placenta mediates communication between mother and fetus (Griffiths & Campbell, 2014; Gude et al., 2004), the stress-responsive fetal-placental Corticotrophin-releasing hormone (CRH) system has been proposed as a mechanistic pathway for these associations (Moog et al., 2016; Sandman, 2018). CRH is a neuropeptide that plays a key role in regulating the activity of the hypothalamus-pituitary-adrenal (HPA) axis, the major neuroendocrine stress response system. While typically barely detectable in human plasma, CRH is synthesized and secreted by the placenta during pregnancy (pCRH), with maternal levels rising progressively from around 20 weeks gestation and exponentially increasing during the last 5–6 gestational weeks, before rapidly returning to pre-pregnancy levels postpartum (Campbell et al., 1987; Perkins et al., 1995). Although increases in pCRH levels are normal during pregnancy, comparatively higher levels and rate of increase have been linked to adverse perinatal outcomes, including maternal preeclampsia, pregnancy-induced hypertension (Harville et al., 2009; Laatikainen et al., 1991) and preterm birth (Lee, 2014; Wadhwa et al., 2004), as well as fetal growth restriction (Wadhwa et al., 2004) and physical and muscular maturation (Ellman et al., 2008). Moreover, higher pCRH levels have been linked to greater risk of maternal psychiatric outcomes, including postpartum depression (Glynn & Sandman, 2014; Yim et al., 2009), and to early life risk phenotypes and related psychiatric outcomes within her children, including higher levels of fear and distress (Davis et al., 2005), structural alterations of the brain, externalizing behaviors (Sandman et al., 2018), and depression and anxiety symptoms (Howland et al., 2016), the latter of which have been found to predict psychiatric outcomes, symptom trajectories, and academic functioning later in life (Ialongo et al., 2001). A more comprehensive understanding of environmental factors contributing to pCRH level and rate of rise, especially potentially modifiable risk factors, is needed.
A growing number of studies have examined the association between psychosocial stress and pCRH level and rise. While some reported positive associations of pCRH with stressors in the form of perceived stress (Hobel et al., 1999) and perceived inadequacy of income (Latendresse & Ruiz, 2010), others have reported negative (Harville et al., 2009) or no associations (Kramer et al., 2009; Petraglia et al., 2001) with a wide range of stressors including perceived stress, job strain, daily hassles and interpersonal violence. Others have reported mixed findings, including positive associations of pCRH levels with only pregnancy-specific, but not general measures of stress (Mancuso et al., 2004), and positive associations between intimate partner violence, discrimination, neighborhood violence and pCRH levels among Black women only, but not among White women (Tse et al., 2012). Differences across studies in terms of stressor type, pCRH protocols, and sample characteristics may partially explain mixed findings. However, the failure of previous studies to include stressors experienced during childhood—the developmentally sensitive time period during which exposure to severe stressors produce pervasive effects on brain development, neuroendocrine stress response systems, behavior, and health (Agorastos et al., 2019; Carr et al., 2018; Lupien et al., 2009; McEwen, 2003; Pechtel & Pizzagalli, 2011)—may also play a role. Childhood traumatic events (CTE) such as childhood physical-, sexual- or emotional abuse, emotional neglect, and family dysfunction, have been linked to abnormal stress physiology and HPA-axis functioning in both pregnant (Buss et al., 2016; Thomas et al., 2018) and non-pregnant women (Heim et al., 2000); to gestational diabetes, preeclampsia, shorter gestational length, and postpartum depression in pregnant women (Leigh & Milgrom, 2008; Roberts et al., 2013; M. V. Smith et al., 2016); and to developmental disorders, birth weight, brain anatomy and stress physiology in offspring (Moog et al., 2018; Roberts et al., 2013). Moreover, maternal exposure to traumatic or adverse events in childhood are well-established risk factors for traumatic life events in adulthood, pregnancy conditions, and health behaviors that have been linked to, or may be linked to, higher pCRH levels, including interpersonal violence (Ports et al., 2016), gestational diabetes, preeclampsia (Roberts et al., 2013), obesity (Danese & Tan, 2013), smoking during pregnancy (Hughes et al., 2017), socioeconomic status (Fergusson et al., 2013), and preterm birth (Leeners et al., 2010). Yet, only two previous studies have examined associations between maternal CTE and pCRH levels during pregnancy.
Moog and colleagues (2016), in a racially diverse sample of 295 women, found no association between CTE (physical/emotional/sexual abuse, physical/emotional neglect) and pCRH levels in early pregnancy, but women with CTE showed greater pCRH rise across gestation) compared to women without CTE. The pCRH increase was approximately double for one or more CTE compared to none, with differences emerging around 19 weeks gestation (Moog et al., 2016). This study, however, did not examine other lifetime or current stressor domains. In contrast, Chen and colleagues (2010) found no association between maternal CTE (physical and sexual abuse) and pCRH levels in a racially diverse sample of 1216 women (Y. Chen et al., 2010); however pCRH was measured at a single, second trimester time point, precluding the potential to detect associations arising later in pregnancy or the increase over gestation. In fact, the majority of previous maternal stress studies have included only a single measure of pCRH (Kramer et al., 2009; Latendresse & Ruiz, 2010; Tse et al., 2012) and focused only on a single stressor domain (Moog et al., 2016; Ramos et al., 2019). Of the few studies examining multiple stressor domains, all measured pCRH at a single (often second trimester) time point (Kramer et al., 2009; Latendresse & Ruiz, 2010; Tse et al., 2012). Moreover, most previous studies were conducted in small samples (e.g. < 200) (Hobel et al., 1999; Latendresse & Ruiz, 2010; Tse et al., 2012) or samples with limited sociodemographic diversity (Harville et al., 2009; Latendresse & Ruiz, 2010), with few examining the role of sociodemographic (Harville et al., 2009) and neighborhood/community stressors (Tse et al., 2012), limiting the generalizability to socioeconomically and racially diverse populations. The latter is particularly relevant given racial/ethnic differences in birth-related outcomes and stress exposure during pregnancy, particularly higher rates of preterm birth (Orchard & Price, 2017; Schaaf et al., 2013) and chronic stress during pregnancy (Borders et al., 2015) among Black women. Furthermore, several studies have reported lower levels of pCRH among Black women compared to White women (Y. Chen et al., 2010; Glynn et al., 2007; Harville et al., 2009), while other studies did not find such differences by race (Borders et al., 2015; Mancuso et al., 2004). These findings raise the question of whether race may be a factor affecting the association between stress exposures and pCRH levels. In sum, comprehensive examination of the role of multiple domains of stress exposures across the life course for pCRH levels is needed, particularly predicting measurements of both the second and third trimester pCRH, and from large, sociodemographically diverse samples.
Based on the above, the aims of this study were threefold: 1) To conceptually replicate the previously reported association between CTE and pCRH levels and rate of rise (Moog et al., 2016) by examining whether such association could be detected in a larger sample, specifically a sociodemographically diverse sample of 1303 pregnant Black and White women, adjusting for some of the same obstetric, clinical, and health related factors known to be associated with pCRH levels, as well as additional potential confounders not included in their model (specifically, maternal age, maternal education level, marital status, fetal sex, gestational week, and maternal social support); 2) To leverage our diverse sample to test whether associations between CTE and pCRH would vary by race; 3) To examine the role of multiple other, formerly understudied, domains of maternal psychosocial stressors across the life course, specifically maternal traumatic life events in adulthood, interpersonal violence victimization during pregnancy, and neighborhood crime and deprivation during pregnancy. Based on literature demonstrating biological embedding of early life adversity (Bublitz & Stroud, 2012; Buss et al., 2016; Heim et al., 2000; Thomas et al., 2018) and previous studies examining associations between CTE and pCRH levels (Y. Chen et al., 2010; Moog et al., 2016), we hypothesized that CTE would predict pCRH levels measured during later stages of pregnancy (i.e., during the 3rd pregnancy trimester) as well as the rate of rise of pCRH during pregnancy (Moog et al., 2016), but not pCRH levels measured during earlier stages of pregnancy (i.e., during the 2nd pregnancy trimester) (Y. Chen et al., 2010). Based on previous prenatal stress exposure studies (Harville et al., 2009), we also hypothesized that interpersonal violence victimization during pregnancy would predict pCRH levels and rate of rise during pregnancy. Due to a lack of previous pCRH studies examining neighborhood factors or lifetime exposure to traumatic events, no hypotheses were made for those exposures.
Methods and Materials
Participants
The data used in this study came from participants in the University of Tennessee Health Science Center’s Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study (for a comprehensive methodological overview and baseline sample description, see Sontag-Padilla et al., 2015). From December 2006-July 2011, 1503 women with low-medical risk pregnancies were recruited from prenatal clinics with intent to deliver at one of the five participating health care settings in Shelby County, Tennessee, USA. Inclusion and exclusion criteria are detailed in Supplemental Material. Of the initial study sample, pCRH data was available for a subsample of 1303 women.
Procedures
Participants were recruited between 16–29 weeks gestation, and were assessed again between 22–39 weeks gestation, which predominantly corresponded to 2nd and 3rd pregnancy trimester (Sontag-Padilla et al., 2015). The study was approved by the Institutional Review Board at the University of Tennessee Health Science Center. Written informed consent was obtained from all participants. Analyses were conducted as part of the CANDLE DOHaD study and the ECHO PATHWAYS Consortium.
Measures
Placental CRH
pCRH was measured via blood samples collected using lavender top (EDTA) plasma separator tubes obtained at two study visit time points during pregnancy. After blood collection, tubes were centrifuged, processed, and frozen at −80. Plasma pCRH assays were completed by Dr. Roger Smith’s laboratory at the University of Newcastle, Australia. The pCRH concentrations (pg/mL) were analyzed by radioimmunoassay as previously described (R. Smith et al., 2009). Extraction recovery was 87%. Inter- and intra-assay coefficients of variance were 8.7% and 7.3%, respectively, which compares favorably with previous studies using this methodology, providing superior sensitivity to other approaches. Data reduction for the radioimmunoassay was performed with a computer-assisted logistics program. Analyses were conducted with log-transformed scores to prevent violations of model assumptions for ordinary least squares regression. Raw data are presented in nanomoles per liter (nmol/L) in Table 1.
Table 1.
Descriptive Statistics of Sample and Main Variables used in the Study
| Total Sample (N=1322) | Whites (n=475) | Blacks (n=847) | * p<.05 | |||||||
|
|
||||||||||
| Continuous Variables | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
|
| ||||||||||
| Maternal age at recruitment | 26.19 | 5.46 | 16 –40 | 28.64 | 4.94 | 16 – 39 | 24.83 | 5.25 | 16 –40 | * |
| Gestational age, time 1 assessment (weeks) | 23.07 | 3.07 | 16.0 – 29.6 | 23.01 | 3.13 | 16.1 – 29.6 | 23.10 | 3.02 | 16.0 – 28.4 | ns |
| Gestational age, time 2 assessment (weeks) | 31.74 | 1.83 | 22.4 – 39.2 | 31.91 | 1.59 | 25.6 – 39.2 | 31.64 | 1.94 | 22.4 – 39.2 | * |
| Income/education composite score | 0.03 | 0.92 | −0.9 – 2.5 | 0.70 | 0.91 | −0.9 – 2.5 | −0.35 | 0.69 | −0.9 – 1.9 | * |
| Parity | 2.56 | 1.63 | 1 – 12 | 2.25 | 1.34 | 1 – 10 | 2.74 | 1.74 | 1 – 12 | * |
| Pre-pregnancy Body Mass Index (BMI) | 27.56 | 7.50 | 14.2 – 72.4 | 25.73 | 5.98 | 14.2 – 50.8 | 28.59 | 8.06 | 14.2 – 72.4 | * |
| Brief Symptom Inventory depression t-score | 48.27 | 7.67 | 36 – 74 | 48.07 | 7.52 | 36 – 74 | 48.39 | 7.77 | 36 – 74 | ns |
| Social support summary score | 3.58 | 1.87 | 0 – 9 | 4.39 | 1.91 | 0 – 9 | 3.13 | 1.69 | 0 – 9 | * |
| Maternal childhood traumatic events | 0.52 | 0.78 | 0 – 3 | 0.42 | 0.73 | 0 – 3 | 0.58 | 0.80 | 0 – 3 | * |
| Maternal traumatic life events in adulthood | 3.16 | 2.33 | 0 – 14 | 3.04 | 2.30 | 0 – 14 | 3.23 | 2.34 | 0 – 12 | ns |
| Interpersonal violence victimization | 0.99 | 0.89 | 0 – 4 | 0.82 | 0.69 | 0 – 4 | 1.08 | 0.97 | 0 – 4 | * |
| Neighborhood crime and deprivation | −0.03 | 0.96 | −2.1 – 1.7 | −0.83 | 0.72 | −2.1 – 1.4 | 0.41 | 0.78 | −2.1 – 1.7 | * |
| pCRH level, time 1 | 58.53 | 74.91 | 1.1 – 1443.0 | 73.55 | 75.87 | 4.3 – 788.9 | 50.21 | 73.14 | 1.1 – 1443.0 | * |
| pCRH level, time 2 | 364.39 | 425.63 | 4.5 – 6385.0 | 451.41 | 424.73 | 18.2 – 3515.0 | 316.30 | 418.87 | 4.5 – 6385.0 | * |
| pCRH rate of rise from time 1 to time 2 | 305.86 | 392.72 | 3.4 – 6276.0 | 377.87 | 386.65 | 13.5 – 3304.0 | 266.09 | 390.86 | 3.4 – 6276.0 | * |
| Gestational age (at birth) | 38.95 | 1.34 | 31.5 – 42.0 | 38.96 | 1.43 | 28.5 – 42.0 | 38.65 | 2.10 | 22.4 – 41.6 | * |
| Birth weight (grams) | 3277.8 | 498.1 | 1227.0 – 4954.0 | 3420.7 | 525.3 | 930.0 – 4954.0 | 3128.4 | 561.2 | 200.0 – 4685 | * |
| Categorical Variables | N (%) | N (%) | N (%) | |||||||
|
| ||||||||||
| Marital status | ||||||||||
| Married/living together with partner | 764 | (57.7%) | 427 | (89.9%) | 337 | (39.8%) | * | |||
| Single/widowed/divorced/separated/never married | 560 | (35.3%) | 48 | (10.1%) | 510 | (60.2%) | ||||
| Gestational diabetes | ns | |||||||||
| Yes | 75 | (5.7%) | 33 | (6.9%) | 42 | (5.0%) | ||||
| No | 1235 | (94.3%) | 442 | (93.1%) | 791 | (95.0%) | ||||
| Gestational hypertension or preeclampsia | ns | |||||||||
| Yes | 124 | (9.5%) | 38 | (8.0%) | 85 | (10.2%) | ||||
| No | 1185 | (90.5%) | 436 | (92.0%) | 748 | (89.8%) | ||||
| Tobacco use during pregnancy | ||||||||||
| Yes | 176 | (13.3%) | 66 | (13.9%) | 109 | (12.9%) | ns | |||
| No | 1148 | (86.7%) | 409 | (86.1%) | 738 | (87.1%) | ||||
Note:
p<.05 column indicates differences between Whites and Blacks.
Ns= Non-statistically significant.
Maternal Stress exposures
Childhood Traumatic Events (CTE).
Exposure to CTE was measured during the second trimester using the three items from the Traumatic Life Events Questionnaire (TLEQ) (Kubany et al., 2000), assessing whether women were subjected to 1) physical abuse or 2) witnessing family violence “while growing up” or 3) sexual abuse “before age thirteen”. A count of the number of potentially traumatic childhood adversities experienced was created (range 0–3) (Slopen et al., 2018).
Traumatic Life Events in Adulthood.
Maternal exposure to traumatic events in adulthood was assessed using 17 items from the TLEQ pertaining to adulthood (Kubany et al., 2000; Slopen et al., 2018). A count of the number of potentially traumatic events experienced (e.g., warfare, serious accidents, natural disaster, sudden death of a loved one) was created (range 0–17).
Interpersonal Violence Victimization during Pregnancy.
Maternal exposure to physical, sexual or emotional abuse and/or injury during pregnancy was measured during the second trimester using the short form of the Conflict Tactics Scale (CTS) (Straus & Douglas, 2004), which measures the extent to which romantic partners engage in physical and psychological attacks on each other. Here, four dichotomous items assessing maternal victimization (yes/no) from physical/sexual/emotional abuse and injury were summed to calculate a victimization score (range 0–4).
Neighborhood Crime and Deprivation.
To assess potential stress exposure from neighborhood adversities, a composite variable comprising geospatially-linked objectively-measured information about neighborhood violent crime (derived from the Neighborhood Scout Violent Crime Index)(Goldman-Mellor et al., 2016), and neighborhood quality (derived from the Childhood Opportunity Index (COI), a geocode-linked database of a nationally-validated index that compares social and economic risks and opportunities relative to nearby neighborhoods) (Acevedo-Garcia et al., 2014). A detailed description of these measures and the creation of this composite variable is provided in Supplementary Material.
Inter-correlations between the four stress exposure variables were small to moderate, ranging from .05 to .40. Multiple maternal obstetric/clinical/health related factors were included as covariates, based on the existing literature. An overview of all covariates is provided in Table 2, and a description of their sources are provided in Supplementary Material.
Table 2.
Maternal Childhood Traumatic Events and Covariate Variables predicting pCRH Levels and Rate of Rise across Pregnancy.
| GLM pCRH level, time 1 | GLM pCRH level, time 2 | Mixed Model pCRH rise from time 1 to time 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| β | SE | t-value | p | β | SE | t-value | p | β | SE | t-value | p | |
|
|
|
|||||||||||
| Maternal childhood traumatic events (CTE) (CTE) | .04 | .02 | 1.45 | .06 | .03 | 2.10 | * | −.07 | .05 | −1.32 | ||
| Maternal CTE x Gestational Weeks | - | - | - | - | - | - | .00 | .00 | 2.38 | * | ||
| Gestational weeks (time 1) | .17 | .01 | 29.69 | *** | - | - | - | - | - | - | ||
| Gestational weeks (time 2) | - | - | - | - | .23 | .01 | 20.81 | *** | - | - | - | |
| Repeated measures gestational weeks T1–2 | - | - | - | - | - | - | - | - | .21 | .00 | 125.32 | *** |
| Maternal age | .01 | .01 | 2.01 | * | .00 | .00 | 1.74 | .01 | .01 | 2.01 | * | |
| Married/cohabiting 1 | −.00 | .04 | −.03 | −.01 | .05 | −.18 | −.00 | .05 | −.05 | |||
| White 2 | .41 | .05 | 8.48 | *** | .33 | .05 | 6.20 | *** | .38 | .05 | 7.69 | *** |
| Parity | −.03 | .01 | −2.33 | * | −.04 | .02 | −2.80 | * | −.04 | .01 | −2.89 | * |
| Socioeconomic status | .01 | .03 | .20 | .02 | .04 | .67 | .02 | .03 | .52 | |||
| Smoking during pregnancy3 | −.06 | .06 | .−1.06 | −.00 | .06 | −.06 | −.04 | .06 | −.69 | |||
| Pre-pregnancy BMI | −.02 | .00 | 6.75 | *** | −.02 | .00 | −5.47 | *** | −.02 | .00 | −6.32 | *** |
| BSI depression score | .00 | .00 | .23 | −.00 | .00 | −.57 | −.00 | .00 | −.29 | |||
| Gestational diabetes 4 | .08 | .08 | 1.07 | .27 | .09 | 3.00 | ** | .17 | .08 | 2.18 | * | |
| Gestational hypertension/preeclampsia 5 | .22 | .06 | 3.48 | *** | .45 | .07 | 6.47 | *** | .34 | .06 | 5.44 | *** |
| Fetal sex 6 | −.00 | .04 | −.12 | −.03 | .04 | −.71 | −.02 | .03 | −.58 | |||
| Maternal social support | .01 | .01 | .62 | .00 | .01 | .21 | .00 | .01 | .40 | |||
Note: Level 1 and Level 2 models used GLM regression. Rise/change model used a mixed model approach, and the effect of CTE on rise is interpreted from the interaction coefficient.
‘Single/widowed/divorced/separated/never married’ used as reference group.
‘Black’ used as reference group.
‘No smoking’ used as reference group.
‘No gestational diabetes’ used as reference group.
‘No gestational hypertension/preeclampsia’ used as reference group.
‘Female’ used as reference group.
β= Standardized regression coefficients. SE= Standard errors for standardized regression coefficient.
p< .05
p< .01
p< .001.
Statistical Analyses
SAS 9.4 was used to conduct all analyses (SAS, 2008). Descriptive analyses for continuous and categorical variables for the overall sample and by race were conducted using PROC MEANS and PROC FREQ. General linear models, using PROC GLM, were fitted to the data for the analyses looking at level (time 1 and time 2 pCRH). For the models examining change in pCRH, we used a mixed model (PROC MIXED) with a random intercept. Models were run in three steps: 1) the three primary models including CTE and all covariates, 2) adding an interaction term for CTE with race, and 3) adding the three additional stress exposure variables, each in their own model. Models predicting pCRH levels (at time 1 and time 2) were adjusted for gestational weeks at the time of the collection. In the mixed models, we examined the interaction between stress exposure (e.g., CTE) and continuous gestational weeks (timing) to understand the effect of CTE exposure on pCRH rate of rise across pregnancy.
Results
Figure 1 shows log-transformed pCRH levels as a function of gestational age. Descriptive statistics for all main study variables, including raw pCRH levels, are displayed in Table 1, by total sample and by race given limited existing reference data on longitudinal pCRH in diverse samples. Notably, pCRH levels and rise were lower for Black women.
Figure 1:
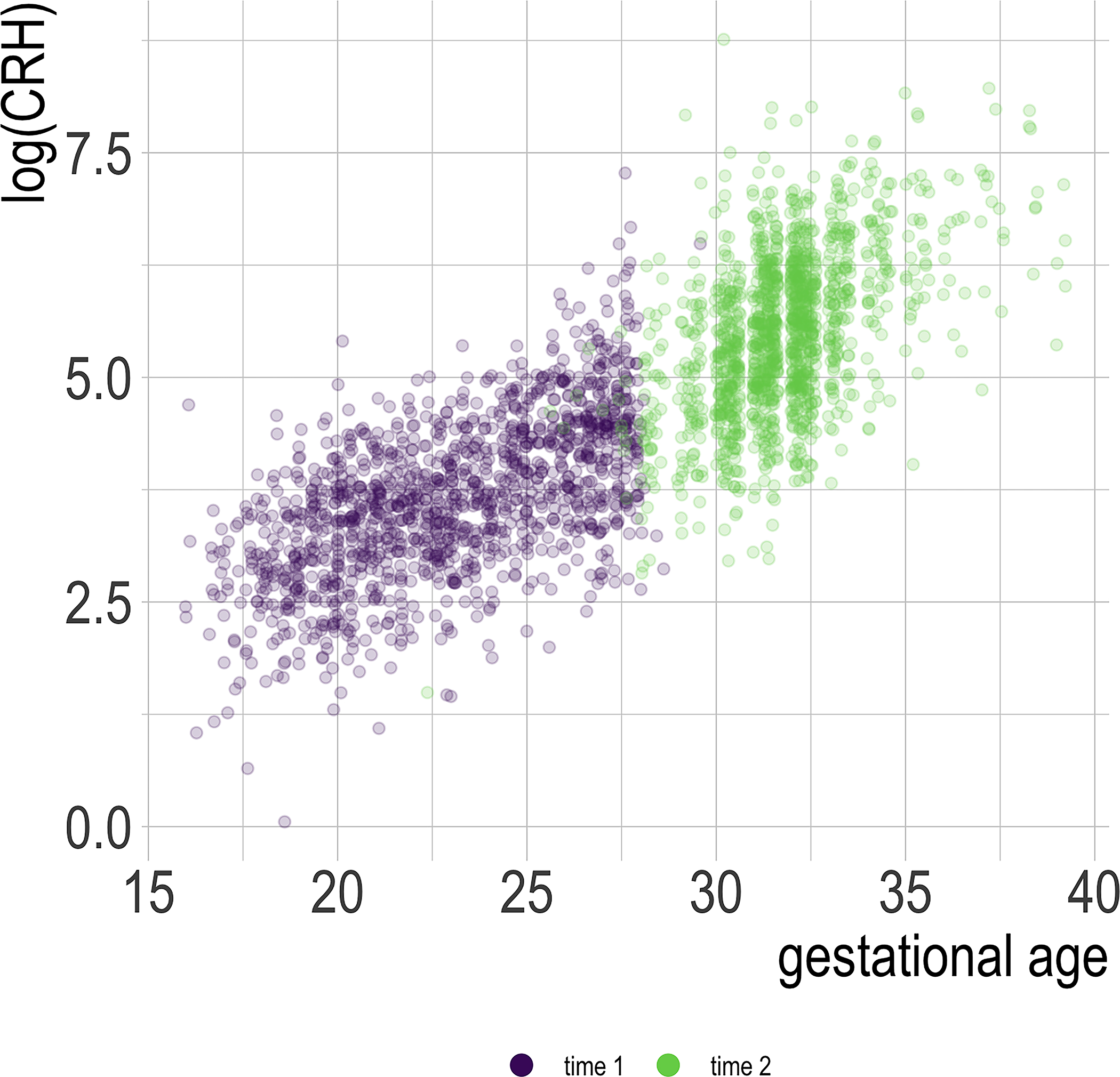
Plot of log-transformed pCRH by gestational age
Maternal CTE
Results of primary regression analyses and mixed models are displayed in Table 2. Adjusting for all covariates, maternal CTE did not predict pCRH levels at time 1 (β=.04, p=.15), but did predict pCRH levels at time 2 (β=.06, p=.04). In the mixed models testing the association between CTE and repeated measure of pCRH over time, the interaction term between CTE and gestational weeks (time) was significant, showing a positive association with the magnitude of the rise in pCRH between participants’ two time points of assessment (β=.005, p=.02). The significant covariance parameter estimate (0.37, p<.001) indicated considerable between-participant variation in the starting value, with the calculated the intraclass coefficient indicating that 77% of the variability in pCRH is accounted for by the participant. Examination of the simple slopes for each level of CTE endorsement indicated a slight increase in rise for each additional traumatic event (β ACES =0 = 0.2055, p =<.001, β ACES =1 = 0.2099, p =<.001, β ACES =2 = 0.2143, p =<.001, βACES =3 = 0.2188, p =<.001).
Although not the focus of this study, the covariates that most strongly predicted pCRH across pregnancy were being White, gestational diabetes, and gestational hypertension/preeclampsia (see Table 2).
Effect modification by race.
No statistically significant interaction effects were found between CTE and race, predicting pCRH, at any time points or rate of rise in pCRH (results not shown).
Additional Multilevel and Exposure Period Stressors
Adjusting for all covariates and CTE, maternal traumatic life events in adulthood (.01<p<.69), interpersonal violence victimization during pregnancy (.02<p<.39), or neighborhood crime and deprivation (.02<p<.27), did not significantly associate with pCRH levels or rate of rise (nonsignificant interaction term in mixed model) in the models (results not shown).
Discussion
In a large, racially and sociodemographically diverse sample of women, the present study examined the association of maternal CTE with the level and rate of rise of pCRH during pregnancy, whether such association varied by race, and whether the consideration of traumatic events in adulthood, interpersonal violence, or neighborhood risk during pregnancy, would provide additional predictive value. As hypothesized, even after rigorous confounder adjustment, CTE predicted pCRH level in later pregnancy and rate of rise across pregnancy. No other stressors tested added to the prediction of pCRH.
Maternal CTE, in the form of exposure to sexual or physical abuse or family violence during childhood, did not predict levels of pCRH at time 1 (largely corresponding to the 2nd trimester), which is consistent with Chen and colleagues’ (Y. Chen et al., 2010) finding of no association between CTE and pCRH levels measured between 15–27 weeks gestation. CTE did predict pCRH levels at time 2 (predominantly during the 3rd trimester), with an effect corresponding to a 5.5% increase in pCRH levels for each additional CTE. This timing-specific finding indicates that differences in pCRH levels as a function of CTE emerge during later stages of pregnancy. CTE also predicted as greater rate of pCRH rise between assessments, although the increase was small. The pattern of associations found here for level and rise are consistent with those of Moog and colleagues (Moog et al., 2016) and were detected after controlling for the same obstetric, clinical, and health related potential confounders shown to be associated with both CTE and pCRH levels, and additional variables not included in their model (maternal age, maternal education level, marital status, fetal sex, gestational week, and maternal social support). Further, although CTE increases the risk for gestational diabetes and preeclampsia (Roberts et al., 2013), smoking during pregnancy (Roberts et al., 2013), prenatal depression (Wosu et al., 2015), and lower education levels (Fergusson et al., 2013)—all factors associated with pCRH levels (Y. Chen et al., 2010; Ellman et al., 2008; Holzman et al., 2001; Justus et al., 2004; Kramer et al., 2010; Laatikainen et al., 1991; Mancuso et al., 2004; Sandman et al., 2006)— CTE remained significant despite inclusion of these variables as covariates in the present study. This increases confidence in the association found and suggests CTE-effects are not fully mediated by those health behaviors and problems in adulthood. Our findings also expand upon previous work (Moog et al., 2016) by showing that inclusion of additional lifetime and pregnancy period psychosocial stressors did not add predictive value, even within a large sample with broad exposure across those domains. Taken together, these findings highlight the potency of exposure to adverse events early in life in predisposing the individual to persistent, measurable changes in the pCRH-system. Subsequently, this implicates the placental-fetal pCRH-system as a biologically plausible mechanism linking CTE to adverse pregnancy-related, maternal psychiatric, and child developmental outcomes.
The overall study findings suggest that childhood exposure to traumatic events may affect female development in a manner that relates to their later life pregnancy levels of pCRH - one of the most salient signals shaping human fetal development (Sandman, 2018). Higher pCRH levels have potentially profound consequences for both the mother and the child, as established by studies linking higher pCRH levels to biomedical risks such as preeclampsia and pregnancy-induced hypertension (Harville et al., 2009; Laatikainen et al., 1991), adverse pregnancy outcomes such as fetal growth restriction and pre-term birth (Lee, 2014; Wadhwa et al., 2004), psychiatric health in mothers including postpartum depression (Glynn & Sandman, 2014; Yim et al., 2009), and to early life development and phenotypes of psychiatric outcomes in the child, including infant fear and distress (Davis et al., 2005), cortical thinning, externalizing behaviors (Sandman et al., 2018), and depression and anxiety symptoms (Howland et al., 2016). Of note, post hoc analyses showed that both pCRH levels at time 2 and the rate of rise in pCRH were correlated with gestational weeks at birth (Pearson’s r = .50 and .52 for time 2 level and rate of rise, respectively, p< .0001) and birth weight (Pearson’s r = −.08 and −.07, for time 2 level and rate of rise, respectively, p< .05) in the present sample, confirming relevance for such outcomes in this sample. Building on the collective evidence from other studies, our findings here point to the possibility that maternal childhood trauma exposure may affect her pregnancy, birth outcomes, and postpartum mental health, as well as her child’s development and mental health across its lifecourse, via influence on pCRH levels. The apparent transdiagnostic and intergenerational implications of pCRH associations with childhood trauma exposure suggest further study in this area may be of high public health value.
While our study does not provide information about how CTE lead to higher pCRH levels, several non-mutually exclusive mechanistic pathways are plausible. For example, CTE might affect pCRH via influences on brain development and life-long patterns of emotionality and stress responsivity. Specifically, exposure to CTE or other childhood adversities are linked to structural and functional alterations of brain areas and neuroendocrine systems involved in stress response regulation, cognition, and emotion (McEwen, 2007), including hyper-reactivity of the amygdala (Hein & Monk, 2017; Malter Cohen et al., 2013), a brain area crucially involved in threat detection, fear arousal and conditioning, and HPA-axis reactivity and regulation (Pruessner et al., 2010; Roozendaal et al., 2009). CTE-related HPA-axis hyperactivity has been shown in both pregnant (Bublitz & Stroud, 2012; Buss et al., 2016; Thomas et al., 2018) and non-pregnant women (Heim et al., 2000) and may result, in part, from impaired glucocorticoid-induced negative feedback inhibition of hypothalamic CRH-secretion due to altered glucocorticoid receptor functioning (Frodl & O’Keane, 2013; Ladd et al., 2004), possibly caused by epigenetic modifications of the glucocorticoid receptor gene (McGowan et al., 2009; Palma-Gudiel et al., 2015). Veritably, animal experimental studies have linked exposure to stressful events early in life to increased basal levels of CRH in the brain (Coplan et al., 1996; Coplan et al., 2001), and to increased CRH gene expression and lower methylation of the CRH gene promoter (J. Chen et al., 2012; A. Wang et al., 2014), demonstrating that early life stress can persistently alter CRH gene expression and overall levels. In humans, exposure to CTE or a broader range of childhood adversities strongly predict developing PTSD and depression (Hughes et al., 2017), both of which have been linked to higher CRH levels in the brain (Bremner et al., 1997; Nemeroff et al., 1984). Thus, CTE-induced neurobiological alterations associated with the function of key stress response systems within women’s bodies could affect the stress-responsive fetal-placental CRH system, and- via the glucocorticoid-induced positive feedback mechanism characterizing this particular system (B. G. Robinson et al., 1988; Sirianni et al., 2005)- contribute to higher pCRH levels among CTE-exposed women.
Childhood adversity might also influence pCRH levels via early life programming of immune phenotypes (Carroll et al., 2011). CTE and a broader range of childhood adversities have been linked to elevated inflammation levels in both cross-sectional population studies (Lin et al., 2016) and longitudinal studies of youth- (Slopen et al., 2013) and adults (Baumeister et al., 2016). Pro-inflammatory markers, in turn, have been shown to stimulate expression of CRH in both the placenta (Uh et al., 2008) and in human endometrial cell cultures (Makrigiannakis et al., 1999), and reversely, CRH has pro-inflammatory effects on the intrauterine environment (You et al., 2014). Pro-inflammatory markers have also been shown to suppress the expression of 11-hydroxysteroid dehydrogenase type 2 (HSD11B2) (Kossintseva et al., 2006), a placental barrier enzyme that buffers the impact of maternal glucocorticoid exposure, and suppression of which may result in high fetal exposure to maternal cortisol (Christiaens et al., 2008; Kossintseva et al., 2006). Thus, early life programming of immune phenotypes could be another developmental mediator translating CTE into biological risk, via their downstream effects on maternal prenatal stress physiology and gestational biology.
Race did not moderate the association between CTE and pCRH. White women exhibited higher levels and rise of pCRH, relative to Black women, consistent with previous reports (Y. Chen et al., 2010; Harville et al., 2009; Holzman et al., 2001). Yet, given evidence that Black women experience more stress during pregnancy (Borders et al., 2015), and the positive correlation between stress and pCRH (Hobel et al., 1999; Latendresse & Ruiz, 2010; B. G. Robinson et al., 1988), the direction of this difference appears incongruent. One potential explanation could be racial differences in chronic stress, which has been linked to lower pCRH levels (Guendelman et al., 2008), and is more prevalent among Black women (Borders et al., 2015). Another possible explanation comes from biomedical studies implicating a potential role of the placental barrier enzyme HSD11B2 in ancestry-specific differences in pCRH levels. Maternal distress has been shown to suppress placental HSD11B2 in animal experimental studies (Jensen Peña et al., 2012; Mairesse et al., 2007) and in pregnant women (O’Donnell et al., 2012; Seth et al., 2015), although only for White women (Capron et al., 2018), which potentially could translate to racial differences in downstream effects on the cortisol-responsive pCRH system. Finally, there may be cultural differences in the exposure to and appraisal of stressors (Brown et al., 2020), making counts of exposure types less predictive than reports of distress. Future studies in diverse populations are warranted in order to understand the role of racial differences in pCRH and maternal and child health disparities.
Interpersonal violence victimization, neighborhood adversity, and traumatic events in adulthood, did not add to the prediction of pCRH in the present study. Given that CTE-exposure increases the risk of interpersonal violence victimization, adulthood traumatic events, and lower socioeconomic status (Fergusson et al., 2013), these variables were potential mediators in the association between CTE and pCRH levels, making their effects difficult to untangle in models adjusting for CTE. However, the low correlation of CTE with these variables in the present sample makes this explanation unlikely. Taken together with the finding that only CTE predicted pCRH levels and rise, a parsimonious interpretation of the overall study findings is that the timing of exposure to stressful events is a key determining factor of whether maternal stress becomes biologically embedded in her prenatal stress physiology, with childhood events being particularly potent. Such interpretation is in line with a large empirical literature demonstrating biological embedding of childhood adversities specifically (Berens et al., 2017; Danese & Lewis, 2017; Heim et al., 2019; Miller et al., 2011), but should be further tested.
Although not the focus of this study, the large, diverse cohort provides important information about maternal factors relevant for pCRH levels and rise. Alongside race, gestational diabetes and hypertension were the strongest predictors of pCRH levels at time 2 and the magnitude of pCRH rise across pregnancy. These findings are consistent with previous studies linking preeclampsia and pregnancy-induced hypertension to higher pCRH levels (Emanuel et al., 1994; Harville et al., 2009; Laatikainen et al., 1991), but to our knowledge provide the first evidence of an association with gestational diabetes. In this Urban South sample with many overweight women, BMI was inversely associated with pCRH. Increased BMI has been associated with defective uterine contraction at delivery and post-dates delivery (McLean et al., 1995), and low pCRH has also previously been associated with post-dates delivery (McLean et al., 1995).
Clinical and Policy Implications
A massive empirical literature has established CTE and a broader range of childhood adversities as a major public health concern and root cause underlying an extensive range of psychosocial and somatic health problems (Hughes et al., 2017), mortality (Bellis et al., 2015), socioeconomic- (Fergusson et al., 2013; Pinto Pereira et al., 2016), birth related- (Leigh & Milgrom, 2008; Roberts et al., 2013; M. V. Smith et al., 2016), and offspring developmental outcomes (Moog et al., 2018; Roberts et al., 2013), and immense societal costs (Bellis et al., 2017; C. T. Wang & Holton, 2007). The present study indicates, advancing previous evidence (Moog et al., 2016), that effects of maternal CTE may extend intergenerationally to offspring via shifts in the gestational environment related to higher circulating pCRH. Given the large literature linking higher pCRH levels to biomedical risks (Emanuel et al., 1994; Laatikainen et al., 1991), adverse pregnancy outcomes (Wadhwa et al., 2004), psychiatric health in mothers (Yim et al., 2009), and to early life development and phenotypes of psychiatric outcomes in the child (Davis et al., 2005; Howland et al., 2016; Sandman et al., 2018), this work calls for primary prevention to reduce the occurrence of CTE and secondary prevention to disrupt adverse developmental trajectories and the intergenerational transmission of their effects. This work also points to windows of opportunity to interrupt negative developmental trajectories and prevent intergenerational cascades of childhood adversity (Borja et al., 2019), which can include universal screening for childhood adversities among pregnant women, specifically. The majority of women are comfortable with childhood adversity screenings during pregnancy and with discussing screening results with their doctors (Flanagan et al., 2018). Consequently, physicians should routinely inquire about physical/sexual/psychological abuse as a part of their patients’ medical history; consider such exposures as possible factors in the presentation of medical complaints; and collectively provide leadership in raising awareness about the need for such assessments (American Medical Association). Critical in this path is development of reliable referral networks to and provision of psychiatric treatment, where indicated (Finkelhor, 2018). Though findings should be replicated using broader measures of childhood adversity, the predictive value of even simple screening tools is suggested by the present study, where the association of CTE with pCRH levels was detected using a simple, three-item CTE-screening tool.
Study strengths and limitations
Key strengths of the present study include the large, racially diverse sample, the control for multiple obstetric, clinical, and health related factors in the statistical analyses, and the examination of a wide range of stress exposures. Additional strengths include the thorough, state of the art assay of the pCRH samples ensuring precise, reliable CRH values. Limitations include having only two time points of pCRH with which to estimate rise over pregnancy. Moreover, there were some cases of overlap in the assessment time windows. Although sensitivity analyses showed that removal of these cases did not change the pattern of association between CTE and pCRH (data not shown), future studies should consider including more assessment time points to enable more complex statistical modeling that may capture nuances in the temporality of pCRH levels over time. The recall of CTE items was limited to three domains of traumatic events, and a richer assessment across a broader range of exposures would be informative. The recall of CTE items was limited to three domains of traumatic events, and a richer assessment across a broader range of exposures would be informative. Specifically, the three CTE-items used in the present study all captured exposure to sexual or physical violence, which may be classified as a threatening, whereas no items on our measure captured deprivation adversity (McLaughlin et al., 2014; Sheridan & McLaughlin, 2014). Given studies indicating that experiences of threat and deprivation may differentially influence neurodevelopment and psychobiology (Lambert et al., 2017; Machlin et al., 2019; McLaughlin et al., 2019), it is possible that a broader measure that also captured deprivation adversity (such as the CDC ACEs 10-item scale) may have produced different results. Future studies using a broader measure capturing of both threat- and deprivation CTE will be informative in terms of ascertaining whether the associations found here are specific to violence exposure in childhood, or adversity more broadly. Furthermore, although measures of perceived stress have shown low correlations with exposure to stressful life events (Bush et al., 2017) and stress biomarkers (see for example Harville et al., 2009), the present study did not include assessment of psychological distress, and such measures may have provided additional information of value in these models, thus future studies should consider their inclusion. Finally, although enrolling only women with low-medical risk pregnancies improved retention and limited fetal loss for the cohort, and still resulted in a sample broadly representative of the county it was drawn from, analyses within this cohort are not fully representative of all types of pregnancies, and effects of CTE may be most pronounced in women who were excluded at enrollment due to health conditions.
Conclusions
The present study found an association between experiencing traumatic events in childhood, such as physical or sexual abuse or domestic violence, and higher levels and rise of pCRH across pregnancy in a large, sociodemographically and racially diverse sample of women, after adjusting for multiple obstetric, clinical, and health related factors associated with pCRH levels. Taken together with the extant literature, the findings implicate the placental-fetal unit as a mechanistic pathway through which intergenerational transmission of the adverse effects of childhood trauma may take place, and add to the growing literature calling for systematic efforts to prevent childhood trauma and its adverse effects.
Supplementary Material
Acknowledgements
This work was funded by the Urban Child Institute (awarded to Tylavsky, Bush, and LeWinn), the ECHO PATHWAYS consoritium (NIH: 1UG3OD023271–01 and 4UH3OD023271–03, awarded to Bush, LeWinn, Tylavsky, Sathyarayana, and Karr) and the CANDLE Developmental Origins of Health and Disease study (CIHR award number MWG-146331, awarded to Kobor). Dr. Bush is the Lisa and John Pritzker Distinguished Professor of Developmental and Behavioral Health and receives support from the John and Lisa Pritzker Family Foundation. Dr. Steine is funded by the Norwegian Dam Foundation (2018/FO187445) and the Meltzer Foundation. Dr. R. Smith is a University of Newcastle Laureate Professor and an Australian National Health and Medical Research Senior Principal Research Fellow. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript; its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders. The authors would like to thank the CANDLE study participants for their valuable contributions to this work; CANDLE study team members Maureen Sorrells, Lauren Sims Taylor, and Anum Abass for their leadership in data collection and processing; the ECHO PATHWAYS team Christine Loftus, Marnie Hazlehurst, and Ellen Kersten for their support in data compilation and prep; Meaghan Jones for her contributions to obtaining some of the funding for this work; and Michael Coccia for his support with figure development
Footnotes
Disclosure and conflicts of interest: The authors have no disclosures or conflict of interest.
References
- Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, et al. (2014). The Child Opportunity Index: Improving Collaboration Between Community Development And Public Health. Health Affairs, 33, 1948–1957. [DOI] [PubMed] [Google Scholar]
- Agorastos A, Pervanidou P, Chrousos GP, & Baker DG (2019). Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front Psychiatry, 10, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Medical Association. Preventing, Identifying & Treating Violence & Abuse. pp. AMA Principles of Medical Ethics: I, III): AMA Principles of Medical Ethics: I, III. [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry, 21, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis MA, Hughes K, Hardcastle K, Ashton K, Ford K, Quigg Z, et al. (2017). The impact of adverse childhood experiences on health service use across the life course using a retrospective cohort study. Journal of Health Services Research & Policy, 22, 168–177. [Google Scholar]
- Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, & Lowey H (2015). Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. Journal of public health (Oxford, England), 37, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens AE, Jensen SKG, & Nelson CA 3rd (2017). Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Medicine, 15, 135–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, & Glover V (2007). Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry, 46, 1454–1463. [DOI] [PubMed] [Google Scholar]
- Borders AEB, Wolfe K, Qadir S, Kim KY, Holl J, & Grobman W (2015). Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. Journal of perinatology : official journal of the California Perinatal Association, 35, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borja S, Nurius PS, Song C, & Lengua LJ (2019). Adverse childhood experiences to adult adversity trends among parents: Socioeconomic, health, and developmental implications. Children and Youth Services Review, 100, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. (1997). Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry, 154, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Mitchell UA, & Ailshire JA (2020). Disentangling the Stress Process: Race/Ethnic Differences in the Exposure and Appraisal of Chronic Stressors Among Older Adults. J Gerontol B Psychol Sci Soc Sci, 75, 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublitz MH, & Stroud LR (2012). Childhood sexual abuse is associated with cortisol awakening response over pregnancy: preliminary findings. Psychoneuroendocrinology, 37, 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa G, Dahan S, Sinclair I, St-Pierre M, Roofigari N, Mutran D, et al. (2018). Prenatal stress and child development: A scoping review of research in low- and middle-income countries. PLOS ONE, 13, e0207235–e0207235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, et al. (2017). Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Dev Psychopathol, 29, 1553–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Wadhwa PD, Adam E, Culhane J, Grobman W, et al. (2016). Maternal childhood trauma and cortisol during pregnancy. American Journal of Obstetrics & Gynecology, 214, S350–S351. [Google Scholar]
- Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, & Lowry PJ (1987). Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab, 64, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Capron LE, Ramchandani PG, & Glover V (2018). Maternal prenatal stress and placental gene expression of NR3C1 and HSD11B2: The effects of maternal ethnicity. Psychoneuroendocrinology, 87, 166–172. [DOI] [PubMed] [Google Scholar]
- Carr A, Duff H, & Craddock F (2018). A Systematic Review of Reviews of the Outcome of Noninstitutional Child Maltreatment. Trauma, Violence, & Abuse, 1524838018801334. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, & Marsland AL (2011). Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun, 25, 1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, & Aguilera G (2012). Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J Neuroendocrinol, 24, 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Holzman C, Chung H, Senagore P, Talge NM, & Siler-Khodr T (2010). Levels of maternal serum corticotropin-releasing hormone (CRH) at midpregnancy in relation to maternal characteristics. Psychoneuroendocrinology, 35, 820–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, & Olson DM (2008). Inflammatory processes in preterm and term parturition. J Reprod Immunol, 79, 50–57. [DOI] [PubMed] [Google Scholar]
- Class QA, Lichtenstein P, Långström N, & D’Onofrio BM (2011). Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom Med, 73, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. (1996). Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A, 93, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Smith EL, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, et al. (2001). Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiatry, 50, 200–204. [DOI] [PubMed] [Google Scholar]
- Danese A, & Lewis SJ (2017). Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology, 42, 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & Tan M (2013). Childhood maltreatment and obesity: systematic review and meta-analysis. Molecular psychiatry, 19, 544. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, & Sandman CA (2005). Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci, 27, 299–305. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of child psychology and psychiatry, and allied disciplines, 52, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, & Sandman CA (2008). Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol, 50, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel RL, Robinson BG, Seely EW, Graves SW, Kohane I, Saltzman D, et al. (1994). Corticotrophin releasing hormone levels in human plasma and amniotic fluid during gestation. Clinical Endocrinology, 40, 257–262. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, & Wust S (2009). Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav, 55, 292–298. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, McLeod GFH, & Horwood LJ (2013). Childhood sexual abuse and adult developmental outcomes: Findings from a 30-year longitudinal study in New Zealand. Child Abuse & Neglect, 37, 664–674. [DOI] [PubMed] [Google Scholar]
- Finkelhor D (2018). Screening for adverse childhood experiences (ACEs): Cautions and suggestions. Child Abuse Negl, 85, 174–179. [DOI] [PubMed] [Google Scholar]
- Flanagan T, Alabaster A, McCaw B, Stoller N, Watson C, & Young-Wolff KC (2018). Feasibility and Acceptability of Screening for Adverse Childhood Experiences in Prenatal Care. J Womens Health (Larchmt), 27, 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, & O’Keane V (2013). How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of Disease, 52, 24–37. [DOI] [PubMed] [Google Scholar]
- Glynn LM, & Sandman CA (2014). Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med, 76, 355–362. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, & Sandman CA (2007). Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides, 28, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Goldman-Mellor S, Margerison-Zilko C, Allen K, & Cerda M (2016). Perceived and Objectively-Measured Neighborhood Violence and Adolescent Psychological Distress. J Urban Health, 93, 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths SK, & Campbell JP (2014). Placental structure, function and drug transfer. BJA Education, 15, 84–89. [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, & King RG (2004). Growth and function of the normal human placenta. Thrombosis Research, 114, 397–407. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Lang Kosa J, Pearl M, Graham S, & Kharrazi M (2008). Exploring the relationship of second-trimester corticotropin releasing hormone, chronic stress and preterm delivery. The Journal of Maternal-Fetal & Neonatal Medicine, 21, 788–795. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, & Thorp JM (2009). Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt), 18, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Entringer S, & Buss C (2019). Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology, 105, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport D, Heit S, & et al. (2000). Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Jama, 284, 592–597. [DOI] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. J Child Psychol Psychiatry, 58, 222–230. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, & Arora CP (1999). Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol, 180, S257–263. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, & Barrett ES (2008). Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol, 51, 333–348. [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Siler-Khodr T, Fisher R, & Rip T (2001). Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol, 97, 657–663. [DOI] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, Glynn LM, Crippen C, & Davis EP (2016). Fetal exposure to placental corticotropin-releasing hormone is associated with child self-reported internalizing symptoms. Psychoneuroendocrinology, 67, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. (2017). The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health, 2, e356–e366. [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Edelsohn G, & Kellam SG (2001). A Further Look at the Prognostic Power of Young Children’s Reports of Depressed Mood and Feelings. Child Development, 72, 736–747. [DOI] [PubMed] [Google Scholar]
- Jensen Peña C, Monk C, & Champagne FA (2012). Epigenetic Effects of Prenatal Stress on 11β-Hydroxysteroid Dehydrogenase-2 in the Placenta and Fetal Brain. PLOS ONE, 7, e39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus K, Arora C, Sandhu M, & Hobel C (2004). The effect of smoking on the time course release of catecholamines and corticotropin releasing (CRH) hormone. American Journal of Obstetrics & Gynecology, 191, S21. [Google Scholar]
- Kossintseva I, Wong S, Johnstone E, Guilbert L, Olson DM, & Mitchell BF (2006). Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. Am J Physiol Endocrinol Metab, 290, E282–288. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, et al. (2009). Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol, 169, 1319–1326. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, et al. (2010). Non-stress-related factors associated with maternal corticotrophin-releasing hormone (CRH) concentration. Paediatric and Perinatal Epidemiology, 24, 390–397. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, et al. (2000). Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess, 12, 210–224. [DOI] [PubMed] [Google Scholar]
- Laatikainen T, Virtanen T, Kaaja R, & Salminen-Lappalainen K (1991). Corticotropin-releasing hormone in maternal and cord plasma in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol, 39, 19–24. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, & Plotsky PM (2004). Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mrna and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological Psychiatry, 55, 367–375. [DOI] [PubMed] [Google Scholar]
- Lambert HK, King KM, Monahan KC, & McLaughlin KA (2017). Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Development and Psychopathology, 29, 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse G, & Ruiz RJ (2010). Maternal coping style and perceived adequacy of income predict CRH levels at 14–20 weeks of gestation. Biol Res Nurs, 12, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C (2014). Intergenerational health consequences of in utero exposure to maternal stress: Evidence from the 1980 Kwangju uprising. Social Science & Medicine, 119, 284–291. [DOI] [PubMed] [Google Scholar]
- Leeners B, Stiller R, Block E, Görres G, & Rath W (2010). Pregnancy complications in women with childhood sexual abuse experiences. Journal of Psychosomatic Research, 69, 503–510. [DOI] [PubMed] [Google Scholar]
- Leigh B, & Milgrom J (2008). Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry, 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JE, Neylan TC, Epel E, & O’Donovan A (2016). Associations of childhood adversity and adulthood trauma with C-reactive protein: A cross-sectional population-based study. Brain Behav Immun, 53, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomans EM, van Dijk AE, Vrijkotte TGM, van Eijsden M, Stronks K, Gemke RJBJ, et al. (2012). Psychosocial stress during pregnancy is related to adverse birth outcomes: results from a large multi-ethnic community-based birth cohort. Eur J Public Health, 23, 485–491. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci, 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Machlin L, Miller AB, Snyder J, McLaughlin KA, & Sheridan MA (2019). Differential Associations of Deprivation and Threat With Cognitive Control and Fear Conditioning in Early Childhood. Frontiers in Behavioral Neuroscience, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, et al. (2018). A Meta-Analysis of Maternal Prenatal Depression and Anxiety on Child Socioemotional Development. Journal of the American Academy of Child & Adolescent Psychiatry, 57, 645–657.e648. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, et al. (2007). Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab, 292, E1526–1533. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Margioris AN, Zoumakis E, Stournaras C, & Gravanis A (1999). The Transcription of Corticotropin-Releasing Hormone in Human Endometrial Cells Is Regulated by Cytokines. Neuroendocrinology, 70, 451–459. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, & Casey BJ (2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A, 110, 18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, & Hobel CJ (2004). Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med, 66, 762–769. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2003). Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev, 9, 149–154. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiological Reviews, 87, 873–904. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience, 12, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and biobehavioral reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, & Bitrán D (2019). Childhood Adversity and Neural Development: A Systematic Review. Annual Review of Developmental Psychology, 1, 277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, & Smith R (1995). A placental clock controlling the length of human pregnancy. Nat Med, 1, 460–463. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull, 137, 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, et al. (2016). Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol Psychiatry, 79, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Rasmussen JM, Styner M, Gilmore JH, Kathmann N, et al. (2018). Intergenerational Effect of Maternal Exposure to Childhood Maltreatment on Newborn Brain Anatomy. Biol Psychiatry, 83, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. (1984). Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science, 226, 1342–1344. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, & Glover V (2012). Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology, 37, 818–826. [DOI] [PubMed] [Google Scholar]
- Orchard J, & Price J (2017). County-level racial prejudice and the black-white gap in infant health outcomes. Social Science & Medicine, 181, 191–198. [DOI] [PubMed] [Google Scholar]
- Palma-Gudiel H, Córdova-Palomera A, Leza JC, & Fañanás L (2015). Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neuroscience & Biobehavioral Reviews, 55, 520–535. [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl), 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AV, Linton EA, Eben F, Simpson J, Wolfe CDA, & Redman CWG (1995). Corticotrophin-releasing hormone and corticotrophin- releasing hormone binding protein in normal and pre-eclamptic human pregnancies. BJOG: An International Journal of Obstetrics & Gynaecology, 102, 118–122. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Hatch MC, Lapinski R, Stomati M, Reis FM, Cobellis L, et al. (2001). Lack of Effect of Psychosocial Stress on Maternal Corticotropin-Releasing Factor and Catecholamine Levels at 28 Weeks’ Gestation. Journal of the Society for Gynecologic Investigation, 8, 83–88. [PubMed] [Google Scholar]
- Pinto Pereira SM, Li L, & Power C (2016). Child Maltreatment and Adult Living Standards at 50 Years. Pediatrics. [DOI] [PubMed] [Google Scholar]
- Ports KA, Ford DC, & Merrick MT (2016). Adverse childhood experiences and sexual victimization in adulthood. Child Abuse Negl, 51, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, et al. (2010). Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology, 35, 179–191. [DOI] [PubMed] [Google Scholar]
- Qobadi M, Collier C, & Zhang L (2016). The Effect of Stressful Life Events on Postpartum Depression: Findings from the 2009–2011 Mississippi Pregnancy Risk Assessment Monitoring System. Matern Child Health J, 20, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos IF, Guardino CM, Mansolf M, Glynn LM, Sandman CA, Hobel CJ, et al. (2019). Pregnancy anxiety predicts shorter gestation in Latina and non-Latina white women: The role of placental corticotrophin-releasing hormone. Psychoneuroendocrinology, 99, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, & Weisskopf MG (2013). Association of Maternal Exposure to Childhood Abuse With Elevated Risk for Autism in Offspring. JAMA Psychiatry, 70, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Emanuel RL, Frim DM, & Majzoub JA (1988). Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A, 85, 5244–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Oddy WH, Li J, Kendall GE, de Klerk NH, Silburn SR, et al. (2008). Pre- and postnatal influences on preschool mental health: a large-scale cohort study. J Child Psychol Psychiatry, 49, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, & Chattarji S (2009). Stress, memory and the amygdala. Nat Rev Neurosci, 10, 423–433. [DOI] [PubMed] [Google Scholar]
- Sandman CA (2018). Prenatal CRH: An integrating signal of fetal distress. Development and Psychopathology, 30, 941–952. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, & Baram TZ (2018). Cortical Thinning and Neuropsychiatric Outcomes in Children Exposed to Prenatal Adversity: A Role for Placental CRH? Am J Psychiatry, 175, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. (2006). Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides, 27, 1457–1463. [DOI] [PubMed] [Google Scholar]
- SAS (2008). SAS/STAT® 9.2 User’s Guide.
- Schaaf JM, Liem SMS, Mol BWJ, Abu-Hanna A, & Ravelli ACJ (2013). Ethnic and Racial Disparities in the Risk of Preterm Birth: A Systematic Review and Meta-Analysis. Amer J Perinatol, 30, 433–450. [DOI] [PubMed] [Google Scholar]
- Seth S, Lewis AJ, Saffery R, Lappas M, & Galbally M (2015). Maternal Prenatal Mental Health and Placental 11beta-HSD2 Gene Expression: Initial Findings from the Mercy Pregnancy and Emotional Wellbeing Study. Int J Mol Sci, 16, 27482–27496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: deprivation and threat. Trends in cognitive sciences, 18, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirianni R, Rehman KS, Carr BR, Parker CR Jr., & Rainey WE (2005). Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J Clin Endocrinol Metab, 90, 279–285. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, & Koenen KC (2013). Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology, 38, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Roberts AL, LeWinn KZ, Bush NR, Rovnaghi CR, Tylavsky F, et al. (2018). Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology, 98, 168–176. [DOI] [PubMed] [Google Scholar]
- Smith MV, Gotman N, & Yonkers KA (2016). Early Childhood Adversity and Pregnancy Outcomes. Maternal and child health journal, 20, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, et al. (2009). Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab, 94, 2066–2074. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla LM, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, et al. (2015). The Urban Child Institute CANDLE Study Methodological Overview and Baseline Sample Description: RAND Corporation. [Google Scholar]
- Straus MA, & Douglas EM (2004). A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence Vict, 19, 507–520. [DOI] [PubMed] [Google Scholar]
- Thomas JC, Magel C, Tomfohr-Madsen L, Madigan S, Letourneau N, Campbell TS, et al. (2018). Adverse childhood experiences and HPA axis function in pregnant women. Horm Behav, 102, 10–22. [DOI] [PubMed] [Google Scholar]
- Tse AC, Rich-Edwards JW, Koenen K, & Wright RJ (2012). Cumulative stress and maternal prenatal corticotropin-releasing hormone in an urban U.S. cohort. Psychoneuroendocrinology, 37, 970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uh A, Nicholson RC, Gonzalez GV, Simmons CF, Gombart A, Smith R, et al. (2008). Lipopolysaccharide stimulation of trophoblasts induces corticotropin-releasing hormone expression through MyD88. Am J Obstet Gynecol, 199, 317.e311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. (2004). Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. Am J Obstet Gynecol, 191, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Wang A, Nie W, Li H, Hou Y, Yu Z, Fan Q, et al. (2014). Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PLOS ONE, 9, e94394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, & Holton J (2007). Total estimated cost of child abuse and neglect in the United States. Economic impact study. Retrieved April. Chicago, Illinois: : Prevent Child Abuse America. [Google Scholar]
- Wosu AC, Gelaye B, & Williams MA (2015). History of childhood sexual abuse and risk of prenatal and postpartum depression or depressive symptoms: an epidemiologic review. Arch Womens Ment Health, 18, 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, & Sandman CA (2009). Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry, 66, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Liu J, Xu C, Liu W, Zhu X, Li Y, et al. (2014). Corticotropin-Releasing Hormone (CRH) Promotes Inflammation in Human Pregnant Myometrium: The Evidence of CRH Initiating Parturition? The Journal of Clinical Endocrinology & Metabolism, 99, E199–E208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


