Abstract
Purpose
This study aimed to investigate the relationship between monocyte/high-density lipoprotein cholesterol ratio (MHR) and 25-hydroxyvitamin D [25(OH) D] level in patients with type 2 diabetes mellitus (T2DM), the risk factors for vitamin D deficiency, and the clinical value of MHR as a predictor of vitamin D deficiency in this population.
Patients and Methods
This was a cross-sectional study of 260 patients with T2DM from May 2021 to October 2021. Based on internationally used criteria for defining vitamin D levels, the patients were divided according to sex and levels of vitamin D into the following four groups: Group A1 (male patients with vitamin D levels <20 ng/mL), group A2 (male patients with vitamin D levels ≥20 ng/mL), group B1 (female patients with vitamin D levels <20 ng/mL), and group B2 (female patients with vitamin D levels≥20 ng/mL). The MHR was calculated as a monocyte/high-density cholesterol lipoprotein ratio.
Results
The vitamin D level was independently and negatively correlated with the MHR in male patients with T2DM, but not in female patients. The MHR was an independent risk factor and predictor for the development of vitamin D deficiency in male patients, but not in female patients, with T2DM. High-density lipoprotein (HDL) was an independent protective factor for vitamin D deficiency in female patients with T2DM.
Conclusion
This study suggested that the MHR was a new marker for predicting vitamin D deficiency in male patients with T2DM. Alleviating inflammation, improving lipid metabolism, and increasing HDL levels in patients with T2DM might help improve vitamin D levels, which might be important for preventing and managing T2DM. The MHR might help as a new marker to predict vitamin D deficiency in China, where primary hospitals lack the capacity for vitamin D testing on a large scale.
Keywords: monocyte/high-density lipoprotein cholesterol ratio, type 2 diabetes mellitus, vitamin D
Introduction
The prevalence of diabetes has increased dramatically worldwide in the last few decades. Type 2 diabetes (T2DM) is the most prevalent type of diabetes. In China, the prevalence of diabetes among people aged 18 years and older is 11.2%.1 Insulin resistance is the most common cause of type 2 diabetes,2 but inflammation, oxidative stress, and abnormal lipid metabolism are also important causes.
Vitamin D deficiency is a common condition, affecting approximately one billion people worldwide.3 It is not only associated with osteoporosis, but also with metabolic diseases, respiratory diseases, immune system disorders, and inflammatory conditions.4–7 Patients with T2DM are more likely to experience vitamin D deficiency8 than those without T2DM. Therefore, investigating the factors underlying vitamin D deficiency in this population is important. Unfortunately, however, most primary care hospitals in China do not have the capacity to test for vitamin D deficiency on a large scale, underscoring the need to find an indicator that can predict vitamin D deficiency.
The monocyte/high-density lipoprotein (HDL) cholesterol ratio (MHR) is a new and inexpensive marker in inflammation, which can easily be obtained by calculations using blood cell analysis and lipid analysis. Previous studies on the value of MHR focused on cardiovascular diseases and also on the role of MHR as a predictor of mortality and major adverse cardiovascular events among patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention.9 The relationship between MHR and vitamin D is largely unknown. This study aimed to investigate the relationship between MHR and vitamin D and the predictive value of MHR for the development of vitamin D deficiency in this population so as to provide a basis for the prevention and early treatment of vitamin D deficiency in patients with T2DM.
Materials and Methods
Study Participants
Basic information was collected on 260 patients diagnosed with T2DM, including 186 male and 74 female patients aged 18–65 years. Based on the internationally used criteria for defining vitamin D levels, the patients were divided according to sex and levels of vitamin D into the following four groups: Group A1 (male patients with vitamin D levels <20 ng/mL), group A2 (male patients with vitamin D levels ≥20 ng/mL), group B1 (female patients with vitamin D levels <20 ng/mL), and group B2 (female patients with vitamin D levels ≥20 ng/mL). All participants met the 2011 World Health Organization diagnostic criteria for diabetes mellitus. Pregnant women, patients with type 1 diabetes, steroid-induced diabetes, and other types of diabetes, patients with complications of acute diabetes, patients with malignancies, patients with hepatic or renal insufficiency, patients with thyroid disease or hyperparathyroidism, patients with previous fractures and a diagnosis of severe osteoporosis, patients taking medications that could affect vitamin D levels, patients with acute or chronic infections, and patients with co-morbid cardiovascular diseases were excluded. This study followed the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Hebei General Hospital, China. All participants signed an informed consent form. The participant information was kept strictly confidential.
Clinical Assessment and Biochemical Measurements
The patients were interviewed by a health care professional from the endocrinology department. The collected information included age, sex, residence, clinical history, family history, history of smoking and alcohol consumption, history of menstruation, history of allergies, and details on the patient’s daily outdoor activity, diet, and sleep habits. The height (cm), weight (kg), and body mass index (BMI) were measured automatically using an ultrasound height and weight measuring device. The blood pressure was measured three times using an electronic sphygmomanometer in the sitting position after the patient had rested for 15 min. The blood pressure was measured three times, and the average of the last two measurements (in mm Hg) was used. The morning blood samples were obtained from patients after 8–12 h of fasting.Blood biomarkers were assessed using a fully automated biochemical analyzer and included total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), HDL, serum uric acid (SUA), serum creatinine (SCr), glomerular filtration rate (GFR), fasting blood glucose (FBG), and fasting insulin (FINS). Also, the present study measured the neutrophil count (NC), lymphocyte count (LC), and blood platelet count (PLT) using a hematology analyzer. 25-Hydroxyvitamin D3 was measured via an electrochemiluminescence method by laboratory physicians in the Department of Nuclear Medicine, Hebei General Hospital, China. The HbA1c level was assessed in the whole blood using ion-exchange high-performance liquid chromatography. NLR, LMR, PLR, MHR, and HOMA-IR were calculated manually, where
NLR = neutrophil count/lymphocyte count
LMR = lymphocyte count/monocyte count
PLR = platelet count/lymphocyte count
MHR = monocyte count/high-density lipoprotein
HOMA-IR = fasting blood glucose (FBG, mmol/L) × fasting blood insulin (FINS, μU/mL)/22.5
Statistical Analysis
The images were drawn with GraphPad Prism 9 software, and the data were analyzed using Statistical Product and Service Solutions 24.0 (SPSS 24). Descriptive statistical analysis of the study sample was performed. Normally distributed continuous variables were expressed as mean ± standard deviation and compared using the Student’s t-test. Non-normally distributed continuous variables were expressed as median and interquartile range (25–75%) and compared using the Mann–Whitney test. Spearman correlation analysis was used for correlation analysis. The logistic regression analysis and linear regression analysis were used for multi-factor analysis. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to calculate the accuracy of MHR in predicting vitamin D deficiency in patients with T2DM. The differences were considered statistically significant at P <0.05.
Results
Clinical Characteristics of Distinct Groups of Patients
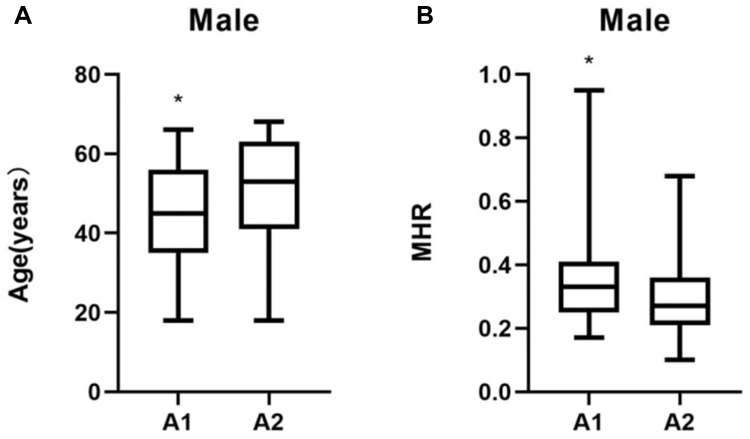
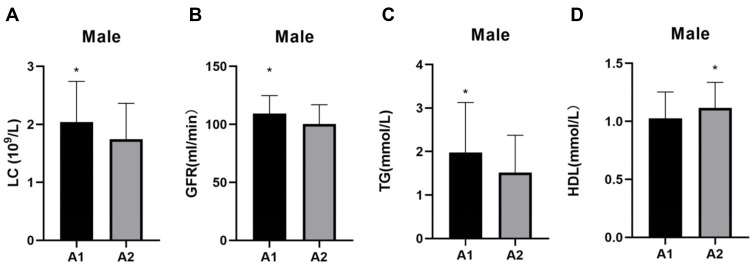
The patients in group A1 had significantly higher levels of MHR, LC, GFR, and TG (P < 0.05) but lower levels of HDL, and were younger (P < 0.05) than those in group A2 (P < 0.05) (Table 1). The differences in age and MHR, and the differences in the GFR and the levels of LC, TG, and HDL between group A1 and group A2 are shown in Figures 1 and 2, respectively.
Table 1.
Characteristics of Male Patients with T2DM (n = 186)
| Variable | A1 Group (n=123) | A2 Group (n=63) | Statistic | P |
|---|---|---|---|---|
| Age (years) | 45 (30, 56) | 53 (41, 63) | −2.964u | 0.003 |
| Course (mon) | 12 (1, 60) | 24 (1, 120) | −1.784u | 0.074 |
| BMI (kg/m2) | 27.46 (24.66, 29.89) | 27.06 (24.45, 28.41) | −0.942u | 0.346 |
| SBP (mmHg) | 132.61±17.48 | 132.89±14.92 | −0.078t | 0.938 |
| DBP (mmHg) | 85.13±11.48 | 85.5±10.94 | −0.180t | 0.857 |
| NC (109/L) | 3.46±2.16 | 3.59±1.32 | 1.588t | 0.114 |
| LC (109/L) | 2.04±0.70 | 1.75±0.62 | 2.803t | 0.006 |
| MO (109/L) | 0.35±0.13 | 0.31±0.10 | 1.941t | 0.054 |
| PLT (109/L) | 235.24±60.55 | 219.05±52.75 | 1.799t | 0.074 |
| NLR (%) | 2.51±0.75 | 2.17±0.77 | 0.693t | 0.489 |
| LMR (%) | 6.28±2.35 | 5.77±1.47 | 1.927t | 0.056 |
| PLR (%) | 132.13±82.13 | 135.16±38.95 | −0.365t | 0.715 |
| SUA (µmol/L) | 343.44±111.77 | 314.68±93.11 | 1.795t | 0.058 |
| SCr (µmol/L) | 61.8 (55.00, 69.40) | 63.7 (57.6, 71.2) | −1.532u | 0.125 |
| GFR (mL/min) | 109.31±15.57 | 100.34±16.70 | 3.642t | <0.001 |
| FBG (mmol/L) | 9.26 (7.53, 12.34) | 8.58 (6.14, 11.6) | −2.891u | 0.059 |
| TC (mmol/L) | 4.26±0.90 | 4.20±0.79 | 0.683t | 0.495 |
| TG (mmol/L) | 1.98±1.15 | 1.52±0.86 | 2.588t | 0.006 |
| HDL (mmol/L) | 1.02±0.23 | 1.11±0.22 | −2.569t | 0.011 |
| LDL (mmol/L) | 3.21±0.94 | 3.16±1.02 | 0.446t | 0.656 |
| MHR (%) | 0.33 (0.25, 0.40) | 0.27 (0.21, 0.36) | −2.865u | 0.004 |
| FINS (µU/mL) | 8.49 (4.21, 13.96) | 7.5 (5.33, 14.18) | −0.324u | 0.746 |
| HbA1c (%) | 9.8 (8.4, 11.75) | 9.35 (7, 11.2) | −1.800u | 0.072 |
| HOMA-IR | 3.12 (1.67, 6.58) | 6.23 (3.33, 10.08) | −0.022u | 0.983 |
| Vitamin D (ng/mL) | 14.73 (12.69, 17.44) | 24.37 (21.81, 29.87) | −11.150u | <0.001 |
Note: umann–Whitney U-test; tt-test.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; FINS, fasting insulin; GFR, glomerular filtration rate; HbA1c, hemoglobin Alc; HDL, high-density lipoprotein cholesterol; HOMA-IR, homa insulin-resistance; LC, lymphocyte count; LDL, low-density lipoprotein cholesterol; LMR, lymphocyte to monocyte ratio; MHR, monocyte/high-density lipoprotein cholesterol ratio; MO, monocyte count; NC, neutrophil count; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, blood platelet count; SBP, systolic blood pressure; SCr, serum creatinine; SUA, serum uric acid; TC, total cholesterol; TG, triglyceride.
Figure 1.
Comparisons of age and MHR in groups A1 and A2. (A) Age comparisons; (B) MHR comparisons. *P < 0.05.
Figure 2.
Comparisons of LC, GFR, TG, and HDL in groups A1 and A2. (A) LC comparisons; (B) GFR comparisons; (C) TG comparisons; and (D) HDL comparisons. *P < 0.05.
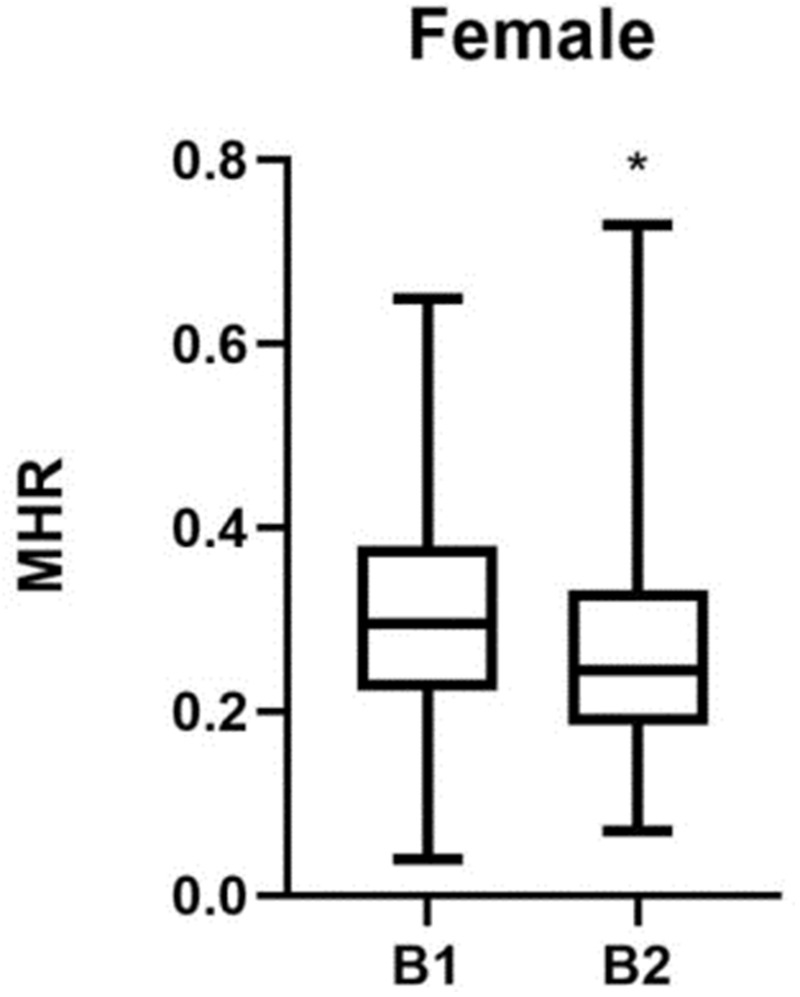
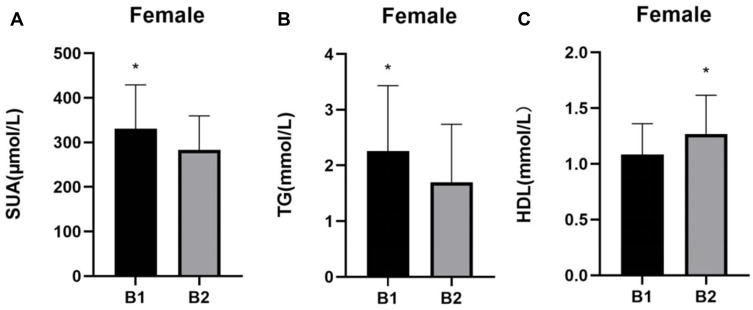
The patients in group B1 had higher levels of MHR, SUA, and TG than those in group B2 (P < 0.05), but lower levels of HDL (P < 0.05) (Table 2). The differences in MHR and the differences in the levels of SUA, TG, and HDL between group B1 and group B2 are shown in Figures 3 and 4, respectively.
Table 2.
Characteristics of Female Patients with T2DM (n = 74)
| Variable | B1 Group (n=44) | B2 Group (n=30) | Statistic | P |
|---|---|---|---|---|
| Age (years) | 56.00 (48, 62) | 56.00 (52, 63) | −0.281u | 0.778 |
| Course (mon) | 12.00 (1, 132) | 12.00 (5.00, 66.00) | −0.992u | 0.321 |
| BMI (kg/m2) | 26.70 (23.99, 30.18) | 25.16 (23.23, 28.87) | −1.773u | 0.076 |
| SBP (mmHg) | 133.77±19.54 | 136.80±19.33 | −0.548t | 0.568 |
| DBP (mmHg) | 82.3±11.97 | 84.17±14.75 | −0.363t | 0.717 |
| NC (109/L) | 4.13±1.18 | 3.65±1.05 | 1.764t | 0.082 |
| LC (109/L) | 1.95±0.53 | 1.95±0.46 | 0.061t | 0.951 |
| MO (109/L) | 0.32±0.08 | 0.30±0.08 | 0.594t | 0.554 |
| PLT (109/L) | 248.95±78.14 | 244.60±62 | 0.346t | 0.730 |
| NLR (%) | 2.27±0.91 | 2.01±0.97 | 1.119t | 0.267 |
| LMR (%) | 6.53±2.19 | 6.57±1.42 | 0.109t | 0.913 |
| PLR (%) | 135.30±49.04 | 131.45±42.56 | 0.348t | 0.729 |
| SUA (µmol/L) | 330.41±99.48 | 283.09±76.31 | 2.230t | 0.029 |
| SCr (µmol/L) | 56.7 (49.05, 67.95) | 53.85 (48.4, 66.5) | −0.495u | 0.620 |
| GFR (mL/min) | 104.57±21.68 | 100.96±14.96 | 0.894t | 0.274 |
| FBG (mmol/l) | 9.28 (7.74, 11.25) | 9.36 (6.8, 10.28) | −1.107u | 0.269 |
| TC (mmol/l) | 4.35±0.97 | 4.21±0.85 | −1.451t | 0.151 |
| TG (mmol/l) | 2.26±1.17 | 1.70±1.04 | −2.290t | 0.038 |
| HDL (mmol/l) | 1.08±0.28 | 1.27±0.35 | −2.566t | 0.012 |
| LDL (mmol/l) | 3.27±0.90 | 3.68±1.04 | −1.828t | 0.072 |
| MHR (%) | 0.29 (0.23, 0.38) | 0.25 (0.19, 0.33) | −2.990u | 0.043 |
| FINS (µU/mL) | 9.97 (4.52, 13.82) | 8.02 (4.13, 9.93) | −1.360u | 0.174 |
| HbA1c (%) | 10.00 (8.4, 10.85) | 9.80 (8.70, 11.80) | −0.666u | 0.505 |
| HOMA-IR | 3.69 (2.24, 6.91) | 2.54 (1.53, 4.46) | −1.673u | 0.092 |
| Vitamin D (ng/mL) | 13.72 (11.04, 16.85) | 23.15 (21.51, 25.27) | −7.267u | <0.001 |
Note: umann–Whitney U-test; tt-test.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; FINS, fasting insulin; GFR, glomerular filtration rate; HbA1c, hemoglobin Alc; HDL, high-density lipoprotein cholesterol; HOMA-IR, homa insulin-resistance; LC, lymphocyte count; LDL, low-density lipoprotein cholesterol; LMR, lymphocyte to monocyte ratio; MHR, monocyte/high-density lipoprotein cholesterol ratio; MO, monocyte count; NC, neutrophil count; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, blood platelet count; SBP, systolic blood pressure; SCr, serum creatinine.
Figure 3.
MHR comparisons in groups B1 and B2. *P < 0.05.
Figure 4.
Comparisons of SUA, TG, and HDL in groups B1 and B2. (A) SUA comparisons; (B) TG comparisons; and (C) HDL comparisons. *P < 0.05.
Correlation Between Vitamin D Levels and MHR in Patients with T2DM
In male patients with T2DM, the vitamin D level was positively correlated with age, SCr, and HDL (r = 0.190, r = 0.160, and r = 0.198, respectively, P < 0.05), and negatively correlated with NC, MO, PLT, FBG, GFR, TG, HbA1c, and MHR (r = –0.211, r = –0.240, r = –0.174, r = –0.163, r = –0.232, r = –0.154, r = –0.194, r = –0.300, respectively, P < 0.05) (Table 3).
Table 3.
Correlation Between Other Factors and Vitamin D Level in Male Patients with T2DM
| Factor | r | P |
|---|---|---|
| Age | 0.190 | 0.009 |
| NC | −0.211 | 0.004 |
| MO | −0.240 | 0.001 |
| PLT | −0.174 | 0.018 |
| FBG | −0.163 | 0.027 |
| SCr | 0.160 | 0.029 |
| GFR | −0.232 | 0.001 |
| TG | −0.154 | 0.035 |
| HDL | 0.198 | 0.007 |
| HbA1c | −0.194 | 0.008 |
| MHR | −0.300 | <0.000 |
Abbreviations: FBG, fasting blood glucose; GFR, glomerular filtration rate; HbA1c, hemoglobin Alc; HDL, high-density lipoprotein cholesterol; MHR, monocyte/high-density lipoprotein cholesterol ratio; MO, monocyte count; NC, neutrophil count; PLT, blood platelet count; SCr, serum creatinine; TG, triglyceride.
In female patients with T2DM, the vitamin D level was negatively correlated with BMI, NC, and TG (r = –0.234, r = –0.243, r = –0.246, respectively, P < 0.05) (Table 4).
Table 4.
Correlation Between Other Factors and Vitamin D Level in Female Patients with T2DM
| Factor | r | P |
|---|---|---|
| BMI | −0.234 | 0.045 |
| NC | −0.243 | 0.037 |
| TG | −0.246 | 0.035 |
Abbreviations: BMI, body mass index; NC, neutrophil count; TG, triglyceride.
Multivariate Linear Correlation Analysis of Vitamin D Level and MHR in Patients with T2DM
The crude model 1 suggested that MHR was negatively correlated with the vitamin D level in male participants (β = −0.291, P < 0.05). The results for the other models were as follows: Model 2 (adjusted for age, course, BMI, SBP, and DBP) (β = −0.269, P < 0.05); Model 3 (adjusted for age, course, BMI, SBP, DBP, NC, LC, MO, PLT, NLR, and LMR) (β = −0.373, P < 0.05); Model 4 (adjusted for age, course, BMI, SBP, DBP, NC, LC, MO, PLT, NLR, LMR, SUA, SCr, and GFR) (β = −0.355, P < 0.05); and Model 5 (adjusted for age, course, BMI, SBP, DBP, NC, LC, MO, PLT, NLR, LMR, SUA, SCr, GFR, FBG, TC, TG, HDL, LDL, HbA1c, and HOMA-IR) (β = −0.441, P < 0.05) (Table 5).
Table 5.
Association Between Vitamin D Levels and MHR in Male Patients with T2DM
| B (95% CI) | SE | β | T | P | |
|---|---|---|---|---|---|
| Model1 | −15.23 (−22.526, −7.934) | 3.698 | −0.291 | −4.118 | 0.000 |
| Model2 | −15.497 (−22.917, −8.078) | 3.760 | −0.269 | −4.122 | 0.000 |
| Model3 | −19.560 (−31.822, −7.298) | 6.213 | −0.373 | −3.148 | 0.002 |
| Model4 | −18.690 (−31.142, −6.076) | 6.349 | −0.355 | −2.931 | 0.004 |
| Model5 | −23.106 (−43.501, −2.170) | 10.329 | −0.441 | −2.237 | 0.027 |
Regardless of the model used, the MHR was not associated with the vitamin D level in female patients with T2DM (P > 0.05) (Table 6).
Table 6.
Association Between Vitamin D Levels and MHR in Female Patients with T2DM
| B (95% CI) | SE | β | T | P | |
|---|---|---|---|---|---|
| Model1 | −9.619 (−23.275, 4.037) | 6.850 | −0.613 | −1.404 | 0.165 |
| Model2 | –11.482 (−26.735, 3.878) | 7.669 | −0194 | −1.490 | 0.141 |
| Model3 | −15.249 (−38.619, 8.121) | 11.687 | −0.259 | −1.305 | 0.197 |
| Model4 | −13.318 (−38.061, 11.300) | 12.330 | −0.227 | −1.085 | 0.282 |
| Model5 | 22.172 (−29.987, 74.331) | 25.981 | 0.376 | 0.853 | 0.397 |
Logistic Regression Analysis of Risk Factors for Vitamin D Deficiency
Table 7 shows the presence or absence of vitamin D deficiency as the dependent variable (yes = 1, no = 0) and age, NC, LC, MO, PLT, FBG, SCr, GFR, TG, HDL, HbA1c, and MHR as independent variables. A one-way binary logistic regression was performed; statistically significant variables were further analyzed by multifactorial binary logistic regression. The present analysis revealed that the MHR was an independent risk factor for vitamin D deficiency in male patients with T2DM [OR = 54.040, 95% CI (1.895–1541.103), P < 0.05].
Table 7.
Logistic Regression Analysis of the Association Between Male Patients with T2DM and Vitamin D Deficiency
| Factor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Age | 0.007 | 0.966 (0.942–0.991) | 0.156 | 0.980 (0.952–1.008) |
| NC | 0.119 | 1.197 (0.955–1.499) | – | – |
| LC | 0.007 | 2.020 (1.213–3.363) | 0.380 | 1.299 (0.725–2.326) |
| MO | 0.057 | 20.122 (0.911–444.363) | – | – |
| PLT | 0.076 | 1.005 (0.999–1.011) | – | – |
| FBG | 0.125 | 1.074 (0.980–1.177) | – | – |
| SCr | 0.166 | 0.988 (0.970–1.005) | – | – |
| GFR | 0.001 | 1.035 (1.015–1.056) | 0.057 | 1.024 (0.999–1.049) |
| TG | 0.007 | 1.574 (1.130–2.194) | 0.111 | 1.337 (0.936–1.911) |
| HDL | 0.013 | 0.176 (0.045–1.691) | 0.676 | 1.489 (0.230–9.639) |
| HbA1c | 0.063 | 1.136 (0.993–1.299) | – | – |
| MHR | 0.003 | 63.387 (4.036–995.472) | 0.020 | 54.040 (1.895–1541.103) |
Abbreviations: FBG, fasting blood glucose; GFR, glomerular filtration rate; HbA1c, hemoglobin Alc; HDL, high-density lipoprotein cholesterol; LC, lymphocyte count; MHR, monocyte/high-density lipoprotein cholesterol ratio; MO, monocyte count; NC, neutrophil count; PLT, blood platelet count; SCr, serum creatinine; TG, triglyceride.
Similarly, Table 8 shows the presence or absence of vitamin D deficiency as the dependent variable and BMI, NC, SUA, TG, HDL, and MHR as independent variables in female patients with T2DM. Using the same methods as in male patients, it was observed that HDL was an independent protective factor for vitamin D deficiency in female patients [OR = 0.149, 95% CI (0.026–0.859), P < 0.05].
Table 8.
Logistic Regression Analysis of the Association Between Female Patients with T2DM and Vitamin D Deficiency
| Factor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| BMI | 0.085 | 1.080 (0.986–1.246) | – | – |
| NC | 0.088 | 1.493 (0.942–2.367) | – | – |
| SUA | 0.037 | 1.007 (1.000–1.013) | 0.193 | 1.005 (0.998–1.012) |
| TG | 0.044 | 1.621 (1.013–2.593) | 0.275 | 1.327 (0.798–2.208) |
| HDL | 0.018 | 0.140 (0.028–0.711) | 0.033 | 0.149 (0.026–0.859) |
| MHR | 0.096 | 35.054 (0.532–2310.403) | – | – |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein cholesterol; MHR, monocyte/high-density lipoprotein cholesterol ratio; NC, neutrophil count; SUA, serum uric acid; TG, triglyceride.
ROC Analysis of MHR in Predicting Vitamin D Deficiency
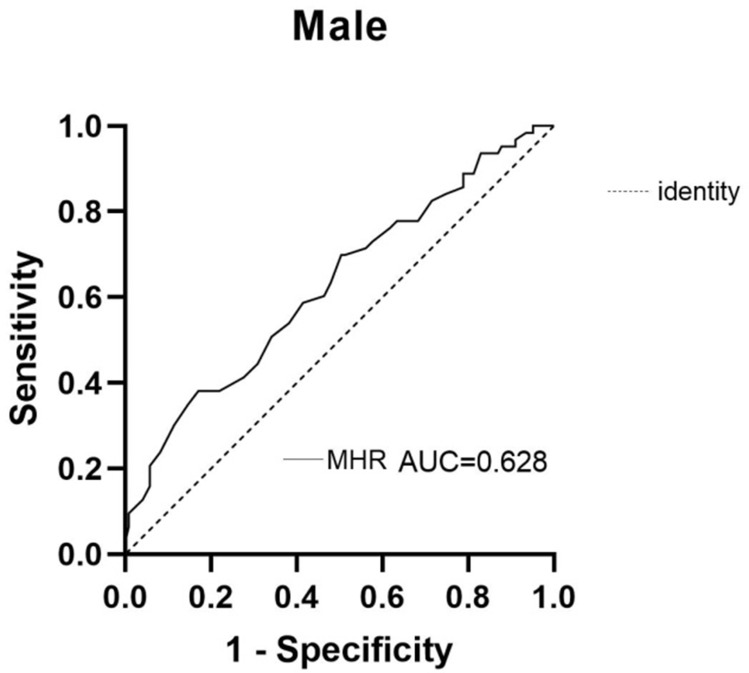
In the ROC curve display, the AUC of MHR in the male group was 0.628, indicating that the MHR predicted vitamin D deficiency in male patients with T2DM (P > 0.05; sensitivity of 87.8%, specificity of 34.9%, Youden index of 0.227, and a cutoff value of 0.221) (Table 9). The ROC curve of MHR in male patients with T2DM and vitamin D deficiency is shown in Figure 5.
Table 9.
ROC Curve Analysis of MHR in Predicting Vitamin D Deficiency in Male Patients with T2DM
| Factor | AUC | 95% CI | Youden Index | Cut-Off | Sensitivity | Specificity | P |
|---|---|---|---|---|---|---|---|
| MHR | 0.628 | 0.543–0.714 | 0.227 | 0.221 | 0.878 | 0.349 | 0.004 |
Abbreviation: MHR, monocyte/high-density lipoprotein cholesterol ratio.
Figure 5.
ROC curve of MHR in male patients with T2DM and vitamin D deficiency.
Discussion
The number of adults with diabetes worldwide has quadrupled in the last 40 years. The International Diabetes Federation estimated the global diabetes population to be 463 million in 2019, a figure that was expected to rise to 700 million by 2045.10,11 Diabetes and complications related to diabetes have enormous burdens on individuals, families, health systems, and national economies. The estimated global direct health expenditure on diabetes in 2019 was of 760 billion USD. The United States of America had the highest estimated expenditure in the world (294.6 billion USD), followed by China (109.0 billion USD).12 Early detection, diagnosis, and access to treatment are absolutely essential in the face of a disease that is both a threat to one’s own life and a burden on the economy of a country.
Vitamin D is a fat-soluble vitamin involved in the metabolism of calcium and phosphorus. Low serum vitamin D levels are a common condition worldwide. Many factors contribute to this deficiency, including old age, race, winter, limited sunlight hours, poor diet, obesity, and medications that interfere with vitamin D metabolism. Vitamin D deficiency in adults can lead to osteoporosis, and deficiency in children can lead to rickets.13,14 Almost 40% of the European population presents with vitamin D deficiency, and more than 20% of people from India, Afghanistan, and Pakistan have severe depletion of vitamin D.15 Severe vitamin D deficiency can also increase the risk of many diseases, including cancer, cardiovascular disease, and neurodegenerative disorders. These diseases are often characterized by underlying chronic inflammation, which can exacerbate the rate of disease progression.
The links between vitamin D levels and metabolic disease have been an essential research topic in recent years, with new evidence suggesting that vitamin D is involved in blood glucose homeostasis and the pathophysiological processes of insulin resistance and diabetes. The exact pathophysiological mechanisms are not known. However, evidence suggests that vitamin D improves β-cell function through molecular repair mechanisms. It also increases insulin sensitivity by inhibiting inflammatory responses, reducing oxidative damage, and promoting insulin signaling.16 Previous studies identified the presence of vitamin D receptors on pancreatic β-cells. The binding of 1,25(OH)2D3 led to the activation of the L-type calcium channel on β-cells and the initiation of downstream signaling pathways. It promoted insulin secretion by regulating intra- and extracellular calcium balance and tyrosine phosphorylation of the insulin receptor substrate.17 Early vitamin D supplementation reduced the incidence of T2DM by improving inflammation in KK-Ay mice.18 Early detection of vitamin D deficiency can be an important factor in preventing T2DM. In addition, the timely detection of vitamin D deficiency and supplementation in people who already have diabetes may be beneficial in improving insulin resistance, maintaining blood glucose homeostasis, and reducing the risk of complications. Unfortunately, however, most primary hospitals in China do not have a strong capacity to test for vitamin D deficiency. Therefore, it is important to find markers that can help predict this condition.
The MHR is a novel predictor that combines inflammation and lipid metabolism. Monocytes are the main source of pro-inflammatory cytokines. HDL has anti-inflammatory and antioxidant effects by inhibiting monocyte activation, macrophage migration, and LDL oxidation. HDL also increases the expression of endothelial nitric oxide and improves vasodilation.19,20 The prognostic value of MHR in cardiovascular diseases has been well studied previously.21,22 However, few studies explored the link between MHR and vitamin D. A negative association between vitamin D and MHR in women, independent of the metabolic status, has been shown. However, this relationship is observed only in men with abdominal obesity.23 The MHR can be obtained by a simple calculation using blood cell analysis and lipid analysis. It is inexpensive and easy to obtain, and hence may have a value in predicting vitamin D deficiency.
Among male patients, MHR, LC, GFR, and TG were significantly higher in group A1 than in group A2, whereas the HDL levels and age were lower in group A1 than in group A2. In female patients, the levels of TG, MHR, and SUA were significantly higher in group B1 than in group B2, whereas the levels of HDL were lower in group B1 than in group B2. The MHR was found to be statistically significant in both male and female patients. The HDL level was lower in groups A1 and B1 than in groups A2 and B2; the level was lower in male patients than in female patients. The TG level was higher in groups A1 and B1 than in groups A2 and B2. The Spearman correlation analysis showed that vitamin D level was positively correlated with the HDL level and negatively correlated with the TG level in male patients, which was in agreement with previous findings.24 Vitamin D supplementation has been also reported to increase HDL levels.25 In the present study, a negative correlation was not observed between vitamin D and LDL levels in male patients. It is widely appreciated that anti-dyslipidemia drugs can affect LDL cholesterol concentrations. In addition, smoking and alcohol consumption can also affect LDL levels. A limitation of the present study was that it did not investigate these factors, which were potential confounders.
This study was novel in reporting that the vitamin D level was negatively correlated with MHR in male patients with T2DM. The multivariate linear regression analysis demonstrated that vitamin D level was significantly and independently and negatively correlated with the MHR in male patients with or without adjustment for confounders [B = –23.106, 95% CI (–43.501 to –2.170), P < 0.05]. This study did not exclude people taking lipid-lowering medications and controlled for a relatively large number of confounding factors. A correlation was not observed between MHR and vitamin D levels in the female patient group, although this might be related to the smaller sample size of female patients.
The results of the present study were consistent with the findings of Mousa et al,26 although the study population in that study comprised healthy adults and the sample size was much larger than the one included in our study. Several mechanisms might underlie the link between vitamin D levels and diabetes. Vitamin D suppresses endoplasmic reticulum stress, leading to the downregulation of adhesion molecules such as PSGL-1, β(1)-integrin, and β(2)-integrin, ultimately reducing monocyte activation.27 In addition, vitamin D has immunomodulatory effects via the regulation of monocyte inflammatory response by attenuating cell signaling and the activation of pro-inflammatory genes, further preventing the release of cytokines. For example, vitamin D leads to reduced TNFα release and less activation of intracellular inflammatory pathways, such as the p38 and NF-κB pathways, by attenuating TLR2 and TLR4-mediated signaling.28 It has been shown that NLR is strongly influenced by serum vitamin D levels, and patients with pre-diabetes and vitamin D deficiency have higher levels of NLR.29 Another study has shown that PLR is an independent predictor of vitamin D levels.30 In the present study, higher NLR levels in patients with T2D are not a risk factor for vitamin D deficiency. Also, sample selection might have been a confounder. Hence, larger studies are needed.
This study was also novel in reporting that the MHR was an independent risk factor for vitamin D deficiency in male patients with T2DM [OR = 54.040, 95% CI (1.895–1541.103), P < 0.05]. The risk of developing T2DM increased 54.04 times for each increased unit of MHR. The median MHR was 0.33 in group A1 and 0.27 in group A2. In most cases, it is less than 1, suggesting that MHR might be an independent risk factor for developing vitamin D deficiency. An OR >1 indicated that the MHR contributed to vitamin D deficiency. HDL was an independent protective factor for vitamin D deficiency in female patients with T2DM [OR = 0.149, 95% CI (0.026–0.859), P < 0.05]. The risk of T2DM decreased by 0.149 times for each increased unit of HDL. The findings suggested that a better lipid control in female patients with T2DM could lower the risk of vitamin D deficiency, and were in agreement with most previous studies.31
The analysis of the working characteristic curve of the MHR of participants in this study showed an AUC of 0.628 for the MHR in male patients, which had a predictive value in vitamin D deficiency. The greater the AUC, the higher the predictive accuracy. The optimal critical point of MHR was 0.221, indicating that the risk of vitamin D deficiency was greatly increased if MHR ≥0.221. The sensitivity was 87.8%, and specificity was 34.9%. Although the sensitivity of MHR as a predictive factor was high, it had a low specificity and a high false-positive rate.
This study highlighted the negative correlation between vitamin D levels and MHR in male patients with T2DM, and showed that MHR could be used as a marker to predict vitamin D deficiency in this population. Examining MHR in patients with T2DM could help identify those at high risk of developing vitamin D deficiency and help minimize this risk. These measures could help in glycemic management in these patients and reduce the risk of long-term complications. This study had several limitations, one of them being its cross-sectional design. Therefore, the causality could not be established. In addition, the study did not account for confounders related to personal habits, socioeconomic status, dietary habits, and duration of sun exposure, as access to this information was limited. This study was conducted on patients with T2DM aged between 18 and 65 years. Older populations might have a higher MHR, and therefore the correlation between MHR and vitamin D level might be stronger in this population. Larger-scale studies are required to confirm the correlation between MHR and vitamin D level, especially in female patients and older patients with T2DM.
Conclusions
The vitamin D level was independently and negatively correlated with the MHR in male patients with T2DM; however, its significance was lost in the female group. One of the independent risk factors for vitamin D deficiency in male patients with T2DM was the MHR. Alleviating inflammation or increasing the HDL level might improve vitamin D levels. HDL was an independent protective factor for vitamin D deficiency in female patients with T2DM. Improving lipid metabolism might increase vitamin D levels, and this might be an important strategy in female patients with T2DM. The MHR was a predictor of vitamin D deficiency in male patients with T2DM, and could potentially be used as a predictive biomarker for vitamin D deficiency in primary hospitals in China, so as to help improve the testing capacity.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White PJ, McGarrah RW, Herman MA, et al. Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab. 2021;52:101261. doi: 10.1016/j.molmet.2021.101261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Vitamin D. Evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4–18. doi: 10.2174/138945011793591635 [DOI] [PubMed] [Google Scholar]
- 4.Castillo-Oti JM, Galvan-Manso AI, Callejas-Herrero MR, et al. Vitamin D deficiency is significantly associated with retinopathy in type 2 diabetes mellitus: a case-control study. Nutrients. 2021;14(1):84. doi: 10.3390/nu14010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andujar-Espinosa R, Salinero-Gonzalez L, Illan-Gomez F, et al. Effect of vitamin D supplementation on asthma control in patients with vitamin D deficiency: the ACVID randomised clinical trial. Thorax. 2021;76(2):126–133. doi: 10.1136/thoraxjnl-2019-213936 [DOI] [PubMed] [Google Scholar]
- 6.Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55(4):1103–1111. doi: 10.1002/hep.24737 [DOI] [PubMed] [Google Scholar]
- 7.Fyfe I. Multiple sclerosis: vitamin D deficiency leads to excessive B-cell responses in multiple sclerosis. Nat Rev Neurol. 2016;12(5):252. doi: 10.1038/nrneurol.2016.49 [DOI] [PubMed] [Google Scholar]
- 8.Inoue D. Diabetes mellitus and osteoporosis. Role for vitamin D in glucose and energy metabolism. Clin Calcium. 2012;22(9):1391–1397. Japanese. [PubMed] [Google Scholar]
- 9.Villanueva D, Tiongson MD, Ramos JD, et al. Monocyte to High-Density Lipoprotein Ratio (MHR) as a predictor of mortality and Major Adverse Cardiovascular Events (MACE) among ST Elevation Myocardial Infarction (STEMI) patients undergoing primary percutaneous coronary intervention: a meta-analysis. Lipids Health Dis. 2020;19(1):55. doi: 10.1186/s12944-020-01242-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaboration NR. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi: 10.1016/j.ophtha.2021.04.027 [DOI] [PubMed] [Google Scholar]
- 12.Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. doi: 10.1016/j.diabres.2020.108072 [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Rosen CJ. Clinical practice postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254–262. doi: 10.1056/NEJMcp1513724 [DOI] [PubMed] [Google Scholar]
- 14.Kruger DM, Lyne ED, Kleerekoper M. Vitamin D deficiency rickets. A report on three cases. Clin Orthop Relat Res. 1987;1987(224):277–283. [PubMed] [Google Scholar]
- 15.Lips P, Cashman KD, Lamberg-Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–P54. doi: 10.1530/EJE-18-0736 [DOI] [PubMed] [Google Scholar]
- 16.Yaribeygi H, Maleki M, Sathyapalan T, et al. The molecular mechanisms by which vitamin D improve glucose homeostasis: a mechanistic review. Life Sci. 2020;244:117305. doi: 10.1016/j.lfs.2020.117305 [DOI] [PubMed] [Google Scholar]
- 17.Kjalarsdottir L, Tersey SA, Vishwanath M, et al. 1,25-Dihydroxyvitamin D3 enhances glucose-stimulated insulin secretion in mouse and human islets: a role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J Steroid Biochem Mol Biol. 2019;185:17–26. doi: 10.1016/j.jsbmb.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Tian LQ, Yu YT, Jin MD, et al. Early 1,25-Dihydroxyvitamin D3 Supplementation Effectively Lowers the Incidence of Type 2 Diabetes Mellitus via Ameliorating Inflammation In KK-A(y) Mice. J Nutr Sci Vitaminol. 2021;67(2):84–90. doi: 10.3177/jnsv.67.84 [DOI] [PubMed] [Google Scholar]
- 19.Ganjali S, Gotto AJ, Ruscica M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. 2018;233(12):9237–9246. doi: 10.1002/jcp.27028 [DOI] [PubMed] [Google Scholar]
- 20.Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. doi: 10.1016/j.jacc.2013.07.043 [DOI] [PubMed] [Google Scholar]
- 21.Zhang DP, Baituola G, Wu TT, et al. An elevated monocyte-to-high-density lipoprotein-cholesterol ratio is associated with mortality in patients with coronary artery disease who have undergone PCI. Biosci Rep. 2020;40(8). doi: 10.1042/BSR20201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao Y, Xia N, Fu Y, et al. Application of monocyte-to-HDL-C ratio in diagnosis of elderly patients with type 2 diabetes mellitus complicated with coronary heart disease and in the evaluation of patients’ coronary artery disease severity. Guangxi Med J. 2021;43(21):2539–2543. [Google Scholar]
- 23.De Matteis C, Crudele L, Cariello M, et al. Monocyte-to-HDL Ratio (MHR) predicts vitamin D deficiency in healthy and metabolic women: a cross-sectional study in 1048 subjects. Nutrients. 2022;14(2):347. doi: 10.3390/nu14020347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X, Peng M, Chen S, et al. Vitamin D deficiency is associated with dyslipidemia: a cross-sectional study in 3788 subjects. Curr Med Res Opin. 2019;35(6):1059–1063. doi: 10.1080/03007995.2018.1552849 [DOI] [PubMed] [Google Scholar]
- 25.Hirschler V, Maccallini G, Sanchez MS, et al. Improvement in high-density lipoprotein cholesterol levels in Argentine Indian school children after vitamin D supplementation. Horm Res Paediatr. 2013;80(5):335–342. doi: 10.1159/000355511 [DOI] [PubMed] [Google Scholar]
- 26.Mousa H, Islam N, Ganji V, et al. Serum 25-hydroxyvitamin D is inversely associated with monocyte percentage to HDL cholesterol ratio among young healthy adults in Qatar. Nutrients. 2020;13(1):127. doi: 10.3390/nu13010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riek AE, Oh J, Sprague JE, et al. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287(46):38482–38494. doi: 10.1074/jbc.M112.386912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36(2):361–370. doi: 10.1002/eji.200425995 [DOI] [PubMed] [Google Scholar]
- 29.Wang SY, Shen TT, Xi BL, et al. Vitamin D affects the neutrophil-to-lymphocyte ratio in patients with type 2 diabetes mellitus. J Diabetes Investig. 2021;12(2):254–265. doi: 10.1111/jdi.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbas EM, Gungor A, Ozcicek A, et al. Vitamin D and inflammation: evaluation with neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Arch Med Sci. 2016;12(4):721–727. doi: 10.5114/aoms.2015.50625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Tong CH, Rowland CM, et al. Association of changes in lipid levels with changes in vitamin D levels in a real-world setting. Sci Rep. 2021;11:21536. doi: 10.1038/s41598-021-01064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]