Folate is the generic term for vitamin B9, a water-soluble vitamin that includes chemically similar compounds essential in periods of rapid cell growth and division, in the maintenance of new cells, and in the making of DNA and RNA. While best recognized for pregnancy and fertility support, recognition is growing that it also benefits cardiovascular health, mood, and cognition.
Recent data suggest the need to distinguish between naturally occurring folates and folic acid (FA), terms often mistaken and used interchangeably, both by practitioners and consumers, causing considerable confusion.1
In particular, 5-Methyltetrahydrofolate [methylfolate, 5-MTHF, or (6S)-5-MTHF], has been evaluated as a better alternative to folic-acid supplementation.2-5 In this article, I endeavor to clearly identify the difference between folic acid and folate, pointing out why Quatrefolic, the 5-MTHF glucosamine salt from Gnosis by Lesaffre, is the ideal choice, suitable for anyone but in particular for those expressing methylenetetrahydrofolatereductase (MTHFR) polymorphism, which is approximately 40% of the global population.6-10
FOLATE: SOURCES AND DEFICIENCY
People can’t synthesize folate, and due to its water-soluble nature, the body stores it to a limited extent. Therefore, folate must be obtained from their diets.
Although naturally occurring folates are found in various foods, such as green leafy vegetables, sprouts, fruits, brewer’s yeast, and animal liver (Figure 1), it’s exceedingly difficult for most people to get the daily recommended amount of folate through food alone. Furthermore, food folates are unstable, and can be oxidized by heat, light, and/or metal ions, so cooking can reduce bioavailability.
Figure 1.
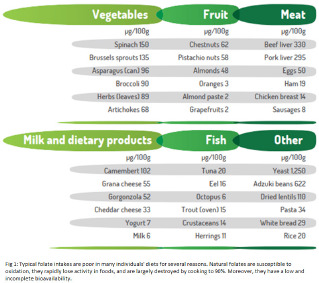
Folate intakes are typically poor in many individuals’ diets for several reasons. Natural folates: (1) are susceptible to oxidation, (2) can rapidly lose activity in foods, and (3) are largely destroyed by cooking, to 90%. Moreover, they have a low and incomplete bioavailability.
While implementing specific food-processing procedures can minimize folate degradation, the absorption of folate is much more effective with fortified foods and supplements, such as 5-MTHF/folate and folic acid. In fact, in the last decades, the main form of folate supplementation has been FA. Prescription of FA to women in the preconception period and during pregnancy is a consolidated practice.11-14
The recommended dietary allowances (RDAs) for folate are 400 μg/day for adults and 600 μg/day for women of childbearing age.15 Furthermore, many countries have initiated mandatory food fortification with FA to compensate for the losses and maintain adequate dietary intakes.
Still, folate deficiency is incredibly common and may occur when: (1) dietary intake is inadequate; (2) an increased need isn’t matched by increased intake, as in physiological conditions such as pregnancy, lactation, and children’s growth; (3) absorption or excretion is altered or losses occur; or (4) metabolism or drug use interfere with the body’s ability to use folate.
Deficiency of folate may be asymptomatic or present with the symptoms of anemia, diarrhea, loss of appetite, and weight loss. Additional signs are weakness, sore tongue, headaches, heart palpitations, irritability, and behavioral disorders.6,16
Folate Forms and Metabolization
The bioavailability and metabolism of the different folate forms vary due to their respective chemical structures. All forms of folates, natural or synthetic, must be converted to the circulating form 5-MTHF to exert their biological activity. The structurally related compounds included in the folate group are FA, natural folates, and 5-MTHF.
FA
FA is the oxidized, monoglutamate precursor form of folate that was synthesized in pure crystalline form for the first time in the 1940s. Many dietary supplements include it as do fortified foods, such as cereal-based products, pasta, enriched bread, and fruit juice. FA doesn’t occur in nature and has no biological functions. To be utilized, the human body must metabolize and reduce it to 5-MTHF using a multistep enzymatic conversion.
Natural folates
Natural folates occur in foods and also exist in many chemical forms of polyglutamate. Food folates are hydrolyzed to the monoglutamate form in the gut before absorption by active transport across the intestinal mucosa. Therefore, before entering the bloodstream, the monoglutamate form is reduced to tetrahydrofolate (THF) and converted into methyl forms (5-MTHF).
5-MTHF
The biologically active form 5-MTHF, the predominant physiological form of folate found in blood and in umbilical cord blood, is also available in small amounts in foods. It’s widely available as a food ingredient and doesn’t require metabolization.
Drawbacks of FA
FA is first reduced to dihydrofolate by the enzyme dihydrofolate reductase (DHFR) and then to tetrahydrofolate (THF). This is a rate-limiting step, leading to DHFR’s weak activity in humans, with considerable interindividual variations. High doses of FA can lead to a rapid saturation or inhibition of the DHFR enzyme, resulting in an accumulation of unmetabolized FA (UMFA) and the UMFA syndrome.13-17
Additionally, some people have genetic variations that decrease the activity of DHFR. Levels of circulating UMFA in the population are persistent in countries where the FA fortification of grains and cereals has been implemented.18 UMFA may compete with natural folate (5-MTHF) for the folate transporter and the folate receptor, thus depleting active folate for participation in the metabolic cycles.
A 2014 published study clearly showed that 86% of FA in the hepatic portal vein is unmetabolized, while almost all the natural folate was converted correctly.19,20 Detectable levels of UMFA occur temporarily in plasma after the consumption of >200 μg FA, with concentrations increasing in parallel to that of total FA after supplementation. UMFA has been detected in cord and infant blood, a source of concern due to potential adverse effects on health, as I will further describe.18,21
While it’s ideal that people obtain nutrients from food, the population can’t universally assume that it can rely on the diet for food folates. THF is a critical player in folate metabolism as a folate-acceptor molecule. THF is first converted to 5,10-methylene-THF and then later is reduced to 5-MTHF by MTHFR.22
Genetic polymorphism may impair the MTHFR activity and the related metabolization of food folates and folic acid in 5-MTHF. MTHFR is highly polymorphic in the general population, with multiple MTHFR gene alterations having been identified. Today, 35 rare but deleterious mutations in MTHFR, polymorphisms, and nine common variants have been reported. The two most common are C677T and A1298C. The numbers refer to their location on the gene.
A polymorphic MTHFR enzyme may function with approximately 55% to 70% efficiency as compared to a normal MTHFR enzyme. The incidence of people presenting a form of polymorphism of MTHFR is about 40% worldwide. This polymorphism is associated with an increased thermolability and reduced specific activity of MTHFR in vivo, resulting in a residual enzyme activity of 65% for heterozygous carriers and only 30% for homozygous carriers.23,24
The limited conversion of FA may jeopardize folate availability and increase the risk of adverse health outcomes. Cutting-edge scientific research has shed light on how much the MTHFR polymorphism is implicated in chronic disease states and how folate nutrition may contribute to replacing methylation adequately and improving overall health.
Supplementation with active folate 5-MTHF bypasses the entire folate metabolization, which is potentially impaired by MTHFR polymorphism, and 5-MTHF is directly absorbed to exert the biological activity. Therefore, using 5-MTHF as a food supplement instead of FA is strongly recommended for external supplementation.13
Among the available ingredients, Quatrefolic, the glucosamine salt of 5-MTHF, patented by Gnosis by Lesaffre, offers a significant advantage over previous generations of folates (Figure 2). Thanks to its high solubility and bioavailability, the supplement delivers finished folate directly used by an organism without any specific form of metabolism, which makes it the ideal choice because it’s suitable for everyone regardless of MTHFR polymorphism.
Figure 2.
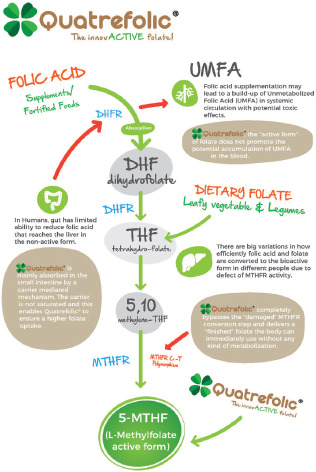
Quatrefolic, the Glucosamine Salt of 5-MTHF With High Solubility and Bioavailability
ACTIVE FOLATE AND HOMOCYSTEINE
Regardless of how the active form of folate 5-MTHF has been obtained, the methylfolate, in concert with vitamin B12, enters the one-carbon metabolism. This metabolism is a network of interrelated biochemical reactions that occurs in all of the body’s cells, and it’s vital for various functions, including detoxification, energy production, immune function, maintenance and regulation of genes, mood balancing, and control of inflammation. It’s essential for sustaining life and for inhibiting or slowing the development of age-associated diseases.
Perturbations of one-carbon metabolism, owing to low levels of 5-MTHF, critically contribute to increasing the circulating homocysteine (Hcy) levels and to a toxic accumulation in the bloodstream (Figure 3).22
Figure 3.
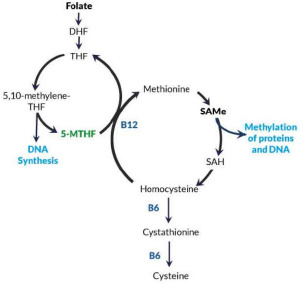
In the folate-dependent, one-carbon metabolism, the 5MTHF provides methyl groups to the S-adenosylmethionine (SAMe, SAM) cycle through homocysteine (Hcy) conversion to methionine. SAMe is the primary methyl donor to the methyl groups in the body and is required in the methylation of a diverse number of targets, including phospholipids, DNA, ribonucleic acid, neurotransmitters, and proteins. Hcy is a byproduct of the one-carbon cycle and is accumulated in the bloodstream when it can’t be remethylated to methionine. Plasma Hcy levels are an indirect indicator of folate levels.18
Abbreviations: 5MTHF, 5-methyltetrahydrofolate; DHF, dihydrofolate; SAH, S adenosylhomocysteine; THF, tetrahydrofolate.
Prolonged exposure to high Hcy, hyperhomocysteinemia (HCys), can damage the blood vessels, contributing to blood clots, and can lead to the onset of cardiovascular disease, including atherosclerosis, stroke, and inflammatory syndromes as well as neuronal pathologies. Because healthy blood vessels and folate levels are essential for fertility and pregnancy, high Hcy can make it difficult to conceive and maintain a pregnancy.25
Mazza et al demonstrated the capacity of 400 μg of Quatrefolic, in conjunction with B6 and B12, to lower Hcy serum levels better than conventional vitamin supplementation with highly dosed folic acid (5 mg/day). Quatrefolic was tested on hypertensive people with a low cardiovascular risk—104 patients with HCys of ≥15 μmol/L.
Significant HCys reduction occurred as compared to baseline, from 21.5 μmol/L to 10.0 μmol/L. Moreover, the treatment was significantly effective. The ideal HCys level was reached in 55.8% of cases in the Quatrefolic group, and it was substantially higher than that of the control. Polymorphisms in folate pathway genes could be one reason for fertility complications in some women with unexplained infertility.26
ACTIVE FOLATE OVER FOLIC ACID
Averting Birth Defects
Pregnant women have a five-to-ten-fold higher requirement for folate than do women who aren’t pregnant. Folate is needed in cell growth, cell division, cell synthesis, and repair of DNA. During pregnancy, folate requirements increase not only to support embryonic and fetal development and maternal tissue growth but also to reduce the risk of low birth weight, preterm birth, elevated Hcy levels, and related adverse pregnancy outcomes.
The indication is to take folate for 3 months before conception and to continue taking it for at least the first 3 months of pregnancy to prevent having a baby with neural tube defects (NTDs), a malformation that develops in the first 28 days of pregnancy, before many women even know that they’re pregnant.22,23
Due to an inability to properly process FA, pregnant women with MTHFR gene polymorphisms may have an increased risk of many deleterious defects. Moreover, elevated Hcy caused by folate deficiency is one of the independent risk factors for many pregnancy-related disorders.
Some studies have explored the effects of 5-MTHF supplementation for individuals with MTHFR polymorphisms—wild-type CC or homozygous TT. Prinz-Langenohl et al27 demonstrated that 5-MTHF supplementation isn’t affected by MTHFR gene polymorphism. In another chronic, bioavailability study, Litynski et al have shown a significant prolonged effect in reducing Hcy levels at six months after ceasing treatment with 5-MTHF in homozygous individuals (TT), at 12.1 ± 2.5 as compared to 16.9 ± 6.8 for folic acid (P < .01).28
Other studies suggest that folate insufficiency due to MTHFR deficiency is bypassed by 5-MTHF supplementation.27-29 Hence, active 5-MTHF, such as Quatrefolic, appears to be a preferred option for folate supplementation in individuals with MTHFR polymorphism.
Many studies have reported that hyperhomocysteinemia was associated with numerous pregnancy complications, including recurrent pregnancy loss,30 NTDs,31 preeclampsia,32 preterm delivery,33 placental abruption,34,35 fetal growth restriction,36 and gestational diabetes mellitus.37 Cawley et al also observed that neonatal birth weight and maternal Hcy levels were negatively correlated.38 While the beneficial effects of folate supplementation in reduction of the risks of NTDs have been clearly demonstrated,39-41 more robust meta-analyses are needed to assess its efficacy in preventing other congenital disabilities.42-44
Interestingly, many studies have observed folate-and-Hcy metabolism anomalies in children affected by autism spectrum disorder (ASD).45,46 More important, folate supplementation during pregnancy may reduce the risk of delivering newborns affected by ASD22,47 and may improve children’s language competency.48
But a clinically validated option exists. Supplementation for hypertensive individuals with 400 μg of Quatrefolic for two months was found to be more effective in reducing Hcy blood levels than folic acid.26 Additionally, the supplementation of pregnant women with Quatrefolic until the twenty-fourth week of pregnancy was more efficient in increasing 5-MTHF blood levels than the same dose of folic acid.49 The study also confirmed the importance of folate supplementation to avoid developing NTDs.
In a recent retrospective study of 269 individuals, Cirillo et al investigated the role of a vitamin B complex supplement—Quatrefolic, vitamin B12, and vitamin B6 (Inpha Duemila, Normocis)—compared to FA supplementation only, in relation to clinical pregnancy and live birth in infertile women undergoing homologous assisted reproductive technology (ART).
A higher percentage of women in the vitamin B complex group had a clinical pregnancy and live birth when compared to the percentage of the FA group. The researchers concluded that women supplemented with Quatrefolic and vitamin B12 have a higher chance of clinical pregnancy and live birth. Supplementation with vitamin B complex might be considered in clinical practice with women undergoing ART.50
Efficacy of 5-MTHF in Infertility
The level of folate and Hcy status is critical in the early stages of human reproduction. Investigation of the role of Hcy metabolism in patients with unexplained female sterility or secondary sterility due to recurrent pregnancy loss shows a positive association. Women and men with fertility problems may have low folate availability, which is often related to the MTHFR enzyme polymorphism. Preconceptional folate supplementation has been linked to beneficial reproductive outcomes in both natural pregnancies and those after ART treatment.
An increasing volume of publications related to folates and fertility problems have been published over the past few years, showing that 5-MTHF is a better option than folic acid to correct metabolic defects in gametes and embryos. In patients with repeated miscarriages and ART failures, Servy and Menezo observed a strong impact for the C677T MTHFR isoform.51 Both partners could be responsible for the failure; it isn’t restricted to women.
Based on these observations, couples with fertility problems, especially if at least one of the partners is a carrier of one of the two main MTHFR isoforms (C677T or A1298C) and if he or she has a folate-deficient diet, should be supplemented with 5-MTHF rather than folic acid to bypass the bottleneck created by the deficiency of MTHFR.51-56
Observational studies have been conducted with Quatrefolic in couples with fertility problems and have shown promising results.22,57 In Servy et al’s case series, seven couples with more than five miscarriages and a history of inefficient supplementation with high doses of FA (5 mg/day) to prevent pregnancy loss, were supplemented for four months with Quatrefolic.57 Among seven couples, both partners in five carried the main MTHFR isoform (C677T). After the supplementation with 400 μg/day of Quatrefolic, six couples achieved pregnancy. Three were spontaneous pregnancies and deliveries, two had pregnancies at 16 and 25 weeks, and one had just started a pregnancy.
Another larger case series included 33 couples with fertility problems that had lasted for at least four years, such as recurrent fetal loss, premature ovarian insufficiency, or abnormal sperm parameters. Two-thirds of them had failed ART attempts.52 For all the couples, at least one of the partners was a carrier of one of the two main MTHFR isoforms (C677T or A1298C), and the women had been previously treated unsuccessfully with high doses of FA (5 mg/day).
In that study, before attempting conception or ART, all couples were supplemented with 800 μg/day of Quatrefolic, either Impryl or Tetrafolic, for 74 days, which corresponds to the entire cycle of spermatogenesis (Figure 4). This led to successful pregnancy spontaneously for 13 couples and with ART for 17 couples. The researchers concluded that a physiological dose of 800 μg/day of Quatrefolic bypasses the MTHFR block, and they suggest that it’s an effective treatment for such couples.
Figure 4.

Quatrefolic Can Bypass the Methylenetetra-hydrofolatereductase (MTHFR) Block
More recently, Clement et al showed that couples with at least 3 years of fertility problems, with one of the partners being homozygous for MTHFR isoforms, have higher circulating Hcy levels than the heterozygous or wild-type patients.53 The supplementation of 89 couples with 600 μg/day Quatrefolic (either Impryl or Tetrafolic) for 3 months was effective in significantly decreasing plasma Hcy levels. The authors concluded that couples with a long history of infertility should be analyzed for MTHFR single-nucleotide polymorphisms and Hcy and should be treated with a physiological dose of 5-MTHF instead of high doses of FA.
No UMFA Risk
Several studies have reported an increase in serum UMFA levels after implementing FA fortification, with possible concerns about potential overdoses and adverse effects.1,20 Variability in the presence or persistence of UMFA in the population that it may be accumulated in the blood due to different conditions, such as uncontrolled FA intake or impairment of the FA reduction pathway. High doses of FA can lead to UMFA syndrome, which is suspected of causing immune dysfunction and other adverse pathological effects, such as cancer, especially colorectal and prostate.61-63 However, the causal effect between UMFA and those pathologies has yet to be demonstrated.64
A dose of about 100 to 200 μg of FA is known to be unlikely to cause the appearance of UMFA in the blood but FA can be detected after a supplementation period of 14 weeks at 400 μg and can lead to UMFA appearance in serum.24 In contrast, daily supplementation with the reduced active form 5-MTHF, Quatrefolic, seems not to produce UMFA because it’s directly used by cells, and enters the one-carbon cycle required to synthesize DNA and red blood cells.17,23,27,52,57 Bailey et al’s study supported that finding, showing that 5-MTHF enables folate repletion more quickly and uniformly than FA and without exposure to UMFA.17
CONCLUSIONS
Testing every pregnant woman for the existence of a mutated MTHFR gene isn’t a standard medical protocol, but women expressing MTHFR polymorphism may not experience the perceived advantage of FA supplementation and can be at potential risk because they are less able to transform FA. Because the association between the MTHFR polymorphism and a low folate concentration has been assessed, the direct supplementation of an active form, such as Quatrefolic (5-MTHF), through fertility supplements, prenatal vitamins, and dietary supplements, should be strongly considered as being universally beneficial.
Biography
Lorena Carboni, MSc, Senior Scientific Communication Specialist, Product manager, Gnosis by Lesaffre, Desio, Italy. Earning her Master’s degree in Pharmaceutical Chemistry and Technology and having over 20 years of experience in the nutraceutical and pharmaceutical industries, Lorena Carboni has a specific background in technical and scientific aspects of Gnosis by Lesaffre’s ingredients, with a particular focus on the One Carbon metabolites, including folate (5-MTHF), S-adenosylmethionine, and glutathione. At Gnosis by Lesaffre, she has specialized in the scientific and clinical aspects of natural ingredients as a Scientific Communication Specialist, through close and proactive collaborations with several universities, the University of Modena and Reggio Emilia, Polytechnic University of Marche, University of Milan, and others and with academic panels of experts with whom she has conducted specific educational projects and publications.
REFERENCES
- 1.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006;15(2):189-193. doi:10.1158/1055-9965.EPI-06-0054 [DOI] [PubMed] [Google Scholar]
- 2.Henderson AM, Aleliunas RE, Loh SP, et al. L-5-methyltetrahydrofolate supplementation increases blood folate concentrations to a greater extent than folic acid supplementation in Malaysian women. J Nutr. 2018;148(6):885-890. doi:10.1093/jn/nxy057 [DOI] [PubMed] [Google Scholar]
- 3.Lamers Y, Prinz-Langenohl R, Brämswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr. 2006;84(1):156-161. doi:10.1093/ajcn/84.1.156 [DOI] [PubMed] [Google Scholar]
- 4.Venn BJ, Green TJ, Moser R, Mann JI. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo-controlled study. Am J Clin Nutr. 2003;77(3):658-662. doi:10.1093/ajcn/77.3.658 [DOI] [PubMed] [Google Scholar]
- 5.Lamers Y, Prinz-Langenohl R, Moser R, Pietrzik K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am J Clin Nutr. 2004;79(3):473-478. doi:10.1093/ajcn/79.3.473 [DOI] [PubMed] [Google Scholar]
- 6.Ferrazzi E, Tiso G, Di Martino D. Folic acid versus 5- methyl tetrahydrofolate supplementation in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2020;253:312-319. doi:10.1016/j.ejogrb.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 7.Maulik D, van Haandel L, Allsworth J, Chaisanguanthum KS, Yeast JD, Leeder JS. The effect of race and supplementation on maternal and umbilical cord plasma folates. J Matern Fetal Neonatal Med. 2019;1-9. [DOI] [PubMed] [Google Scholar]
- 8.Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25(5):901-911. doi:10.1007/ s10897-016-9956-7 [DOI] [PubMed] [Google Scholar]
- 9.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862-877. doi:10.1093/oxfordjournals.aje.a010290 [DOI] [PubMed] [Google Scholar]
- 10.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87(3):517-533. doi:10.1093/ajcn/87.3.517 [DOI] [PubMed] [Google Scholar]
- 11.Stover PJ. Folic acid. In: Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 12.Singh J. Vitamin B9 in Dark Green Vegetables: Deficiency Disorders, Bio-Availability, and Fortification Issues. In: Guy LeBlanc J, ed. B-Complex Vitamins - Sources, Intakes and Novel Applications. IntechOpen; 2022. doi:10.5772/ intechopen.100318 [Google Scholar]
- 13.Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488. doi:10.3109/00498254.2013.845705 [DOI] [PubMed] [Google Scholar]
- 14.Bailey LB, Gregory JF, III. Folate metabolism and requirements. J Nutr. 1999;129(4):779-782. doi:10.1093/jn/129.4.779 [DOI] [PubMed] [Google Scholar]
- 15.EFSA Scientific Opinion. Scientific opinion on dietary reference values for folate. EFSA J. 2014. [Google Scholar]
- 16.Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development-folate review. J Nutr. 2015;145(7):1636S-1680S. doi:10.3945/jn.114.206599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey S W, Ayling JE. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci Rep. 2018;8(1):4096. doi:10.1038/ s41598-018-22191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menezo Y, Elder K, Clement A, Clement P. Folic acid, folinic acid, 5 methyl tetrahydrofolate supplementation for mutations that affect epigenesis through the folate and one-carbon cycles. Biomolecules. 2022;12(2):197. doi:10.3390/ biom12020197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patanwala I, King MJ, Barrett DA, et al. Folic acid handling by the human gut: implications for food fortification and supplementation. Am J Clin Nutr. 2014;100(2):593-599. doi:10.3945/ajcn.113.080507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010;49(8):535-548. doi:10.2165/11532990-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 21.Sweeney MR, McPartlin J, Weir DG, et al. Evidence of unmetabolised folic acid in cord blood of newborn and serum of 4-day-old infants. Br J Nutr. 2005;94(5):727-730. doi:10.1079/BJN20051572 [DOI] [PubMed] [Google Scholar]
- 22.Tinelli C, Di Pino A, Ficulle E, Marcelli S, Feligioni M. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front Nutr. 2019;6:49. doi:10.3389/fnut.2019.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Put NM, Gabreëls F, Stevens EM, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62(5):1044-1051. doi:10.1086/301825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoef H, Veenemans J, Mwangi MN, Prentice AM. Safety and benefits of interventions to increase folate status in malaria-endemic areas. Br J Haematol. 2017;177(6):905-918. doi:10.1111/bjh.14618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34(1):75-81. doi:10.1007/s10545-010-9177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazza A, Cicero AF, Ramazzina E, et al. Nutraceutical approaches to homocysteine lowering in hypertensive subjects at low cardiovascular risk: a multicenter, randomized clinical trial. J Biol Regul Homeost Agents. 2016;30(3):921-927. [PubMed] [Google Scholar]
- 27.Prinz-Langenohl R, Brämswig S, Tobolski O, et al. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C-->T polymorphism of methylenetetrahydrofolate reductase. Br J Pharmacol. 2009;158(8):2014-2021. doi:10.1111/j.1476-5381.2009.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litynski P, Loehrer F, Linder L, Todesco L, Fowler B. Effect of low doses of 5-methyltetrahydrofolate and folic acid on plasma homocysteine in healthy subjects with or without the 677C-->T polymorphism of methylenetetrahydrofolate reductase. Eur J Clin Invest. 2002;32(9):662-668. doi:10.1046/j.1365-2362.2002.01055.x [DOI] [PubMed] [Google Scholar]
- 29.Vidmar Golja M, Šmid A, Karas Kuželički N, Trontelj J, Geršak K, Mlinarič-Raščan I. Folate insufficiency due to MTHFR deficiency is bypassed by 5-methyltetrahydrofolate. J Clin Med. 2020;9(9):9. doi:10.3390/jcm9092836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Zhao Y, Ping Y, Chen L, Feng X. Association between gene polymorphism of folate metabolism and recurrent spontaneous abortion in Asia: A Meta-analysis. Medicine (Baltimore). 2020;99(40):e21962. doi:10.1097/MD.0000000000021962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav U, Kumar P, Rai V. Maternal biomarkers for early prediction of the neural tube defects pregnancies. Birth Defects Res. 2021;113(7):589-600. doi:10.1002/ bdr2.1842 [DOI] [PubMed] [Google Scholar]
- 32.Serrano NC, Quintero-Lesmes DC, Becerra-Bayona S, et al. Association of preeclampsia risk with maternal levels of folate, homocysteine and vitamin B12 in Colombia: A case-control study. PLoS One. 2018;13(12):e0208137. doi:10.1371/ journal.pone.0208137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu X, Gao F, Qiu Y, et al. Association of maternal serum homocysteine concentration levels in late stage of pregnancy with preterm births: a nested case-control study. J Matern Fetal Neonatal Med. 2018;31(20):2673-2677. doi:10.1080/14767058.2017.1351534 [DOI] [PubMed] [Google Scholar]
- 34.Dai C, Fei Y, Li J, Shi Y, Yang X. A novel review of homocysteine and pregnancy complications. BioMed Res Int. 2021;2021:6652231. doi:10.1155/2021/6652231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goddijn-Wessel TA, Wouters MG, van de Molen EF, et al. Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur J Obstet Gynecol Reprod Biol. 1996;66(1):23-29. doi:10.1016/0301-2115(96)02383-4 [DOI] [PubMed] [Google Scholar]
- 36.Jiang HL, Cao LQ, Chen HY. Blood folic acid, vitamin B12, and homocysteine levels in pregnant women with fetal growth restriction. Genet Mol Res. 2016;15(4):4. doi:10.4238/gmr15048890 [DOI] [PubMed] [Google Scholar]
- 37.Guven MA, Kilinc M, Batukan C, Ekerbicer HC, Aksu T. Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch Gynecol Obstet. 2006;274(6):333-337. doi:10.1007/s00404-006-0191-6 [DOI] [PubMed] [Google Scholar]
- 38.Cawley S, O’Malley EG, Kennedy RAK, Reynolds CME, Molloy AM, Turner MJ. The relationship between maternal plasma homocysteine in early pregnancy and birth weight. J Matern Fetal Neonatal Med. 2020;33(18):3045-3049. doi:10.1080/ 14767058.2019.1567705 [DOI] [PubMed] [Google Scholar]
- 39.De-Regil LM, Peña-Rosas J P, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;12(12):CD007950. doi:10.1002/14651858.CD007950.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blom HJ. Folic acid, methylation and neural tube closure in humans. Birth Defects Res A Clin Mol Teratol. 2009;85(4):295-302. doi:10.1002/bdra.20581 [DOI] [PubMed] [Google Scholar]
- 41.Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. 2010;39(suppl 1):i110-i121. doi:10.1093/ije/dyq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai C, Fei Y, Li J, Shi Y, Yang X. A novel review of homocysteine and pregnancy complications. BioMed Res Int. 2021;2021:6652231. doi:10.1155/2021/6652231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Liu C, Wang Q, Zhang Z. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: a meta-analysis. Arch Gynecol Obstet. 2018;298(4):697-704. doi:10.1007/s00404-018-4823-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali A, Waly MI, Al-Farsi YM, Essa MM, Al-Sharbati MM, Deth RC. Hyperhomocysteinemia among Omani autistic children: a case-control study. Acta Biochim Pol. 2011;58(4):547-551. doi:10.18388/abp.2011_2223 [PubMed] [Google Scholar]
- 45.Paşca S P, Nemeş B, Vlase L, et al. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78(19):2244-2248. doi:10.1016/j.lfs.2005.09.040 [DOI] [PubMed] [Google Scholar]
- 46.DeVilbiss EA, Gardner RM, Newschaffer CJ, Lee BK. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. Br J Nutr. 2015;114(5):663-672. doi:10.1017/S0007114515002470 [DOI] [PubMed] [Google Scholar]
- 47.Roth C, Magnus P, Schjølberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306(14):1566-1573. doi:10.1001/ jama.2011.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B, Zhang X, Peng X, Zhang S, Wang X, Zhu C. Folic acid and risk of preterm birth: A meta-analysis. Front Neurosci. 2019;13:1284. doi:10.3389/ fnins.2019.01284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dell’Edera D, Sarlo F, Allegretti A, et al. Prevention of neural tube defects and maternal gestational diabetes through the inositol supplementation: preliminary results. Eur Rev Med Pharmacol Sci. 2017;21(14):3305-3311. [PubMed] [Google Scholar]
- 50.Cirillo M, Fucci R, Rubini S, Coccia ME, Fatini C. 5-methyltetrahydrofolate and vitamin b12 supplementation is associated with clinical pregnancy and live birth in women undergoing assisted reproductive technology. Int J Environ Res Public Health. 2021;18(23):18. doi:10.3390/ijerph182312280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Servy E, Menezo Y. The methylene tetrahydrofolate reductase (MTHFR) isoform challenge. high doses of folic acid are not a suitable option compared to 5 methyltetrahydrofolate treatment. Clin Obstet Gynecol Reprod Med. 2017;3(6). doi:10.15761/COGRM.1000204 [Google Scholar]
- 52.Servy EJ, Jacquesson-Fournols L, Cohen M, Menezo YJR. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: a key to pregnancy outcome: a case series. J Assist Reprod Genet. 2018;35(8):1431-1435. doi:10.1007/s10815-018-1225-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clément A, Menezo Y, Cohen M, Cornet D, Clément P. 5-Methyltetrahydrofolate reduces blood homocysteine level significantly in C677T methyltetrahydrofolate reductase single-nucleotide polymorphism carriers consulting for infertility. J Gynecol Obstet Hum Reprod. 2020;49(1):101622. doi:10.1016/j.jogoh.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 54.House SH, Nichols JAA, Rae S. Folates, folic acid and preconception care - a review. JRSM Open. 2021;12(5):2054270420980875. doi:10.1177/2054270420980875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ménézo Y, Patrizio P, Alvarez S, et al. MTHFR (methylenetetrahydrofolate reductase: EC 1.5.1.20) SNPs (single-nucleotide polymorphisms) and homocysteine in patients referred for investigation of fertility. J Assist Reprod Genet. 2021;38(9):2383-2389. doi:10.1007/s10815-021-02200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta P, Vishvkarma R, Singh K, et al. MTHFR 1298A>C substitution is a strong candidate for analysis in recurrent pregnancy loss: evidence from 14 289 subjects. Reprod Sci. 2021. [DOI] [PubMed] [Google Scholar]
- 57.Servy E, Menezo Y. The Methylene Tetrahydrofolate Reductase (MTHFR) isoform challenge. High doses of folic acid are not a suitable option compared to 5 Methyltetrahydrofolate treatment. Clin Obstet Gynecol Reprod Med. 2017;3(6). doi:10.15761/COGRM.1000204 [Google Scholar]
- 58.Cornet D, Cohen M, Clement A, et al. Association between the MTHFR-C677T isoform and structure of sperm DNA. J Assist Reprod Genet. 2017;34(10):1283-1288. doi:10.1007/s10815-017-1015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sweeney MR, McPartlin J, Scott J. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health. 2007;7(1):41. doi:10.1186/1471-2458-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim Y-I. Folic acid supplementation and cancer risk: point. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2220-2225. doi:10.1158/1055-9965.EPI-07-2557 [DOI] [PubMed] [Google Scholar]
- 61.Maruvada P, Stover PJ, Mason JB, et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr. 2020;112(5):1390-1403. doi:10.1093/ajcn/nqaa259 [DOI] [PMC free article] [PubMed] [Google Scholar]


