Abstract
BACKGROUND:
Unfractionated heparin administered immediately after traumatic brain injury (TBI) reduces brain leukocyte (LEU) accumulation, and enhances early cognitive recovery, but may increase bleeding after injury. It is unknown how non-anticoagulant heparins, such as 2,3-O desulfated heparin (ODSH), impact post-TBI cerebral inflammation and long-term recovery. We hypothesized that ODSH after TBI reduces LEU-mediated brain inflammation and improves long-term neurologic recovery.
METHODS:
CD1 male mice (n = 66) underwent either TBI (controlled cortical impact [CCI]) or sham craniotomy. 2,3-O desulfated heparin (25 mg/kg [25ODSH] or 50 mg/kg [50ODSH]) or saline was administered for 48 hours after TBI in 46 animals. At 48 hours, intravital microscopy visualized rolling LEUs and fluorescent albumin leakage in the pial circulation, and the Garcia Neurologic Test assessed neurologic function. Brain edema (wet/dry ratio) was evaluated post mortem. In a separate group of animals (n = 20), learning/memory ability (% time swimming in the Probe platform quadrant) was assessed by the Morris Water Maze 17 days after TBI. Analysis of variance with Bonferroni correction determined significance (p < 0.05).
RESULTS:
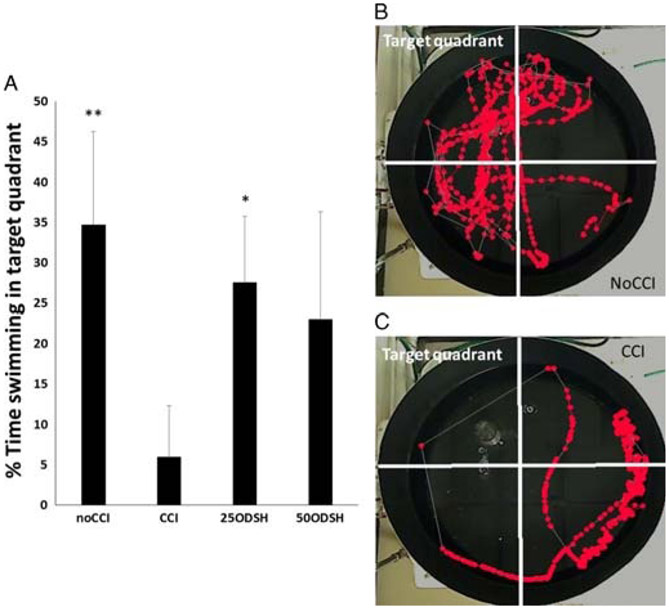
Compared with CCI (LEU rolling: 32.3 ± 13.7 LEUs/100 μm per minute, cerebrovascular albumin leakage: 57.4 ± 5.6%), both ODSH doses reduced post-TBI pial LEU rolling (25ODSH: 18.5 ± 9.2 LEUs/100 μm per minute, p = 0.036; 50ODSH: 7.8 ± 3.9 LEUs/100 μm per minute, p < 0.001) and cerebrovascular albumin leakage (25ODSH: 37.9 ± 11.7%, p = 0.001, 50ODSH: 32.3 ± 8.7%, p < 0.001). 50ODSH also reduced injured cerebral hemisphere edema (77.7 ± 0.4%) vs. CCI (78.7 ± 0.4 %, p = 0.003). Compared with CCI, both ODSH doses improved Garcia Neurologic Test at 48 hours. Learning/memory ability (% time swimming in target quadrant) was lowest in CCI (5.9 ± 6.4%) and significantly improved in the 25ODSH group (27.5 ± 8.2%, p = 0.025).
CONCLUSION:
2,3-O desulfated heparin after TBI reduces cerebral LEU recruitment, microvascular permeability and edema. 2,3-O desulfated heparin may also improve acute neurologic recovery leading to improved learning/memory ability weeks after injury.
Keywords: ODSH, TBI, leukocyte, Morris Water Maze, heparin
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in young patients with over 10 million patients affected every year.1,2 In the past few decades, despite intense efforts invested in developing neuroprotective therapies for TBI, none has been consistently implemented at the bedside. In predicting outcome following TBI, the initial cerebral tissue deforming impact is of utmost importance. However, the host and the environment the host is exposed to after injury have also been implicated in promoting the ongoing tissue injury and destruction that persist days and even weeks after severe TBI. This ongoing process is thought to be driven by multiple cellular, metabolic and molecular processes.3-6
Several reports have demonstrated that heparinoids may reduce tissue inflammation after injury by blunting the activation of circulating leukocytes (LEUs) regionally and reducing their sequestration in injured tissue. For example, both unfractionated heparin (UFH) and low molecular weight heparin (LMWH) reduce neutrophil and endothelial cell (EC) activation and adhesion receptor expression.7,8 Specifically in TBI models, heparinoids evidence similar effects on activation of cerebral endothelium and circulating LEUs but also, concurrently reduce microvascular permeability and improve subsequent neurologic recovery.9-12 Unfortunately, heparinoids are invariably withheld when managing patients early after TBI as their anticoagulant effects may augment cerebral hemorrhage. Manipulating the sulfation of heparin may eliminate the majority of heparin’s anticoagulant effects, a process that has been leveraged to use heparin’s anti-LEU effects in other contexts including in the formulation of chemotherapeutic agents addressing liquid and bone marrow tumors.13,14 One such compound, 2,3-O desulfated heparin (ODSH) exerts less than 15% the anticoagulant properties of UFH.8,15,16 Nonetheless, some heparin-related anti-inflammatory effects appear to be preserved with this manipulation, at least with respect to the ability to inhibit complement activation, as well as binding to certain adhesion molecules.8,17 In several injury models, including central nervous system (CNS) infection and stroke, ODSH reduces brain tissue edema, and improves neurologic recovery.
To our knowledge, no published studies have evaluated whether ODSH affects microvascular cerebral inflammation after TBI nor subsequent neurologic recovery. In the current study, we sought to determine whether ODSH specifically reduced in vivo LEU-mediated cerebrovascular inflammation after TBI and hypothesized that early, repeated administration of ODSH following TBI reduces pial EC/LEU interactions and decreases microvascular permeability and cerebral edema. We further postulated that ODSH treatment would lead to enhanced neurologic recovery beyond 2 weeks after injury.
MATERIALS AND METHODS
Reagents
Normal saline (0.9%, NS) was purchased from Baxter Healthcare Corporation (Deerfield, IL), ketamine from Hospira (Lake Forest, IL), xylazine from Akorn (Decatur, IL) and acepromazine from Boehringer Ingelheim (St. Joseph, MO). Bovine fluorescein isothiocyanate (FITC)-labeled albumin and 0.3% rhodamine 6G were purchased from Sigma-Aldrich (St. Louis, MO). 2-O, 3-O desulfated heparin (ODSH) was supplied from Cantex Pharmaceuticals (Weston, FL) and per the company is manufactured as a pharmaceutical grade compound under Good Manufacturing Practices.
Experimental Design
All experimental procedures were conducted with approval of the University of Pennsylvania Institutional Animal Care and Use Committee (Fig. 1). CD1 adult male mice (25–30 g) (Charles River Laboratories, Wilmington, MA) were kept in standard housing with water and chow ad libitum, for 5 days to 7 days before experiments. On day 1, all 66 animals were anesthetized with intraperitoneal ketamine (100 mg/kg), xylazine (10 mg/kg), and acepromazine (1 mg/kg). As described below, animals were subjected to controlled cortical impact (CCI) or sham craniotomy and then received subcutaneous injections of ODSH (25 or 50 mg/kg) or an equal volume of 0.9% saline (vehicle [VEH], 1 mL/kg) at 2 hours, 10 hours, 26 hours, and 36 hours after CCI. This regimen was used to reflect human UFH dosages as well as per the manufacturer's recommendations to mirror prior ODSH dosing regimens.11,12 Series 1 animals (n = 46) underwent the 48-hour intravital microscopy protocol while series 2 animals (n = 20) were kept in their cages with unimpeded mobility for 14 days to 17 days when they were subjected to the Morris Water Maze protocol (Fig. 1). Overall, 66 animals were randomized into four groups: negative control (no CCI + VEH: noCCI), positive control (CCI + VEH: CCI), CCI + 25 mg/kg ODSH (25ODSH), and CCI + 50 mg/kg ODSH (50ODSH).
Figure 1.

Experimental design and procedures. (A) Timeline of SERIES 1 experimental protocol. (B) Timeline of SERIES 2 experimental protocol. IVM, intravital microscopy; NS, normal saline.
Severe TBI Model
As we described previously, a validated CCI model was used to establish severe TBI.18,19 In brief, prone anesthetized animals were secured in a stereotactic frame, and using a 4-mm trephine, a left-sided craniotomy was created between the bregma and lambda sutures to expose the dura overlying the parieto-temporal cortex. The CCI device (AMS 201; AmScien Instruments, Richmond, VA) was armed with a 3-mm diameter impactor tip, discharged at an impact velocity of 6 m/s which created a 1.0-mm cortical deformation.19 The resultant cerebral injury is reproducible and consistent with severe TBI.
SERIES 1
Intravital Microscopy
Forty-six (46) animals were randomized as follows: noCCI (n = 13), CCI (n = 13), 25ODSH (n = 10), 50ODSH (n = 10) (Fig. 1A). After receiving the above regimen of ODSH or VEH for 36 hours, in vivo pial intravital microscopy assessment of the cerebral microcirculation was conducted as described elsewhere.9-11 Animals were again anesthetized with the same regimen; the right internal jugular vein was cannulated for the administration of rhodamine and FITC-labeled albumin, and the animal was again secured in the stereotactic frame before the second craniotomy. This second 2.5-mm craniotomy was created adjacent to the first using a dental drill (Henry Schein, Melville, NY) and was then covered with a 5-mm coverslip (Fisher Scientific, Waltham, MA). Live animals were then transferred to an intravital microscope (ECLIPSE FN1; Nikon Instruments, Melville, NY) and received a slow 50-μL intrajugular injection of 0.3% rhodamine 6G to fluorescently label circulating LEUs visible at a 590-nm epi-illumination emission exposure. In each animal one, randomly selected, non-branching pial venules measuring 25 μm to 50 μm in diameter was videorecorded for 1 minute using a digital camera (QuantEM; Photometrics, Tucson, AR). Intravenous FITC-labeled albumin (100-mg/kg) was then administrated for visualization of albumin leakage as a surrogate for microvascular permeability. The same venule was observed under a different, 488-nm fluorescent filter and 10-second footage was captured and recorded for offline determination of microvascular permeability.
Microcirculatory Analysis
Video recordings and still images were imported into a digital analysis software (NIS-Elements, Nikon Instruments Melville, NY) and LEU-EC interactions were quantified by a blinded observer documenting the following parameters: (1) PMN rolling: mean number of labeled cells crossing a 100-μm venular segment per 60 s; (2) PMN adhesion: number of PMN stationary for at least 30 seconds during the recording period. Fluorescently labeled spherical cells measuring 7 μm to 12 μm were counted as LEU, and interactions were reported as number of cells/100 μm per minute (Fig. 2A). Images captured under the 488-nm filter were evaluated for FITC-labeled albumin fluorescence intensity. This was measured in three distinct regions within the vessel (venular intensity) and outside the vessel wall (perivenular intensity) (Fig. 2B). The ratio of mean venular intensity to mean perivenular intensity was averaged to determine the permeability index for a given vessel indicating the degree of vessel wall leakage.
Figure 2.
In vivo LEU/EC interactions and microvascular permeability. (A) Representative image showing LEU interacting with endothelium. White arrows indicate fluorescently labeled LEU rolling on endothelium. (B) Representative image showing FITC-albumin leakage in the cerebral microcirculation. The microvascular permeability index is expressed as the ratio of mean fluorescence of three separate locations outside the vessel wall (perivenular intensity, IP) to mean fluorescence of three separate locations within the venule (venular intensity, IV). (C) LEU rolling and FITC-albumin leakage in the pial penumbral microcirculation 48 hours after CCI. Compared with positive controls (CCI), both ODSH regimens reduced post-TBI LEU rolling and cerebrovascular albumin leakage. *p < 0.05, **p < 0.01 vs. CCI, #p < 0.05, ##p < 0.01 vs. 25ODSH.
Body Weight Loss, Neurologic Recovery
Body weight loss after injury is a widely used marker of illness and physical impairment. Animal body weights (W) were obtained before (W0h) and 24 hours and 48 hours after CCI with weight loss expressed as the ratio [(W0h – W24h or 48 hours)/W0h × 100%].
Animal neurologic function as assessed by the modified Garcia Neurologic Test (GNT) was determined 24 hours and 48 hours after TBI.20,21 This validated scale scores the animal’s motor, sensory, reflex, and balance ability to a maximum sum score of 18 points.
Brain and Lung Water Content
After 48-hour intravital microscopy, animals were sacrificed; their brains were excised and divided into injured (ipsilateral) and contralateral hemispheres. In the same setting, the left lung was excised via sternotomy. Organs were immediately weighed (wet weight, WW) and again after 72 hours of drying at 70°C (dry weight, DW). Tissue water content in each organ was calculated using wet-to-dry ratios (% water content =100 × [WW − DW]/WW).
SERIES 2
Morris Water Maze
Twenty animals were evaluated in the second Morris Water Maze (MWM) series and were randomized equally (n = 5 in each) into the same four groups (CCI, noCCI, 25ODSH, 50ODSH) as for series 1 (Fig. 1B) and underwent either CCI or sham craniotomy and then received subcutaneous injections of ODSH (25 or 50 mg/kg) or an equal volume of 0.9% saline at 2 hours, 10 hours, 26 hours, and 36 hours after CCI. Animals were then returned to their cages in standard housing and were monitored for 14 days after which they were first introduced to daily spatial learning and memory exercises using a MWM (until day 17). The MWM consists of a standard construct with a 100-cm diameter circular pool divided into 4 equal quadrants with a surrounding 50-cm wall (Fig. 6, view from above). The pool is filled with 21°C water and is fitted with a 23.5-cm cylindrical plexiglass platform placed 1.0 cm below the water surface at the center of the northwest (target) quadrant for animals to rest from swimming. Visual cues are displayed on the surrounding walls to aid spatial learning and memory. Starting on day 14 after CCI, mice underwent six trials per day for four consecutive days. Each time, they were placed in the water, facing the wall in one of four starting locations (north east, south west, east, south) and allowed a maximum of 60 seconds to find and mount the submerged platform. If the mouse failed to reach the platform within this time frame, it was lifted onto the platform by the operator and left there for 15 seconds to allow the animal to form memory of its location. Mice were then warmed under a heating lamp until the next trial. Latency to the platform (time needed to reach the platform), and swimming velocity (average in max of 60 s of swimming time) were recorded using an overhead video-tracking system (Ethovision, Noldus, Leesburg, VA). On the 17th day, after the final trial, a probe trial was performed to assess spatial memory by removing the submerged platform and allowing mice to swim freely for 60 seconds. Spatial memory was assessed by the time animals spent in the quadrant where the platform had been previously located with improved performance indicated by a greater proportion of time spent in the target quadrant.
Figure 6.
Spacial learning and memory. (A) % animal swimming time in the target quadrant where platform was previously (probe trial). Compared to positive CCI controls, noCCI and 25ODSH animals spent significantly more time in the target quadrant. (B) Representative image sequence of the geographical position of a CCI animal in the 60-s observation period. Each dot is the position of the animal at 33-ms intervals. (C) Representative image sequence of the geographical position of a noCCI animal in the 60-second observation period.
Statistical Analysis
All data are presented as mean ± SD. Statistical analyses were performed using SPSS (version 19, SPSS, Chicago, IL). Differences between group means were compared using analysis of variance with Bonferroni correction. A p value less than 0.05 determined significance.
RESULTS
Series 1
In Vivo LEU-EC Interactions and Microvascular Permeability (48 hours)
Forty-eight hours after CCI, the number of rolling LEUs and degree of microvascular permeability was directly visualized in the penumbral pial brain surface in vivo. CCI animals demonstrated the greatest number of rolling LEUs and microvascular leakage, while noCCI controls demonstrated the lowest levels of both parameters (Fig. 2). Leukocyte rolling in CCI animals (32.3 ± 13.7 LEUs/100 μm per minute) was significantly reduced with both 25ODSH (18.5 ± 9.2 LEUs/100 μm per minute, p = 0.036) and 50ODSH (7.8 ± 3.9 LEUs/100 μm per minute, p < 0.001). There were no significant differences in LEU rolling between ODSH groups. Leukocyte adhesion was rare without any differences among any of the groups (noCCI: 1.0 ± 1.3LEUs/100 μm per minute, CCI: 2.8 ± 2.6LEUs/100 μm per minute, 25ODSH: 1.1 ± 1.0LEUs/100 μm per minute, 50ODSH: 1.4 ± 2.0 LEUs/100 μm per minute, p > 0.05 for all comparisons).
Live cerebrovascular FITC-albumin leakage was significantly greater in CCI animals (57.4 ± 5.6%) compared with low-dose (37.9 ± 11.7%, p = 0.001), high-dose (32.3 ± 8.7%, p < 0.001) ODSH groups or noCCI animals (23.3 ± 4.2%, p < 0.001) (Fig. 2C). High-dose ODSH microvascular permeability was similar to that of uninjured animals.
Brain and Lung Edema (48 Hours)
Compared with positive controls (78.7 ± 0.4%), 50ODSH (77.7 ± 0.4%, p = 0.003) reduced injured cerebral hemisphere edema to that of negative control levels (77.6 ± 0.1%, p = 0.004 vs. CCI) (Fig. 3A). There were no significant differences between 25ODSH (78.2% ± 0.5%) and positive controls. Contralateral cerebral hemisphere edema was low and similar in all groups. Lung edema was similar in all groups (Fig. 3B).
Figure 3.

Tissue edema. (A) High-dose ODSH (50ODSH) reduced brain water content in the injured (ipsilateral) cerebral hemisphere to levels nearing negative controls (noCCI). **p < 0.01 vs. CCI in the ipsilateral hemisphere. Contralateral cerebral edema was similar in all groups. (B) There were no statistical differences in lung water content among groups.
Body Weight Loss, Neurologic Function (24 and 48 Hours)
At 24 hours and 48 hours after TBI, animal weight loss was similar in all injured animals with an average of 9.3 ± 2.9% of body weight.
Neurologic functional recovery (GNT) was complete (max points = 18) in negative controls and significantly better than any of the injured animals at both 24-hour and 48-hour timeframes (Fig. 4). Compared to GNT scores in positive controls (CCI, 24 h; 14.85 ± 1.1, 48 hours; 15.7 ± 0.9), both ODSH regimens significantly improved GNT scores at both time frames (25ODSH 24 hours; 15.9 ± 0.9, 48 hours; 16.6 ± 1.0 p < 0.05 for both, 50ODSH 24 hours; 16.0 ± 0.7, p < 0.01, 48 hours; 16.7 ± 0.8 p < 0.01). There were no differences between 25ODSH and 50ODSH animals at either time frame.
Figure 4.

Neurologic function was normal in negative controls at both 24-hour and 48-hour timeframes. Compared to positive CCI controls, both ODSH treatments improved GNT at both time frames after injury. *p < 0.05, **p < 0.01 vs. CCI, ##p < 0.01 vs. 25ODSH, ◊ ◊ p < 0.01 vs. 50ODSH.
Series 2
Morris Water Maze
Swimming Velocity (Motor Function)
There were no differences among groups in the velocity with which animals swam to find and locate the hidden submerged platform on day 14 (Fig. 5). On days 15, 16, and 17, however, uninjured (noCCI) animals consistently showed greater swimming velocity compared with untreated, injured (CCI) animals. On day 17, noCCI animals also swam faster (44.9 ± 23.5 cm/s) than both ODSH-treated injured groups. High-dose 50ODSH animals swam more rapidly than untreated (CCI) animals on both day 16 (33.6 ± 10.1 cm/s vs. 23.7 ± 9.2 cm/s, p = 0.012) and day 17 (34.0 ± 15.1 cm/s vs. 22.3 ± 7.8 cm/s, p = 0.021).
Figure 5.
Uninjured animals recovered normal swimming velocity by day 15. Only 50ODSH injured animals recovered normal swimming velocity as compared with untreated injured (CCI) animals by day 16 and 17. *p < 0.05, **p < 0.01 vs. CCI, #p < 0.05, ##p < 0.01 vs. 25ODSH.
Latency to the Platform (Learning)
Mean latency to reach the platform steadily decreased in NoCCI animals from day 15 (35.6 ± 24.9 seconds), day 16 (26.2 ± 21.2 seconds) to day 17 (21.8 ± 21.3 seconds). At both days 16 and 17, this was a significantly lower latency time than that of any of the injured (treated or untreated) animals. On both days 16 and 17 after injury, 50ODSH animals (d16: 41.0 ± 21.7 seconds; d17: 43.9 ± 21.5 seconds) demonstrated a significantly shorter latency time than that of CCI animals (d16: 56.5 ± 11.6 seconds, p < 0.05 vs 50ODSH; d17: 59.4 ± 3.4 seconds, p < 0.05 vs. 50ODSH). Though this may suggest improved learning ability in 50ODSH animals over CCI controls, this finding is difficult to interpret in light of the greater swimming velocities observed in 50ODSH animals over CCI counterparts during 2 days.
Percent Swimming Time Spent in Target Quadrant (Probe Trial)
Both noCCI negative controls (34.7 ± 11.6%) and 25ODSH animals (27.5 ± 8.2%) spent more time swimming in the target quadrant than untreated CCI animals (5.9 ± 6.4%), indicating greater memory ability (Fig. 6).
DISCUSSION
In the present study, we report how early administration of a non-anticoagulant heparin preparation reduces in vivo pericontusional LEU mobilization 48 hours after TBI, concurrently reducing local microvascular permeability. While both ODSH doses improved 48-hours neurologic recovery, only high-dose ODSH reduced cerebral edema. Furthermore, ODSH also improved animal learning and memory 17 days after TBI. Our data suggests that ODSH administration after TBI mitigates against the functional deficits that accrue from secondary brain injury after cortical impact.
While the nature of the initial deforming force caused by TBI on cerebral tissue is undeniably the utmost determinant of ultimate neurologic recovery, the pervasive tissue swelling and disruption that follows in the subsequent hours and days is thought to have an equally profound effect on recovery. Indeed, it is during the hours and days after TBI that injured brain tissue may hemorrhage further and/or progressively swell leading to intracranial hypertension, cerebral herniation and sometimes brain death.4,22 A well-known initiating event in the development of cerebral edema relates to cerebrovascular disruption and subsequent loss of microvascular integrity. The blood-brain barrier (BBB) may temporarily become more porous or “leaky” and allow extravasation of fluid and activated immune cells into the adjacent cerebral parenchyma.23 In the brain injured host, locally activated circulating LEUs may act both as a cause and an effect in the BBB disruption that follows injury, otherwise termed “secondary” brain injury. In this setting circulating LEUs interact with activated ECs a sequential process initiated by LEU rolling on endothelium and ultimately leading to LEU migration into tissue.24-26 There the activated LEU will continue secreting inflammatory cytokines and chemokines while releasing activated proteinases and free oxygen radicals. This, in turn, activates local resident astrocytes and microglia, leading to additional tissue injury and BBB disruption, and further promote leakage of activated plasma and circulating cells into the interstitial milieu.3 In TBI, several reports have demonstrated how cerebral accumulation of LEUs is intimately associated with ongoing secondary tissue destruction and swelling.27,28 Thus, slowing or disrupting this self-perpetuating sequence may be a tangible opportunity for therapeutic intervention to slow or ameliorate the progression of secondary brain injury.
We and others have previously shown that heparin, a standard anticoagulant used to prevent venous thromboembolism, has additional, poorly elucidated anti-inflammatory side effects. These other properties appear to particularly affect circulating LEUs and microvascular EC activation. For example, heparin administered in hemorrhagic shock results in improved microvascular function and rheology, directly reducing subsequent ischemic organ injury and improving cardiac, hepatic and renal function.24,29-31 In animal CNS injury models, heparin preparations reduce ischemic territory size after stroke.32 In a rodent pneumococcal meningitis model, heparin administration reduced cerebral edema and intracranial hypertension, and reduced LEU influx identified as fewer rolling and “sticking” LEUs in the pial circulation.33
In TBI, our group has shown how UFH and LMWH reduced early and delayed LEU sequestration in pericontusional tissue and subsequent cerebral edema, findings that were associated with improved short-term animal neurologic recovery and improved activities of daily living.9-12 Unfortunately, these benefits may have a dose limitation in that higher doses failed to yield additional neurologic gains, and in some cases, worsened outcome principally related to hemorrhage promotion as a consequence of heparin's anticoagulant properties (data under review for publication). Relatedly, in humans, heparinoid administration after injury, particularly brain injury, is often withheld for fear of promoting cerebral hemorrhage, often resulting in delayed administration for days and sometimes weeks after TBI.12
Based on the irreconcilable association of heparin and bleeding risk in the early post-TBI period, we explored a molecularly altered heparin known to possess less than one fifth the anticoagulant properties of UFH. The ODSH preparation has a low affinity for antithrombin III, low anti-Xa, and anti-IIa anti-coagulant activity and does not activate Hageman factor.8,16 The ODSH is created by removing the 2-O and 3-O sulfates of the iduronate and glucosamine moieties of standard UFH, and replacing them with hydroxyl groups while maintaining sulfation at the key 6-0 active locus.34 Several acute lung injury models demonstrate reduced pulmonary thrombin/EC activation and alveolar permeability with ODSH therapy.35,36 Myocardial reperfusion injury is also reduced with ODSH treatment as measured by reduced infarct size in dog and pig models subjected to coronary artery occlusion.37,38 In a rat stroke cerebral ischemia model using middle cerebral artery occlusion/reperfusion, ODSH reduced infarct size and improved neurologic function (foot fault performance) without an increased hemorrhage risk.34
One of the proposed mechanisms for ODSH-mediated reductions in LEU and EC interaction is through modulation of neutrophil elastase-induced high mobility group box 1 (HMGB-1) secretion in the perivascular space.8,39 In ALI specifically caused by TBI (neurogenic pulmonary edema) HMGB-1 production is increased in the pulmonary circulation.40-42 Curiously, we previously demonstrated similar reductions in lung edema when either LMWH or anti-HMGB-1 monoclonal antibody blockade is administered after TBI without a separate direct pulmonary injury.10 In the current study, we found a trend in reduced lung edema in animals subjected to ODSH treatment but this was not statistically significant.
Similar to our evaluation with UFH and LMWH, ODSH reduced in vivo LEU rolling and venular leakage in the penumbral microcirculation after brain injury.9,11 These results mirror reports by others in cerebral ischemic stroke and meningitis models where heparinoids similarly reduced cerebral edema.33,43,44 We also demonstrated that ODSH directly improved animal neurologic recovery, a finding that concurs with other investigators' results demonstrating improved foot fault test 5 days after middle cerebral artery occlusion in rodents receiving ODSH.34 However, in our study, we additionally identified increased swimming velocity with high-dose ODSH, approaching that of uninjured controls indicating a beneficial effect on functional motor recovery.
The current report has important limitations. First, it was conducted in mice, and there is no assurance that the pathophysiology of TBI recovery or modulation by heparins is directly translatable to mechanisms involved in human brain injury However, pathway elucidation leading to targeted human trials is an important prerequisite to clinical intervention. Second, only two different doses of ODSH were used. We did not perform a dose response curve and as such cannot be certain that 25 mg/kg or 50 mg/kg represent the optimal risk/benefit effect on LEU-mediated cerebral edema and cognitive recovery. Nonetheless, these doses were selected to maintain some parallel with the doses used in other trials to support comparison across investigations. Lastly, we did not gauge bleeding risk in each group. Evaluation and grading of injured or surgical fields for quantifiable hemorrhage would have improved the precision analysis of the non-anticoagulant properties of ODSH in injured and uninjured tissue. Nonetheless, several previous evaluations in cardiac, pulmonary and stroke injury models have demonstrated ODSH to cause little to no bleeding when administered in the doses used.
In conclusion, the current study demonstrates novel findings indicating that early, post-TBI administration of ODSH reduces in vivo penumbral recruitment of LEUs and local microvascular permeability that is associated with reduced cerebral edema. Furthermore, it demonstrates a concurrent ODSH-related improvement in cognitive recovery, learning, and memory that persists for more than 2 weeks after injury. Additional exploration of ODSH safety and efficacy in brain injured patients will be necessary to determine if such heparin preparations can improve human learning and functional recovery to mitigate against the poor outcomes frequently encountered after severe TBI.
ACKNOWLEDGMENTS
We would like to relate our gratitude to Ms. Robin Armstrong for her technical and organizational assistance.
The reagent, 2,3-O desulfated heparin (ODSH) was kindly provided as a gift, free of charge by Dr. Stephen Marcus CEO, Cantex Pharmaceuticals. No other support, financial or otherwise was provided by the Company.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
Presented at the 76th Annual Meeting of AAST and Clinical Congress of Acute Care Surgery to be held in Baltimore, MD, September 13–16 2017.
Contributor Information
Katsuhiro Nagata, Division of Traumatology, Surgical Critical Care & Emergency Surgery, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania; Department of Emergency and Critical Care Medicine, Tokyo Medical University Hachioji Medical Center, Tokyo, Japan.
Yujin Suto, Division of Traumatology, Surgical Critical Care & Emergency Surgery, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
John Cognetti, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
Kevin D. Browne, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania
Kenichiro Kumasaka, Department of Emergency and Critical Care Medicine, Tokyo Medical University Hachioji Medical Center, Tokyo, Japan.
Victoria E. Johnson, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
Lewis Kaplan, Division of Traumatology, Surgical Critical Care & Emergency Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
Joshua Marks, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pennsylvania.
Douglas H. Smith, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
Jose L. Pascual, Division of Traumatology, Surgical Critical Care & Emergency Surgery, Department of Neurosurgery Center for Brain Injury, and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
REFERENCES
- 1.Kerr ZY, Harmon KJ, Marshall SW, Proescholdbell SK, Waller AE. The epidemiology of traumatic brain injuries treated in emergency departments in North Carolina, 2010-2011. N C Med J. 2014;75(1):8–14. [DOI] [PubMed] [Google Scholar]
- 2.Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, Maas AI. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015;157(10):1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholz M, Cinatl J, Schadel-Hopfner M, Windolf J. Neutrophils and the blood-brain barrier dysfunction after trauma. Med Res Rev. 2007;27(3):401–416. [DOI] [PubMed] [Google Scholar]
- 4.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang ZG, Sun X, Zhang QZ, Yang H. Neuroprotective effects of ultra-low-molecular-weight heparin on cerebral ischemia/reperfusion injury in rats: involvement of apoptosis, inflammatory reaction and energy metabolism. Int J Mol Sci. 2013;14(1):1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logsdon AF, Lucke-Wold BP, Turner RC, Huber JD, Rosen CL, Simpkins JW. Role of microvascular disruption in brain damage from traumatic brain injury. Compr Physiol. 2015;5(3):1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borsig L. Heparin as an inhibitor of cancer progression. Prog Mol Biol Transl Sci. 2010;93:335–349. [DOI] [PubMed] [Google Scholar]
- 8.Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, Prestwich GD, MacArthur RB, Walters BB, Hoidal JR, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299(1):C97–C110. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, Holena DN, Allen SR, Browne KD, Smith DH, et al. Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg. 2015;79(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Eisenstadt R, Kumasaka K, Johnson VE, Marks J, Nagata K, Browne KD, Smith DH, Pascual JL. Does enoxaparin interfere with HMGB1 signaling after TBI? A potential mechanism for reduced cerebral edema and neurologic recovery. J Trauma Acute Care Surg. 2016;80(3):381–387; discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata K, Kumasaka K, Browne KD, Li S, St-Pierre J, Cognetti J, Marks J, Johnson VE, Smith DH, Pascual JL. Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J Trauma Acute Care Surg. 2016;81(6):1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata K, Browne KD, Suto Y, Kumasaka K, Cognetti J, Johnson VE, Marks J, Smith DH, Pascual JL. Early heparin administration after traumatic brain injury: Prolonged cognitive recovery associated with reduced cerebral edema and neutrophil sequestration. J Trauma Acute Care Surg. 2017;83:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Lotze MT, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25(1):23–31. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Mori S, Wake H, Zhang J, Liu K, Izushi Y, Takahashi HK, Peng B, Nishibori M. Establishment of in vitro binding assay of high mobility group box-1 and S100A12 to receptor for advanced glycation endproducts: heparin's effect on binding. Acta Med Okayama. 2009;63(4):203–211. [DOI] [PubMed] [Google Scholar]
- 15.Joglekar MV, Quintana Diez PM, Marcus S, Qi R, Espinasse B, Wiesner MR, Pempe E, Liu J, Monroe DM, Arepally GM. Disruption of PF4/H multimolecular complex formation with a minimally anticoagulant heparin (ODSH). Thromb Haemost. 2012;107(4):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryer A, Huang YC, Rao G, Jacoby D, Mancilla E, Whorton R, Piantadosi CA, Kennedy T, Hoidal J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J Pharmacol Exp Ther. 1997;282(1):208–219. [PubMed] [Google Scholar]
- 17.Lever R, Hoult JR, Page CP. The effects of heparin and related molecules upon the adhesion of human polymorphonuclear leucocytes to vascular endothelium in vitro. Br J Pharmacol. 2000;129(3):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual JL, Murcy MA, Li S, Gong W, Eisenstadt R, Kumasaka K, Sims C, Smith DH, Browne K, Allen S, et al. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg. 2013;206(6):840–845; discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12(2):169–178. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–634; discussion 635. [DOI] [PubMed] [Google Scholar]
- 21.Manaenko A, Lekic T, Ma Q, Ostrowski RP, Zhang JH, Tang J. Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pop V, Badaut J. A neurovascular perspective for long-term changes after brain trauma. Transl Stroke Res. 2011;2(4):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukaszewicz AC, Soyer B, Payen D. Water, water, everywhere: sodium and water balance and the injured brain. Curr Opin Anaesthesiol. 2011;24(2):138–143. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs after hemorrhage in nonheparinized but not in preheparinized models. J Surg Res. 1993;54(5):499–506. [DOI] [PubMed] [Google Scholar]
- 25.Kumasaka K, Marks JA, Eisenstadt R, Murcy MA, Samadi D, Li S, Johnson V, Browne KD, Smith DH, Schwab CW, et al. In vivo leukocyte-mediated brain microcirculatory inflammation: a comparison of osmotherapies and progesterone in severe traumatic brain injury. Am J Surg. 2014;208(6):961–968; discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Marks J, Sanati P, Eisenstadt R, Gong W, Browne K, Smith D, Becker L, Pascual J. 2: In vivo evolution of microvascular inflammation after traumatic brain injury: an intravital microscopy study. Crit Care Med. 2011;39(12):1. [Google Scholar]
- 27.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61(3):279–285. [DOI] [PubMed] [Google Scholar]
- 28.Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD. Importance of post-traumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20(3):531–542. [DOI] [PubMed] [Google Scholar]
- 29.Rana MW, Singh G, Wang P, Ayala A, Zhou M, Chaudry IH. Protective effects of preheparinization on the microvasculature during and after hemorrhagic shock. J Trauma. 1992;32(4):420–426. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Singh G, Rana MW, Ba ZF, Chaudry IH. Preheparinization improves organ function after hemorrhage and resuscitation. Am J Physiol. 1990;259(3 Pt 2):R645–R650. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Ba ZF, Reich SS, Zhou M, Holme KR, Chaudry IH. Effects of nonanticoagulant heparin on cardiovascular and hepatocellular function after hemorrhagic shock. Am J Physiol. 1996;270(4 Pt 2):H1294–H1302. [DOI] [PubMed] [Google Scholar]
- 32.Yanaka K, Spellman SR, McCarthy JB, Oegema TR Jr, Low WC, Camarata PJ. Reduction of brain injury using heparin to inhibit leukocyte accumulation in a rat model of transient focal cerebral ischemia. I. Protective mechanism. J Neurosurg. 1996;85(6):1102–1107. [DOI] [PubMed] [Google Scholar]
- 33.Weber JR, Angstwurm K, Rosenkranz T, Lindauer U, Freyer D, Burger W, Busch C, Einhaupl KM, Dirnagl U. Heparin inhibits leukocyte rolling in pial vessels and attenuates inflammatory changes in a rat model of experimental bacterial meningitis. J Cereb Blood Flow Metab. 1997;17(11):1221–1229. [DOI] [PubMed] [Google Scholar]
- 34.Mocco J, Shelton CE, Sergot P, Ducruet AF, Komotar RJ, Otten ML, Sosunov SA, Macarthur RB, Kennedy TP, Connolly ES Jr. O-desulfated heparin improves outcome after rat cerebral ischemia/reperfusion injury. Neurosurgery. 2007;61(6):1297–1303; discussion 1303–4. [DOI] [PubMed] [Google Scholar]
- 35.Gonzales JN, Kim KM, Zemskova MA, Rafikov R, Heeke B, Varn MN, Black S, Kennedy TP, Verin AD, Zemskov EA. Low anticoagulant heparin blocks thrombin-induced endothelial permeability in a PAR-dependent manner. Vascul Pharmacol. 2014;62(2):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma L, Wu J, Patel V, Sitapara R, Rao NV, Kennedy TP, Mantell LL. Partially-desulfated heparin improves survival in Pseudomonas pneumonia by enhancing bacterial clearance and ameliorating lung injury. J Immunotoxicol. 2014;11(3):260–267. [DOI] [PubMed] [Google Scholar]
- 37.Barry WH, Kennedy TP. Heparins with reduced anti-coagulant activity reduce myocardial reperfusion injury. Recent Pat Cardiovasc Drug Discov. 2011;6(2):148–157. [DOI] [PubMed] [Google Scholar]
- 38.Barry WH, Zhang XQ, Halkos ME, Vinten-Johansen J, Saegusa N, Spitzer KW, Matsuoka N, Sheets M, Rao NV, Kennedy TP. Nonanticoagulant heparin reduces myocyte Na + and Ca2+ loading during simulated ischemia and decreases reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298(1):H102–H111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin KL, Fischer BM, Kummarapurugu AB, Zheng S, Kennedy TP, Rao NV, Foster WM, Voynow JA. 2-O, 3-O-desulfated heparin inhibits neutrophil elastase-induced HMGB-1 secretion and airway inflammation. Am J Respir Cell Mol Biol. 2014;50(4):684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand. 2007;51(4):447–455. [DOI] [PubMed] [Google Scholar]
- 41.Gajic O, Manno EM. Neurogenic pulmonary edema: another multiple-hit model of acute lung injury. Crit Care Med. 2007;35(8):1979–1980. [DOI] [PubMed] [Google Scholar]
- 42.Bratton SL, Davis RL. Acute lung injury in isolated traumatic brain injury. Neurosurgery. 1997;40(4):707–712; discussion 712. [DOI] [PubMed] [Google Scholar]
- 43.Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Rev. 2002;8(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanaka K, Nose T. Heparin ameliorates brain injury by inhibiting leukocyte accumulation. Stroke. 1996;27(11):2146–2147. [PubMed] [Google Scholar]