Abstract
Mild (mTBI) traumatic brain injury (TBI) accounts for the majority of all TBI cases. Evidence has suggested that patients with mTBI can suffer from long-lasting cognitive deficits, persistent symptoms, and decreased quality of life. Sleep disorders are commonly observed after TBI, with the prevalence rate of sleep disturbances in persons with TBI being much higher than that in the general population. Poor sleep quality can impair cognitive functions in the general population. This effect of sleep disturbances may impede the recovery processes in the population with TBI. The objective of this study is to add to our understanding of the relationship between self-reported sleep problems and other post-concussion symptoms and look at the association between early sleep problems and long-term outcomes in mTBI. Post-concussion symptoms, neurocognitive functions, level of global outcomes, and rating of satisfaction of life were assessed in 64 patients with mTBI. The results revealed that the presence of sleep disturbances co-occur with an increased level of overall post-concussion symptoms at the subacute stage of mTBI, particularly with symptoms including poor concentration, memory problems, and irritability. In addition, sleep disturbance at the subacute stage is associated with persistent poor concentration and memory problems, as well as worse neurocognitive function, slower overall recovery, and lower satisfactory of life at the long term. Our findings suggest that sleep disturbance can be a prognostic factor of long-term outcomes after mTBI. Early interventions to improve sleep quality can have potential benefits to facilitate the recovery process from mTBI.
Keywords: mild traumatic brain injury, neurocognitive functions, post-concussion symptoms, sleep disturbances
Introduction
Traumatic brain injury (TBI) is the leading cause of the death and disabilities among all trauma-related injuries.1 Mild TBI (mTBI) accounts for ∼70–90% of all TBI cases.2 Despite being labeled as “mild” injuries, evidence has suggested that, in a subset of patients, mTBI may lead to long-lasting cognitive deficits, persistent symptoms, and decreased quality of life.3–5 Sleep disorders are commonly reported after TBI, with reports of 30–70% of TBI patients experiencing short- or long-term sleep problems post-injury.6–8 The prevalence rate of sleep disturbances in persons post-TBI is much higher than that in the general population6; however, the severity of sleep disturbances is not associated with severity of TBI.9 Patients with TBI across the entire range of severities are at risk. Sleep problems with insomnia and waking up too early were more frequently reported by mTBI patients than moderate or severe TBI patients.10–12 On the other hand, hypersomnia was found to be more profound in moderate-severe TBI patients.13,14
Previous studies showed that mTBI patients experiencing sleep problems often suffered from more severe overall post-concussion symptoms no matter the post-injury stage (i.e., acute, subacute, or chronic).15,16 However, the relationship between sleep and other individual post-concussion symptoms has not been widely investigated. Poor sleep quality is known to impair cognitive functions in the general population.17,18 These detrimental effects can delay the recovery process and exacerbate unfavorable outcomes post-TBI. For instance, mTBI patients experiencing poor sleep quality had a longer recovery period,19 and mTBI patients with persistent sleep problems at 1 year post-injury had significantly poorer long-term functional and social outcomes compared to those whose acute sleep problems recovered by 1 year post-injury.20
A study on neuropsychiatric outcomes reported that sleep disturbance presenting within 3 months post-injury was associated with increased depression, apathy, and anxiety 1 year after injury.21 Another study revealed a bidirectional relationship between sleep disturbances and poor global function across the first 6 months post-injury in a group of patients where the majority were mTBI patients.22 Sleep difficulties within 2 weeks of injury were shown to be highly associated with more severe post-concussion symptoms, poorer neuropsychiatric outcomes, and reduced overall neurocognitive functions 1 year after injury.23
The objective of this study is to examine sleep symptoms of mTBI patients and further our understanding of the relationship between sleep difficulties and post-concussion symptoms, as well as investigate the association between subacute sleep difficulties and long-term outcomes. We hypothesize that early sleep disruptions are associated with unfavorable long-term outcomes post-mTBI, including overall level of post-concussion symptoms, global neurocognitive function, recovery status, and satisfaction with life. In addition, we evaluated the association of early sleep disruptions and secondary measures, including individual post-concussion symptoms and neurocognitive functions.
Methods
Participants
mTBI patients were recruited from the R. Adam Cowley Shock Trauma Center at the University of Maryland Medical Center as part of the MagNeTs study (Magnetic Resonance Imaging of NeuroTraumaStudy), which included longitudinal behavioral and imaging assessment that spanned over 18 months.24 mTBI was defined as having an admission Glasgow Coma Scale of 13–15 and 1) a positive clinical computed tomography or 2) a mechanism of injury consistent with trauma and reporting loss of consciousness, alteration of consciousness, or post-traumatic amnesia. Exclusion criteria were: 1) history of neurological disorders; 2) pre-existing psychological disorders not including depression and anxiety disorders; 3) seizure disorders; 4) history of previous brain injury requiring hospitalization; and 5) contraindications to magnetic resonance imaging (MRI) since this study. Sixty-four mTBI patients were included in this study. A summary of patient information is shown in Table 1. All study procedures were approved by the institutional review board at the University of Maryland, and all participants provided written informed consent and Health Insurance Portability and Accountability Act (HIPAA) compliance.
Table 1.
Summary of Patients with and without Sleep Disruption at Subacute Stage Post-mTBI
| |
All | Sleep disruption |
|
|---|---|---|---|
| Yes | No | ||
| N (male) | 64 (47) | 15 (9) | 49 (38) |
| Age, years | 43.8 ± 2.1 | 43.1 ± 3.5 | 43.9 ± 2.5 |
| Education, years (mean ± SE) | 13.8 ± 0.3 | 12.9 ± 0.7 | 14.0 ± 0.4 |
| Long-term total RPQ (mean ± SE) | 15.0 ± 2.1 | 31.9 ± 5.0 | 9.2 ± 1.8 |
| Long-term ANAM WT (mean ± SE) | 199.5 ± 5.5 | 174.0 ± 11.8 | 207.3 ± 5.8 |
| Long-term GOSE score (mean ± SE) | 7.3 ± 0.1 | 6.6 ± 0.3 | 7.5 ± 0.1 |
| Long-term GOSE recovered (GOSE, >6) | 49 (76.6%) | 7 (46.7%) | 42 (87.5%) |
| Long-term SWLS score (mean ± SE) | 23.6 ± 1.0 | 18.9 ± 2.3 | 20.1 ± 1.0 |
mTBI, mild traumatic brain injury; SE, standard error; RPQ, Rivermead Post Concussion Symptoms Questionnaire; ANAM WT, Automated Neuropsychological Assessment Metrics weighted throughput score; GOSE, Extended Glasgow Outcome Scale; SWLS, Satisfaction with Life Scale.
Measures
Modified Rivermead Post Concussion Symptoms Questionnaire
Severity of sleep problems and other post-concussion symptoms were measured using the modified Rivermead Post Concussion Symptoms Questionnaire (RPQ) at the subacute stage (mean ± standard error [SE], 37.0 ± 1.3 days) and the chronic stage (6–18 months; mean ± SE, 220.0 ± 12.2 days) post-injury.25 Each symptom is rated on a scale from 0 to 4 (0 = not experienced at all; 1 = no more of a problem; 2 = a mild problem; 3 = a moderate problem; and 4 = a severe problem). The modified RPQ used in the present study was adapted from the original 16-item RPQ by adding six additional items that were occasionally reported as “other difficulties” by mTBI patients (i.e., vertigo, loss of balance, hearing difficulty, numbness or tingling, changes in taste and/or smell, and difficulty making decisions). In the present study, the presence of sleep problems or any other post-concussion symptoms was defined as a rating >2 (moderate-severe) on the symptoms.
Automated Neuropsychological Assessment Metrics
Neurocognitive functions were assessed using the Automated Neuropsychological Assessment Metrics (ANAM) at the chronic stage of mTBI.26,27 The ANAM consists of seven subtests, that is, simple reaction time (SR), code substitution–learning (CS), procedural reaction time (PRT), mathematical processing (MATH), matching to sample (MTS), code substitution–delayed (CSD), and repeated simple reaction time (SR2).26 Accuracy and reaction time in each subtest were measured by a throughput score, which is the number of correct responses per minute. Overall performance was assessed using a weighted throughput score.24
Extended Glasgow Outcome Scale
The Extended Glasgow Outcome Scale (GOSE) was administered at the chronic stage of mTBI, which is used for measuring the level of disability and global outcomes post-TBI.28 According to the response in GOSE, subjects were assigned to one of eight categories: dead, vegetative state, lower severe disability, upper severe disability, lower moderate disability, upper moderate disability, lower good recovery, and upper good recovery. A dichotomization threshold was applied to divide patients into two groups: favorable (GOSE, >6; lower good recovery and upper good recovery) and unfavorable (GOSE, < = 6; the other six categories).
Satisfaction with Life Scale
Life satisfaction was measured with the Satisfaction with Life Scale (SWLS), which is a five-item, seven-point scale questionnaire at the chronic stage of mTBI.29 On each item, participants rate their agreement from strongly disagree (point 1) to strongly agree (point 7). Higher total score is indicative of higher satisfaction with life.
Pittsburgh Sleep Quality Index
Detailed sleep quality was assessed in a subset of patients (n = 30) using the Pittsburgh Sleep Quality Index (PSQI).30 A summary of patient information is shown in Table 2. The PSQI contains 19 self-rated items that measure seven components of sleep quality (i.e., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction). A global score >8 was applied to distinguish patients having good versus poor sleep as proposed by Fichtenberg and colleagues.31
Table 2.
Summary of Patients in the Subset with PSQI Assessment
| |
|
All | Sleep disruption |
|
|---|---|---|---|---|
| Yes | No | |||
| N (male) | 30 (23) | 9 (3) | 21 (20)*** | |
| Age, years | 47.2 ± 2.9 | 42.1 ± 5.8 | 49.4 ± 3.3 | |
| Education, years (mean ± SE) | 14.0 ± 0.5 | 13.1 ± 0.8 | 14.4 ± 0.7 | |
| Long-term total RPQ (mean ± SE) | 16.9 ± 3.1 | 27.3 ± 6.7 | 12.4 ± 3.1 | |
| Long-term ANAM WT (mean ± SE) | 194.6 ± 7.3 | 187.6 ± 14.3 | 197.6 ± 8.6 | |
| Long-term GOSE score (mean ± SE) | 7.0 ± 0.2 | 6.2 ± 0.4 | 7.4 ± 0.2 | |
| Long-term GOSE recovered (GOSE, >6) | 22 (73.3%) | 4 (44.4%) | 18 (85.7%) | |
| Long-term SWLS score (mean ± SE) | 23.3 ± 1.4 | 19.3 ± 2.6 | 25.1 ± 1.6 | |
p < 0.001, Fisher's exact test comparing patients with and without sleep disruption during the subacute injury stage.
PSQI, Pittsburgh Sleep Quality Index; SE, standard error; RPQ, Rivermead Post Concussion Symptoms Questionnaire; ANAM WT, Automated Neuropsychological Assessment Metrics weighted throughput score; GOSE, Extended Glasgow Outcome Scale; SWLS, Satisfaction with Life Scale.
Statistical analysis
Data analysis was performed using R Statistical Software (version 4.0.2; The R Foundation for Statistical Computing, Vienna, Austria). All patients completed the RPQ, ANAM, GOSE assessment, and SWLS. Years of education of 3 patients were missing. These 3 patients were not included in the regression analysis described below.
Demographic information
Demographic information was compared between patients with and without sleep problem at subacute stage of injury. Fisher's exact test was used to compare the sex composition between the two groups. A two-sample t-test was performed to compare age and years of education.
Sleep problems and post-concussion symptoms
RPQ total score without the sleep scale was used to reflect the overall level of post-concussion symptoms. Linear regressions were performed to assess the relationship between sleep problems at the subacute stage and overall post-concussion symptoms at the subacute and chronic stages. Headaches, dizziness, trouble concentrating, memory problems, fatigue, and irritability were used to define post-concussion syndrome together with sleep problems.32 Sleep difficulties often co-occur with post-concussion symptoms.15,16 Logistic regression was performed to assess the relationship between the presence of sleep problems and the additional six post-concussion symptoms at the subacute stage of injury. Another logistic regression analysis was performed to investigate whether early sleep problems impose an extra risk of persistent post-concussion symptoms at the chronic stage after controlling for the corresponding symptoms at the subacute stage. Age, sex, and years of education were added to the analysis as independent variables.
Early sleep problem and long-term outcomes
Linear regressions were performed using the presence of sleep problem at the subacute stage, age, years of education, and sex as independent variables and outcome measures at the chronic stage of injury as dependent variables (i.e., ANAM weighted throughput score, GOSE score, and SWLS total score). The association between sleep problems and recovery status (favorable vs. unfavorable as assessed by the GOSE) was assessed using a logistic regression, with the dichotomized GOSE as the dependent variable and the same set of independent variables as in the linear regression. In addition, a profile analysis across the seven ANAM subtests was performed to assess whether patients with and without subacute sleep problems would demonstrate different performance patterns in the tests. Linear regressions were then performed on the throughput score of each subtest to identify subtests that are highly associated with early sleep problems.
Sleep-quality evaluation with the Rivermead Post Concussion Symptoms Questionnaire and Pittsburgh Sleep Quality Index
To evaluate the consistency of the RPQ and PSQI in detecting the presence of sleep problems at the subacute stage, a Fisher's exact test was performed to compare the number of patients with and without sleep problems as detected with the RPQ and PSQI. To characterize the association between the RPQ sleep scale and each component in the PSQI, cumulative link models were performed on the score of each of the seven components, with the RPQ sleep score and sex as independent variables. Years of education was not significant for any of the components and was not expected to affect the association between the RPQ and PSQI and therefore was excluded from the model.
Results
Demographic information
Sex composition (p = 0.197), age (p = 0.848), and years of education (p = 0.188) were not significantly different between patients with and without sleep problem at the subacute stage (Table 1). In the subset of patient with the PSQI assessment, significantly more females presented sleep problems compared to males (p < 0.001; Table 2). Age (p = 0.252) and years of education (p = 0.268) were not significantly different between patients with and without sleep problem in this subset (Table 2).
Relationship between sleep problems and post-concussion symptoms
Patients with moderate-severe sleep problems at the subacute stage suffered from more severe post-concussion symptoms during the subacute stage of injury according to the RPQ total score (p < 0.001; Table 3). They also had a significantly higher odds of concurrently experiencing trouble concentrating (p = 0.020), memory problems (p = 0.015), and irritability (p = 0.009) and a trend for increased risk of headaches (p = 0.071) and fatigue (p = 0.05; Table 3). Similarly, patients who reported early sleep problems were more likely to experience more severe chronic post-concussion symptoms (p = 0.032) regardless of the level of overall post-concussion symptoms at the subacute stage (Table 4). The presence of early sleep problems at the subacute stage significantly increased the chance of experiencing trouble concentrating (p = 0.002) and memory problems (p = 0.038) at the chronic stage whether or not these symptoms were presented at the subacute stage (Table 4).
Table 3.
Relationship between Sleep Disruption and Post-Concussion Symptoms at Subacute Stage Post-Injury
| RPQ | Sleep disruption |
Age |
Sex |
Years of education |
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| RPQ totala | 19.36 (11.83, 26.89) | <0.001 | 0.06 (−0.14, 0.26) | 0.553 | –6.21 (−13.34, 0.92) | 0.086 | –0.83 (−2.06, 0.40) | 0.184 |
| Headaches | 1.43 (−0.13, 3.04) | 0.071 | –0.01 (−0.06, 0.04) | 0.816 | –0.26 (−1.90, 1.52) | 0.755 | –0.28 (−0.73, 0.06) | 0.148 |
| Dizziness | 0.10 (−2.06, 1.94) | 0.917 | 0.00 (−0.05, 0.05) | 0.970 | –1.15 (−3.01, 0.68) | 0.203 | –0.31 (−0.91, 0.09) | 0.194 |
| Trouble concentrating | 1.62 (0.26, 3.03) | 0.020 | 0.00 (−0.04, 0.04) | 0.993 | –0.93 (−2.33, 0.47) | 0.185 | –0.07 (−0.36, 0.20) | 0.644 |
| Memory problems | 2.20 (0.52, 4.18) | 0.015 | –0.01 (−0.07, 0.04) | 0.666 | 0.69 (−1.10, 2.93) | 0.489 | 0.07 (−0.26, 0.41) | 0.662 |
| Fatigue | 1.38 (−0.01, 2.80) | 0.050 | –0.01 (−0.05, 0.04) | 0.725 | –0.63 (−2.03, 0.82) | 0.373 | –0.03 (−0.32, 0.23) | 0.825 |
| Irritability | 3.57 (1.33, 7.04) | 0.009 | –0.04 (−0.13, 0.03) | 0.316 | 0.60 (−1.58, 3.27) | 0.616 | 0.15 (−0.29, 0.65) | 0.505 |
Measurements from linear regression. Other measurements were from logistic regression.
RPQ, Rivermead Post Concussion Symptoms Questionnaire; 95% CI, 95% confidence interval.
Table 4.
Relationship between Subacute Sleep Disruption with Other Post-Concussion Symptoms and Outcome Measures at Chronic Stage
| Assessments | Sleep disruption |
Age |
Sex |
Years of education |
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| RPQ | ||||||||
| RPQ totala | 8.20 (0.72, 15.68) | 0.032 | 0.15 (−0.01, 0.31) | 0.073 | 1.28 (−4.71, 7.28) | 0.670 | –0.43 (−1.46, 0.59) | 0.399 |
| Headaches | 2.09 (−0.49, 5.65) | 0.144 | –0.03 (−0.15, 0.06) | 0.483 | –1.06 (−4.55, 1.87) | 0.473 | 0.07 (−0.58, 0.71) | 0.827 |
| Dizziness | 1.61 (−0.16, 3.49) | 0.074 | 0.02 (−0.04, 0.08) | 0.538 | 0.27 (−1.53, 2.41) | 0.780 | –0.06 (−0.46, 0.30) | 0.753 |
| Trouble concentrating | 3.16 (1.38, 5.50) | 0.002 | –0.02 (−0.10, 0.04) | 0.472 | 0.80 (−1.22, 3.15) | 0.463 | –0.10 (−0.53, 0.27) | 0.600 |
| Memory problems | 1.80 (0.13, 3.62) | 0.038 | 0.05 (0.00, 0.12) | 0.070 | 0.83 (−0.88, 2.84) | 0.369 | –0.21 (−0.58, 0.07) | 0.188 |
| Fatigue | 2.11 (−0.05, 4.77) | 0.072 | –0.03 (−0.12, 0.05) | 0.461 | 0.04 (−2.31, 2.65) | 0.976 | 0.04 (−0.50, 0.54) | 0.858 |
| Irritability | 1.78 (−0.33, 4.08) | 0.096 | 0.03 (−0.03, 0.11) | 0.356 | –0.81 (−2.98, 1.25) | 0.432 | –0.32 (−0.93, 0.10) | 0.198 |
| ANAMa | ||||||||
| WT | –29.21 (−48.67, −9.75) | <0.001 | –1.62 (−2.13, −1.12) | 0.004 | 1.12 (−17.30, 19.55) | 0.903 | 4.34 (1.16, 7.53) | 0.008 |
| CS | –6.61 (−13.01, −0.20) | 0.043 | –0.54 (−0.71, −0.37) | <0.001 | –4.02 (−10.08, 2.04) | 0.189 | 0.86 (−0.19, 1.91) | 0.106 |
| CSD | –6.96 (−15.14, 1.22) | 0.094 | –0.681 (−0.89, −0.47) | <0.001 | –3.15 (−10.89, 4.60) | 0.420 | 0.775 (−0.56, 2.11) | 0.251 |
| MTS | –4.21 (−9.80, 1.39) | 0.138 | –0.24 (−0.39, −0.10) | 0.001 | –0.12 (−5.42, 5.18) | 0.963 | 0.78 (−0.13, 1.70) | 0.092 |
| MATH | –2.82 (−7.02, 1.38) | 0.183 | –0.03 (−0.14, 0.08) | 0.552 | 3.09 (−0.89, 7.07) | 0.125 | 0.91 (0.23, 1.60) | 0.01 |
| PRT | –5.79 (−17.02, 5.44) | 0.306 | –0.35 (−0.64, −0.06) | 0.02 | 1.93 (−8.70, 12.57) | 0.717 | 1.64 (−0.20, 3.48) | 0.079 |
| SR | –37.66 (−61.68, −13.64) | 0.003 | –1.41 (−2.04, −0.79) | <0.001 | 7.17 (−15.58, 29.91) | 0.530 | 1.86 (−2.07, 5.79) | 0.35 |
| SR2 | –39.95 (−63.16, −16.75) | 0.001 | –1.62 (−2.22, −1.01) | <0.001 | 3.78 (−18.19, 25.76) | 0.731 | 4.65 (0.86, 8.45) | 0.017 |
| GOSE | ||||||||
| Scorea | –0.75 (−1.38, −0.13) | 0.020 | –0.01 (−0.03, 0.00) | 0.101 | 0.47 (−0.13, 1.06) | 0.121 | 0.06 (−0.04, 0.16) | 0.263 |
| Favorable recovery | –1.79 (−3.25, −0.44) | 0.011 | –0.03 (−0.08, 0.01) | 0.132 | 1.07 (−0.31, 2.49) | 0.127 | 0.16 (−0.11, 0.48) | 0.267 |
| SWLS totala | –5.59 (−10.35, −0.84) | 0.005 | –0.04 (−0.17, 0.08) | 0.516 | 0.30 (−4.20, 4.80) | 0.894 | 0.67 (−0.11, 1.44) | 0.092 |
Measurements from linear regression. Other measurements were from logistic regression.
RPQ, Rivermead Post Concussion Symptoms Questionnaire; ANAM WT, Automated Neuropsychological Assessment Metrics weighted throughput score; CS, code substitution–learning; CSD, code substitution–delayed; MATH, mathematical processing; MTS, matching to sample; PRT, procedural reaction time; SR, simple reaction time; SR2, repeated simple reaction time; GOSE, Extended Glasgow Outcome Scale; SWLS, Satisfaction with Life Scale.
Association between early sleep problems and long-term outcomes
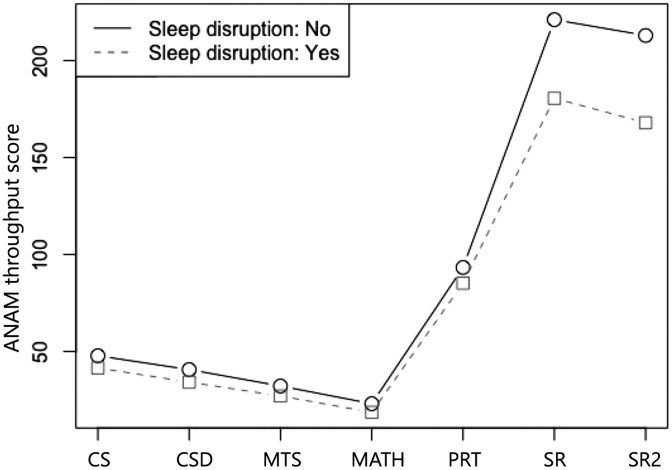
Global neurocognitive performance, as reflected by the ANAM weighted throughput at the chronic stage, was significantly lower in patients reporting early sleep problems (p < 0.001; Table 4). Overall performance on the ANAM battery was also affected by age and years of education, with younger, more highly educated patients performing better. The profile analysis showed a trend of interaction between the patient group and subtests (test of parallelism, p = 0.086), indicating possible differences in the performance pattern between patients with and without early sleep problems. Test of equality suggested significant differences in subtests between the two groups of patients (p = 0.003; Fig. 1). Specifically, significant negative effects of early sleep problems were observed on the CS (p = 0.043), SR (p = 0.003), and SR2 (p = 0.001) subtests, indicating lower functions in attention, visual perception, visuomotor response, and mental fatigue (Table 4). Significant negative effects of age were observed on all but the MATH subtests, where older patients had worse neurocognitive performance. Years of education showed positive effects on MATH and SR2, where patients with higher education performed better in MATH and SR2 (Table 4).
FIG. 1.
Profile plot showing throughput scores of the seven ANAM subtests for patients with and without early sleep problems. ANAM, Automated Neuropsychological Assessment Metrics; CS, code substitution–learning; CSD, code substitution–delayed; MATH, mathematical processing; MTS, matching to sample; PRT, procedural reaction time; SR, simple reaction time; SR2, repeated simple reaction time.
Subacute moderate-severe sleep problems were significantly associated with lower GOSE score (p = 0.02) and lower SWLS (p = 0.005) at the chronic stage. Logistic regression on recovery status showed that patients with subacute sleep problems were less likely to have favorable recovery at the chronic stage (p = 0.011; Table 4).
Consistency between Rivermead Post Concussion Symptoms Questionnaire Sleep Scale and Pittsburgh Sleep Quality Index
Results showed a consistency between the presence of moderate-severe sleep problems reported on the RPQ sleep scale and PSQI (p = 0.003; Table 5). Among the seven components of the PSQI, the presence of sleep problem as detected by the RPQ was significantly associated with issues in sleep duration (p = 0.016), sleep disturbances (p = 0.029), sleep latency (p = 0.006), habitual sleep efficiency (p = 0.031), and subjective sleep quality (p = 0.003; Table 6). The PSQI components that showed no association with RPQ-detected sleep problems were daytime dysfunction (p = 0.079) and sleep medications (p = 0.378; Table 6).
Table 5.
Co-occurrence of Sleep Problem as Detected with RPQ and PSQI at Subacute Stage Post-Injury
| RPQ vs. PSQI | Sleep disruption (PSQI) |
||
|---|---|---|---|
| No | Yes | ||
| Sleep disruption (RPQ) | No | 19 | 3 |
| Yes | 2 | 6 | |
RPQ, Rivermead Post Concussion Symptoms Questionnaire; PSQI, Pittsburgh Sleep Quality Index.
Table 6.
Association between Sleep Disruption as Detected with RPQ and PSQI Components at Subacute Stage Post-Injury
| PSQI components | Sleep disruption (RPQ) |
Age |
Sex |
|||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| Sleep duration | 2.19 (0.49, 4.12) | 0.016 | –0.01 (−0.06, 0.04) | 0.729 | –1.13 (−3.02, 0.64) | 0.214 |
| Sleep disturbances | 2.67 (0.50, 5.46) | 0.029 | 0.07 (0.01, 0.15) | 0.038 | 0.82 (−1.44, 3.30) | 0.484 |
| Sleep latency | 2.61 (0.83, 4.64) | 0.006 | –0.02 (−0.07, 0.02) | 0.337 | –0.69 (−2.64, 1.16) | 0.465 |
| Daytime dysfunctions | 1.80 (−0.09, 4.05) | 0.079 | 0.00 (−0.04, 0.05) | 0.844 | –1.87 (−4.15, 0.04) | 0.071 |
| Habitual sleep efficiency | 1.82 (0.20, 3.57) | 0.031 | –0.01 (−0.05, 0.04) | 0.734 | –1.13 (−2.93, 0.58) | 0.195 |
| Subjective sleep quality | 4.06 (1.74, 7.44) | 0.003 | –0.03 (−0.08, 0.02) | 0.253 | –3.35 (−6.59, −1.08) | 0.011 |
| Sleep medications | 0.91 (−1.21, 2.97) | 0.378 | 0.05 (−0.01, 0.13) | 0.120 | –2.30 (−4.86, −0.11) | 0.049 |
RPQ, Rivermead Post Concussion Symptoms Questionnaire; PSQI, Pittsburgh Sleep Quality Index; 95% CI, 95% confidence interval.
Discussion
Sleep loss or prolonged wakefulness can affect cognitive capacities and impair quality of life. Previous studies reported attention deficits, memory problems, and impaired executive functions after either total or partial sleep deprivation.17,33,34 It is known that patients with mTBI can suffer from long-term cognitive difficulties and lower quality of life. This study showed that moderate-severe sleep disturbances are associated with delayed recovery of cognitive functions and unfavorable long-term outcomes post-mTBI.
In the present study, presence of sleep disturbances was accompanied by an increased level of overall post-concussion symptoms at the subacute stage of mTBI. In particular, sleep disturbances tend to occur simultaneously with poor concentration, memory problems, and irritability. In addition, the presence of subacute sleep disturbances is also associated with persistent poor concentration and memory problems, as well as reduced neurocognitive performance, global recovery, and satisfaction with life in the chronic stage.
The role of sleep in memory has been widely investigated in the general population. Current theory suggests that sleep actively enhances the consolidation of memory in addition to passively protecting memory from interfering stimuli.35 Different stages of sleep have unique contributions to memory consolidation. For example, slow-wave sleep (SWS) can enhance declarative memory consolidation whereas rapid eye movement (REM) sleep can improve implicit memory processes.36–38 Other studies have suggested that SWS and REM play complementary roles in neural reactivation and synaptic remodeling for the transition from short- to long-term storage of memory.35,39 Reduced REM and increased SWS percentages have been reported in patients at the subacute to chronic stages of TBI compared to the population without a history of TBI.40–42 This impaired balance between REM and SWS may disrupt sleep-dependent memory consolidation, which could contribute to long-term memory problems.
In addition to memory problems, the present study shows that sleep difficulty at the subacute stage may predict long-term difficulties in cognitive functions, such as attention, visual perception, and visuomotor function. This can be related with disrupted sleep-dependent synaptic regulation and plasticity.43,44 Sleep-dependent plasticity plays an important role in cognitive recovery after brain injuries. Sleep deprivation can lead to reduced axonal sprouting and neurogenesis in rats after focal cerebral ischemia.45 In contrast, enhancing slow waves during sleep can increase local axonal sprouting and cortical circuit reorganization in mice during stroke recovery.46 Good sleep quality can facilitate neural plasticity in response to brain injuries; however, having poor sleep quality is likely to impede this process and delay the recovery of cognitive functions such as attention and memory.
Besides neural plasticity, poor sleep quality may contribute to poor outcomes through disrupting the function of the glymphatic system. The sleep-dependent glymphatic system, most active during non-rapid-eye movement and SWS sleep,47,48 is a glial-dependent perivascular network.49 It communicates with meningeal lymphatic channels to drain brain waste through an exchange between cerebrospinal fluid and interstitial fluid.50 Impaired glymphatic clearance can lead to the accumulation of waste metabolites, such as tau protein and amyloid-beta, and contribute to the development of cognitive impairments.49,51 TBI is known to directly affect the glymphatic and meningeal lymphatic systems through vascular disruption and aquaporin-4 channel alteration.52–54
Recent studies suggested that sleep can play a moderator role on the effect of TBI.55 Sleep deprivation or restriction can result in the accumulation of amyloid-beta in both humans and rodents, indicating possible dysfunction of the glymphatic system.56,57 A study on veterans showed that mTBIs are more likely to result in glymphatic dysfunction in subjects with poor sleep, as evidenced by an increase in the number and volume of MRI-visible perivascular spaces, a part of the glymphatic pathway, compared to those with normal sleep.58 Disrupted glymphatic function could therefore be another factor connecting sleep difficulties and long-term outcomes observed in the present study.
Several limitations should be noted in this study. The sleep problems in this study were identified using the single self-reported item on the RPQ. Therefore, the sleep problems in this study can only be taken as a general description of subjective sleep quality, which is not as comprehensive as the PSQI. However, this study demonstrated an association between self-reported sleep problems reflected in the sleep scale of the RPQ and those detected with the PSQI in the subset of patients for which the PSQI was collected. Although this association was only measured in a small subset of participants, which may not be ideal for representing the overall mTBI population, it suggests that the RPQ can be a potential quick assessment of subjective sleep quality when a comprehensive evaluation is not feasible. Another limitation is that this study included only subjective measures of sleep. Future studies using objective measures (i.e., overnight sleep studies using polysomnography) should be performed to further investigate what aspects of sleep are disrupted. Further, the present study only includes patients with mTBI, and therefore the relationships between sleep disturbances and chronic outcomes were not investigated in subjects who had been injured but without TBI (i.e., orthopedic controls). It is not clear whether the pattern of relationships is common in the general population or is moderated by mTBI.
Future studies on objective sleep quality and physiological processes, such as neural plasticity and glymphatic function, in both mTBI and the general population are needed. In addition, our study is based on a limited sample size and therefore needs to be interpreted with caution. To evaluate the magnitude of the effect of subacute-stage sleep disruptions on our primary measures (i.e., total RPQ, ANAM weighted throughput, GOSE, and SWLS), we calculated the effect size using Cohen's f2. Cohen's f2 for total RPQ at the subacute and chronic stages were 0.47 and 0.09, respectively, indicating large- and small-to-medium effects. Cohen's f2 also indicated medium effects on ANAM weighted throughput (f2 = 0.16) and GOSE recovery status (f2 = 0.13) and small-to-medium effects on the SWLS (f2 = 0.1). The effect sizes suggest that the impact of subacute-stage sleep disruptions detected in this study is meaningful. However, future studies with larger sample sizes are needed to verify our findings in large populations.
Conclusion
This study revealed relationships between early sleep problems and both short- and long-term outcomes in patients with mTBI. The results agree with previous reports that sleep disturbances are linked with impeded post-injury recovery. In addition, this study showed that the single scale of sleep quality from the RPQ could be a useful tool for the quick assessment of overall sleep quality and that it can be predictive to long-term neurocognitive function and overall recovery. Improvement of sleep quality early post-injury may facilitate recovery and increase the efficacy of other interventions. Future studies on the mechanisms underlying this sleep-injury interaction will provide therapeutic targets and contribute to treatment optimization.
Acknowledgments
We thank the Core for Translational Research in Imaging @ Maryland, part of the University of Maryland School of Medicine Center for Innovative Biomedical Resources (Baltimore, MD). The abstract was previously published in Abstracts form for “The 38th Annual National Neurotrauma Symposium,” July 11–14, 2021 Virtual Conference (https://doi.org/10.1089/neu.2021.29111.abstracts).
Abbreviations Used
- ANAM
Automated Neuropsychological Assessment Metrics
- CS
code substitution – learning
- CSD
code substitution–delayed
- GOSE
Extended Glasgow Outcome Scale
- MATH
mathematical processing
- MRI
magnetic resonance imaging
- mTBI
mild traumatic brain injury
- MTS
matching to sample
- PRT
procedural reaction time
- PSQI
Pittsburgh Sleep Quality Index
- REM
rapid eye movement
- RPQ
Rivermead Post Concussion Symptoms Questionnaire
- SE
standard error
- SR
simple reaction time
- SR2
repeated simple reaction time
- SWLS
Satisfaction with Life Scale
- SWS
slow-wave sleep
- TBI
traumatic brain injury
Authors' Contributions
All authors discussed the results and contributed to the final manuscript. S.T. analyzed and interpreted data and prepared the manuscript. C.S.R. carried out the data acquisition. L.J., H.C., and S.R. assisted with the data analysis. C.S.R., N.B., P.R., J.Z., and R.P.G. contributed significantly to the interpretation of the study results.
Funding Information
This study was supported by NIH Research Project Grant R01 NS105503-01A1 and DOD grant W81XWH-08-1-0725.
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Tang, S, Sours Rhodes, C, Jiang, L, Chen, H, Roys, S, Badjatia, N, Raghavan, P, Zhuo, J, Gullapalli, RP (2022) Association between sleep disturbances at subacute stage of mild traumatic brain injury and long-term outcomes. Neurotrauma Reports 3:1, 276–285, DOI:10.1089/neur.2022.0004.
References
- 1. Rubiano, A.M., Carney, N., Chesnut, R., and Puyana, J.C. (2015). Global neurotrauma research challenges and opportunities. Nature 527, S193–S197. [DOI] [PubMed] [Google Scholar]
- 2. Cassidy, J.D., Carroll, L.J., Peloso, P.M., Borg, J., von Holst, H., Holm, L., Kraus, J., and Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43 Suppl., 28–60. [DOI] [PubMed] [Google Scholar]
- 3. Daneshvar, D.H., Riley, D.O., Nowinski, C.J., McKee, A.C., Stern, R.A., and Cantu, R.C. (2011). Long-term consequences: effects on normal development profile after concussion. Phys. Med. Rehabil. Clin. N. Am. 22, 683–700, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McInnes, K., Friesen, C.L., MacKenzie, D.E., Westwood, D.A., and Boe, S.G. (2017). Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One 12, e0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Theadom, A., Parag, V., Dowell, T., McPherson, K., Starkey, N., Barker-Collo, S., Jones, K., Ameratunga, S., and Feigin, V.L.; BIONIC Research Group. (2016). Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br. J. Gen. Pract. 66, e16–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathias, J.L., and Alvaro, P.K. (2012). Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 13, 898–905. [DOI] [PubMed] [Google Scholar]
- 7. Castriotta, R.J., Wilde, M.C., Lai, J.M., Atanasov, S., Masel, B.E., and Kuna, S.T. (2007). Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep Med. 3, 349–356. [PMC free article] [PubMed] [Google Scholar]
- 8. Kempf, J., Werth, E., Kaiser, P.R., Bassetti, C.L., and Baumann, C.R. (2010). Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 81, 1402–1405. [DOI] [PubMed] [Google Scholar]
- 9. Baumann, C.R., Werth, E., Stocker, R., Ludwig, S., and Bassetti, C.L. (2007). Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 130, 1873–1883. [DOI] [PubMed] [Google Scholar]
- 10. Beetar, J.T., Guilmette, T.J., and Sparadeo, F.R. (1996). Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch. Phys. Med. Rehabil. 77, 1298–1302. [DOI] [PubMed] [Google Scholar]
- 11. Clinchot, D.M., Bogner, J., Mysiw, W.J., Fugate, L., and Corrigan, J. (1998). Defining sleep disturbance after brain injury. Am. J. Phys. Med. Rehabil. 77, 291–295. [DOI] [PubMed] [Google Scholar]
- 12. Ouellet, M.C., Beaulieu-Bonneau, S., and Morin, C.M. (2006). Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J. Head Trauma Rehabil. 21, 199–212. [DOI] [PubMed] [Google Scholar]
- 13. Hou, L., Han, X., Sheng, P., Tong, W., Li, Z., Xu, D., Yu, M., Huang, L., Zhao, Z., Lu, Y., and Dong, Y. (2013). Risk factors associated with sleep disturbance following traumatic brain injury: clinical findings and questionnaire based study. PLoS One 8, e76087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watson, N.F., Dikmen, S., Machamer, J., Doherty, M., and Temkin, N. (2007). Hypersomnia following traumatic brain injury. J. Clin. Sleep Med. 3, 363–368. [PMC free article] [PubMed] [Google Scholar]
- 15. Towns, S.J., Silva, M.A., and Belanger, H.G. (2015). Subjective sleep quality and postconcussion symptoms following mild traumatic brain injury. Brain Inj. 29, 1337–1341. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan, K.A., Berndt, S.L., Edmed, S.L., Smith, S.S., and Allan, A.C. (2016). Poor sleep predicts subacute postconcussion symptoms following mild traumatic brain injury. Appl. Neuropsychol. Adult 23, 426–435. [DOI] [PubMed] [Google Scholar]
- 17. Alhola, P., and Polo-Kantola, P. (2007). Sleep deprivation: impact on cognitive performance. Neuropsychiatr. Dis. Treat. 3, 553–567. [PMC free article] [PubMed] [Google Scholar]
- 18. Zavecz, Z., Nagy, T., Galko, A., Nemeth, D., and Janacsek, K. (2020). The relationship between subjective sleep quality and cognitive performance in healthy young adults: evidence from three empirical studies. Sci. Rep. 10, 4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffman, N.L., O'Connor, P.J., Schmidt, M.D., Lynall, R.C., and Schmidt, J.D. (2020). Relationships between post-concussion sleep and symptom recovery: a preliminary study. J. Neurotrauma 37, 1029–1036. [DOI] [PubMed] [Google Scholar]
- 20. Chan, L.G., and Feinstein, A. (2015). Persistent sleep disturbances independently predict poorer functional and social outcomes 1 year after mild traumatic brain injury. J. Head Trauma Rehabil. 30, E67–E75. [DOI] [PubMed] [Google Scholar]
- 21. Rao, V., McCann, U., Han, D., Bergey, A., and Smith, M.T. (2014). Does acute TBI-related sleep disturbance predict subsequent neuropsychiatric disturbances? Brain Inj. 28, 20–26. [DOI] [PubMed] [Google Scholar]
- 22. Kalmbach, D.A., Conroy, D.A., Falk, H., Rao, V., Roy, D., Peters, M.E., Van Meter, T.E., and Korley, F.K. (2018). Poor sleep is linked to impeded recovery from traumatic brain injury. Sleep 41, zsy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Theadom, A., Cropley, M., Parmar, P., Barker-Collo, S., Starkey, N., Jones, K., and Feigin, V.L.; BIONIC Research Group. (2015). Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 16, 926–932. [DOI] [PubMed] [Google Scholar]
- 24. Sours, C., Zhuo, J., Roys, S., Shanmuganathan, K., and Gullapalli, R.P. (2015). Disruptions in resting state functional connectivity and cerebral blood flow in mild traumatic brain injury patients. PLoS One 10, e0134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King, N.S., Crawford, S., Wenden, F.J., Moss, N.E., and Wade, D.T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592. [DOI] [PubMed] [Google Scholar]
- 26. Reeves, D.L., Bleiberg, J., Roebuck-Spencer, T., Cernich, A.N., Schwab, K., Ivins, B., Salazar, A.M., Harvey, S.C., Brown, F.H.Jr., and Warden, D. (2006). Reference values for performance on the Automated Neuropsychological Assessment Metrics V3.0 in an active duty military sample. Mil. Med. 171, 982–994. [DOI] [PubMed] [Google Scholar]
- 27. Vincent, A.S., Roebuck-Spencer, T.M., Cox-Fuenzalida, L.E., Block, C., Scott, J.G., and Kane, R. (2018). Validation of ANAM for cognitive screening in a mixed clinical sample. Appl. Neuropsychol. Adult 25, 366–375. [DOI] [PubMed] [Google Scholar]
- 28. Teasdale, G.M., Pettigrew, L.E., Wilson, J.T., Murray, G., and Jennett, B. (1998). Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma 15, 587–597. [DOI] [PubMed] [Google Scholar]
- 29. Diener, E., Emmons, R.A., Larsen, R.J., and Griffin, S. (1985). The Satisfaction With Life Scale. J. Pers. Assess. 49, 71–75. [DOI] [PubMed] [Google Scholar]
- 30. Buysse, D.J., Reynolds, C.F. III, Monk, T.H., Berman, S.R., and Kupfer, D.J. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. [DOI] [PubMed] [Google Scholar]
- 31. Fichtenberg, N.L., Zafonte, R.D., Putnam, S., Mann, N.R., and Millard, A.E. (2002). Insomnia in a post-acute brain injury sample. Brain Inj. 16, 197–206. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization. (2010). International Classification of Disease, 10th Review. World Health Organization: Geneva, Switzerland. [Google Scholar]
- 33. Killgore, W.D. (2010). Effects of sleep deprivation on cognition. Prog. Brain Res. 185, 105–129. [DOI] [PubMed] [Google Scholar]
- 34. Mao, T., Dinges, D., Deng, Y., Zhao, K., Yang, Z., Lei, H., Fang, Z., Yang, F.N., Galli, O., Goel, N., Basner, M., and Rao, H. (2021). Impaired vigilant attention partly accounts for inhibition control deficits after total sleep deprivation and partial sleep restriction. Nat. Sci. Sleep 13, 1545–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rasch, B., and Born, J. (2013). About sleep's role in memory. Physiol. Rev. 93, 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plihal, W., and Born, J. (1997). Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 9, 534–547. [DOI] [PubMed] [Google Scholar]
- 37. Wagner, U., Hallschmid, M., Verleger, R., and Born, J. (2003). Signs of REM sleep dependent enhancement of implicit face memory: a repetition priming study. Biol. Psychol. 62, 197–210. [DOI] [PubMed] [Google Scholar]
- 38. Gais, S., and Born, J. (2004). Declarative memory consolidation: mechanisms acting during human sleep. Learn. Mem. 11, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ribeiro, S., Shi, X., Engelhard, M., Zhou, Y., Zhang, H., Gervasoni, D., Lin, S.C., Wada, K., Lemos, N.A., and Nicolelis, M.A. (2007). Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front. Neurosci. 1, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parcell, D.L., Ponsford, J.L., Redman, J.R., and Rajaratnam, S.M. (2008). Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch. Phys. Med. Rehabil. 89, 843–850. [DOI] [PubMed] [Google Scholar]
- 41. Schreiber, S., Barkai, G., Gur-Hartman, T., Peles, E., Tov, N., Dolberg, O.T., and Pick, C.G. (2008). Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med. 9, 481–487. [DOI] [PubMed] [Google Scholar]
- 42. Mantua, J., Mahan, K.M., Henry, O.S., and Spencer, R.M. (2015). Altered sleep composition after traumatic brain injury does not affect declarative sleep-dependent memory consolidation. Front. Hum. Neurosci. 9, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ribeiro, S. (2012). Sleep and plasticity. Pflugers Arch. 463, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gorgoni, M., D'Atri, A., Lauri, G., Rossini, P.M., Ferlazzo, F., and De Gennaro, L. (2013). Is sleep essential for neural plasticity in humans, and how does it affect motor and cognitive recovery? Neural Plast. 2013, 103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zunzunegui, C., Gao, B., Cam, E., Hodor, A., and Bassetti, C.L. (2011). Sleep disturbance impairs stroke recovery in the rat. Sleep 34, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Facchin, L., Schone, C., Mensen, A., Bandarabadi, M., Pilotto, F., Saxena, S., Libourel, P.A., Bassetti, C.L.A., and Adamantidis, A.R. (2020). Slow waves promote sleep-dependent plasticity and functional recovery after stroke. J. Neurosci. 40, 8637–8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fultz, N.E., Bonmassar, G., Setsompop, K., Stickgold, R.A., Rosen, B.R., Polimeni, J.R., and Lewis, L.D. (2019). Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hauglund, N.L., Kusk, P., Kornum, B.R., and Nedergaard, M. (2020). Meningeal lymphangiogenesis and enhanced glymphatic activity in mice with chronically implanted EEG electrodes. J. Neurosci. 40, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iliff, J.J., Wang, M., Liao, Y., Plogg, B.A., Peng, W., Gundersen, G.A., Benveniste, H., Vates, G.E., Deane, R., Goldman, S.A., Nagelhus, E.A., and Nedergaard, M. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Louveau, A., Plog, B.A., Antila, S., Alitalo, K., Nedergaard, M., and Kipnis, J. (2017). Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 127, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harrison, I.F., Ismail, O., Machhada, A., Colgan, N., Ohene, Y., Nahavandi, P., Ahmed, Z., Fisher, A., Meftah, S., Murray, T.K., Ottersen, O.P., Nagelhus, E.A., O'Neill, M.J., Wells, J.A., and Lythgoe, M.F. (2020). Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain 143, 2576–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iliff, J.J., Chen, M.J., Plog, B.A., Zeppenfeld, D.M., Soltero, M., Yang, L., Singh, I., Deane, R., and Nedergaard, M. (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bolte, A.C., Dutta, A.B., Hurt, M.E., Smirnov, I., Kovacs, M.A., McKee, C.A., Ennerfelt, H.E., Shapiro, D., Nguyen, B.H., Frost, E.L., Lammert, C.R., Kipnis, J., and Lukens, J.R. (2020). Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 11, 4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ren, Z., Iliff, J.J., Yang, L., Yang, J., Chen, X., Chen, M.J., Giese, R.N., Wang, B., Shi, X., and Nedergaard, M. (2013). ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 33, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sullan, M.J., Asken, B.M., Jaffee, M.S., DeKosky, S.T., and Bauer, R.M. (2018). Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 84, 316–324. [DOI] [PubMed] [Google Scholar]
- 56. Shokri-Kojori, E., Wang, G.J., Wiers, C.E., Demiral, S.B., Guo, M., Kim, S.W., Lindgren, E., Ramirez, V., Zehra, A., Freeman, C., Miller, G., Manza, P., Srivastava, T., De Santi, S., Tomasi, D., Benveniste, H., and Volkow, N.D. (2018). beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. U. S. A. 115, 4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kang, J.E., Lim, M.M., Bateman, R.J., Lee, J.J., Smyth, L.P., Cirrito, J.R., Fujiki, N., Nishino, S., and Holtzman, D.M. (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Piantino, J., Schwartz, D.L., Luther, M., Newgard, C., Silbert, L., Raskind, M., Pagulayan, K., Kleinhans, N., Iliff, J., and Peskind, E. (2021). Link between mild traumatic brain injury, poor sleep, and magnetic resonance imaging: visible perivascular spaces in veterans. J. Neurotrauma 38, 2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]