Abstract
Although mostly unaware, we constantly navigate a complex landscape of airborne molecules. The perception of these molecules helps us navigate, shapes our social life, and can trigger emotionally charged memories transporting us back to the past within a split second. While the processing of olfactory information in early sensory areas is well understood, how the sense of smell affects cognition only recently gained attention in the field of neuroscience. Here, we review links between olfaction and cognition and explore the idea that the activity in olfactory areas may be critical for coordinating cognitive networks. Further, we discuss how olfactory activity may shape the development of cognitive networks and associations between the decline of olfactory and cognitive abilities in aging. Olfaction provides a great tool to study large-scale networks underlying cognitive abilities and bears the potential for a better understanding of cognitive symptoms associated with many mental disorders.
Keywords: networks, odor, olfaction, oscillations, respiration
Zusammenfassung
Obwohl meist nicht bewusst, navigieren wir ständig durch eine komplexe Landschaft von Duftstoffen. Die Wahrnehmung dieser Duftstoffe hilft uns bei der Orientierung, prägt unser soziales Leben und kann emotionale Erinnerungen auslösen, die uns innerhalb von Sekundenbruchteilen in die Vergangenheit zurückversetzen. Während die Verarbeitung olfaktorischer Informationen in frühen sensorischen Arealen gut verstanden ist, ist die Frage wie der Geruchssinn die Kognition beeinflusst erst vor kurzem in das Blickfeld der neurowissenschaftlichen Forschung gerückt. Wir diskutieren hier das Zusammenspiel zwischen Geruchssinn und Kognition und untersuchen die Idee, dass die Aktivität in olfaktorischen Arealen entscheidend zur Koordination kognitiver Netzwerkaktivität beiträgt. Darüber hinaus diskutieren wir, wie olfaktorische Aktivität die Entwicklung kognitiver Netzwerke beeinflussen kann, sowie Assoziationen zwischen dem Rückgang olfaktorischer und kognitiver Fähigkeiten im Alter. Der Geruchssinn bietet ein großartiges Werkzeug zur Untersuchung weitläufiger Netzwerke, die kognitiven Fähigkeiten zugrunde liegen, und birgt das Potenzial für ein besseres Verständnis kognitiver Symptome, die mit vielen psychischen Störungen einhergehen.
Introduction
Most people are familiar with the phenomenon that a particular smell can trigger strong memories. However, due to the dominance of vision and hearing in our day-to-day life, olfaction has long been overlooked in the field of cognition. The term cognition refers to mental processes as diverse as perception, memory, working memory, decision making, and more. Despite substantial differences, all these tasks require coordinated activity in distributed brain areas centered around a core network consisting of the hippocampal formation and frontal cortical areas (Uhlhaas and Singer, 2006). In this article, we will refer to this network with the term ‘cognitive system’.
Only recently, olfaction gained attention in studying behaviors in the context of cognition. In rodents, cognitive functions are strongly influenced by the sense of smell as they use odor cues for memory, decision making, and navigation (Fischler-Ruiz et al., 2021; Igarashi et al., 2014; Symanski et al., 2021). Human behavior, even though we are not always consciously aware of it, is also influenced by odor cues, and the performance in olfactory and cognitive tests correlates in healthy adults (Yahiaoui-Doktor et al., 2019) and elderly (Uchida et al., 2020). The strong influence of olfaction on cognitive functions can be explained by a tight anatomical and functional coupling between the olfactory and the cognitive system.
In mammals, rhythmic inhalations evoke temporally structured activity in the olfactory bulb, which is termed as respiration rhythm. This prominent rhythm spreads not only within the olfactory system but also organizes activity in the cognitive system (Heck et al., 2019; Karalis and Sirota, 2022; Mori et al., 2013; Tort et al., 2018). The coordination of activity across spatially distributed areas facilitates communication within large-scale brain networks (Uhlhaas and Singer, 2006). Impaired coordination of activity in distributed brain networks centered on the hippocampal formation and the frontal cortex is thought to be the central network dysfunction underlying cognitive symptoms in mental disorders (Uhlhaas and Singer, 2006). Of note, the respiration rhythm coordinates activity in the cognitive system during early development (Gretenkord et al., 2019). Impaired synchronization of activity in the hippocampal-prefrontal networks in mental disorders manifests during neonatal ages when mice rely on olfaction as their main source of sensory input (Chini et al., 2020; Richter et al., 2019). At an older age, the decline in cognitive performance associated with mental disorders is accompanied by a decline in olfactory performance, and deficits in olfaction have been proposed as an early biomarker for disorders such as Alzheimer’s disease and Parkinson’s disease (Murphy, 2019).
With this review, we aim to link the concept of olfactory-driven coordination of widespread network activity with the correlation between olfactory and cognitive performance and discuss potential implications for mental disorders from the beginning until the end of life.
Lateral entorhinal cortex links the olfactory and cognitive system
The olfactory sensory neurons in the nasal cavity detect odor molecules and relay the information to the olfactory bulb; this is the initial processing stage of the olfactory information in the central nervous system. Mitral and tufted cells are the output neurons of the olfactory bulb and project to a range of brain areas considering the olfactory system, including the piriform cortex, lateral entorhinal cortex, and many more (Igarashi et al., 2012). The cognitive system consists of higher associative areas centered around the hippocampal formation and the frontal cortical networks, particularly the prefrontal cortex (Uhlhaas and Singer, 2006). The entorhinal cortex provides the main input to the hippocampus and is considered the gateway to the hippocampal-prefrontal network (Witter et al., 2017).
In contrast to all other senses, the olfactory system lacks a first-order thalamic relay resulting in a short pathway to the hippocampal formation, critical for cognitive functions (Figure 1). The lateral entorhinal cortex plays a prominent role in the interaction between the olfactory and the cognitive system. In addition to the direct input from the olfactory bulb, the lateral entorhinal cortex receives indirect olfactory input through the piriform cortex and sends outputs to a range of hippocampal subdivisions, as well as direct projections to the prefrontal cortex. Inputs from the olfactory bulb and piriform cortex arrive in superficial layers of the lateral entorhinal cortex and preferentially target reelin-expressing fan cells in layer 2a (Bitzenhofer et al., 2022). Nonoverlapping populations of fan cells project to the dentate gyrus and the medial prefrontal cortex, whereas pyramidal cells in layers 2b and 3 project to the hippocampal subdivision CA1 (Bitzenhofer et al., 2022; Leitner et al., 2016; Li et al., 2017; Xu et al., 2021). Projection neurons in the hippocampus project to deep layers of the lateral entorhinal cortex and the medial prefrontal cortex, among other areas (Witter et al., 2017). Further, the lateral entorhinal cortex is reciprocally connected with the orbitofrontal cortex that also receives input from the piriform cortex and is associated with the learning of odor value (Wang et al., 2020). Projections to the amygdala are thought to be involved in triggering emotional memory aspects. This information is also mediated to the hippocampus via the lateral entorhinal cortex which acts as a gateway for amygdala influences on memory consolidation (Roesler and McGaugh, 2022).
Figure 1:
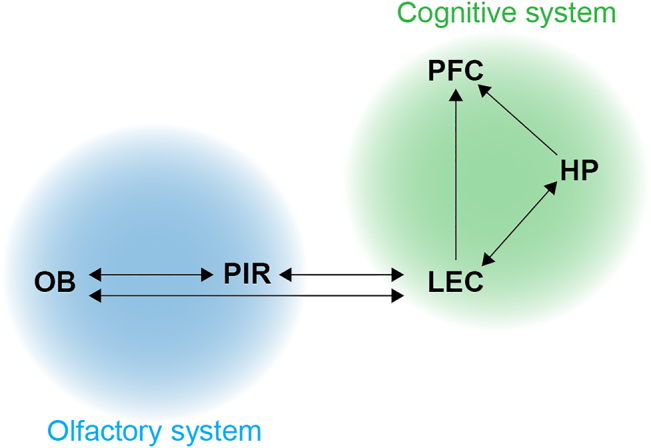
Simplified schematic showing the main connectivity between the olfactory system and the cognitive system. The lateral entorhinal cortex acts as a gateway between the two systems. (HP–hippocampus, LEC–lateral entorhinal cortex, OB–olfactory bulb, PFC–prefrontal cortex, and PIR–piriform cortex).
The strong connectivity between the olfactory and the cognitive system has also been observed functionally. Odor exposure triggers a transient increase in firing rate in the olfactory system, but also in the lateral entorhinal cortex and the hippocampus (Bitzenhofer et al., 2022; Leitner et al., 2016), and learned odor value representations have been described in the prefrontal cortex (Wang et al., 2020). Further, direct projections from lateral entorhinal cortex pyramidal neurons to CA1 were shown to be important for associative odor memories (Li et al., 2017), and lateral entorhinal cortex lesions impair odor-associative memory (Persson et al., 2022; Wilson et al., 2013), object-place-context memory (Vandrey et al., 2020), and social memory (Lopez-Rojas et al., 2022). This emphasizes the important role of the lateral entorhinal cortex for the interaction between the olfactory and the cognitive system.
The communication between the olfactory and the cognitive system is not merely a unidirectional transfer of sensory information to higher associative areas. In addition to the bottom-up projections, there is a range of top-down projections within and between the two systems with the potential to modulate activity in early olfactory areas (Boyd et al., 2012; Chen and Padmanabhan, 2022). In fact, most areas that receive input from the olfactory bulb send direct feedback projections, including the piriform cortex and the lateral entorhinal cortex (Padmanabhan et al., 2019). While projections from the hippocampus to the olfactory bulb and piriform cortex do exist, they are sparse and their functions are not clear. However, the olfactory system receives a strong feedback from the cognitive system through the lateral entorhinal cortex, recently proposed to underlie the formation of spatial maps in the posterior piriform cortex during navigation (Poo et al., 2022) and to enable flexible coding strategies (Chen and Padmanabhan, 2022).
Olfactory activity synchronizes cognitive networks
In mammals, respiration results in the rhythmic sampling of olfactory information. Evoked activity in the olfactory sensory neurons during this repetitive sampling induces an oscillatory rhythm in the olfactory bulb, which is termed as respiration rhythm (Ackels et al., 2020). In rodents, this rhythm is typically in the range of 2–4 Hz, but can increase up to 12 Hz during sniffing and running (Kay et al., 2009). In humans, respiration is typically in the range of 0.16–0.33 Hz (Zelano et al., 2016). In rodents, nasal respiration modulates local field potentials and membrane oscillations in several cortical and subcortical brain areas including the piriform cortex, the lateral entorhinal cortex, the hippocampus, and the prefrontal cortex (Figure 2) (Biskamp et al., 2017; Lockmann et al., 2018; Tort et al., 2018).
Figure 2:
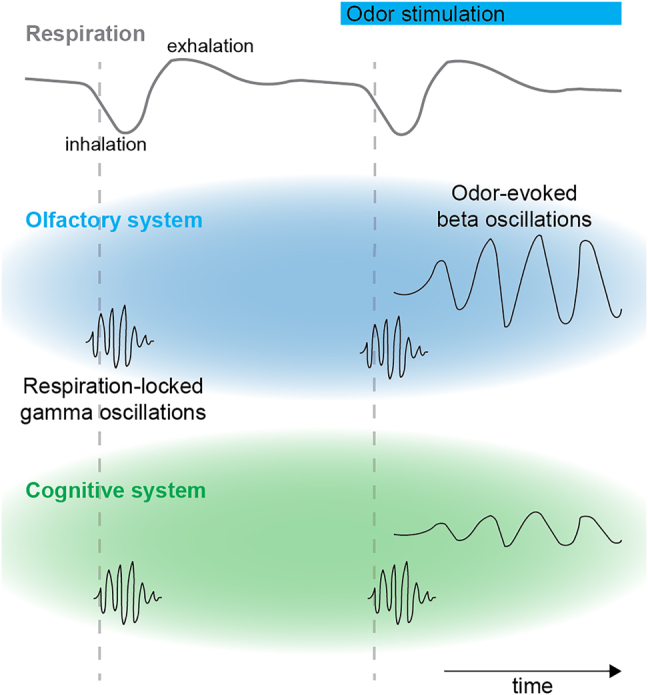
Olfactory activity synchronizes the olfactory system and the cognitive system in oscillatory rhythms. Respiration-related activity temporally organizes periods of high and low excitability that spread from the olfactory to the cognitive system and results in synchronized activity in the gamma frequency range. Odor stimulation induces synchronous activity in the beta frequency range in both systems.
The respiration rhythm is not the only rhythm associated with olfaction, but each inhalation induces a temporal sequence of rhythmic network activity on a faster timescale. Initially, odor inhalation induces activity in the gamma frequency range, followed by slower oscillations in the beta frequency range. Although brief gamma activity mainly reflects the coordination of odor-evoked activity in local networks, sustained activity in beta frequency synchronizes across the olfactory system and spreads into the cognitive system (Mori et al., 2013). Interestingly, the olfactory bulb gamma activity persists when the olfactory bulb output via the lateral olfactory tract is lesioned, but the odor-induced beta activity is disrupted, indicating that it depends on the feedback from downstream areas (Neville and Haberly, 2003). These rhythms are not only prominent in the olfactory system during odor sampling but also occur in the cognitive system during odor-guided memory and decision-making tasks.
During working memory and decision-making tasks, the phase of the respiration rhythm modulates the local neuronal firing as well as the gamma and beta oscillations in several areas of the cognitive system, such as the hippocampus, the lateral entorhinal cortex, and the prefrontal cortex (Biskamp et al., 2017; Karalis and Sirota, 2022; Lockmann et al., 2018). The synchronization of remote brain areas is a hallmark of cognitive functions (Uhlhaas and Singer, 2006). Similar to the coordination of fast gamma activity by slower oscillations in the theta frequency range which is important for several cognitive functions such as working memory, the respiratory rhythm was suggested to coordinate the interactions between distant networks during cognitive processing (Heck et al., 2019; Karalis and Sirota, 2022).
Although studies causally testing the importance of this synchronization for cognitive performance are missing so far, first correlative studies in humans suggest that olfactory-driven synchronization of the cortical systems is important during memory processes and decision-making. In line with the rodent literature, recent studies in humans found a nasal breathing-induced respiratory rhythm in brain areas such as the piriform cortex, the hippocampus, and the amygdala. They show that the respiratory rhythm drops when participants switch to oral breathing and that the respiration rhythm modulates memory recall and task performance, indicating that nasal breathing directly influences cognitive performance (Nakamura et al., 2018; Zelano et al., 2016). In line with this, Arshamian and colleagues showed that memory consolidation is improved during nasal breathing compared to oral breathing in humans (Arshamian et al., 2018). Similar correlations of perception and cognition have been reported for other physiological rhythms, such as the heartbeat. How these other rhythms interact with the respiratory rhythm and its influence on cognition is not clear.
In addition to the respiratory rhythm, beta and gamma oscillations in brain areas such as the piriform cortex, the lateral entorhinal cortex, the hippocampus, and the prefrontal cortex have been implicated in the processing of olfactory information related to cognitive functions. Oscillations in the beta frequency range in the piriform cortex, the lateral entorhinal cortex, and the hippocampus arise during odor sensation and odor-guided learning tasks (Gourévitch et al., 2010; Igarashi et al., 2014). The lateral entorhinal cortex synchronizes fast oscillatory activity in distributed cortical areas critical for memory encoding in a hippocampus-dependent manner (Luo et al., 2022). Furthermore, oscillations in beta frequency synchronize across olfactory and cognitive networks during olfactory learning tasks and within hippocampus-prefrontal cortex networks during memory-guided decision-making tasks (Igarashi et al., 2014; Symanski et al., 2021). Of note, the firing of beta-entrained interneurons in hippocampal CA1 is linked to accurate performance during associative odor memory and decision-making tasks, suggesting that beta oscillations enable the recruitment and coordination of neurons that are involved in the processing of task-relevant odor information (Rangel et al., 2016; Symanski et al., 2021). Intracranial recordings in the human piriform cortex revealed similar odor-induced beta and gamma oscillations, and their presence was shown to correlate with accurate odor perception (Yang et al., 2022).
Thus, during olfactory-guided cognitive processing, the respiratory rhythm entrains the widely distributed brain areas of the olfactory and the cognitive system in beta and gamma oscillations.
Early entrainment of olfactory-cognitive networks
Rodents are born with limited sensory abilities. While their eyes and ears remain closed until the end of the second postnatal week (Martini et al., 2021), newborn rodents smell from birth, and their ability to survive depends strongly on the functionality of the sense of olfaction (Logan et al., 2012). Besides using olfaction for early cue-directed behaviors such as finding the nipple of the dam, newborn mice are also able to perform easy odor discriminations and form associative odor memories (Armstrong et al., 2006; Logan et al., 2012). In line with the early functionality of olfaction, mitral and tufted cells, the sole output neurons of the olfactory bulb, develop prenatally between embryonic day 11 and 16, and their afferent connectivity is largely established at birth (Hirata et al., 2019; Walz et al., 2006). By contrast, glutamatergic feedback connections to the olfactory bulb develop postnatally in an area-specific manner (Kostka and Bitzenhofer, 2022). Moreover, interneurons in the olfactory bulb are generated only during late embryonic to neonatal ages (Batista-Brito et al., 2008), and the biophysical properties of mitral cells are still maturing postnatally (Yu et al., 2015). Therefore, even though functional from birth, the olfactory bulb microcircuitry is still undergoing developmental changes during the first postnatal weeks. In line with this, the neonatal network activity in the developing olfactory bulb lacks the characteristic fast gamma oscillations observed in adults during odor stimulation (Fletcher et al., 2005). An activity in the neonatal olfactory bulb is instead characterized by the respiratory rhythm as well as discontinuous oscillatory events in the theta/beta frequency range during passive sniffing and increased oscillatory activity in the beta frequency range during odor sampling (Gretenkord et al., 2019).
Axons of olfactory bulb mitral cells reach the lateral entorhinal cortex as well as the other regions of the olfactory cortex around embryonic day 16 (Walz et al., 2006). At neonatal ages, calbindin-expressing pyramidal neurons in layers 2b and 3 of the lateral entorhinal cortex project to the CA1 region of the hippocampus, which in turn projects to the prefrontal cortex (Hartung et al., 2016; Xu et al., 2021). Moreover, reelin-positive fan cells of layer 2a project to the dentate gyrus and prefrontal cortex at the neonatal age (Xu et al., 2021). Thus, the anatomical bottom-up connectivity between the olfactory and the cognitive system is established in neonatal mice. Besides the anatomical connectivity, the functional interaction between the olfactory system and the cognitive system is established early in life. Mitral cells in the developing olfactory bulb mediate the entrainment of lateral entorhinal cortex oscillations in the respiration rhythm as well as in theta/beta oscillations during passive sniffing (Gretenkord et al., 2019). Further, the activation of mitral cells by olfactory or optogenetic stimulation entrains the hippocampal-prefrontal network in beta oscillations by the end of the first postnatal week in mice (Gretenkord et al., 2019; Kostka and Hanganu-Opatz, 2021). Thus, olfactory inputs can influence coordinated activity patterns in developing cognitive networks.
Whether early synchronization of the olfactory and the cognitive system in oscillatory rhythms influences the maturation of these networks is unknown. However, considering the importance of oscillatory coupling in beta frequency between the hippocampus and the prefrontal cortex during neonatal development for the emergence of later cognitive abilities (Bitzenhofer et al., 2021; Chini et al., 2020; Richter et al., 2019; Xu et al., 2021), it is tempting to speculate that the olfactory experience during neonatal age may contribute to the functional development of these networks.
Correlated decline of olfaction and cognition in aging
Although many questions remain to be answered about the role of olfaction in the development of cognitive networks, a clearer picture starts to emerge at the other end of the life span. A decline in both olfactory sensation and cognitive abilities is common in old age (Attems et al., 2015; Murman, 2015). Although the performance in olfactory and cognitive tests correlates during adulthood, particularly strong associations can be found for the elderly (Uchida et al., 2020; Yahiaoui-Doktor et al., 2019). This association is not only present in healthy individuals, but a reduction in olfactory performance also correlates with cognitive impairments in Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment, and others (Attems et al., 2015). Therefore, the olfactory function has been suggested as an early biomarker for cognitive decline in neurological disorders, particularly promising for Alzheimer’s disease.
Impaired olfaction is among the most common nonmotor dysfunction in Parkinson’s disease and often appears before movement deficits (Doty, 2012). Pathological changes related to Parkinson’s disease appear early in the olfactory bulb, but the mechanisms of olfactory dysfunction remain unclear. Interestingly, the olfactory dysfunction correlates with cognitive impairment and has been suggested as a biomarker for disease progression for Parkinson’s disease (Cecchini et al., 2019). However, it is still unclear to what extent the olfactory dysfunction, and a potentially resulting lack of coordination in cognitive networks, contributes to cognitive symptoms in Parkinson’s disease.
Structural and functional changes in the entorhinal cortex and hippocampus underlie cognitive impairment in dementia (Murphy, 2019). A functional disconnection between the hippocampus and the prefrontal cortex has been reported in patients with Alzheimer’s disease (Grady et al., 2001). This dysfunction may underlie reduced olfactory working memory capacity reported in a mouse model for Alzheimer’s disease (Huang et al., 2020). The hippocampal atrophy is correlated with olfactory impairment in patients with Alzheimer’s disease (Murphy et al., 2003), and the disrupted connectivity in the olfactory bulb–lateral entorhinal cortex–hippocampus network is associated with deficits in recognition memory in a rat model for Alzheimer’s disease (Salimi et al., 2022).
Due to its prominent position in the connection between the olfactory and the cognitive system, the lateral entorhinal cortex is the most likely candidate to link olfactory and cognitive deficits. The lateral entorhinal cortex is one of the first areas to show morphological abnormalities in Alzheimer’s disease (Khan et al., 2014; Murphy, 2019; Stranahan and Mattson, 2010). Further, the selective vulnerability of neurons in layer 2 of the entorhinal cortex, the layer that receives most olfactory input and sends strong projections to the hippocampus, has been reported during aging and Alzheimer’s disease (Stranahan and Mattson, 2010).
Of interest, the loss of olfaction (anosmia) and cognitive impairments are associated with SARS-CoV2 infections. A recent study on patients with SARS-CoV2 infections found tissue damage in the primary olfactory cortex, a reduction of gray matter thickness in the parahippocampal gyrus, including the entorhinal cortex, as well as cognitive impairments (Douaud et al., 2022). Further studies are required to clarify how these alterations relate to each other.
Conclusions
Growing evidence points toward a powerful link between olfaction and cognition. Odor-evoked activity spreads across the entire olfactory and cognitive system, resulting in the modulation of neuronal activity by respiration and the synchronization of network activity in the beta frequency range in several brain areas. Thus, breathing provides a temporal framework of alternating activity levels that are able to synchronize distant brain areas. Strong connectivity between olfactory areas and the cognitive network underlies this phenomenon, with the lateral entorhinal cortex representing the major area linking the two systems. Only recently, the link between olfaction and cognition gained more attention in the field of neuroscience, and several findings point toward tight coupling of both systems across life. However, several open questions remain about the role of olfaction in cognition and its diagnostic potential (Box 1).
Box 1: Open questions.
| Does olfactory experience during early development influence the functional maturation of the cognitive system and the development of cognitive abilities? |
| Is the coordination of activity in the cognitive system by the respiration rhythm necessary for cognitive processing? |
| Is olfactory dysfunction specific enough as a biomarker for Alzheimer’s disease and Parkinson’s disease? Does olfactory dysfunction and the lack of coordination in olfactory rhythms contribute to cognitive dysfunctions? |
| Does olfactory dysfunction contribute to cognitive symptoms in other mental and neurodevelopmental disorders? |
Due to the early functionality of olfaction compared to hearing and seeing and the early drive of the cognitive system, we hypothesize that the olfactory experience at the neonatal age might influence the development of the cognitive network. It remains to be investigated whether the olfactory input at a young age has a causal influence on the development of the hippocampal-cortical network and thus the emergence of cognitive abilities later in life. How the sense of smell influences network synchronization in the cognitive systems during development is also interesting in the light of the early manifestation of disrupted network synchronization in mental disorders such as schizophrenia long before symptoms emerge (Chini et al., 2020). If a causal link between olfactory dysfunction and impaired maturation of the cognitive network exists, then an impaired sense of smell during development could act as a possible biomarker for the presymptomatic diagnosis of mental disorders.
The influence of sensory systems on cognitive systems is not unique to olfaction. Other sensory systems are associated with cognitive abilities and mental disorders; however, the influence of olfaction seems to be particularly strong and shows the greatest promise as an early biomarker for Alzheimer’s disease and Parkinson’s disease (Doty, 2012; Murphy, 2019). The strong and direct connectivity between the olfactory and cognitive system, as well as the rhythmic sampling during respiration coordinating activity in widespread networks, could be the reason for the crucial role of olfaction. However, it is unclear whether the olfactory dysfunction is specific enough to dissociate between diseases and whether the link between the association of olfactory and cognitive dysfunctions is causal or merely correlational. Nevertheless, including olfactory dysfunction as a biomarker for Alzheimer’s disease and Parkinson’s disease bears great potential for the detection and prediction of disease progression at early stages, critical for therapeutic intervention. Whether the diagnostic potential transfers to cognitive symptoms in other disease types needs further investigation.
Acknowledgments
We thank Dr. Ileana L. Hanganu-Opatz for feedback to the present manuscript and financial support.
Biographies

Johanna K. Kostka is currently completing her Ph.D. at the Institute of Developmental Neurophysiology at the Center of Molecular Neurobiology in Hamburg-Eppendorf. She received her bachelor’s degree in Biophysics at the Humboldt University of Berlin and her master’s degree in Brain and Cognitive Science at the University of Amsterdam. Her research focuses on the role of olfactory processing for the development of cortical and hippocampal networks. She is using in vivo extracellular electrophysiology as well as opto- and chemogenetics to investigate network interactions between the olfactory bulb and brain areas, such as the entorhinal cortex, the hippocampus, and the prefrontal cortex.

Sebastian H. Bitzenhofer is a Junior Research Group Leader associated with the Institute of Developmental Neurophysiology at the University Medical Center Hamburg-Eppendorf. He received his Dr. rer. nat. at the University of Hamburg in 2017, before he did a postdoc at the Center for Neural Circuits and Behavior at the University of California San Diego. He uses a mix of electrophysiological and optogenetic approaches to study neural circuits and population dynamics in mice. In 2021, he was awarded with the Bernard Katz Lecture for his work on the development of the prefrontal cortex.
Footnotes
Author contributions: Both authors equally contributed to the manuscript.
Research funding: This work was supported by grants of the European Research Council (ERC-2015-CoG 681577 to Prof. Dr. Ileana L. Hanganu-Opatz) and the German Research Foundation (Ha4466/11-1 and 178316478 - B5 to Prof. Dr. Ileana L. Hanganu-Opatz).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
Contributor Information
Johanna K. Kostka, Email: johanna.kostka@zmnh.uni-hamburg.de.
Sebastian H. Bitzenhofer, Email: sebastian.bitzenhofer@zmnh.uni-hamburg.de.
References
- Ackels T., Jordan R., Schaefer A.T., Fukunaga I. Respiration-locking of olfactory receptor and projection neurons in the mouse olfactory bulb and its modulation by brain state. Front. Cell. Neurosci. 2020;14:1662–5102. doi: 10.3389/fncel.2020.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M., DeVito L.M., Cleland T.A. One-trial associative odor learning in neonatal mice. Chem. Senses. 2006;31:343–349. doi: 10.1093/chemse/bjj038. [DOI] [PubMed] [Google Scholar]
- Arshamian A., Iravani B., Majid A., Lundström J.N. Respiration modulates olfactory memory consolidation in humans. J. Neurosci. 2018;38:10286–10294. doi: 10.1523/JNEUROSCI.3360-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J., Walker L., Jellinger K.A. Olfaction and aging: a mini-review. GER. 2015;61:485–490. doi: 10.1159/000381619. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R., Close J., Machold R., Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biskamp J., Bartos M., Sauer J.-F. Organization of prefrontal network activity by respiration-related oscillations. Sci. Rep. 2017;7:45508. doi: 10.1038/srep45508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzenhofer S.H., Pöpplau J.A., Chini M., Marquardt A., Hanganu-Opatz I.L. A transient developmental increase in prefrontal activity alters network maturation and causes cognitive dysfunction in adult mice. Neuron. 2021;109:1350–1364.e6. doi: 10.1016/j.neuron.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzenhofer S.H., Westeinde E.A., Zhang H.-X.B., Isaacson J.S. Rapid odor processing by layer 2 subcircuits in lateral entorhinal cortex. Elife. 2022;11:e75065. doi: 10.7554/eLife.75065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A.M., Sturgill J.F., Poo C., Isaacson J.S. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76:1161–1174. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini M.P., Federico A., Zanini A., Mantovani E., Masala C., Tinazzi M., Tamburin S. Olfaction and taste in Parkinson’s disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J. Neural. Transm. 2019;126:585–595. doi: 10.1007/s00702-019-01996-z. [DOI] [PubMed] [Google Scholar]
- Chen Z., Padmanabhan K. Top-down feedback enables flexible coding strategies in the olfactory cortex. Cell Rep. 2022;38:110545. doi: 10.1016/j.celrep.2022.110545. [DOI] [PubMed] [Google Scholar]
- Chini M., Pöpplau J.A., Lindemann C., Carol-Perdiguer L., Hnida M., Oberländer V., Xu X., Ahlbeck J., Bitzenhofer S.H., Mulert C., et al. Resolving and rescuing developmental miswiring in a mouse model of cognitive impairment. Neuron. 2020;105:60–74.e7. doi: 10.1016/j.neuron.2019.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R.L. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 2012;46:527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler-Ruiz W., Clark D.G., Joshi N.R., Devi-Chou V., Kitch L., Schnitzer M., Abbott L.F., Axel R. Olfactory landmarks and path integration converge to form a cognitive spatial map. Neuron. 2021;109:4036–4049.e5. doi: 10.1016/j.neuron.2021.09.055. [DOI] [PubMed] [Google Scholar]
- Fletcher M.L., Smith A.M., Best A.R., Wilson D.A. High-frequency oscillations are not necessary for simple olfactory discriminations in young rats. J. Neurosci. 2005;25:792–798. doi: 10.1523/JNEUROSCI.4673-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourévitch B., Kay L.M., Martin C. Directional coupling from the olfactory bulb to the Hippocampus during a Go/No-Go odor discrimination task. J. Neurophysiol. 2010;103:2633–2641. doi: 10.1152/jn.01075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C.L., Furey M.L., Pietrini P., Horwitz B., Rapoport S.I. Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Gretenkord S., Kostka J.K., Hartung H., Watznauer K., Fleck D., Minier-Toribio A., Spehr M., Hanganu-Opatz I.L. Coordinated electrical activity in the olfactory bulb gates the oscillatory entrainment of entorhinal networks in neonatal mice. PLoS Biol. 2019;17:e2006994. doi: 10.1371/journal.pbio.2006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H., Brockmann M.D., Pöschel B., Feo V.D., Hanganu-Opatz I.L. Thalamic and entorhinal network activity differently modulates the functional development of prefrontal–hippocampal interactions. J. Neurosci. 2016;36:3676–3690. doi: 10.1523/JNEUROSCI.3232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck D.H., Kozma R., Kay L.M. The rhythm of memory: how breathing shapes memory function. J. Neurophysiol. 2019;122:563–571. doi: 10.1152/jn.00200.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T., Shioi G., Abe T., Kiyonari H., Kato S., Kobayashi K., Mori K., Kawasaki T. A novel birthdate-labeling method reveals segregated parallel projections of mitral and external tufted cells in the main olfactory system. ENeuro. 2019;6:ENEURO.0234-19.2019. doi: 10.1523/ENEURO.0234-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.-D., Jiang L.-X., Su F., Wang H.-L., Zhang C., Yu X. A novel paradigm for assessing olfactory working memory capacity in mice. Transl. Psychiatry. 2020;10:1–16. doi: 10.1038/s41398-020-01120-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K.M., Ieki N., An M., Yamaguchi Y., Nagayama S., Kobayakawa K., Kobayakawa R., Tanifuji M., Sakano H., Chen W.R., et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 2012;32:7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K.M., Lu L., Colgin L.L., Moser M.-B., Moser E.I. Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- Karalis N., Sirota A. Breathing coordinates cortico-hippocampal dynamics in mice during offline states. Nat. Commun. 2022;13:467. doi: 10.1038/s41467-022-28090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay L.M., Beshel J., Brea J., Martin C., Rojas-Líbano D., Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan U.A., Liu L., Provenzano F.A., Berman D.E., Profaci C.P., Sloan R., Mayeux R., Duff K.E., Small S.A. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostka J.K., Bitzenhofer S.H. Postnatal development of centrifugal inputs to the olfactory bulb. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.815282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostka J.K., Hanganu-Opatz I.L. Olfactory-driven beta band entrainment of limbic circuitry during neonatal development. . 2021. BioRxiv 2021.10.04.463041. [DOI] [PubMed]
- Leitner F.C., Melzer S., Lütcke H., Pinna R., Seeburg P.H., Helmchen F., Monyer H. Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nat. Neurosci. 2016;19:935–944. doi: 10.1038/nn.4303. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu J., Liu Y., Zhu J., Liu N., Zeng W., Huang N., Rasch M.J., Jiang H., Gu X., et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 2017;20:559–570. doi: 10.1038/nn.4517. [DOI] [PubMed] [Google Scholar]
- Lockmann A.L.V., Laplagne D.A., Tort A.B.L. Olfactory bulb drives respiration-coupled beta oscillations in the rat hippocampus. Eur. J. Neurosci. 2018;48:2663–2673. doi: 10.1111/ejn.13665. [DOI] [PubMed] [Google Scholar]
- Logan D.W., Brunet L.J., Webb W.R., Cutforth T., Ngai J., Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Curr. Biol. 2012;22:1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rojas J., de Solis C.A., Leroy F., Kandel E.R., Siegelbaum S.A. A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron. 2022;110:1559–1572.e4. doi: 10.1016/j.neuron.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Yun D., Hu Y., Tian M., Yang J., Xu Y., Tang Y., Zhan Y., Xie H., Guan J.-S. Acquiring new memories in neocortex of hippocampal-lesioned mice. Nat. Commun. 2022;13:1601. doi: 10.1038/s41467-022-29208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini F.J., Guillamón-Vivancos T., Moreno-Juan V., Valdeolmillos M., López-Bendito G. Spontaneous activity in developing thalamic and cortical sensory networks. Neuron. 2021;109:2519–2534. doi: 10.1016/j.neuron.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Manabe H., Narikiyo K., Onisawa N. Olfactory consciousness and gamma oscillation couplings across the olfactory bulb, olfactory cortex, and orbitofrontal cortex. Front. Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murman D.L. The impact of age on cognition. Semin. Hear. 2015;36:111–121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019;15:11–24. doi: 10.1038/s41582-018-0097-5. [DOI] [PubMed] [Google Scholar]
- Murphy C., Jernigan T.L., Fennema-Notestine C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: a structural MRI study. J. Int. Neuropsychol. Soc. 2003;9:459–471. doi: 10.1017/S1355617703930116. [DOI] [PubMed] [Google Scholar]
- Nakamura N.H., Fukunaga M., Oku Y. Respiratory modulation of cognitive performance during the retrieval process. PLoS One. 2018;13:e0204021. doi: 10.1371/journal.pone.0204021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville K.R., Haberly L.B. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J. Neurophysiol. 2003;90:3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K., Osakada F., Tarabrina A., Kizer E., Callaway E.M., Gage F.H., Sejnowski T.J. Centrifugal inputs to the main olfactory bulb revealed through whole brain circuit-mapping. Front. Neuroanat. 2019;12:115. doi: 10.3389/fnana.2018.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B.M., Ambrozova V., Duncan S., Wood E.R., O’Connor A.R., Ainge J.A. Lateral entorhinal cortex lesions impair odor-context associative memory in male rats. J. Neurosci. Res. 2022;100:1030–1046. doi: 10.1002/jnr.25027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C., Agarwal G., Bonacchi N., Mainen Z.F. Spatial maps in piriform cortex during olfactory navigation. Nature. 2022;601:595–599. doi: 10.1038/s41586-021-04242-3. [DOI] [PubMed] [Google Scholar]
- Rangel L.M., Rueckemann J.W., Riviere P.D., Keefe K.R., Porter B.S., Heimbuch I.S., Budlong C.H., Eichenbaum H. Rhythmic coordination of hippocampal neurons during associative memory processing. Elife. 2016;5:e09849. doi: 10.7554/eLife.09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M., Murtaza N., Scharrenberg R., White S.H., Johanns O., Walker S., Yuen R.K.C., Schwanke B., Bedürftig B., Henis M., et al. Altered TAOK2 activity causes autism-related neurodevelopmental and cognitive abnormalities through RhoA signaling. Mol. Psychiatr. 2019;24:1329–1350. doi: 10.1038/s41380-018-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler R., McGaugh J.L. The entorhinal cortex as a gateway for amygdala influences on memory consolidation. Neuroscience. 2022;S0306-4522:00037–9. doi: 10.1016/j.neuroscience.2022.01.023. [DOI] [PubMed] [Google Scholar]
- Salimi M., Tabasi F., Abdolsamadi M., Dehghan S., Dehdar K., Nazari M., Javan M., Mirnajafi-Zadeh J., Raoufy M.R. Disrupted connectivity in the olfactory bulb-entorhinal cortex-dorsal hippocampus circuit is associated with recognition memory deficit in Alzheimer’s disease model. Sci. Rep. 2022;12:4394. doi: 10.1038/s41598-022-08528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A.M., Mattson M.P. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symanski C.A., Bladon J.H., Kullberg E.T., Jadhav S.P. Rhythmic coordination of hippocampal-prefrontal ensembles for odor-place associative memory and decision making. . 2021. BioRxiv 2020.06.08.140939. [DOI] [PMC free article] [PubMed]
- Tort A.B.L., Brankačk J., Draguhn A. Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci. 2018;41:186–197. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Uchida S., Shimada C., Sakuma N., Kagitani F., Kan A., Awata S. The relationship between olfaction and cognitive function in the elderly. J. Physiol. Sci. 2020;70:48. doi: 10.1186/s12576-020-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vandrey B., Garden D.L.F., Ambrozova V., McClure C., Nolan M.F., Ainge J.A. Fan cells in layer 2 of the lateral entorhinal cortex are critical for episodic-like memory. Curr. Biol. 2020;30:169–175.e5. doi: 10.1016/j.cub.2019.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Omura M., Mombaerts P. Development and topography of the lateral olfactory tract in the mouse: imaging by genetically encoded and injected fluorescent markers. J. Neurobiol. 2006;66:835–846. doi: 10.1002/neu.20266. [DOI] [PubMed] [Google Scholar]
- Wang P.Y., Boboila C., Chin M., Higashi-Howard A., Shamash P., Wu Z., Stein N.P., Abbott L.F., Axel R. Transient and persistent representations of odor value in prefrontal cortex. Neuron. 2020;108:209–224.e6. doi: 10.1016/j.neuron.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.I.G., Watanabe S., Milner H., Ainge J.A. Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus. 2013;23:1280–1290. doi: 10.1002/hipo.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M.P., Doan T.P., Jacobsen B., Nilssen E.S., Ohara S. Architecture of the entorhinal cortex A review of entorhinal anatomy in rodents with some comparative notes. Front. Syst. Neurosci. 2017;11:46. doi: 10.3389/fnsys.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Song L., Kringel R., Hanganu-Opatz I.L. Developmental decrease of entorhinal-hippocampal communication in immune-challenged DISC1 knockdown mice. Nat. Commun. 2021;12:6810. doi: 10.1038/s41467-021-27114-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui-Doktor M., Luck T., Riedel-Heller S.G., Loeffler M., Wirkner K., Engel C. Olfactory function is associated with cognitive performance: results from the population-based LIFE-Adult-Study. Alzheimer’s Res. Ther. 2019;11:43. doi: 10.1186/s13195-019-0494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Zhou G., Noto T., Templer J.W., Schuele S.U., Rosenow J.M., Lane G., Zelano C. Smell-induced gamma oscillations in human olfactory cortex are required for accurate perception of odor identity. PLoS Biol. 2022;20:e3001509. doi: 10.1371/journal.pbio.3001509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Burton S.D., Tripathy S.J., Urban N.N. Postnatal development attunes olfactory bulb mitral cells to high-frequency signaling. J. Neurophysiol. 2015;114:2830–2842. doi: 10.1152/jn.00315.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C., Jiang H., Zhou G., Arora N., Schuele S., Rosenow J., Gottfried J.A. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 2016;36:12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


