Abstract
Objectives
To evaluate the relationships between adipose tissue distribution, insulin secretion and sensitivity, sleep-disordered breathing, and inflammation in obese adolescents.
Methods
Cross-sectional study of 56 obese adolescents who underwent anthropometric measures, dual-energy X-ray absorptiometry, overnight polysomnography, oral glucose tolerance test (OGTT) and frequently sampled intravenous glucose tolerance test. Correlation and regression analyses were used to assess relationships between adiposity, insulin secretion and sensitivity, measures of sleep-disordered breathing (oxyhemoglobin nadir, SpO2; apnea hypopnea index, AHI; arousal index, AI; maximum end-tidal CO2; non-REM sleep duration), and inflammation (high-sensitivity C-reactive protein, hsCRP).
Results
Subjects (55% female) were mean (SD) 14.4 (2.1) years, with BMI Z-score of 2.3 (0.4). AHI was >5 in 10 (18%) subjects and 1< AHI ≤5 in 22 (39%). Visceral adipose tissue area (VAT) was positively correlated with OGTT 1 and 2 h insulin and 1 h glucose, and hsCRP (r=0.3–0.5, p≤0.007 for each). VAT was negatively correlated with sensitivity to insulin (r=−0.4, p=0.005) and SpO2 nadir (r=−0.3, p=0.04) but not with other sleep measures. After adjustment for BMI-Z, sex, population ancestry, age, and sleep measures, VAT remained independently associated with insulin measures and 1 h glucose, but no other measures of glycemia. SAT was not associated with measures of glycemia or insulin resistance.
Conclusions
Among adolescents with obesity, visceral adiposity was associated with insulin resistance, SpO2 nadir, and inflammation. The independent association of visceral adiposity with insulin resistance highlights the potential role of VAT in obesity-related chronic disease.
Keywords: adolescent, obesity, sleep, inflammation, adiposity, insulin resistance
Introduction
Approximately 17% of youth ages 2–19 in the United States have obesity, placing them at risk for several comorbidities, including, but not limited to, prediabetes or type 2 diabetes mellitus, dyslipidemia, hypertension, and obstructive sleep apnea (OSA) [1]. While adult studies indicate that visceral fat is strongly associated with insulin resistance, limited data is available for children [2]. One potentially modifiable risk factor for visceral adiposity and insulin resistance is sleep. In adults, intermittent hypoxia, a consequence of obstructive apneas, is thought to mediate pancreatic beta-cell dysfunction and increase insulin resistance in liver, skeletal muscle, and adipose tissue through induction of a pro-inflammatory environment in visceral adipose tissue [3, 4]. Although studies in youth with obesity have separately documented relationships between sleep disordered breathing and either inflammation [5], [6], [7] or insulin resistance [8, 9], simultaneous assessment of the interrelationships between adiposity, sleep disordered breathing, insulin resistance, and inflammation has been limited in children [10]. In addition, pediatric research in this area has been largely based on proxy measures of adiposity such as body mass index and waist circumference rather than measured visceral adiposity [11], a proposed mediator of inflammatory changes in sleep disordered breathing [7].
We previously demonstrated a U-shaped relationship between sleep duration and measures of short- and long-term glycemia in a cohort of adolescents with obesity [12]. In the present report, we utilized contemporary software to examine abdominal visceral fat from dual-energy X-ray absorptiometry (DXA) in this cohort. The primary aim of this study was to examine associations between abdominal visceral fat and sleep, insulin secretion and sensitivity, and inflammation. We hypothesized that visceral adiposity would be associated with (a) sleep apnea and hypoxia (as measured by apnea-hypopnea index and oxyhemoglobin saturation nadir during sleep), (b) insulin secretion and sensitivity, and (c) inflammation. In addition, we sought to explore the independence of visceral adiposity and sleep indices as predictors of glycemia and insulin resistance, after adjustment for degree of obesity as measured by body mass index Z-score, as well as demographic and clinical characteristics.
Materials and methods
A total of 70 pubertal adolescents with obesity (body mass index, BMI, >95th percentile for age and sex) were recruited from the Endocrine clinic at Children’s Hospital of Philadelphia [12]. Subjects were excluded if they had diagnosed obstructive sleep apnea, diabetes, genetic syndromes affecting glucose tolerance, major organ system illness, or were on medications affecting insulin or glucose metabolism. Fifty-six subjects completed DXA, thereby comprising the cohort used for the current study. The Children’s Hospital of Philadelphia Institutional Review Board approved this study. All subjects provided assent, and their parents or guardians provided informed consent.
Anthropometrics
Detailed descriptions of data collection were previously reported [12]. Briefly, medical history and demographic data were obtained from participants or their guardians, and physical examination including Tanner staging for pubertal maturation was performed by a study investigator. Weight was measured using a digital scale (Scaletronix, white Plains, NY) and height by a wall-mounted stadiometer (Holtain Inc., Crymych, UK). Body mass index (BMI) was calculated using the equation weight/height squared (kg/m2). BMI Z-scores were assessed using age- and sex-specific reference data [13].
Glucose, metabolic testing, and inflammatory markers
Subjects underwent oral glucose tolerance test (OGTT) and frequently sampled intravenous glucose tolerance test (FSIGT) as well as hemoglobin A1c (HbA1c) measurement. OGTT was performed after a 12 h overnight fast; 1.75 g/kg (max 75 g) oral glucose solution was given, and blood samples for glucose and insulin were obtained at −10, 0, 10, 30, 60, 90, 120, 150, and 180 min. Plasma glucose was measured by the glucose dehydrogenase method and plasma insulin by radioimmunoassay. Thresholds for impaired fasting glucose (100–125 mg/dL), impaired glucose tolerance (2 h plasma glucose on OGTT 140–199 mg/dL), prediabetes-range HbA1c (5.7–6.4%), or diabetes (fasting glucose ≥126 mg/dL, 2 h plasma glucose ≥200, or HbA1c ≥6.5%) were based on American Diabetes Association standards [14]. Whole-body insulin sensitivity index (WBISI), a validated insulin sensitivity measure in children and adolescents with obesity [15], was calculated using data from the 180 min OGTT as follows:
On morning 2, fasting subjects were administered an infusion of 0.25 g/kg 25% dextrose intravenously over 30 s, regular human insulin (0.015 units/kg IV) over 5 min at 20 min, and blood samples for glucose and insulin were drawn at −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min. To estimate insulin sensitivity, the MINMOD Millenium software program was used [16]. Sensitivity to insulin (SI) is a measure of the net capacity of insulin to promote glucose disposal and inhibit endogenous glucose production and was calculated using FSIGT data [16]. High-sensitivity CRP (hsCRP) was used to assess systemic inflammation (enzyme-linked immunosorbent assay; ALPCO, Wind-ham, NH, USA; detection range 1.9–150 ng/mL, sensitivity 0.124 ng/mL).
Polysomnography
Polysomnography (PSG) was performed the night between OGTT and FSIGT for all subjects. Electroencephalogram, right and left electro-oculograms, submental electromyogram, tibial electromyogram, and electrocardiogram were recorded. In addition, inductance plethysmography was used for chest and abdominal wall motion, nasal pressure cannula with oral thermistor bead for oronasal pressure/airflow, infrared capnometry (Nellcor N-1000) for end-tidal PCO2 at the nose, and pulse oximetry for arterial oxygen saturation. PSGs were reviewed by a single sleep Board-certified investigator (LJB) who was unaware of the subjects’ metabolic status. Variables of interest for this study included apnea-hypopnea index (AHI, total number of central and obstructive apneas plus hypopneas per hour of sleep), oxyhemoglobin saturation (SpO2) nadir, arousal index (AI, number of awakenings per hour), highest end-tidal carbon dioxide level (maxETCO2), time spent in non-rapid eye movement sleep (non-REM), and total sleep time (TST). Standard pediatric criteria were used for assessment of respiratory disturbances [17].
Dual-energy X-ray absorptiometry (DXA)
Whole body dual-energy X-ray absorptiometry scans were performed using the Hologic Discovery A (Marlborough, MA, USA) device. Scans were performed by a trained technician using instrument-specific guidelines for participant preparation and scan acquisition, which were subsequently analyzed using Apex software (version 5.5.3). Abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) area (cm2) were assessed using Hologic software [18]. A ratio of visceral to subcutaneous adipose tissue (VAT/SAT), which has been shown to correlate with cardiometabolic risk above and beyond absolute visceral adipose tissue and BMI in adults [19], was calculated as VAT divided by SAT.
Statistical analysis
Statistical analyses were performed using Stata software (Stata Corporation, TX; version 14). Continuous variables were visually inspected for normality; nearly all biochemical measures were right-skewed so were log-transformed for linear regression analysis. Descriptive analyses included means and standard deviations or medians and interquartile ranges of continuous variables, and distributions of categorical variables. Differences in continuous variables across groups were assessed using Student’s t-test and ANOVA or the Wilcoxon rank sum test and Kruskal–Wallis test by ranks depending on normal or skewed distribution. Group differences in categorical variables were assessed using Fisher’s exact test.
Spearman’s correlation was used to assess the relationship between adipose tissue indices; sleep measures; measures of insulin resistance or secretion and glycemia; and hsCRP. Multivariable linear regression was used to determine the independent association of adipose tissue indices and sleep indices with insulin and glycemia outcomes, adjusting for BMI-Z, sex, population ancestry, and age. Sleep indices were evaluated using separate models and were retained if significant at p<0.05. hsCRP was retained in the final multivariable models if significant at p<0.05. The original study was powered to evaluate relationships of sleep parameters with glucose homeostasis; in this current exploratory analysis, no adjustment for multiple testing was made.
Results
Cohort characteristics
The cohort consisted of 56 (31 female) subjects with a mean age of 14.4 (SD 2.1) years (Table 1). Approximately half (n=30, 54%) were African American, and 7 (13%) were Hispanic. More than half had completed puberty (at Tanner Stage 5 on exam; n=29, 53%). Median HbA1c, fasting plasma glucose, and 2 h post-load glucose were in the normal range (Table 2). However, 39% (n=22) had at least one of impaired fasting glucose, impaired glucose tolerance, or prediabetes-range HbA1c, and 1 subject (2%) had diabetes-range fasting glucose and 2 h post-load glucose. Over half (32/56, 57%) had an apnea–hypopnea index (AHI) greater than 1, including 22 (39%) with 1<AHI≤5, 6 (7%) with 5<AHI≤10, and 4 (11%) with AHI>10. The majority of apneas and hypopneas were obstructive (69% of all, and 77% of all with AHI>5). SpO2 nadir ranged from 81 to 100%, with a median of 93%.
Table 1:
Cohort characteristics. n=56.
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 25 (45%) |
| Female | 31 (55%) |
| Age, yearsa | 14.4 (2.1) |
| Race | |
| White | 21 (38%) |
| African American | 30 (54%) |
| Asian American | 1 (2%) |
| >1 race or other | 4 (7%) |
| Ethnicity | |
| Hispanic | 7 (13%) |
| Non-hispanic | 46 (82%) |
| Other | 3 (5%) |
| Tanner stage by breast/genital (1 missing) | |
| 2 | 5 (9%) |
| 3 | 10 (18%) |
| 4 | 11 (20%) |
| 5 | 29 (53%) |
| BMI, kg/m2a | 36.0 (5.6) |
| BMI Z-scorea | 2.34 (0.36) |
| Visceral adipose tissue area, cm2b | 98.4 (76.6–118.4) |
| Subcutaneous adipose tissue area, cm2b | 520.5 (459.2–623.4) |
aMean (SD); bMedian (IQR).
Table 2:
Polysomnography and glucose or insulin measures.
| Variable | Value |
|---|---|
| Total sleep time, h | 7.1 (0.9) |
| Time in non-rapid eye movement sleep, h | 5.7 (0.8) |
| Apnea–hypopnea index, n (%) | |
| <1 | 24 (43%) |
| 1 < AHI ≤ 5 | 22 (39%) |
| >5–10 | 6 (11%) |
| >10 | 4 (7%) |
| SpO2 nadir, % | 93 (91–94.5) |
| Maximum end-tidal CO2, mmHg | 53.2 (3.4) |
| Arousal/awakening index (events per hour of sleep) | 12.1 (9.6–15.5) |
| Hemoglobin A1c, % | 5.5 (5.3–5.6) |
| <5.7%, n (%) | 45 (80%) |
| 5.7–6.4%, n (%) | 11 (20%) |
| Fasting plasma glucose, mg/dL | 90 (85–98) |
| <100 mg/dL, n (%) | 46 (82%) |
| 100–125 mg/dL, n (%) | 9 (16%) |
| ≥126 mg/dL, n (%) | 1 (2%) |
| 2 h post-load glucose, mg/dL | 124 (110–138) |
| <140 mg/dL, n (%) | 43 (77%) |
| 140–199 mg/dL, n (%) | 12 (21%) |
| ≥200 mg/dL, n (%) | 1 (2%) |
| WBISI | 2.2 (1.3–3.4) |
| Sensitivity to insulin | 1.7 (1.1–3.3) |
All values are median (IQR) except sleep times and maximum end-tidal CO2, which are mean (SD).
Adiposity, inflammation, and sleep parameters by age, sex, population ancestry
VAT, SAT, and VAT/SAT were not associated with age. VAT and SAT, but not VAT/SAT, differed by sex and were higher in females than males (VAT: median (IQR) 106.8 (85.9–130.0) vs. 77.7 (71.1–104.6) cm2, p=0.03; SAT: median (IQR) 534.4 (479.3–673.5) vs. 479.1 (397.3–560.0) cm2, p=0.006). BMI-Z did not differ by sex. VAT and VAT/SAT differed by population ancestry and were lowest in African American subjects (VAT, median (IQR): white: 118.3 (99.4–131.5), African American: 84.0 (75.0–103.6), others: 104.6 (62.9–112.5), p=0.01; VAT/SAT, median (IQR): white 0.22 (0.17–0.26), African American: 0.17 (0.13–0.21), others: 0.21 (0.19–0.23); p=0.01). No significant differences in hsCRP were found by age, sex, or population ancestry. Out of six sleep parameters of interest (AHI, SpO2, AI, maxETCO2, non-REM, TST), only maxETCO2 differed significantly by age and had an inverse association (r=−0.27, p=0.04). No other sleep parameters varied by sex, and none differed by population ancestry.
Interrelationships between adiposity, insulin secretion and sensitivity, measures of sleep disordered breathing, and inflammation
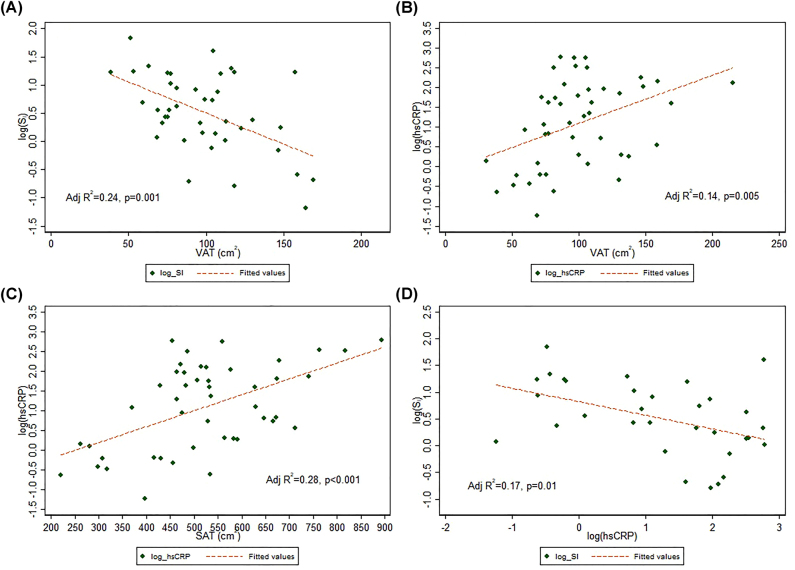
As shown in Table 3, VAT and SAT were strongly correlated (r=0.67, p=0.003). VAT and VAT/SAT were correlated with several measures of insulin resistance (both VAT and VAT/SAT: SI, 1 h-insulin on OGTT; VAT only: 2 h-insulin on OGTT). The inverse relationship between VAT and log(SI) is depicted in Figure 1A. No significant correlations were found between WBISI and adiposity, inflammation, or sleep indices. Positive associations were found between VAT and 1 h-glucose on OGTT, SAT and 2 h-glucose on OGTT, and VAT/SAT and HbA1c. AHI was not correlated with VAT, SAT, or VAT/SAT. SpO2 nadir was negatively correlated with VAT but not SAT or VAT/SAT. TST, non-REM time, AI, and MaxETCO2 were not associated with any adiposity indices. As we previously demonstrated [12], TST was negatively correlated with 2 h plasma glucose on OGTT as well as HbA1c but was not correlated with measures of insulin resistance. VAT (Figure 1B) and SAT (Figure 1C) were each positively correlated with hsCRP, but VAT/SAT was not. Log-transformed hsCRP was inversely associated with (log) SI (Figure 1D). None of the assessed sleep measures were correlated with hsCRP.
Table 3:
Spearman’s correlations between adipose tissue, sleep indices, inflammation, and insulin resistance.
| VAT | SAT | VAT/SAT | hsCRP | AHI | SpO2 | Total sleep time | Non-REM time | Max ETCO2 | AI | |
|---|---|---|---|---|---|---|---|---|---|---|
| SAT | 0.39b | |||||||||
| VAT/SAT | 0.67b | −0.33a | ||||||||
| hsCRP | 0.45b | 0.47b | 0.003 | |||||||
| AHI | −0.03 | 0.07 | −0.06 | 0.13 | ||||||
| SpO2 nadir | −0.27a | −0.07 | −0.21 | −0.18 | −0.46b | |||||
| Total sleep time | 0.03 | −0.02 | 0.06 | −0.24 | −0.18 | −0.04 | ||||
| Non-REM time | −0.06 | −0.17 | 0.04 | −0.22 | −0.29a | 0.03 | 0.81 | |||
| maxETCO2 | 0.22 | 0.07 | 0.14 | 0.21 | −0.0034 | 0.01 | 0.10 | −0.02 | ||
| Arousal index | −0.1541 | 0.04 | −0.16 | 0.07 | 0.42b | −0.02 | −0.45b | −0.36a | −0.11 | |
| WBISI | −0.25 | −0.08 | −0.16 | 0.06 | −0.15 | 0.15 | 0.13 | 0.23 | 0.04 | −0.17 |
| SI | −0.43a | −0.23 | −0.21 | −0.48a | −0.23 | 0.23 | 0.09 | 0.27 | −0.28 | −0.20 |
| 1 h-insulin | 0.36a | 0.02 | 0.30a | 0.11 | 0.10 | −0.26 | −0.16 | −0.28a | 0.00 | 0.08 |
| 2 h-insulin | 0.42b | 0.27a | 0.16 | 0.22 | 0.17 | −0.20 | −0.14 | −0.25 | 0.07 | 0.12 |
| Fasting glucose | −0.13 | −0.07 | −0.01 | −0.09 | −0.11 | 0.12 | −0.17 | −0.09 | −0.17 | 0.06 |
| 1 h-glucose | 0.38b | 0.21 | 0.24 | 0.24 | −0.18 | −0.01 | −0.19 | −0.25 | 0.05 | −0.13 |
| 2 h-glucose | 0.26 | 0.40 c | −0.03 | 0.31a | −0.05 | 0.00 | −0.32a | −0.34a | 0.02 | 0.12 |
| HbA1c | −0.13 | 0.16 | −0.27a | 0.08 | −0.03 | 0.21 | −0.26a | −0.23 | −0.01 | 0.23 |
ap<0.05, bp<0.005. WBISI, whole-body insulin sensitivity index; SI, insulin sensitivity; 1 h and 2 h- insulin/glucose, from OGTT.
Figure 1:
Linear relationships between adiposity indices, inflammation, and sensitivity to insulin.
Visceral adipose tissue (VAT) was inversely associated with SI (A) and positively associated with hsCRP (B). Subcutaneous adipose tissue (SAT) was also positively associated with hsCRP (C), which was inversely related to SI (D).
Multivariable linear regression (Table 4) adjusted for BMI-Z, sex, population ancestry, and age revealed VAT as a significant independent predictor of insulin resistance, with greater VAT associated with lower (log) SI and higher (log) 1 and 2 h insulin on OGTT. In contrast to measures of insulin resistance, measures of glycemia (fasting glucose, 1 and 2 h glucose, HbA1c) were not, or were only weakly, independently associated with VAT. AI, maxETCO2, and AHI were not independently associated with measures of insulin resistance or glycemia when VAT was included as a covariate.
Table 4:
Multivariable linear regression models, adjusted for BMI-Z, sex, population ancestry, and age.
| Outcome (log-transformed) | Predictors: | Adjusted R2 | ||||
|---|---|---|---|---|---|---|
| Adiposity index | Sleep index (evaluated in separate models) | |||||
| VAT (β) | SpO2 nadir (β) | TST (β) | Non-REM (β) | Max ETCO2 (β) | ||
| SI | −0.012a | 0.31 | ||||
| 1 h-insulin | 0.011b | 0.08 | ||||
| 2 h-insulin | 0.010b | 0.19 | ||||
| 1 h-glucose | 0.002 | −0.068b | 0.06 | |||
| 1 h-glucose | 0.002 | −0.0014b | 0.07 | |||
| 2 h-glucose | 0.0007 | −0.086a | 0.21 | |||
| 2 h-glucose | 0.0008 | −0.0013b | 0.14 | |||
| HbA1c | −0.0004 | −0.024b | 0.05 | |||
|
Outcome (log-transformed)
|
SAT (β)
|
SpO2 nadir (β)
|
TST (β)
|
Non-REM (β)
|
Max ETCO2 (β)
|
Adjusted R2
|
| SI | −0.0005 | −0.0737b | 0.13 | |||
| 1 h-insulin | −0.0007 | −0.076b | 0.04 | |||
| 1 h-glucose | 0.00004 | −0.072b | 0.02 | |||
| 1 h-glucose | 0.00003 | −0.0014b | 0.02 | |||
| 2 h-glucose | 0.0002 | −0.086a | 0.2 | |||
| 2 h-glucose | 0.0001 | −0.0013b | 0.13 |
|
Outcome (log-transformed)
|
VAT/SAT (β)
|
SpO2 nadir (β)
|
TST (β)
|
Non-REM (β)
|
Max ETCO2 (β)
|
Adjusted R2
|
| SI | −6.34a | 0.34 | ||||
| 1 h-insulin | 4.87b | 0.07 | ||||
| 1 h-glucose | 0.83 | −0.071b | 0.05 | |||
| 1 h-glucose | 0.83 | −0.0014b | 0.05 | |||
| 2 h-glucose | 0.22 | −0.087a | 0.2 | |||
| 2 h-glucose | 0.23 | −0.0013b | 0.13 |
ap<0.01, bp<0.05. SI, insulin sensitivity; 1 h and 2 h- insulin/glucose, from OGTT; HbA1c, hemoglobin A1c.
In contrast to VAT, SAT was not independently associated with insulin resistance in multivariable linear regression (Table 4). In multivariable models including SAT as a covariate, maxETCO2 was inversely associated with (log) SI. Non-REM and TST were inversely associated with (log) 1 and 2 h glucose on OGTT. AI and AHI were not independent predictors of insulin or glycemia outcomes in models including SAT.
In multivariable regression, VAT/SAT was a significant independent predictor of insulin resistance, inversely associated with (log) SI and directly associated with (log) 1 h insulin on OGTT (Table 4). hsCRP was not significant in any multivariable model including VAT/SAT so was not retained as a covariate. No sleep measures were associated with (log) SI or 1 h insulin on OGTT. AI, AHI, and maxETCO2 were not independently associated with measures of insulin resistance or glycemia when VAT/SAT was included as a covariate.
Discussion
In this study of adolescents with obesity, visceral adiposity was associated with several measures of insulin resistance, independent of BMI-Z, sex, population ancestry, and age, as well as measures of sleep disordered breathing and inflammation. In addition, youth with increased abdominal visceral fat exhibited lower minimum oxygen saturation during sleep, as well as increased inflammation as measured by hsCRP. However, obstructive sleep apnea, as measured by AHI, was not independently associated with measures of insulin resistance or glycemia.
Most studies investigating the relationships between abdominal fat depots and glycemia utilize static fasting measures, so our use of OGTT and FSIGT to assess insulin and glucose response over time is a key strength of this study. We observed a positive relationship between abdominal visceral fat and 1 h insulin and glucose, as well as 2 h insulin, during an OGTT. To our knowledge, the relationships between visceral adiposity and 1 h insulin and glucose have not previously been reported. Although the 2 h glucose on OGTT is used to define impaired glucose tolerance [14, 20], the 1 h glucose has been associated with diabetes complications and mortality and is a predictor of future type 2 diabetes risk in adults and progression to prediabetes in youth with obesity [21], [22], [23], [24].
The correlation between WBISI and VAT did approach significance (rho=−0.25, p=0.06), but SI was clearly more strongly correlated with VAT (rho=−0.43, p=0.005). WBISI and SI were correlated with each other (Spearman’s rho=0.61, p<0.0001), similar to a study of peri-pubertal Latino overweight children [25]. These authors also found that the correlation between adiposity (as measured by total body fat mass and BMI) was nearly twice as strong for SI as it was for WBISI, with no significant relationship between BMI and WBISI. They theorize that 2 h post load insulin better reflects the peripheral insulin resistance of puberty than fasting insulin and with stronger associations with FSIGT-derived SI. It is possible that relative over-weighting of fasting as opposed to stimulated insulin in the WBISI may thus limit the ability to detect peripheral insulin resistance in the setting of our relatively small sample size. The WBISI may also display greater variability due to alterations in absorption and incretin effect [26].
The association of visceral fat with lower minimal oxygen saturation is consistent with cell and animal models: in adipose-derived stem cells isolated from visceral adipose tissue of obese adults, hypoxic exposure leads to a deregulated pro-inflammatory response [26], and in murine models, intermittent hypoxia leads to visceral adipose tissue inflammation and insulin resistance [27, 28]. Among non-obese men with OSA, greater abdominal adiposity as measured by waist circumference was associated with lower minimal oxygen saturation, independent of AHI, similar to our findings in adolescents [29]. In contrast to prior studies, we did not observe a relationship between visceral adiposity and AHI [7, 9, 10]. Notably, the majority of adolescents in our study did not have moderate nor severe sleep apnea (AHI ≥5), and we excluded those previously diagnosed with OSA. In addition, our cohort had higher mean and greater variability in VAT area, possibly limiting detection of this previously-described relationship.
Arousal index was positively associated with several measures of insulin resistance, but not after adjustment for visceral adiposity or pubertal stage. The relationship between insulin resistance and sleep fragmentation, as measured by spontaneous arousals, has been previously demonstrated. In a large cohort of obese and non-obese children aged 5–12 years, sleep fragmentation defined by spontaneous arousals was found to be an independent risk factor for insulin resistance, beyond contributions from obesity [30]. In the present study, we adjusted for degree of obesity as measured by BMI-Z, but also assessed the independent contribution of adipose tissue distribution. Our finding that AI was not independently associated with insulin or glycemic outcomes after adjustment for VAT suggests that the relationship between sleep fragmentation and insulin resistance may depend on the degree of visceral adiposity.
Our study has several strengths, including the measurement of visceral and subcutaneous adiposity via DXA, careful assessment of pubertal stage, our extensive biochemical assessment of insulin and glucose response as well as a marker of inflammation, and multiple sleep parameters assessed by the “gold standard” of overnight polysomnography. In addition, our cohort was diverse with respect to population ancestry, sex, and ethnicity. Our results provide a detailed reference for future studies of body composition and glucose homeostasis in adolescents, including additional evidence of sex differences in adipose tissue distribution in adolescents with obesity. In our cohort, female adolescents had greater VAT and SAT than male adolescents, despite similar BMI-Z, pubertal stage, and age distribution. This observation is notable because although visceral adiposity tends to be greater in adult men, the relationship between sex and adipose tissue distribution is less clear in youth [31]. For example, in a study of 7–16 year-old children, Benfield et al. found that magnetic resonance imaging-derived visceral adipose tissue area was greater for girls than boys, but that boys had a higher VAT/SAT ratio than girls [32]. During puberty, differences in VAT accumulation by sex emerge, with boys tending to accumulate a greater amount of VAT in proportion to SAT growth [33]. Our findings that VAT was greatest among adolescent girls and that VAT was independently associated with measures of insulin resistance are consistent with the observation of higher incidence of type 2 diabetes in girls than in boys during childhood [34]. Our adjustment for additional potential confounders, including BMI-Z, population ancestry, and age allowed for assessment of the independent contribution of VAT and SAT toward insulin and glycemia measures. The adjustment for BMI-Z, in particular, allowed for an assessment of the independent impact of visceral and subcutaneous adiposity on insulin resistance and dysglycemia, above and beyond overall obesity.
We acknowledge limitations, including our study’s cross-sectional design, which precludes the ability to determine causal relationships from the correlations reported or to assess changes within individuals longitudinally. In addition, our study findings are limited to adolescents with obesity; the relationships between adiposity indices and insulin and glycemia measures may not be generalizable to adolescents of normal weight. The proportion of patients with AHI >5 (n=10) was low, which may have limited our ability to detect a relationship between visceral adiposity and AHI. There was little sleep time with oxyhemoglobin saturation <90%. Although this study population did not have a very significant hypoxic burden, there was a statistically significant relationship between the lowest oxygen saturation and measures of adiposity. We suspect this relationship would be even stronger in a sample with more significant OSA. Finally, because of the study inclusion criteria, we are unable draw conclusions about glucose and insulin secretory values in the diabetes range, which may have limited our ability to detect associations between sleep apnea and insulin resistance or hyperglycemia.
In summary, results from this study provide novel insights regarding the relationship between specific abdominal fat depots, insulin resistance and glycemia, sleep and inflammation among youth with obesity, independent of key confounders. Our findings highlight the important relationship between visceral adiposity and insulin resistance. Visceral adiposity was associated with minimum oxygen saturation but not with other measures of sleep quantity and sleep-disordered breathing. Longitudinal studies of obese adolescents throughout puberty are required to more clearly define the role of pathogenic fat stores in sleep quality and risk for cardiometabolic disease.
Footnotes
Research funding: Dr. Vajravelu is supported by grant NIH 7K23DK125719-03. Dr. Katz is supported by UL1-TR000003. The study was supported by the PA State Tobacco Settlement Fund (LLK) and 5T32DK063688 (DK).
Author contributions: All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission. MEV, LLK, and LJB were responsible for conception and design of the study. MEV and LLK carried out analysis, interpretation of data, and drafting and critical revision of the article. JK, BZ, and AJ assisted with study conception and design, analysis and interpretation, and critical revision of the article for important intellectual content. JR assisted with data analysis and critical revision of the article. LLK, DK and PB carried out the study visits. DK, PB and LJB critically revised the article for important intellectual content. study, and takes responsibility for the integrity of the data and accuracy of the data analysis.
Competing interests: The funding organizations played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: The Children’s Hospital of Philadelphia Institutional Review Board approved this study.
References
- 1.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:709–57. doi: 10.1210/jc.2016-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tershakovec AM, Kuppler KM, Zemel BS, Katz L, Weinzimer S, Harty MP, et al. Body composition and metabolic factors in obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:19–24. doi: 10.1038/sj.ijo.0802185. [DOI] [PubMed] [Google Scholar]
- 3.Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol. 2017;595:2423–30. doi: 10.1113/jp273312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad Med. 2010;85:693–8. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 5.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.cir.0000161819.76138.5e. [DOI] [PubMed] [Google Scholar]
- 6.Deboer MD, Mendoza JP, Liu L, Ford G, Yu PL, Gaston BM. Increased systemic inflammation overnight correlates with insulin resistance among children evaluated for obstructive sleep apnea. Sleep Breath. 2012;16:349–54. doi: 10.1007/s11325-011-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Calhoun SL, He F, Liao D, et al. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab. 2016;311:E851–8. doi: 10.1152/ajpendo.00249.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S, Storfer-Isser A, Rosen CL, Johnson NL, Kirchner HL, Emancipator J, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannon TS, Lee S, Chakravorty S, Lin Y, Arslanian SA. Sleep-disordered breathing in obese adolescents is associated with visceral adiposity and markers of insulin resistance. Int J Pediatr Obes. 2011;6:157–60. doi: 10.3109/17477166.2010.482156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7:268–73. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber DR, Levitt Katz LE, Zemel BS, Gallagher PR, Murphy KM, Dumser SM, et al. Anthropometric measures of abdominal adiposity for the identification of cardiometabolic risk factors in adolescents. Diabetes Res Clin Pract. 2014;103:e14–7. doi: 10.1016/j.diabres.2013.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koren D, Levitt Katz LE, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34:2442–7. doi: 10.2337/dc11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 14.American Diabetes A 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S15–33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 15.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 16.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Therapeut. 2003;5:1003–15. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 17.Iber CAASM . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes. 2015;10:172–9. doi: 10.1111/ijpo.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–30. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41((Suppl 1)):S13–27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 21.Pareek M, Bhatt DL, Nielsen ML, Jagannathan R, Eriksson KF, Nilsson PM, et al. Enhanced predictive capability of a 1-hour oral glucose tolerance test: a prospective population-based cohort study. Diabetes Care. 2018;41:171–7. doi: 10.2337/dc17-1351. [DOI] [PubMed] [Google Scholar]
- 22.Trico D, Galderisi A, Mari A, Santoro N, Caprio S. One-hour post-load plasma glucose predicts progression to prediabetes in a multi-ethnic cohort of obese youths. Diabetes Obes Metabol. 2019;21:1191–8. doi: 10.1111/dom.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serbis A, Giapros V, Challa A, Chaliasos N, Siomou E. Elevated 1-hour post-load plasma glucose identifies obese youth with abnormal glucose metabolism and an unfavourable inflammatory profile. Clin Endocrinol (Oxf). 2018;89:757–64. doi: 10.1111/cen.13859. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281–6. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigensberg MJ, Cruz ML, Goran MI. Association between insulin sensitivity and post-glucose challenge plasma insulin values in overweight latino youth. Diabetes Care. 2003;26:2094–9. doi: 10.2337/diacare.26.7.2094. [DOI] [PubMed] [Google Scholar]
- 26.Muniyappa RMR, Varghese RT. Assessing insulin sensitivity and resistance in humans. South Dartmouth, MA: MDText.com, Inc; 2021. https://www.ncbi.nlm.nih.gov/books/NBK278954/ Available from. [Google Scholar]
- 27.Petrangeli E, Coroniti G, Brini AT, de Girolamo L, Stanco D, Niada S, et al. Hypoxia promotes the inflammatory response and stemness features in visceral fat stem cells from obese subjects. J Cell Physiol. 2016;231:668–79. doi: 10.1002/jcp.25113. [DOI] [PubMed] [Google Scholar]
- 28.Gileles-Hillel A, Almendros I, Khalyfa A, Nigdelioglu R, Qiao Z, Hamanaka RB, et al. Prolonged exposures to intermittent hypoxia promote visceral white adipose tissue inflammation in a murine model of severe sleep apnea: effect of normoxic recovery. Sleep. 2017;40:1–10. doi: 10.1093/sleep/zsw074. [DOI] [PubMed] [Google Scholar]
- 29.Poulain L, Thomas A, Rieusset J, Casteilla L, Levy P, Arnaud C, et al. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur Respir J. 2014;43:513–22. doi: 10.1183/09031936.00019913. [DOI] [PubMed] [Google Scholar]
- 30.Borel AL, Monneret D, Tamisier R, Baguet JP, Faure P, Levy P, et al. The severity of nocturnal hypoxia but not abdominal adiposity is associated with insulin resistance in non-obese men with sleep apnea. PLoS One. 2013;8:e71000. doi: 10.1371/journal.pone.0071000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koren D, Gozal D, Philby MF, Bhattacharjee R, Kheirandish-Gozal L. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. Eur Respir J. 2016;47:1152–61. doi: 10.1183/13993003.01430-2015. [DOI] [PubMed] [Google Scholar]
- 32.Suliga E. Visceral adipose tissue in children and adolescents: a review. Nutr Res Rev. 2009;22:137–47. doi: 10.1017/s0954422409990096. [DOI] [PubMed] [Google Scholar]
- 33.Benfield LL, Fox KR, Peters DM, Blake H, Rogers I, Grant C, et al. Magnetic resonance imaging of abdominal adiposity in a large cohort of British children. Int J Obes. 2008;32:91–9. doi: 10.1038/sj.ijo.0803780. [DOI] [PubMed] [Google Scholar]
- 34.Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int J Obes. 2012;36:1261–9. doi: 10.1038/ijo.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]