Abstract
The field of neurofeedback training (NFT) has seen growing interest and an expansion of scope, resulting in a steadily increasing number of publications addressing different aspects of NFT. This development has been accompanied by a debate about the underlying mechanisms and expected outcomes. Recent developments in the understanding of psychophysiological regulation have cast doubt on the validity of control systems theory, the principal framework traditionally used to characterize NFT. The present article reviews the theoretical and empirical aspects of NFT and proposes a predictive framework based on the concept of allostasis. Specifically, we conceptualize NFT as an adaptation to changing contingencies. In an allostasis four-stage model, NFT involves (a) perceiving relations between demands and set-points, (b) learning to apply collected patterns (experience) to predict future output, (c) determining efficient set-points, and (d) adapting brain activity to the desired (“set”) state. This model also identifies boundaries for what changes can be expected from a neurofeedback intervention and outlines a time frame for such changes to occur.
Keywords: allostasis framework, neurofeedback training, psychophysiological regulation, self-regulation
Introduction
The study of neurofeedback (NF) began with initial explorations in the 1960s, which showed that both humans and animals can acquire the ability to alter their electroencephalographic (EEG) signals when given appropriate instructions and suitable feedback (Kamiya 1962; Wyrwicka and Sterman 1968). In the following half century, neurofeedback training (NFT) has become the most widely applied psychophysiological procedure enabling individuals to self-regulate specific characteristics of the EEG. Mounting evidence shows that NFT prompts measurable clinical and performance benefits (see the reviews by Arns et al. 2009; Coben et al. 2010; Gruzelier 2014a; Linhartova et al. 2019; Moore 2000; Trambaiolli et al. 2021; Yeh et al. 2020). However, NFT has not escaped criticism; first, concerns have been raised regarding the widely used framework of homeostasis and its ability to explain the effects of NFT on EEG signals (Reiner et al. 2018). A second issue relates to the understanding of the neural mechanisms thought to underlie NFT. Although extensive research has shown positive behavioral outcomes for NFT, this is in stark contrast to the fact that the neural mechanisms underlying NFT are still poorly understood (Micoulaud Franchi et al. 2020). In fact, several authors have suggested that the effects of NFT may simply reflect the placebo effects (Schabus 2017; Thibault et al. 2016; Thibault and Raz 2017). Finally, the lack of understanding of NFT’s mechanisms has limited researchers’ abilities to predict specific training outcomes. For example, there is an ongoing debate regarding the extent to which NFT prompts changes in resting brain activity across training sessions (Schabus 2018; Witte et al. 2018). To better leverage the potential of NFT, the field would thus benefit from (a) a heightened understanding of the underlying neurophysiology and (b) precise definitions of brain-behavior interactions during self-regulation, as discussed in Papo (2019).
This article addresses these challenges in three ways: First, we develop a theoretical framework for NFT, grounded in the concept of allostasis and based on the current understanding of psychophysiological regulation. We outline the key concepts of physiological regulation, including homeostasis and control theory. Learning to control one’s own neural signals involves operant conditioning, which is also discussed. Whereas more traditional control approaches have difficulty in explaining how learning and experience shape physiological regulation; our allostatic framework characterizes the ability of complex organisms to learn and implement self-regulation by dynamically forming and updating predictions and expectations regarding neural states and associated outcomes. Second, we review the research study into neural mechanisms underlying self-regulation based on NF and relate it to the allostasis-based framework. Third, we discuss about unresolved issues in NFT research and how these issues can be addressed in the context of our framework.
A psychophysiological framework of NF
NFT aims to enable a person to self-regulate neurophysiological processes such as electrocortical activity measured by EEG. The current understanding of this self-regulation is based on the tenets of homeostatic psychophysiological regulation (Gruzelier and Egner 2004; Micoulaud Franchi et al. 2020). Since the time of Claude Bernard (Cooper 2008), it has been believed that physiological regulation encompasses various processes that all seek to maintain an organism in a stable (internal) state by detecting and countering fluctuations in or perturbations to the state of the organism—a process known as homeostasis.
Homeostasis and the negative feedback loop
In 1929, Walter Cannon, expanding on Bernard’s ideas, introduced the term homeostasis, the processes and mechanisms that maintain a stable and optimal physiological state of the organism. Cannon also introduced negative feedback as a mechanism to explain how homeostasis functions (Figure 1): In response to maladaptive perturbation (for example, when getting too hot), a physiological process (for example, sweating) rectifies the function and restores an optimal level based on feedback. Thus, homeostatic regulation connects sensors (thermoreceptors in our example) to effectors (sweat glands), allowing the system to reset to pre-perturbation levels (Ramsay and Woods 2014).
Figure 1:
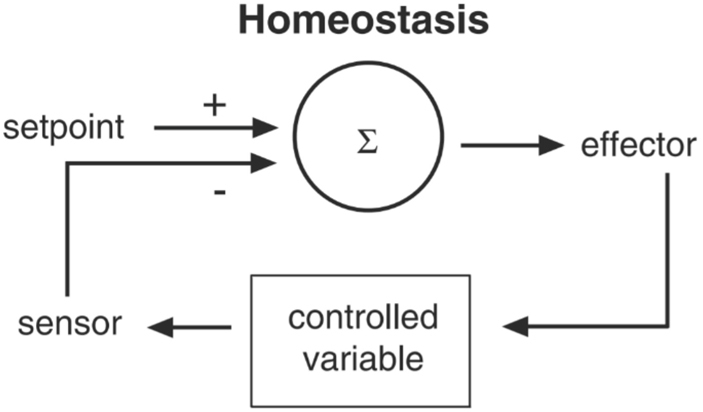
Homeostasis.
What stands out in this model is the general pattern of maintaining a controlled parameter constant by recognizing its divergence from a “set-point” and then transferring this information to correct the error (taken from Sterling 2012).
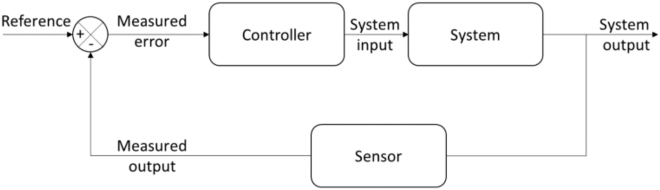
The negative feedback loop within homeostasis was later more formally modeled within control systems theory (Wiener 2019). It has been argued that all systems, “living and mechanical, are both information and feedback control systems” (Shinners 1998, p. 6). As depicted in Figure 2, the control theory is suitable for describing regulation at the system level, irrespective of individual processes, which often cannot be specified in the context of complex systems. The focus is on the transformation of information as it flows through the system and on the effects exerted by this information on the system’s output. A controller adjusts the system’s behavior according to the real-time comparison between the output sensor and the input reference value, referred to as the systems’ set-point (denoted as ± in Figure 2). Paralleling the homeostatic process outlined above, this control circuit thus minimizes the difference between the set-point and the system’s observed state. One common example of such a control system is the thermostat on a heater (Gopal 2002); similarly, the regulation of body temperature around the set-point of 37 °C is readily modeled within control systems theory (Hensel 1981). Although there are powerful explanatory devices, homeostatic control theories are limited in their ability to characterize NFT, as discussed below.
Figure 2:
Basic control theory model (adapted version from LeBlanc and Coughanowr 2008).
This model is a closed-loop system, where the output is sent back, as feedback, to the input to regulate the system.
Learning from reward and punishment
In physiological regulation, beyond phylogenetically determined mechanisms (Dworkin 1986), a control over central and autonomic nervous system functions can be acquired through instrumental learning, which is also referred to as operant conditioning. In this type of learning, the likelihood that a behavior or response is shown depends on contingencies experienced in the past. Success (reward) and failure (punishment) act to, respectively, reinforce or suppress the behavior. For example, in terms of physiological regulation, the increase in heart rate before a 100 m dash will be associated with higher success, in turn leading to a higher likelihood of an increase in heart rate before the next event.
Using feedback signals for operant learning involves the association between (a) a neural target signal, (b) contingent rewards or punishments, and (c) knowledge of outcomes. Although feedback effects themselves are often considered automatic (Gruzelier 2014c), the voluntary control or self-regulation of physiological states is widely thought to require that the feedback stimulus is actively perceived (Miller 1978). Indeed, most people are unable to perceive certain physiological responses such as current blood pressure, which is analogous to a blindfolded beginner trying to learn to shoot baskets. External measurement devices that index a biological process in real time can “remove the blindfold” by supplying an appropriate feedback, known as biofeedback—allowing operant learning to take place, and thus enabling the participant to acquire self-regulation of the targeted physiological state.
Self-regulation through biofeedback
Self-regulation refers to processes of managing one’s own thoughts, feelings, and behaviors, often in the context of accomplishing certain goals (de Ridder and de Wit 2006), and physiological self-regulation can be considered a sub-process of this broader notion. Biofeedback is traditionally understood to rely on the principles of physiological regulation discussed above: feedback and operant conditioning (Gruzelier 2014c; Pandria et al. 2018; Ros et al. 2014). A biological system is constantly fluctuating. In the context of biofeedback, a physiological process is considered well-controlled when it can be shifted from an undesirable or unhealthy state to a desired or healthy state, while minimizing random variations and nearly in real time. By contrast, an unregulated physiological process is one in which a maladaptive state cannot be changed, has too much random variation, and/or changes too slowly or too rapidly (Mulholland 1984).
The concept of biofeedback is to use operant principles to shape the regulatory processes of these fluctuations. Biological functions are not only fed back, but fluctuations in the desired direction are also reinforced by setting appropriate contingencies or instructions. Today, a wide spectrum of physiological signals are used for feedback, including brain activity (NF). NFT has been defined as a noninvasive brain stimulation technique that trains individuals to modulate their own brain activity toward functionally desirable states (Gruzelier 2014b). The most common NFT is based on the feedback of electrical activity of the cerebral cortex via EEG-NFT, in which the participant aims to heighten or suppress the EEG band power within a specific frequency range. Similar approaches based on other neural signals are now widely used, including NFT based on hemodynamic (functional magnetic resonance imaging, fMRI) signals or optical signals as are captured using near-infrared spectroscopy (Sitaram et al. 2017). By framing NFT within homeostasis, this can be understood as a therapist providing an (external) set-point to which the current state is compared. Deviation from this set-point is fed back via the feedback signal, and the state of the system is subsequently regulated in the direction of the set-point.
Psychophysiological regulation—from homeostasis to allostasis
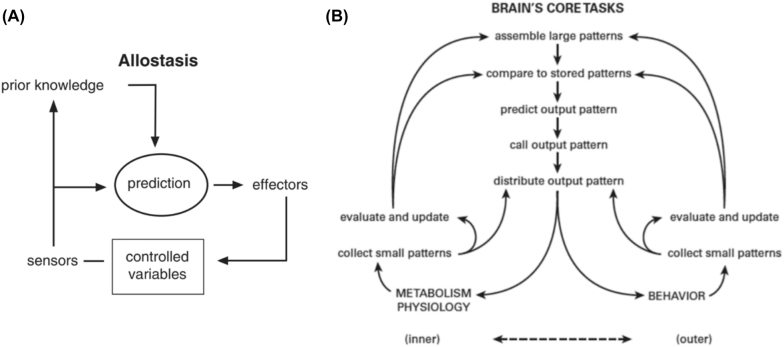
A more recent development in the study of physiological regulation has been the widespread recognition that homeostasis, and especially negative feedback, does not completely explain physiological regulation. One obvious problem is that complex physiological systems do not possess a fixed, rigid set-point for regulation, and thus the state of a biological system may indeed be stable, yet far from constant. Rather, physiological systems fluctuate adaptively and dynamically, according to the demands of the environment or the organism (Ramsay and Woods 2014). A second problem is that homeostasis is considered to rely on a reflexive response to a perturbation, after the fact. However, physiological regulation also clearly relies on anticipatory responses (Ramsay and Woods 2014). For example, with respect to thermoregulation, it has been shown that thermosensors on the skin produce a feedforward disturbance signal in ambient heat which eventually leads to preventive cold-defense behaviors like shivering even before the core temperature drops (Kanosue et al. 2010). Such anticipatory responses are thought to require a learning process—a third aspect that is not considered within the concept of homeostasis. “The conclusion seems almost inescapable: the central nervous system (CNS) anticipates present and future needs on the basis of past experience. By having successfully corrected errors, the CNS learns how to prevent them” Somjen (1992, p. 184). Figure 3 (A and B) illustrates the main characteristics of general allostasis models.
Figure 3:
(A) General allostasis model (taken from Sterling 2018). This model describes a mechanism in which the brain merges sensory data with the previous knowledge to predict adaptations that are likely to be required. [….] (B) Predictive regulation (allostasis). Adapting internal states of the body (bottom left) with external demands is a crucial regulatory task of the brain. [….] What is striking in this model is that the brain, before deciding on a course of action, likens larger input patterns to stored patterns to obtain historical background (what happened previously?).
In its original form, allostasis differs from homeostasis in which the level of a requisite parameter (the system’s target level or set-point) can vary between situations (i.e., there is no fixed set-point). Whereas homeostasis is based on stability (stasis) via constancy (homeo), allostasis is based on achieving stability (stasis) through change (allo; Gerdes et al. 2013). Thus, allostasis describes the process of achieving stability through adaptive adjustment of the internal milieu to meet perceived and anticipated (new) demands (Zsoldos and Ebmeier 2016). Such predictive processes are now considered a hallmark of neurophysiological functioning (Friston and Kiebel 2009). Thus, a number of allostasis-based models have recently been proposed to account for a wide spectrum of cognitive and behavioral processes (Barrett et al. 2016; Saxbe et al. 2020). The basic configuration of a system-maintaining state can still be presumed to be based on homeostasis, whereas the configuration of a system-changing output is based on goal-setting or allostasis (Collura 2014, p. 48). Figure 4 illustrates how the framework proposed here accounts for these processes: Allostasis enables an organism to adaptively respond to its physical state (e.g., awake and asleep) and to flexibly cope with its physiological state and external demands (e.g., hunger, temperature extremes, and psychosocial stress; McEwen 2016; Sterling 2012). Importantly, the allostasis model accounts for the changing of “set-points” and other control boundaries in response to changing requirements or conditions (McEwen 2016). Anticipatory changes of target states in a given situation require the use of previous experience (i.e., learning). In our allostasis-based model, learning is incorporated into the control process, putting the system into an altered, “allostatic state”, optimal for the anticipated challenge. Thus, an organism may use earlier experiences in a given environment (e.g., Florida summer afternoons) in order to properly anticipate future challenges to the internal milieu (e.g., implement anticipatory heat-reduction strategies; Sterling 2012; Sterling et al. 1988). Readers interested in allostasis as a model of predictive regulation are referred to the papers by Sterling (Sterling 2012, 2018).
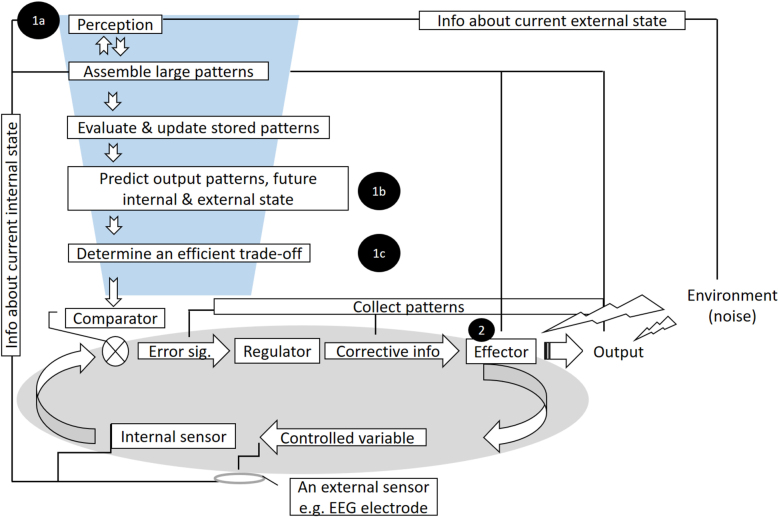
Figure 4:
Allostasis four-stage NFT model for neurophysiological regulation and adaptation.
(1) Brain as a regulator: A: Perceive (current internal and external state), B: Predict (output patterns, future internal & external state), and C: Regulate (determine an efficient trade-off or set-point). (2) Brain as an effector: Adapt (respond efficiently and flexibly to demands).
A new allostasis-based framework for understanding NFT
The concepts of homeostasis and classic control system theory have long been used to describe physiological self-regulation through biofeedback. However, it has become clear that homeostasis, at least as understood by the control system theory, is unable to explain how control is acquired and how experience contributes to self-regulation. Yet, this is paramount for an understanding of the mechanisms of biofeedback in general, and of NF in particular. We, therefore, propose to frame NFT within the concept of allostasis. As Sterling (2020) highlights, physiological regulation based on allostasis does not come cheaply; “a brain must collect, process, store, and retrieve immense quantities of information from the external environment and also from the internal environment—from the body itself”. In this review, we argue that successful self-regulation via NFT involves processes that can be organized into four subsequent stages, each of which builds upon its predecessor(s).
In our framework (Figure 4), until an initial (current) set-point is valid, a system based on homeostasis (gray box) maintains its stability, that is, a trade-off between demands and the current internal state. For example, during a brisk walk, the heart rate is kept at roughly 100 bpm, with occasional fluctuations around this set-point. However, when there is a new demand due to new conditions, a system founded on allostasis—by applying prior knowledge—introduces a new physiological set-point in accordance with the new conditions. In the model developed by Sterling (Sterling 2018; Sterling and Laughlin 2015), the detection of new demands prompts a process in which information (internal [sensory data] and external [disturbance in ambient heat and/or behavioral outcomes]) is compared to the patterns (the last set-point successfully determined for such conditions) stored in memory to obtain a historical background. If a difference between this information and the existing data and patterns is found, then the memory is updated. These processes allow the organism to predict the internal and external state of the body and its future output. Following these predictions, the brain determines an efficient trade-off between demands to help the body remain in balance. Returning to the above example (i.e., a brisk walk), seeing a steep incline ahead leads to an adaptive, anticipatory change in heart rate, and a new set-point of 130 bpm. The system tries to maintain this new set-point (through homeostasis) until a new demand is encountered or anticipated. In the following, we discuss how these notions may inform the conceptualization of NFT.
Importantly, the brain plays two roles during NFT: as an effector (defined as an organ or cell that acts in response to a stimulus) and as a regulator (by integrating the information of the internal and external milieu to adapt a set-point to meet a trade-off between demand(s) and the current internal state). During biofeedback, the ability to regulate the functions of an effector, such as the heart, lungs, or sweat glands, is acquired by first measuring and then feeding back the activity of this effector. Thus, in NFT, the brain plays two roles: first as a regulator of the body (and itself), which might occur by perceiving the error signal(s) and sending corrective information for regulation, and, second and simultaneously, as an organ or effector, which may occur by responding to the corrective information by up- and/or downregulating the brain activity.
This framework also allows us to distinguish two different time scales of adaptation that relate to within-session and between-session changes. Regarding the immediate, short-term adaptations, the framework predicts that, during an NFT session, the system tends to regulate based on an allostatic state by reinforcing fluctuations in brain activity that follow a desired direction. This reinforcement can be understood as an adaptive change of the set-point in response to new demands. Once an NFT session is over, the system is expected to return to the previous allostatic state; accordingly, the power of the trained frequency band might be similar to its presession measurement (Kober et al. 2015). However, the system might also adapt to a new allostatic state matching the new demand(s), which would be a response to the new internal and/or external demands induced by conditions after an NFT session.
During the initial stages of an NFT session, the regulation process consists of (a) perception and (b) adaptation. A new set-point is introduced into the system externally (by setting the feedback goal; e.g., lowering alpha power), and the brain, as the regulator, perceives the error signal(s) and then, as an effector response to corrective information, modifies its function (i.e., adaptation), which, in the case of EEG-NFT, occurs by up- and/or downregulation of power at the targeted frequency or frequencies. In this step, when a new set-point is defined for the system, the stability of the system’s neurophysiological states is based on the homeostasis framework (this step is shown in the gray box in Figure 4). Gradually, the brain—as the regulator—acquires the ability to predict the outcome based on the error signal(s), corrective information (i.e., a command sent to an effector), and the resulting adaptation(s). The regulation process develops to perception, prediction, and adaptation accordingly.
Regarding long-term adaptations, NFT can be conceived of as optimizing neurophysiological regulation to meet new demands in four stages. We have already explained the three stages (perception, prediction, and adaptation) of the regulation process that are thought to occur during NFT under laboratory conditions. However, a permanent adaptation after the intervention process has ended might occur if the brain, as regulator, determines a suitable set-point with respect to the internal and/or external state of the body and its future output. Thus, the perception, prediction, and adaptation model requires further development in the form of an additional step that leads the brain as the regulator after its (a) perception, (b) prediction, to (c) determining an efficient trade-off between demands to help the body remain in balance, and finally, (d) as an effector adapting its function to meet the new demands (accordingly, the regulation process develops to perception, prediction, determination, and adaptation). In this article, the name “allostasis four-stage model of NFT” will be used to address the aforementioned stages for neurophysiological regulation and adaptation.
One prediction from the model is that in the initial stages of NFT, the power fluctuations at a trained frequency are assumed to be spontaneous, without any volitional control from participants, yet meeting the current intrinsic set-point. It would then be possible for the brain, by connecting the reward to some specific physical or mental state, to gradually predict the next rewarding outcome, using mechanisms of associative or operant learning. Thus, extensive NFT aims to enable a participant to predict and regulate physiological and mental states, in the light of expected external demands and future outcomes. A final prediction of the four-stage allostasis framework is that repeated NFT and the associated volitional changing of set-points facilitates future shifts in set-points through practice effects: Within and across NFT sessions, new set-points are expected to be more readily implemented as training progresses because of learned flexibility in coping (adaptation) and increased application of prior knowledge (experience). Thus, passing through the hypothetical four stages (i.e., perception, prediction, determination, and adaptation) is expected to assist participants in regulating their behavior more and more easily and quickly.
Previous studies (Kamiya 1962) of NFT mechanisms have also focused on the participants’ ability to better (a) perceive the current mental state, (b) predict whether they are in the required mental state, (c) determine a set-point which meets the required demand, and (d) quickly adapt brain activities to the desired (“set”) state. These four stages (perceive, predict, determine, and adapt) are incorporated into the present allostasis four-stage NFT framework.
Support for the four-stage framework comes from several sources. For example, in a seminal study of NFT, Kamiya (1962) first gave participants the opportunity to be aware of fluctuations in their own EEG alpha activity (spectral power changes in the range between 8 and 12 Hz) and eventually found that participants could readily perceive changes in their EEG alpha activity without feedback. Thus, providing feedback on a specific neurophysiological state appears to facilitate the perception of that state, potentially along with its cognitive correlates. Afterward, the majority of participants were able to voluntarily increase the spectral power of the alpha rhythm based on instruction (Gruzelier and Egner 2005). These findings support the notion that the ability of participants to deal with a new demand can be developed and improved by NFT. Such a regulation can best be explained within the framework of allostasis; brain activity not only fluctuated around a given set-point (“homeostasis”) but participants learned to change the set-point upon request and based on their experience (“allostasis”).
A broader perspective was adopted by Sorger et al. (2018), who showed that a protocol combining mental strategies and continuous feedback of their BOLD signal level (real-time fMRI [rtfMRI] NFT) further improved the ability of participants to learn self-regulation. Participants were instructed to consecutively self-regulate the level of regional brain activation to reach 30, 60, or 90% of their maximal capacity by implementing a selected activation strategy. The task was conducting a mental task, such as inner speech, in concomitance with modulation strategies (e.g., applying different speech rates). Most of the participants gradually showed the ability to self-regulate so as to change the regional brain activation in at least two of the target levels, even in the absence of NF (Sorger et al. 2018).
In line with these findings, the present framework states that NFT involves learning relations between demands and set-points (“perception and prediction”) by learning to apply memorized patterns (experience), to determine efficient set-points, and to quickly adapt brain activity to the desired (“set”) state. To the extent that the model is focused on the level of behavioral and physiological processes, it should not depend on a particular measurement on which the NFT is based. In fact, based on the allostasis four-stage model of NFT, the structural and functional plasticity/changes, which are the expected consequences of NFT, can now be better specified and examined, ideally using a range of neural measurement modalities. When the brain (as an effector) adapts to new set-points, this adaptation leads to structural plasticity. When the brain (as a regulator) is involved in perception and prediction by applying prior knowledge gained through NFT, functional plasticity develops by refining and improving neural networks. The following sections present and discuss the theoretical framework around neural plasticity and the evidence regarding its development through NFT.
Neural mechanisms underlying NF
Among the substantial research efforts devoted to the application of NF, there are very few investigations into the neural changes accompanying NF interventions (Bluschke et al. 2016; Papo 2019). Neural plasticity is a broad term and is often applied to a wide range of processes, by means of which neurons (but also glia or blood vessels) change in form (structure) and/or function in response to experience, including changes in the environment or damage to the brain itself (Kaas 2001, p. 10,542). Thus far, based on the allostasis four-stage model of NFT, we have argued that, when training the brain during NFT sessions to adapt to new set-points, these adaptations might lead to different types of structural plasticity. Moreover, we argue that the functional plasticity may develop during NFT when the brain perceives and predicts changes to the internal organs and the environment by applying its previous knowledge to more efficiently determine a new set-point.
Structural plasticity
Adaptive changes in brain structure in response to internal (e.g., learning) and/or external (e.g., changes in the environment) challenges, which is assumed as a result of physiological regulation, can be observed in the activity of the synapses, the activity of neuromodulators (neurotransmitters), and in microstructural changes of the neurons and their sheaths (Cohen et al. 2017). In the case of NFT, structural plasticity would be supported by findings of neural change following intervention: For example, NFT may prompt alteration of the neuron firing rate.
Synaptic modification
Neuronal activity is itself a key physiological factor which is subject to both homeostatic and allostatic regulation. In order to attain a state in which they can form and maintain constant activity patterns throughout an organism’s life, billions of neurons wire themselves into intricate networks during brain network development. These circuits are not static, but rather constantly adapt to enable organisms to store information and modify their behavior in a changing environment (Turrigiano 2012). One major mechanism mediating these adaptive network dynamics is represented by the synaptic currents transferred between neurons (Sanei and Chambers 2007). Importantly, these currents as an excitatory or inhibitory postsynaptic potential strengthen or weaken over time in response to changes in the temporal rate and changes in spatial and temporal patterns of engagement. Together, many processes prompting changes in synaptic processing are broadly referred to as synaptic plasticity, which has been extensively discussed as a potential basis for learning (Gerstner 2011). Most contemporary conceptions of synaptic changes and learning build upon the principles first proposed by Hebb (1949), which stated “neurons that fire together wire together” (Markram et al. 2011).
The literature on associative learning (of which classical and operant conditioning are two major types) holds that, in associative synaptic plasticity, concurrent or rapid consecutive activation of two synaptically joined neurons result in a modification in the strength of the synapses binding them. Synaptic plasticity has been suggested as a foundation for acquiring knowledge and memory (Feldman 2012). Long-term potentiation (LTP) is a candidate mechanism for explaining the processes underlying associative learning (Sitaram et al. 2017). Recent studies have focused on a form of LTP called spike timing-dependent plasticity (STDP). In essence, STDP is an asymmetric function that relies on the progression of firing times of presynaptic and postsynaptic neurons (Sitaram et al. 2017).
NFT could also be framed within Hebbian forms of plasticity, such as firing rate and synchronization (Ossadtchi et al. 2017). Using intracranial recordings—not susceptible to some of the problems associated with measuring neural mass activity through scalp, skull, and cerebrospinal fluid, as is the case in EEG—animal studies have shed insights on the neuronal basis of learning as a result of NFT. For example, in a study with rats, Arduin et al. (2013) recorded the activities of neurons in the motor cortical area. The aim was to monitor and control the neuronal activity in the motor cortical area by using a linear actuator connected to a water bottle. To obtain the reward (i.e., water), the rats had to maintain the firing rate of a neuron above a certain level. The firing rates of conditioned neurons immediately rose after a trial had begun and, in a very short time, a bottle entered the drinking zone. Moreover, the conditioned neurons fired almost simultaneously, more often, and stronger than the adjacent neurons that were recorded at the same time close to the conditioned neurons. The authors determined that only the neurons that were rewarded (operant-conditioned) showed a significant rise in firing rate, and they also prompted pronounced modulations of firing in neighboring neurons, forming a local neural network (Arduin et al. 2013). In studies using human participants, Ossadtchi et al. (2017), for the first time, investigated discrete structural characteristics of EEG patterns and showed that NFT leads to an increase in the incidence rate of spindles at the trained frequency.
To conclude, first, it is now widely accepted that cortical plasticity (either in short-, medium-, or long-term) enables the brain to flexibly adapt to changes, optimizing its ability to meet environmental and behavioral demands. Such modifications could be framed within the allostatic framework where the brain (acting as the system’s regulator) is highlighted as a dynamically adapting interface between the changing environment and physiological regulation and adaptation. Second, by considering the allostasis four-stage model of NFT (due to frequent adaptive response to new set-points), we have argued that NFT affects neuronal activity (through parameters such as LTP and STDP) in the short-term but is also thought to change the strength of synaptic connections (referred to as “synaptic weight”), a form of neuroplasticity which has been linked to associative learning. The following section addresses the roles of neurotransmitters in reinforcement learning (operant conditioning) and, more specifically, in NFT.
Neurotransmitters: interaction and modification
One conclusion frequently drawn from the research on STDP is that, if a synapse active just prior to a spike event increases in efficacy, then a synapse that is only active after the spike therefore decreases efficacy. A question arises whether the mere association of presynaptic input and postsynaptic spiking activity is sufficient to induce synaptic efficacy. One possible answer to this question has been proposed in the context of reward-mediated learning (Miller 1981; Wickens 1990). Beyond pre- and postsynaptic activity, some theoretical studies have suggested that a “third factor” might be involved in the network that enabled both the temporal and the spatial selection of specific inputs (Pawlak et al. 2010).
Theoretical and computational studies have proposed that neuromodulators represent such a third factor for selecting particular active inputs to a neuron in an active network. One of the most important neuromodulators that has been investigated is dopamine, which appears to impact timing-dependent plasticity within a number of areas of the brain (Bissiere et al. 2003; Pawlak et al. 2010).
Dopamine links STDP to behavioral modifications by inducing plasticity at corticostriatal and cortical synapses (Gallistel and Matzel 2013; Sitaram et al. 2017). Behavioral research on animals and humans in which dopamine transmission was experimentally restricted has specifically linked motivational effects to dopaminergic projections from the nucleus accumbens to the frontal cortex. This network appears to be crucially involved in the use of reward information for learning, maintaining, and consummatory behavior (Schultz 2002). In classical and operant conditioning tasks, when visual and auditory stimuli are conditioned, between 55 and 70% of dopamine neurons are activated (Miller et al. 1981; Schultz 2002). For more on dopaminergic effects on operant behavior, see Kringelbach and Berridge (2016).
As discussed earlier, NFT rewards neural fluctuations in a desired direction and, consequently, positively reinforces these fluctuations. Conversely, the fluctuations in an undesired direction are punished. Thus, dopaminergic signaling may play an important role in associative learning during NFT.
Evidence from animal studies suggests that NFT effects spike the activity of brainstem dopaminergic neurons. For example, Kulichenko et al. (2009) reported that the EEG alpha/theta ratio changed during NFT in cats due to an increase in the alpha-band (8–13 Hz) and a decrease in the theta-band (4–8 Hz) of spectral power density in feline EEG recordings. The authors also observed an augmentation of the spike activity of dopaminergic neurons (Kulichenko et al. 2009). In human studies, the effect of NFT on substantia nigra/ventral tegmental area activation was tested directly by Sulzer et al. (2013). The authors reported that only participants with veridical feedback (compared to a group with sham feedback) improved their ability to upregulate dopaminergic signaling in the substantia nigra/ventral tegmental area complex. Feedback also prompted coactivation of other dopaminergic regions and augmented connectivity along the nigrostriatal pathway when compared to the control condition (Sulzer et al. 2013). In the same vein, Ros et al. (2020b) investigated the capacity of biofeedback training and NFT to release dopamine, as revealed by positron emission tomography, and reported a significant effect of both interventions in dopamine release. In this experiment, healthy participants were randomly assigned to either an NFT or electromyography biofeedback training group and were trained to downregulate cortical alpha power or facial muscle tone, respectively. Task-induced effects led to significant endogenous dopamine release in the frontal cortex and anterior cingulate cortex (ACC), but not in the thalamus (Ros et al. 2020b).
In addition to establishing the role of dopamine in neuroplasticity, research has highlighted an essential role for glutamatergic signals. Glutamate has been recognized as the major excitatory neurotransmitter in the CNS of mammals and is, therefore, essential for all mammalian behaviors, particularly with regard to learning and memory (McEntee and Crook 1993). Recent studies show that postsynaptic glutamate receptors can be regulated dynamically by excitatory synapses, displaying time-varying changes in synaptic efficacy as seen, for example, in LTP and long-term depression (Purves et al. 2001). Experimental evidence has shown location-specific increases in glutamate and glutamine concentration when transcranial Direct Current Stimulation (tDCS) was applied during a challenging visual search task (Clark and Parasuraman 2014). This is indirect proof for other kinds of neural stimulation techniques, such as NFT, which have changed behavior.
In summary, due to contingent feedback, dopaminergic projections to the striatum might enable a behavior to be modified in reaction to relevant stimuli and contingent feedback. In addition, an increase in glutamatergic transmission, a major excitatory transmission of the brain, has been reported to be a consequence of neural stimulation. Overall, this evidence provides direct insights into reinforcement- and NF-learning mechanisms, meaning that synaptic changes are involved in the learning process. The section below examines the changes in gray and white matter due to NFT, another element of structural plasticity.
Gray and white matter modification
For a long time, it was widely believed that the brain, having reached adulthood, was an anatomically and physiologically static organ (van Boxtel and Gruzelier 2014). However, evidence became available that the brain possesses self-organizing principles, which means that neural systems are modifiable networks, and training in adults can lead to changes in neural structure (Hölzel et al. 2011). These structural changes are evident in the case of self-regulation (Hölzel et al. 2011; Tang et al. 2012), as well as in the acquisition of abstract information (Draganski et al. 2006), motor skills (Draganski et al. 2004), cognitive skills (Ilg et al. 2008), and physical training, such as aerobics, over a period of time (Colcombe et al. 2006). Regarding the nature of adaptive structural plastic changes in the neuronal circuits of the brain caused by self-regulation, neuroimaging studies showing changes in white and gray matter are driving factors of self-regulation (e.g., here mindfulness training was applied to demonstrate the structural changes in the brain, Hölzel et al. 2011; Tang et al. 2012). In NF studies, increases were also reported in fractional anisotropy in white matter pathways and gray matter volume. For example, in a study by Marins et al. (2019), participants were trained to brain patterns related to motor execution while performing a motor imagery task, with no overt movement. Although (rtfMRI) NFT lasted less than 1 h, results showed an increase in fractional anisotropy in the sensorimotor segment of the corpus callosum and increased functional connectivity of the sensorimotor resting state network. The authors, however, did not observe these results in the control group, which was trained with sham feedback (Marins et al. 2019; Ghaziri et al. 2013). It has now been established that there is an inseparable connection between the components of neural regulation, that is, brain function and structure (Ros et al. 2014). Thus, a successful NFT which causes functional changes in the brain is expected to positively induce some structural changes in the brain. To date, however, there has been relatively little evidence supporting the notion that changes in brain activity after NFT are accompanied by microstructural changes in the white matter pathways and gray matter volume (Ghaziri et al. 2013; Hohenfeld et al. 2017; Munivenkatappa et al. 2014; Papoutsi et al. 2018). This point has also been made in a recent special issue on NF, “despite the mounting evidence of the impact of NF on brain function and behavior, the impact of NF on brain structure remains to be fully explored” (Hampson et al. 2020).
This section has examined another level of neural plasticity—structural adaptive neuroplasticity—which could be caused by NFT. These changes seem to be necessary for at least a functional rewiring. It has been argued that NFT involves multiple aspects of mental functions that use multiple complex interactive networks in the brain (Gaume et al. 2016; Sitaram et al. 2017), which will be discussed in the following.
Functional plasticity
Adaptive plasticity in brain function in response to internal (e.g., brain damage) and/or external (e.g., changes in the environment) demands can be observed through strengthening, weakening, adding, or pruning in the connectivity of different brain regions (Cohen et al. 2017).
With reference to our model, and based on other studies that synthesize anatomical and functional brain studies and provide evidence that a large-scale brain system supports allostasis (see e.g., Kleckner et al. 2017), we argue that a better self-regulation induced by NFT can be explained by its reliance on modifying the required structural and functional plasticity. In the case of NFT, functional plasticity would be supported by findings of modification in the connectivity between different regions following intervention. For example, NFT may result in an altered network configuration.
Neural underpinnings of self-regulation
Recent developments in the field of neuroscience have helped to expand our understanding of the neural underpinnings of self-regulation. Early examples of research on this topic addressed the possibility that the functions assumed for the supervisory attentional system (controlled processing) correspond to the prefrontal areas described by Luria 1966 as responsible for the execution and regulation of behavior (Banfield et al. 2004). We will explore two key functions involved in NFT: executive functions and memory. Executive functions include high-order cognitive abilities such as working memory, inhibitory control, and the flexible volitional shifting of the focus of attention, all of which provide a foundation for reflection on experience, reasoning, and the purposeful regulation of behavior (Blair 2016). We have examined data that help to determine which brain areas are involved in these functions, how these areas are implicated in the self-regulation process, and how these areas interact during NFT.
Executive function
All cognitive processes related to self-regulation, monitoring, initiation of activity, use of feedback, and more, are thought to be an enveloping process of the executive functions (Cannon et al. 2007; Sohlberg and Mateer 1989). It has long been known that various sectors of the prefrontal cortex (PFC) circuits are implicated in executive functions: the dorsolateral PFC (DLPFC), the ventromedial PFC (VMPFC), and the ACC (Banfield et al. 2004). Thus, the mapping of anatomical connectivity patterns underlying regions of the PFC is crucial to comprehend how these regions work together to make self-regulation feasible.
The DLPFC has been shown to play an important role in addressing the issue of cognitive processes, for example, actively retaining information in working memory (Duncan and Owen 2000), changing behavior according to task demands (MacDonald et al. 2000), or representing past events, current goals, and future predictions (Miller 2000). Given these issues, research suggests that activation in DLPFC is linked with behavioral self-regulation, for example, the selection and initiation of actions (Banfield et al. 2004; Spence and Frith 1999). This idea is supported by evidence that shows elevated activation in DLPFC when participants successfully engage in self-control (Hare et al. 2009).
The VMPFC demarcation in neuroimaging studies shows its strong interconnection with the limbic structures involved in emotional processing, and the orbitofrontal cortex (OFC), which is a part of VPFC, has been suggested as one contributor to emotional processing (Pandya and Barnes 2019), reward and inhibition processes, real-life decision making (Hernandez et al. 2009; Rolls 2000), self-awareness (Stuss 1991; Stuss and Levine 2002), and strategic regulation (Levine et al. 1998; Miller and Cohen 2001). The ACC is a specialized medial prefrontal region that consistently interacts with the PFC in monitoring and guiding behavior (Gehring and Knight 2000) and is thought to be part of a circuit that regulates both cognitive and emotional processing.
The ACC is functionally split into ventral (affective) and dorsal (cognitive) regions, which have distinct cytoarchitectures, connectivities, and functions (Vogt et al. 2005). Located between the neocortex and the limbic system, the ACC is well-positioned to serve as an interface between cognition and emotion. This region contains spindle-shaped neurons allowing for widespread connections to other brain areas. The ACC areas have extensive connections with the insula, PFC, amygdala, hypothalamus, and brainstem. Via these projections, the ACC controls sympathetic and parasympathetic functions (Hurley et al. 1991; Ter Horst et al. 1996; Terreberry and Neafsey 1987; Verberne and Owens 1998). Accordingly, the ACC is strongly involved in issues of self-regulation (Awh and Gehring 1999; Botvinick et al. 1999; Carter et al. 2000; Posner and Rothbart 1998). Research has established a role for the ACC in decision making and behavior monitoring (Bush et al. 2002; Elliott and Dolan 1998; Liddle et al. 2001), reward-punishment assessment (Knutson et al. 2000), and initiating the selection of an appropriate novel response among several alternatives (Raichle et al. 1994), performance monitoring (MacDonald et al. 2000), action monitoring (Gehring and Knight 2000; Paus 2001), detecting or processing response conflict (Gehring and Fencsik 2001), detecting and processing errors (Carter et al. 1998; Kiehl et al. 2000; Menon et al. 2001), error outcome and predictability (Paulus et al. 2002), and internal cognitive control (Wyland et al. 2003). Clearly, dysfunction within the ACC can disrupt self-regulatory processes on several levels.
This section has focused on three different sectors of the PFC and their role in self-regulation. First, the DLPFC has been shown to play a crucial role in key aspects of executive function essential for planning behavior and maintaining regulatory goals. Second, the VMPFC shows a strong interconnection with the limbic structures involved in emotion, receiving rewards, the inhibition process, and strategic regulation. Third, the ACC has been shown to be involved in monitoring signals that are required for control systems to regulate behavior. The function of memory in self-regulation will be investigated in the following.
Memory function
One further factor that must be considered when investigating brain function and self-regulation is the role of memory as key to predictive regulation (i.e., regulation based on the concept of allostasis). For instance, it has been argued that the hypothalamus, as “mission control” for allostasis, receives not only its information from myriad sensors of the external and internal states, but also information from memory (Sterling 2020, p. 75). With respect to reinforcement learning, research has also suggested that the ubiquitous and diverse roles of memory may function as a part of an integrated learning system (Gershman and Daw 2017).
As pointed out earlier, working memory operations, such as maintenance and updating of relevant information, are an essential element for executive functions. Data from several studies suggest that successful self-regulation entails the representation of goals and goal-relevant information (Kane et al. 2001; Miller and Cohen 2001). Working memory directly serves the active mental representation of an individual’s self-regulatory goals (retrieved from long-term memory) and the means by which these goals can be attained (Hofmann et al. 2012; Miller and Cohen 2001). Researchers have argued that, without an active representation of such goal-related information, self-regulation is directionless and bound to fail (Baumeister and Heatherton 1996), unless individuals have fully habitualized and have automatic self-regulatory routines at their disposal (Fishbach and Shah 2006; Gollwitzer and Brandstätter 1997).
In the following, the empirical evidence on how these regions are activated and modified during and after NFT will be reviewed.
Empirical evidence for functional plasticity due to NFT
Turning now to the experimental evidence on the regulation of brain functioning through NFT, preliminary evidence suggests that learning self-regulation of brain activity through NFT can lead to changes in functional connectivity. This view is supported by the hemodynamic response in different brain regions, such as the dorsal ACC, the thalamus, and the lateral PFC, which have been associated with implementing (trying to voluntarily control feedback from various brain signals) and/or learning NF (see Emmert et al. 2016 for review). By way of illustration, Paret et al. (2018) investigated the neural signatures of feedback monitoring and controlling when participants were provided with continuous rtfMRI NFT from the amygdala. During feedback monitoring, the researchers reported activation in the thalamus, VMPFC, ventral striatum, and rostral PFC. Feedback controlling, on the other hand, engaged the ACC, lateral PFC, and insula. Moreover, Paret et al. (2018) observed an overlap in the thalamus and ventral striatum activations, meaning they are also involved in feedback controlling. Similarly, Zotev et al. (2011) showed how the functional connectivity between a single region of interest and regions that were interacting changed significantly across the rtfMRI NFT. Participants in the intervention group were provided with ongoing information about blood oxygen level dependent (BOLD) activity in the left amygdala and were instructed to raise the BOLD rtfMRI signal by contemplating positive memories. A control group was given the identical activity. However, participants received sham feedback based on the activity of the left horizontal segment of the intraparietal sulcus, a region not thought to play a role in emotion regulation. A significant increase in the BOLD signal due to rtfMRI NFT at the left amygdala was reported only for the experimental group. This effect persisted during the transfer run without NF. A functional connectivity analysis of the amygdala network also revealed significant widespread correlations in a fronto-temporo-limbic network. Additionally, the authors detected six regions—the right medial frontal polar cortex, bilateral dorsomedial PFC, left ACC, and bilateral superior frontal gyrus—where the functional connectivity with the left amygdala rose substantially during the rtfMRI NFT runs and the transfer run. These activation patterns have also been observed with slow cortical potential NFT. Raised BOLD responses have been found in the dorsal anterior cingulate gyrus, the anterior insula, middle frontal gyrus, and the supplementary motor area when participants experience NFT in the form of surface-negative slow cortical potentials (increased cortical excitation), whereas positivity (decreased cortical excitation) was associated with widespread deactivations (Hinterberger et al. 2003). These responses have also been reported in the case of sham NFT. In a recent fMRI study by Ninaus et al. 2013, participants thought that they were receiving a valid feedback but, unbeknownst to them, were instead watching a realistic video of an NFT session inside the fMRI scanner. In a passive viewing condition, participants were ordered to only watch the bar movements but not to attempt to control them. Participants in the active condition were expressly requested to control their brain activation so that the moving bar remained as high as possible—a normal task in NFB studies. When differentiating between the passive and active task conditions, the ACC, the anterior insula, the middle frontal gyrus, and the supplementary motor area were strongly activated in a bilateral manner, which reveals the areas of the brain involved in supervisory control. Regarding the function of memory, some have argued that, when participants explore different cognitive strategies in an attempt to control the NF signal, they must remember a history of behaviors over time and determine which behavior was responsible for influencing the feedback signal (Oblak et al. 2017). The hippocampus is apparently involved in recalling information, particularly for episodic memories (Shirvalkar 2009). The consolidation of the long-term memory, stored in cortical networks, results from the reactivation of the assembly due to operant conditioning. In this phase, the VMPFC and the hippocampus may work together to form schema and possibly represent semiconsolidated schemata (van Kesteren et al. 2010). Finally, a recent meta-analysis literature review (including 12 experiments that investigated 9 different target regions for a total of 175 participants and 899 NFT sessions) suggested that the anterior insula and the basal ganglia, in particular, the striatum, were constantly active throughout the regulation of brain activation across the experiments. Moreover, this study showed additional activations in the ACC, the dorsolateral and ventrolateral PFC, the temporo-parietal area, and the visual association areas, including the temporo-occipital junction (Emmert et al. 2016).
Surprisingly, these kinds of activation do not seem to be limited to methods of NFT; for example, a newly emerging NF protocol for ADHD utilizes functional near-infrared spectroscopy (fNIRS) based on oxygenated hemoglobin (O2Hb) activity in the PFC, an area traditionally implicated in the disorder. O2Hb activity also reflects activation of the underlying brain regions where chromophores are most strongly correlated with the BOLD response synonymous with fMRI studies (Hudak et al. 2018). In accordance with these findings, recent consensus about NFT has indicated that, regardless of which signal is used for feedback (e.g., blood flow, oxygen consumption, or electrical activity), mechanisms other than NF-specific factors account for the effects of NFT (Ros et al. 2020a).
As mentioned above, the aim of this section was to track the effects of NFT on the regions crucial for self-regulation. These effects could be considered as being functional plasticity in interaction with the change in the level of self-regulation. As expected, the brain regions, such as VMPFC and ACC, which are identified as playing a role in executive and memory functions, regardless of trained frequency and location, showed activation during and after NFT termination. The evidence suggests that NFT enables the manipulation of neural activity in circumscribed regions in the form of trained regions and, accordingly, might drive some functional connection activities in other brain regions. The ability to enhance neural dynamics at a network level with NFT may be a better method for neural regulation than NFT involving one area. This widespread activation by NFT appears to be required for brain and behavioral regulation (Gaume et al. 2016; Ros et al. 2014; Sitaram et al. 2017).
Intermediate summary—neural mechanisms underlying NFT
We have proposed that NFT should be framed within the theoretical framework of allostasis: NFT may alter the state (set-point) of brain activity. The alterations in brain oscillatory activity and connectivity induced by NFT may be produced by structural and functional plasticity at different levels of analysis, ranging from molecular- to system-level changes: NFT-based plastic changes may include synaptic modification, alterations in gray and white matter, and may also promote measurable changes taken to indicate functional plasticity. This assumption is in line with the recent publications suggesting that behavioral changes induced by rtfMRI NFT can be explained through two plasticity models: One refers to the targeted neural plasticity model, which postulates plasticity at the neuronal level within a target area for rtfMRI NFT. The other refers to the learned modulation model, which proposes modulation of the target area by other nontarget areas learned through rtfMRI NFT (Shibata 2021; Watanabe et al. 2017). Quantifying such changes is needed in order to rigorously investigate the neural mechanisms and, ultimately, the effectiveness of NFT. Such an objective and quantitative approach may also address some concerns regarding the external and internal validity of NFT (Papo 2019; Schabus 2017; Thibault and Raz 2017).
In the neuropsychophysiological literature, the association between self-regulation and the activation of different brain regions is discussed, and the activations and modifications of these regions are also examined in the NFT literature. Interestingly, the brain regions discussed earlier in the section on neural mechanisms underlying NF involved in neurophysiological regulation are similar to those that other researchers (such as Stephan et al. 2016) have suggested to be involved in homeostasis and allostasis regulation. For example, in their analysis of neuroanatomical circuits, Stephan et al. (2016) proposed that the anterior insular cortex, ACC, subgenual cortex, and OFC play an important role in homeostasis, allostasis, and interoception. The authors argued that these regions, which they call “visceromotor areas”, are situated at the top of this circuit, embodying a generative model of (potentially different types of) viscerosensory inputs enabling a biological agent to infer current bodily states and predict future states. These visceromotor areas form the basis for allostatic predictions.
However, the research in this field is generally limited to specifying whether changes in the structures and functions of the brain lead to self-regulation and, if so, to what extent the concept of self-regulation can be attained, or whether participants have no control over alterations of the brain after NFT. We should bear in mind that structural and functional alterations of the brain may also happen through techniques that externally stimulate neurons (vs. NFT that internally stimulates the neurons), such as rTMS and tDCS, without volitional control on up- or downregulation of neural activity as a consequence of learning self-regulation. Historically, the term “self-regulation” has been used to describe volitional control of one’s own thoughts, feelings, and behaviors to reach certain goals. Thus, it is assumed that the alterations in the brain oscillatory activity induced by NFT should be under the volitional control of the participants after the termination of training. In the field of NFT, it has thus far been shown (by Joe Kamiya) that participants were, after the termination of NFT, able to voluntarily increase the incidence of the trained frequency (i.e., alpha waves) based on demands (e.g., a researcher’s request). However, the participants were not able to say how they produced that mental state (Thompson 2004). Similarly, in a magnetoencephalography (MEG) NF study, Bagherzadeh et al. (2020) sought to shed light on the causal role of alpha synchrony in attention and showed a decrease in alpha corresponding to the enhanced sensory processing; the participants were trained to manipulate the ratio of alpha power over the left versus right parietal cortex and found that a comparable alpha asymmetry developed over the visual cortex, such that a persistent bias in attention in the expected directions was observed after termination of the training. Volitional control of up- and downregulation, however, was not reported if it was tested.
Regarding the neural mechanisms of NFT, we have provided examples and evidence from studies applying EEG and fMRI-NFT as well as from studies applying the less used MEG and fNIRS-NFT. We showed that NFT is able to trigger synaptic modifications that lead to a firming of neural circuitry (Davelaar et al. 2018; Niv 2013; Ros et al. 2014). These modifications (contraction and/or expansion) are required for functional changes at the neural network level, for example, the default mode network (Mayeli et al. 2020; Russell-Chapin et al. 2013), and/or regions that are involved in the executive function, such as DLPFC, the region that is involved with changing behavior through incorporating past events, current goals, and future predictions. Based on our model, we argue that changes on the structural and functional levels are due to an adaptation to new set-points. Such changes are generally required for predictive regulation (based on the concept of allostasis) and efficient self-regulation.
From mechanisms to outcomes—effects of NFT
The aim of this article is to introduce a new framework for NFT. In an allostasis four-stage model, NFT involves (a) learning relations between external demands (first, the feedback stimulus; later, the internal and external cues) and set-points (desired brain states), (b) learning to apply learned patterns (experience), (c) determining efficient set-points, and (d) adapting brain activity to the desired (“set”) state. As reviewed above, successful NFT appears to improve perception of internal states, as well as their prediction, and ultimately, adaptation through structural and functional plasticity. When the brain, as an effector, adapts to new set-points, this leads to structural plasticity such as synaptic modification and alterations in gray and white matter. In addition, once the brain (as a regulator) applies prior knowledge (experience) for perception, anticipation, and adaptation, this promotes measurable changes taken to indicate functional plasticity. This theoretical framework of NFT has not previously been described, and its main goal is to provide novel hypotheses in the pursuit of issues currently debated in NFT research. The most obvious conclusion emerging from this review is that NFT enables the system to implement appropriate set-points. This ability can form on the basis of more precise perception and prediction. In addition, NFT enables the system to deal and adapt more efficiently to a new set-point by mechanisms of learning, that is, by applying the experience gained and developed during the training.
In the following section, we discuss how framing NFT within the allostasis four-stage model can further our understanding of NFT and how the model can address currently debated issues. We will also present predictions derived from the model that may be used to test the model.
Changes in baseline (sustained changes) or adaptive changes?
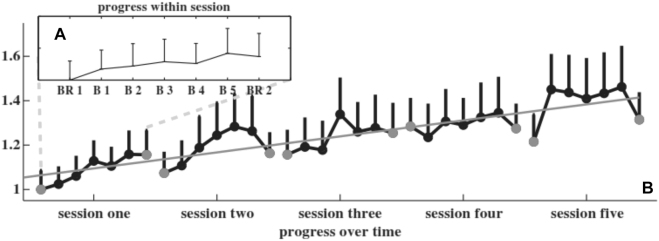
Traditionally, the effectiveness of NFT has been expected to be shown through sustained changes in the power of the trained frequency across intervention sessions, even in resting baseline measurements. That is, when a trained frequency is upregulated, the increase in the power of that frequency remains stable; conversely a reduction in the power remains stable when a trained frequency is downregulated. By way of illustration, Zoefel et al. (2011) applied NFT to reinforce an individually determined upper alpha frequency, ultimately to improve cognitive performance. In the course of the training sessions, they recorded a substantial linear increase in the upper alpha amplitude (Figure 5). In the final training session in both pre- and postintervention, the alpha amplitude was higher than that in the very first pre- and postintervention, respectively. Doubt has recently been cast on the validity of this continuous progression or increase as a valid marker for the effects of NFT (Witte et al. 2018). The authors argued that the nature of NFT is reflected in participants having attained the ability to (self-) regulate instantaneously. Thus, a trainee in an EEG-NFT intervention may learn to quickly regulate brain activations with less conscious cognitive effort (Witte et al. 2018), but not to constantly regulate these activations.
Figure 5:
Progress within and across sessions.
Taken from Zoefel et al. (2011).
The four-stage model of NFT can now more precisely explain these changes in the rest condition. One key assumption of allostasis is that a set-point changes as a result of demands. However, in the rest condition, which is an inherently stable condition with few variations in the environment, there are no such changing demands. Why should the set-point then change?
To answer this question, two different conditions for NFT should be considered. We assume that the aims and outcomes of NFT differ between allostatic (over)load states (e.g., clinical applications) and allostasis states (e.g., nonclinical applications). In this regard, in an allostatic (over)load state, such as in ADHD or a major depressive disorder, the brain activity appears to be dysregulated. By contrast, in an allostasis state (e.g., a performance-enhancing application), the brain activity is generally well-regulated. Accordingly, when NFT is applied, the researchers should consider the condition of the participants and the purpose of the intervention. Skouras and Scharnowski (2019) have also recently highlighted whether self-regulating brain oscillations and function would be identical between individuals with a history of psychiatric pathology and healthy participants. These conditions will be explained in more detail below.
NFT to modify allostatic (over)load states (improving clinical conditions)
When NFT is applied to treat a patient, the aim is to ameliorate the symptoms and to modify brain functioning toward a healthy condition. Framed within the allostasis model, the person’s brain activity is in a condition of allostatic (over)load in which brain activity is not adaptively changing according to demands (McEwen and Wingfield 2003). Whereas, in a healthy system, changes to the set-point occur adaptively; maladaptive functioning leads to health problems. For example, it has been argued that perceptions of (chronic) stress lead to “allostatic load” such that the set-point or allostatic state is not reset or turned off after some time. This can be illustrated briefly by continued elevated blood pressure in response to continued or repeated perceptions of stress which can lead to maladaptive changes like hypertension and subsequent atherosclerosis. Allostatic load can occur as a result of continuous demands or repeated “hits”, a failure to reset, or a failure to respond to demands at all (Sterling 2012). The dysregulated brain activity in persons with ADHD can be seen as an allostatic (over)load. Although the elevated theta/beta ratio may be adaptive to demands in an inattentive, unfocused state, the prolonged or permanently elevated pattern is maladaptive. In such a state, for example, Monastra (2008) has argued that theta waves predominate over the prefrontal and frontal cortex, as well as at certain midline locations, such as the vertex. Thus, in a clinical application, NFT is supposed to restore the flexibility of a system’s regulator (i.e., the brain) to vary parameters of its internal milieu and match them to environmental demands by breaking down the allostatic (over)load that has caused an unhealthy condition. The breaking down of an allostatic (over)load should be considered an extra step, or a prerequisite for an unhealthy system to become healthy, as compared to what exists in nonclinical (or optimizing) applications of NFT. In clinical applications, the focus of NFT within the four-stage model thus lies on enhancing the perception of current internal and external states, the prediction of output patterns, future internal and external states, and the determination of an efficient trade-off or set-point.
NFT to increase allostasis competence (improving performance)
In nonclinical applications, for example, to enhance the performance of athletes or artists, the system is not generally dysregulated and has flexibility to react to demands. The principal aim is, therefore, to enhance the capacity for adapting the system’s state to task demands and do so as accurately and quickly as possible. Thus, based on the allostasis model, the changes in the brain oscillatory activity in this scenario should be specific to the training sessions and time on task. In the rest condition, the brain functioning of the participant, as a nonpatient, should not be far outside the norm. These alterations in brain oscillatory activity, for example, during the execution time, are responses to changes in condition and, as a consequence, new demands.
Therefore, when NFT is employed to optimize performance, the aim is to change the brain’s oscillatory activity only during NFT sessions and while a specific task is being executed, but not apart from these conditions. For a healthy participant (with an intact brain and without any mental disorder) who can handle daily affairs without any problems, a long-term change in brain activity and function could lead to unwanted outcomes. A protocol applied to healthy participants is generally driven by brain activity linked to an optimal performance of a desired task. For example, changes have been reported in event-related synchronization/desynchronization studies regarding optimal and nonoptimal performance of a specific task (Landers et al. 1991; Ring et al. 2015). Thus, there is no need for any changes in brain oscillatory activity in other conditions, like in the resting condition. Researchers (Mirifar et al. 2019) instead theorize that participants initially learn (consciously or not) to modify their brain activity according to the aim of the intervention during training sessions, and then transfer this ability of flexible regulation to the execution time. The assumption that participants can learn to modify brain activity can probably be explained by a flexible and precise regulation function that can be induced by NFT and which accords with the allostasis framework. Thus, the focus of NFT within the four-stage model in nonclinical applications lies on enhancing efficient adaptation.
In clinical applications, by contrast, researchers can expect to observe sustained changes in a trained frequency across time. That is, gradual changes in the trained frequency occur within sessions but are also sustained between sessions even under rest conditions. In nonclinical applications, however, changes in the trained frequency should only be observed during the time of training (within sessions) and at the time of task execution—when specific demands (of a task) are high. In principle, prolonged and sustained changes of trained frequencies in the resting state (between sessions) are not expected. However, there is one exception to this rule: due to the neural efficiency induced by NFT, prolonged changes in brain activity after NFT to enhance alpha activity (7–13 Hz) should be expected. Alpha activity is indicative of the inhibition and suppression of unnecessary or irrelevant information processing, especially during the resting state. After NFT, inhibition should generally be more evident in resting conditions. Therefore, the key outcome of nonclinical applications is instead the flexibility of the system to respond to demands and the accuracy and the rate of change—efficient adaptation.
The four-stage model of NFT predicts that NFT induces a more flexible ability to self-regulate. Thus, it would be useful if researchers were to demonstrate how flexible self-regulation by means of NFT can be with regard to volitionally alternating between up- and downregulation of a trained frequency. In light of the expectations for an NFT outcome, the other issue that probably should be addressed is the relation between neurophysiological and behavioral changes that might occur due to NFT.
An explanation of the relation between the neurophysiological and behavioral changes induced by NFT
Another issue that remains controversial in the field of NFT is the interaction of neurophysiological and behavioral changes and whether neurophysiological changes are behind behavioral changes. In addition, how much do these changes depend on each other? After scrutinizing the evidence in the field of NFT, researchers (Micoulaud-Franchi and Fovet 2018; Thibault and Raz 2018) have argued that there is an ambiguous relationship between the mechanisms underlying NFT, which are (a) psychosocial, (b) cognitive, and (c) neurophysiological. A broader perspective on the discrepancy between neurophysiological and behavioral changes has recently been revealed by Tinga et al. (2019), who showed that the effect sizes of neurophysiological outcomes are smaller than those of behavioral outcomes. With reference to our model, we will now explain the interaction between the neurophysiological and behavioral outcomes.
Regarding brain and behavioral plasticity, researchers have argued that, if behavior changes, “there must be some change in organization or properties of the neural circuitry that produces the behavior” (Kolb et al. 2003). Our model incorporates a bidirectional relation between neurophysiological and behavioral changes in an NFT session, which is fundamental in operant conditioning. Our model indicates that these changes will occur simultaneously. However, they might not be proportionate over a given period of time. During an NFT session, structural and functional changes (due to plasticity) occur in the brain that are influenced by perceptions, predictions, a set-point determination, and adaptation to the set-point (demands). Simultaneously, in an NFT session, a behavior could be modified as a result of positive feedback (or reinforcement) and/or negative feedback (punishment). Studies have shown changes at microlevels (e.g., by looking at the parameters such as LTP and STDP) in the short-term, even within a single session. Significant changes at macrolevels (or the functional level), however, may only occur after multiple training sessions. In this respect, Davelaar (2020) has argued that “[a change in the functional level] operates on a timescale that covers multiple training sessions and is sensitive to consolidation processes that unfold during sleep. This stage involves updating striatal-thalamic and thalamo-cortical connections.”
Commenting on the current debate, Thibault and Raz (2018) and Micoulaud-Franchi and Fovet (2018) suggested that the effects of NFT should be interpreted through three distinct mechanisms: (a) psychosocial, (b) cognitive, and (c) neurophysiological. Psychosocial refers to “the elements involved in the motivation for and expectation associated with participating in a clinical procedure, interacting with a practitioner, and interfacing with neurotechnology”; cognition refers to “the process of actively engaging in a form of mental or behavioral training, regardless of the type or contingency of the feedback provided” (Thibault and Raz 2018).
With respect to our model, we argue that the immediate effects of NFT on the micro- and behavioral levels can be observed, although functional changes will only be observed after multiple training sessions. However, researchers should bear in mind that the initial behavioral changes are not stable and would partially be due to psychosocial and cognitive factors.
In conclusion, in an NFT intervention, the functional changes that researchers expect to observe as the specific effects of a trained protocol require time to become established in the brain. Some recent studies even suggest that effects of NFT, at neurophysiological levels, might be delayed (Linhartova et al. 2019; Rance et al. 2018). This means that the initial changes in the neural circuitry which generate a behavior are not yet well-established. The behavioral outcomes in the initial stage thus encompass unspecific and/or less well-established neurophysiological changes, as well as psychosocial and cognitive factors. Tinga et al. (2019) have recently shown that, in general, neurophysiological outcomes have smaller effect sizes than those of behavioral outcomes. This discrepancy between neurophysiological and behavioral evidence has been reported in the field of NFT. Now, with respect to the prediction that our model makes about NFT mechanisms, the brain, as a regulator, develops patterns to meet demands placed on it. Therefore, we predict that a longer intervention not only leads to specific structural changes but also to the functional changes required to (develop patterns to) modify a particular behavior. Longer interventions then enable researchers to observe changes at the neurophysiological level that are comparable to the behavioral changes.
Third conclusion—expectations about NFT process and outcomes
The third aim of this article was to discuss outstanding issues regarding expected outcomes of NFT and the reasons that might differentiate the consequences of NFT between the medical treatments (or clinical application) and optimized performance applications, and to provide ideas on how our framework may aid in solving these issues. From a theoretical perspective, we can conclude that, when NFT is applied to optimize performance, there is no reason to assume any changes in brain activity in a rest condition in the first place. Moreover, such changes would most likely even cause probable negative side effects. This may, for example, be observed in a situation in which, following NFT and in a resting condition, a healthy participant shows a consistently high level in the power/amplitude of a fast frequency band such as beta (15–30 Hz) compared to their baseline. However, we have also explained that, when NFT is applied to optimize performance, there is one exception to this rule: due to the neural efficiency induced by NFT, one can expect to observe prolonged changes in brain activity after NFT is applied to enhance alpha activity (7–13 Hz). Alpha activity is indicative of the inhibition and suppression of unnecessary or irrelevant information processing, especially during the resting state. We also argued that, after learning to modify their brain activity, a person should be able to up- and downregulate the trained frequency more freely based on physiological demands. Furthermore, with respect to our model, we comment on the current debate regarding the interaction between neurophysiological and behavioral changes that can be induced by NFT. We argued that the immediate (and nonstable) effects of NFT on behavior can be observed after a few sessions, even though functional changes might not be well-established. However, longer interventions are required to stabilize behavioral changes and enable researchers to observe changes at the neurophysiological level, which are comparable to the behavioral level. These theoretical concerns could provide insights for future research.
Conclusions and outlook
NFT is continuing to gain widespread interest both in clinical and performance-related disciplines. It has, therefore, become necessary to define a theoretical framework and the neural mechanisms associated with NFT, along with potential outcomes. This article has identified the importance of self-regulation and the role of operant conditioning for inducing physiological self-regulation and, consequently, optimizing a behavior or function of interest. In addition, we discussed relations among control system theory, hemostasis, and allostasis and NFT to develop a new theoretical model of the neural mechanisms underlying NFT.
The most important contribution that this article makes is based on the framework of allostasis, and that NFT may optimize adaptation to new demands in four stages, which we have named as the allostasis four-stage model of NFT: (A) by more accurately perceiving internal and external demands; (B) by predicting the internal and external state of the body and its future output; (C) by more appropriately determining new set-points (which is an efficient trade-off between new demands and the current internal state); and (D) by more efficiently responding to new set-points (when the brain assumes the role of an effector). This perspective review also supports the idea that the greatest benefit of NFT is that changes resulting from interventions occur under physiologically normal conditions, which are clearly required for clinical applications. However, this perspective review proposes that neurophysiological changes resulting from NFT interventions occurring under physiologically abnormal conditions may differ for nonclinical applications. Compared to pharmacotherapy and noninvasive brain stimulation (such as rTMS and tDCS), NFT is purely an endogenous technique, whereby physiological regulation is invoked by the mechanism of action itself, that is, from the “inside out” rather than from the “outside in” (Ros et al. 2014). It is, therefore, assumed (as evident in the seminal study by Joe Kamiya) that the alteration induced by NFT is under volitional control when the process of learning is complete. More evidence on volitional control on brain oscillatory activity following the termination of NFT would help us to establish a greater degree of accuracy on this matter. In general, the theoretical implications of these findings offer promise for NFT as a means of influencing learning and self-regulation across a variety of normative and clinical groups.
Footnotes
Author contributions: AM conceived and developed the idea and frame of the manuscript. AM and FE developed the model and AK provided feedback on it. AM wrote the first draft of the manuscript and FE and AK commented on it and revised it. All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: AM and FE received no financial support for the research, authorship, and/or publication of this article. AK was supported by grant R01MH112558, by the National Institute of Mental Health.
Conflict of interest statement: None.
References
- Arduin P.J., Fregnac Y., Shulz D.E., Ego-Stengel V. “Master” neurons induced by operant conditioning in rat motor cortex during a brain-machine interface task. J. Neurosci. 2013;33:8308–8320. doi: 10.1523/jneurosci.2744-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M., de Ridder S., Strehl U., Breteler M., Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin. EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Awh E., Gehring W.J. The anterior cingulate cortex lends a hand in response selection. Nat. Neurosci. 1999;2:853. doi: 10.1038/13145. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh Y., Baldauf D., Pantazis D., Desimone R. Alpha synchrony and the neurofeedback control of spatial attention. Neuron. 2020;105:577–587 e575. doi: 10.1016/j.neuron.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Banfield J.F., Wyland C.L., Macrae C.N., Münte T.F., Heatherton T.F. Handbook of self-regulation: research, theory, and applications . The Guilford Press; New York, NY, US: 2004. The cognitive neuroscience of self-regulation; pp. 62–83. [Google Scholar]
- Barrett L.F., Quigley K.S., Hamilton P. An active inference theory of allostasis and interoception in depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20160011. doi: 10.1098/rstb.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R.F., Heatherton T.F. Self-regulation failure: an overview. Psychol. Inq. 1996;7:1–15. doi: 10.1207/s15327965pli0701_1. [DOI] [Google Scholar]
- Bissiere S., Humeau Y., Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat. Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Blair C. Developmental science and executive function. Curr. Dir. Psychol. Sci. 2016;25:3–7. doi: 10.1177/0963721415622634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluschke A., Broschwitz F., Kohl S., Roessner V., Beste C. The neuronal mechanisms underlying improvement of impulsivity in ADHD by theta/beta neurofeedback. Sci. Rep. 2016;6:31178. doi: 10.1038/srep31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L.E., Fissell K., Carter C.S., Cohen J.D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bush G., Vogt B.A., Holmes J., Dale A.M., Greve D., Jenike M.A., Rosen B.R. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U. S. A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon R., Lubar J., Congedo M., Thornton K., Towler K., Hutchens T. The effects of neurofeedback training in the cognitive division of the anterior cingulate gyrus. Int. J. Neurosci. 2007;117:337–357. doi: 10.1080/00207450500514003. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Macdonald A.M., Botvinick M., Ross L.L., Stenger V.A., Noll D., Cohen J.D. Parsing executive processes: strategic versus evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V.P., Parasuraman R. Neuroenhancement: enhancing brain and mind in health and in disease. Neuroimage. 2014;85:889–894. doi: 10.1016/j.neuroimage.2013.08.071. [DOI] [PubMed] [Google Scholar]
- Coben R., Linden M., Myers T.E. Neurofeedback for autistic spectrum disorder: a review of the literature. Appl. Psychophysiol. Biofeedback. 2010;35:83–105. doi: 10.1007/s10484-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Cohen E.J., Quarta E., Bravi R., Granato A., Minciacchi D. Neural plasticity and network remodeling: from concepts to pathology. Neuroscience. 2017;344:326–345. doi: 10.1016/j.neuroscience.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E., Elavsky S., Marquez D.X., Hu L., Kramer A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Biol. Med. Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Collura T.F. Technical foundations of neurofeedback. Taylor & Francis; New York, US: 2014. [Google Scholar]
- Cooper S.J. From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite. 2008;51:419–427. doi: 10.1016/j.appet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Davelaar E.J. A multi-stage theory of neurofeedback learning. In: Schmorrow D.D., Fidopiastis C.M., editors. Augmented cognition. Theoretical and technological approaches . Springer International Publishing; Cham: 2020. pp. 118–128. [Google Scholar]
- Davelaar E.J., Barnby J.M., Almasi S., Eatough V. Differential subjective experiences in learners and non-learners in frontal alpha neurofeedback: piloting a mixed-method approach. Front. Hum. Neurosci. 2018;12:402. doi: 10.3389/fnhum.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder D.T.D., de Wit J.B.F. Self-regulation in health behavior. Wiley; Chichester, West Sussex, UK: 2006. Self-regulation in health behavior: concepts, theories, and central issues; pp. 1–23. [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Kempermann G., Kuhn H.G., Winkler J., Buchel C., May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 2006;26:6314–6317. doi: 10.1523/jneurosci.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dworkin B.R. Learning and long-term physiological regulation. In: Davidson R.J., Schwartz G.E., Shapiro D., editors. Consciousness and self-regulation: Advances in research and theory . Springer US; Boston, MA: 1986. pp. 163–182. [Google Scholar]
- Elliott R., Dolan R.J. Neural response during preference and memory judgments for subliminally presented stimuli: a functional neuroimaging study. J. Neurosci. 1998;18:4697–4704. doi: 10.1523/jneurosci.18-12-04697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert K., Kopel R., Sulzer J., Bruhl A.B., Berman B.D., Linden D.E.J., Horovitz S.G., Breimhorst M., Caria A., Frank S., et al. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: how is brain regulation mediated? Neuroimage. 2016;124:806–812. doi: 10.1016/j.neuroimage.2015.09.042. [DOI] [PubMed] [Google Scholar]
- Feldman D.E. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbach A., Shah J.Y. Self-control in action: implicit dispositions toward goals and away from temptations. J. Pers. Soc. Psychol. 2006;90:820–832. doi: 10.1037/0022-3514.90.5.820. [DOI] [PubMed] [Google Scholar]
- Friston K., Kiebel S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1211–1221. doi: 10.1098/rstb.2008.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel C.R., Matzel L.D. The neuroscience of learning: beyond the Hebbian synapse. Annu. Rev. Psychol. 2013;64:169–200. doi: 10.1146/annurev-psych-113011-143807. [DOI] [PubMed] [Google Scholar]
- Gaume A., Vialatte A., Mora-Sánchez A., Ramdani C., Vialatte F.B. A psychoengineering paradigm for the neurocognitive mechanisms of biofeedback and neurofeedback. Neurosci. Biobehav. Rev. 2016;68:891–910. doi: 10.1016/j.neubiorev.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Fencsik D.E. Functions of the medial frontal cortex in the processing of conflict and errors. J. Neurosci. 2001;21:9430–9437. doi: 10.1523/jneurosci.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gerdes L., Gerdes P., Lee S.W., Tegeler C.H. HIRREM: a noninvasive, allostatic methodology for relaxation and auto-calibration of neural oscillations. Brain Behav. 2013;3:193–205. doi: 10.1002/brb3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman S.J., Daw N.D. Reinforcement learning and episodic memory in humans and animals: an integrative framework. Annu. Rev. Psychol. 2017;68:101–128. doi: 10.1146/annurev-psych-122414-033625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner W. Hebbian learning and plasticity. In: Arbib M.A., Bonaiuto J.J., editors. From neuron to cognition via computational neuroscience. MIT Press Cambridge; London: 2011. pp. 0–25. [Google Scholar]
- Ghaziri J., Tucholka A., Larue V., Blanchette-Sylvestre M., Reyburn G., Gilbert G., Levesque J., Beauregard M. Neurofeedback training induces changes in white and gray matter. Clin. EEG Neurosci. 2013;44:265–272. doi: 10.1177/1550059413476031. [DOI] [PubMed] [Google Scholar]
- Gollwitzer P.M., Brandstätter V. Implementation intentions and effective goal pursuit. J. Pers. Soc. Psychol. 1997;73:186. doi: 10.1037/0022-3514.73.1.186. [DOI] [PubMed] [Google Scholar]
- Gopal M. Control systems: principles and design. McGraw-Hill Education (India) Pvt Limited; New Delhi: 2002. [Google Scholar]
- Gruzelier J., Egner T. Critical validation studies of neurofeedback. Child Adolesc. Psychiatr. Clin. North Am. 2005;14:83–104. doi: 10.1016/j.chc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H., Egner T. Musical excellence. Oxford University Press; London, UK: 2004. Physiological self-regulation: biofeedback and neurofeedback; pp. 197–219. [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. I: a review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014a;44:124–141. doi: 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. II: creativity, the performing arts and ecological validity. Neurosci. Biobehav. Rev. 2014b;44:142–158. doi: 10.1016/j.neubiorev.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. III: a review of methodological and theoretical considerations. Neurosci. Biobehav. Rev. 2014c;44:159–182. doi: 10.1016/j.neubiorev.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Hampson M., Ruiz S., Ushiba J. Neurofeedback. Neuroimage. 2020;218:116473. doi: 10.1016/j.neuroimage.2019.116473. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. The organization of behavior . Vol. 65. Wiley; New York: 1949. [Google Scholar]
- Hensel H. Thermoreception and temperature regulation. Monogr. Physiol. Soc. 1981;38:1–321. [PubMed] [Google Scholar]
- Hernandez M., Denburg N.L., Tranel D. A neuropsychological perspective on the role of the prefrontal cortex in reward processing and decision-making. In: Dreher J.-C., Tremblay L., editors. Handbook of reward and decision making . Academic Press; New York: 2009. pp. 291–306. [Google Scholar]
- Hinterberger T., Veit R., Strehl U., Trevorrow T., Erb M., Kotchoubey B., Flor H., Birbaumer N. Brain areas activated in fMRI during self-regulation of slow cortical potentials (SCPs) Exp. Brain Res. 2003;152:113–122. doi: 10.1007/s00221-003-1515-4. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Schmeichel B.J., Baddeley A.D. Executive functions and self-regulation. Trends Cognit. Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hohenfeld C., Nellessen N., Dogan I., Kuhn H., Muller C., Papa F., Ketteler S., Goebel R., Heinecke A., Shah N.J., et al. Cognitive improvement and brain changes after real-time functional MRI neurofeedback training in healthy elderly and prodromal Alzheimer’s disease. Front. Neurol. 2017;8:384. doi: 10.3389/fneur.2017.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Carmody J., Vangel M., Congleton C., Yerramsetti S.M., Gard T., Lazar S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatr. Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak J., Rosenbaum D., Barth B., Fallgatter A.J., Ehlis A.C. Functionally disconnected: a look at how study design influences neurofeedback data and mechanisms in attention-deficit/hyperactivity disorder. PLoS One. 2018;13:e0200931. doi: 10.1371/journal.pone.0200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley K.M., Herbert H., Moga M.M., Saper C.B. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ilg R., Wohlschlager A.M., Gaser C., Liebau Y., Dauner R., Woller A., Zimmer C., Zihl J., Muhlau M. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J. Neurosci. 2008;28:4210–4215. doi: 10.1523/jneurosci.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H. Neural plasticity. In: Smelser N.J., Baltes P.B., editors. International encyclopedia of the social & behavioral sciences . Pergamon; Oxford: 2001. pp. 10542–10546. [Google Scholar]
- Kamiya J. The western psychological . The Western Psychological; San Francisco, California: 1962. Conditioned discrimination of the EEG alpha rhythm in humans. [Google Scholar]
- Kane M.J., Bleckley M.K., Conway A.R., Engle R.W. A controlled-attention view of working-memory capacity. J. Exp. Psychol. Gen. 2001;130:169–183. doi: 10.1037/0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kanosue K., Crawshaw L.I., Nagashima K., Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur. J. Appl. Physiol. 2010;109:5–11. doi: 10.1007/s00421-009-1256-6. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Liddle P.F., Hopfinger J.B. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. doi: 10.1111/1469-8986.3720216. [DOI] [PubMed] [Google Scholar]
- Kleckner I.R., Zhang J., Touroutoglou A., Chanes L., Xia C., Simmons W.K., Quigley K.S., Dickerson B.C., Barrett L.F. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 2017;1:0069. doi: 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kober S.E., Witte M., Stangl M., Väljamäe A., Neuper C., Wood G. Shutting down sensorimotor interference unblocks the networks for stimulus processing: an SMR neurofeedback training study. Clin. Neurophysiol. 2015;126:82–95. doi: 10.1016/j.clinph.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Kolb B., Gibb R., Robinson T.E. Brain plasticity and behavior. Curr. Dir. Psychol. Sci. 2003;12:1–5. doi: 10.1111/1467-8721.01210. [DOI] [Google Scholar]
- Kringelbach M.L., Berridge K.C. Recent developments in neuroscience research on human motivation. Emerald Group Publishing Limited; Bingley, UK: 2016. Neuroscience of reward, motivation, and drive; pp. 23–35. [Google Scholar]
- Kulichenko A.M., Fokina Y.O., Pavlenko V.B. Changes in EEG rhythms and spike activity of brainstem dopaminergic neurons induced by neurofeedback sessions in cats. Neurophysiology. 2009;41:196. doi: 10.1007/s11062-009-9093-0. [DOI] [Google Scholar]
- Landers D.M., Petruzzello S.J., Salazar W., Crews D.J., Kubitz K.A., Gannon T.L., Han M. The influence of electrocortical biofeedback on performance in pre-elite archers. Med. Sci. Sports Exerc. 1991;23:123–129. doi: 10.1249/00005768-199101000-00018. [DOI] [PubMed] [Google Scholar]
- LeBlanc S.E., Coughanowr D. Process systems analysis and control. McGraw-Hill Education; New York: 2009. [Google Scholar]
- Levine B., Stuss D.T., Milberg W.P., Alexander M.P., Schwartz M., Macdonald R. The effects of focal and diffuse brain damage on strategy application: evidence from focal lesions, traumatic brain injury and normal aging. J. Int. Neuropsychol. Soc. 1998;4:247–264. doi: 10.1017/s1355617798002471. [DOI] [PubMed] [Google Scholar]
- Liddle P.F., Kiehl K.A., Smith A.M. Event-related fMRI study of response inhibition. Hum. Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::aid-hbm1007>3.0.co;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhartova P., Latalova A., Kosa B., Kasparek T., Schmahl C., Paret C. fMRI neurofeedback in emotion regulation: a literature review. Neuroimage. 2019;193:75–92. doi: 10.1016/j.neuroimage.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Luria A.R. Higher cortical functions in man . Basic; New York: 1966. [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marins T., Rodrigues E.C., Bortolini T., Melo B., Moll J., Tovar-Moll F. Structural and functional connectivity changes in response to short-term neurofeedback training with motor imagery. Neuroimage. 2019;194:283–290. doi: 10.1016/j.neuroimage.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Markram H., Gerstner W., Sjöström P.J. A history of spike-timing-dependent plasticity. Front. Synaptic Neurosci. 2011;3:4. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeli A., Misaki M., Zotev V., Tsuchiyagaito A., Al Zoubi O., Phillips R., Smith J., Stewart J.L., Refai H., Paulus M.P., et al. Self-regulation of ventromedial prefrontal cortex activation using real-time fMRI neurofeedback-Influence of default mode network. Hum. Brain Mapp. 2020;41:342–352. doi: 10.1002/hbm.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee W.J., Crook T.H. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology. 1993;111:391–401. doi: 10.1007/bf02253527. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Chapter 5 – central role of the brain in stress and adaptation: allostasis, biological embedding, and cumulative change. In: Fink G., editor. Stress: concepts, cognition, emotion, and behavior . Academic Press; San Diego: 2016. pp. 39–55. [Google Scholar]
- McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::aid-hbm1010>3.0.co;2-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micoulaud-Franchi J.A., Fovet T. A framework for disentangling the hyperbolic truth of neurofeedback: comment on Thibault and Raz (2017) Am. Psychol. 2018;73:933–935. doi: 10.1037/amp0000340. [DOI] [PubMed] [Google Scholar]
- Micoulaud Franchi J.A., Jeunet C., Lotte F. Neurofeedback: a challenge for integrative clinical neurophysiological studies. Neurophysiol. Clin. 2020;50:1–3. doi: 10.1016/j.neucli.2020.01.001. [DOI] [PubMed] [Google Scholar]
- Miller E.K. The prefontral cortex and cognitive control. Nat. Rev. Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller J.D., Sanghera M.K., German D.C. Mesencephalic dopaminergic unit activity in the behaviorally conditioned rat. Life Sci. 1981;29:1255–1263. doi: 10.1016/0024-3205(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Miller N.E. Biofeedback and visceral learning. Annu. Rev. Psychol. 1978;29:373–404. doi: 10.1146/annurev.ps.29.020178.002105. [DOI] [PubMed] [Google Scholar]
- Miller R. Meaning and purpose in the intact brain: a philosophical, psychological, and biological account of conscious processes. Oxford University Press; New York City, US: 1981. [Google Scholar]
- Mirifar A., Keil A., Beckmann J., Ehrlenspiel F. No effects of neurofeedback of beta band components on reaction time performance. J. Cognit. Enhanc. 2019;3:251–260. doi: 10.1007/s41465-018-0093-0. [DOI] [Google Scholar]
- Monastra V.J. Quantitative electroencephalography and attention-deficit/hyperactivity disorder: implications for clinical practice. Curr. Psychiatr. Rep. 2008;10:432–438. doi: 10.1007/s11920-008-0069-3. [DOI] [PubMed] [Google Scholar]
- Moore N.C. A review of EEG biofeedback treatment of anxiety disorders. Clin. Electroencephalogr. 2000;31:1–6. doi: 10.1177/155005940003100105. [DOI] [PubMed] [Google Scholar]
- Mulholland T.B. Concepts of control in biofeedback. In: Elbert T., Rockstroh B., Lutzenberger W., Birbaumer N., editors. Self-regulation of the brain and behavior . Springer Berlin Heidelberg; Berlin, Heidelberg: 1984. pp. 277–295. [Google Scholar]
- Munivenkatappa A., Rajeswaran J., Indira Devi B., Bennet N., Upadhyay N. EEG neurofeedback therapy: can it attenuate brain changes in TBI? NeuroRehabilitation. 2014;35:481–484. doi: 10.3233/nre-141140. [DOI] [PubMed] [Google Scholar]
- Ninaus M., Kober S.E., Witte M., Koschutnig K., Stangl M., Neuper C., Wood G. Neural substrates of cognitive control under the belief of getting neurofeedback training. Front. Hum. Neurosci. 2013;7:914. doi: 10.3389/fnhum.2013.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv S. Clinical efficacy and potential mechanisms of neurofeedback. Pers. Indiv. Differ. 2013;54:676–686. doi: 10.1016/j.paid.2012.11.037. [DOI] [Google Scholar]
- Oblak E.F., Lewis-Peacock J.A., Sulzer J.S. Self-regulation strategy, feedback timing and hemodynamic properties modulate learning in a simulated fMRI neurofeedback environment. PLoS Comput. Biol. 2017;13:e1005681. doi: 10.1371/journal.pcbi.1005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossadtchi A., Shamaeva T., Okorokova E., Moiseeva V., Lebedev M.A. Neurofeedback learning modifies the incidence rate of alpha spindles, but not their duration and amplitude. Sci. Rep. 2017;7:3772. doi: 10.1038/s41598-017-04012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandria N., Kovatsi L., Vivas A.B., Bamidis P.D. Resting-state abnormalities in heroin-dependent individuals. Neuroscience. 2018;378:113–145. doi: 10.1016/j.neuroscience.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Pandya D.Ν., Barnes C.L. The frontal lobes revisited. Psychology Press; New York, US: 2019. Architecture and connections of the frontal lobe; pp. 41–72. [Google Scholar]
- Papo D. Neurofeedback: principles, appraisal, and outstanding issues. Eur. J. Neurosci. 2019;49:1454–1469. doi: 10.1111/ejn.14312. [DOI] [PubMed] [Google Scholar]
- Papoutsi M., Weiskopf N., Langbehn D., Reilmann R., Rees G., Tabrizi S.J. Stimulating neural plasticity with real-time fMRI neurofeedback in Huntington’s disease: a proof of concept study. Hum. Brain Mapp. 2018;39:1339–1353. doi: 10.1002/hbm.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Zahringer J., Ruf M., Gerchen M.F., Mall S., Hendler T., Schmahl C., Ende G. Monitoring and control of amygdala neurofeedback involves distributed information processing in the human brain. Hum. Brain Mapp. 2018;39:3018–3031. doi: 10.1002/hbm.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Hozack N., Frank L., Brown G.G. Error rate and outcome predictability affect neural activation in prefrontal cortex and anterior cingulate during decision-making. Neuroimage. 2002;15:836–846. doi: 10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pawlak V., Wickens J., Kirkwood A., Kerr J. Timing is not everything: neuromodulation opens the STDP gate. Front. Synaptic Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. Attention, self-regulation and consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Augustine G.J., Fitzpatrick D., Katz L.C., LaMantia A.S., McNamara J.O., Williams S.M. Neuroscience. 3rd ed. Sinauer Associates; Sunderland, Massachusetts, US: 2001. Chapter 24 - Neurotransmitters; pp. 275–610. [Google Scholar]
- Raichle M.E., Fiez J.A., Videen T.O., MacLeod A.M., Pardo J.V., Fox P.T., Petersen S.E. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebr. Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Ramsay D.S., Woods S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 2014;121:225–247. doi: 10.1037/a0035942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance M., Walsh C., Sukhodolsky D.G., Pittman B., Qiu M., Kichuk S.A., Wasylink S., Koller W.N., Bloch M., Gruner P., et al. Time course of clinical change following neurofeedback. Neuroimage. 2018;181:807–813. doi: 10.1016/j.neuroimage.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner M., Gruzelier J., Bamidis P.D., Auer T. The science of neurofeedback: learnability and effects. Neuroscience. 2018;378:1–10. doi: 10.1016/j.neuroscience.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Ring C., Cooke A., Kavussanu M., McIntyre D., Masters R. Investigating the efficacy of neurofeedback training for expediting expertise and excellence in sport. Psychol. Sport Exerc. 2015;16:118–127. doi: 10.1016/j.psychsport.2014.08.005. [DOI] [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cerebr. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Ros T., Baars B.J., Lanius R.A., Vuilleumier P. Tuning pathological brain oscillations with neurofeedback: a systems neuroscience framework. Front. Hum. Neurosci. 2014;8:1008. doi: 10.3389/fnhum.2014.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Enriquez-Geppert S., Zotev V., Young K.D., Wood G., Whitfield-Gabrieli S., Wan F., Vuilleumier P., Vialatte F., Van De Ville D., et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist) Brain. 2020a;143:1674–1685. doi: 10.1093/brain/awaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Kwiek J., Andriot T., Michela A., Vuilleumier P., Garibotto V., Ginovart N. PET imaging of dopamine neurotransmission during EEG neurofeedback. Front. Physiol. 2020b;11:590503. doi: 10.3389/fphys.2020.590503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Chapin L., Kemmerly T., Liu W.-C., Zagardo M.T., Chapin T., Dailey D., Dinh D. The effects of neurofeedback in the default mode network: pilot study results of medicated children with ADHD. J. Neurother. 2013;17:35–42. doi: 10.1080/10874208.2013.759017. [DOI] [Google Scholar]
- Sanei S., Chambers J.A. EEG signal processing. John Wiley & Sons; Chichester, West Sussex, UK: 2007. [Google Scholar]
- Saxbe D.E., Beckes L., Stoycos S.A., Coan J.A. Social allostasis and social allostatic load: a new model for research in social dynamics, stress, and health. Perspect. Psychol. Sci. 2020;15:469–482. doi: 10.1177/1745691619876528. [DOI] [PubMed] [Google Scholar]
- Schabus M. Reply: on assessing neurofeedback effects: should double-blind replace neurophysiological mechanisms? Brain. 2017;140:e64. doi: 10.1093/brain/awx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M. Reply: noisy but not placebo: defining metrics for effects of neurofeedback. Brain. 2018;141:e41. doi: 10.1093/brain/awy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Shibata K. Mechanisms of fMRI neurofeedback. In: Hampson M., editor. fMRI neurofeedback. Academic Press; London, UK: 2021. pp. 287–313. [Google Scholar]
- Shinners S.M. Modern control system theory and design. Wiley; New York, US: 1998. [Google Scholar]
- Shirvalkar P.R. Hippocampal neural assemblies and conscious remembering. J. Neurophysiol. 2009;101:2197–2200. doi: 10.1152/jn.91363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R., Ros T., Stoeckel L., Haller S., Scharnowski F., Lewis-Peacock J., Weiskopf N., Blefari M.L., Rana M., Oblak E., et al. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 2017;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- Skouras S., Scharnowski F. The effects of psychiatric history and age on self-regulation of the default mode network. Neuroimage. 2019;198:150–159. doi: 10.1016/j.neuroimage.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg M.M., Mateer C.A. Introduction to cognitive rehabilitation: theory and practice . Guilford Press; New York, NY, US: 1989. [Google Scholar]
- Somjen G.G. The missing error signal—regulation beyond negative feedback. Physiology. 1992;7:184–185. doi: 10.1152/physiologyonline.1992.7.4.184. [DOI] [Google Scholar]
- Sorger B., Kamp T., Weiskopf N., Peters J.C., Goebel R. When the brain takes ‘BOLD’ steps: real-time fMRI neurofeedback can further enhance the ability to gradually self-regulate regional brain activation. Neuroscience. 2018;378:71–88. doi: 10.1016/j.neuroscience.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S.A., Frith C.D. Towards a functional anatomy of volition. J. Conscious. Stud. 1999;6:11–29. [Google Scholar]
- Stephan K.E., Manjaly Z.M., Mathys C.D., Weber L.A., Paliwal S., Gard T., Tittgemeyer M., Fleming S.M., Haker H., Seth A.K., et al. Allostatic self-efficacy: a metacognitive theory of dyshomeostasis-induced fatigue and depression. Front. Hum. Neurosci. 2016;10:550. doi: 10.3389/fnhum.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. Allostasis: a model of predictive regulation. Physiol. Behav. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sterling P. Predictive regulation and human design. eLife. 2018;7:e36133. doi: 10.7554/eLife.36133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. What is health?: Allostasis and the evolution of human design. MIT Press; Cambridge, Massachusetts, US: 2020. [DOI] [PubMed] [Google Scholar]
- Sterling P., Eyer J., Fisher S., Reason J. Handbook of life stress, cognition and health. Allostasis; a new paradigm to explain arousal pathology . Wiley; New York: 1988. pp. 629–649. [Google Scholar]
- Sterling P., Laughlin S. Principles of neural design . MIT Press; 2015. [Google Scholar]
- Stuss D.T. Self, awareness, and the frontal lobes: a neuropsychological perspective. In: Strauss J., Goethals G.R., editors. The self: interdisciplinary approaches . Springer New York; New York, NY: 1991. pp. 255–278. [Google Scholar]
- Stuss D.T., Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu. Rev. Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Sulzer J., Sitaram R., Blefari M.L., Kollias S., Birbaumer N., Stephan K.E., Luft A., Gassert R. Neurofeedback-mediated self-regulation of the dopaminergic midbrain. Neuroimage. 2013;83:817–825. doi: 10.1016/j.neuroimage.2013.05.115. [DOI] [PubMed] [Google Scholar]
- Tang Y.Y., Lu Q., Fan M., Yang Y., Posner M.I. Mechanisms of white matter changes induced by meditation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst G.J., Hautvast R.W., De Jongste M.J., Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur. J. Neurosci. 1996;8:2029–2041. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Terreberry R.R., Neafsey E.J. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res. Bull. 1987;19:639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Thibault R.T., Lifshitz M., Raz A. The self-regulating brain and neurofeedback: experimental science and clinical promise. Cortex. 2016;74:247–261. doi: 10.1016/j.cortex.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Thibault R.T., Raz A. The psychology of neurofeedback: clinical intervention even if applied placebo. Am. Psychol. 2017;72:679–688. doi: 10.1037/amp0000118. [DOI] [PubMed] [Google Scholar]
- Thibault R.T., Raz A. A consensus framework for neurofeedback research (and the perils of unfounded neuroreductionism): reply to Micoulaud-Franchi and Fovet (2018) Am. Psychol. 2018;73:936–937. doi: 10.1037/amp0000366. [DOI] [PubMed] [Google Scholar]
- Thompson L. Electroencephalographic applications . AAPB; Wheat Ridge Colorado: 2004. [Google Scholar]
- Tinga A.M., de Back T.T., Louwerse M.M. Non-invasive neurophysiological measures of learning: a meta-analysis. Neurosci. Biobehav. Rev. 2019;99:59–89. doi: 10.1016/j.neubiorev.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Trambaiolli L.R., Kohl S.H., Linden D.E.J., Mehler D.M.A. Neurofeedback training in major depressive disorder: a systematic review of clinical efficacy, study quality and reporting practices. Neurosci. Biobehav. Rev. 2021;125:33–56. doi: 10.1016/j.neubiorev.2021.02.015. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor Perspect. Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel G.J., Gruzelier J.H. Neurofeedback: introduction to the special issue. Biol. Psychol. 2014;95:1–3. doi: 10.1016/j.biopsycho.2013.11.011. [DOI] [PubMed] [Google Scholar]
- van Kesteren M.T., Fernandez G., Norris D.G., Hermans E.J. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne A.J.M., Owens N.C. Cortical modulation of the cardiovascular system. Prog. Neurobiol. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Vogt L., Farber N.B., Bush G. Architecture and neurocytology of monkey cingulate gyrus. J. Comp. Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Sasaki Y., Shibata K., Kawato M. Advances in fMRI real-time neurofeedback. Trends Cognit. Sci. 2017;21:997–1010. doi: 10.1016/j.tics.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens J. Striatal dopamine in motor activation and reward-mediated learning: steps towards a unifying model. J. Neural Transm. Gen. Sect. 1990;80:9–31. doi: 10.1007/bf01245020. [DOI] [PubMed] [Google Scholar]
- Wiener N. Cybernetics or control and communication in the animal and the machine. MIT Press; Cambridge, Massachusetts, US: 2019. [Google Scholar]
- Witte M., Kober S.E., Wood G. Noisy but not placebo: defining metrics for effects of neurofeedback. Brain. 2018;141:e40. doi: 10.1093/brain/awy060. [DOI] [PubMed] [Google Scholar]
- Wyland C.L., Kelley W.M., Macrae C.N., Gordon H.L., Heatherton T.F. Neural correlates of thought suppression. Neuropsychologia. 2003;41:1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Wyrwicka W., Sterman M.B. Instrumental conditioning of sensorimotor cortex EEG spindles in the waking cat. Physiol. Behav. 1968;3:703–707. doi: 10.1016/0031-9384(68)90139-x. [DOI] [Google Scholar]
- Yeh W.H., Hsueh J.J., Shaw F.Z. Neurofeedback of alpha activity on memory in healthy participants: a systematic review and meta-analysis. Front. Hum. Neurosci. 2020;14:562360. doi: 10.3389/fnhum.2020.562360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel B., Huster R.J., Herrmann C.S. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. Neuroimage. 2011;54:1427–1431. doi: 10.1016/j.neuroimage.2010.08.078. [DOI] [PubMed] [Google Scholar]
- Zotev V., Krueger F., Phillips R., Alvarez R.P., Simmons W.K., Bellgowan P., Drevets W.C., Bodurka J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One. 2011;6:e24522. doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsoldos E., Ebmeier K.P. Chapter 38 – aging and psychological stress. In: Fink G., editor. Stress: concepts, cognition, emotion, and behavior . Academic Press; San Diego: 2016. pp. 311–323. [Google Scholar]