Abstract
Background
There is a scarcity of research regarding the effectiveness of the mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) COVID-19 vaccines in patients taking immunosuppressant medications, and no data are published to date pertaining to their effectiveness against omicron (B.1.1.529) variant SARS-CoV-2 infection and hospitalisation. We aimed to assess the relationship between immunosuppressive medications, mRNA vaccination, omicron infection, and severe COVID-19 outcomes (ie, hospitalisation, ICU admission, death).
Methods
We did a retrospective cohort study and included vaccinated and unvaccinated people aged 18 years or older in the Michigan Medicine health-care system, USA, during the omicron-dominant period of the pandemic (Dec 16, 2021–March 4, 2022). We collected data from electronic health records (demographics, diagnoses, medications) combined with immunisation data from the Michigan State Registry to determine vaccination status, and we collected COVID-19-related hospitalisation data by chart review. We used a Cox proportional hazards model based on calendar time to assess the effectiveness of the mRNA-1273 and BNT162b2 vaccines in people taking immunosuppressive medications (conventional synthetic disease-modifying antirheumatic drugs [DMARDs], biologic DMARDs, or glucocorticoids within the past 3 months), while controlling for participant characteristics. Using the same model, we assessed the effect of different classes of medication such as immunosuppressive DMARDs, immunomodulatory DMARDs, and glucocorticoids on SARS-CoV-2 infection and hospitalisation due to COVID-19. All analyses were done using complete cases after removing participants with missing covariates.
Findings
209 492 people were identified in Michigan Medicine, including 165 913 who were vaccinated and 43 579 who were unvaccinated. 41 078 people were excluded because they were younger than 18 years, partially vaccinated, had received a vaccine other than the two vaccines studied, or had incomplete covariate data. 168 414 people were included in the analysis; 97 935 (58%) were women, 70 479 (42%) were men, and 129 816 (77%) were White. 5609 (3%) people were taking immunosuppressive medications. In patients receiving immunosuppressants, three doses of BNT162b2 had a vaccine effectiveness of 50% (95% CI 31–64; p<0·0001) and three doses of mRNA-1273 had a vaccine effectiveness of 60% (42–73; p<0·0001) against SARS-CoV-2 infection. Three doses of either vaccine had an effectiveness of 87% (95% CI 73–93; p<0·0001) against hospitalisation due to COVID-19. Receipt of immunosuppressive DMARDs (hazard ratio 2·32, 95% CI 1·23–4·38; p=0·0097) or glucocorticoids (2·93, 1·77–4·86; p<0·0001) and a history of organ or bone marrow transplantation (3·52, 2·01–6·16; p<0·0001) were associated with increased risk of hospitalisation due to COVID-19 compared with those who had not received immunosuppressive medications or transplant.
Interpretation
People taking immunosuppressive DMARDs or glucocorticoids are at substantially higher risk of hospitalisation due to COVID-19 than the general population. However, the mRNA-1273 and BNT162b2 vaccines remain effective within this group, and it is important that patients taking these medications remain up to date with vaccinations to mitigate their risk.
Funding
National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Introduction
The BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines have been shown to be safe and highly effective against SARS-CoV-2 infection or severe COVID-19 outcomes in clinical trials and became the first two COVID-19 vaccines to be approved in the USA. However, patients taking immunosuppressive medications were excluded from these trials.1, 2 The paucity of data regarding the effectiveness of COVID-19 vaccines in patients who are immunosuppressed has persisted throughout the pandemic, as observational studies have mostly not included a sufficient sample size or the necessary medication data to conduct analyses in this population. Following WHO designation on Nov 26, 2021, the omicron (B.1.1.529) variant spread rapidly and became the dominant SARS-CoV-2 variant worldwide. Initial findings among immunocompetent people showed that two doses of the BNT162b2 or mRNA-1273 vaccines provides low protection against symptomatic omicron infection, although receipt of a third dose substantially increases protection.3, 4 Multiple studies in patients who are immunosuppressed have shown mRNA vaccines to be effective against infection and hospitalisation due to the previously dominant delta (B.1.617.2) variant, but there are few data regarding their effectiveness against the omicron variant.5, 6, 7, 8, 9 Patients taking immunosuppressive medications have reduced immune responses following vaccination compared with immunocompetent people, but granular data about classes of immunosuppressive medications and dose level are still scarce.10, 11, 12, 13, 14, 15 In addition to vaccine effectiveness, there is little knowledge on how taking immunosuppressive medications affects risk of SARS-CoV-2 infection and hospitalisation due to COVID-19. There is evidence of increased risk of hospitalisation and severe outcomes from COVID-19 for some specific immunosuppressive medications, such as rituximab, JAK inhibitors, and glucocorticoids, but no studies have investigated all SARS-CoV-2 infections.16
Research in context.
Evidence before this study
We searched major rheumatology journals (including The Lancet Rheumatology , Annals of the Rheumatic Diseases, Arthritis and Rheumatology , and others) and PubMed on April 5, 2022, for studies published in English, with no date restrictions, using the terms “COVID-19”, “immunosuppressed”, “immunocompromised”, “rheumatic diseases”, “auto-immune disease”, SARS-CoV-2”, “infection”, and “hospitalisation” to identify studies on COVID-19 risk in patients taking immunosuppressive medications, and using combinations of the same search terms in addition to “vaccines”, “efficacy”, “immunogenicity”, “omicron”, “delta”, and “effectiveness” to identify studies on vaccine effectiveness in patients taking immunosuppressive medications. Previous research on vaccine effectiveness in people who are immunosuppressed is scarce, although COVID-19 vaccines have been shown to be effective against previously circulating variants in this populaton. Some immunosuppressive medications, including glucocorticoids, rituximab, and JAK inhibitors, have been shown to increase risk of hospitalisation due to COVID-19 and death.
Added value of this study
To our knowledge, this is the first study to assess the effectiveness of COVID-19 vaccines against the omicron (B.1.1.529) variant in patients taking immunosuppressive medications. Moreover, we performed detailed analysis on the risk of SARS-CoV-2 infection and hospitalisation due to COVID-19 associated with different types of immunosuppressive medications in a larger study population than in most previous studies. Access to chart-reviewed COVID-19 hospitalisation data is also a major strength of this study, as this reduces bias due to the inclusion of patients hospitalised for other reasons. In patients taking immunosuppressive medications, we found strong protection against COVID-19 hospitalisation due to the omicron variant among recipients of two doses or three doses of either the mRNA-1273 or BNT162b2 vaccine. We also found that three doses of BNT162b2 had a vaccine effectiveness of 50% (95% CI 31–64) and three doses of mRNA-1273 had a vaccine effectiveness of 60% (42–73) against SARS-CoV-2 omicron infection. We found increased risk of hospitalisation due to COVID-19 in patients taking glucocorticoids or immunosuppressive disease-modifying antirheumatic drugs, and in transplant recipients compared with those not taking these medications or who had not received a transplant.
Implications of all the available evidence
In people who are immunosuppressed, COVID-19 vaccines can provide some protection against omicron infection and are highly effective against hospitalisation caused by this variant. Nonetheless, people taking immunosuppressive medications remain at higher risk of severe COVID-19 outcomes, such as hospitalisation due to COVID-19, relative to the general population. It is crucially important that people in this group are prioritised and that they remain up to date on vaccinations.
We aimed to assess the relationship between immunosuppressive medications, mRNA vaccination, omicron infection, and severe COVID-19 outcomes, such as hospitalisation or intensive care unit (ICU) admission due to COVID-19, in a large cohort of patients in the USA.
Methods
Study design and participants
In this retrospective cohort study, we included vaccinated and unvaccinated people aged 18 years or older in the Michigan Medicine health-care system, USA, during the omicron-dominant period of the pandemic (Dec 16, 2021–March 4, 2022). We used electronic health records (EHRs) at Michigan Medicine (including patient demographics, International Classification of Diseases [ICD-10] codes, medications, and laboratory tests for SARS-CoV-2), combined with immunisation data from the Michigan State Registry and chart-reviewed COVID-19 hospitalisation data. EHR data were linked to the immunisation database at the Michigan Care Improvement Registry (MCIR) to determine vaccination status (including dose number and date) for each patient. MCIR documents immunisations given to Michigan residents throughout life and so includes all statewide immunisations, even those administered outside of Michigan Medicine. We included patients who were registered with a primary care physician at Michigan Medicine and had at least one primary care physician visit within the past 18 months. We excluded patients who were younger than 18 years or were vaccinated with a vaccine other than mRNA-1273 or BNT162b2. Comorbidities and number of hospital visits were assessed based on diagnosis codes in the 18 months before baseline (defined as Dec 16, 2021). Transplantation status was determined on the basis of the presence of ICD-10 code Z94 within the 12 months before baseline. The study was approved by the University of Michigan Institutional Review Board (HUM00164771) with a waiver of patient consent, given the use of deidentified EHR data.
Procedures
We identified patients who were immunosuppressed as those taking conventional synthetic disease-modifying antirheumatic drugs (DMARDs), biologic DMARDs, or glucocorticoids (appendix p 3) within 3 months before baseline. Patients were required to have a prescription that covered at least half of this 3-month period, or two separate prescriptions at least 30 days apart (to account for immunosuppressants given via injection).
We identified SARS-CoV-2 infection on the basis of ICD-10 code U07.1 and laboratory test results. Reinfections were SARS-CoV-2 infections that occurred at least 90 days after any previously identified infection (appendix p 2). Hospitalisation was identified using chart review and only included patients in whom COVID-19 was the cause of hospitalisation. ICU stay and death data were identified using chart review.
Statistical analysis
The primary objective was to estimate vaccine effectiveness in patients taking immunosuppressive medications, with SARS-CoV-2 infection and hospitalisation due to COVID-19 as primary endpoints. Secondary objectives were to assess the COVID-19 risk associated with different types of immunosuppressive medication and transplantation status, and vaccine effectiveness among immunocompetent people. All analyses were conducted using complete cases after removing patients with missing covariates. Statistical analyses for SARS-CoV-2 infection, hospitalisation due to COVID-19, and ICU admission due to COVID-19 were conducted on a calendar time scale, with time to infection, hospitalisation, or ICU admission measured from Dec 16, 2021, for all patients, to control for time-varying confounders, such as the community prevalence of COVID-19.17 Dec 16 was chosen as the study start date to correspond with the timepoint at which omicron became the dominant SARS-CoV-2 variant in the USA.18 If infection or hospitalisation occurred twice in the same patient within the study period, only the first instance of each was included in the analysis. We studied vaccination as a time-varying covariate, with vaccinated individuals included in the unvaccinated group before receipt of their first dose and excluded from the analysis from date of their first dose until 14 days after receipt of their second dose. Patients were censored at the date of receiving a fourth dose of vaccine, death, or March 4, 2022, whichever occurred first. To balance patient covariates between vaccine groups and immunosuppressive medication groups, the inverse propensity weighted (IPW)19 Kaplan-Meier method was used to estimate cumulative incidence curves, and IPW Cox proportional hazards regression was used for comparison between curves. Propensity scores were estimated using a multinomial logistic regression with the dependent variable comprised of four groups: vaccinated immunosuppressed, vaccinated immunocompetent, unvaccinated immunosuppressed, and unvaccinated immunocompetent. The independent variables were age, previous SARS-CoV-2 infection, sex, race, number of hospital visits, number of negative SARS-CoV-2 tests recorded before baseline, transplantation status, and Charlson comorbidity index. The Charlson comorbidity index is a weighted sum of 19 conditions, with a weight of 1–6 for each condition, based on the estimated 1-year mortality hazard ratio (HR) from a Cox proportional hazards model, and was obtained using the R package comorbidity.20, 21 All covariates were categorised to account for non-linear effects, and group balance was assessed based on differences between proportions in the weighted sample. The number of hospital visits and negative SARS-CoV-2 tests before baseline were included to reduce informed presence bias,22 which occurs due to differing degrees of interaction with the health-care system between comparison groups. Controlling for negative tests is particularly important as it reflects an individual's propensity to seek testing and mitigates the potential bias from different rates of testing between immunosuppressed and immunocompetent patients or vaccinated and unvaccinated patients.
The IPW Kaplan-Meier method was used to visually display the differences between groups after removing the confounding effects, but all further analysis was conducted in the unweighted sample using Cox regression adjusted for the aforementioned covariates, and the vaccine effectiveness was calculated as 1 – HR. The assumption of proportional hazards was assessed using Schoenfeld residuals. We used Cox regression to compare patients who received two or three doses of vaccine with unvaccinated patients with the same medication status. Patients were included in the two-dose group 14 days after receipt of their second dose and included in the three-dose group 7 days after receipt of their third dose. Given the strong possibility that covariates, such as previous SARS-CoV-2 infection, would have different effects between immunosuppressed and immunocompetent patients, we fit separate Cox models for the two groups when assessing vaccine effectiveness. We also used a Cox model to assess the effect of different immunosuppressant medication types on infection and hospitalisation, controlling for the aforementioned covariates, including vaccination status.
We performed a sensitivity analysis excluding patients who had cancer in the year before baseline, on the basis of ICD-10 codes.
All statistical analyses were performed using R (version 4.1.2).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
209 492 people were identified in Michigan Medicine, including 165 913 who were vaccinated and 43 579 who were unvaccinated. 41 078 people were excluded because they were younger than 18 years, partially vaccinated (ie, received one dose), had received a vaccine other than the two vaccines studied, or had incomplete covariate data. The frequency of missing data was low, with 2597 people missing race data, and 11 missing sex data. 168 414 people were included in the analysis, 5609 (3%) of whom were taking immunosuppressive medications. The population for analysis had a median age of 49 years (IQR 33–63), 97 935 (58%) were women and 70 479 (42%) were men, and 129 816 (77%) were White (table ).
Table.
Characteristics of the study population by vaccination status and immunosuppression
|
Immunocompetent (n=162 805)* |
Immunosuppressed (n=5609)* |
||||
|---|---|---|---|---|---|
| Unvaccinated (n=34 430) | Vaccinated (n=128 375) | Unvaccinated (n=746) | Vaccinated (n=4863) | ||
| Age, years | |||||
| Median (IQR) | 39 (28–54) | 51 (35–65) | 48 (35–59) | 59 (44–69) | |
| ≤30 | 10 928 (32%) | 23 695 (18%) | 130 (17%) | 457 (9%) | |
| 31–50 | 12 968 (38%) | 39 882 (31%) | 289 (39%) | 1202 (25%) | |
| 51–64 | 6581 (19%) | 32 421 (25%) | 217 (29%) | 1458 (30%) | |
| ≥65 | 3953 (11%) | 32 377 (25%) | 110 (15%) | 1746 (36%) | |
| Date of second vaccine dose | NA | April 10 (March 7–April 30) | NA | April 7 (March 13–April 27) | |
| Date of third vaccine dose | NA | Nov 18 (Oct 25–Dec 15) | NA | Oct 28 (Sept 13–Dec 4) | |
| Sex* | |||||
| Male | 14 730 (43%) | 53 649 (42%) | 280 (38%) | 1820 (37%) | |
| Female | 19 700 (57%) | 74 726 (58%) | 466 (62%) | 3043 (63%) | |
| Race* | |||||
| White | 25 389 (74%) | 99 934 (78%) | 574 (77%) | 3919 (81%) | |
| Black | 5008 (15%) | 10 305 (8%) | 122 (16%) | 536 (11%) | |
| Asian | 2454 (7%) | 13 411 (10%) | 17 (2%) | 251 (5%) | |
| American Indian or Alaska Native | 146 (<1%) | 389 (<1%) | 5 (1%) | 18 (<1%) | |
| Native Hawaiian and other Pacific Islander | 34 (<1%) | 118 (<1%) | 1 (<1%) | 3 (<1%) | |
| Other | 1399 (4%) | 4218 (3%) | 27 (4%) | 136 (3%) | |
| Number of previous negative SARS-CoV-2 tests | |||||
| 0 | 26 938 (78%) | 83 083 (65%) | 346 (46%) | 1940 (40%) | |
| 1 | 4752 (14%) | 25 627 (20%) | 151 (20%) | 1085 (22%) | |
| 2–4 | 2389 (7%) | 16 978 (13%) | 184 (25%) | 1311 (27%) | |
| ≥5 | 351 (1%) | 2687 (2%) | 65 (9%) | 527 (11%) | |
| Weighted comorbidity index† | |||||
| 0 | 31 905 (93%) | 107 976 (84%) | 449 (60%) | 2194 (45%) | |
| 1–2 | 2010 (6%) | 15 648 (12%) | 222 (30%) | 1799 (37%) | |
| 3–4 | 315 (1%) | 3068 (2%) | 49 (7%) | 478 (10%) | |
| ≥5 | 200 (1%) | 1683 (1%) | 26 (3%) | 392 (8%) | |
| Immunosuppressant medication type | |||||
| None | 34 430 (100%) | 128 375 (100%) | NA | NA | |
| Glucocorticoid | NA | NA | 290 (39%) | 1681 (35%) | |
| Immunosuppressive DMARD | NA | NA | 240 (32%) | 1612 (33%) | |
| Immunomodulatory DMARD | NA | NA | 100 (13%) | 784 (16%) | |
| Multiple types | NA | NA | 116 (16%) | 786 (16%) | |
| Previous bone marrow or organ transplantation‡ | 41 (<1%) | 355 (<1%) | 80 (11%) | 496 (10%) | |
| Number of hospital visits§ | 2 (0–7) | 6 (3–13) | 15 (7–30) | 19 (10–36) | |
| SARS-CoV-2 infection¶ | 872 (3%) | 3058 (2%) | 73 (10%) | 242 (5%) | |
| COVID-19-related hospitalisation | 62 (<1%) | 94 (<1%) | 20 (3%) | 27 (1%) | |
Data are n (%) or median (IQR). NA=not applicable. DMARD=disease-modifying antirheumatic drug.
Table shows participants with complete data after excluding those with missing covariates.
Comorbidity index was calculated using diagnosis codes reported within the 18 months before Dec 16, 2021.
Transplantation status determined on the basis of the presence of International Classification of Diseases-10 code Z94 within the 12 months before Dec 16, 2021.
Number of visits was defined as the number of in-person hospital visits for the patient within the 18 months before Dec 16, 2021.
SARS-CoV-2 infections and COVID-19-related hospitalisations for vaccinated individuals are listed as long as they occurred during the study period, regardless of whether they occurred before vaccination, after partial vaccination, or after full vaccination.
Second vaccine doses were received across a range of dates in March and April, 2021, whereas third doses were received from October to December, 2021, with people taking immunosuppressive medications generally receiving third doses earlier than people who were immunocompetent, due to prioritisation. Third doses of mRNA-1273 were half doses relative to the initial two vaccinations (50 mcg vs 100 mcg). Patients who were immunosuppressed were more likely to seek SARS-CoV-2 testing (3323 [59%] of 5609 patients who were immunosuppressed had at least one negative test in the 18 months before baseline, compared with 52 784 [32%] of 162 805 patients who were immunocompetent). Patient covariates by vaccination status and immunosuppression are shown in the table.
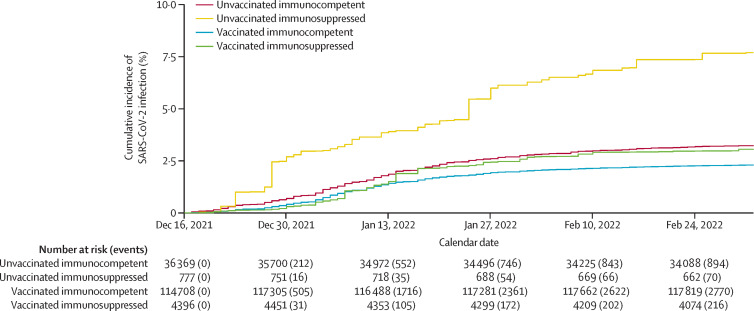
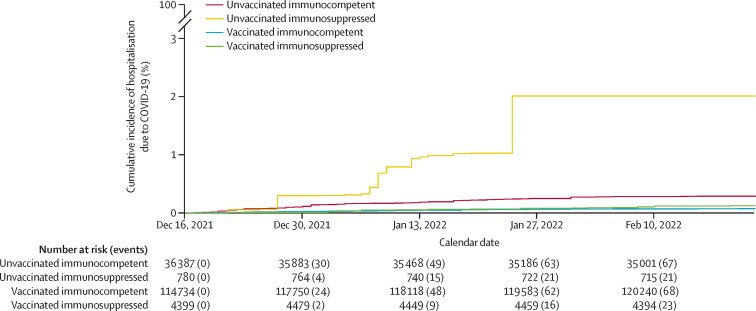
In all patients, after weighting based on inverse propensity scores, covariates were well balanced between vaccination (vaccinated vs unvaccinated) and medication (immunosuppressed vs immunocompetent) groups. Covariate balance before and after weighting is shown in the appendix (pp 14–15). SARS-CoV-2 infection rates were higher in patients who were immunosuppressed than in those who were immunocompetent in both the vaccinated (p=0·030 in IPW Cox regression) and unvaccinated groups (p=0·0003) after adjusting for confounders (figure 1 ). Rates of hospitalisation due to COVID-19 were higher in patients who were immunosuppressed than in those who were immunocompetent in the unvaccinated group (p=0·0003) after adjusting for confounders, whereas the difference was not significant in the vaccinated group (p=0·10; figure 2 ).
Figure 1.
Inverse propensity weighted cumulative incidence of SARS-CoV-2 infection over calendar time by immunosuppression and vaccination status
Vaccinated participants had received two or three doses of either vaccine. We studied vaccination as a time-varying covariate, with vaccinated individuals included in the unvaccinated group before receipt of their first dose and excluded from the analysis from date of their first dose until 14 days after receipt of their second dose.
Figure 2.
Inverse propensity weighted cumulative incidence of hospitalization due to COVID-19 over calendar time by immunosuppression and vaccination status
Vaccinated participants had received two or three doses of either vaccine. We studied vaccination as a time-varying covariate, with vaccinated individuals included in the unvaccinated group before receipt of their first dose and excluded from the analysis from date of their first dose until 14 days after receipt of their second dose.
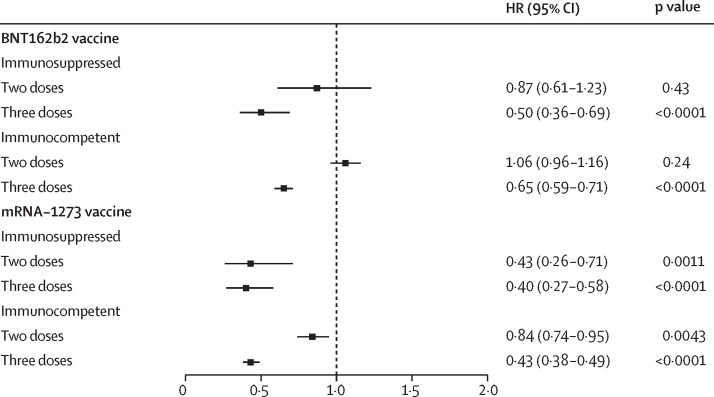
Two doses of the BNT162b2 vaccine provided no protection against SARS-CoV-2 infection in either patients who were immunosuppressed (vaccine effectiveness 13%, 95% CI –19 to 39; p=0·43) or immunocompetent (−6%, –14 to 4; p=0·24; figure 3 ). By contrast, three doses of the BNT162b2 had a vaccine effectiveness of 50% (95% CI 31 to 64; p<0·0001) in patients who were immunosuppressed and 35% in those who were immunocompetent (29 to 41; p<0·0001). Two doses of the mRNA-1273 vaccine was protective against SARS-CoV-2 infection in both patients who were immunosuppressed (vaccine effectiveness 57%, 95% CI 29 to 74; p=0·0011) and those who were immunocompetent (16%, 5 to 26; p=0·0043). Three doses of mRNA-1273 vaccine had a vaccine effectiveness of 60% (95% CI 42 to 73; p<0·0001) in patients who were immunosuppressed and 57% (51 to 62; p<0·0001) in those who were immunocompetent. Full Cox regression results for SARS-CoV-2 infection are provided in the appendix (pp 5–6).
Figure 3.
HRs for SARS-CoV-2 infection by vaccine product and immunosuppression, adjusted by Cox regression for age, previous SARS-CoV-2 infection, sex, race, number of hospital visits, previous negative tests, and Charlson comorbidity index
HRs for SARS-CoV-2 infection are relative to unvaccinated participants with the same medication status. HR=hazard ratio.
Due to the low sample size in the immunosuppressed group, it was not feasible to provide estimates of vaccine effectiveness against hospitalisation due to COVID-19 by vaccine product. Three doses of either vaccine was strongly protective against hospitalisation due to COVID-19 in patients who were immunosuppressed (vaccine effectiveness 87%, 95% CI 74–93; p<0·0001) and in those who were immunocompetent (92%, 87–95; p<0·0001). Two doses of either vaccine also showed substantial protection against hospitalisation due to COVID-19 in both patients who were immunosuppressed (vaccine effectiveness 85%, 95% CI 62–94; p<0·0001) and those who were immunocompetent (68%, 53–78; p<0·0001). Full Cox regression results for hospitalisation are shown in the appendix (pp 7–8).
1852 patients were taking immunosuppressive DMARDs only (tacrolimus, mycophenolate mofetil, methotrexate, and others), 884 were taking immunomodulatory DMARDs only (hydroxychloroquine, mesalamine, sulfasalazine and others), and 1971 were taking glucocorticoids only (prednisone, dexamethasone, hydrocortisone, and others; appendix pp 3–4). 902 patients were taking medications from multiple categories and were considered as a separate group. After controlling for patient characteristics, including vaccination status, immunosuppressive DMARDs (HR 1·39, 95% CI 1·13–1·72; p=0·0022), glucocorticoids (1·48, 1·21–1·80; p=0·0001), and a combination of immunosuppressant medications from multiple categories (1·58, 1·21–2·06; p=0·0008) were associated with an increased risk of SARS-CoV-2 infection compared with not taking any immunosuppressant. However, no increased risk of SARS-CoV-2 infection was observed with immunomodulatory DMARDs (HR 1·04, 95% CI 0·72–1·49; p=0·85) compared with taking no immunosuppressant (appendix p 9). Patients who had received a bone marrow or organ transplant were at higher risk of SARS-CoV-2 infection (HR 1·70, 95% CI 1·33–2·18; p<0.0001) than patients who had not received a transplant, after adjusting for immunosuppressive medication type and patient characteristics.
Immunosuppressive DMARDs (HR 2·32, 95% CI 1·23–4·38; p=0·0097) and glucocorticoids (2·93, 1·77–4·86; p<0·0001) were associated with an increased risk of hospitalisation due to COVID-19 compared with not taking any immunosuppressant. Patients who had received a bone marrow or organ transplant were again at higher risk of hospitalisation due to COVID-19 (HR 3·52, 95% CI 2·01–6·16; p<0·0001) than patients who had not received a transplant. Due to small sample size in these groups, there was less certainty in our estimates of COVID-19-related hospitalisation risk for patients taking immunomodulatory DMARDs (HR 2·02, 95% CI 0·64–6·37; p=0·23) and those taking multiple immunosuppressant medication types (1·90, 95% CI 0·90–4·01; p=0·090). Full Cox regression results for COVID-19-related hospitalisation risk by immunosuppressant type are shown in the appendix (p 10).
Among those who were immunocompetent, male sex was associated with a lower risk of SARS-CoV-2 infection compared with female sex (HR 0·87, 95% CI 0·81–0·93; p<0·0001; appendix p 5). There was no association between SARS-CoV-2 infection risk and sex among patients who were immunosuppressed (HR 0·99, 95% CI 0·78–1·27; p=0·94; appendix p 6), and no association between sex and hospitalisation due to COVID-19 in either those who were immunocompetent (1·02, 0·73–1·43; p=0·91; appendix p 7) or those who were immunosuppressed (0·93, 0·51–1·71; p=0·83; appendix p 8). Black patients were at higher risk of hospitalisation due to COVID-19 than patients of other races in both the immunosuppressed (HR 2·35, 95% CI 1·23–4·50; p=0·010) and immunocompetent (1·77, 1·14–2·73; p=0·011) groups (appendix pp 7–8).
Nine deaths (0·5 per 10 000 patients) due to COVID-19 were recorded during the study period. 25 patients had an admission to the ICU during the study period, with a higher rate of ICU admission among patients who were immunosuppressed (four patients; 7·1 ICU admissions per 10 000 patients) than among those who were immunocompetent (21; 1·3 per 10 000). Based on analysis including all patients in the study, receiving either two (vaccine effectiveness 92%, 95% CI 66–98; p=0·0007) or three doses (91%, 75–97; p<0·0001) of either the BNT162b2 vaccine or the mRNA-1273 vaccine was highly effective in reducing the risk of ICU admission (appendix p 11). The proportional hazards assumption was satisfied for all covariates in all models based on Schoenfeld residual plots.
In the sensitivity analysis excluding patients with cancer (n=9115), the findings remained the same regarding vaccine effectiveness in relation to immunosuppressant medication (data not shown).
Discussion
We found that the mRNA-1273 and BNT162b2 vaccines provided strong protection against COVID-19-related hospitalisation in patients who were immunosuppressed during the omicron-dominant SARS-CoV-2 wave in the USA. Protection against SARS-CoV-2 infection was not as substantial, but we found that patients who were immunosuppressed who received two doses of the mRNA-1273 vaccine or three doses of either vaccine had significant protection. Despite higher effectiveness estimates in patients who were immunosuppressed than those who were immunocompentent, those who were immunosuppressed were at higher risk of infection and hospitalisation due to COVID-19 than were those who were immunocompetent. It has been hypothesised that reduced antibody responses would lead to lower vaccine effectiveness in patients who are immunosuppressed. Our findings contradict this hypothesis, possibly due to higher baseline (pre-vaccine) vulnerability in patients who were immunosuppressed leading to a larger relative increase in immune protection, or differences in wider immune response (eg, T-cells). Moreover, our estimates for vaccine effectiveness are uncertain, and for all vaccine series, except two doses of mRNA-1273, the differences in effectiveness between patients who were immunosuppressed and those who were immunocompetent were not significant. Controlling for patient characteristics, including vaccination status, we found that patients taking glucocorticoids or immunosuppressive DMARDs were at higher risk of COVID-19-related hospitalisation and SARS-CoV-2 infection than patients not taking immunosuppressants. Patients who had received a bone marrow or organ transplant were particularly vulnerable to both hospitalisation and infection and remained at high risk even after vaccination with either vaccine.
Two large studies conducted before mass vaccination occurred found no increased risk of COVID-19 hospitalisation, critical care admission, or death associated with most immunosuppressant medications.23, 24 A study that assessed severe outcomes (ie, mechanical ventilation, death) found that immunosuppressant medications were not associated with higher risk of severe COVID-19 outcomes, with some exceptions.25 All of these studies analysed more severe endpoints (mechanical ventilation and death) than did our study (infection and hospitalisation). Some of these studies accounted for underlying immune-mediated inflammatory diseases24, 25 or used a standard therapy control group rather than the general population.24 The major advantages of our study are that we analysed data obtained during the omicron-dominant SARS-CoV-2 wave rather than timeframes dominated by previous variants, and that—unlike other post-vaccination studies25—we controlled for vaccination status, which is an important confounder (unbalanced between immunosuppressed and immunocompetent patients). Another study did not find increased risk of SARS-CoV-2 infection among vaccinated immunosuppressed people compared with the immunocompetent vaccinated population.26 Compared with our study, that study had information on underlying immune-mediated inflammatory diseases but used a less restrictive definition of immunosuppression and did not account for a higher incidence of third vaccine doses in the immunosuppressed population. Nonetheless, all of these findings could be consistent with increased risk of SARS-CoV-2 infection associated with some specific potent immunosuppressants but not with other immunosuppressant medications. Further studies in larger patient populations, which can account for vaccination status while studying individual immunosuppressants, are needed to determine the true effect of individual medications.
Our findings regarding vaccine effectiveness in immunosuppressed patients show a substantial reduction in effectiveness relative to previous studies that looked at the delta (B.1.617.2) variant.5, 6, 7, 8, 9 This difference is consistent with a similar reduction in the immunocompetent population, as seen in this study and in previous work.3, 4 The higher effectiveness observed for the mRNA-1273 vaccine compared with the BNT162b2 vaccine is also in accordance with previous findings in people who were immunocompetent.3, 4 However, the reduced risk of infection observed in mRNA-1273 recipients might be primarily explained by higher doses in the initial two vaccinations compared with BNT162b2, rather than the reduced third dose (50 mcg vs 100 mcg).
On March 29, 2022, the US Food and Drug Administration recommended a fourth dose for people older than 50 years or people older than 12 years who have received a solid organ transplant or have “an equivalent level” of immunocompromising condition.25 Our analysis confirmed that patients who had received a bone marrow or organ transplant were at substantially higher risk of COVID-19-related hospitalisation than those who had not, and indicated that a similar prioritisation for a fourth dose might be justified for all patients taking glucocorticoids or immunosuppressive DMARDs. With the advent of more transmissible variants, such as the BA.2 and BA.5 subvariants of omicron, the protection afforded by two or three vaccine doses is likely to deteriorate further and leave people taking immunosuppressants at high risk of SARS-CoV-2 infection and hospitalisation due to COVID-19. Our findings underscore the importance of vaccination in patients taking immunosuppressants, especially given the robust evidence that vaccines are safe in this group.27, 28
Previous research has suggested that there are minimal differences in rates of SARS-CoV-2 infection between male and female sex, with patients of male sex having a higher risk of ICU admission and death.29 We found similar risks of omicron infection and COVID-19-related hospitalisation between males and females but did not have sufficient sample size to assess differences in risk of ICU admission or death. The slightly elevated risk of SARS-CoV-2 infection among females in the immunocompetent population might be explained by region-specific factors, such as a large, majority-female population of health-care workers, or by differences in willingness to seek testing between males and females. Our finding that Black patients were at elevated risk of COVID-19-related hospitalisation compared with White patients is similar to findings from other studies.30, 31
Our study has several strengths that allow for more detailed analysis relative to previous work. We linked EHR data with immunisation data from the Michigan State Registry, allowing us to capture data on vaccinations received outside of Michigan Medicine. We considered a comprehensive list of immunosuppressant medications, allowing us to study different types of immunosuppressants, including immunosuppressive DMARDs, immunomodulatory DMARDs, and glucocorticoids. The availability of chart-reviewed COVID-19 hospitalisation data allowed us to accurately assess vaccine effectiveness during the omicron-dominant period of the pandemic, when high infection rates might have led to many incidental positive SARS-CoV-2 test results among patients who were hospitalised for other reasons.
There are several limitations to this study, mostly related to using EHR data from a single institution and under-reporting of SARS-CoV-2 infections. The sample size from a single institution limits our ability to examine individual immunosuppressant medications and mortality rate. Michigan Medicine has 15 primary care sites across a four-county area and the catchment area for these clinics crosses several more counties, so patients might seek care at other facilities outside the Michigan Medicine system, leading to missing infection data. However, the use of the number of negative tests before the study period and the number of hospital visits as covariates is an effective method of controlling for the propensity to seek care and testing. We found that the previous number of negative SARS-CoV-2 tests and hospital visits were highly predictive of observed infections during the study period. This allowed us to reduce bias due to differing degrees of interaction with the Michigan Medicine system between those who were immunosuppressed and those who were immunocompetent, or between the vaccinated and unvaccinated groups. The requirement of at least one visit with a primary care physician within the past 18 months ensured that patients had more complete follow-up data, but this might have led to selection of a slightly older population with more comorbidities than the general population; therefore, incidence of hospitalisation might be overestimated. However, this should not have affected comparisons between vaccine groups and immunosuppressant medication groups after correction for confounders.
Immunosuppressants are given for a wide variety of conditions, including autoimmune diseases, pulmonary fibrosis, and gastrointestinal or endocrine disorders. Previous studies indicate that autoimmune diseases can increase risk of severe COVID-19 outcomes, such as ICU admission, mechanical ventilation, and death, although the degree of effect depends on the severity of the underlying condition.24, 32, 33, 34 Therefore, we cannot determine whether higher infection or hospitalisation rates are due to the medications themselves, target organ involvement, or the underlying conditions they are prescribed for. Due to sample size constraints, we aggregated medications into groups and so could not investigate individual medications, the effects of which might differ depending on mode of action or potency. Given the absence of sequencing data, we could not fully guarantee that all recorded infections in our study period were caused by the omicron variant. However, given the rapid rise of omicron, it is likely that a large majority of cases after the study start date (Dec 16, 2021) were caused by this variant.
Despite these limitations, our study fills an important gap in knowledge regarding the associations between vaccination, immunosuppressant medications, and the omicron variant. In patients taking immunosuppressants, the mRNA-1273 and BNT162b2 vaccines can provide some protection against omicron infection and are highly effective against COVID-19-related hospitalisation caused by the omicron variant. It is crucially important that patients taking immunosuppressants are prioritised and remain up to date on vaccinations.
Data sharing
Data are not available without approval from the University of Michigan Institutional Review Board. Requests should be sent to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI158543. XS was also supported by NIH/NIGMS grant R01GM139926. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We used deidentified EHR data, the use of which was approved by the Institutional Review Board. We thank the University of Michigan Data Office for assistance with data extraction from electronic medical records.
Contributors
MR contributed to writing (first draft of the manuscript), review and editing of the manuscript, visualisation, formal analysis, methodology, software, and data curation. SSH contributed to review and editing of the manuscript, investigation, data curation, and conceptualisation. ES contributed to review and editing of the manuscript and conceptualisation. LY contributed to methodology, data curation, and software use. CS contributed to methodology, data curation, and software use. XS contributed to conceptualisation and methodology. GF contributed to review and editing of the manuscript and conceptualisation. LZ contributed to review and editing of the manuscript, conceptualisation, methodology, project administration, supervision, and funding acquisition. MR and LZ accessed and verified the underlying data and were responsible for the decision to submit the manuscript for publication.
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen C, Risk M, Schiopu E, et al. Efficacy of COVID-19 vaccines in patients taking immunosuppressants. Ann Rheum Dis. 2022;81:875–880. doi: 10.1136/annrheumdis-2021-222045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a Veterans Affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161:827–836. doi: 10.1053/j.gastro.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74:472–478. doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74:1515–1524. doi: 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widdifield J, Kwong JC, Chen S, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol. 2022;4:e430–e440. doi: 10.1016/S2665-9913(22)00096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-COV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett AM, Greenberg RS, Boyarsky BJ, et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant. 2021;40:1579–1588. doi: 10.1016/j.healun.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speer C, Töllner M, Benning L, et al. Third COVID-19 vaccine dose with BNT162b2 in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2022;81:593–595. doi: 10.1136/annrheumdis-2021-221747. [DOI] [PubMed] [Google Scholar]
- 14.Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4:e338–e350. doi: 10.1016/S2665-9913(22)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandl P, Tobudic S, Haslacher H, et al. Response to SARS-CoV-2 vaccination in systemic autoimmune rheumatic disease depends on immunosuppressive regimen: a matched, prospective cohort study. Ann Rheum Dis. 2022;81:1017–1022. doi: 10.1136/annrheumdis-2021-221788. [DOI] [PubMed] [Google Scholar]
- 16.Kroon FPB, Najm A, Alunno A, et al. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis. 2022;81:422–432. doi: 10.1136/annrheumdis-2021-221575. [DOI] [PubMed] [Google Scholar]
- 17.Lin D-Y, Gu Y, Zeng D, Janes HE, Gilbert PB. Evaluating vaccine efficacy against severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2022;74:544–552. doi: 10.1093/cid/ciab630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Monitoring variant proportions. 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 19.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24:3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Gasparini A. Comorbidity: an R package for computing comorbidity scores. J Open Source Softw. 2018;3:648. [Google Scholar]
- 22.Goldstein BA, Bhavsar NA, Phelan M, Pencina MJ. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol. 2016;184:847–855. doi: 10.1093/aje/kww112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis. 2021;80:1086–1093. doi: 10.1136/annrheumdis-2021-219845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKenna B, Kennedy NA, Mehrkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. 2022;4:e490–e506. doi: 10.1016/S2665-9913(22)00098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen KM, Bates BA, Rashidi ES, et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4:e33–e41. doi: 10.1016/S2665-9913(21)00325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boekel L, Stalman EW, Wieske L, et al. Breakthrough SARS-CoV-2 infections with the delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies. Lancet Rheumatol. 2022;4:e417–e429. doi: 10.1016/S2665-9913(22)00102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration FDA authorizes second booster dose of two COVID-19 vaccines for older and immunocompromised individuals. 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-second-booster-dose-two-covid-19-vaccines-older-and
- 28.Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2022;81:695–709. doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
- 29.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.England BR, Roul P, Yang Y, et al. Risk of COVID-19 in rheumatoid arthritis: a National Veterans Affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73:2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Silva KM, Jorge A, Cohen A, et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2021;73:914–920. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available without approval from the University of Michigan Institutional Review Board. Requests should be sent to the corresponding author.