Abstract
Heterogeneity in the clinical presentation of SARS-CoV-2 infection and COVID-19 progression underscores the urgent need to identify individual-level susceptibility factors that affect infection vulnerability and disease severity. Tobacco product use is a potential susceptibility factor. In this Personal View, we provide an overview of the findings of peer-reviewed, published studies relating tobacco product use to SARS-CoV-2 infection and COVID-19 outcomes, with most studies focusing on cigarette smoking in adults. Findings pertaining to the effects of tobacco product use on the incidence of SARS-CoV-2 infection are inconsistent. However, evidence supports a role for cigarette smoking in increasing the risk of poor COVID-19 outcomes, including hospital admission, progression in disease severity, and COVID-19-related mortality. We discuss the potential effects of tobacco use behaviour on SARS-CoV-2 transmission and infection, and highlight the pathophysiological changes associated with cigarette smoking that could promote SARS-CoV-2 infection and increased disease severity. We consider the biological mechanisms by which nicotine and other tobacco product constituents might affect immune and inflammatory responses to SARS-CoV-2 infection. Finally, we identify current knowledge gaps and suggest priorities for research to address acute and post-acute health outcomes of COVID-19 during and after the pandemic.
Introduction
Despite vaccination and social precautions, SARS-CoV-2 continues to spread, particularly in areas with low vaccination rates. Similar to influenza, COVID-19 might become endemic as infections spread around the globe, but not all individuals are equally susceptible to SARS-CoV-2 infection or disease. Early data on disease susceptibility showed that individuals of advanced age and those with hypertension, obesity, diabetes, or chronic obstructive pulmonary disease (COPD) have more severe symptoms and require more intensive medical care than do younger individuals or those without pre-existing conditions.1, 2, 3 In view of the strong association with pulmonary disease, cigarette smoking is considered to be a likely susceptibility factor for poor COVID-19 outcomes.4 The use of other tobacco and nicotine products has also been posited as a risk factor. However, more than 2 years after the pandemic began, the relationship between tobacco, nicotine, and COVID-19 remains unclear or contradictory.
Extensive previous research has shown that smoking induces substantial lung injury. Harmful substances in tobacco smoke damage the lung epithelium as well as the pulmonary vascular endothelium. Damage to epithelial cells compromises the epithelial barrier and mucociliary clearance. Modified molecules released by injured cells activate specific receptors in the lung that activate both innate and acquired immune responses.5 Therefore, since the beginning of the COVID-19 pandemic, there has been a keen interest in understanding whether smokers are more susceptible to SARS-CoV-2 infection and, when infected, whether they are at increased risk of more serious clinical outcomes than non-smokers. A study of Middle East respiratory syndrome coronavirus (MERS-CoV) infection found that the case fatality rate among smokers was double that of non-smokers.6 Smoking and the use of other tobacco products might therefore be expected to affect SARS-CoV-2 infection and COVID-19 outcomes.
Given the overwhelming number of infections and the severity of illness among patients with COVID-19 during the pandemic, systematic and detailed assessment of tobacco use has often been unfeasible as those providing clinical care have focused on the immediate challenges of managing large numbers of critically ill patients. However, numerous clinical questions have been raised in relation to the use of tobacco products—including electronic nicotine-delivery systems—and COVID-19, including, but not limited to, the question of whether tobacco users have different susceptibility to infection and get tested at different rates; whether they have higher propensity for symptomatic infection, greater risk of severe disease or mortality, or more severe or prolonged post-acute sequelae of COVID-19; and whether they have different responses to vaccination. Understanding the impact of tobacco and nicotine use on COVID-19—including the behavioural and biological mechanisms by which tobacco product use affects susceptibility to infection, development and course of disease (including underlying inflammatory and immune responses), and interactions with medications—and identifying specific modifiable risk factors could have implications for both the development of public health recommendations and the clinical care of patients infected with SARS-CoV-2. In addition, tobacco use will be increasingly relevant as the pandemic evolves and we consider the course of recovery after SARS-CoV-2 infection; for example, does SARS-CoV-2 infection trigger changes in the body that increase the risk of conditions such as chronic lung, heart, or brain disorders, which are also affected by tobacco use?
Key messages.
-
•
Results from epidemiological studies of the effects of cigarette smoking on SARS-CoV-2 infection are conflicting, and further research is needed to establish whether or not smoking affects susceptibility to SARS-CoV-2 infection
-
•
Smokers are at greater risk of developing severe disease following SARS-CoV-2 infection than are non-smokers, with an increased risk of hospital admission, ICU admission, and death; evidence for the effects of other tobacco products, including electronic nicotine-delivery systems, on COVID-19 incidence or progression is lacking
-
•
Mechanisms underlying the association between cigarette smoking and more severe disease might include upregulation of ACE2 receptors, immune suppression, oxidative stress, inflammation, and vascular injury; cigarette smoking increases the risk of chronic lung disease, cardiovascular disease, and diabetes, which are associated with poorer outcomes in SARS-CoV-2-infected individuals
-
•
Sharing of tobacco products could lead to SARS-CoV-2 transmission; environments in which users or bystanders are exposed to second-hand smoke and e-cigarette aerosols or spitting might also increase the risk of SARS-CoV-2 transmission
-
•
The COVID-19 pandemic has led to changes in patterns of tobacco use, with some people decreasing and others increasing or maintaining use of tobacco; long-term follow-up is needed to establish whether or not the pandemic has promoted tobacco cessation and lasting abstinence among those who expressed an intention to quit
-
•
Research is needed to establish the impact of nicotine and non-nicotine constituents of tobacco products on the risk of COVID-19, including effects on immune and inflammatory responses to SARS-CoV-2; evidence from preclinical studies suggests that nicotine might exert an anti-inflammatory effect, but there is insufficient epidemiological or experimental evidence to support the assertion that nicotine might decrease the hyperinflammatory response in people with COVID-19
ACE2=angiotensin-converting enzyme 2. ICU=intensive care unit.
As for investigations of any emerging disease, early studies are frequently based on inherently limited clinical series or public health data, but a growing body of research is now addressing the effects of tobacco use on SARS-CoV-2 infection and COVID-19 outcomes. In this Personal View, we evaluate evidence for the effects of tobacco product use on COVID-19 incidence and outcomes, including disease progression and severity, as well as changes in tobacco use behaviour during the pandemic. We highlight pathophysiological changes associated with cigarette smoking that could affect COVID-19 incidence and severity, and we outline biologically plausible mechanisms that might link nicotine and other constituents of tobacco products to SARS-CoV-2 infection susceptibility and the clinical sequelae of COVID-19. Finally, we identify current knowledge gaps and provide recommendations for future research.
Links between tobacco use and COVID-19: epidemiological evidence
We reviewed 236 epidemiological studies of tobacco use and COVID-19 published from the start of the COVID-19 pandemic to Aug 30, 2021. These epidemiological studies included evidence from many countries—but most frequently from China, the UK, and the USA—and were based mainly on data from clinical or health-care systems, although a few population-based studies had been done (see appendix pp 3–6; epidemiological studies are organised by COVID-19 disease stage, and according to whether associations were reported in multivariable or univariate models or as frequencies). Sample sizes of these studies ranged from 39 to more than 7 278 000. Most examined cigarette smoking, and only a few reported information on the use of e-cigarettes7, 8, 9, 10, 11, 12 or other tobacco products, such as smokeless tobacco13, 14 or hookah.10, 15 Because there were only a few epidemiological studies on non-cigarette tobacco products, we cannot comment on the relationships between use of tobacco products other than cigarettes and COVID-19.
Incidence of SARS-CoV-2 infection
We identified conflicting evidence for the effects of cigarette smoking on the incidence of SARS-CoV-2 infection (appendix pp 3–6). Specifically, we identified 12 multivariable and eight univariate or frequency-reporting studies that found a positive effect of cigarette smoking on SARS-CoV-2 infection rates, and 14 multivariable and 12 univariate or frequency-reporting studies that found a negative effect. In addition, we found seven multivariable studies that did not find any association. Many studies did not control for relevant confounding factors such as comorbidities, symptoms, or access to care, and most lacked detailed measures of smoking status such as smoking history or frequency of smoking. One intriguing study conducted in a controlled setting that performed SARS-CoV-2 tests and assessed smoking status for all who participated (rather than for only those with symptoms) was a study of an outbreak of COVID-19 on a French Navy aircraft carrier.16 All crew members were tested and multivariable models included age, sex, and body-mass index, but not comorbidities or medication use. The study reported that of the 76% of the crew who became infected, 48% were current cigarette smokers, and the odds ratio (OR) for infection among current smokers versus former smokers and never-smokers was 0·59 (95% CI 0·45–0·78; p<0·001). Although the odds of infection were significantly lower in smokers, 45% of those infected were smokers, suggesting that smoking did not offer complete protection. A limitation of this study is that the crew were young and healthy, and the findings might not, therefore, be generalisable to other populations of smokers. Interestingly, another aircraft carrier study that was conducted in a similar controlled environment and tested the whole population did not report any associations between tobacco and nicotine use and SARS-CoV-2 infection.17 Furthermore, although outside the scope of our review to Aug 30, 2021, a recent retrospective cohort study of more than 2·4 million adults, of whom 44 270 developed SARS-CoV-2 infection, found that compared with never-smokers, current smokers had a significantly lower adjusted rate of infection (adjusted hazard ratio [HR] 0·64, 95% CI 0·61–0·67). Infection rates were also slightly lower in former smokers than in never-smokers (0·96, 0·94–0·99).18 Despite these few positive studies, as indicated above, our review identified many studies suggesting that smoking was not protective against COVID infection, in addition to many that indicated that it was protective, so the evidence remains conflicting (see appendix pp 3–6). It is also important to note that almost all the epidemiological studies were limited to adult participants, which limits our ability to comment on younger populations. We found only one study that focused on adolescents and young adults,7 which reported a five-times or more increased likelihood of a COVID-19 diagnosis among ever-users of e-cigarettes only (OR 5·05, 95% CI 1·82–13·96), ever-users of both e-cigarettes and cigarettes (6·97, 1·98–24·55), and past 30-day users of both e-cigarettes and cigarettes (6·84, 2·40–19·55).
COVID-19 outcomes
Owing to the challenges of conducting research in the context of caring for huge numbers of critically ill patients during the pandemic, most studies on the contribution of smoking to COVID-19 outcomes have reported only crude descriptive information on tobacco use, such as ever-smoking or never-smoking, or classified participants as current, former, or never-smokers, and have not included definitions for these terms or specific frequencies of use. Moreover, only a few studies have reported differences in COVID-19-related outcomes according to current versus past smoking status or duration of abstinence in past smokers. Four studies19, 20, 21, 22 provided more detailed quantitative information on smoking. One of these was a retrospective case study of 954 patients admitted to Wuhan Red Cross Hospital, Wuhan, China, with PCR-confirmed COVID-19 between December 2019 and March 2020, which assessed current and past smoking, pack-years of smoking, and length of smoking history.19 This study reported a significantly increased risk of severe disease—intensive care unit (ICU) treatment or death—in patients with a history of smoking compared with those without a history of smoking (OR 5·5, 95% CI 3·1–9·9; p<7·3 × 10−8). An Italian study that found a positive association between the Human Development Index (an integrated index of healthy long life, education, and living standards, measured according to life expectancy at birth, mean and expected years of schooling, and gross national income per capita) and SARS-CoV-2 infection and COVID-19-related death also showed, in multivariable analysis including current cigarette smokers aged 14 years and older and frequency of smoking, a positive association between cigarettes smoked per day and incident SARS-CoV-2 infection and death.20
The third study was a review of 102 patients with lung cancer and COVID-19 in New York City, USA, in March to May 2020, which measured smoking in pack-years and, in univariate analyses comparing median smoking (23·5 pack-years) to never-smoking (0 pack-years), showed increased odds of hospital admission (OR 2·38, 95% CI 1·08–5·40), ICU admission or intubation (1·72, 0·75–4·21), and death (2·90, 1·07–9·44).21 Lastly, a study that combined large-scale observation and mendelian randomisation analyses—to address the issue of confounding in observational studies—examined smoking behaviour (documented in primary care health records) and SARS-CoV-2 infections, COVID-19-related hospital admissions, and deaths recorded in electronic health records.22 The study found an increased risk of COVID-19-related death for heavy smokers (≥20 cigarettes per day; OR 6·11, 95% CI 3·59–10·42) and moderate smokers (10–19 cigarettes per day; 5·91, 3·66–9·54) compared with never-smokers in fully adjusted models. Former smokers also had significantly increased odds of hospital admission and death in adjusted models, but light smokers did not. The investigators also conducted mendelian randomisation analyses using genetic proxies for smoking behaviours from the UK Biobank, and found significant positive associations for SARS-CoV-2 infection, hospital admission, and death with genetically predicted higher rates of cigarette smoking per day. The authors concluded that the consistent observational and genetic analyses supported a causal relationship between smoking and adverse COVID-19 outcomes.22
The overall weight of evidence was stronger for more advanced COVID-19 outcomes, suggesting that cigarette smoking is associated with more severe COVID-19 symptoms, as evidenced by the need for hospital admission. Importantly, smoking also appeared to adversely affect progression in severity of COVID-19 outcomes (eg, admission to the ICU, need for intubation), and most studies found a positive association between smoking and COVID-19-related mortality, with only a few studies showing no significant association. Of note, a study of more than 17 million patient records in England found that the increase in COVID-19-related mortality associated with smoking was no longer significant after controlling for the presence of chronic lung disease, suggesting that smoking-induced comorbidities might explain the overall increase in mortality associated with smoking.23 In addition, a recent retrospective cohort study of 44 270 SARS-CoV-2-infected individuals18 reported that, compared with never-smokers, current smokers had lower rates of hospital admission (adjusted HR 0·48, 95% CI 0·40–0·58), ICU admission (0·62, 0·42–0·87), and death (0·52, 0·27–0·89). However, former smokers had higher rates of hospital admission (1·10, 1·03–1·08) and death (1·32, 1·11–1·56).18 The findings highlight the need for further understanding of the mechanisms by which smoking affects the risk of worse outcomes in patients with COVID-19.
Summary of epidemiological findings
In summary, we found insufficient evidence to conclude whether or not smoking affects susceptibility to SARS-CoV-2 infection, but good evidence for a negative effect of smoking on the progression and severity of symptoms and on COVID-19-related mortality. However, it is important to note that the effect of smoking was not the main research question in many epidemiological studies. Moreover, because much of this research was done in the context of caring for overwhelming numbers of critically ill patients with respiratory distress, most studies did not quantify smoking frequency or intensity; therefore, we cannot draw strong conclusions about associations between patterns of use and COVID-19 outcomes. Studies were primarily cross-sectional, and most did not differentiate or compare current versus former smokers, frequency and intensity of tobacco use among users, age groups, presence or type of comorbidities, or any differences in self-protection practices, or specify the types of tobacco products used, and were focused on cigarette use among adults.
We chose not to conduct a meta-analysis owing to variation in methodological rigour, variation and lack of specificity in assessments of tobacco use status and COVID-19 outcomes, and high variability in sample size. Although we have included sample sizes of the studies listed in the appendix (pp 3–6), it should be noted that those with larger sample sizes were not always better, owing to methodological limitations, such as crude and outdated assessment of smoking status. Furthermore, although a living review24 and multiple meta-analyses25, 26, 27, 28 on this topic have been published, many include studies that have been published without peer review on pre-publishing sites, and the large methodological and measurement variations between studies suggest that it might not be appropriate to report this heterogeneous body of literature as a meta-analysis. Methodological variability and limitations might partially explain the mixed results that we observed for tobacco use and initial infection rates. Nevertheless, despite the variability and limitations, there was broad consensus across studies that cigarette smoking was significantly associated with progression of COVID-19 following initial infection, similar to the conclusions of the living review;24 however, in contrast to the living review, which currently suggests that smoking is protective against SARS-CoV-2 infection,24 the evidence reviewed here does not allow definitive conclusions regarding incidence of infection.
Tobacco product use behaviours during the pandemic
Shared use and risk of SARS-CoV-2 transmission
In addition to the potential for direct effects of tobacco, nicotine, or smoke constituents, behavioural aspects of tobacco product use could have an impact on the risk of SARS-CoV-2 infection. The communal nature of e-cigarette use and waterpipe smoking often leads to the sharing of a single device or mouthpiece between users, especially among young people in social settings,7, 29, 30 which could increase the potential for transmission of infectious agents. Previous studies have shown that waterpipe use is associated with an increased risk of transmission of respiratory viruses, hepatitis C virus, Epstein-Barr virus, and herpes simplex virus.31, 32 Particles of SARS-CoV-2 have been found in saliva from COVID-19-positive individuals, making saliva a potential route for SARS-CoV-2 transmission among users who share tobacco products.33
Second-hand smoke or aerosol exposure and risks to bystanders
Passive smokers are exposed to the toxicants present in tobacco smoke, and several studies have shown that people living with smokers have increased susceptibility to infection. A systematic review of studies assessing the effect of passive smoking on the risk of lower respiratory tract infections in children under 2 years of age found that exposure to smoking by both parents, as well as prenatal and postnatal smoke exposure, significantly increased the risk of infants developing lower respiratory tract infections.34 Nevertheless, whether second-hand exposure to tobacco smoke or e-cigarette aerosols exhaled by SARS-CoV-2-positive tobacco users constitutes a significant risk of infection is unclear. At least in principle, exhaled particles in aerosols can transport viral particles between people.35 Of particular concern is the use of tobacco products in small or poorly ventilated indoor spaces, which increases exposure to particulates, given that particulates in ambient outdoor air appear to increase the risk of spreading SARS-CoV-2 infection.36 Studies showing a significant association between air pollution and COVID-1937 suggest that exposure to fine particles, such as those in tobacco smoke and e-cigarette aerosols, increases the risk of SARS-CoV-2 infection.38 Nevertheless, SARS-CoV-2 viral loads in exhaled tobacco smoke and e-cigarette aerosols have not been determined, and it is unclear how long SARS-CoV-2 particles remain viable after they have been exhaled with cigarette smoke or e-cigarette aerosols.39
Droplets expelled in the context of face-to-face exposure during talking, coughing, or sneezing are the most common mode of SARS-CoV-2 transmission. Even brief exposure to individuals who are symptomatic (eg, coughing) is associated with higher risk for SARS-CoV-2 transmission.40 Because chronic cough is a common respiratory symptom among tobacco users,41 bystanders in close proximity to tobacco users infected with SARS-CoV-2 could be at particularly high risk of infection. The absence of mask wearing when smoking or using e-cigarettes creates additional opportunities for transmission of SARS-CoV-2. Moreover, the use of smokeless tobacco products that are often held in the mouth for some time and then spat out along with saliva generates salivary droplets that could promote airborne infection,42 and thus increase the risk of infection among bystanders.
Changes in tobacco product use behaviours
Owing to its widespread social, economic, health, and behavioural impacts, COVID-19 is likely to have multiple influences on tobacco use behaviour. Studies of changes in and predictors of tobacco use during the COVID-19 pandemic have had mixed results, with most showing that, within the same sample, some participants reduced their tobacco use, while others increased or maintained use. Across all studies, including cross-sectional and a few longitudinal designs, about 5–70% of the participants reported trying to quit or reduce tobacco use, or reported quitting altogether.43, 44, 45, 46, 47, 48, 49, 50, 51, 52 Approximately 7–55% of participants increased use or relapsed,46, 47, 48, 51, 53, 54, 55, 56 and 14–93% of participants reported no change in tobacco use.46, 47, 48, 51, 53, 54, 57, 58 Importantly, although some studies have reported a decrease in the overall prevalence or amount of cigarette smoking during the pandemic,59, 60 others report no change in use frequency or even an increase in the number of cigarettes smoked per day60, 61, 62, 63 or an increase in the amount of other tobacco products used (eg, cigars, cigarillos).64
Similar to studies of conventional cigarettes, those focused on e-cigarette use have shown increases, decreases, and no change in use within study samples,54, 65 although many other studies showed decreases in e-cigarette use.49, 50, 61, 66, 67, 68, 69, 70, 71, 72 One longitudinal study that followed adolescents over time both before and during the pandemic showed that a reduction in youth e-cigarette use in young adults was occurring even before the pandemic.50 Another study showed reductions in amount and frequency of use during the pandemic.69 Studies of cigar users have shown similar results to those for smoking, with many participants intending to quit and a third to a half reporting attempting to quit, although about half of cigar users continued to use as before the pandemic.73, 74, 75, 76 More than half of waterpipe users stopped using or wanted to quit.77
Changes in tobacco use during the pandemic did not vary much by age. The proportion of adolescents and young adults quitting or attempting to quit ranged from about 7% to 30%,49, 50, 66, 78 and the proportion of adults ranged from 1·1% to 50%.46, 47, 48, 53, 79, 80, 81, 82 Furthermore, some studies showed that men were less likely than women to stop using cigarettes or e-cigarettes during the pandemic;54, 58, 80 however, other studies showed that men were more likely than women to quit tobacco use.69 Some of the same studies showed that alcohol use increased and physical activity decreased during the pandemic,50, 61, 76, 82, 83, 84 although others have reported declines in alcohol use.72, 85
Perceptions and reasons for changes in tobacco product use
Across studies, adolescents, young adults, and adults were concerned about COVID-19 and perceived that use of cigarettes and e-cigarettes increased their risk of infection or severe COVID-19 symptoms, or both.45, 47, 52, 77, 86, 87 Such perceived risk of infection or disease severity, or concern over lung health more generally, was associated with reduced tobacco use.49, 79, 80, 86, 88, 89, 90, 91, 92 Additional reasons for reduced tobacco use included wanting to help others who might have COVID-19;88 concerns that parents might learn about their use;49 and closure of bars and pubs, suspension of classes, and reduced socialising with friends.52 Those with chronic illness were also more likely to reduce tobacco use during the pandemic.44, 93 Reasons reported for greater tobacco use included response to stress or anxiety,43, 64, 88, 94 depression,48, 65, 95, 96, 97 boredom,88, 98 fewer smoking bans,88 and feeling social isolation.88 Adolescents, young adults, and adults also reported reducing tobacco use because of reduced access to e-cigarettes49, 66, 70, 78 and cigarettes98, 99 during the pandemic. Several participants reported stocking up on tobacco66, 78 or rationing supplies.78 Berg and colleagues66 found that about a quarter of adolescent and young adult participants worried that vape shops would close, decreasing their access, but in reality, access was not as difficult as they expected.
Public health messaging and industry activities
Public health authorities such as WHO and the US National Cancer Institute,100, 101 and anti-smoking campaigns such as US state tobacco campaigns,102, 103 included messages warning about a smoking-related increased risk of COVID-19 in their media campaigns in early 2020.104 One study testing COVID-19 messaging reported that messages with both COVID-19 health warnings and traditional health warnings were perceived to be effective in discouraging use among current smokers and e-cigarette users.104 The recommendations of the US Advisory Committee on Immunization Practices in December 2020 included smokers as a priority population for early COVID-19 vaccinations.105
Tobacco industry promotional and policy activities during the pandemic might also have influenced perceptions, behaviour, and structural factors affecting tobacco use. To preserve access, the tobacco industry adapted to provide delivery and pick-up options for tobacco products. Independent reports have documented that the tobacco industry cited the pandemic in lobbying efforts against implementation of tobacco control policies,106 mirrored health authority COVID-19 messaging in promotional campaigns,107 and developed corporate social responsibility activities to gain access to governmental authorities,108, 109, 110 similar to past behaviour.111 Tobacco marketing and promotional activities included arguments that vape shops were essential businesses,112 sale of e-cigarette-branded masks linked to device purchase,113 offers of COVID-19-related promotion codes,114 and co-opting hashtags such as #StayAtHome and #FlattenTheCurve in tobacco advertising.107 Tobacco companies used the pandemic to argue successfully for a deadline extension to submit premarket review applications for new tobacco products to the US Food and Drug Administration.115 Pandemic-associated economic losses were also used to oppose policies relating to flavoured tobacco products in the EU and the USA.106, 110 Furthermore, despite the lack of clear evidence, vaping websites questioned the risks of e-cigarette use for COVID-19, and unsupported claims that vaping and nicotine can be protective against COVID-19 proliferated on social media.114, 116, 117
Biological relationships between tobacco product use and COVID-19
Smoking-associated pathophysiology
Many biological factors are likely to have a role in determining the link between tobacco product use and COVID-19. Cigarette smoking is associated with increased susceptibility to and severity of respiratory infections, including influenza and tuberculosis.118, 119, 120 Mechanisms for this association include both immune suppression and physical changes, such as abnormal ciliary function.5 Combustion products of tobacco smoke induce oxidative stress, promote inflammation and vascular injury, and establish a prothrombotic state121—changes that could promote SARS-CoV-2 infection and contribute to increased severity of COVID-19. Furthermore, cigarette smoking causes cardiovascular disease and chronic lung disease, which are associated with poorer outcomes in SARS-CoV-2-infected individuals.1, 2, 3 Here, we describe biological mechanisms related to cigarette smoking and tobacco product use that might influence the clinical sequelae of COVID-19, including the potential effects of smoking on viral binding and the possible role of nicotine and non-nicotine constituents of tobacco products in determining the susceptibility and response to SARS-CoV-2 and the progression of disease in individual users.
Cigarette smoking and viral binding
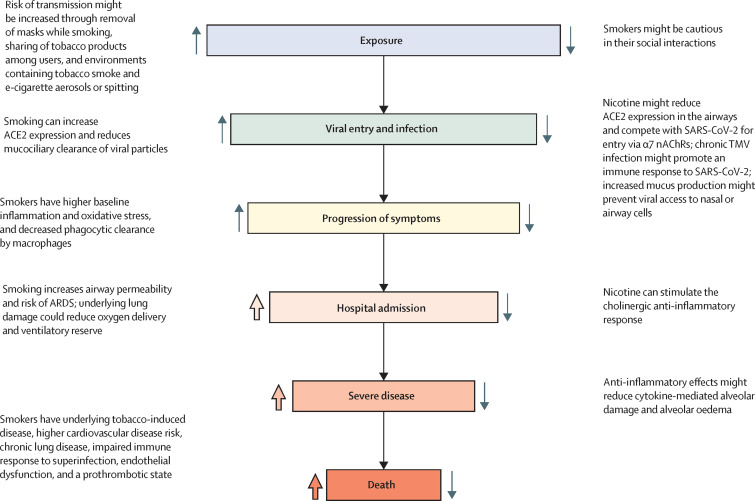
Multiple theories have been proposed in describing the mechanisms that link cigarette smoking to COVID-19. One theory relates to viral binding. The first stage of SARS-CoV-2 infection is the binding of its spike protein to angiotensin-converting enzyme 2 (ACE2) in the lung or the nasopharynx. This leads to entry of the virus into the cell, following proteolytic cleavage and activation of the spike protein by transmembrane protease serine 2 (TMPRSS2).122 Individuals with a history of cigarette smoking have been found to have significant overexpression of the ACE2 protein in lung epithelial cells123 and significant increases in TMPRSS2 expression.122 Moreover, current smokers and those with COPD have significantly higher expression of ACE2 in small airways compared with non-smokers.124, 125 Similarly, mice exposed to cigarette smoke express nearly 80% more ACE2 in their lungs compared with sham-treated mice, suggesting that smoking-induced upregulation of ACE2 in the lung126 might increase the risk of infection and disease severity (figure 1 ).
Figure 1.
Stages at which cigarette smoking might affect the risk of SARS-CoV-2 infection and the course of COVID-19
Upward-facing arrows indicate potential increased risk and downward-facing arrows indicate decreased risk. Wide, coloured arrows are used to indicate mechanisms for which evidence in humans is strong; narrow, grey arrows indicate mechanisms for which evidence is mixed or lacking. Note that some mechanisms might affect more than one stage of infection and disease progression. α7 nAChRs=α7 nicotinic acetylcholine receptors. ACE2=angiotensin-converting enzyme 2. ARDS=acute respiratory distress syndrome. TMV=tobacco mosaic virus.
Potential role of nicotine
In contrast to cigarette smoking, the effects of nicotine are less clear. Nicotine has been shown to downregulate ACE2 expression in experimental animals,127 but to elevate ACE2 expression in human bronchial epithelial cells.128 However, exposure to smoke has recently been reported to decrease membrane ACE2 expression in human bronchial epithelial cells, and this reduction was directly correlated with nicotine delivery.129 In another study, smoking but not e-cigarette use upregulated ACE2 expression,130 suggesting that overexpression of ACE2 in smokers is not due to nicotine. Regardless of the stimulus, the theory that increased ACE2 expression elevates the risk of developing SARS-CoV-2 infection and disease progression conflicts with the observation that drugs that increase ACE2 expression, such as ACE inhibitors and angiotensin receptor blockers, do not seem to increase the likelihood of a positive test for SARS-CoV-2131 or the risk of severe COVID-19.131, 132 Another theory, based on amino acid homologies between the SARS-CoV-2 virus spike glycoprotein and a snake venom toxin that binds to nicotinic acetylcholine receptors (nAChRs), posits that nAChRs might also serve as an entry binding site for SARS-CoV-2.133, 134 If correct, nicotine might compete with the virus for surface binding, and thereby reduce SARS-CoV-2 entry. Additionally, some researchers have speculated that the tobacco mosaic virus, which is found frequently in smokers' airways, and which stimulates adaptive and innate immunity, might have a protective effect against SARS-CoV-2 infection.135 However, at present, any protective role for nicotine is speculative and any potential effects of nicotine must be viewed in the context of the range of factors at play in determining disease risk in tobacco product users, including smokers.
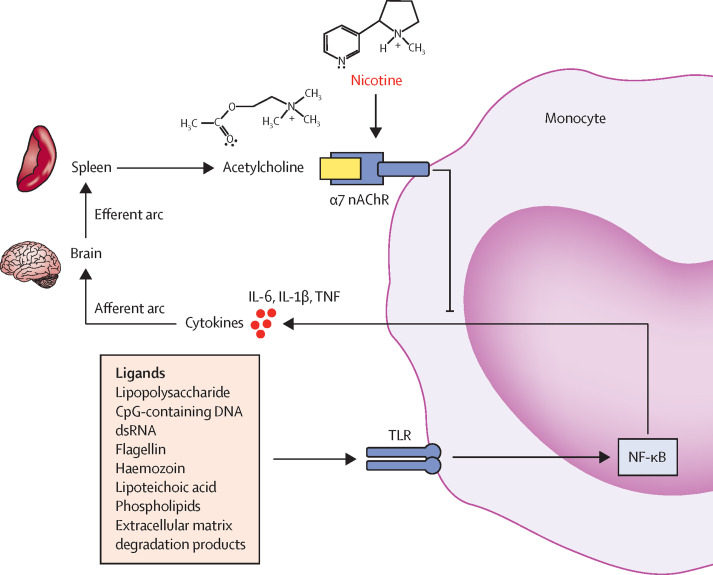
Acute lung injury and life-threatening complications of SARS-CoV-2 infection are accompanied by a hyperinflammatory immune response,136 and clinical trials have shown that fluvoxamine, an antidepressant drug that downregulates cytokine production, protects symptomatic adults who have not been admitted to hospital with COVID-19 from clinical deterioration, supporting a potential therapeutic effect of immune suppression early in the course of SARS-CoV-2 infection.137 Activation of the cholinergic anti-inflammatory pathway could also antagonise the development of such hyperinflammatory responses.138, 139 Products of inflammation act on the brain to activate the parasympathetic nervous system to release acetylcholine, which then acts on circulating immune cells to modulate the immune response. Because nAChRs (primarily α7) mediate the cholinergic anti-inflammatory response, nicotine or vagal stimulation might reduce systemic inflammation.139 Activation of the α7 nAChR suppresses nuclear factor-κB-dependent transcriptional events, which in turn leads to downregulation of cytokine production and an attenuated response to inflammatory triggers such as lipopolysaccharides and double-stranded RNA (figure 2 ).140, 141, 142, 143, 144, 145, 146 In addition, nicotine acts on macrophages to inhibit the release of proinflammatory but not anti-inflammatory cytokines and chemokines.140
Figure 2.
The cholinergic anti-inflammatory reflex
Several products of infection, tissue injury, or ischaemia (eg, lipopolysaccharides, dsRNA, extracellular matrix degradation products) activate the immune response, which results in the release of cytokines from innate immune cells, such as monocytes and macrophages in the spleen. These cytokines act on afferent nerves in the parasympathetic nervous system, generating signals that are transmitted via the vagus nerve or act directly on the brain, which in turn activate efferent anti-inflammatory pathways in the brainstem. Efferent neural pathways travel via the vagus nerve to the spleen, where acetylcholine is released. Acetylcholine acts on α7 nAChRs on the surface of immune cells to downregulate the immune response via a mechanism involving TLRs, which activate the nuclear transcription factor NF-κB. NF-κB downregulates transcription of genes that control the production of proinflammatory cytokines. The reflex control of immunity is described by Tracey.140 Nicotine activates α7 nAChRs and has been shown to have anti-inflammatory effects in animal models of immune-mediated disease;141, 142, 143, 144, 145, 146 however, direct evidence for a protective effect of nicotine in humans is weak. α7 nAChR=α7 nicotinic acetylcholine receptor. dsRNA=double-stranded RNA. IL-1β=interleukin-1β. IL-6=interleukin-6. NF-κB=nuclear factor-κB. TLR=Toll-like receptor. TNF=tumour necrosis factor.
In animal studies, activation of the cholinergic pathway has been shown to protect against immune-mediated disease, including acute lung injury,141, 142 sepsis,143, 144, 145 viral myocarditis,146 acute kidney injury,147 and brain injury.148 These studies have shown that nicotine might have the potential to dampen immune responses and reduce tissue injury under conditions similar to those of the initial stages of COVID-19. In mouse and rat models of acid-induced acute lung injury, nicotine substantially decreased lung vascular permeability.141 And in a mouse model of acute lung injury induced by intra-tracheal administration of lipopolysaccharide, which simulates acute respiratory distress syndrome similar to that seen in severely ill patients with COVID-19,142 nicotine substantially decreased leukocyte infiltration and proinflammatory cytokines concentrations. Notably, in the same model, cigarette smoke exposure increased lung injury.149 Thus, at least in animal models, nicotine by itself seems to exert an anti-inflammatory effect; however, insufficient epidemiological or experimental evidence exists at present to support the assertion that nicotine might decrease the hyperinflammatory response in people with COVID-19. Moreover, in cigarette smoke, the effects of nicotine are likely to be modified by other constituents and toxicants generated during combustion; similarly, in the case of other tobacco products, nicotine will be one of a number of constituents that might influence the effects of SARS-CoV-2 and the host response to infection (table ). Nicotine has several adverse cardiopulmonary effects172 and is highly addictive, so any recreational use of nicotine products should be strongly discouraged; however, the possibility that nicotine-containing medications or nicotine replacement drugs might be therapeutic at doses without strong cardiopulmonary effects and with little risk of addiction warrants further investigation.
Table.
Potential mechanisms by which non-nicotine constituents of tobacco products could affect susceptibility to SARS-CoV-2 infection or severity of COVID-19
| Tobacco product constituent | Mechanism of toxicity relevant to viral infections | Potential implications for COVID-19 | |
|---|---|---|---|
| Polyaromatic hydrocarbons | Produced from the incomplete combustion of organic compounds, polyaromatic hydrocarbons are present in smoke emitted from combustible tobacco products, including cigarettes, cigars, and waterpipes | Exposure to polyaromatic hydrocarbons has been shown to result in immune suppression or immune potentiation, depending on variables such as timing of testing or dosage, but immune suppression is the most frequently reported effect;150 underlying mechanisms remain unclear, but immunomodulatory effects of polyaromatic hydrocarbons might be due to their reactive epoxide metabolites151 | Exposure to polyaromatic hydrocarbons from combustible tobacco products might result in immune suppression, thereby increasing the severity of COVID-19152 |
| Acrolein | Acrolein, a carbonyl, is a toxic product of combustible tobacco products; acrolein has also been found in emissions from e-cigarettes | Acrolein might be responsible for the suppression of cytokine production by cigarette smoke;153 acute high-dose acrolein exposure might suppress innate immunity and inflammatory responses, thereby increasing susceptibility to infection, whereas chronic low-dose exposures might increase inflammation, leading to tissue injury153, 154, 155, 156 | Acrolein could potentially diminish defences against SARS-CoV-2 infection by inhibiting macrophage responses and their function via suppression of NF-κB or direct alkylation of signalling proteins, or by suppressing M1 macrophage proinflammatory reactions and favouring anti-inflammatory M2 macrophage responses, consistent with observations in smokers154 |
| Carbon monoxide | Carbon monoxide is a gaseous toxicant generated by combustible tobacco products | When inhaled, carbon monoxide reacts with haemoglobin to produce carboxyhaemoglobin, thereby diminishing the oxygen-carrying capacity of blood; at low concentrations, carbon monoxide induces vasodilation, and can differentially and selectively inhibit the expression of proinflammatory cytokines TNF, IL-1β, and MIP-1β, and increase lipopolysaccharide-induced expression of the anti-inflammatory cytokine IL-10157 | Research efforts are underway to validate treatments based on the delivery of pharmacological amounts of carbon monoxide to patients infected with other viruses; because a protective effect against viral infections has been reported primarily in experimental models,158, 159, 160 whether carbon monoxide delivery from tobacco smoke could modulate the development of COVID-19 remains unknown161 |
| Humectants | Vegetable glycerin and propylene glycol are commonly used in e-cigarettes; both chemicals are also frequently employed as humectants in combustible cigarettes | Animal studies have shown that inhalation of vegetable glycerin and propylene glycol, followed by virus inoculation, was associated with increased lung viral titres,162 downregulated innate immunity against influenza virus,163 and disrupted immune homoeostasis164, 165 | Exposure to humectants commonly used in e-cigarettes and combustible cigarettes could potentially impair innate and adaptive immune defences against SARS-CoV-2 |
| Flavourings | Flavouring chemicals commonly used in flavoured cigars, e-cigarettes, and waterpipe tobacco include aliphatic aldehydes (for fruity flavours) and aromatic aldehydes (for sweet and spicy flavours) | Emerging evidence from in-vitro and in-vivo animal models suggests that at least some flavourings used in tobacco products (cinnamaldehyde, vanillin, ethyl maltol) cause oxidative stress in respiratory cells, induce release of proinflammatory cytokines, and have immunosuppressive effects;166, 167, 168 however, there is little direct evidence of toxicity in humans | Because inhaled flavouring chemicals can potentially alter airway epithelial functions such as inflammatory responses and innate immune defence against respiratory viruses, users of flavoured tobacco products might be at a higher risk of SARS-CoV-2 infection and more severe COVID-19 |
| Particulate matter | Tobacco smoke contains respirable particles that can be deposited in the lungs | Inhalation of particulates induces oxidative stress in the lungs, leading to cell damage and stimulation of several inflammatory pathways;169 it is speculated that inhaled particulates could inhibit antiviral responses by inducing inflammation and cytotoxicity in the lungs and decreasing virus-induced cytokine production by alveolar macrophages170, 171 | Inhalation of particulate matter with tobacco smoke can cause lung cell inflammation and negatively affect the antiviral response, thereby potentially increasing sensitivity to SARS-CoV-2 infection and the severity of COVID-19 symptoms in people who smoke or in those exposed to second-hand tobacco smoke or e-cigarette aerosol |
IL-1β=interleukin-1β. IL-10=interleukin-10. MIP-1β=macrophage inflammatory protein-1β. NF-κB=nuclear factor-κB. TNF=tumour necrosis factor.
Despite the known harms of nicotine use, if anti-inflammatory effects of nicotine were to be demonstrated in humans, this might have potential implications with respect to smoking cessation in the context of COVID-19 (see appendix p 16 for current approaches to smoking cessation). For example, an anti-inflammatory effect of nicotine might provide a rationale for encouraging smokers with COVID-19 to stop smoking with the use of nicotine replacement therapy or drugs such as varenicline or cytisine, all of which activate α7 nAChRs, as a step towards tobacco and nicotine use cessation. Note that the use of commercially available electronic nicotine-delivery systems is not advised for those attempting to stop smoking.
Potential role of non-nicotine constituents of tobacco products
Emissions from inhaled tobacco products contain not only nicotine, but also many respiratory toxicants and irritants, such as benzene, formaldehyde, acrolein, 1,3-butadiene, aromatic amines, polyaromatic hydrocarbons, and a range of other volatile organic compounds. Inhalation of these toxicants could potentially affect the progression and severity of COVID-19,173 in part because the pathological changes induced by tobacco smoke constituents are similar to the pathophysiological mechanisms of SARS-CoV-2 infection. Chronic exposure to toxicants in cigarette smoke promotes inflammation121 and, similarly, systemic inflammatory responses contribute to severe COVID-19, as evidenced by elevated plasma concentrations of proinflammatory cytokines (similar to the so-called cytokine storm described in other conditions174, 175). The proinflammatory cytokines produced during COVID-19 are similar to those that are elevated by smoking.176 Moreover, smoking increases the permeability of epithelial cells and mucus production, and impairs mucociliary clearance.177 Such changes, in concert with increased inflammation, could potentially increase the severity of COVID-19-associated lung injury in smokers (figure 1).
Most human studies cannot isolate the effects of exposure to a single ingredient in a tobacco product, but rather report effects of a mixture, such as tobacco smoke, e-cigarette aerosol, or waterpipe smoke. Many of the immunotoxicology studies that have focused on a single chemical were done in vitro or in animals with variable dosing regimens, routes, and durations of exposure. This variability in experimental conditions creates challenges for extrapolating laboratory results to human exposure and human epidemiology. Nevertheless, several chemicals present in tobacco products have been shown to affect various inflammatory signalling pathways in cells, and thus potentially modulate immunological responses to viral infection in users (table). Some tobacco constituents have been shown to induce immune suppression responses, whereas others cause proinflammatory responses.155, 156, 178, 179 At certain stages of SARS-CoV-2 infection, immune suppression in tobacco users might theoretically prevent inflammation-related organ injury, but this theory remains to be tested. By contrast, exposure to tobacco product ingredients that exhibit strong proinflammatory activity might exacerbate harm caused by SARS-CoV-2 infection.
The use of tobacco products other than combustible cigarettes could also affect SARS-CoV-2 infection and COVID-19 progression, but the effects of these products are as yet unknown. For example, it is unclear how e-cigarette use affects the risk of SARS-CoV-2 infection and disease progression. Although e-cigarette aerosols contain lower numbers and concentrations of most toxicants than do combustible tobacco products, they still expose users to nicotine as well as several other potentially harmful chemicals. Emerging evidence suggests that inhaling oxidants, chemicals, particulates, and flavourings that are present in e-cigarette aerosols could induce inflammation, bronchoconstriction, and airway remodelling.180, 181 In experimental animals, exposure to e-cigarette aerosols has been found to trigger systemic inflammation and to increase the abundance of macrophages, T-lymphocytes, and proinflammatory cytokines in bronchoalveolar lavage fluid. When infected with the flu virus, mice exposed to e-cigarette aerosols show delayed immune responses and persistent lung inflammation and oedema, as well as increased haemorrhage and mortality.163, 181 Studies in humans suggest that e-cigarette use is associated with increased inflammation and elevated plasma IgE concentrations, which are consistent with e-cigarette users reporting a higher prevalence of chronic cough, bronchitis, asthma, and wheezing.181
The effects of smokeless tobacco on viral infections are largely unknown, and data on the effects of smokeless tobacco on systemic inflammation are conflicting. Chronic use of smokeless tobacco has been associated with systemic inflammation and a prothrombotic state in some studies,182 but not in others.183, 184 Systemic dysregulation of immune responses (decrease in CD4+ and CD8+ T cells and CD11b monocytes) by smokeless tobacco has been shown in animals.185 A cross-sectional study from Sweden of more than 26 000 users of snus (an oral form of tobacco) found that snus use was associated with a higher prevalence of chronic bronchitis, chronic rhinosinusitis, and sleep disturbances,186 all of which could affect COVID-19 severity.
Summary of mechanistic findings
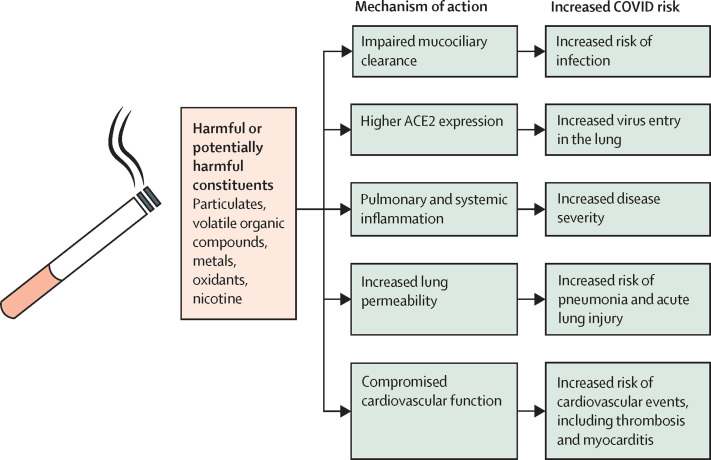
Tobacco products contain a large number of potentially toxic chemicals; additional chemicals are generated during aerosolisation due to heating or combustion. Although the contributions of individual chemicals and constituents remain unclear, the use of tobacco products can cause substantial injury leading to impaired mucociliary clearance in the lung, higher expression of ACE2, immune dysregulation, cardiovascular dysfunction, and an increase in lung permeability, which can collectively increase the risk of viral infection and severe disease (figure 3 ). Nicotine, by activating the cholinergic response, exerts an anti-inflammatory effect in a variety of pathological states; however, its role in the context of the complex milieu of tobacco product constituents remains unclear.
Figure 3.
Potential mechanisms by which smoking could increase COVID-19 risk
Exposure to the harmful substances present in or produced by cigarettes and other tobacco products leads to substantial pulmonary and cardiovascular dysfunction and injury that could collectively increase the risk of SARS-CoV-2 infection and progression to severe COVID-19.
Conclusions and future directions
In our review of the evidence, we found that epidemiological studies of the effects of cigarette smoking on SARS-CoV-2 infection have produced conflicting results, and further research is needed to ascertain whether or not smoking or the use of other tobacco products affects susceptibility to SARS-CoV-2 infection. However, the evidence indicates that smokers are at greater risk of poor outcomes from COVID-19, including hospital admission and progression to severe disease, than are non-smokers. We identified biological mechanisms that might underpin the link between cigarette smoking and the severity and course of COVID-19; however, further studies are needed to establish the role of nicotine and non-nicotine constituents of tobacco products in determining disease susceptibility.
It should be noted that we have focused mainly on published research that was done during the initial pandemic outbreak in 2020–21, and that more recent developments, such as the effects of tobacco use in relation to vaccination and new SARS-CoV-2 variants, are not covered here. As is the case for other scientific investigations conducted as part of a rapid response to a large-scale adverse event, the studies discussed in this Personal View have heterogeneous methods and, in some cases, lack measurement specificity—eg, lack of detailed information on frequency and intensity of smoking history and types of tobacco products used. This is commensurate with the need to rapidly respond with information about a new virus and its health consequences, without the time to design and conduct large resource-intensive studies. Consequently, there is a need for additional studies with more systematic assessment and more detailed measurements of smoking behaviour. Increased methodological rigour and innovation in experimental design are required to identify and quantify the effects of tobacco products such as e-cigarettes, smokeless tobacco, cigars, and hookahs on COVID-19 incidence and outcomes. Future studies using longitudinal designs or comparing cohorts over different time periods to examine changes in tobacco use behaviour during the pandemic are also warranted, as well as studies relating changes in restriction policies and self-protection policies during spikes in infection rates.
As the pandemic has evolved, additional important research questions have emerged. One such question relates to the long-term health effects of COVID-19—the so-called post-COVID-19 condition or long COVID—and how these long-term sequelae might be affected by tobacco product use. A second question relates to changes in tobacco use behaviour post-COVID-19. A third pressing issue relates to whether smoking affects immune responses to anti-SARS-CoV-2 vaccination. Smoking could reduce vaccine efficacy either by reducing immune responses or by creating a pre-vaccination hyperinflammatory response. For example, Godoy and colleagues187 did a case-control study of patients over the age of 65 years with influenza and found that influenza vaccination prevented 21% of hospital admissions in smokers and ex-smokers compared with 39% of hospital admissions in never-smokers. The efficacy of vaccination was modest in general and was non-significantly higher in non-smokers when compared with smokers. In patients with COPD, the effectiveness of influenza vaccination does not appear to be modified by current smoking status.188 However, results from a randomized, double-blind, placebo-controlled trial on the efficacy of influenza vaccination189 found a greater antibody titre rise due to vaccination in smokers compared with non-smokers. By contrast, hepatitis B vaccination has been found to be less immunogenic in smokers versus non-smokers.190, 191 A recent meta-analysis found that 17 of 23 studies of responses to SARS-CoV-2 vaccines showed much lower antibody titres or more rapid lowering of vaccine-induced IgG in smokers than in non-smokers.192 Given such disparate responses to different vaccines, an important research priority is to assess and follow immunogenic responses and clinical protection in smokers who have received anti-SARS-CoV-2 vaccines.
Finally, studies on the relationship between tobacco use status and COVID-19 outcomes done after 2021 need to consider the SARS-CoV-2 vaccination status of the population being studied as well as the effect of vaccination on COVID-19 screening rates (see appendix p 17 for a brief survey of tobacco use history). Another important research question concerns how the relationship between tobacco use and COVID-19 might change as new variants of SARS-CoV-2 with different infectivity and virulence emerge. These and other recommendations for future research during and after the COVID-19 pandemic are listed in the panel . A deeper understanding of the links between tobacco product use and disease risk could help to shape public health recommendations and to improve the clinical care of those with a history of tobacco dependence. To achieve meaningful progress in future studies, it will be imperative to obtain sufficient information regarding tobacco product use, and to develop a validated and harmonised approach for the systematic evaluation of the effects of tobacco product use on the susceptibility to and severity of COVID-19 and other future microbial threats.
Panel. Priorities for future research.
-
•
Conduct randomised sampling of the general population to understand the association between SARS-CoV-2 infection and tobacco product use in symptomatic and asymptomatic individuals
-
•
In studies of SARS-CoV-2 infection, COVID-19, and other infectious agents and diseases, use rigorous research methods, including longitudinal designs with precise measures of current tobacco use, more precise identification and stratification of types of tobacco products used, timing of use, and frequency and intensity of use
-
•
Perform multivariable analyses or case-control studies with propensity matching to examine whether tobacco use is associated with SARS-CoV-2 infection or COVID-19 severity independent of tobacco-induced comorbidities
-
•
Identify modifiers and mediators of the relationship between tobacco use and COVID-19 risk, severity, and outcomes; systematically include demographic, psychosocial, and health variables such as age, gender, and comorbidities, and other variables such as anxiety, access to mental health care, perceptions of risk, misperceptions about COVID-19, and testing hesitancy
-
•
Assess the effect of vaccination on the association between tobacco use and COVID-19 outcomes and delineate the pathophysiological mechanisms by which smoking and use of other tobacco products affects the humoral response to SARS-CoV-2 vaccines
-
•
Conduct well controlled animal studies to differentiate the effects of tobacco smoke inhalation and nicotine on the risk of SARS-CoV-2 infection and disease severity and outcomes
-
•
Conduct studies in humans to specifically compare the effects of inhaled and non-inhaled tobacco product use and to establish the effects of different tobacco products on the risk of SARS-CoV-2 infection and COVID-19 severity and outcomes
-
•
Further investigate the effects of ACE2 inhibitors and angiotensin receptor blockers on the relationship between tobacco product use and COVID-19 risk, severity, and outcomes
-
•
Further evaluate the efficacy of nicotine-replacement therapy, varenicline, or cytisine for the treatment of patients with COVID-19 who have a history of tobacco product use, in terms of tobacco cessation and COVID-19 outcomes
-
•
Examine changes in tobacco product use, sustainability of changes, and coping and resilience after COVID-19; determine the immediate and long-term effects of the pandemic on increases, decreases, switching, and quitting of cigarettes and other tobacco products
-
•
Conduct long-term studies to investigate the effects of tobacco product use on the post-COVID-19 condition
ACE2=angiotensin-converting enzyme 2.
Search strategy and selection criteria
On Dec 9, 2020, we searched PubMed and Embase using a comprehensive set of search terms (appendix pp 1–2) for articles on COVID-19 and tobacco. Similar searches were conducted on Web of Science, CINAHL, PsycINFO, and Sociological Abstracts, after which duplicates and opinion pieces were removed. On Aug 30, 2021, we conducted a second search of PubMed, Embrace, Web of Science, CINAHL, PsychINFO, and Sociological Abstracts, using nearly identical search terms to those used in the first search, for articles on COVID-19 and tobacco published between Dec 9, 2020, and Aug 30, 2021. The final list consisted of 2151 articles. Articles were screened to remove those not presenting original research findings. Further screening, detailed assessment, data extraction, and classification by co-authors (BH-F, SK-S, PML, MAP, and RMR) resulted in two sets of references: 236 epidemiological studies on the relationship between tobacco use and COVID-19 incidence and outcomes, and 87 behavioural studies on tobacco use and perceptions during the COVID-19 pandemic. Papers on biological mechanisms were selected on the basis of their relevance to the aims of the paper. Full details of the search strategy and selection criteria are included in the appendix (pp 1–2). We included all relevant studies that met criteria in our literature review. Papers were cited within the narrative on the basis of relevance to the topics covered and the aims of this Personal View, and the complete set of studies is included in the appendix (categorised by outcome, pp 3–6, and full reference details, pp 7–15). After our original searches and preparation of this Personal View, several key reports were published and identified by the authors, including some high-quality studies and papers relevant to our discussion, which were cited in the text as the paper was revised and prepared for publication.
Declaration of interests
NLB reports honoraria from McGraw Hill (for a chapter on “Antihypertensive agents” in Basic and Clinical Pharmacology) and Wolters Kluwer (for a chapter on the “Cardiovascular effects of nicotine” in UpToDate), payment for expert testimony against tobacco companies, and consulting fees from Pfizer and Achieve Life Sciences. NLB and MLG were speakers at the 2017 Global Nicotine Forum; they received no reimbursement from the tobacco industry for this role and the conference received no sponsorship from tobacco companies, e-cigarette companies, or the pharmaceutical industry. MLG reports ad-hoc consultancy fees from WHO, the US Food and Drug Administration (FDA), the Campaign for Tobacco-Free Kids, the University of South Carolina, and the University of North Carolina; honoraria for lectures from the University of Rochester, Memorial Sloan Kettering Cancer Center, and the University of South Carolina; reimbursement for travel from WHO and the International Association for the Study of Lung Cancer; and consultancy fees from Johnson & Johnson. MLG has a leadership role as a member of the Tobacco Products and Cancer Subcommittee of the American Association for Cancer Research and is a member of the Tobacco Control and Cessation Committee of the International Association for the Study of Lung Cancer. SK-S is the President of the Society for Research on Nicotine and Tobacco. PML is a member of the California Tobacco Education and Research Oversight Committee. BH-F reports payment for expert testimony in e-cigarette litigations and volunteer membership on the board for Parents Against Vaping E-cigarettes. RJO'C reports consultancy fees from the National Institutes of Health, WHO, Georgetown University, and the FDA; he also reports payment from the British Medical Journal for his work as Senior Editor, and support for travel and meeting attendance from WHO, the FDA, and Georgetown University. MAP, RMR, and AB declare no competing interests.
Acknowledgments
Acknowledgments
All authors are in leadership positions at Tobacco Centers of Regulatory Science, funded by the US Food and Drug Administration (FDA) and the National Institutes of Health (NIH). Work in the laboratories of the authors is supported by grants to NLB (HL147127), MLG (CA228110, HL142511, CA20749, CA210625, DA051446, DA037446, DA039264), BH-F (HL147127), PML (HL147127, TRDRP 27IR-0042, CA141661), RJO'C (CA228110), MAP (CA180905), and AB and RMR (HL120163). We thank Evans Whitaker (Health Sciences Library, University of California, San Francisco) for designing and conducting the search queries, and Anshula Kesh (American Heart Association), Francesca Vescia (Stanford University), and Nicholas Franco and Thomas Liss (Yale University) for their assistance with the bibliography. The content of this paper is solely the responsibility of the authors and does not represent the official views of the FDA or the NIH.
Contributors
All authors contributed equally to the writing and revision of this Personal View. All authors approved the final version of the manuscript.
Supplementary Material
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyunoya T, Mebratu Y, Contreras A, Delgado M, Chand HS, Tesfaigzi Y. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am J Respir Cell Mol Biol. 2014;50:471–482. doi: 10.1165/rcmb.2013-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam HS, Park JW, Ki M, Yeon MY, Kim J, Kim SW. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaiha SM, Cheng J, Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J Adolesc Health. 2020;67:519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Croft DP, Ossip DJ, Xie Z. The association between statewide vaping prevalence and COVID-19. Prev Med Rep. 2020;20 doi: 10.1016/j.pmedr.2020.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meini S, Fortini A, Andreini R, Sechi LA, Tascini C. The paradox of the low prevalence of current smokers among COVID-19 patients hospitalized in nonintensive care wards: results from an Italian multicenter case–control study. Nicotine Tob Res. 2021;23:1436–1440. doi: 10.1093/ntr/ntaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mostafa A, El-Sayed MH, El-Meteini M, et al. SARS-Co-V2 infection in never, former, and current tobacco/nicotine users: a cohort study of 4040 Egyptian healthcare workers. BMC Public Health. 2021;21 doi: 10.1186/s12889-021-11290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aung S, Vittinghoff E, Nah G, et al. Characteristics and behaviors associated with prevalent SARS-CoV-2 infection. Int J Gen Med. 2021;14:1063–1067. doi: 10.2147/IJGM.S305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams SH, Park MJ, Schaub JP, Brindis CD, Irwin CE., Jr Medical vulnerability of young adults to severe COVID-19 illness—data from the National Health Interview Survey. J Adolesc Health. 2020;67:362–368. doi: 10.1016/j.jadohealth.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam MZ, Riaz BK, Islam A, et al. Risk factors associated with morbidity and mortality outcomes of COVID-19 patients on the 28th day of the disease course: a retrospective cohort study in Bangladesh. Epidemiol Infect. 2020;148:e263. doi: 10.1017/S0950268820002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantele A, Laaveri T, Kareinen L, et al. SARS-CoV-2 infections among healthcare workers at Helsinki University Hospital, Finland, spring 2020: serosurvey, symptoms and risk factors. Travel Med Infect Dis. 2021;39 doi: 10.1016/j.tmaid.2020.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirsoleymani S, Nekooghadam SM, Marzaleh MA, et al. Assessment of risk factors for severe coronavirus disease 2019 among Iranian patients. Iran Red Crescent Med J. 2020;22:e72. [Google Scholar]
- 16.Paleiron N, Mayet A, Marbac V, et al. Impact of tobacco smoking on the risk of COVID-19. A large scale retrospective cohort study. Nicotine Tob Res. 2021;23:1398–1404. doi: 10.1093/ntr/ntab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper MR, Geibe JR, Sears CL, et al. An outbreak of Covid-19 on an aircraft carrier. N Engl J Med. 2020;383:2417–2426. doi: 10.1056/NEJMoa2019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young-Wolff KC, Slama N, Alexeeff SE, et al. Cigarette smoking and risk of SARS-CoV-2 infection and disease severity among adults in an integrated health care system in California. Nicotine Tob Res. 2022 doi: 10.1093/ntr/ntac090. published online April 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Long X, Zhang Q, et al. Tobacco smoking confers risk for severe COVID-19 unexplainable by pulmonary imaging. J Intern Med. 2021;289:574–583. doi: 10.1111/joim.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K, He M, Zhuang Z, He D, Li H. Unexpected positive correlation between human development index and risk of infections and deaths of COVID-19 in Italy. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clift AK, von Ende A, Tan PS, et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax. 2022;77:65–73. doi: 10.1136/thoraxjnl-2021-217080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7) Addiction. 2021;116:1319–1368. doi: 10.1111/add.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22:1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93:1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulsen A, Yigitbas BA, Uslu B, Dromann D, Kilinc O. The effect of smoking on COVID-19 symptom severity: systematic review and meta-analysis. Pulm Med. 2020;2020 doi: 10.1155/2020/7590207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatnagar A, Maziak W, Eissenberg T, et al. Water pipe (hookah) smoking and cardiovascular disease risk: a scientific statement from the American Heart Association. Circulation. 2019;139:e917–e936. doi: 10.1161/CIR.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKelvey K, Baiocchi M, Halpern-Felsher B. Adolescents' and young adults' use and perceptions of pod-based electronic cigarettes. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urkin J, Ochaion R, Peleg A. Hubble bubble equals trouble: the hazards of water pipe smoking. ScientificWorldJournal. 2006;6:1990–1997. doi: 10.1100/tsw.2006.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knishkowy B, Amitai Y. Water-pipe (narghile) smoking: an emerging health risk behavior. Pediatrics. 2005;116:e113–e119. doi: 10.1542/peds.2004-2173. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Ren B, Peng X, et al. Saliva is a non-negligible factor in the spread of COVID-19. Mol Oral Microbiol. 2020;35:141–145. doi: 10.1111/omi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayes L, Haslam PL, Gratziou CG, et al. SmokeHaz: systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest. 2016;150:164–179. doi: 10.1016/j.chest.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 35.Mahabee-Gittens EM, Merianos AL, Matt GE. Letter to the Editor regarding: “An Imperative Need for Research on the Role of Environmental Factors in Transmission of Novel Coronavirus (COVID-19)” —secondhand and thirdhand smoke as potential sources of COVID-19. Environ Sci Technol. 2020;54:5309–5310. doi: 10.1021/acs.est.0c02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azuma K, Yanagi U, Kagi N, Kim H, Ogata M, Hayashi M. Environmental factors involved in SARS-CoV-2 transmission: effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ Health Prev Med. 2020;25:66. doi: 10.1186/s12199-020-00904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu G, Li X, Hu L, Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ Sci Technol. 2020;54:3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- 40.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 41.Dicpinigaitis PV. Effect of tobacco and electronic cigarette use on cough reflex sensitivity. Pulm Pharmacol Ther. 2017;47:45–48. doi: 10.1016/j.pupt.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Gaunkar RB, Nagarsekar A, Carvalho KM, Jodalli PS, Mascarenhas K. COVID-19 in smokeless tobacco habitues: increased susceptibility and transmission. Cureus. 2020;12 doi: 10.7759/cureus.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carreras G, Lugo A, Stival C, et al. Impact of COVID-19 lockdown on smoking consumption in a large representative sample of Italian adults. Tob Control. 2021 doi: 10.1136/tobaccocontrol-2020-056440. published online March 29. [DOI] [PubMed] [Google Scholar]
- 44.Gold AK, Hoyt DL, Milligan M, et al. The role of fear of COVID-19 in motivation to quit smoking and reductions in cigarette smoking: a preliminary investigation of at-risk cigarette smokers. Cogn Behav Ther. 2021;50:295–304. doi: 10.1080/16506073.2021.1877340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordon M, Eyestone E, Hutchison S, et al. A qualitative study exploring older smokers' attitudes and motivation toward quitting during the COVID-19 pandemic. Prev Med Rep. 2021;22 doi: 10.1016/j.pmedr.2021.101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koopmann A, Georgiadou E, Reinhard I, et al. The effects of the lockdown during the COVID-19 pandemic on alcohol and tobacco consumption behavior in Germany. Eur Addict Res. 2021;27:242–256. doi: 10.1159/000515438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rigotti NA, Chang Y, Regan S, et al. Cigarette smoking and risk perceptions during the COVID-19 pandemic reported by recently hospitalized participants in a smoking cessation trial. J Gen Intern Med. 2021;36:3786–3793. doi: 10.1007/s11606-021-06913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knell G, Robertson MC, Dooley EE, Burford K, Mendez KS. Health behavior changes during COVID-19 pandemic and subsequent “stay-at-home” orders. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17176268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaiha SM, Lempert LK, Halpern-Felsher B. Underage youth and young adult e-cigarette use and access before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaffee BW, Cheng J, Couch ET, Hoeft KS, Halpern-Felsher B. Adolescents' substance use and physical activity before and during the COVID-19 pandemic. JAMA Pediatr. 2021;175:715–722. doi: 10.1001/jamapediatrics.2021.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson SE, Brown J, Shahab L, Steptoe A, Fancourt D. COVID-19, smoking and inequalities: a study of 53 002 adults in the UK. Tob Control. 2021;30:e111–e121. doi: 10.1136/tobaccocontrol-2020-055933. [DOI] [PubMed] [Google Scholar]
- 52.Ho LLK, Li WHC, Cheung AT, et al. Impact of COVID-19 on the Hong Kong Youth Quitline service and quitting behaviors of its users. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17228397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqi K, Siddiqui F, Khan A, et al. The impact of COVID-19 on smoking patterns in Pakistan: findings from a longitudinal survey of smokers. Nicotine Tob Res. 2021;23:765–769. doi: 10.1093/ntr/ntaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kale D, Herbec A, Perski O, Jackson SE, Brown J, Shahab L. Associations between vaping and Covid-19: cross-sectional findings from the HEBECO study. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peckham E, Allgar V, Crosland S, et al. Investigating smoking and nicotine dependence among people with severe mental illness during the COVID-19 pandemic: analysis of linked data from a UK Closing the Gap cohort. BJPsych Open. 2021;7:e86. doi: 10.1192/bjo.2021.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benschop A, van Bakkum F, Noijen J. Changing patterns of substance use during the coronavirus pandemic: self-reported use of tobacco, alcohol, cannabis, and other drugs. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.633551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zajacova A, Jehn A, Stackhouse M, Denice P, Ramos H. Changes in health behaviours during early COVID-19 and socio-demographic disparities: a cross-sectional analysis. Can J Public Health. 2020;111:953–962. doi: 10.17269/s41997-020-00434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanafi E, Siste K, Limawan AP, et al. Alcohol- and cigarette-use related behaviors during quarantine and physical distancing amid COVID-19 in Indonesia. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.622917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cransac-Miet A, Zeller M, Chague F, et al. Impact of COVID-19 lockdown on lifestyle adherence in stay-at-home patients with chronic coronary syndromes: towards a time bomb. Int J Cardiol. 2021;323:285–287. doi: 10.1016/j.ijcard.2020.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malta DC, Szwarcwald CL, Barros MBA, et al. The COVID-19 pandemic and changes in adult Brazilian lifestyles: a cross-sectional study, 2020. Epidemiol Serv Saude. 2020;29 doi: 10.1590/S1679-49742020000400026. [DOI] [PubMed] [Google Scholar]
- 61.Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67:354–361. doi: 10.1016/j.jadohealth.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12 doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odone A, Lugo A, Amerio A, et al. COVID-19 lockdown impact on lifestyle habits of Italian adults. Acta Biomed. 2020;91:87–89. doi: 10.23750/abm.v91i9-S.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen-Sankey JC, Broun A, Duarte DA, et al. Exploring changes in cigar smoking patterns and motivations to quit cigars among Black young adults in the time of COVID-19. Addict Behav Rep. 2020;12 doi: 10.1016/j.abrep.2020.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romm KF, Patterson B, Crawford ND, et al. Changes in young adult substance use during COVID-19 as a function of ACEs, depression, prior substance use and resilience. Subst Abus. 2022;43:212–221. doi: 10.1080/08897077.2021.1930629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berg CJ, Callanan R, Johnson TO, et al. Vape shop and consumer activity during COVID-19 non-essential business closures in the USA. Tob Control. 2021;30:e41–e44. doi: 10.1136/tobaccocontrol-2020-056171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tattan-Birch H, Perski O, Jackson S, Shahab L, West R, Brown J. COVID-19, smoking, vaping and quitting: a representative population survey in England. Addiction. 2021;116:1186–1195. doi: 10.1111/add.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez M, Epperson AE, Halpern-Felsher B, Halliday DM, Song AV. Smokers are more likely to smoke more after the COVID-19 California lockdown order. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18052582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hopkins DB, Al-Hamdani M. Young Canadian e-cigarette users and the COVID-19 pandemic: examining vaping behaviors by pandemic onset and gender. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.620748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreslake JM, Simard BJ, O'Connor KM, Patel M, Vallone DM, Hair EC. E-cigarette use among youths and young adults during the COVID-19 pandemic: United States, 2020. Am J Public Health. 2021;111:1132–1140. doi: 10.2105/AJPH.2021.306210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sokolovsky AW, Hertel AW, Micalizzi L, White HR, Hayes KL, Jackson KM. Preliminary impact of the COVID-19 pandemic on smoking and vaping in college students. Addict Behav. 2021;115 doi: 10.1016/j.addbeh.2020.106783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorisdottir IE, Asgeirsdottir BB, Kristjansson AL, et al. Depressive symptoms, mental wellbeing, and substance use among adolescents before and during the COVID-19 pandemic in Iceland: a longitudinal, population-based study. Lancet Psychiatry. 2021;8:663–672. doi: 10.1016/S2215-0366(21)00156-5. [DOI] [PubMed] [Google Scholar]
- 73.Kowitt SD, Cornacchione Ross J, Jarman KL, et al. Tobacco quit intentions and behaviors among cigar smokers in the United States in response to COVID-19. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17155368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rebollar Alvarez A, Nuez Vicente C, Lozano Polo A, et al. Tobacco use in Spain during COVID-19 lockdown: an evaluation through social media. Rev Esp Salud Publica. 2021;95 (in Spanish). [PubMed] [Google Scholar]
- 75.Guignard R, Andler R, Quatremere G, et al. Changes in smoking and alcohol consumption during COVID-19-related lockdown: a cross-sectional study in France. Eur J Public Health. 2021;31:1076–1083. doi: 10.1093/eurpub/ckab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolokotroni O, Mosquera MC, Quattrocchi A, Heraclides A, Demetriou C, Philippou E. Lifestyle habits of adults during the COVID-19 pandemic lockdown in Cyprus: evidence from a cross-sectional study. BMC Public Health. 2021;21:786. doi: 10.1186/s12889-021-10863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalan ME, Ghobadi H, Taleb ZB, et al. COVID-19 and beliefs about tobacco use: an online cross-sectional study in Iran. Environ Sci Pollut Res Int. 2021;28:40346–40354. doi: 10.1007/s11356-020-11038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soule EK, Mayne S, Snipes W, Guy MC, Breland A, Fagan P. Impacts of COVID-19 on electronic cigarette purchasing, use and related behaviors. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17186762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naik BN, Biswas B, Singh C, Pandey S, Nirala SK, Chaudhary N. Tobacco use pattern and quitting behaviour among healthcare professionals during the COVID-19 pandemic: Insights from a pan India online survey. Clin Epidemiol Glob Health. 2021;12 doi: 10.1016/j.cegh.2021.100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gravely S, Craig LV, Cummings KM, et al. Smokers' cognitive and behavioural reactions during the early phase of the COVID-19 pandemic: findings from the 2020 ITC four country smoking and vaping survey. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18:229. doi: 10.1186/s12967-020-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koyama S, Tabuchi T, Okawa S, et al. Changes in smoking behavior since the declaration of the COVID-19 state of emergency in Japan: a cross-sectional study from the Osaka health app. J Epidemiol. 2021;31:378–386. doi: 10.2188/jea.JE20200533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez-Bueno R, Calatayud J, Casana J, et al. COVID-19 confinement and health risk behaviors in Spain. Front Psychol. 2020;11 doi: 10.3389/fpsyg.2020.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson SE, Garnett C, Shahab L, Oldham M, Brown J. Association of the COVID-19 lockdown with smoking, drinking and attempts to quit in England: an analysis of 2019–20 data. Addiction. 2021;116:1233–1244. doi: 10.1111/add.15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kusuma D, Pradeepa R, Khawaja KI, et al. Low uptake of COVID-19 prevention behaviours and high socioeconomic impact of lockdown measures in South Asia: evidence from a large-scale multi-country surveillance programme. SSM Popul Health. 2021;13 doi: 10.1016/j.ssmph.2021.100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chertok IRA. Perceived risk of infection and smoking behavior change during COVID-19 in Ohio. Public Health Nurs. 2020;37:854–862. doi: 10.1111/phn.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Streck JM, Kalkhoran S, Bearnot B, et al. Perceived risk, attitudes, and behavior of cigarette smokers and nicotine vapers receiving buprenorphine treatment for opioid use disorder during the COVID-19 pandemic. Drug Alcohol Depend. 2021;218 doi: 10.1016/j.drugalcdep.2020.108438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bommele J, Hopman P, Walters BH, et al. The double-edged relationship between COVID-19 stress and smoking: implications for smoking cessation. Tob Induc Dis. 2020;18:63. doi: 10.18332/tid/125580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogel EA, Henriksen L, Schleicher NC, Prochaska JJ. Perceived susceptibility to and seriousness of COVID-19: associations of risk perceptions with changes in smoking behavior. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Y, Lindblom EN, Salloum RG, Ward KD. Perceived health risks associated with the use of tobacco and nicotine products during the COVID-19 pandemic. Tob Induc Dis. 2021;19:46. doi: 10.18332/tid/136040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein EG, Koopman Gonzalez S, Pike Moore S, Bohnert EJ, Quisenberry AJ, Trapl ES. Pulling your mask down to smoke: qualitative themes from young adults on nicotine use during a pandemic. Subst Use Misuse. 2021;56:437–441. doi: 10.1080/10826084.2020.1869264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Philip K, Cumella A, Farrington-Douglas J, Laffan M, Hopkinson N. Respiratory patient experience of measures to reduce risk of COVID-19: findings from a descriptive cross-sectional UK wide survey. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bar-Zeev Y, Shauly M, Lee H, Neumark Y. Changes in smoking behaviour and home-smoking rules during the initial COVID-19 lockdown period in Israel. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bourion-Bedes S, Tarquinio C, Batt M, et al. Stress and associated factors among French university students under the COVID-19 lockdown: the results of the PIMS-CoV 19 study. J Affect Disord. 2021;283:108–114. doi: 10.1016/j.jad.2021.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J, Lee HJ, Hong Y, Shin YW, Chung S, Park J. Risk perception, unhealthy behavior, and anxiety due to viral epidemic among healthcare workers: the relationships with depressive and insomnia symptoms during COVID-19. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.615387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tavolacci MP, Wouters E, Van de Velde S, et al. The impact of COVID-19 lockdown on health behaviors among students of a French university. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18084346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kar P, Tomfohr-Madsen L, Giesbrecht G, Bagshawe M, Lebel C. Alcohol and substance use in pregnancy during the COVID-19 pandemic. Drug Alcohol Depend. 2021;225 doi: 10.1016/j.drugalcdep.2021.108760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giovenco DP, Spillane TE, Maggi RM, Lee EY, Philbin MM. Multi-level drivers of tobacco use and purchasing behaviors during COVID-19 “lockdown”: a qualitative study in the United States. Int J Drug Policy. 2021;94 doi: 10.1016/j.drugpo.2021.103175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klemperer EM, West JC, Peasley-Miklus C, Villanti AC. Change in tobacco and electronic cigarette use and motivation to quit in response to COVID-19. Nicotine Tob Res. 2020;22:1662–1663. doi: 10.1093/ntr/ntaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.@WHO April 22, 2020. https://twitter.com/who/status/1252879486606913536
- 101.@NCICancerCtrl May 5, 2020. https://twitter.com/NCICancerCtrl/status/1257665358498009089
- 102.Office of Attorney General Maura Healey Smoking, vaping & COVID-19. https://www.mass.gov/doc/covid-vaping-advisory-english-and-spanish/download
- 103.UNDO COVID-19 and tobacco: what you need to know now. Feb 23, 2022. https://www.undo.org/disease/covid-19-and-tobacco/
- 104.Grummon AH, Hall MG, Mitchell CG, et al. Reactions to messages about smoking, vaping and COVID-19: two national experiments. Tob Control. 2020;31:402–410. doi: 10.1136/tobaccocontrol-2020-055956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices' updated interim recommendation for allocation of COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657–1660. doi: 10.15585/mmwr.mm695152e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.STOP The tobacco industry's subversive lobbying during COVID-19. June 18, 2020. https://exposetobacco.org/news/ti-subversive-lobbying/
- 107.The Conversation Coronavirus: big tobacco sees an opportunity in the pandemic. May 14, 2020. https://theconversation.com/coronavirus-big-tobacco-sees-an-opportunity-in-the-pandemic-138188/
- 108.Tobacco Tactics. Tobacco Control Research Group. University of Bath COVID-19. June 15, 2021. https://tobaccotactics.org/wiki/covid-19/#COVID19-briefings/
- 109.Zatoński M, Gilmore AB, Hird TR. The two faces of the tobacco industry during the COVID-19 pandemic. May 10, 2020. https://blogs.bmj.com/tc/2020/05/10/the-two-faces-of-the-tobacco-industry-during-the-covid-19-pandemic/
- 110.John Dunham & Associates Senate Bill 810's flavor ban will decimate Florida small businesses. https://vaportechnology.org/wp-content/uploads/2020/03/Flavor-Ban-Economic-Impact-FLORIDA-3-19-20-FINAL.pdf
- 111.Fooks GJ, Gilmore AB, Smith KE, Collin J, Holden C, Lee K. Corporate social responsibility and access to policy elites: an analysis of tobacco industry documents. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Globally reported tobacco industry interference during COVID-19 pandemic. http://www.tobaccounmaskedsouth.asia/Globally_Reported_Tobacco_Industry_Interference_during_COVID-19_Pandemic TobaccoUnmaskedSouth.Asia.
- 113.Campaign for Tobacco-Free Kids Big Tobacco is exploiting COVID-19 to market its harmful products. https://www.tobaccofreekids.org/media/2020/2020_05_covid-marketing/
- 114.Ramamurthi D, Chau C, Jackler RK. Exploitation of the COVID-19 pandemic by e-cigarette marketers. Tob Control. 2021;30:e56–e59. doi: 10.1136/tobaccocontrol-2020-055855. [DOI] [PubMed] [Google Scholar]
- 115.US Food and Drug Administration Coronavirus (COVID-19) update: court grants FDA's request for extension of premarket review submission deadline for certain tobacco products because of impacts from COVID-19. April 23, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-court-grants-fdas-request-extension-premarket-review-submission-deadline/
- 116.Majmundar A, Allem JP, Cruz TB, Unger JB. Public health concerns and unsubstantiated claims at the intersection of vaping and COVID-19. Nicotine Tob Res. 2020;22:1667–1668. doi: 10.1093/ntr/ntaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaminski M, Muth A, Bogdanski P. Smoking, vaping, and tobacco industry during COVID-19 pandemic: Twitter data analysis. Cyberpsychol Behav Soc Netw. 2020;23:811–817. doi: 10.1089/cyber.2020.0384. [DOI] [PubMed] [Google Scholar]
- 118.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 119.Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect. 2013;67:169–184. doi: 10.1016/j.jinf.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Lopez-Hernandez Y, Rivas-Santiago CE, Lopez JA, Mendoza-Almanza G, Hernandez-Pando R. Tuberculosis and cigarette smoke exposure: an update of in vitro and in vivo studies. Exp Lung Res. 2018;44:113–126. doi: 10.1080/01902148.2018.1444824. [DOI] [PubMed] [Google Scholar]
- 121.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 122.Piva F, Sabanovic B, Cecati M, Giulietti M. Expression and co-expression analyses of TMPRSS2, a key element in COVID-19. Eur J Clin Microbiol Infect Dis. 2021;40:451–455. doi: 10.1007/s10096-020-04089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu A, Zhang X, Li R, et al. Overexpression of the SARS-CoV-2 receptor ACE2 is induced by cigarette smoke in bronchial and alveolar epithelia. J Pathol. 2021;253:17–30. doi: 10.1002/path.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang H, Rostami MR, Leopold PL, et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med. 2020;202:219–229. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev Cell. 2020;53:514. doi: 10.1016/j.devcel.2020.05.012. 29.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315:R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Russo P, Bonassi S, Giacconi R, Malavolta M, Tomino C, Maggi F. COVID-19 and smoking: is nicotine the hidden link? Eur Respir J. 2020;55 doi: 10.1183/13993003.01116-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caruso M, Distefano A, Emma R, et al. Role of cigarette smoke on angiotensin-converting enzyme-2 protein membrane expression in bronchial epithelial cells using an air-liquid interface model. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee AC, Chakladar J, Li WT, et al. Tobacco, but not nicotine and flavor-less electronic cigarettes, induces ACE2 and immune dysregulation. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21155513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farsalinos K, Eliopoulos E, Leonidas DD, Papadopoulos GE, Tzartos S, Poulas K. Nicotinic cholinergic system and COVID-19: in silico identification of an interaction between SARS-CoV-2 and nicotinic receptors with potential therapeutic targeting implications. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21165807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Changeux JP, Amoura Z, Rey FA, Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. C R Biol. 2020;343:33–39. doi: 10.5802/crbiol.8. [DOI] [PubMed] [Google Scholar]
- 135.de Bernardis E, Busa L. A putative role for the tobacco mosaic virus in smokers' resistance to COVID-19. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee TC, Vigod S, Bortolussi-Courval E, et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 139.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 140.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Su X, Lee JW, Matthay ZA, et al. Activation of the α7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37:186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mabley J, Gordon S, Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 2011;34:231–237. doi: 10.1007/s10753-010-9228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 144.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 145.van Westerloo DJ, Giebelen IA, Florquin S, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 146.Cheng Z, Li-Sha G, Jing-Lin Z, et al. Protective role of the cholinergic anti-inflammatory pathway in a mouse model of viral myocarditis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sadis C, Teske G, Stokman G, et al. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS One. 2007;2:e469. doi: 10.1371/journal.pone.0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ. Nicotinic attenuation of central nervous system inflammation and autoimmunity. J Immunol. 2009;182:1730–1739. doi: 10.4049/jimmunol.182.3.1730. [DOI] [PubMed] [Google Scholar]
- 149.Gotts JE, Abbott J, Fang X, et al. Cigarette smoke exposure worsens endotoxin-induced lung injury and pulmonary edema in mice. Nicotine Tob Res. 2017;19:1033–1039. doi: 10.1093/ntr/ntx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin Immunol. 2001;98:2–10. doi: 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]
- 151.Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. Mechanism of 7,12-dimethylbenz[a]anthracene-induced immunotoxicity: role of metabolic activation at the target organ. Jpn J Pharmacol. 2001;86:302–309. doi: 10.1254/jjp.86.302. [DOI] [PubMed] [Google Scholar]
- 152.Jerzynska J, Podlecka D, Polanska K, Hanke W, Stelmach I, Stelmach W. Prenatal and postnatal exposure to polycyclic aromatic hydrocarbons and allergy symptoms in city children. Allergol Immunopathol (Madr) 2017;45:18–24. doi: 10.1016/j.aller.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Lambert C, McCue J, Portas M, et al. Acrolein in cigarette smoke inhibits T-cell responses. J Allergy Clin Immunol. 2005;116:916–922. doi: 10.1016/j.jaci.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 154.Moghe A, Ghare S, Lamoreau B, et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Conklin DJ, Malovichko MV, Zeller I, et al. Biomarkers of chronic acrolein inhalation exposure in mice: implications for tobacco product-induced toxicity. Toxicol Sci. 2017;158:263–274. doi: 10.1093/toxsci/kfx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sithu SD, Srivastava S, Siddiqui MA, et al. Exposure to acrolein by inhalation causes platelet activation. Toxicol Appl Pharmacol. 2010;248:100–110. doi: 10.1016/j.taap.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 158.Seehase S, Lauenstein HD, Schlumbohm C, et al. LPS-induced lung inflammation in marmoset monkeys — an acute model for anti-inflammatory drug testing. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fredenburgh LE, Perrella MA, Barragan-Bradford D, et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight. 2018;3 doi: 10.1172/jci.insight.124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mitchell LA, Channell MM, Royer CM, Ryter SW, Choi AM, McDonald JD. Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L891–L897. doi: 10.1152/ajplung.00366.2009. [DOI] [PubMed] [Google Scholar]
- 161.Dattilo M. The role of host defences in Covid 19 and treatments thereof. Mol Med. 2020;26:90. doi: 10.1186/s10020-020-00216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129:4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Szafran BN, Pinkston R, Perveen Z, et al. Electronic-cigarette vehicles and flavoring affect lung function and immune responses in a murine model. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Q, Khan NA, Muthumalage T, et al. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadv. 2019;1:609–623. doi: 10.1096/fba.2019-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clapp PW, Pawlak EA, Lackey JT, et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol. 2017;313:L278–L292. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gerloff J, Sundar IK, Freter R, et al. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol. 2017;3:28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS) Tob Control. 2016;25:ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mantecca P, Sancini G, Moschini E, et al. Lung toxicity induced by intratracheal instillation of size-fractionated tire particles. Toxicol Lett. 2009;189:206–214. doi: 10.1016/j.toxlet.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 170.Farina F, Sancini G, Longhin E, Mantecca P, Camatini M, Palestini P. Milan PM1 induces adverse effects on mice lungs and cardiovascular system. Biomed Res Int. 2013;2013 doi: 10.1155/2013/583513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kaan PM, Hegele RG. Interaction between respiratory syncytial virus and particulate matter in guinea pig alveolar macrophages. Am J Respir Cell Mol Biol. 2003;28:697–704. doi: 10.1165/rcmb.2002-0115OC. [DOI] [PubMed] [Google Scholar]
- 172.Bhatnagar A. E-cigarettes and cardiovascular disease risk: evaluation of evidence, policy implications, and recommendations. Curr Cardiovasc Risk Rep. 2016;10:24. [Google Scholar]
- 173.Margaritopoulos GA, Harari S, Caminati A, Antoniou KM. Smoking-related idiopathic interstitial pneumonia: a review. Respirology. 2016;21:57–64. doi: 10.1111/resp.12576. [DOI] [PubMed] [Google Scholar]
- 174.Lukan N. “Cytokine storm”, not only in COVID-19 patients. Mini-review. Immunol Lett. 2020;228:38–44. doi: 10.1016/j.imlet.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Monneret G, Benlyamani I, Gossez M, et al. COVID-19: what type of cytokine storm are we dealing with? J Med Virol. 2021;93:197–198. doi: 10.1002/jmv.26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ Toxicol Pharmacol. 2020;78 doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Malovichko MV, Abplanalp WT, McFall SA, et al. Subclinical markers of cardiovascular toxicity of benzene inhalation in mice. Toxicol Appl Pharmacol. 2021;431 doi: 10.1016/j.taap.2021.115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abplanalp WT, Wickramasinghe NS, Sithu SD, et al. Benzene exposure induces insulin resistance in mice. Toxicol Sci. 2019;167:426–437. doi: 10.1093/toxsci/kfy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaur G, Pinkston R, McLemore B, Dorsey WC, Batra S. Immunological and toxicological risk assessment of e-cigarettes. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0119-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Keith R, Bhatnagar A. Cardiorespiratory and immunologic effects of electronic cigarettes. Curr Addict Rep. 2021;8:336–346. doi: 10.1007/s40429-021-00359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dwivedi S, Goel A, Khattri S, Sharma P, Pant KK. Aggravation of inflammation by smokeless tobacco in comparison of smoked tobacco. Indian J Clin Biochem. 2015;30:117–119. doi: 10.1007/s12291-014-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sgambato JA, Jones BA, Caraway JW, Prasad GL. Inflammatory profile analysis reveals differences in cytokine expression between smokers, moist snuff users, and dual users compared to non-tobacco consumers. Cytokine. 2018;107:43–51. doi: 10.1016/j.cyto.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 184.Arimilli S, Madahian B, Chen P, Marano K, Prasad GL. Gene expression profiles associated with cigarette smoking and moist snuff consumption. BMC Genomics. 2017;18:156. doi: 10.1186/s12864-017-3565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Malovichko MV, Zeller I, Krivokhizhina TV, et al. Systemic toxicity of smokeless tobacco products in mice. Nicotine Tob Res. 2019;21:101–110. doi: 10.1093/ntr/ntx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gudnadottir AY, Olafsdottir IS, Middelveld R, et al. An investigation on the use of snus and its association with respiratory and sleep-related symptoms: a cross-sectional population study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Godoy P, Castilla J, Soldevila N, et al. Smoking may increase the risk of influenza hospitalization and reduce influenza vaccine effectiveness in the elderly. Eur J Public Health. 2018;28:150–155. doi: 10.1093/eurpub/ckx130. [DOI] [PubMed] [Google Scholar]
- 188.Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;125:2011–2020. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- 189.Cruijff M, Thijs C, Govaert T, Aretz K, Dinant GJ, Knottnerus A. The effect of smoking on influenza, influenza vaccination efficacy and on the antibody response to influenza vaccination. Vaccine. 1999;17:426–432. doi: 10.1016/S0264-410X(98)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Winter AP, Follett EA, McIntyre J, Stewart J, Symington IS. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine. 1994;12:771–772. doi: 10.1016/0264-410x(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 191.Shaw FE, Jr, Guess HA, Roets JM, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine. 1989;7:425–430. doi: 10.1016/0264-410x(89)90157-6. [DOI] [PubMed] [Google Scholar]
- 192.Ferrara P, Gianfredi V, Tomaselli V, Polosa R. The effect of smoking on humoral response to COVID-19 vaccines: a systematic review of epidemiological studies. Vaccines (Basel) 2022;10:303. doi: 10.3390/vaccines10020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.