Abstract
Introduction
Fibrin glue is widely used in spine surgery. Nevertheless, no report has demonstrated the feasibility of completely autologous fibrin glue (CAFG) in spine surgery. This study aims to investigate the safety, efficacy, and effect of bone fusion of CAFG on spine surgery.
Methods
We retrospectively extracted data of patients who underwent primary spine surgery with preoperatively prepared CAFG. Primary outcomes were the incidence of wound-related unplanned reoperations within 90 days following primary surgery and the occurrence of reoperation for the management of cerebrospinal fluid (CSF) leakage in patients who had been treated with CAFG used as dural sealants. The effect of CAFG on bone fusion was also assessed by detecting implant failure at one year postoperatively in patients aged 25 years or less undergoing primary fusion for idiopathic scoliosis.
Results
We identified 131 eligible patients (47 males and 84 females) with a mean age of 32.3 years. CAFG was used most frequently as an adhesive for fixation of graft bone (110 patients), followed by as a dural sealant for CSF leakage in 17 patients, and as a local hemostatic agent in four patients. Wound-related reoperations were identified in four patients (3.1%), which included three for surgical site infection, and one for postoperative epidural hematoma. There was no reoperation required for the management of CSF leakage among 17 patients with dural incision or incidental durotomy. Compared with the control cohort, the use of CAFG was not associated with early wound-related reoperations or implant failure in patients with spinal deformity.
Conclusions
We demonstrated the clinical feasibility of CAFG in spine surgery. The use of CAFG was not associated with the incidence of reoperations for wound-related complications. CAFG worked effectively as a dural sealant for preventing CSF leakage. CAFG had no beneficial or adverse effect on spinal bone fusion.
Keywords: autologous fibrin glue, fibrin glue, fibrin sealant, bone fusion, dural sealant, cerebrospinal fluid leakage, cryoprecipitate, spine surgery
Introduction
Fibrin glue (FG) is widely used in spine surgery. Generally, FG is a human blood product consisting of two components: cryoprecipitate and thrombin. Cryoprecipitate is the fraction of plasma that contains concentrated coagulation factors such as fibrinogen, and thrombin is an enzyme that facilitates the conversion of fibrinogen into fibrin. FG is used as a sealant for cerebrospinal fluid (CSF) leakage, hemostatic agent, and bone enhancer for fusion surgery as well as an adhesive for bone grafts. Its effectiveness in spine surgery has been well documented1-5). However, commercially available FG has potential risks for infection such as human parvovirus B19, allogenic immunity, allergic reaction, and prion transmission because it is made from pooled human plasma and bovine aprotinin6-8). To avoid these risks, “autologous” FG had been developed and reported in the last few decades; nevertheless, this conventional autologous FG produced using a manual production method also possesses potential risks for infection or allergic reaction because only cryoprecipitate is prepared from patients' own blood and is used with commercially available thrombin due to a lack in the technical ability to refine the thrombin component from the patient's plasma3,9).
Recently, CryoSealⓇ FS System (Asahi Kasei Medical Co. Ltd., Tokyo, Japan) has been introduced as an automated device for the production of completely autologous FG (CAFG), which enabled us to prepare the cryoprecipitate and thrombin simultaneously in 90 min preoperatively from the patient's own blood10-12). CAFG produced using the CryoSealⓇ FS system contains no allogenic component as exogenous additives; thus, it can completely eliminate the risk of viral or prion transmission and allogenic immunity. However, no report describes the feasibility of CAFG in spine surgery. Thus, this study aimed to demonstrate the safety and efficacy of CAFG in spine surgery and to elucidate the effect of CAFG on bone fusion in spine surgery, since the effect of FG on bone fusion is controversial and there has been no previous study that has investigated the effect of CAFG on spinal bone fusion, even in an animal model13-18).
Materials and Methods
Data source and patients
We retrospectively obtained data from the prospective cohort of our institute for a total of 66 months, from May 1, 2015, to October 31, 2020. Among the patients who underwent spine surgery during this period, we collected data from patients in whom CAFG was prepared preoperatively. The exclusion criteria were as follows: 1) patients undergoing revision surgery, 2) patients whose prepared CAFGs were not used during surgery, and 3) patients with a follow-up period of less than 90 days. This research has been approved by the Research Ethics Committee of the authors' affiliated institutions.
Collected baseline data of patients
We collected the baseline data of the patients from medical records, including sex, age at surgery, body mass index, and diagnosis for surgery. For patients undergoing surgery for spinal deformity, we further collected the data of preoperative Cobb angle, the number of fused vertebrae, and the etiology of spinal deformity. Regarding the etiology of spinal deformity other than L5 spondylolisthesis, we classified the etiology into four categories according to the previous work by Taniguchi et al.: (a) congenital or structural, (b) neuromuscular, (c) syndromic, and (d) idiopathic curves19,20).
Preparation and use of CAFG
CAFG was prepared for patients undergoing preoperative autologous blood donation. The decision of preoperative autologous blood donation or preparation of CAFG was made by each surgeon accordingly. Written informed consent was obtained from patients or patients' parents for minor patients before preoperative autologous blood donation and CAFG preparation. The donated whole blood was immediately stored at 4°C after donation, and plasma was separated from it within 6 h and stored below −20°C as fresh frozen plasma (FFP). CAFG was prepared from FFP using CryoSealⓇ FS System within 1 week before surgery and stored below −20°C. The detail of CryoSealⓇ FS System was described previously11,12). CAFG was thawed at 37°C and applied to the surgical site with the spray tip or the dot tip within 1 h after thawing in principle.
Surgical procedure and application of CAFG
CAFG was used either as a sealant for CSF leakage, as an adhesive of bone graft, or as a local hemostatic agent. CAFG was used as a sealant in patients requiring a dural incision for resection of intradural spinal tumors or untethering of the filum terminale. CAFG was also used as a sealant in patients undergoing incidental durotomy during surgery. When used as a sealant, CAFG was routinely applied with a polyglycolic acid sheet (NeoveilⓇ sheet, Gunze, Ltd., Tokyo, Japan) after the closure of the dura mater as previously described2). In patients who underwent fusion surgery and did not require a dural sealant, we used CAFG as an adhesive of graft bone to the lamina. By pressing graft bone to the decorticated posterior elements with CAFG, we aimed to reduce bleeding as well as to stabilize graft bone. In all patients with fusion surgery, we only used autologous bone as a bone graft. In the remaining patients, we used CAFG as a local hemostatic agent at the final phase of surgery.
Evaluation of safety and efficacy of CAFG
To determine the safety of using CAFG at the surgical site, we first investigated the occurrence of postoperative wound-related complications, which was defined as unplanned reoperation in the operating theater within 90 days following primary surgery for surgical site infection (SSI), postoperative hematoma, or wound dehiscence. To assess the impact of CAFG, we compared the incidence of unplanned reoperation in patients aged 25 years or less who underwent primary fusion for spinal deformity with CAFG to that in a control cohort. The control cohort was prepared by extracting the same number of consecutive patients aged 25 years or less undergoing primary fusion for spinal deformity at our institute just before the introduction of CryoSealⓇ FS System. We excluded a few patients whose conventional autologous cryoprecipitate was prepared preoperatively from the control cohort.
To evaluate the efficacy of CAFG as a sealant, we retrospectively investigated the occurrence of reoperation for the management of CSF leakage in patients who had been treated with CAFG used as a sealant for intentional dural incision or incidental durotomy.
Evaluation of the effect of CAFG on bone fusion
To evaluate the effect of CAFG on bone fusion, we investigated the occurrence of implant failure at one year postoperatively in patients aged 25 years or less who underwent primary fusion for idiopathic scoliosis. Implant failure was defined as the loosening of pedicle screws, dislodgment of hooks, or rod breakage. As a control cohort, we extracted the same number of consecutive patients aged 25 years or less undergoing primary fusion for idiopathic scoliosis at our institute just before the introduction of CryoSealⓇ FS System. For assessment for spinal fusion, we utilized plain radiography and regarded implant failure as proof of non-union, because implant failure following fusion surgery is usually a consequence of pseudoarthrosis and we do not routinely take CT for the assessment of bone fusion for avoiding patients' high radiation exposure21). An assessment of implant failure on plain radiography was conducted by two attending spine surgeons. The final decision of the existence of implant failure was made after an agreement from the two surgeons was obtained.
Statistical analysis
The chi-square test was used to compare categorical data. The Student's t-test was used to compare continuous variables. The kappa statistic was used to verify the interobserver reliability as a reliability analysis for the judgment of implant failure. The threshold for significance was set at p<0.05. All statistical analyses were performed using JMP Pro version 15.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic data and use of CAFG during surgery
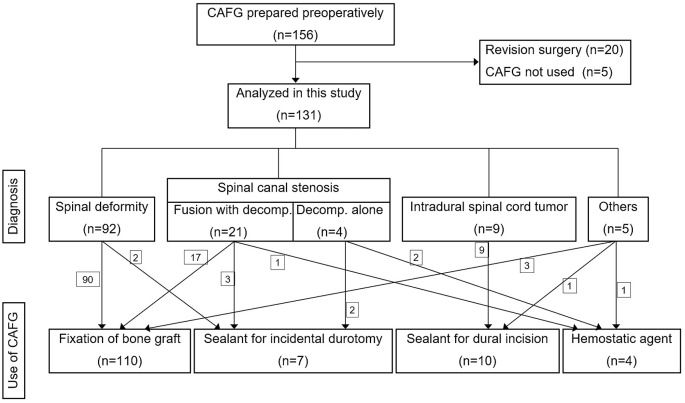
Among the 156 patients whose CAFG had been prepared preoperatively, 131 eligible patients were identified for analysis (Fig. 1, Table 1). The patients comprised 47 men (35.9%) and 84 women (64.1%) with a mean age of 32.3 years. The diagnosis for surgery was a spinal deformity in 92 patients (70.2%), spinal canal stenosis in 25 patients (19.1%), intradural spinal cord tumor in nine patients (6.9%), and others in five patients (3.8%), which included three cases with tethered cord syndrome and two cases with spinal tumors (Table 1). In patients with spinal canal stenosis, decompression with fusion was performed in 21 patients. CAFG has been used most frequently as an adhesive for fixation of graft bone (110 patients), which was probably because preoperative autologous blood donation was conducted mainly in patients undergoing fusion surgery (Table 2). CAFG was used as a sealant for CSF leakage in 17 patients, including 10 patients with intentional dural incision and seven patients with incidental durotomy (Fig. 1, Table 2). CAFG was used as a hemostatic agent only in the remaining four patients (Table 2). No patients developed allergic reactions or systemic complications associated with the use of CAFG.
Figure 1.
Flow diagram for study inclusion and use of completely autologous fibrin glue (CAFG).
decomp., decompression
Table 1.
Demographic Data of Patients Undergoing Primary Spine Surgery with CAFG.
| Number of patients | 131 | |
| Age, years (mean [SD]) | 32.3 | [22.9] |
| Sex | ||
| Male (%) | 47 | (35.9) |
| Female (%) | 84 | (64.1) |
| Body mass index, kg/m2 (mean [SD]) | 20.1 | [5.0] |
| Diagnosis for operation | ||
| Spinal deformity (%) | 92 | (70.2) |
| Spinal canal stenosis (%) | 25 | (19.1) |
| Decompression with fusion | (21) | |
| Decompression alone | (4) | |
| Intradural spinal cord tumor (%) | 9 | (6.9) |
| Others (%) | 5 | (3.8) |
CAFG, completely autologous fibrin glue
Table 2.
Purpose for Using CAFG during Surgery and Incidence for Wound-related Reoperation within 90 Days following Primary Spine Surgery.
| Purpose for using CAFG | Number of
patients |
Incidence of reoperation
(cause for reoperation) |
(%) |
|---|---|---|---|
| Fixation of bone graft | 110 | 3
(deep SSI: 2) (superficial SSI: 1) |
(2.7) |
| Sealant for dural incision | 10 | 0 | (0) |
| Sealant for incidental durotomy | 7 | 1
(epidural hematoma: 1) |
(14.3) |
| Hemostatic agent | 4 | 0 | (0) |
CAFG, completely autologous fibrin glue; SSI, surgical site infection
Incidence of reoperation for wound-related complications
Wound-related reoperations within postoperative 90 days were identified in four patients, which included two reoperations for deep SSI, one for superficial SSI, and one for postoperative epidural hematoma (Table 2). Three reoperations for SSI occurred in patients whose CAFG was used as an adhesive for graft bone in fusion surgery (Table 2). There was no reoperation required for the management of CSF leakage among 17 patients with dural incision or incidental durotomy, indicating the efficacy of CAFG as a dural sealant (Table 2).
Assessment of safety for using CAFG
To assess the impact of CAFG on safety, we compared the incidence of wound-related reoperations in patients aged 25 years or less who underwent primary fusion for spinal deformity with CAFG to that in a control cohort. This was done since, in this study, CAFG was prepared most frequently in adolescent or young adult patients with a diagnosis of spinal deformity. The control cohort was prepared by extracting the same number of consecutive patients undergoing surgery for spinal deformity at our institute just before the introduction of CryoSealⓇ FS System. We identified 76 eligible cases in the CAFG group, comprising 32 men and 44 women with a mean age of 16.4 years (Table 3). There was no significant difference in baseline data between the CAFG group and the control group (Table 3). No patients in the control group were treated with commercially available FG during surgery. Wound-related reoperations were identified in two patients (2.6%) in the CAFG group and in four patients (5.2%) in the control group, bearing no significant difference (Table 3). This result suggested that CAFG has no beneficial or adverse effect on wound-related complications, indicating the safety of CAFG.
Table 3.
Incidence of Wound-related Reoperations within 90 Days following Primary Posterior Fusion for Spinal Deformity with or without CAFG in Patients Aged 25 years or Less.
| CAFG group | Control group | p | |||
|---|---|---|---|---|---|
| Number of patients | 76 | 76 | |||
| Male:Female | 32:44 | 21:55 | 0.06 | ||
| Age, years (mean [SD]) | 16.4 | [3.7] | 15.5 | [3.4] | 0.12 |
| Body mass index, kg/m2 (mean [SD]) | 17.9 | [3.6] | 18.2 | [3.3] | 0.55 |
| Number of fused vertebras (mean [SD]) | 11.3 | [3.8] | 10.9 | [3.1] | 0.40 |
| Cobb angle of major curve, ° (mean [SD]) | 62.3 | [24.3] | 59.7 | [19.3] | 0.47 |
| Etiology of spinal deformity (%) | 0.11 | ||||
| Congenital or structural | 7 | (9.2) | 4 | (5.3) | |
| Neuromuscular | 16 | (21.1) | 9 | (11.8) | |
| Syndromic | 11 | (14.4) | 8 | (10.5) | |
| Idiopathic | 40 | (52.6) | 55 | (72.4) | |
| L5 spondylolisthesis | 2 | (2.6) | |||
| Perioperative allogenic blood transfusion (%) | 1 | (1.3) | 3 | (3.9) | 0.62 |
| Wound-related reoperations within 90 days (%) | 2 | (2.6) | 4 | (5.2) | 0.68 |
| (deep SSI: 1) (superficial SSI: 1) |
(deep SSI: 3) (wound dehiscence: 1) |
||||
CAFG, completely autologous fibrin glue; SSI, surgical site infection
Effect of CAFG on bone fusion
To evaluate the effect of CAFG on bone fusion, we investigated the occurrence of implant failure at one year postoperatively in patients aged 25 years or less who underwent primary fusion for idiopathic scoliosis with CAFG, because implant failure is usually a consequence of pseudoarthrosis. We identified 36 eligible patients with a follow-up period of a minimum one year in the CAFG group; hence, we extracted the same number of consecutive patients undergoing primary fusion for idiopathic scoliosis at our institute just before the introduction of CryoSealⓇ FS System as a control cohort. No patients in the control group were treated with commercially available FG during surgery. There was no significant difference in baseline data between the CAFG group and the control group (Table 4). Implant failure was identified in three patients (8.3%) in the CAFG group and in two patients (5.6%) in the control group, respectively, bearing no significant difference. Kappa value for the judgment of implant failure between two surgeons was 0.45, revealing moderate interobserver agreement (Table 4). These results suggested that CAFG had no beneficial or adverse effect on spinal bone fusion.
Table 4.
Incidence of Implant Failure at One-year Postoperatively following Primary Posterior Fusion for Idiopathic Scoliosis with or without CAFG in Patients Aged 25 years or Less.
| Case | Control | p | |||
|---|---|---|---|---|---|
| Number of patients | 36 | 36 | |||
| Male:Female | 12:24 | 9:27 | 0.61 | ||
| Age, years (mean [SD]) | 16.9 | [3.7] | 16.2 | [3.6] | 0.42 |
| Body mass index, kg/m2 (mean [SD]) | 18.2 | [1.8] | 18.5 | [1.9] | 0.55 |
| Number of fused vertebras, (mean [SD]) | 10.0 | [3.1] | 10.8 | [3.1] | 0.31 |
| Cobb angle of major curve, ° (mean [SD]) | 52.8 | [9.5] | 53.4 | [9.9] | 0.77 |
| Number of patients with IF (%) | 3 | (8.3) | 2 | (5.6) | 1.00 |
| Loosening of PS at UIV | 1 | ||||
| Loosening of PS at LIV | 1 | 2 | |||
| Dislodgment of the transverse hook at UIV | 1 | ||||
CAFG, completely autologous fibrin glue; IF, implant failure; PS, pedicle screw; UIV, upper instrumented vertebra; LIV, lowest instrumented vertebra
Discussion
This study provides two novel pieces of information. First, this study elucidated the clinical feasibility of CAFG in spine surgery. Second, this study investigated the effect of CAFG on spinal bone fusion. There have been only three studies that described the use of CAFG in clinical practice, and all these studies reported the safety and efficacy of CAFG produced by CryoSealⓇ FS System, although these studies discussed CAFG use in maxillofacial surgery, neurosurgery, or thoracic surgery10-12). Thus, this is the first report that elucidated the feasibility of CAFG in spine surgery.
Although the previous meta-analysis demonstrated that the use of commercially available FG in spine surgery was not associated with the incidence of SSI, concerns about the use of CAFG remain, because the possibility of contamination cannot be ruled out in the process of producing CAFG by CryoSealⓇ FS System22). Conversely, Kinaci et al. demonstrated that the use of dural sealants following craniotomy reduced the risk of SSI in cranial surgery23). These ideas and previous findings motivated us to investigate the association between the use of CAFG and the incidence of SSI in spine surgery. Hence, it was noteworthy that we demonstrated the safety of using CAFG during spine surgery (Table 3). However, considering the low incidence of SSI, further investigation with a larger number of cases will be necessary to elucidate the actual relationship between CAFG use and the incidence of SSI.
Regarding the efficacy of CAFG as a sealant following dural incision or incidental durotomy, none of the 17 patients required unplanned reoperation for CSF leakage. Although there have been several previous studies reporting the usefulness of FG as a sealant for CSF leakage, its effectiveness remains controversial3,4,22,24,25). Considering that CSF leakage was identified in 9.1% of the patients treated with commercially available FG for durotomy in the previous meta-analysis, the present study indicates that CAFG can work effectively as a dural sealant to prevent CSF leakage22).
In the present study, we were unable to draw any conclusion about the efficacy of CAFG as a hemostatic agent because estimation of postoperative blood loss was technically difficult (Table 2). The utility of FG as a hemostatic agent has been reported in many fields, including spine surgery; thus, it is reasonable to assume that CAFG may also work as a good hemostatic agent5,26-29). However, this hypothesis needs further investigation because CAFG produced by CryoSealⓇ FS System requires a slightly longer coagulation time (3-4 s) than the commercially available FG3,10).
The present study demonstrated that CAFG had no beneficial or adverse effects on spinal bone fusion. To date, the effect of FG on bone fusion has been controversial. A few animal studies have reported negative effects of FG on bone fusion13,15,17,18). Conversely, Santos et al. demonstrated a positive effect of FG on bone formation in a rat calvarial defect model16). Furthermore, because FG used in previous animal studies was allogenic, local immunity reaction might affect the process of bone fusion. Therefore, CAFG, which can completely eliminate the effect of local immunity reaction, can potentially positively affect bone fusion; however, there have been no reports investigating the effect of CAFG on bone fusion, even in an animal model. In this respect, the results of the present study are intriguing. However, it would be premature to arrive at the conclusion regarding the effect of CAFG on bone fusion, because the present study is based on the analysis of implant failure detected on plain radiography similar to previous studies instead of directly assessing bone fusion on CT30,31). Although CT is widely accepted as the gold standard for the assessment of bone fusion, it is not recommended to routinely take CT for the assessment of bone fusion, especially in young patients following fusion surgery for idiopathic scoliosis, because CT inevitably causes high radiation exposure in patients21). In any case, the present study failed to demonstrate the beneficial effect of CAFG on bone fusion; hence, further investigation, including animal studies or cost effective analysis, will be necessary to support the use of CAFG in fusion surgery hereafter.
This study had some limitations. First, because the present study was retrospective, there might be some bias in this study. Second, we did not conduct a cost-effectiveness analysis; thus, the actual efficacy of CAFG was not determined. Third, the sample size may not be sufficient to discuss the relatively rare complications, such as SSI, CSF leakage, or pseudoarthrosis.
Conclusions
We demonstrated for the first time the clinical feasibility of CAFG in spine surgery. The use of CAFG was not associated with the incidence of reoperations for wound-related complications. CAFG worked effectively as a dural sealant to prevent CSF leakage. CAFG seemed to have no beneficial or adverse effects on spinal bone fusion.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Sources of Funding: None.
Author Contributions: Y.T. and T.I. designed the study; Y.T., Y.M., T.D., and S.K. analyzed the data; S.T. and H.O. supervised the study; Y.T. wrote the original manuscript; Y.O. and T.I. revised the manuscript.
Ethical Approval: This study was approved by the research ethics committee of the University of Tokyo. (#2674-(4), #3312)
Informed Consent: Consent for publication was not required because this study is fully anonymized.
References
- 1.Fortuna A, Palatinsky E, Di Lorenzo N. Anterior cervical arthrodesis with heterologous bone graft and human fibrin glue in the surgical treatment of myelopathy due to spondylosis. Preliminary note. Clin Neurol Neurosurg. 1988;90(2):125-9. [DOI] [PubMed] [Google Scholar]
- 2.Masuda S, Fujibayashi S, Otsuki B, et al. The dural repair using the combination of polyglycolic acid mesh and fibrin glue and postoperative management in spine surgery. J Orthop Sci. 2016;21(5):586-90. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura H, Matsuyama Y, Yoshihara H, et al. The effect of autologous fibrin tissue adhesive on postoperative cerebrospinal fluid leak in spinal cord surgery: a randomized controlled trial. Spine. 2005;30(13):E347-51. [DOI] [PubMed] [Google Scholar]
- 4.Ono K, Shikata J, Shimizu K, et al. Bone-fibrin mixture in spinal surgery. Clin Orthop Relat Res. 1992;(275):133-9. [PubMed] [Google Scholar]
- 5.Yeom JS, Buchowski JM, Shen HX, et al. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: a retrospective matched pair analysis. Spine. 2008;33(16):E543-7. [DOI] [PubMed] [Google Scholar]
- 6.Hino M, Ishiko O, Honda KI, et al. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000;108(1):194-5. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura M, Sawafuji M, Watanabe M, et al. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg. 2002;73(4):1098-100. [DOI] [PubMed] [Google Scholar]
- 8.Orsel I, Guillaume A, Feiss P. [Anaphylactic shock caused by fibrin glue]. Ann Fr Anesth Reanim. 1997;16(3):292-3. [DOI] [PubMed] [Google Scholar]
- 9.Kjaergard HK, Weis-Fogh US, Sørensen H, et al. Autologous fibrin glue--preparation and clinical use in thoracic surgery. Eur J Cardiothorac Surg. 1992;6(1):52-4. [DOI] [PubMed] [Google Scholar]
- 10.Kawashima M, Kohno T, Fujimori S, et al. Feasibility of autologous fibrin glue in general thoracic surgery. J Thorac Dis. 2020;12(3):484-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouketsu A, Nogami S, Yamada-Fujiwara M, et al. Clinical evaluations of complete autologous fibrin glue, produced by the CryoSeal(Ⓡ) FS system, and polyglycolic acid sheets as wound coverings after oral surgery. J Craniomaxillofac Surg. 2017;45(9):1458-63. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama N, Yano H, Egashira Y, et al. Efficacy, reliability, and safety of completely autologous fibrin glue in neurosurgical procedures: single-center retrospective large-number case study. World Neurosurg. 2018;109:e819-28. [DOI] [PubMed] [Google Scholar]
- 13.Jarzem P, Harvey EJ, Shenker R, et al. The effect of fibrin sealant on spinal fusions using allograft in dogs. Spine. 1996;21(11):1307-12. [DOI] [PubMed] [Google Scholar]
- 14.Khodakaram-Tafti A, Mehrabani D, Shaterzadeh-Yazdi H. An overview on autologous fibrin glue in bone tissue engineering of maxillofacial surgery. Dent Res J (Isfahan). 2017;14(2):79-86. [PMC free article] [PubMed] [Google Scholar]
- 15.Lucht U, Bünger C, Møller JT, et al. Fibrin sealant in bone transplantation. No effects on blood flow and bone formation in dogs. Acta Orthop Scand. 1986;57(1):19-24. [DOI] [PubMed] [Google Scholar]
- 16.Santos T de S, Abuna RP, Almeida AL, et al. Effect of collagen sponge and fibrin glue on bone repair. J Appl Oral Sci. 2015;23(6):623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turgut M, Erkuş M, Tavus N. The effect of fibrin adhesive (Tisseel) on interbody allograft fusion: an experimental study with cats. Acta Neurochir (Wien). 1999;141(3):273-8. [DOI] [PubMed] [Google Scholar]
- 18.Zárate-Kalfópulos B, Estrada-Villaseñor E, Lecona-Buitrón H, et al. [Use of fibrin glue in combination with autologous bone graft as bone enhancer in posterolateral spinal fusion. An experimental study in New Zealand rabbits]. Cir Cir. 2007;75(3):201-5. [PubMed] [Google Scholar]
- 19.Taniguchi Y, Ohara T, Suzuki S, et al. Incidence and risk factors for unplanned return to the operating room following primary definitive fusion for pediatric spinal deformity: a multicenter study with minimum two-year follow-up. Spine. 2021;46(8):E498-E504. [DOI] [PubMed] [Google Scholar]
- 20.Williams BA, Matsumoto H, McCalla DJ, et al. Development and initial validation of the Classification of Early-Onset Scoliosis (C-EOS). J Bone Joint Surg Am. 2014;96(16):1359-67. [DOI] [PubMed] [Google Scholar]
- 21.Selby MD, Clark SR, Hall DJ, et al. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703. [DOI] [PubMed] [Google Scholar]
- 22.Kinaci A, Moayeri N, van der Zwan A, et al. Effectiveness of sealants in prevention of cerebrospinal fluid leakage after spine surgery: a systematic review. World Neurosurg. 2019;127:567-75. [DOI] [PubMed] [Google Scholar]
- 23.Kinaci A, Algra A, Heuts S, et al. Effectiveness of dural sealants in prevention of cerebrospinal fluid leakage after craniotomy: a systematic review. World Neurosurg. 2018;118:368-76. [DOI] [PubMed] [Google Scholar]
- 24.Jankowitz BT, Atteberry DS, Gerszten PC, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. 2009;18(8):1169-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HR, Cao SS, Jiang YQ, et al. A comparison between “sandwich” and conventional methods of repairing spinal dura rupture. Orthop Surg. 2012;4(4):233-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutignani M, Seerden T, Tringali A, et al. Endoscopic hemostasis with fibrin glue for refractory postsphincterotomy and postpapillectomy bleeding. Gastrointest Endosc. 2010;71(4):856-60. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner MG, Maniscalco-Theberge ME, Champion HR. Fibrin glue as a hemostatic agent in hepatic and splenic trauma. J Trauma. 1990;30(7):884-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Merchan EC. Fibrin glue for local haemostasis in haemophilia surgery. Hosp Pract (1995). 2017;45(5):187-91. [DOI] [PubMed] [Google Scholar]
- 29.Vaiman M, Eviatar E, Shlamkovich N, et al. Use of fibrin glue as a hemostatic in endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2005;114(3):237-41. [DOI] [PubMed] [Google Scholar]
- 30.Crostelli M, Mazza O, Mariani M, et al. Adolescent idiopathic scoliosis correction by instrumented vertebral arthrodesis with autologous bone graft from local harvesting without bone substitute use: results with mean 3 year follow-up. Eur Spine J. 2018;27(Suppl 2):175-81. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Xu H, Li M, et al. Clinical and radiographic outcomes of the treatment of adolescent idiopathic scoliosis with segmental pedicle screws and combined local autograft and allograft bone for spinal fusion: a retrospective case series. BMC Musculoskelet Disord. 2010;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]