Abstract
SecY and SecE are integral cytoplasmic membrane proteins that form an essential part of the protein translocation machinery in Escherichia coli. Sites of direct contact between these two proteins have been suggested by the allele-specific synthetic phenotypes exhibited by pairwise combinations of prlA and prlG signal sequence suppressor mutations in these genes. We have introduced cysteine residues within the first periplasmic loop of SecY and the second periplasmic loop of SecE, at a specific pair of positions identified by this genetic interaction. The expression of the cysteine mutant pair results in a dominant lethal phenotype that requires the presence of DsbA, which catalyzes the formation of disulfide bonds. A reducible SecY-SecE complex is also observed, demonstrating that these amino acids must be sufficiently proximal to form a disulfide bond. The use of cysteine-scanning mutagenesis enabled a second contact site to be discovered. Together, these two points of contact allow the modeling of a limited region of quaternary structure, establishing the first characterized site of interaction between these two proteins. This study proves that actual points of protein-protein contact can be identified by using synthetic phenotypes.
Gram-negative bacteria, such as Escherichia coli, must transport proteins to several different compartments, including the inner membrane, the outer membrane, and the periplasmic space. Catalyzing these processes are a number of cytoplasmic, peripheral, and integral membrane proteins, including SecY (also known as PrlA; see below), SecE (PrlG), SecA (PrlD), SecG (PrlH), SecB, SecD, and SecF (4, 11). SecA and SecB recognize the hydrophobic N-terminal signal peptide and/or portions of the mature sequence of the secretory protein. SecA then interacts with integral membrane proteins, including SecY and SecE, and this interaction triggers ATP-dependent conformational changes in SecA (12, 13, 20, 27). ATP binding leads to the periplasmic exposure of a portion of the secretory protein N terminus, as well as the periplasmic exposure of SecA itself. SecA subsequently hydrolyzes ATP and loses its exposure to the periplasm. The translocation of the remaining portions of the secretory protein can be catalyzed by repeated cycles of SecA insertion and deinsertion or by the proton motive force, possibly through a channel comprised of SecY, SecE, and SecG. The signal peptide is processed by a leader peptidase during the translocation reaction.
The process of bacterial protein translocation has several features in common with the translocation across the endoplasmic reticular membrane of eukaryotes. For example, both processes employ cleavable signal peptides, the sequences of which are virtually indistinguishable (39). Bacterial proteins can be translocated by eukaryotes (23) and vice versa (37). Moreover, several components of the translocation machinery are conserved in all of the domains of life (33). The function and interactions of these common proteins have thus drawn intense interest.
SecY and SecE are among the translocation proteins that have been conserved across domains. Moreover, in E. coli, yeast, and mammals, homologues of SecY and SecE can be found as part of a protein complex (11, 34). It has been established genetically and biochemically that SecY and SecE are in direct contact (5–7, 14). Altogether there has been much attention paid to the interaction between these extremely hydrophobic proteins (1, 16, 19, 32, 41), including the recent description of the complex with electron microscopy (25). Unfortunately, many techniques for assessing protein structure are not available for the analysis of membrane proteins like SecY and SecE. Understanding the structure of this protein complex before and during translocation likely will require new technologies.
In E. coli, certain alleles of secY and secE, the prlA and prlG mutations, respectively, suppress defective signal peptides enabling the translocation of mutant secretory proteins (4). It was noted from a previous study that one combination of these suppressor prlA and prlG alleles results in a synthetic phenotype (5). A synthetic phenotype is one created by alleles of two different genes in combination, but not by either allele by itself (17, 18). Often the phenotype is lethality: a cell containing both mutant genes cannot grow. Synthetic phenotypes can suggest a direct interaction between the products of the two genes.
A subsequent study of prlA and prlG allelic combinations provided a glimpse into synthetic phenomena at a molecular level (16). As membrane proteins, the residues of SecY and SecE are found within three distinct compartments: the cytoplasm, membrane, and periplasm. Of 88 different pairwise combinations of prlA and prlG alleles examined, only five produced synthetic phenotypes. Intriguingly, the positions of the mutations were topologically juxtaposed. Three pairs of mutations mapped to transmembrane helices, and two pairs of mutations mapped to periplasmic loops. It was proposed that the loops and helices containing these pairs of mutations interact directly.
A narrower hypothesis is that the sites of mutations identified in the prlA and prlG study are positions of direct contact between the two proteins. Here we test and verify this hypothesis by exploiting the oxidizing environment of the periplasm to produce a SecY-SecE complex that is specifically disulfide bonded between these sites.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli strains are ara+ derivatives of MC4100 (F− araD139 Δ[argF-lac]U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR). For protein analysis, an ompT::Kn mutation was introduced into these strains to prevent the proteolysis of SecY. Plasmid pAF26 has been previously described (16). Plasmids pSecE and pSecY are derivatives of pACYC177 into which wild-type copies of secE and secY have been inserted, respectively. The secE gene in pSecE is under the control of its own promoter. The secY gene in pSecY is under control of the trc promoter, which was obtained from pTrc99a (Pharmacia). Media were prepared as described previously (36).
Arabinose sensitivity assays.
The strain of interest was grown to saturation in Luria-Bertani (LB) broth, washed three times in 10 mM MgSO4–5 mM CaCl, and then mixed with 3 ml of F-top agar at 47°C. This suspension was then poured onto M63 minimal agar containing glycerol and ampicillin. After the top agar had solidified, a small disc of filter paper was placed in the middle of the dish, and 15 μl of 20% arabinose was added to the disc. The plates were incubated at 37°C for 16 h, and then zones of growth inhibition were measured.
Site-directed mutagenesis.
Single point mutations were generated in the secE gene encoded by plasmid pAF26 (16) by using the unique site elimination method (9).
Nonreducing PAGE.
E. coli strains were grown to saturation in LB broth and then subcultured into M9 media containing glycerol and ampicillin supplemented with 1% LB broth. After 5 h, arabinose was added to a concentration of 0.2%, and cultures were grown for 90 min. Iodoacetamide was added to a concentration of 25 mM (10), and then trichloroacetic acid (TCA) was added to a concentration of 5%. Protein pellets were washed in 5% TCA, washed in acetone, and then resuspended in 1% sodium dodecyl sulfate (SDS)–50 mM Tris (pH 7.5)–1 mM EDTA–50 mM iodoacetamide. Samples were normalized for cell number and then mixed with an SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer containing either dithiothreitol (DTT) or iodoacetamide. Samples were heated to 55°C for 30 min prior to electrophoresis. Proteins were electrophoresed in a gel consisting of SDS–12.5% polyacrylamide by using a Bio-Rad Protean II miniapparatus. Antibodies raised against SecE, β-lactamase, murein lipoprotein, or the N terminus of SecY were used to visualize proteins. Chemiluminescence was performed by using solutions supplied by Amersham Corp.
Incorporation of [35S]methionine into polypeptide.
E. coli strains were grown to saturation in LB broth and then subcultured into M9 media containing glycerol and ampicillin supplemented with 1% LB broth. After 2 h, arabinose was added to a concentration of 0.2%, and cultures were further grown for between 10 and 180 min. A short pulse of [35S]methionine was applied and then quenched by the addition of TCA to a concentration of 5%. Protein pellets were washed twice in 5% TCA and once in acetone and then were resuspended in 1% SDS–50 mM Tris (pH 7.5)–1 mM EDTA. Samples were normalized for cell number, and then the [35S]methionine incorporated into acid-precipitable protein was measured with a Beckman LS-1701 liquid scintillation counter.
Flow cytometry.
Cultures of the prlA3 secE(S120C) strain, as well as isogenic control strains, were exposed to arabinose for 1 or 4 h, pelleted by microcentrifugation, and then washed twice in 10 mM MgSO4–5 mM CaCl2. Cells were mixed with SYTOX Green or DIBAC4 as previously described (24, 35). A permeabilized control was also generated, by resuspending the cells in 70% ethanol for 5 min prior to washing in 10 mM MgSO4–5 mM CaCl2. Flow cytometry was performed by using a FACScan instrument (Becton Dickinson). Fluorescence was excited at 488 nm, and then emission measurements were collected at 530 nm (SYTOX Green) or 516 nm (DIBAC4).
Measurement of CFU.
E. coli strains were grown to saturation in LB broth and then subcultured into M9 media containing glycerol and ampicillin supplemented with 1% LB broth. After 2 h, arabinose was added to a concentration of 0.2%, and the optical density at 600 nm was measured. An aliquot of cells was removed, diluted 105-fold, and plated directly onto LB agar containing ampicillin. Platings were repeated every 30 min for 4 h. Plates were incubated at 37°C overnight, and colonies were counted.
RESULTS
A synthetic phenotype that requires the function of DsbA.
prlG3, which causes a substitution of phenylalanine for serine at position 120 (S120F) of SecE, was previously shown to be synthetically lethal with prlA3, which causes a substitution of cysteine for phenylalanine at position 67 (F67C) of SecY (16, 28). Both residues localize to the periplasm, an oxidizing environment in which disulfide bonds can form. We reasoned that if these two residues are normally points of direct contact between the two proteins, then replacing serine-120 of SecE with cysteine might enable disulfide bonding to the cysteine substitution in PrlA3.
The cysteine mutant of SecE, secE(S120C), was created on a plasmid from which the expression of the gene could be controlled conditionally. The gene is controlled by a promoter, PBAD, such that expression is only induced in the presence of the sugar arabinose. This plasmid was used to transform a strain of E. coli in which prlA3 and secE+ were present on the chromosome.
While it is possible to transform the plasmid encoding secE(S120C) into the prlA3 strain under noninducing conditions, the addition of arabinose results in a severe growth defect. This is best illustrated on a petri dish as a zone of arrested growth surrounding a filter paper disc containing arabinose (Table 1). The secE(S120C) gene alone is not responsible for this zone of arrested growth, because a secY+ strain containing this plasmid does not display the same phenotype (Table 1); nor does the overproduction of secE+ in the prlA3 strain result in a growth defect. Since only the combination of secE(S120C) expression in the prlA3 background results in a growth defect, this defect is a synthetic phenotype.
TABLE 1.
Arabinose-dependent synthetic phenotype of secE(S120C) prlA3
| secE allele on plasmid | secY allele on chromosome | Other allele or plasmid | Cystine added | Diam (mm) of zone of growth inhibitiona |
|---|---|---|---|---|
| secE(S120C) | prlA3 | None | None | 24 |
| secE(S120C) | secY+ | None | None | 0 |
| secE+ | prlA3 | None | None | 0 |
| secE(S120C) | prlA3 | dsbA::kan | None | 0 |
| secE(S120C) | prlA3 | dsbA::kan | 0.005% | 12 |
| secE(S120C) | prlA205 | None | None | 0 |
| secE(S120C) | prlA300 | None | None | 0 |
| secE(S120C) | prlA3 | pSecE | None | 24 |
| secE(S120C) | prlA3 | pSecY | None | 25 |
Arabinose sensitivity assays are described in Materials and Methods. Zero indicates no growth inhibition.
Given that other mutations at these two positions in SecE and SecY have previously been shown to yield synthetic combinations (16), we were not surprised by this growth defect. However, there is a novel and very striking phenotype specific to the strain containing both cysteine mutants. As discussed above, if the two cysteine residues are closely apposed within the native SecY-SecE protein complex, they might form a disulfide bond when both proteins are expressed, and the formation of this disulfide bond might account for the dramatic growth defect. This reasoning predicts that the growth defect would be suppressed by knocking out the gene encoding DsbA, the enzyme responsible for catalyzing disulfide bond formation in the periplasm of E. coli (3). This turns out to be the case. In the prlA3 dsbA::kan strain background, the expression of secE(S120C) does not confer a growth defect (Table 1). Moreover, the presence of cystine, an oxidizing agent that partially suppresses the loss of dsbA (2), also partially inhibits the growth of the secE(S120C) prlA3 dsbA::kan strain (Table 1).
As a negative control to show that the dsbA gene disruption has no effect upon synthetic phenotypes in general, this null allele was introduced into a prlG8 prlA726 strain. These alleles combine to give a strong synthetic phenotype (16). However, neither mutation encodes a cysteine, and the dsbA gene disruption has no effect upon the growth defect of either the prlG3 prlA3 strain or the prlG8 prlA726 strain (data not shown). Thus, the growth problems of the secE(S120C) prlA3 strain depend upon disulfide bond formation.
Altogether the results presented in this section indicate that positions 120 of SecE and 67 of SecY are close enough to permit disulfide bond formation between them. Moreover they suggest that this cross-linked SecY-SecE complex causes a growth defect.
A high degree of allele specificity.
Data in the previous section indicate that positions 120 of SecE and 67 of SecY are linked covalently when replaced by cysteine residues. This suggests that the amino acids are extremely proximal within the protein complex. However, it could also represent a more nonspecific association. For example, the two amino acids could be within domains sufficiently flexible to occasionally slide close to one another. DsbA would then act to fix the interaction covalently. We sought to rule out this possibility by examining position dependency. If the domains are flexible, then most amino acid substitutions in the nearby region should result in cross-links. On the other hand, if the domain interaction is relatively static, we would expect that cross-linking would be specific to the original pair of cysteine mutants.
The synthetic phenotype created by the secE(S120C)-prlA3 gene pair turns out to be highly position specific. The original plasmid carrying secE(S120C) was tested to see if it can confer a growth defect on a strain expressing either prlA205, which causes a substitution of cysteine for glycine at position 69, or prlA300, which causes a substitution of cysteine for phenylalanine at position 64 of SecY (28). Even though the mutations are tightly linked to the F67C substitution in prlA3, secE(S120C) expression does not affect the growth of these strains (Table 1).
Four more alleles of secE, also plasmid localized and under arabinose control, were created in which cysteines replace amino acids 118, 121, 122, and 124. The results of expressing each new mutant in the wild-type, prlA3, prlA205, and prlA300 backgrounds are shown in Table 2. As measured by the aforementioned filter disc assay, none of the new alleles confers arabinose sensitivity upon a wild-type strain, and nearly all allelic combinations are benign. One novel synthetic combination was discovered: secE(G124C) expression in a prlA300 strain leads to a growth defect. prlA300 causes a substitution of cysteine for phenylalanine at position 64 of SecY. As was previously the case, knocking out dsbA overcomes the growth defect of the prlA300 secE(G124C) strain. It therefore appears that positions 124 of SecE and 64 of SecY, like positions 120 of SecE and 67 of SecY, are sufficiently proximal to form a disulfide bond. Moreover these results further strengthen the causal relationship between cross-linked SecY and SecE and the observed growth defect.
TABLE 2.
Cysteine scanning for arabinose-dependent synthetic phenotypesa
| Form of secE | Diam (mm) of zone of growth inhibition with secY
|
|||
|---|---|---|---|---|
| Wild type | F64C | F67C | G69C | |
| Wild type | 0 | 0 | 0 | 0 |
| L118C | 0 | 0 | 0 | 0 |
| S120C | 0 | 0 | 24 | 0 |
| F121C | 0 | 0 | 0 | 0 |
| I122C | 0 | 0 | 0 | 0 |
| G124C | 0 | 25 | 0 | 0 |
Arabinose sensitivity assays are described in Materials and Methods. The position of the cysteine point mutation for each allele of secE and secY is indicated. Zero indicates no growth inhibition.
A reducible SecY-SecE complex.
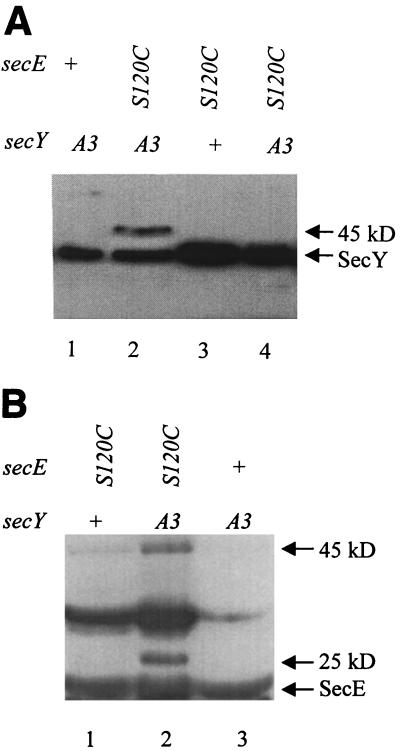
If position 67 of SecY and position 120 of SecE are points of direct contact, it should be possible to detect a reducible SecY-SecE complex when both of these residues are replaced by cysteine. Western blots presented in Fig. 1 show the disulfide-bonded complex formed by PrlA3 and SecE(S120C). When anti-SecY sera are used as a probe, a novel 45-kDa band appears under oxidizing conditions only in extracts from the strain expressing both cysteine mutant proteins (Fig. 1A). A band of precisely the same size, 45 kDa, also appears when anti-SecE sera are used as a probe (Fig. 1B). Upon reduction with DTT, the 45-kDa band disappears, demonstrating that the complex is disulfide bonded (Fig. 1A, lane 4). Thus, the 45-kDa band contains both SecY and SecE, is present only in strains producing both PrlA3 and SecE(120C), and disappears when DTT is added. Such data could be obtained only if position 67 of SecY and position 120 of SecE were sufficiently close in the SecY-SecE complex to allow disulfide bond formation.
FIG. 1.
Disulfide bonding of PrlA3 and SecE(S120C). Alleles are shown, including the wild type (+), the prlA3 gene, which codes for an F67C point mutation (A3), and secE(S120C) (S120C). (A) Total protein from strains expressing the secE and secY alleles indicated was TCA precipitated in the presence of iodoacetamide and then electrophoresed in the oxidized state (lanes 1 to 3) or reduced with DTT (lane 4). SecY antisera were used to visualize SecY and SecY protein complexes. (B) Total protein was precipitated in the presence of iodoacetamide and then electrophoresed in the oxidized state. SecE antisera were used to visualize SecE and SecE protein complexes.
In strains producing both PrlA3 and SecE(120C) an additional 25-kDa band that disappears upon reduction is recognized by the SecE antibody (Fig. 1B). This band is also recognized by the SecY antisera, although the band intensity is much weaker (data not shown). Unlike the SecE antisera, which were generated against whole protein, the SecY antisera recognize only the N-terminal 20 amino acids of the protein. We suspect that the 25-kDa band represents a degradation product of the 45-kDa SecE(S120C)-PrlA3 covalent complex and that part of the SecY N terminus is removed by the proteolysis event. This explanation would account for both the smaller size and the poor recognition of the 25-kDa band by the SecY antibody.
One band, of approximately 30 kDa, is detected only by the SecE antibody (Fig. 1B). This band disappears upon reduction (data not shown), but it is present in all of the strains producing SecE(120C), not just the secE(S120C) prlA3 strain. Thus, it cannot account for the toxicity of the latter.
We have searched for a specific disulfide-bonded complex in strains expressing the synthetic lethal pair prlA300 and secE(G124C). Unfortunately, for this particular combination of alleles, the results obtained with Western blots are not conclusive. In any strain expressing prlA300, including the secE+ background, there is a band(s) recognized by the SecY antibody in the 45-kDa range (data not shown). Thus, we cannot be sure that any of the bands that we see in the double mutant with SecE antisera really contain PrlA300.
Strains that do not demonstrate a synthetic phenotype have been examined, and we were unable to see a covalent SecY-SecE complex in any of them. For example, when secE(L118C) or secE(I122C) is expressed in the prlA3 background, a 45-kDa band cannot be seen under nonreducing conditions when probed with either SecY or SecE antiserum (data not shown). Thus, the side chains of residues 118 and 122 of SecE and 67 of SecY do not appear to be within disulfide-bonding distance.
Biochemical data presented in this section verify an important prediction of the genetic results presented above: SecE(S120C) and PrlA3 can form a disulfide-bonded complex. Although we have looked, we have not found a reducible SecY-SecE complex in strains that do not exhibit a synthetic phenotype. This latter result is consistent with the view that the cross-linked complex causes the observed growth defects.
Expression of secE(S120C) is toxic in prlA3 strains.
It is not immediately obvious why cross-linking SecY and SecE at a point of normal contact would cause a growth defect. In an effort to address this issue and in the hope of learning more about the functional role(s) of SecY and SecE, we have examined some of the changes in cellular physiology caused by the expression of secE(S120C) in a prlA3 strain.
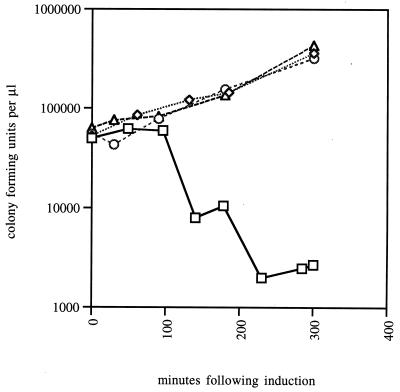
First, we tested whether secE(S120C) expression in a prlA3 strain causes cell death or simply results in a growth arrest. As shown in Fig. 2, there is a strong effect on cell viability 2 h following secE(S120C) induction. By 3.5 h postinduction, 99% of cells have lost the ability to form colonies on permissive media. Thus, SecE(S120C) is toxic in prlA3 strains; after a period of time or at some critical concentration it causes cell death.
FIG. 2.
Toxicity of secE(S120C) expression in prlA3 strain. At 0 min, arabinose was added to secE(S120C) prlA3 (squares), secE(S120C) secY+ (diamonds), and secE+ prlA3 (circles) strains. Aliquots were removed at various times postinduction, and CFU were measured. Also shown are CFU of the secE(S120C) prlA3 strain culture in the absence of arabinose (triangles).
We considered the possibility that a covalent linkage would inactivate SecY and SecE, thus depleting the cells of these essential proteins. Several lines of evidence argue against this simple model. First, strains depleted of most Sec proteins, including SecY and SecE, become cold sensitive (31), whereas the arabinose sensitivity of the prlA3 secE(S120C) strain is lessened rather than increased by incubation at lower temperatures (data not shown). More convincing evidence is provided by diploid analysis. We call attention to the last strain listed in Table 1. This strain contains secE(S120C) and prlA3, but it also contains secE+ on the chromosome and secY+ on a second, compatible plasmid. It is difficult to imagine how cross-linking could deplete this diploid strain of functional SecY and SecE, yet toxicity is still observed. We conclude that toxicity is dominant, and this suggests that cell death is precipitated by some acquired novel function.
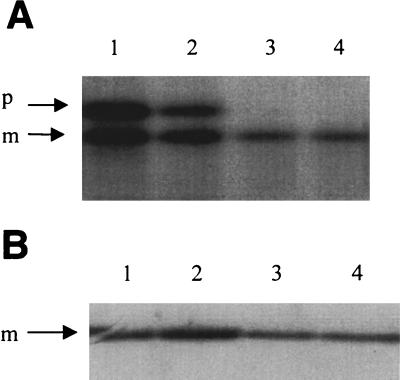
One altered function that the cross-linked complex might acquire would be the ability to titrate some other Sec protein or another secretion factor such as a component of the prokaryotic signal recognition particle (pSRP). To test this possibility assays were performed to determine if the expression of secE(S120C) in prlA3 strains leads to a block in translocation. The failure to cleave signal peptides, resulting in the accumulation of the higher-molecular-weight precursor species, can be used to indicate translocation defects. When β-lactamase translocation is analyzed in this way, the amount of β-lactamase precursor clearly increases (Fig. 3). However, β-lactamase translocation is known to be sensitive to changes in cellular physiology other than a defect in the secretion machinery, such as lowered levels of GroEL and GroES (21, 30). Indeed, under identical conditions the translocation of other proteins, including murein lipoprotein (Fig. 3), OmpF, and PhoA, is unaffected (data not shown). Moreover, using the assay of Ulbrandt et al. (38), we find that the membrane insertion of α-ketoglutarate permease, which was shown to be strongly dependent upon pSRP, is unaffected as well (data not shown). Thus, we can find no evidence for a secretion defect severe enough to kill cells.
FIG. 3.
Protein translocation in prlA3 secE(S120C) cells. (A) prlA3 secE(S120C) cells were induced for secE(S120C) expression with arabinose (lanes 1 and 2) for 2 h or were left uninduced (lanes 3 and 4). Cultures were pulse labeled for 30 s with [35S]methionine and chased for 30 s (lanes 1 and 3) or 4 min (lanes 2 and 4), and then the protein was TCA precipitated. β-Lactamase was collected by immunoprecipitation and separated by SDS-PAGE. The positions of precursor (p) and mature (m) β-lactamase are shown. (B) prlA3 secE(S120C) cells were induced for secE(S120C) expression with arabinose (lanes 3 and 4) for 2 h or were left uninduced (lanes 1 and 2). Cultures were pulse labeled for 30 s with [35S]methionine and chased for 30 s (lanes 1 and 3) or 4 min (lanes 2 and 4), and then the protein was TCA precipitated. Lipoprotein was collected by immunoprecipitation and separated by SDS-PAGE. Only mature lipoprotein was present. The lipoprotein precursor could not be detected.
As noted in the introduction, SecY and SecE are part of a large complex that is thought to form a protein-conducting channel in the inner membrane (25, 34). A covalent bond between these two proteins might lock the complex in an open or partially open conformation, which would result in the depolarization of the membrane. Since this would be an acquired function, it would explain the dominance observed in the diploid strain.
We tested for permeability changes as follows: prlA3 cells that were induced for secE(S120C) expression for as long as 4 h were exposed to DIBAC4, a compound that fluoresces in the presence of a membrane potential, and SYTOX Green, a compound that fluoresces upon membrane rupture. Using flow cytometry as previously described (24, 35), we could find no decrease in DIBAC4 fluorescence or any increase in SYTOX Green fluorescence (data not shown). Therefore, there is no indication of either membrane depolarization or rupture. The channel does not appear to be locked open.
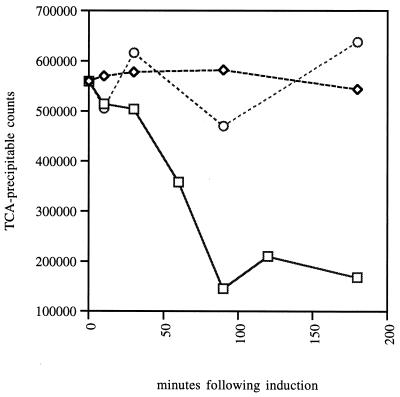
While performing the translocation assays described above, we discovered a significant but unexpected defect in the prlA3 secE(S120C) strain. As shown in Fig. 4, the levels of labeled protein begin to decrease shortly after the induction of the secE(S120C) gene. By 90 min postinduction total protein labeling is reduced by 80%.
FIG. 4.
PrlA3 and SecE(S120C) cross-linking inhibits the incorporation of the radioactive methionine into polypeptide. At 0 min, arabinose was added to the strains with the following genotypes: secE(S120C) prlA3 (squares), secE(S120C) secY+ (diamonds), and secE+ prlA3 (circles). At various time points samples were removed, and the ability of cells to incorporate [35S]methionine into polypeptide was measured by pulse labeling followed by TCA precipitation and radioactivity measurement as described in Materials and Methods.
We do not understand how the cross-linking of SecE(S120C) to PrlA3 can result in the decreased incorporation of methionine into protein. Because the membranes remain polarized (see above), the defect is probably not due to amino acid uptake. Rather it is likely to be related to transcription, translation, or protein stability. We have not investigated this defect further, because we are not certain whether this change in cellular physiology is the primary cause of death. Indeed, for reasons described in the following section, we suspect that the cytotoxicity is the cumulative effect of a complex cascade of cellular defects.
DISCUSSION
In a previous study, it was demonstrated that allele-specific combinations of prlA and prlG give rise to synthetic phenotypes (16). Strikingly, the pairs of mutations that produce these synthetic phenotypes always mapped to the same cellular compartment; either both resided within the periplasm or both resided within the membrane. This combination of allele specificity and topological coincidence led to the proposal that the domains containing these residues interact directly. In particular, since the synthetic defect in all cases was recessive to secY+ or secE+ provided in trans and since recessive behavior implies a loss of function, it was concluded that the prl mutations in question disrupt a normal, functionally important interaction between SecY and SecE.
Here we have directly tested the hypothesis that the synthetic pair, prlA3 and prlG3, define a site of contact between SecY and SecE. Both of these mutations alter amino acid residues in periplasmic domains. If residues 67 of SecY and 120 of SecE are in close contact, then the oxidizing environment of this cellular compartment should allow disulfide bond formation if both residues are replaced by cysteine. Genetic and biochemical experiments demonstrate disulfide bond formation between PrlA3 and SecE(S120C). This proves that actual points of protein-protein contact can be identified by using allele-specific synthetic phenotypes.
The genetic data demonstrating disulfide bond formation between PrlA3 and SecE(S120C) are compelling. The synthetic lethality observed with these cysteine substitutions is dominant to both secY+ and secE+ provided in trans, and this allows us to distinguish it from the recessive synthetic lethality observed previously by Flower et al. (16) with other amino acid substitutions at these positions. Dominant synthetic lethality requires cysteines at position 67 of SecY and 120 of SecE. No other amino acid at either position will do. This is a case of extreme allele specificity. Dominant synthetic lethality is also unique in that it requires DsbA, the periplasmic enzyme that catalyzes disulfide bond formation. In the absence of this enzyme, no synthetic phenotypes are detectable with the cysteine substitutions unless an oxidizing agent is added to the growth media. Alternative models for dominant synthetic lethality that account for all of these facts invariably invoke a hypothetical molecule(s), and these complex scenarios fail to adequately explain the recessive synthetic lethality observed by Flower et al. (16). We conclude that dominant synthetic lethality is due to disulfide-bonded PrlA3-SecE(S120C).
Biochemical support for a disulfide-bonded PrlA3-SecE(S120C) is provided by Western blot analysis. SDS-PAGE with nonreduced samples reveals a new band of 45 kDa that is recognized by both SecY and SecE antibodies. This band disappears when DTT is added, and it is specific to strains that synthesize PrlA3 and SecE(S120C). These results provide direct evidence that residues 67 of SecY and 120 of SecE are sufficiently close to allow disulfide bond formation.
The amount of cross-linked PrlA3-SecE(S120C) is relatively small compared with the total amount of SecE present (Fig. 1B), and this merits further comment. Since secE is an essential gene and the gene pair secE(S120C)-prlA3 confers a dominant toxic phenotype, maintaining the prlA3 secE(S120C) strain requires the repression of secE(S120C) and the presence of the secE+ gene. Prior to the induction of secE(S120C), all of the PrlA3 is thus partnered with wild-type SecE, and this complex will not be disulfide bonded. Upon induction, most of the SecE protein is SecE(S120C). However, since PrlA3 is not co-overexpressed, most of the SecE(S120C) is likely to lack a PrlA3 partner and therefore cannot yield a cross-linked complex. Moreover, there is evidence that preformed SecYE complexes are stable and do not mix with newly synthesized molecules of either protein (19). Thus overexpressing SecE(S120C) would not be expected to contribute to disulfide bonding within existing PrlA3-SecE+ complexes. It may be possible to optimize conditions for the formation of the cross-linked product in order to study its properties in vitro, such as by co-overexpressing PrlA3 with SecE(S120C). However, there are expression problems in this strain (Fig. 4), and we also see a 25-kDa band that is likely a degradation product (Fig. 1B). Both the expression defect and degradation may contribute in a negative fashion to the overall yield.
A second issue raised by the Western blots is the presence of cross-linked species in addition to the PrlA3-SecE(S120C) complex. For instance, there is a prominent band at 30 kDa in Fig. 1B. Either this represents a SecE homodimer or else there is a high degree of cross-linking between SecE(S120C) and another cysteine-containing protein of the periplasm. Indeed, upon further exposure of the gels in Fig. 1, we can see several other discrete cross-linked products (data not shown). Apparently cysteine residues in the periplasm are quite active chemically. Whatever the composition of these disulfide-bonded complexes, they seem to have no effect on cell growth and viability, even when prlA3, prlA205, or prlA300 is the sole copy of the secY gene in the cell. However, these additional bands can interfere with biochemical analysis, as was seen with the prlA300 strain.
The synthetic lethality observed with the SecY and SecE cysteine substitution mutations is exquisitely position dependent. In total 15 different pairwise combinations of SecY and SecE cysteine substitution mutations were tested, and all of these substitutions were confined to tightly clustered regions: three in SecY at codons 64, 67, and 69 and five in SecE at codons 118, 120, 121, 122, and 124. DsbA-dependent, dominant synthetic lethality was only observed in 2 of the 15: prlA3 secE(S120C) and prlA300 secE(G124C). In all of the other cases, no synthetic phenotypes were observed. We conclude that these two points of contact define small interactive domains in both SecY and SecE.
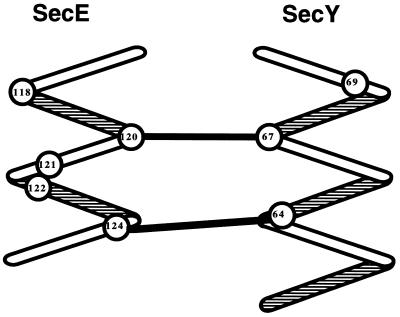
From these two points of contact, a model for the local quaternary structure may be gleaned. Model building indicates that arranging these two periplasmic loops as antiparallel α-helices places both pairs of contacting side chains, SecY-64–SecE-124 and SecY-67–SecE-120, within bonding distance (Fig. 5). Model building rules out an antiparallel β-conformation, since both points of contact cannot be brought together. Secondary structure algorithms also suggest that this region of SecY is helical, although no prediction is made with respect to the region of SecE. The model shown in Fig. 5 is also supported by the benign phenotypes of most of the cysteine mutant pairs, since in this model the relevant side chains are not within covalent-bonding distance. This approach of scanning a local region with cysteine point mutants and looking for disulfide bonding has previously revealed interactive faces within domains of a single protein or within proteins that form homomeric complexes (15, 22, 26, 29, 40). We show that the approach can also be applied to heteromeric protein complexes.
FIG. 5.
Molecular modeling of the regions subjected to cysteine scanning mutagenesis. The second periplasmic loop of the SecE protein and the first periplasmic loop of the SecY protein are depicted as antiparallel α-helices. The positions of the amino acids subjected to cysteine scanning are shown as numbers within circles. We have linked the amino acids at points sufficiently proximal to form disulfide bonds when replaced by cysteines.
While this study identifies specific contact sites between SecY and SecE, it is by no means an exhaustive search. Other interactions between these proteins must exist. Preliminary data indicate that periplasmic loop 1 of SecY, which includes the contact points shown in Fig. 5, can be deleted without eliminating SecY function (27a). Thus, assuming that the SecY-SecE interaction is essential, this region cannot be the only domain in which the two proteins are in contact. There are data suggesting that SecY and SecE have a cytoplasmic contact, with one of the sites on cytoplasmic loop 5 of SecY and another site (which is not necessarily the partner of the first) on cytoplasmic loop 2 of SecE (1, 33). At a finer level, other synthetic phenotypes suggest interactions between specific amino acids within transmembrane helices 7 and 10 of SecY and transmembrane helix 3 of SecE (16).
As noted in Results, it is not immediately obvious why cross-linking SecY and SecE at a point of normal contact would cause cell death. Since the synthetic lethality observed with these cysteine substitutions is dominant to both secY+ and secE+ provided in trans and since protein secretion is largely unperturbed in dying cells, it is clear that death is not caused by a lack of functional SecY or SecE. Rather, the cross-linked complex must actively kill cells.
It appears that the covalent linkage between PrlA3 and SecE(S120C) activates a novel function for the SecYE protein complex. Exactly what this function might be is a mystery. Ordinarily, we might expect to find an answer within the physiology of the afflicted cells. However, it appears that the cause of death in these strains is quite complicated. We have managed to rule out the more obvious possibilities that would be consistent with a dominant phenotype: both membrane integrity and potential remain intact; the cold resistance of the cytotoxicity and the lack of translocation defects for most of the proteins tested argue against the titration of other Sec proteins; and the lack of an effect on the membrane insertion of α-ketoglutarate permease indicates that pSRP is not limiting. Of the changes in physiology that do occur, the most striking is a reduced ability to label newly synthesized proteins. However, this defect is apparent within 1 h of induction of the secE(S120C) gene, whereas most cell death occurs much later. Since hours elapse between this change in cellular physiology and the onset of cell death, we cannot be certain if it is the direct cause. We suspect that SecY-SecE cross-linking triggers a complex series of events that progressively weaken the cell to the point of death.
An understanding of the mechanism of toxicity would be germane, were we attempting to analyze the functional role of SecY and SecE. Here we probe the structure of the SecY-SecE complex, not its function, and our conclusions about structure will not change regardless of the mechanism by which the cross-linked complex kills the cell. It is important to note that genetic analysis can provide very specific information even when the phenotypes in question are complex and poorly understood. For example Crick et al. (8) used genetics to provide key insights into the nature of the genetic code and gene structure long before the phenotypes of T4 rII mutants were understood.
Our results demonstrate that allele-specific synthetic phenotypes can identify points of direct contact between interacting proteins. They also show that DsbA can catalyze the formation of nonnative disulfide bonds among the subunits of multimeric protein complexes. We believe that these approaches will prove to be generally useful.
ACKNOWLEDGMENTS
We are grateful to Robert Osborne and Ann Flower, who contributed strains and plasmids and conducted seminal experiments leading to this study, to Masayori Inouye and Koreaki Ito for the generous gift of antisera, to Harris Bernstein for the α-ketoglutarate permease plasmids, to Andrew Beavis, who helped with flow cytometry, and to Richard Ebright and Austin Newton for helpful suggestions.
This work was supported by NIGMS grant GM34821 to T.J.S.
REFERENCES
- 1.Baba T, Taura T, Shimoike T, Akiyama Y, Yoshihisa T, Ito K. A cytoplasmic domain is important for the formation of a SecY-SecE translocator complex. Proc Natl Acad Sci USA. 1994;91:4539–4543. doi: 10.1073/pnas.91.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardwell J C, Lee J O, Jander G, Martin N, Belin D, Beckwith J. A pathway for disulfide bond formation in vivo. Proc Natl Acad Sci USA. 1993;90:1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 4.Bieker K L, Phillips G J, Silhavy T J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990;22:291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- 5.Bieker K L, Silhavy T J. PrlA (SecY) and PrlG (SecE) interact directly and function sequentially during protein translocation in E. coli. Cell. 1990;61:833–842. doi: 10.1016/0092-8674(90)90193-i. [DOI] [PubMed] [Google Scholar]
- 6.Bieker-Brady K, Silhavy T J. Suppressor analysis suggests a multistep, cyclic mechanism for protein secretion in Escherichia coli. EMBO J. 1992;11:3165–3174. doi: 10.1002/j.1460-2075.1992.tb05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundage L, Hendrick J P, Schiebel E, Driessen A J M, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 8.Crick F H C, Barnett L, Brenner S, Watts-Tobin R J. General nature of the genetic code for proteins. Nature. 1961;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- 9.Deng W P, Nickoloff J A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 10.Derman A I, Prinz W A, Belin D, Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993;262:1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- 11.Duong F, Eichler J, Price A, Leonard M R, Wickner W. Biogenesis of the Gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- 12.Economou A. Bacterial preprotein translocase: mechanism and conformational dynamics of a processive enzyme. Mol Microbiol. 1998;27:511–518. doi: 10.1046/j.1365-2958.1998.00713.x. [DOI] [PubMed] [Google Scholar]
- 13.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 14.Esnault Y, Feldheim D, Blondel M-O, Schekman R, Kepes F. SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J Biol Chem. 1994;269:27478–27485. [PubMed] [Google Scholar]
- 15.Falke J J, Koshland D E., Jr Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science. 1987;237:1596–1600. doi: 10.1126/science.2820061. [DOI] [PubMed] [Google Scholar]
- 16.Flower A M, Osborne R S, Silhavy T J. The allele-specific lethality of prlA-prlG double mutants predicts interactive domains of SecY and SecE. EMBO J. 1995;14:884–893. doi: 10.1002/j.1460-2075.1995.tb07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarente L. Synthetic enhancement in gene interaction: a genetic tool come of age. Trends Genet. 1993;9:362–366. doi: 10.1016/0168-9525(93)90042-g. [DOI] [PubMed] [Google Scholar]
- 18.Huffaker T C, Hoyt M H, Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- 19.Joly J C, Leonard M R, Wickner W. Subunit dynamics in Escherichia coli preprotein translocase. Proc Natl Acad Sci USA. 1994;91:4703–4707. doi: 10.1073/pnas.91.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y J, Rajapandi T, Oliver D. SecA protein is exposed to the periplasmic surface of the E. coli inner membrane in its active state. Cell. 1994;78:845–853. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 21.Kusukawa N, Yura T, Ueguchi C, Akiyama Y, Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989;8:3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G F, Burrows G G, Lebert M R, Dutton D P, Hazelbauer G L. Deducing the organization of a transmembrane domain by disulfide cross-linking. The bacterial chemoreceptor Trg. J Biol Chem. 1994;269:29920–29927. [PubMed] [Google Scholar]
- 23.Lingappa V R, Chaidez J, Yost C S, Hedgpeth J. Determinants for protein localization: beta-lactamase signal sequence directs globin across microsomal membranes. Proc Natl Acad Sci USA. 1984;81:456–460. doi: 10.1073/pnas.81.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason D J, Power E G M, Talsania H, Phillips I, Gant V A. Antimicrobial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2752–2758. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer T H, Menetret J F, Breitling R, Miller K R, Akey C W, Rapoport T A. The bacterial SecY/E translocation complex forms channel-like structures similar to those of the eukaryotic Sec61p complex. J Mol Biol. 1999;285:1789–1800. doi: 10.1006/jmbi.1998.2413. [DOI] [PubMed] [Google Scholar]
- 26.Milligan D L, Koshland D E., Jr Site-directed cross-linking: establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J Biol Chem. 1992;263:6268–6275. [PubMed] [Google Scholar]
- 27.Nishiyama K, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 27a.Osborne, R. S., and T. J. Silhavy. Unpublished data.
- 28.Osborne R S, Silhavy T J. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 1993;12:3391–3398. doi: 10.1002/j.1460-2075.1993.tb06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pakula A A, Simon M I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci USA. 1992;89:4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips G J, Silhavy T J. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992;359:744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- 31.Pogliano K J, Beckwith J. The cs sec mutants of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics. 1993;133:763–773. doi: 10.1093/genetics/133.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohlschroder M, Murphy C, Beckwith J. In vivo analyses of interactions between SecE and SecY, core components of the Escherichia coli protein translocation machinery. J Biol Chem. 1996;271:19908–19914. doi: 10.1074/jbc.271.33.19908. [DOI] [PubMed] [Google Scholar]
- 33.Pohlschroder M, Prinz W A, Hartmann E, Beckwith J. Protein translocation in the three domains of life: variations on a theme. Cell. 1997;91:563–566. doi: 10.1016/s0092-8674(00)80443-2. [DOI] [PubMed] [Google Scholar]
- 34.Rapoport T A, Jungnickel B, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- 35.Roth B L, Poot M, Yue S T, Millard P J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–2431. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 37.Talmadge K, Stahl S, Gilbert W. Bacteria mature preproinsulin to proinsulin. Proc Natl Acad Sci USA. 1980;77:3369–3373. doi: 10.1073/pnas.77.7.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulbrandt N D, Jewitt J A, Bernstein H D. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- 39.von Heijne G. Signal sequences: the limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 40.Whitley P, Nilsson L, von Heijne G. Three-dimensional model for the membrane domain of Escherichia coli leader peptidase based on disulfide mapping. Biochemistry. 1993;32:8534–8539. doi: 10.1021/bi00084a020. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson B M, Esnault Y, Craven R A, Skiba F, Fieschi J, Kepes F, Stirling C J. Molecular architecture of the ER translocase probed by chemical crosslinking of Sss1p to complementary fragments of Sec61p. EMBO J. 1997;16:4549–4559. doi: 10.1093/emboj/16.15.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]