Abstract
Background:
Only a few studies investigated the association between proton pump inhibitor (PPI) use and pancreatic cancer, with inconsistent results. Moreover, these studies had a number of methodologic limitations. Our objective was to assess this association in a nationwide case–control study.
Methods:
We used the French National Health Data System (SNDS), covering 99% of the French population since 2006. Incident cases of pancreatic cancer, identified between 2014 and 2018, were matched with up to four controls on year of birth, sex, frequency of hospitalization within 8 years prior to index date, and department of residence. Associations between PPIs and pancreatic cancer were estimated using conditional logistic regression models adjusted for sociodemographic characteristics, risk factors of pancreatic cancer (including diabetes mellitus, tobacco-related diseases, and morbid obesity), and other comorbidities.
Results:
A total of 23,321 cases of pancreatic cancer (mean age, 69.8 years; 51.7% males) and 75,937 matched controls were included. Overall, 77.8% of cases and 75.5% of controls were PPI ever users. Ever (vs. never) PPI use was associated with an increased risk of pancreatic cancer [adjusted OR (aOR) = 1.05, 95% confidence interval (CI), 1.01–1.09]. A dose–response relationship was observed [1–30 cumulative defined daily dose (cDDD): aOR = 0.92, 95% CI, 0.87–0.97; 31–180 cDDD: aOR = 1.05, 95% CI, 1.00–1.11; 181–1,080 cDDD: aOR = 1.18, 95% CI, 1.12–1.24; >1,080 cDDD: aOR = 1.17, 95% CI, 1.10–1.23].
Conclusions:
On the basis of these findings, a slight increase in the risk of pancreatic cancer associated with high cumulative doses of PPIs cannot be excluded.
Impact:
Given the overuse of PPIs, efforts should be continued to limit treatments to appropriate indications and durations.
Introduction
Since their market introduction in the late 1980s, proton pump inhibitors (PPI) have proven their efficacy and have become a standard treatment for acid-related conditions such as peptic ulcer disease and gastroesophageal reflux disease (1). Their use has steadily increased, and they are currently one of the most commonly prescribed classes of drug worldwide (2, 3). Misuse, such as coprescribing with nonsteroidal anti-inflammatory drugs (NSAID) without gastrointestinal risk, or prescribing without a clear indication (3), is widespread, reaching on average 50% among outpatients (4). Despite a good overall tolerance at short term, prolonged PPI use has raised safety concerns. Risks of long-term PPI therapy have been extensively explored in the literature, although some of them are still debated (5). Potential gastrointestinal health outcomes include infections (6), inflammatory bowel diseases (7), and malignancies (8–10). However, only a few observational studies have examined the association between PPI use and the risk of pancreatic cancer as main outcome, with inconsistent results and methodologic limitations arising from limited numbers of cases or long-term PPI users, concerns of reverse causality, or inability to capture important confounders (11–18). Plausible mechanisms have been suggested for the potential carcinogenic effect of PPIs in pancreatic cancer, related to induced hypergastrinemia (19) and microbiome alterations (20). While risk factors of pancreatic cancer are still insufficiently known, incidence rates are rising in developed countries, with over seven cases per 100,000 person-years in Northern America and Western Europe. After diagnosis, pancreatic cancer is associated with a poor prognosis, with an estimated 5-year survival rate of less than 5% (21–23). Thus, clarifying the impact of PPI exposure on the risk of pancreatic cancer is of major importance.
The aim of this study was to investigate the association between PPI use and the risk of pancreatic cancer in France, based on a large nationwide, population-based case–control study, addressing methodologic limitations of previous studies.
Materials and Methods
Data sources
This study was conducted using the French National Health Data System (Système National des Données de Santé, SNDS), consisting of comprehensive sociodemographic and medical individual information for 99% of the population living in France (about 67 million people) since 2006. The database contains data about all outpatient services reimbursed by the National Health Insurance, including drugs [coded according to the Anatomical Therapeutic Chemical Classification System (ATC; ref. 24)], and physician visits. Patients with costly chronic diseases (long-term diseases), such as cancer, are fully reimbursed for their health expenditures, and the diagnosis are recorded [coded according to the International Classification of Diseases, 10th Revision (ICD-10; ref. 25)]. The database also contains diagnoses related to hospital admissions, and procedures performed during hospital stays. A detailed presentation of the SNDS databases is available in the Supplementary Data S1.
Study population
Cases
We identified all patients ages 40 to 85 years, with an incident primary pancreatic cancer between January 1, 2014 and December 31, 2018. The index date was the date of first mention of pancreatic cancer (ICD-10 code C25), either as a hospital discharge diagnosis, or as a cause of long-term disease if it was further followed by a hospital discharge diagnosis within the next 3 months. We focused on pancreatic adenocarcinoma, which accounts for about 9 of 10 of all pancreatic cancers (21, 23). Thus, patients with a neuroendocrine neoplasm of the pancreas were not included. They were identified by an ICD-10 code C25.4, and/or an outpatient treatment with somatostatin analogs (ATC codes H01CB02, H01CB03) in the year following index date. To note, cases of pancreatic adenocarcinoma receiving a somatostatin analog in the perioperative setting, to reduce the risk of postoperative pancreatic leaks, were not excluded from the analyses.
Controls
Four controls with no diagnosis of pancreatic cancer at index date were randomly selected after matching on year of birth, sex, frequency of hospitalization within 8 years before index date (figured by the number of calendar years with at least one hospital admission, categorized as: 0, 1, 2, or ≥3), and department of residence. The index date of each case was assigned to the matched controls.
Exclusion criteria
Exclusion criteria for cases and controls were death on index date, absence of outpatient claim 7 or 8 years prior to index date (to assign the same length of observation to cases and to their matched controls, ensuring equal time windows to measure exposure and to identify comorbidities), and history of cancer (all causes) or pancreatic abnormality within 8 years before index date (ICD-10 codes available in the Supplementary Table S1).
Exposure to PPIs
Exposure to PPIs was defined as redeeming at least one prescription of a PPI marketed in France, namely omeprazole, esomeprazole, lansoprazole, pantoprazole, or rabeprazole (ATC codes A02BC01 to A02BC05), between January 1, 2006 and the index date. For ever users, we calculated the cumulative defined daily dose (cDDD), classified in quartile categories based on the distribution of use in cases. There is no consensus on optimal treatment duration or agreed definition of long‐term PPI use (26, 27). On the basis of information contained in the Summary of Product Characteristics, individuals were considered long-term users if they had been exposed to a cumulative dose of 181 DDD, equivalent to a 6-month therapy within the study period.
The SNDS does not contain information neither on inpatient nor on over-the-counter (OTC) PPI use. However, this use accounts for a limited proportion in France. In 2015, 92% of PPI boxes were delivered to outpatients and almost 97% of them were obtained from prescriptions (source: French National Agency for Medicines and Health Products Safety, ANSM).
Exclusion of incident PPI users
To allow for latency, and to minimize reverse causality, incident PPI users within 2 years before index date (defined by at least one redeemed prescription of PPI within 24 months prior to index date, and no redeemed prescription of PPI between 24 and 36 months prior to index date) were excluded from the set of cases and matched controls. Strata containing only cases or controls after exclusion of incident PPI users within 2 years before index date were removed.
Covariates
Sociodemographic characteristics were affiliation to complementary universal health insurance (CMUC, free access to healthcare for low-income people under 65 years old), and social deprivation index (levels of disadvantage calculated across small geographic areas). We also identified medical covariates, defined by a diagnosis, or, if appropriate, by at least three redeemed prescriptions, within 8 years prior to index date (codes available in the Supplementary Table S2): (i) potential risk factors of pancreatic cancer, defined according to the current best available evidence (21, 22, 28, 29): diabetes mellitus, tobacco-related diseases (including COPD diagnosis) or drug use, morbid obesity, alcohol-related diseases or drug use, acute pancreatitis, chronic pancreatitis, pancreatic cyst, gallstones, hepatitis B or C (ii); proxies of potential contexts of PPI treatment: gastroesophageal reflux disease, peptic ulcer, Helicobacter pylori eradication (iii); and other comorbidities or drug use: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, mild liver disease, hemiplegia, moderate to severe liver disease, moderate to severe chronic kidney disease, HIV/AIDS, antihypertensive drug use, NSAID use, statin use.
Statistical analysis
Characteristics of the cases and matched controls were presented using descriptive statistics.
Associations between exposure to PPIs and pancreatic cancer were estimated based on crude and adjusted ORs (aOR) and their 95% confidence intervals (CI) obtained using conditional logistic regression models. In addition to the matching variables (year of birth, sex, history of hospitalizations, and department of residence), and calendar year, accounted for by design, potential risk factors of pancreatic cancer (diabetes mellitus, tobacco-related diseases or drug use, morbid obesity, alcohol-related diseases or drugs use, acute pancreatitis, chronic pancreatitis, pancreatic cyst, gallstones, hepatitis B or C) were forced in the adjusted models. Then, final models were run introducing the remaining covariates through forward selection process.
Complementary analyses stratified by age group, sex, calendar year, and cancer localization were conducted. We also assessed whether the main modifiable risk factors of pancreatic cancer (28, 29), which were reported with a prevalence above 1% among cases and controls, were effect modifier. To this end, we included in the model an interaction term between PPI use and the following covariates: diabetes mellitus, tobacco-related diseases or drug use, or morbid obesity.
The robustness of the main results was assessed in four sensitivity analyses. First, we applied a 2-year and a 4-year lag before the index date, disregarding PPI exposure in these periods, to maximize the control of reverse causality (30). Second, analyses were restricted to new PPI users, excluding patients who received a PPI in 2006. Third, we used inverse probability of treatment weighting (IPTW) to reduce confounding. Stabilized weights were computed from a logistic model using all cases and controls, adjusted for all covariates (except for CMUC, only available among individuals ages under 65 years). Weights were then introduced in a logistic regression model for the outcome, including no other predictor than exposure (31–33). Fourth, a sensitivity analysis considering histamine‐2‐receptor antagonists (H2RA, ATC codes A02BA01 to A02BA04) as an active comparator was conducted (34). These drugs have similar indications to PPIs, but inhibit acid secretion less profoundly than PPIs (35). Because of their well-documented superiority in relieving symptoms and healing mucosal lesions, PPIs have rapidly replaced H2RAs in treating any clinical acid-related condition (4). Most previous studies suggested a lack of association between pancreatic cancer development and H2RAs use (11, 13, 18). Thus, we compared the risk of pancreatic cancer between PPI and H2RA ever users, excluding those with incident PPI or H2RA use within 2 years before index date. Subjects using both PPIs and H2RAs were defined as PPI users.
All analyses were conducted using SAS EG (Copyright 2017 SAS Institute Inc.).
Data availability
The data generated in this study are not publicly available. EPIPHARE (https://www.epi-phare.fr/en/) has a regulatory permanent access to the data from the French National Health Data System (SNDS) via its constitutive bodies ANSM and CNAM. This permanent access is given according the French Decree No. 2016-1871 of December 26, 2016 relating to the processing of personal data called "National Health Data System" [Décret no. 2016–1871 du 26 décembre 2016 relatif au traitement de données à caractère personnel dénommé « système national des données de santé » (Internet). 2016 (cited 2021 Mar 12). Available from: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000033702840&categorieLien=id] and French law articles Art. R. 1461-13 [Article R1461-13 - Code de la santé publique - Légifrance (Internet). (cited 2021 Mar 12). Available from: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000038789574/] and 14 [Article R1461-14 - Code de la santé publique - Légifrance (Internet). (cited 2021 Mar 12). Available from: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000037678676/].
All data were deidentified, thus, informed consent was not necessary.
Results
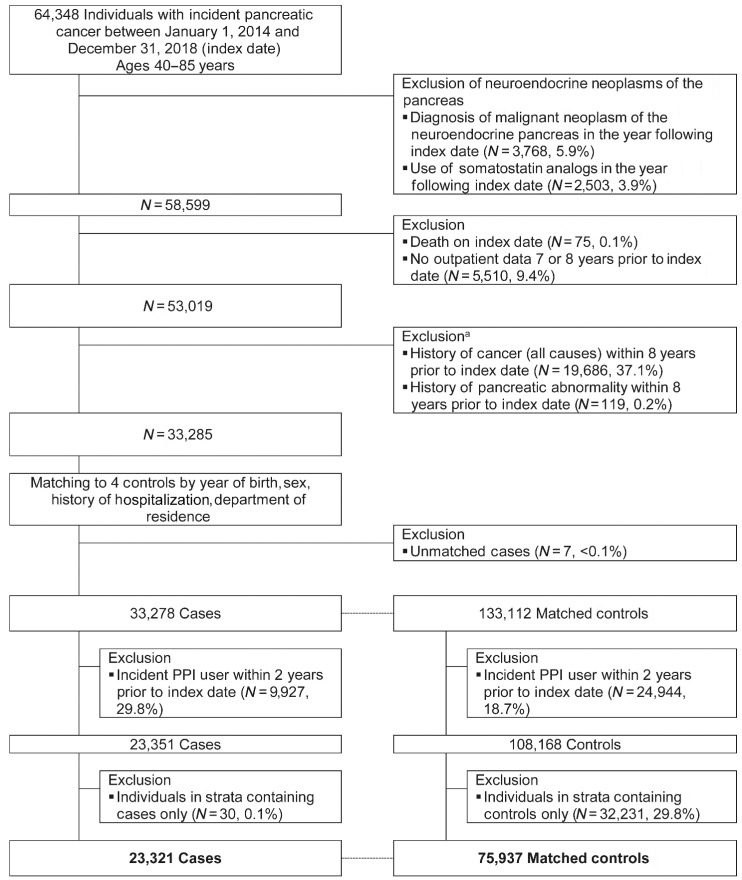
We identified a total of 64,348 individuals ages 40–85 years, with incident pancreatic cancer between January 1, 2014 and December 31, 2018, including 58,599 (91.1%) patients with pancreatic adenocarcinoma. Among them, 37.1% (N = 19,686) were excluded because of history of cancer within 8 years prior to index date. Seven cases failed to be matched with controls. Almost 30% (N = 9,927) of cases and 18.7% (N = 24,944) of matched controls were excluded because of incident PPI use within 2 years prior to index date. Finally, the study population comprised 23,321 cases of pancreatic cancers, and 75,937 matched population controls, with a mean number of 3.3 controls per case (Fig. 1).
Figure 1.
Study flow chart. Figure shows the flowchart of the study and the number of patients included in the case and in the control groups. PPI, proton pump inhibitor. aCodes available in Supplementary Table S1.
The characteristics of cases and controls are shown in Table 1. Among cases, mean age at diagnosis was 69.8 ± 10.1 years, and 51.7% were males. Compared with controls, cases had a higher prevalence of diabetes mellitus, tobacco- and alcohol-related diseases or drug use, morbid obesity, and history of acute or chronic pancreatitis. Cases were also more likely to present with other comorbidities such as chronic obstructive pulmonary disease. Pancreatic cancers were most often localized to the head of the pancreas (Table 2). More than half of the cases died within 1 year after the index date (52.3%, N = 12,202). Overall, 77.8% (N = 18,141) of cases and 75.5% (N = 57,307) of controls were PPI ever users, and 43.9% and 37.9% redeemed prescriptions for 181 cDDD or more (Supplementary Table S3). Cases and controls were respectively exposed to 658.3 ± 1,079.1 and 560.8 ± 1,009.7 cDDD in mean during the study period. Omeprazole was the most frequently prescribed drug (in 50.8% of cases, and 47.9% of controls), followed by esomeprazole (46.0% of cases and 40.6% of controls; Supplementary Table S3).
Table 1.
Baseline characteristics of pancreatic cancer cases and controls.
| Cases | Controls | |
|---|---|---|
| N = 23,321 | N = 75,937 | |
| Sociodemographic characteristics | ||
| Agea (years), mean (SD) | 69.8 ± 10.1 | 70.0 ± 10.0 |
| 40–64 years, n (%) | 6,694 (28.7) | 21,188 (27.9) |
| ≥65 years, n (%) | 16,627 (71.3) | 54,749 (72.1) |
| Mena, n (%) | 12,061 (51.7) | 39,370 (51.8) |
| CMUCb, n (%) | 765 (11.4) | 1,669 (7.9) |
| Social deprivation index (quintiles), n (%) | ||
| 1 (least deprivation) | 4,209 (18.0) | 13,997 (18.4) |
| 2 | 4,136 (17.7) | 13,896 (18.3) |
| 3 | 4,524 (19.4) | 15,007 (19.8) |
| 4 | 4,740 (20.3) | 15,506 (20.4) |
| 5 (highest deprivation) | 4,963 (21.3) | 15,083 (19.9) |
| Missing | 749 (3.2) | 2,448 (3.2) |
| Comorbidities, n (%) | ||
| Diabetes mellitus | 7,177 (30.8) | 13,304 (17.5) |
| Complications of diabetes mellitus | 882 (3.8) | 1,597 (2.1) |
| Tobacco-related diseases or drug use | 3,544 (15.2) | 7,673 (10.1) |
| Morbid obesity | 2,936 (12.6) | 7,531 (9.9) |
| Alcohol-related diseases or drug use | 1,691 (7.3) | 2,711 (3.6) |
| Acute pancreatitis | 825 (3.5) | 439 (0.6) |
| Chronic pancreatitis | 474 (2.0) | 155 (0.2) |
| Pancreatic cyst | 825 (3.5) | 156 (0.2) |
| Gallstones | 1,425 (6.1) | 2,655 (3.5) |
| Hepatitis B or C | 211 (0.9) | 454 (0.6) |
| Gastroesophageal reflux disease | 3,290 (14.1) | 8,459 (11.1) |
| Peptic ulcer | 455 (2.0) | 1,026 (1.4) |
| Helicobacter pylori eradication | 910 (3.9) | 2,407 (3.2) |
| Myocardial infarction | 1,084 (4.6) | 3,129 (4.1) |
| Congestive heart failure | 2,051 (8.8) | 5,609 (7.4) |
| Peripheral vascular disease | 2,006 (8.6) | 4,595 (6.1) |
| Cerebrovascular disease | 1,788 (7.7) | 5,118 (6.7) |
| Dementia | 1,555 (6.7) | 5,300 (7.0) |
| Chronic obstructive pulmonary disease | 5,325 (22.8) | 14,869 (19.6) |
| Connective tissue disease | 449 (1.9) | 1,316 (1.7) |
| Mild liver disease | 895 (3.8) | 1,521 (2.0) |
| Hemiplegia | 588 (2.5) | 1,733 (2.3) |
| Moderate to severe chronic kidney disease | 922 (4.0) | 2,400 (3.2) |
| Moderate to severe liver disease | 259 (1.1) | 347 (0.5) |
| AIDS | 63 (0.3) | 148 (0.2) |
| Comedicationsc, n (%) | ||
| Antihypertensive drugs | 14,547 (62.4) | 43,658 (57.5) |
| Nonsteroidal anti-inflammatory drugs | 17,579 (75.4) | 55,441 (73.0) |
| Statins | 9,527 (40.9) | 28,514 (37.5) |
Abbreviations: CMUC, complementary universal health insurance; SD, standard deviation.
aMatching variables.
bAmong individuals aged less than 65 years only.
cAt least three redeemed prescriptions within 8 years prior to index date.
Table 2.
Characteristics of pancreatic cancer cases at the time of diagnosis.
| Cases | |
|---|---|
| N = 23,321 | |
| Age (years), mean (SD) | 69.8 ± 10.1 |
| Men, n (%) | 12,061 (51.7) |
| Year of diagnosis, n (%) | |
| 2014 | 3,975 (17.0) |
| 2015 | 4,416 (18.9) |
| 2016 | 4,878 (20.9) |
| 2017 | 4,922 (21.1) |
| 2018 | 5,130 (22.0) |
| Cancer localization, n (%) | |
| Head of pancreas | 12,438 (53.3) |
| Body of pancreas | 3,222 (13.8) |
| Tail of pancreas | 3,025 (13.0) |
| Pancreatic duct | 461 (2.0) |
| Neck of pancreas | 769 (3.3) |
| Unspecified | 3,406 (14.6) |
| Region of residence, n (%) | |
| Île-de-France | 3,577 (15.3) |
| Centre-Val de Loire | 1,012 (4.3) |
| Bourgogne-Franche-Comté | 1,135 (4.9) |
| Normandie | 1,217 (5.2) |
| Hauts-de-France | 2,023 (8.7) |
| Grand Est | 1,884 (8.1) |
| Pays de la Loire | 1,255 (5.4) |
| Bretagne | 1,007 (4.3) |
| Nouvelle-Aquitaine | 2,262 (9.7) |
| Occitanie | 2,225 (9.5) |
| Auvergne-Rhône-Alpes | 3,051 (13.1) |
| Provence-Alpes-Côte d'Azur | 2,099 (9.0) |
| Corse | 122 (0.5) |
| Overseas territories | 452 (1.9) |
Abbreviation: SD, standard deviation.
The results of the main analysis are shown in Table 3. Ever use of PPIs was associated with a slightly increased risk of pancreatic cancer when compared with never use [crude OR = 1.15, 95% CI, 1.10–1.19; aOR (final model) = 1.05, 95% CI, 1.01–1.09]. A dose–response relationship was observed (1–30 cDDD: aOR = 0.92, 95% CI, 0.87–0.97; 31–180 cDDD: aOR = 1.05, 95% CI, 1.00–1.11; 181–1,080 cDDD: aOR = 1.18, 95% CI, 1.12–1.24; >1,080 cDDD: aOR = 1.17, 95% CI, 1.10–1.23). Analyses by PPI subtype showed a higher risk with esomeprazole (aOR = 1.18, 95% CI, 1.14–1.22). Similar results were found when covariates included in the model were restricted to potential risk factors of pancreatic cancer only (Table 3).
Table 3.
Association between exposure to PPIs and pancreatic cancer.
| Cases | Controls | ||||
|---|---|---|---|---|---|
| Exposure to PPIs | N = 23,321 | N = 75,937 | Crude OR (95% CI) | Adjusted ORa (95% CI) | Adjusted ORb (95% CI) |
| Ever use, n (%) | |||||
| No | 5,180 (22.2) | 18,630 (24.5) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 18,141 (77.8) | 57,307 (75.5) | 1.15 (1.10–1.19) | 1.10 (1.05–1.14) | 1.05 (1.01–1.09) |
| cDDD, n (%) | |||||
| 0 cDDD | 5,180 (22.2) | 18,630 (24.5) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1–30 cDDD | 3,186 (13.7) | 12,500 (16.5) | 0.92 (0.88–0.97) | 0.92 (0.88–0.97) | 0.92 (0.87–0.97) |
| 31–180 cDDD | 4,720 (20.2) | 16,056 (21.1) | 1.08 (1.03–1.13) | 1.07 (1.02–1.12) | 1.05 (1.00–1.11) |
| 181–1,080 cDDD | 5,087 (21.8) | 14,578 (19.2) | 1.31 (1.25–1.38) | 1.23 (1.17–1.29) | 1.18 (1.12–1.24) |
| >1,080 cDDD | 5,148 (22.1) | 14,173 (18.7) | 1.40 (1.34–1.47) | 1.24 (1.17–1.30) | 1.17 (1.10–1.23) |
| By PPI subtype (ever use), n (%) | |||||
| Omeprazole | |||||
| No | 11,464 (49.2) | 39,548 (52.1) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 11,857 (50.8) | 36,389 (47.9) | 1.13 (1.09–1.16) | 1.11 (1.08–1.15) | 1.08 (1.04–1.12) |
| Esomeprazole | |||||
| No | 12,599 (54.0) | 45,118 (59.4) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 10,722 (46.0) | 30,819 (40.6) | 1.28 (1.24–1.32) | 1.22 (1.18–1.26) | 1.18 (1.14–1.22) |
| Lansoprazole | |||||
| No | 17,035 (73.0) | 56,822 (74.8) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 6,286 (27.0) | 19,115 (25.2) | 1.10 (1.06–1.13) | 1.08 (1.05–1.12) | 1.05 (1.01–1.09) |
| Pantoprazole | |||||
| No | 14,654 (62.8) | 50,341 (66.3) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 8,667 (37.2) | 25,596 (33.7) | 1.17 (1.14–1.21) | 1.14 (1.10–1.17) | 1.09 (1.06–1.13) |
| Rabeprazole | |||||
| No | 18,930 (81.2) | 62,615 (82.5) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 4,391 (18.8) | 13,322 (17.5) | 1.09 (1.05–1.14) | 1.07 (1.03–1.12) | 1.03 (0.99–1.08) |
Abbreviation: CI, confidence interval.
aAdjusted for history of diabetes mellitus, tobacco-related diseases or drug use, morbid obesity, alcohol-related diseases or drug use, acute pancreatitis, chronic pancreatitis, pancreatic cyst, gallstones, and hepatitis B or C.
bFinal model adjusted for deprivation index, history of diabetes mellitus, tobacco-related diseases or drug use, morbid obesity, alcohol-related diseases or drug use, acute pancreatitis, chronic pancreatitis, pancreatic cyst, gallstones, hepatitis B or C, gastroesophageal reflux disease, peptic ulcer, Helicobacter pylori eradication, peripheral vascular disease, dementia, mild liver disease, AIDS, use of anti-hypertensive drugs, nonsteroidal anti-inflammatory drugs, and statin use.
Stratified analyses are shown in Table 4. The magnitude of the association between PPI use and risk of pancreatic cancer remained consistent across all subgroup analyses. Ever use of PPIs was associated with a significantly increased risk of pancreatic cancer among females (aOR = 1.08, 95% CI, 1.02–1.15), subjects without history of diabetes mellitus (aOR = 1.07, 95% CI,1.02–1.12), without history of tobacco-related disease or drug uses (aOR = 1.05, 95% CI, 1.01–1.10), or without morbid obesity (aOR = 1.05, 95% CI, 1.01–1.10). The associations of these risk factors with pancreatic cancer are presented in Supplementary Table S4 (history of diabetes mellitus: aOR = 2.07, 95% CI, 1.99–2.16; tobacco-related diseases or drug use: aOR = 1.35, 95% CI, 1.28–1.42; morbid obesity: aOR = 1.00, 95% CI, 0.95–1.06).
Table 4.
Association between ever use of PPIs and pancreatic cancer, separately by patient characteristics.
| Cases | Controls | |||
|---|---|---|---|---|
| N = 23,321 | N = 75,937 | Crude OR | Adjusted ORa | |
| Exposed/Total | Exposed/Total | (95% CI) | (95% CI) | |
| Ageb, n (%) | ||||
| 40–64 years | 4,971/6,694 (74.3) | 14,963/21,188 (70.6) | 1.21 (1.13–1.29) | 1.05 (0.97–1.13) |
| 65–85 years | 13,170/16,627 (79.2) | 42,344/54,749 (77.3) | 1.11 (1.07–1.17) | 1.04 (0.99–1.10) |
| Sexb, n (%) | ||||
| Male | 9,039/12,061 (74.9) | 28,597/39,370 (72.6) | 1.13 (1.07–1.19) | 1.02 (0.97–1.08) |
| Female | 9,102/11,260 (80.8) | 28,710/36,567 (78.5) | 1.17 (1.11–1.24) | 1.08 (1.02–1.15) |
| Calendar yearb, n (%) | ||||
| 2014 | 2,918/3,975 (73.4) | 9,128/12,897 (70.8) | 1.15 (1.05–1.25) | 1.05 (0.96–1.16) |
| 2015 | 3,347/4,416 (75.8) | 10,560/14,261 (74.0) | 1.10 (1.01–1.19) | 0.97 (0.88–1.06) |
| 2016 | 3,815/4,878 (78.2) | 11,860/15,821 (75.0) | 1.21 (1.12–1.32) | 1.14 (1.04–1.25) |
| 2017 | 3,927/4,922 (79.8) | 12,437/16,083 (77.3) | 1.17 (1.08–1.27) | 1.09 (1.00–1.20) |
| 2018 | 4,134/5,130 (80.6) | 13,322/16,875 (78.9) | 1.11 (1.02–1.21) | 1.00 (0.91–1.09) |
| Cancer localization, n (%) | ||||
| Head of the pancreas | 9,603/12,438 (77.2) | 30,455/40,610 (75.0) | 1.14 (1.08–1.20) | 1.04 (0.98–1.10) |
| Other | 8,538/10,883 (78.5) | 26,852/35,327 (76.0) | 1.16 (1.10–1.22) | 1.06 (1.00–1.13) |
| History of diabetes mellitusc, n (%) | ||||
| No | 12,270/16,144 (76.0) | 46,487/62,633 (74.2) | 1.14 (1.10–1.19)c | 1.07 (1.02–1.12)c |
| Yes | 5,871/7,177 (81.8) | 10,820/13,304 (81.3) | 1.06 (0.98–1.15)c | 0.99 (0.91–1.07)c |
| History of tobacco-related diseases or drug usec, n (%) | ||||
| No | 15,058/19,777 (76.1) | 50,701/68,264 (74.3) | 1.14 (1.09–1.18)c | 1.05 (1.01–1.10)c |
| Yes | 3,083/3,544 (87.0) | 6,606/7,673 (86.1) | 1.12 (0.99–1.26)c | 1.02 (0.90–1.16)c |
| Morbid obesityc, n (%) | ||||
| No | 15,503/20,385 (76.1) | 50,602/68,406 (74.0) | 1.14 (1.10–1.19)c | 1.05 (1.01–1.10)c |
| Yes | 2,638/2,936 (89.9) | 6,705/7,531 (89.0) | 1.12 (0.98–1.29)c | 1.00 (0.86–1.17)c |
Abbreviation: CI, confidence interval.
aFinal model adjusted for deprivation index, history of diabetes mellitus, tobacco-related diseases or drug use, morbid obesity, alcohol-related diseases or drug use, acute pancreatitis, chronic pancreatitis, pancreatic cyst, gallstones, hepatitis B or C, gastroesophageal reflux disease, peptic ulcer, Helicobacter pylori eradication, peripheral vascular disease, dementia, mild liver disease, AIDS, use of anti-hypertensive drugs, nonsteroidal anti-inflammatory drugs, and statin use.
bMatching variables.
cThe risk factor of pancreatic cancer was introduced in the model along with an interaction term with PPI use. The P value for interaction was 0.08 for history of diabetes mellitus, 0.67 for history of tobacco-related diseases or drug use, and 0.55 for morbid obesity.
In sensitivity analyses, a dose–response relationship persisted after introduction of a 2-year or a 4-year lag period on PPI exposure, although the associations were of lower magnitude compared with the main analyses. Statistically significant associations were still observed, above 180 cDDD, with the 2-year lag period (2-year lag analyses: 181–1,080 cDDD: aOR = 1.08, 95% CI, 1.03–1.14; >1,080 cDDD: aOR = 1.11, 95% CI, 1.05–1.19; 4-year lag analyses: 181–1,080 cDDD: aOR = 1.02, 95% CI, 0.97–1.07; >1,080 cDDD: aOR = 1.08, 95% CI, 1.01–1.14; Supplementary Table S5). Restriction to new PPI users (Supplementary Table S6), or IPTW approach (Supplementary Table S7) produced results consistent with those of the main analysis. We observed an increased risk of pancreatic cancer associated with PPI use compared with H2RA use, more marked at high PPI cumulative doses (181–1,080 cDDD: crude OR = 1.43, 95% CI, 1.06–1.92; aOR = 1.15, 95% CI, 0.84–1.56; >1,080 cDDD: crude OR = 1.55, 95% CI, 1.15–2.08; aOR = 1.15, 95% CI, 0.84–1.57; Supplementary Table S8).
Discussion
Principal findings
To our knowledge, the current study is the largest investigation on the risk of pancreatic cancer associated with PPI use, with 23,321 cases included. More than 3 of 4 individuals were PPI users over the study period, with a large proportion exposed to high cumulative doses (>180 cDDD). PPI use was associated with a slightly increased risk of pancreatic cancer, especially for cumulative exposure over 180 DDD. Overall, the results remained robust across subgroups, and in sensitivity analyses.
Comparison with the literature
Three previous observational studies found no association between PPI use and pancreatic cancer (11, 13, 17). Limited power for analyses, or low proportion of long-term PPI users may have explained these null findings. In contrast, five studies reported increased risks, three of them conducted in Asian countries (14, 16, 18), while two set in European countries (12, 15). However, these studies also had limitations. First, regional specificities in the distribution of pancreatic cancer risk factors or patterns of PPI use preclude generalization of their findings (14, 16, 18). In a study conducted in Taiwan (18), the prevalence of viral hepatitis was 10-fold higher than those observed in our study. Second, another study (15) found disproportionate numbers of short-term PPI users in the case group compared with the controls, leading to concerns of reverse causality. Finally, some of these studies were unable to capture major confounders such as tobacco smoking, obesity, or pancreatitis (12, 15, 16, 18). In the present study, we sought to address such limitations through careful study design and various sensitivity analyses.
We observed higher risks of pancreatic cancer among long-term PPI users, or with esomeprazole, one of the most potent PPI in decreasing gastric acidity (36). These findings were consistent with the physiopathology of PPIs described in the literature. Hypergastrinemia, produced as a negative feedback of prolonged PPI use, might stimulate the overgrowth of pancreatic cells via CCK-B/gastrin like receptors. However, although exogenous administration of gastrin promotes pancreatic cancer in animal models, in humans, underlying factors are needed to reactivate CCK-B/gastrin like receptors reexpression from their postnatal silenced state to active state in cancer (37). PPI induced hypochlorhydria can also lead to major changes in the gut microbiome, with consequent potential retrograde microbe migration from the gastrointestinal tract, and modulation of the intratumor microbiome. There is strong evidence for the role of the gut and tumor microbiome in pancreatic cancer, that may impact pancreatic carcinogenesis, progression and resistance to therapy (20, 38).
Strengths and limitations
Our study has a number of strengths. First, it was based on a nationwide database, with comprehensive sociodemographic and medical information on both outpatient and inpatient data, recorded since 2006. This allowed the inclusion of more than 23,000 pancreatic cancers over a 5-year period. Second, this database is a valuable tool for detecting cancers, with expected good predictive value and sensitivity (39, 40), which has been used in several studies (41–45). To identify only primary pancreatic cancers, but not pancreatic metastases or secondary pancreatic cancers, we excluded patients with a history of all causes cancers before the index date, which accounted for about one-third of cases. Nevertheless, one-fifth of diagnoses that led to these exclusions were suggestive of misclassified pancreatic cancers (namely, ICD-10 codes D01: Carcinoma in situ of other and unspecified digestive organs; D37: Neoplasm of uncertain or unknown behavior of oral cavity and digestive organs; C24: Malignant neoplasm of other and unspecified parts of biliary tract), half of them identified in the 2 months preceding the index date. Consequently, our selection procedure was very conservative. However, there is no reason to believe that this could have biased the results or prevented their generalization. Moreover, we found that about 9 of 10 of all pancreatic cancers were adenocarcinoma, most often localized in the head of the pancreas, which is consistent with the epidemiology of the disease (21, 23). Third, many covariates were available in the SNDS, and could have been taken into account in the analyses. Among them, smoking is a major risk factor for pancreatic cancer. In this study, prevalence of tobacco use was consistent with the figures of daily smoking reported within the same age groups in a national survey (46). Moreover, the magnitude of the association with pancreatic cancer was comparable with those of a meta-analysis of 82 studies (47). Fourth, PPI exposure could have been measured during a period of up to 13 years (2006–2018), in a time frame compatible with the development of pancreatic cancer. Finally, the careful implementation of the study design, and numerous sensitivity analyses contributed to the robustness of our results. The case–control and the cohort design are two observational designs relevant for studying drug–cancer associations, with similar underlying concepts (48). Here, a cumulative dose–response investigation was needed for establishing plausibility of a causal effect. Thus, the case–control approach was privileged to compute the exposure level of cases and controls. The results were consistent across several sensitivity analyses. Notably, an increased risk of pancreatic cancer was also observed with PPI compared with H2RA use, suggesting that confounding by indication was likely to be limited.
Our study also has some limitations. Given its observational nature, it is prone to bias (48, 49), including residual confounding, time-related bias, and misclassification of exposure. Residual confounding may have occurred, first, because information on genetic, family history, lifestyle, and environmental risk factors for cancers was not available. Analyses, though, were adjusted for other identifiable potential risk factors of pancreatic cancers, including diabetes mellitus, tobacco, and morbid obesity. Second, the indication for PPI treatment was not recorded in the databases, and thus could not be taken into account in the analyses. Third, the lack of an active comparator in the main analyses may also have led to residual confounding (34). The results of the sensitivity analysis considering H2RAs as an active comparator must be interpreted cautiously, because H2RA use is restricted to a small number of users with specific profiles in France (50). However, they support the finding of an excess risk of pancreatic cancer development associated with PPI exposure as compared to H2RAs. Time-related biases were limited by design. Exclusion of cases and controls with an observation period under 7 years resulted in similar duration of exposure opportunity time, minimizing time-window bias. Nevertheless, even studies with similar observation periods between cases and controls can in some instances, introduce differential drug-treated time window, when the duration of treated disease is different (49). Here, information on the nature, and onset date of the condition that led to the initiation of PPI therapy was not available. Thus, time-window bias cannot be fully ruled out. However, given the careful selection of controls and their matched index dates, such a bias is likely to be limited if any. We employed a very conservative method to address latency time bias and reverse causality (or protopathic bias; ref. 30), excluding new PPI users in 2 years before the index date in the main analysis, and applying 2-year and 4-year lag-times in sensitivity analyses. The lagged analyses tended to decrease the magnitude of the associations with the highest cDDD categories. This may either suggest potential residual reverse causality, or reflect excessive caution in the choice of the delay. Using very long lag periods could tend to unjustifiably consider lower level of exposures, which translates to lower ORs in the case of a dose–response association (51). Finally, potential misclassification of exposure status may have occurred. Cumulative exposure to PPIs was estimated on the basis of the quantity redeemed, but there is no guarantee on patient's adherence to the prescription, or even that the patient actually took the drug. This bias is not expected to affect long-term users, with regularly redeemed prescription. Otherwise, the SNDS does not contain information neither on inpatient nor on OTC PPI use. However, these uses are quantitatively much lower than outpatient use. Moreover, in this study, rates of inpatient or OTC PPI uses were not supposed to be different between cases and controls nor to introduce differential bias.
Conclusion
On the basis of these findings, a slight increase in the risk of pancreatic cancers associated with the use of PPIs at high cumulative doses cannot be excluded. Given the massive PPI use, even a relatively modest association would have important public health implications. Therefore, efforts should be continued to limit PPI treatments to appropriate indications and durations. Regular monitoring and reevaluation of treatment are needed.
Authors' Disclosures
M. Camus reports personal fees from Cook Medical, Boston Scientific, Ambu, and Alfasigma outside the submitted work. J. Kirchgesner reports personal fees from Pfizer, Roche, and Gilead outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
The study was performed at EPI-PHARE in cooperation with clinicians. No external funding was received.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Contributions
M. Lassalle: Conceptualization, software, formal analysis, methodology, writing–original draft, writing–review and editing. T. Le Tri: Software, writing–review and editing. P. Afchain: Methodology, writing–review and editing. M. Camus: Methodology, writing–review and editing. J. Kirchgesner: Methodology, writing–review and editing. M. Zureik: Conceptualization, supervision, validation, methodology, project administration, writing–review and editing. R. Dray-Spira: Conceptualization, supervision, validation, methodology, project administration, writing–review and editing.
References
- 1. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 2017;11:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008;336:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lassalle M, Le Tri T, Bardou M, Biour M, Kirchgesner J, Rouby F, et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol 2020;76:449–57. [DOI] [PubMed] [Google Scholar]
- 4. Savarino V, Dulbecco P, BN de, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med 2017;37:19–24. [DOI] [PubMed] [Google Scholar]
- 5. Salvo EM, Ferko NC, Cash SB, Gonzalez A, Kahrilas PJ. Umbrella review of 42 systematic reviews with meta-analyses: the safety of proton pump inhibitors. Aliment Pharmacol Ther 2021;54:129–43. [DOI] [PubMed] [Google Scholar]
- 6. Hafiz RA, Wong C, Paynter S, David M, Peeters G. The risk of community-acquired enteric infection in proton pump inhibitor therapy: systematic review and meta-analysis. Ann Pharmacother 2018;52:613–22. [DOI] [PubMed] [Google Scholar]
- 7. Xia B, Yang M, Nguyen LH, He Q, Zhen J, Yu Y, et al. Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: pooled analysis of 3 prospective cohorts. Gastroenterology 2021;161:1842–52. [DOI] [PubMed] [Google Scholar]
- 8. Abrahami D, McDonald EG, Schnitzer ME, Barkun AN, Suissa S, Azoulay L. Proton pump inhibitors and risk of colorectal cancer. Gut 2021;71:111–8. [DOI] [PubMed] [Google Scholar]
- 9. Abrahami D, McDonald EG, Schnitzer ME, Barkun AN, Suissa S, Azoulay L. Proton pump inhibitors and risk of gastric cancer: population-based cohort study. Gut 2021;71:16–24. [DOI] [PubMed] [Google Scholar]
- 10. Kamal H, Sadr-Azodi O, Engstrand L, Brusselaers N. Association between proton pump inhibitor use and biliary tract cancer risk: a Swedish Population-Based Cohort Study. Hepatology 2021;74:2021–31. [DOI] [PubMed] [Google Scholar]
- 11. Bradley MC, Murray LJ, Cantwell MM, Hughes CM. Proton pump inhibitors and histamine-2-receptor antagonists and pancreatic cancer risk: a nested case-control study. Br J Cancer 2012;106:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brusselaers N, Sadr-Azodi O, Engstrand L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J Gastroenterol 2020;55:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicks B, Friis S, Pottegård A. Use of proton pump inhibitors and risk of pancreatic cancer. Pharmacoepidemiol Drug Saf 2018;27:926–30. [DOI] [PubMed] [Google Scholar]
- 14. Hwang IC, Chang J, Park SM. Association between proton pump inhibitor use and the risk of pancreatic cancer: a Korean nationwide cohort study. PLoS One 2018;13:e0203918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kearns MD, Boursi B, Yang Y-X. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiol 2017;46:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai S-W, Sung F-C, Lin C-L, Liao K-F. Use of proton pump inhibitors correlates with increased risk of pancreatic cancer: a case-control study in Taiwan. Kuwait Med J 2014;46:44–8. [Google Scholar]
- 17. Lee JK, Merchant SA, Schneider JL, Jensen CD, Fireman BH, Quesenberry CP, et al. Proton pump inhibitor use and risk of gastric, colorectal, liver, and pancreatic cancers in a community-based population. Am J Gastroenterol 2020;115:706–15. [DOI] [PubMed] [Google Scholar]
- 18. Peng Y-C, Lin C-L, Hsu W-Y, Lu I-T, Yeh H-Z, Chang C-S, et al. Proton pump inhibitor use is associated with risk of pancreatic cancer: a nested case-control study. Dose Response 2018;16:1559325818803283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith JP, Fonkoua LK, Moody TW. The role of gastrin and CCK receptors in pancreatic cancer and other malignancies. Int J Biol Sci 2016;12:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McAllister F, Khan MAW, Helmink B, Wargo JA. The tumor microbiome in pancreatic cancer: bacteria and beyond. Cancer Cell 2019;36:577–9. [DOI] [PubMed] [Google Scholar]
- 21. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. [DOI] [PubMed] [Google Scholar]
- 24. WHO Collaborating Centre for Drug Statistics Methodology. WHOCC - ATC/DDD Index. Available from: https://www.whocc.no/atc_ddd_index/.
- 25. World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines; 1992.
- 26. Haastrup PF, Jarbøl DE, Thompson W, Hansen JM, Søndergaard J, Rasmussen S. When does proton pump inhibitor treatment become long term? A scoping review. BMJ Open Gastroenterol 2021;8:e000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raghunath AS, O'morain C, Mcloughlin RC. Review article: the long-term use of proton-pump inhibitors. Aliment Pharmacol Ther 2005;22:55–63. [DOI] [PubMed] [Google Scholar]
- 28. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020;395:2008–20. [DOI] [PubMed] [Google Scholar]
- 29. Khalaf N, El-Serag HB, Abrams HR, Thrift AP. Burden of pancreatic cancer: from epidemiology to practice. Clin Gastroenterol Hepatol 2021;19:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med 1980;68:255–8. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Månsson R, Joffe MM, Sun W, Hennessy S. On the estimation and use of propensity scores in case-control and case-cohort studies. Am J Epidemiol 2007;166:332–9. [DOI] [PubMed] [Google Scholar]
- 33. Platt RW, Delaney JAC, Suissa S. The positivity assumption and marginal structural models: the example of warfarin use and risk of bleeding. Eur J Epidemiol 2012;27:77–83. [DOI] [PubMed] [Google Scholar]
- 34. D'Arcy M, Stürmer T, Lund JL. The importance and implications of comparator selection in pharmacoepidemiologic research. Curr Epidemiol Rep 2018;5:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maton PN. Omeprazole. N Engl J Med 1991;324:965–75. [DOI] [PubMed] [Google Scholar]
- 36. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009;65:19–31. [DOI] [PubMed] [Google Scholar]
- 37. Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol 2013;306:G91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li P, Shu Y, Gu Y. The potential role of bacteria in pancreatic cancer: a systematic review. Carcinogenesis 2020;41:397–404. [DOI] [PubMed] [Google Scholar]
- 39. Ajrouche A, Estellat C, Rycke YD, Tubach F. Evaluation of algorithms to identify incident cancer cases by using French health administrative databases. Pharmacoepidemiol Drug Saf 2017;26:935–44. [DOI] [PubMed] [Google Scholar]
- 40. Doat S, Samson S, Fagot-Campagna A, Tuppin P, Menegaux F. Estimation of breast, prostate, and colorectal cancer incidence using a French administrative database (general sample of health insurance beneficiaries). Rev Epidemiol Sante Publique 2016;64:145–52. [DOI] [PubMed] [Google Scholar]
- 41. Bailly L, Fabre R, Pradier C, Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of french individuals with obesity. JAMA Surg 2020;155:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jabagi MJ, Vey N, Goncalves A, Le Tri T, Zureik M, Dray-Spira R. Evaluation of the incidence of hematologic malignant neoplasms among breast cancer survivors in France. JAMA Netw Open 2019;2:e187147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, et al. Association between use of Thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA 2017;318:1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia 2012;55:1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weill A, Nguyen P, Labidi M, Cadier B, Passeri T, Duranteau L, et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: cohort study. BMJ 2021;372:n37. [DOI] [PubMed] [Google Scholar]
- 46. Pasquereau A, Andler R, Arwidson P, Guignard R, Nguyen-Thanh V. [ Tobacco use among adults: five-year review of the National Tobacco Control Programme, 2014–2019]. Bull Epidémiol Hebd 2020;14:273–81. [Google Scholar]
- 47. Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535–45. [DOI] [PubMed] [Google Scholar]
- 48. Pottegård A, Friis S, Stürmer T, Hallas J, Bahmanyar S. Considerations for pharmacoepidemiological studies of drug-cancer associations. Basic Clin Pharmacol Toxicol 2018;122:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suissa S, Dell'Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2020;29:1101–10. [DOI] [PubMed] [Google Scholar]
- 50. Tuppin P, Rivière S, Deutsch D, Gastaldi-Menager C, Sabaté J-M. Burden of drug use for gastrointestinal symptoms and functional gastrointestinal disorders in France: a national study using reimbursement data for 57 million inhabitants. Therap Adv Gastroenterol 2019;12:1756284819853790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tamim H, Monfared AAT, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf 2007;16:250–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available. EPIPHARE (https://www.epi-phare.fr/en/) has a regulatory permanent access to the data from the French National Health Data System (SNDS) via its constitutive bodies ANSM and CNAM. This permanent access is given according the French Decree No. 2016-1871 of December 26, 2016 relating to the processing of personal data called "National Health Data System" [Décret no. 2016–1871 du 26 décembre 2016 relatif au traitement de données à caractère personnel dénommé « système national des données de santé » (Internet). 2016 (cited 2021 Mar 12). Available from: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000033702840&categorieLien=id] and French law articles Art. R. 1461-13 [Article R1461-13 - Code de la santé publique - Légifrance (Internet). (cited 2021 Mar 12). Available from: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000038789574/] and 14 [Article R1461-14 - Code de la santé publique - Légifrance (Internet). (cited 2021 Mar 12). Available from: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000037678676/].
All data were deidentified, thus, informed consent was not necessary.