Abstract
Background:
Endogenous sex hormones may contribute to higher colorectal cancer incidence rates in men compared with women, but despite an increased number of studies, clear evidence is lacking.
Methods:
We conducted a comprehensive nested case–control study of circulating concentrations of sex hormones, sex hormone precursors, and sex hormone binding globulin (SHBG) in relation to subsequent colon cancer risk in European men. Concentrations were measured using liquid LC/MS-MS in prospectively collected plasma samples from 690 cases and 690 matched controls from the European Prospective Investigation into Cancer and Nutrition (EPIC) and the Northern Sweden Health and Disease Study (NSHDS) cohorts. Multivariable conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI). In addition, we conducted a meta-analysis of previous studies on men.
Results:
Circulating levels of testosterone (OR, 0.68; 95% CI, 0.51–0.89) and SHBG (OR, 0.77; 95% CI, 0.62–0.96) were inversely associated with colon cancer risk. For free testosterone, there was a nonsignificant inverse association (OR, 0.83; 95% CI, 0.58–1.18). In a dose–response meta-analysis of endogenous sex hormone levels, inverse associations with colorectal/colon cancer risk were found for testosterone [relative risks (RR) per 100 ng/dL = 0.98; 95% CI, 0.96–1.00; I2 = 22%] and free testosterone (RR per 1 ng/dL = 0.98; 95% CI, 0.95–1.00; I2 = 0%).
Conclusions:
Our results provide suggestive evidence for the association between testosterone, SHBG, and male colon cancer development.
Impact:
Additional support for the involvement of sex hormones in male colon cancer.
Introduction
Colorectal cancer is the third most common cancer and the second most common cause of cancer-related death worldwide. It is more common in the developed world, but the incidence is rising in low to middle income countries (1). Colorectal cancer is more frequent among men than women, which is unlikely to depend on different risk behaviors given the consistency of the sex difference regardless of geographical location (2). Instead, the higher incidence in men has been hypothesized to relate to differences in levels of circulating endogenous sex hormones and exposure to exogenous hormones (3).
While a role for sex hormones in colorectal cancer development has been extensively investigated in women, with menopausal hormone therapy consistently showing an inverse association with colorectal cancer risk (4–8), the evidence for men is more limited.
Several studies (9–16) using prospectively collected blood samples have investigated endogenous sex hormone levels in relation to subsequent colorectal cancer risk. Of these, one found an inverse association between circulating levels of testosterone and colorectal cancer risk (9), while four reported inverse associations for sex hormone binding globulin (SHBG) (9, 13–15), a glycoprotein responsible for sex hormone transportation (17). Furthermore, the risk of colorectal cancer has been observed to be higher in patients with prostate cancer treated with androgen deprivation therapy (18, 19). Taken together, existing studies on the subject, support the hypothesis that sex hormones may play a role in the etiology of male colorectal cancer.
Studies on colorectal cancer etiology suggest that colon and rectal cancer may represent distinct parts of a colorectal continuum with different associated risk factors (20). When it comes to endogenous hormone levels this distinction is supported by at least one study on postmenopausal women and sex hormone levels, which stratified analyses by tumor location and found an inverse relationship for estrogens and colon, but not rectal, cancer (21). As earlier studies, apart from one (10), have included both colon and rectal cancer cases, the power to detect site specific associations has been small. Furthermore, the sex hormone axis includes a number of hormone precursors that have not been previously evaluated in relation to colon cancer risk.
In the current study we measured circulating levels of endogenous sex hormones (progesterone, estrone, estradiol, and testosterone) as well as two of their precursors (androstenedione and dehydroepiandrosterone) and SHBG. In order to maximize power, and relying on previous results (21), we decided to limit our cases to those with colon cancer. All concentrations were measured in 690 male colon cancer cases and 690 matched control participants of the European Prospective Investigation into Cancer and Nutrition (EPIC) and the Northern Sweden Health and Disease Study (NSHDS) cohorts. Additionally, we conducted a meta-analysis, combining the findings from the current and previous prospective studies (9–12, 14–16) investigating the associations between endogenous sex hormones, SHBG, and colon/colorectal cancer.
Materials and Methods
Study cohorts
The EPIC study is an ongoing prospective cohort study with more than half a million participants recruited from 23 centers across 10 Western European countries (France, Italy, United Kingdom, Spain, the Netherlands, Greece, Germany, Sweden, Denmark, and Norway). Data collection and recruitment have been described in detail elsewhere (22). In short, enrollment of participants, mostly between 35 to 70 years of age, began 1992 and lasted until 2000. Data on diet, lifestyle, anthropometrics, and medical history were collected, and in addition, blood samples were collected from approximately three quarters of the participants. All participants have provided written informed consent. This study was approved by the ethical review board of the International Agency of Research on Cancer (IARC) as well as by the local ethics committees of all contributing EPIC countries.
The NSHDS consists of three cohorts, the Västerbotten Intervention Programme (VIP), the Northern Sweden Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases (MONICA) project, and the Mammography Screening Project (MSP; ref. 23). The VIP invites residents of Västerbotten County to undergo a health examination upon turning 40, 50, and 60 years of age. Participants are given the opportunity to donate blood samples for research and complete an extensive questionnaire on lifestyle and health. VIP participants recruited during 1992 to 1996 are included as a study center in EPIC. The Northern Sweden MONICA project invites a random subset of 2,500 residents in Norrbotten and Västerbotten, between the ages of 25 and 74. MONICA has occurred repeatedly since 1986. Similarly to VIP, MONICA participants are asked to answer questions on lifestyle and health and to donate blood samples for research. The current study included additional participants from VIP and MONICA that were not originally part of EPIC.
Cases and controls
In this study we included 455 men with incident primary colon cancer from EPIC centers located in Italy, Spain, United Kingdom, the Netherlands, and Germany, all of which store participant samples centrally at the IARC biobank in Lyon, France. EPIC cases were identified by a combination of methods, including self-report, medical record review, and linkage with a cancer registry. In addition, all cases had two straws of prospectively collected plasma, baseline information, and no record of diabetes (as it might confound the association between sex hormones and colorectal cancer risk; ref. 24). An additional 235 incident male primary colon cancer cases from the NSHDS were identified through linkage with the national cancer registry and the Swedish colorectal cancer registry and verified by a gastrointestinal pathologist at Umeå University (Umeå, Sweden). For cases with repeated sampling occasions, the blood sample closest to diagnosis was used. Furthermore, the International Classification of Diseases for Oncology was used to code all cases. We included proximal (C18.0 and C18.2–C18.5), distal (C18.6–C18.7), overlapping (C18.8), and unspecified (C18.9) colon cancers.
Controls included in this study were alive and free of cancer (except nonmelanoma skin cancer) at the time of diagnosis of their matched case and had no history of diabetes. Controls were selected using incidence density sampling. Matching criteria for case-control pairs were: study center, age at and date of blood sample (both ±12 months).
Eleven EPIC observations were removed prior to statistical analysis: five due to incomplete case-control sets, four due to lack of all sex hormone and SHBG data (caused by technical issues or empty straws), and two due to rectal cancer misdiagnosis. In addition, eight NSHDS cases were either misdiagnosed or falsely reported as colon cancers and thus removed (together with their matched controls). Our final study population consisted of 1,380 individuals, 690 colon cancer cases, and 690 matched controls. Of these, 40 men (from a different study on methylation and sex hormones within the NSHDS) had repeated blood samples donated 10 years apart. In downstream analysis the samples closest to the diagnosis of the case were used.
Laboratory analysis
Sex hormones (estradiol, estrone, testosterone, androstenedione, dehydroepiandrosterone, and progesterone) were measured in plasma at the IARC, using a validated LC-MS/MS method. In brief, samples were extracted with tert-Butyl Methyl Ether, evaporated to dryness, redissolved in a solution of 40% methanol in water, and injected into a LC-MS/MS system (LC: Agilent 1290, Agilent; MS: QTRAP 5500 SCIEX). A reversed phase column was used (Waters Acquity UPLC CSH C18 1.7 μm 2.1 × 100 mm), with methanol/water mobile phase with a gradient from 55% to 100% methanol in 10 minutes. Stable isotope labeled analogues of each analyte were used as internal standards and two MS/MS transitions were monitored for each compound, one for quantification and another to monitor specificity. SHBG was measured using a commercially available enzyme-linked immunoassays kit (DRG Instruments GmbH). Cases and matched controls, including the small subset of repeated samples, were measured within the same analytical batch. In each batch, three quality control samples were measured in duplicate. Intrabatch coefficients of variation (CV) ranged from 1.4% for testosterone to 8% for estradiol, and interbatch CVs ranged from 2.4% for SHBG to 10% for progesterone.
Free levels of estradiol and testosterone were estimated from total estradiol and testosterone concentrations, SHBG concentrations and an assumed constant concentration of albumin of 43 g/L, using a previously validated algorithm (25).
None of the sex hormone and SHBG measurements were below their lower limits of quantification (LOQ).
Statistical analysis
Correlations between age, body mass index (BMI), sex hormones, and SHBG were assessed by calculating partial Spearman correlation coefficients (adjusted for age, BMI, and batch) among controls.
Prior to multivariable analysis, missing data were observed for estrone (n = 3) and estradiol (n = 2; caused by technical issues or empty straws), which were replaced with the median values. Participants were then categorized into quartiles according to the distribution of each analyte in the control group. In analyses including continuous levels of sex hormone and SHBG levels, we used log2-transformation. For multivariable analysis, conditional logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the associations between sex hormones and SHBG and colon cancer risk. Multivariable models were adjusted for established colon cancer risk factors, including physical activity [classified according to the Cambridge index (26) as inactive, moderately inactive, moderately active, active], smoking status (former, current, never), alcohol consumption (zero, above/below median control intake), and BMI (continuous, kg/m2). We also adjusted for red and processed meat; however, since associations estimates remained nearly identical, we dropped these confounding factors from the adjusted model. Since SHBG binds and transports estradiol and testosterone (17), we further adjusted SHBG models for levels of estradiol and testosterone in order to separate the effect of these from the explanatory variable. Statistical tests for trend were performed by assigning and including the median log2-transformed plasma value for each quartile as a continuous variable in conditional logistic regression models. For potential nonlinear relationships, we also fitted models for each analyte using restricted cubic splines with five knots placed at the 5th, 27.5th, 50th, 72.5th and 95th percentiles.
To assess the possible influence of reverse causation, i.e., preclinical disease influencing our results, we excluded cases diagnosed within the first 2 years of follow-up and investigated study-specific (EPIC/NSHDS) associations. We also performed secondary analyses stratifying by BMI (≥25/<25 kg/m2) and tumor location (proximal/distal) for the sex hormones that were significantly associated with colon cancer in the full study population. Heterogeneity of associations between distal and proximal colon cancer subsites were tested using the Q-statistics with one degree of freedom. For the BMI subgroups, we dissolved the matched case-control pairs and used logistic regression, additionally adjusted for the matching criteria, to estimate associations. Interaction was tested using the likelihood ratio test.
To assess reproducibility of sex hormones and SHBG across measurements, we estimated the intraclass correlation coefficients (ICC), defined as the proportion of total variance accounted for by the between-person variance, by fitting two-way mixed effects models (27). In this step, we utilized the NSHDS subset of 40 men (20 case-control pairs) with repeated samples collected 10 years apart. However, from the same study on methylation and sex hormones, we also utilized rectal cancer cases (n = 12) and additional controls (n = 13) since we only were interested in the reproducibility of the exposure. Mixed effects models included case status and age as fixed factors and participant identification code as a random factor.
Meta-analysis
We systematically searched the MEDLINE-PubMed and Elsevier's Scopus databases between 18 June 2020 and 7 June 2021, thus extending the search from a recent meta-analysis on the associations between sex hormones and colorectal cancer (28). We based our systematic search on the same search algorithm as described in the previous meta-analysis, but limited our results to epidemiologic studies that evaluated associations between sex hormones and colorectal/colon cancer in prediagnostic male blood samples and reported the information needed to conduct a dose-response meta-analysis.
For the dose-response meta-analysis, we used association estimates for colon cancer subgroups when applicable, and chose the most adjusted estimates. In the case multiple studies from the same study population, the study with the most colon cancer cases was included. When continuous estimates were not provided, linear trends for per-category associations were estimated and expressed as per unit increase (100 ng/dL of testosterone, 1 ng/dL of free testosterone, 10 nmol/L of SHBG, and 10 pg/mL of estradiol), as proposed by Greenland & Longnecker (29). In this procedure we selected the mean value of each quantile (tertile, quartile, or quintile) for sex hormones and SHBG levels as the dose value. For the open-ended upper extreme quantile, the dose value was set to the quantile cut-off plus half the range of the foregoing quantile. As for the reference group, the dose was set to zero.
We consequently combined the results of the studies by the inverse variance weighting method, as implemented in the “meta” R-package (30). In the case of heterogeneity between studies, assessed by the Cochran Q test (P < 0.05) and the I2 statistics (>50%), we additionally fitted random-effects models using the DerSimonian and Laird method (31). Pooled associations were expressed as summary relative risks (RR) together with the corresponding 95% CIs.
Statistical analyses were conducted in R v.3.6.0 (R Foundation for Statistical Computing). All statistical tests for significance were two-sided and a P value of below 0.05 was considered statistically significant.
Results
Descriptive statistics
Participant characteristics are shown in Table 1. Comparing colon cancer cases with the control group, cases had higher BMI and alcohol consumptions (g/day). Fewer cases were overweight (BMI; 25–30), but nearly a quarter were obese at enrollment (BMI ≥30), compared with approximately 15% of the controls. This was in line with the higher proportion of physically inactive participants in the case versus control group. In addition, cases had generally lower circulating levels of sex hormones, apart from estrone and free estradiol for which cases had slightly higher levels.
Table 1.
Study participant characteristics from the EPIC study and the NSHDS.
| Study population (n = 1,380) | |||
|---|---|---|---|
| Variables | Cases (n = 690) | Controls (n = 690) | |
| Time to diagnosis, y, median (IQR) | 10.6 (7.4–13.5) | NA | |
| Age, y, median (IQR) | 58.0 (50.2–60.2) | 57.7 (50.1–60.2) | |
| BMI, kg/m2, median (IQR) | 27.1 (25.0–29.8) | 26.5 (24.5–28.7) | |
| BMI groups, n (%) | Underweight (<18.5) | 0 (0.0) | 2 (0.3) |
| Normal weight (18.5–24.9) | 171 (24.8) | 209 (30.3) | |
| Overweight (25.0–29.9) | 357 (51.7) | 374 (54.2) | |
| Obese (>30.0) | 160 (23.2) | 103 (15.1) | |
| Unknown | 2 (0.3) | 1 (0.1) | |
| Smoking status, n (%) | Never | 172 (24.9) | 203 (29.4) |
| Former | 289 (41.9) | 263 (38.1) | |
| Current | 214 (31.0) | 208 (30.2) | |
| Unknown | 15 (2.2) | 16 (2.3) | |
| Alcohol consumption, g/day, median (IQR) | 9.4 (2.3–28.6) | 7.9 (1.9–23.1) | |
| Alcohol consumption groupsa, n (%) | Zero intake | 63 (9.1) | 56 (8.1) |
| Below median intake | 281 (40.7) | 288 (41.7) | |
| Above median intake | 344 (49.9) | 344 (49.9) | |
| Unknown | 2 (0.3) | 2 (0.3) | |
| Physical activity groups, n (%) | Inactive | 170 (24.7) | 128 (18.6) |
| Moderately inactive | 203 (29.4) | 221 (32.0) | |
| Moderately active | 155 (22.5) | 163 (23.6) | |
| Active | 121 (17.5) | 136 (19.7) | |
| Unknown | 41 (5.9) | 42 (6.1) | |
| Serological levels, median (IQR) | Androstenedione, pg/mL | 701.1 (526.7–944.1) | 720.8 (548.2–922.7) |
| Dehydroepiandrosterone, ng/mL | 2.28 (1.45–3.54) | 2.34 (1.50–3.47) | |
| Estrone, pg/mL | 29.1 (22.6–37.9) | 28.2 (22.4–35.8) | |
| Estradiol, pg/mL | 18.9 (15.1–23.9) | 19.1 (14.9–24.1) | |
| Progesterone, pg/mL | 74.1 (53.4–107.5) | 78.3 (56.1–112.1) | |
| Testosterone, ng/mL | 4.10 (3.29–5.27) | 4.41 (3.47–5.50) | |
| Free estradiol, pg/mL | 0.49 (0.39–0.61) | 0.48 (0.38–0.62) | |
| Free testosterone, pg/mL | 75.8 (63.3–90.7) | 77.6 (65.1–92.6) | |
| SHBG, nmol/L | 35.7 (26.2–47.0) | 37.6 (28.6–51.0) | |
Abbreviations: IQR, interquartile range; y, years.
aBased on the control groups median of 7.9 g/day alcohol consumption.
Distal colon cancer (n = 374; 54.2%) was more common compared with proximal colon cancer (n = 296; 42.9%). In 20 (2.9%) cases, tumor subsite was unknown. Median time from sampling to diagnosis was 10.6 years (interquartile range: 7.4–13.5 years).
Partial Spearman correlation coefficients between age, BMI, sex hormones, and SHBG among the controls are presented in Table 2. Strong positive correlations were observed between total estradiol and free estradiol (r = 0.91, P < 0.001), and total testosterone and free testosterone (r = 0.76, P < 0.001). Dehydroepiandrosterone and androstenedione (r = 0.69, P < 0.001), estrone and estradiol (r = 0.67, P < 0.001), and SHBG and testosterone (r = 0.66, P < 0.001) were moderately correlated. Weaker, yet statistically significant, inverse correlations were found between dehydroepiandrosterone and age (r = −0.42, P < 0.001), and SHBG and BMI (r = −0.30, P < 0.001).
Table 2.
Partial Spearman correlation coefficients (adjusted for age, BMI, and batch) between sex hormones, SHBG, agea, and BMIa among controls in EPIC and NSHDS.
| DHEA | E1 | E2 | P4 | T | Free E2 | Free T | SHBG | Age | BMI | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.69b | 0.50b | 0.27b | 0.45b | 0.30b | 0.23b | 0.33b | 0.10c | −0.25b | −0.03d |
| DHEA | 0.29b | 0.11e | 0.32b | 0.13b | 0.10c | 0.15b | 0.02d | −0.42b | −0.09c | |
| E1 | 0.67b | 0.17b | 0.29b | 0.64b | 0.27b | 0.12e | 0.04d | 0.15b | ||
| E2 | 0.17b | 0.52b | 0.91b | 0.49b | 0.23b | 0.06d | 0.12e | |||
| P4 | 0.30b | 0.10c | 0.29b | 0.16b | −0.11e | −0.16b | ||||
| T | 0.27b | 0.76b | 0.66b | 0.03d | −0.22b | |||||
| Free E2 | 0.47b | −0.13b | −0.06f | 0.25b | ||||||
| Free T | 0.08c | −0.23b | −0.04d | |||||||
| SHBG | 0.33b | −0.30b | ||||||||
| Age | 0.03d |
Abbreviations: A, androstenedione; DHEA, dehydroepiandrosterone; E1, estrone; E2, estradiol; P4, progesterone; T, testosterone.
aNot adjusted for itself.
b P < 0.001.
c P < 0.05.
dNonsignificant.
e P < 0.01.
f P < 0.1.
For the subset of 65 men from the NSHDS with repeated samples, SHBG had the highest ICC at 0.74 (0.63–0.84), whereas testosterone had the lowest at 0.29 (0.04–0.54). Additionally, estrone and androstenedione had moderate ICCs of 0.57 (0.39–0.73) and 0.66 (0.50–0.59) respectively. Dehydroepiandrosterone, estradiol, and progesterone were just below the cut-off (0.5) to be considered moderately reliable. ICC estimates looked similar when we only included controls and are therefore not presented. The reproducibility of all sex hormones and SHBG is presented in more detail in Supplementary Table S1.
Sex hormone levels and subsequent risk of colon cancer
Table 3 presents quartile cut-off points, the number of cases and controls per quartile, and ORs with corresponding 95% CIs for the unadjusted and adjusted associations between endogenous levels of sex hormones, SHBG, and risk of colon cancer. In the multivariable analysis (adjusted for BMI, smoking, physical activity, and alcohol), continuous values of total testosterone were inversely associated with the risk of colon cancer (OR per 1-log2 unit increment = 0.68; 95% CI, 0.51–0.89). Levels of free testosterone were also inversely associated with colon cancer risk (ORq4-q1 = 0.77; 95% CI, 0.53–1.10; Ptrend = 0.21; OR per 1-log2 unit increment = 0.83; 95% CI, 0.58–1.18); however, the association did not reach statistical significance in categorical or continuous analysis. For the other sex hormones analyzed (androstenedione, dehydroepiandrosterone, estrone, total and free estradiol, and progesterone), we found little statistical evidence of associations with colon cancer risk. Finally, SHBG was inversely associated with colon cancer (ORq4-q1 = 0.67; 95% CI, 0.46–0.97; Ptrend = 0.07; OR per 1-log2 unit increment = 0.77; 95% CI, 0.62–0.96). However, the OR for the association between SHBG and colon cancer risk was attenuated and no longer statistically significant when additionally adjusted for levels of estradiol and testosterone (ORq4-q1 = 0.80; 95% CI, 0.50–1.26; Ptrend = 0.51; OR per 1-log2 unit increment = 0.83; 95% CI, 0.61–1.13). In Supplementary Table S2, the stepwise adjusted ORs are presented. The associations were fairly similar to the unadjusted models in terms of ORs when adjusting for smoking physical activity and alcohol consumption.
Table 3.
Associations of circulating levels of sex hormones and SHBG (quartiles) with colon cancer in EPIC and NSHDS.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend | Continuousa | |
|---|---|---|---|---|---|---|
| Androstenedione | ||||||
| Quartile cut-off points, pg/mL | ≤548.2 | >548.2–720.8 | >720.8–922.7 | >922.7 | ||
| n (case/control) | 195/173 | 162/172 | 147/172 | 186/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.82 (0.61–1.11) | 0.74 (0.54–1.03) | 0.94 (0.67–1.31) | 0.83 | 0.98 (0.79–1.22) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.80 (0.58–1.09) | 0.74 (0.53–1.03) | 0.99 (0.70–1.41) | 0.87 | 1.03 (0.83–1.29) |
| Dehydroepiandrosterone | ||||||
| Quartile cut-off points, ng/mL | ≤1.50 | >1.50–2.34 | >2.34–3.47 | >3.47 | ||
| n (case/control) | 185/173 | 173/172 | 152/172 | 180/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.93 (0.70–1.25) | 0.81 (0.59–1.12) | 0.95 (0.67–1.33) | 0.79 | 0.99 (0.85–1.14) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.92 (0.68–1.25) | 0.86 (0.62–1.21) | 1.12 (0.78–1.60) | 0.45 | 1.05 (0.91–1.23) |
| Estrone | ||||||
| Quartile cut-off points, pg/mL | ≤22.4 | >22.4–28.2 | >28.2–35.8 | >35.8 | ||
| n (case/control) | 169/172 | 156/172 | 174/171 | 191/172 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.94 (0.69–1.28) | 1.06 (0.78–1.44) | 1.15 (0.83–1.61) | 0.27 | 1.18 (0.92–1.52) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.91 (0.66–1.26) | 1.02 (0.74–1.41) | 1.10 (0.77–1.56) | 0.43 | 1.13 (0.87–1.47) |
| Estradiol | ||||||
| Quartile cut-off points, pg/mL | ≤14.9 | >14.9–19.1 | >19.1–24.1 | >24.1 | ||
| n (case/control) | 165/173 | 189/172 | 167/172 | 167/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 1.16 (0.86–1.57) | 1.01 (0.73–1.39) | 0.99 (0.70–1.41) | 0.76 | 0.83 (0.64–1.09) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 1.14 (0.84–1.56) | 0.96 (0.69–1.34) | 0.89 (0.62–1.38) | 0.36 | 0.76 (0.58–1.01) |
| Progesterone | ||||||
| Quartile cut-off points, pg/mL | ≤56.1 | >56.1–78.3 | >78.3–112.1 | >112.1 | ||
| n (case/control) | 197/173 | 175/172 | 160/172 | 158/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.88 (0.65–1.18) | 0.78 (0.57–1.08) | 0.76 (0.55–1.05) | 0.10 | 0.96 (0.81–1.14) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.92 (0.68–1.24) | 0.90 (0.65–1.26) | 0.89 (0.64–1.26) | 0.58 | 1.06 (0.89–1.28) |
| Testosterone | ||||||
| Quartile cut-off points, ng/mL | ≤3.47 | >3.47–4.41 | >4.41–5.50 | >5.50 | ||
| n (case/control) | 211/173 | 189/172 | 145/172 | 145/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.86 (0.65–1.15) | 0.64 (0.47–0.88) | 0.62 (0.44–0.86) | 0.002 | 0.59 (0.45–0.76) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.92 (0.68–1.24) | 0.74 (0.53–1.02) | 0.75 (0.53–1.08) | 0.08 | 0.68 (0.51–0.89) |
| Free estradiol | ||||||
| Quartile cut-off points, pg/mL | ≤0.38 | >0.38–0.48 | >0.48–0.62 | >0.62 | ||
| n (case/control) | 147/173 | 175/172 | 210/172 | 156/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 1.23 (0.90–1.69) | 1.49 (1.09–2.05) | 1.09 (0.78–1.54) | 0.66 | 1.10 (0.85–1.43) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 1.20 (0.86–1.66) | 1.40 (1.01–1.95) | 0.91 (0.64–1.31) | 0.53 | 0.93 (0.70–1.23) |
| Free testosterone | ||||||
| Quartile cut-off points, pg/mL | ≤65.1 | >65.1–77.6 | >77.6–92.6 | >92.6 | ||
| n (case/control) | 202/173 | 167/172 | 166/172 | 155/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.79 (0.58–1.07) | 0.77 (0.56–1.06) | 0.69 (0.49–0.98) | 0.05 | 0.73 (0.52–1.02) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.79 (0.58–1.09) | 0.83 (0.59–1.15) | 0.77 (0.53–1.10) | 0.21 | 0.83 (0.58–1.18) |
| SHBG | ||||||
| Quartile cut-off points, nmol/L | ≤28.6 | >28.6–37.6 | >37.6–51.0 | >51.0 | ||
| n (case/control) | 222/173 | 153/172 | 173/172 | 142/173 | ||
| Unadjusted model OR (95% CI) | 1.00 | 0.67 (0.50–0.90) | 0.72 (0.53–0.99) | 0.56 (0.40–0.79) | 0.003 | 0.69 (0.57–0.84) |
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.73 (0.54–1.00) | 0.85 (0.61–1.18) | 0.67 (0.46–0.97) | 0.07 | 0.77 (0.62–0.96) |
| Multivariable-adjusted OR (95% CI)c | 1.00 | 0.78 (0.56–1.06) | 0.92 (0.64–1.32) | 0.80 (0.50–1.26) | 0.51 | 0.83 (0.61–1.13) |
aOR per 1-log2 unit increment.
bAdjusted for BMI (continuous), smoking status (current, former, never, unknown), physical activity (active, moderately active, moderately inactive, inactive), and alcohol consumption (zero, below median, above median).
cAdditionally adjusted for levels of estradiol and testosterone.
As for nonlinear relationships between sex hormones, SHBG, and colon cancer, models using restricted cubic splines were generally not significantly different from regular linear models (Pnonlinear > 0.05). Only the restricted cubic spline model for dehydroepiandrosterone was significantly different (Pnonlinear = 0.03); nonetheless, dehydroepiandrosterone itself remained nonsignificant in the model (P > 0.05).
Finally, associations remained largely unchanged when excluding cases (and corresponding controls), with less than 2 years of follow-up (Supplementary Table S3).
Subgroup analyses
In addition to the main analyses, we conducted secondary analyses stratifying by BMI (>25, ≤25; Table 4) and tumor location (proximal, distal colon; Table 5) for testosterone and SHBG, which were inversely associated with colon cancer in the full dataset, as well as free testosterone. Finally, we also investigated study-specific (EPIC, NSHDS) associations for all sex hormones (Supplementary Table S4).
Table 4.
Associations of circulating levels of free and total testosterone and SHBG (tertiles) with colon cancer in EPIC and NSHDS by BMI categories.
| Tertile 1 | Tertile 2 | Tertile 3 | P trend | Continuousa | P interaction | |
|---|---|---|---|---|---|---|
| Testosterone | 0.48 | |||||
| BMI > 25 | ||||||
| n (case/control) | 241/181 | 152/153 | 125/144 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.77 (0.56–1.06) | 0.69 (0.49–0.98) | 0.03 | 0.64 (0.48–0.86) | |
| BMI ≤ 25 | ||||||
| n (case/control) | 43/49 | 56/77 | 73/86 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.65 (0.36–1.17) | 0.82 (0.45–1.50) | 0.66 | 0.91 (0.55–1.49) | |
| Free testosterone | 0.82 | |||||
| BMI > 25 | ||||||
| n (case/control) | 207/160 | 148/162 | 163/156 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.72 (0.52–0.99) | 0.84 (0.60–1.18) | 0.29 | 0.78 (0.53–1.13) | |
| BMI ≤ 25 | ||||||
| n (case/control) | 60/70 | 48/68 | 64/74 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.71 (0.40–1.25) | 0.98 (0.55–1.76) | 0.95 | 1.03 (0.55–1.93) | |
| SHBG | 0.20 | |||||
| BMI > 25 | ||||||
| n (case/control) | 244/185 | 176/163 | 98/130 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.84 (0.62–1.14) | 0.60 (0.42–0.87) | 0.009 | 0.70 (0.55–0.90) | |
| Multivariable-adjusted OR (95% CI)c | 1.00 | 0.95 (0.68–1.31) | 0.79 (0.50–1.24) | 0.39 | 0.78 (0.55–1.12) | |
| BMI ≤ 25 | ||||||
| n (case/control) | 41/45 | 48/67 | 83/100 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.71 (0.38–1.32) | 0.85 (0.46–1.56) | 0.77 | 0.90 (0.60–1.35) | |
| Multivariable-adjusted OR (95% CI)c | 1.00 | 0.70 (0.37–1.32) | 0.81 (0.39–1.66) | 0.74 | 0.87 (0.47–1.61) |
aOR per 1-log2 unit increment.
bAdjusted for BMI (continuous), smoking status (current, former, never, unknown), physical activity (active, moderately active, moderately inactive, inactive), and alcohol consumption (zero, below median, above median).
cAdditionally adjusted for levels of estradiol and testosterone.
Table 5.
Associations of circulating levels of free and total testosterone and SHBG (tertiles) with colon cancer in EPIC and NSHDS by tumor site.
| Tertile 1 | Tertile 2 | Tertile 3 | P trend | Continuousa | P heterogeneity | |
|---|---|---|---|---|---|---|
| Testosterone | 0.43 | |||||
| Proximal colon (n = 592) | ||||||
| n (case/control) | 118/99 | 86/105 | 92/92 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.67 (0.43–1.03) | 0.91 (0.57–1.46) | 0.69 | 0.81 (0.55–1.21) | |
| Distal colon (n = 748) | ||||||
| n (case/control) | 157/123 | 114/118 | 103/133 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.72 (0.48–1.09) | 0.62 (0.39–0.97) | 0.04 | 0.61 (0.41–0.91) | |
| Free testosterone | 0.72 | |||||
| Proximal colon (n = 592) | ||||||
| n (case/control) | 120/96 | 79/102 | 97/98 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.63 (0.41–0.96) | 0.84 (0.52–1.34) | 0.40 | 0.85 (0.50–1.43) | |
| Distal colon (n = 748) | ||||||
| n (case/control) | 135/126 | 111/120 | 128/128 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.86 (0.58–1.26) | 0.96 (0.63–1.46) | 0.87 | 0.89 (0.55–1.46) | |
| SHBG | 0.36 | |||||
| Proximal colon (n = 592) | ||||||
| n (case/control) | 114/94 | 94/103 | 88/99 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.79 (0.53–1.19) | 0.79 (0.49–1.27) | 0.30 | 0.89 (0.65–1.22) | |
| Multivariable-adjusted OR (95% CI)c | 1.00 | 0.79 (0.52–1.20) | 0.79 (0.43–1.43) | 0.52 | 1.02 (0.64–1.64) | |
| Distal colon (n = 748) | ||||||
| n (case/control) | 160/131 | 123/118 | 91/125 | |||
| Multivariable-adjusted OR (95% CI)b | 1.00 | 0.87 (0.59–1.28) | 0.67 (0.42–1.06) | 0.09 | 0.71 (0.52–0.96) | |
| Multivariable-adjusted OR (95% CI)c | 1.00 | 0.94 (0.63–1.40) | 0.78 (0.46–1.35) | 0.38 | 0.69 (0.44–1.07) |
aOR per 1-log2 unit increment.
bAdjusted for BMI (continuous), smoking status (current, former, never, unknown), physical activity (active, moderately active, moderately inactive, inactive), and alcohol consumption (zero, below median, above median).
cAdditionally adjusted for levels of estradiol and testosterone.
For subgroups based on BMI, we found statistically significant inverse associations between testosterone and SHBG and colon cancer risk in overweight and obese men (BMI > 25) similar to the findings in the full data set; subgroup results for testosterone: ORt3-t1 = 0.69; 95% CI, 0.49–0.98; Ptrend = 0.03; OR per 1-log2 unit increment = 0.64; 95% CI, 0.48–0.86; and SHBG: ORt3-t1 = 0.60; 95% CI, 0.42–0.87; Ptrend = 0.009; OR per 1-log2 unit increment = 0.70; 95% CI, 0.55–0.90. However, the OR for SHBG was attenuated slightly after additional adjustment for levels of estradiol and testosterone (ORt3-t1 = 0.79; 95% CI, 0.50–1.24; Ptrend = 0.39; OR per 1-log2 unit increment = 0.78; 95% CI, 0.55–1.20). Furthermore, we found a nonsignificant inverse association between free testosterone and colon cancer in the overweight/obese group (ORt3-t1 = 0.84; 95% CI, 0.60–1.18; Ptrend = 0.29; OR per 1-log2 unit increment = 0.78; 95% CI, 0.53–1.13). In normal weight men (BMI < 25), we found no significant associations between testosterone, free testosterone, or SHBG and colon cancer risk. None of the associations differed significantly by strata of BMI (Pinteraction > 0.05).
In secondary analyses stratifying by tumor site, levels of testosterone were significantly inversely associated with distal colon cancer (ORt3-t1 = 0.62; 95% CI, 0.39–0.97; Ptrend = 0.04; OR per 1-log2 unit increment = 0.61; 95% CI, 0.41–0.91) and SHBG (OR per 1-log2 unit increment = 0.71; 95% CI, 0.52–0.96). Similar to the analysis stratified by BMI, the OR for SHBG was attenuated after additional adjustment for levels of estradiol and testosterone (OR per 1-log2 unit increment = 0.69; 95% CI, 0.44–1.07). We found no evidence of differences in associations for testosterone or SHBG between subsites (Pheterogeneity = 0.43 and 0.36, respectively). For free testosterone we observed inverse associations for both proximal and distal colon cancer (Pheterogeneity = 0.72), but none reached significance.
Finally, point estimates for free testosterone differed significantly between the EPIC and NSHDS study cohorts, although the individual estimates were not statistically significant (Supplementary Table S4).
Meta-analysis
A recent meta-analysis on the associations between endogenous sex hormones and colorectal cancer included results from four studies on men; one case-control study (9) and three prospective cohorts (10–12). Through our extended search we identified 214 additional studies of which we included two (14, 16). One study (32) also measured circulating sex hormones in prospectively collected blood samples; however, was not considered as it had time until event (primarily colorectal cancer–related death) as the outcome of interest. Furthermore, one study, by our coauthors (15), did not appear in our systematic search due to the fact that the search term “colorectal” was not mentioned in the abstract. This study was also included in the meta-analysis. Characteristics of all included studies can be found in Supplementary Table S5.
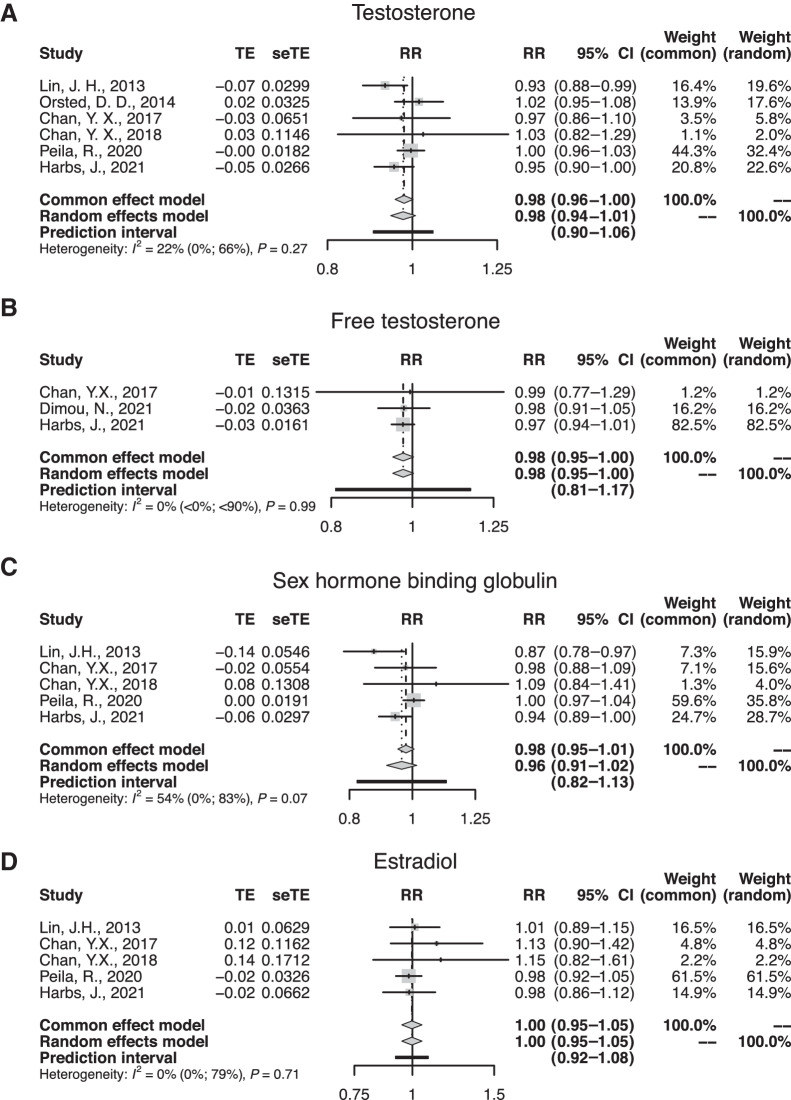
Results from the dose-response meta-analysis are shown in Fig. 1. We report summary RRs and 95% CIs for testosterone, free testosterone, and estradiol derived from the fixed effect models. In the case of SHBG, we report the summary RR and 95% CI derived from the random effects model due to substantial heterogeneity (I2 > 50%). Borderline significant inverse associations with colorectal cancer risk were observed for testosterone (RR per 100 ng/dL = 0.98; 95% CI, 0.96–1.00; I2 = 22%) and free testosterone (RR per 1 ng/dL = 0.98; 95% CI, 0.95–1.00; I2 = 0%). SHBG (RR per 10 nmol/L = 0.96; 95% CI, 0.91–1.02; I2 = 54%) was weakly inversely associated with colorectal cancer risk, although not significantly. Estradiol was neither positively nor negatively associated with colorectal cancer risk (RR per 10 pg/mL = 1.00; 95% CI, 0.95–1.05; I2 = 0%). The remaining sex hormones (androstenedione, dehydroepiandrosterone, estrone, and free estradiol) were not included in the meta-analysis, as none or too few studies included them.
Figure 1.
Pooled RRs and 95% CIs for the associations of colorectal/colon cancer with (A) testosterone, (B) free testosterone, (C) SHBG, and (D) estradiol in men as reported in prospective studies. TE, treatment effect; seTE, standard error of treatment effect.
Discussion
In this prospective analysis of European men, we investigated circulating concentrations of multiple sex hormones in relation to incident colon cancer risk. We found statistically significant inverse associations between colon cancer and circulating levels of total testosterone and SHBG, which were retained after adjustment for established colon cancer risk factors. However, the association for SHBG was attenuated slightly and lost statistical significance when testosterone and estradiol were added to the model. Significant inverse associations were also identified for testosterone and SHBG in the subgroup of overweight and obese men (BMI > 25) and for distal colon cancer, though with no clear evidence of heterogeneity of associations by BMI or tumor site. Free levels of testosterone, which we also included in stratified analyses as a measure to investigate potential effects independent of SHBG levels, were not statistically significantly associated with colon cancer, nor did associations differ significantly by tumor site or BMI. In addition, we performed a meta-analysis of eight prospective studies of endogenous sex hormones and/or SHBG in relation to the risk of colorectal/colon cancer in men. Pooled estimates suggested borderline significant inverse associations between testosterone, free testosterone, and subsequent colon cancer risk.
Our observation of an inverse association between circulating levels of total testosterone and colon cancer risk is concordant with a previous nested case-control study (9) and partly consistent with four studies nested within (or using participants from) the UK Biobank cohort (13–16) as well as one study nested within the Health in Men Study (HIMS; ref. 11), all reporting inverse but statistically nonsignificant associations. In addition, these findings corroborate previous observations that patients with prostate cancer treated with androgen-deprivation therapy have a higher risk of colorectal cancer (18, 19). However, two prospective population-based studies reported null results for testosterone levels in relation to colon/colorectal cancer risk (10, 12).
When further stratifying individuals by BMI the inverse associations between testosterone, SHBG, and colon cancer risk remained significant in overweight and obese men only (BMI > 25) with the same direction of association as in the normal-weight subgroup. However, there was no sign of an interaction effect. The possibility of a stronger inverse association between testosterone, SHBG, and colon cancer risk in men of higher BMI is supported by a recent mediation analysis demonstrating that the association between adiposity and higher colorectal cancer risk in men may be partly explained by lower levels of SHBG and testosterone (33).
Although the observational results to date are suggestive of a possible protective effect of androgens in male colorectal cancer, the experimental evidence is mixed. In vivo and in vitro experimental data have shown antitumorigenic effects of testosterone through the activation of the membrane androgen receptor (AR) in colon cancer tissue (34–36). In contrast, an experimental study on the APC Pirc rat model found that surgically castrated rats had increased susceptibility to colon adenomas after being administered testosterone enanthate compared with those administered a placebo (37). Furthermore, studies on cytosine-adenine-guanine (CAG) repeats in the genes coding for the AR found that men with ≥23 and ≥22 CAG repeats, respectively, had an approximately 30% increase in risk for colorectal/colon cancer (38, 39). Longer CAG repeats are associated with decreased AR activity which in turn, through negative feedback, leads to increased androgen levels (40–42), hence indicating possible adverse effects of testosterone. The lack of consensus between epidemiologic and experimental data and the fact that a Mendelian randomization study (16), using UK Biobank data, has not found evidence that supports a causal relationship between testosterone and colorectal cancer risk, calls for further investigations into the precise mechanisms by which testosterone may play a role in colorectal cancer.
The inverse association between SHBG levels and colon cancer risk that we observed is consistent with results from previous epidemiologic studies (9, 11, 13–15). However, the observational cohort and Mendelian randomization study (16) using participants from the UK Biobank and a cohort study within the Busselton Health Study (12) found nonsignificant positive associations between SHBG levels and future risk of colon/colorectal cancer in men. A variant in the SHBG gene (rs6259) has been associated with an increase in colorectal cancer risk (per A allele OR = 1.26; 95% CI, 1.04–1.60; ref. 43). However, a prospective study found that men with either the AA or AG genotype at rs6259 had higher SHBG plasma levels (44), which would seem to contradict our findings that higher SHBG levels are associated with reduced colorectal cancer risk. SHBG is a glycoprotein produced in the liver and most commonly a strong regulator of sex hormone bioavailability, especially for testosterone and estradiol (17). Although the inverse association between SHBG and colon cancer risk was attenuated after adjustment for circulating estradiol and testosterone levels, the OR point estimates remained roughly the same, suggesting that SHBG might still confer an independent protective effect against colon cancer, which might be related to inflammation or dietary factors (45, 46). Estrone also binds to SHBG, but with such low affinity that the impact on association estimates was negligible and consequently excluded from all adjustments.
For the remaining sex hormones (androstenedione, dehydroepiandrosterone, progesterone, estrone, and estradiol; free and total) there was no clear evidence of statistically significant associations with colon cancer risk in men. These findings are in line with previous studies on men (9, 11–14), and, therefore, based on the current evidence, it is unlikely that these sex hormones play an important role in male colon cancer development.
One major strength of our study is the use of a validated high-performance liquid chromatography (HPLC) tandem mass spectrometry (HPLC-MS/MS) method to measure sex hormones as opposed to immunoassays used by other studies. This method allowed us to detect lower levels of especially estrogens than has been previously recorded in men. In addition, we also included a comprehensive panel of sex hormones, and measured two androgen precursors (dehydroepiandrosterone and androstenedione), not included in previous studies. Furthermore, this large study included European men from several diverse European cohorts covering a large geographical area. All samples were prospectively collected with long follow up (median >10 years) and for all individuals we had access to well harmonized data on lifestyle variables such as smoking status, alcohol consumption, physical activity, and fasting that may be of importance as confounding factors. Finally, our inclusion of a meta-analysis combining results from recent studies of circulating sex hormones and SHBG is an important addition that strengthens our main results.
Our study also has some limitations. For example, contrary to some previous studies (9, 14–16) we did not include adjustments for circulating markers of insulin signaling and inflammation [e.g., levels of insulin or C-peptide and C-reactive protein (CRP), respectively]. These have, individually, been linked to colorectal cancer and sex hormone levels but it remains unclear to what extent they may confound the associations. We also lacked data on family history, one of the strongest predictors of colon cancer risk (47). However, our results are in line with previously reported studies on testosterone, SHBG, and colorectal cancer risk in men (9, 13–15), where family history was included in the final models. Furthermore, sex hormones for most participants were measured at a single time point and may therefore not be representative of longer-term exposures. However, in the small subgroup of repeated NSHDS samples collected 10 years apart, SHBG had an ICC of 0.74 (Supplementary Table S1). The ICC of testosterone in our study on the other hand was poor (0.29), likely due to the 10-year period between samples as testosterone is known to decrease with age (48, 49). Nonetheless, other studies (16, 50) found reproducibility measures ranging from 0.6 to 0.8 for testosterone and SHBG for shorter time periods (around 3 to 4 years), suggesting that single time measurements of sex hormones are sufficiently stable for capturing long-term exposure. In addition, we cannot fully rule out the influence of reverse causality, despite the consistency of findings in sensitivity analyses excluding participants diagnosed within 2 years of follow-up. Studies with very long follow-up, and with sample sizes large enough to allow for stratification by combinations of age and follow-up time are needed.
As previously stated, our study includes a dose-response meta-analysis of endogenous sex hormone levels, adding some additional strength to the associations between testosterone and colorectal cancer. However, in the case of free levels of testosterone, this association is primarily driven by our study as there were only two other studies, both smaller in size, that included free testosterone. Nonetheless, this meta-analysis relies on several assumptions regarding the included studies and also has important limitations that need to be considered. For example, in studies where estimates were presented categorically, we transformed them to continuous estimates under the assumption that the relationship between the exposure and the outcome is linear. We also fitted fixed effects models assuming that the dose-response effect remained identical across studies (supported by the observation that findings do not differ significantly between studies, possibly due to similar, Caucasian, population backgrounds). Furthermore, differences in adjusting factors as well as hormone assessment methods between studies could be a potential source of variation that we have not considered in our analysis. Therefore, the results from the meta-analysis need to be interpreted with caution.
In conclusion, although further validation in larger populations of men is warranted, the results of this prospective case-control study and meta-analysis partly support the hypothesis that endogenous levels of sex hormones, specifically testosterone and SHBG, may play a role in male colon cancer development.
Authors' Disclosures
S. Rinaldi reports grants from Department of Radiation Sciences, Umeå University, through S. Harlid during the conduct of the study. R.C. Travis reports grants from Cancer Research UK during the conduct of the study. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
S. Harlid received grants from the Cancer foundation in Northern Sweden (grant nos. AMP 17-856, AMP 18-915, and AMP 19-967), the Lions Cancer Research Fund in Northern Sweden (grant no. LP 20-2227), and internal funds from the Department of Radiation Sciences, Umeå University. The coordination of EPIC is financially supported by IARC and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM; France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF; Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (the Netherlands); Health Research Fund (FIS) - Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology - ICO (Spain); Swedish Cancer Society, Swedish Research Council and Regions of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford). We would like to thank the participants and staff of the EPIC and NSHDS cohorts for their valuable contribution to this research. We thank the Biobank Research Unit at Umeå University, VIP, the Northern Sweden MONICA study, and the County Council of Västerbotten for providing data and samples and acknowledge the contribution from Biobank Sweden, supported by the Swedish Research Council (VR 2017-00650). Special thanks also to Emmanouil Bouras for assistance on the meta-analysis. Furthermore, we acknowledge the use of data and biological samples from the following cohorts: EPIC-Florence [principal investigator (PI) Dr Domenico Palli], EPIC-Asturias (PI Dr J. Ramón Quirós) and EPIC-Cambridge (PI Professor Nick Wareham). Finally, on behalf of the EPIC-Norfolk study, we are grateful to all the participants who have been part of the project and to the many members of the study teams at the University of Cambridge (Cambridge, United Kingdom) who have enabled this research.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Authors' Contributions
J. Harbs: Formal analysis, visualization, methodology, writing–original draft, writing–review and editing. S. Rinaldi: Investigation, writing–review and editing. A. Gicquiau: Investigation, writing–review and editing. P. Keski-Rahkonen: Investigation, writing–review and editing. N. Mori: Writing–review and editing. X. Liu: Supervision, methodology, writing–review and editing. R. Kaaks: Writing–review and editing. V. Katzke: Writing–review and editing. M.B. Schulze: Writing–review and editing. C. Agnoli: Writing–review and editing. R. Tumino: Writing–review and editing. B. Bueno-de-Mesquita: Writing–review and editing. M. Crous-Bou: Writing–review and editing. M.-J. Sánchez: Writing–review and editing. A. Aizpurua: Writing–review and editing. M.-D. Chirlaque: Writing–review and editing. A. Barricarte Gurrea: Writing–review and editing. R.C. Travis: Writing–review and editing. E.L. Watts: Writing–review and editing. S. Christakoudi: Writing–review and editing. K.K. Tsilidis: Writing–review and editing. E. Weiderpass: Writing–review and editing. M.J. Gunter: Resources, writing–review and editing. B. Van Guelpen: Resources, writing–review and editing. N. Murphy: Conceptualization, resources, supervision, methodology, writing–review and editing. S. Harlid: Conceptualization, resources, supervision, funding acquisition, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- 3. McMichael AJ, Potter JD. Reproduction, endogenous and exogenous sex hormones, and colon cancer: a review and hypothesis. J Natl Cancer Inst 1980;65:1201–7. [PubMed] [Google Scholar]
- 4. Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med 1999;106:574–82. [DOI] [PubMed] [Google Scholar]
- 5. Johnson JR, Lacey JV Jr, Lazovich D, Geller MA, Schairer C, Schatzkin A, et al. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2009;18:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, et al. Postmenopausal hormone therapy and colorectal cancer risk by molecularly defined subtypes among older women. Gut 2012;61:1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mørch LS, Lidegaard Ø, Keiding N, Løkkegaard E, Kjær SK. The influence of hormone therapies on colon and rectal cancer. Eur J Epidemiol 2016;31:481–9. [DOI] [PubMed] [Google Scholar]
- 8. Botteri E, Støer NC, Sakshaug S, Graff-Iversen S, Vangen S, Hofvind S, et al. Menopausal hormone therapy and colorectal cancer: a linkage between nationwide registries in Norway. BMJ Open 2017;7:e017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol 2013;11:419–24.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ørsted DD, Nordestgaard BG, Bojesen SE. Plasma testosterone in the general population, cancer prognosis and cancer risk: a prospective cohort study. Ann Oncol 2014;25:712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan YX, Alfonso H, Chubb SA, Handelsman DJ, Fegan PG, Hankey GJ, et al. Higher dihydrotestosterone is associated with the incidence of lung cancer in older men. Horm Cancer 2017;8:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan YX, Knuiman MW, Divitini ML, Handelsman DJ, Beilby JP, Yeap BB. Lower circulating androgens are associated with overall cancer risk and prostate cancer risk in men aged 25–84 years from the busselton health study. Horm Cancer 2018;9:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMenamin ÚC, Liu P, Kunzmann AT, Cook MB, Coleman HG, Johnston BT, et al. Circulating sex hormones are associated with gastric and colorectal cancers but not esophageal adenocarcinoma in the UK Biobank. Am J Gastroenterol 2021;116:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peila R, Arthur RS, Rohan TE. Sex hormones, SHBG and risk of colon and rectal cancer among men and women in the UK Biobank. Cancer Epidemiol 2020;69:101831. [DOI] [PubMed] [Google Scholar]
- 15. Watts EL, Perez-Cornago A, Knuppel A, Tsilidis KK, Key TJ, Travis RC. Prospective analyses of testosterone and sex hormone-binding globulin with the risk of 19 types of cancer in men and postmenopausal women in UK Biobank. Int J Cancer 2021;149:573–84. [DOI] [PubMed] [Google Scholar]
- 16. Dimou N, Mori N, Harlid S, Harbs J, Martin RM, Smith-Byrne K, et al. Circulating levels of testosterone, sex hormone binding globulin and colorectal cancer risk: Observational and mendelian randomization analyses. Cancer Epidemiol Biomarkers Prev 2021;30:1336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981;53:58–68. [DOI] [PubMed] [Google Scholar]
- 18. Gillessen S, Templeton A, Marra G, Kuo YF, Valtorta E, Shahinian VB. Risk of colorectal cancer in men on long-term androgen deprivation therapy for prostate cancer. J Natl Cancer Inst 2010;102:1760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu Y, Ljung R, Martling A, Lindblad M. Risk of colorectal cancer by subsite in a swedish prostate cancer cohort. Cancer Control 2015;22:263–70. [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Lo CH, He X, Hang D, Wang M, Wu K, et al. Risk factor profiles differ for cancers of different regions of the colorectum. Gastroenterology 2020;159:241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst 2015;107;djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113–24. [DOI] [PubMed] [Google Scholar]
- 23. Hallmans G, Agren A, Johansson G, Johansson A, Stegmayr B, Jansson JH, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort - evaluation of risk factors and their interactions. Scand J Public Health Suppl 2003;61:18–24. [DOI] [PubMed] [Google Scholar]
- 24. Ma W, Song M, Kværner AS, Prescott J, Chan AT, Giovannucci EL, et al. Sex-specific association between family history of diabetes and risk of colorectal cancer: Two prospective cohort studies. Cancer Prev Res 2018;11:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 2002;11:1065–71. [PubMed] [Google Scholar]
- 26. Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407–13. [DOI] [PubMed] [Google Scholar]
- 27. Rosner B. Fundamentals of biostatistics. 4th ed. Belmont, California: Duxbury Press; 1995. [Google Scholar]
- 28. Bouras E, Papandreou C, Tzoulaki I, Tsilidis KK. Endogenous sex steroid hormones and colorectal cancer risk: a systematic review and meta-analysis. Discover Oncology 2021;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 30. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 32. Yang W, Giovannucci EL, Hankinson SE, Chan AT, Ma Y, Wu K, et al. Endogenous sex hormones and colorectal cancer survival among men and women. Int J Cancer 2020;147:920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dashti SG, Viallon V, Simpson JA, Karahalios A, Moreno-Betancur M, English DR, et al. Explaining the link between adiposity and colorectal cancer risk in men and postmenopausal women in the UK Biobank: a sequential causal mediation analysis. Int J Cancer 2020;147:1881–94. [DOI] [PubMed] [Google Scholar]
- 34. Gu S, Papadopoulou N, Gehring EM, Nasir O, Dimas K, Bhavsar SK, et al. Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Mol Cancer 2009;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu S, Papadopoulou N, Nasir O, Föller M, Alevizopoulos K, Lang F, et al. Activation of membrane androgen receptors in colon cancer inhibits the prosurvival signals Akt/bad in vitro and in vivo and blocks migration via vinculin/actin signaling. Mol Med 2011;17:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu S, Honisch S, Kounenidakis M, Alkahtani S, Alarifi S, Alevizopoulos K, et al. Membrane androgen receptor down-regulates c-src-activity and beta-catenin transcription and triggers GSK-3beta-phosphorylation in colon tumor cells. Cell Physiol Biochem 2014;34:1402–12. [DOI] [PubMed] [Google Scholar]
- 37. Amos-Landgraf JM, Heijmans J, Wielenga MC, Dunkin E, Krentz KJ, Clipson L, et al. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci U S A 2014;111:16514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slattery ML, Sweeney C, Murtaugh M, Ma KN, Wolff RK, Potter JD, et al. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol Biomarkers Prev 2005;14:2936–42. [DOI] [PubMed] [Google Scholar]
- 39. Huang R, Wang G, Song Y, Wang F, Zhu B, Tang Q, et al. Polymorphic CAG repeat and protein expression of androgen receptor gene in colorectal cancer. Mol Cancer Ther 2015;14:1066–74. [DOI] [PubMed] [Google Scholar]
- 40. Krithivas K, Yurgalevitch SM, Mohr BA, Wilcox CJ, Batter SJ, Brown M, et al. Evidence that the CAG repeat in the androgen receptor gene is associated with the age-related decline in serum androgen levels in men. J Endocrinol 1999;162:137–42. [DOI] [PubMed] [Google Scholar]
- 41. Huhtaniemi IT, Pye SR, Limer KL, Thomson W, O'Neill TW, Platt H, et al. Increased estrogen rather than decreased androgen action is associated with longer androgen receptor CAG repeats. J Clin Endocrinol Metab 2009;94:277–84. [DOI] [PubMed] [Google Scholar]
- 42. Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab 2007;92:3604–10. [DOI] [PubMed] [Google Scholar]
- 43. Sainz J, Rudolph A, Hein R, Hoffmeister M, Buch SV, Schönfels W, et al. Association of genetic polymorphisms in ESR2, HSD17B1, ABCB1, and SHBG genes with colorectal cancer risk. Endocr Relat Cancer 2011;18:265–76. [DOI] [PubMed] [Google Scholar]
- 44. Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab 2015;26:376–83. [DOI] [PubMed] [Google Scholar]
- 46. Kapoor D, Malkin CJ, Channer KS, Jones TH. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol 2005;63:239–50. [DOI] [PubMed] [Google Scholar]
- 47. Smith T, Muller DC, Moons KGM, Cross AJ, Johansson M, Ferrari P, et al. Comparison of prognostic models to predict the occurrence of colorectal cancer in asymptomatic individuals: a systematic literature review and external validation in the EPIC and UK Biobank prospective cohort studies. Gut 2019;68:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol 2003;149:583–9. [DOI] [PubMed] [Google Scholar]
- 49. Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab 2007;92:196–202. [DOI] [PubMed] [Google Scholar]
- 50. Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev 2005;14:1262–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.