Abstract
Based on the findings that plastids and cyanobacteria have similar group I introns inserted into tRNAUAALeu genes, these introns have been suggested to be immobile and of ancient origin. In contrast, recent evidence suggests lateral transfer of cyanobacterial group I introns located in tRNAUAALeu genes. In light of these new findings, we have readdressed the evolution and lateral transfer of tRNAUAALeu group I introns in cyanobacteral radiation. We determined the presence of introns in 38 different strains, representing the major cyanobacterial lineages, and characterized the introns in 22 of the strains. Notably, two of these strains have two tRNAUAALeu genes, with each of these genes interrupted by introns, while three of the strains have both interrupted and uninterrupted genes. Two evolutionary distinct clusters of tRNA genes, with the genes interrupted by introns belonging to two distinct intron clusters, were identified. We also compared 16S rDNA and intron evolution for both closely and distantly related strains. The distribution of the introns in the clustered groups, as defined from 16S rDNA analysis, indicates relatively recent gain and/or loss of the introns in some of these lineages. The comparative analysis also suggests differences in the phylogenetic trees for 16S rDNA and the tRNAUAALeu group I introns. Taken together, our results show that the evolution of the intron is considerably more complex than previous studies found to be the case. We discuss, based on our results, evolutionary models involving lateral intron transfer and models involving differential loss of the intron.
Group I introns share conserved sequence motifs and secondary structure (3, 20). They occur in a variety of locations: in mitochondrial, chloroplast, cyanellar, and nuclear genetic systems in eukaryotes, as well as in bacteria and bacteriophages. Many of these introns are mobile, either by the action of a homing endonuclease or possibly through reverse splicing and reverse transcription (19, 40). There has been a controversy as to whether group I introns are of early or late evolutionary origin (25, 35). In this regard, the group I introns in tRNAUAALeu genes, widespread both in choloroplasts and cyanobacteria, have attracted substantial attention because of the presumed immobility and ancient origin (1, 26). These introns are anticipated to be older than the divergence of cyanobacteria and chloroplasts more than 1 billion years ago (6, 17, 41).
There is evidence for lateral transfer in the cyanobacterial radiation of group I introns located in tRNACAUfMet genes (1). Recently, it has also been found that group I introns located in tRNAUAALeu anticodon loops may have polyphyletic origin (30). On the basis of these findings, we have readdressed tRNAUAALeu group I intron origin and evolution, both by analyzing the distribution of this intron in the cyanobacterial radiation and by comparative analysis of the 16S rDNA and tRNAUAALeu intron/exon evolution. Our study is based on 16S rDNA and tRNAUAALeu intron and exon sequences from a total of 38 different strains, representing the major cyanobacterial lineages. We have included several evolutionarily tightly clustered groups—based on 16S rDNA analysis—in our study, because the existence of closely related strains with and without introns may imply intron mobility (19).
MATERIALS AND METHODS
Sample and sample preparation.
The organisms used in this work (Table 1) were classified according to the criteria given in Bergey’s Manual of Systematic Bacteriology (4). Most of the strains investigated are maintained at the Norwegian Institute for Water Research (NIVA). The cultures are maintained in a constant temperature room 17 ± 2°C in growth medium Z8 (23). The strains were originally isolated from their natural habitat as single cells or filaments. The cultures at NIVA are routinely examined for contaminations by microscopy. Furthermore, all cultures used in this work have been characterized by direct sequencing of 16S rDNA without cloning. No mixed sequences were observed in these experiments. The cultures are confirmed to be unialgal (containing a single cyanobacterial strain) based on these criteria. However, the existence of uncharacterized heterotrophic bacteria in the cultures cannot be ruled out because these bacteria may be closely associated with or attached to the cyanobacteria (24). The cultivation of strains and DNA extraction for PCR amplification were done as described by Rudi et al. (32). For Southern hybridization analysis, DNA was isolated from approximately 50-mg (wet weight) cell pellets by a standard phenol-chloroform method (31).
TABLE 1.
Strains of cyanobacteria and prochlorophytes used in this study and EMBL accession numbers for tRNAUAALeu intron and 16S rDNA sequences
| Strain | EMBL accession no.
|
|
|---|---|---|
| Intron | 16S rDNA | |
| Cyanobacteria | ||
| Chroococcales | ||
| Microcystis sp. strain NIVA-CYA 324/1 | None | z82708 |
| Microcystis aeruginosa NIVA-CYA 43 | None | z82784 |
| Microcystis aeruginosa NIVA-CYA 57 | y13474 | z82785 |
| Microcystis aeruginosa NIVA-CYA 143 | aj228694 | z82786 |
| Microcystis aeruginosa NIVA-CYA 228/1 | aj228695 | z82783 |
| Cyanothece aeruginosa NIVA-CYA 258/2 | None | z82775 |
| Synechococcus leopoliensis NIVA-CYA 20 | aj228715 | z82780 |
| Synechococcus sp. strain NIVA-CYA 328 | None | z82779 |
| Oscillatoriales | ||
| Planktothrix agardhii NIVA-CYA 29 | aj228702 | z82796 |
| Planktothrix agardhii NIVA-CYA 299 | aj228701 | z82799 |
| Planktothrix mougeotii NIVA-CYA 11 | aj228700 | z82795 |
| Planktothrix prolifica NIVA-CYA 320 | aj228703 | z82798 |
| Phormidium sp. strain NIVA-CYA 203 | None | z82792 |
| Phormidium sp. strain NIVA-CYA 202 | aj228704 | z82794 |
| Phormidium sp. strain NIVA-CYA 177 | None | z82790 |
| Arthrospira fusiformis NIVA-CYA 136/2 | aj228714 | z82793 |
| Tychonema bourrellyi NIVA-CYA 261/1 | None | z82791 |
| Pseudanabaena limnetica NIVA-CYA 276/6 | None | z82778 |
| Spirulina subsalsa NIVA-CYA 163 | None | z82787 |
| Spirulina subsalsa NIVA-CYA 164 | None | z82788 |
| Nostocales | ||
| Anabaena sp. strain NIVA-CYA 267/4 | None | z82802 |
| Anabaena lemmermannii NIVA-CYA 281/1 | None | z82797 |
| Anabaena lemmermannii NIVA-CYA 83/1 | None | z82801 |
| Anabaena lemmermannii NIVA-CYA 266/1 | None | z82800 |
| Aphanizomenon gracile NIVA-CYA 103 | aj228706 | z82806 |
| Aphanizomenon flos-aquae NIVA-CYA 142a | aj228697 | z82809 |
| aj228707 | ||
| Nostoc sp. strain NIVA-CYA 246 | aj228705 | z82803 |
| Nostoc sp. strain NIVA-CYA 124 | aj228711 | z82776 |
| Nostoc sp. strain NIVA-CYA 194 | aj228708 | z82805 |
| Nostoc sp. strain NIVA-CYA 308a | aj228696 | z82804 |
| aj228712 | ||
| Nostoc commune | aj228709 | y12687 |
| Nostoc flagelliforme | aj228710 | y12688 |
| Pleurocapsales | ||
| Chroococcidiopsis thermalis PCC 7203 | None | z82789 |
| Dermocarpella incrassata PCC 7326 | None | z82807 |
| Pleurocapsa minor PCC 7327 | aj228713 | z82810 |
| Dermocarpa violacea PCC 7301 | None | z82777 |
| Prochlorophytes | ||
| Prochlorales | ||
| Prochlorothrix hollandica NIVA-5/89 | aj228698 | z82782 |
| Prochlorothrix sp. strain NIVA-8/90 | aj228699 | z82781 |
Strain with two introns.
PCR amplification and sequencing.
Primer pair 5′GCGGAATGGTAGACGCTACGGA3′ (CA)-5′TGGGGGTGGGGGGACTTGAC3′ (CB) was constructed to selectively amplify cyanobacterial tRNAUAALeu genes (30). Primer 5′CCCGTCGAGTCTCTGCACCTTC3′ (CR; complementary to the conserved intron element R) was constructed for amplification with primer CA. Primer 5′CAGCTCTCAAATTCAGGGAAACC3′ (CS; complementary to the conserved intron element P) was constructed for amplification with primer CB. Primer pairs CA-CR and CS-CB were used to amplify intron-containing genes, while primer pair CR-CS was used to generate intron-specific hybridization probes.
The PCR cycling parameters were as follows: for primer pair CA-CB, 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s; for primer pair CA-CR, 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s; for primer pair CB-CS, 94°C for 30 s, 54°C for 30 s, and 72°C for 30; and for primer pair CR-CS, 94°C for 30 s, 54°C for 30 s, and 72°C for 30 s. Between 30 and 40 cycles were used in the amplification reactions. All reactions were initiated with a 4-min denaturation at 94°C and ended with 7-min extension at 72°C. PCR amplification and direct DNA sequencing of the amplification products were done as described by Rudi et al. (32).
Southern hybridization.
Approximately 1 μg of DNA was digested overnight at 37°C with 1 U of HindIII (Promega, Madison, Wis.). The restricted DNA was then heated to 65°C for 5 min, immediately loaded on a 1.5% agarose gel, and run at 4°C at 45 V for 5 h. The DNA was transferred and cross-linked to GeneScreen hybridization membranes as recommended by the manufacturer (NEN, Boston, Mass.). The membranes were hybridized at 50 to 55°C as described by Galau et al. (11).
Single-stranded 32P-labeled probes were generated from PCR-amplified DNA by using a Random Primer DNA labeling kit (Boehringer Mannheim, GmbH, Mannheim, Germany) as described by Espelund et al. (7). Amplification products with primers CA-CB, not containing introns, from Anabaena lemmermannii NIVA-CYA 266/1 were used as a tRNAUAALeu exon-specific probe. The intron-specific probe was generated by nested amplification with the primer pair CR-CS of a gel-purified intron-containing PCR product amplified with the primer pair CA-CB from Nostoc sp. strain NIVA-CYA 194.
Phylogenetic reconstruction.
The sequences were aligned both manually and by the computer algorithm PILEUP from the Genetics Computer Group (Madison, Wis.) Wisconsin Package (13). Sites that appeared to be ambiguously aligned were not considered in the phylogenetic analysis. For the 16S rDNA data, 489 aligned positions (nucleotides [nt] 346 to 845 relative to the Escherichia coli rDNA sequence) were considered in the phylogenetic analysis; for the tRNAUAALeu intron data, 346 positions were considered. For positions in the intron data set where only a subset of the data could be aligned, the missing characters were substituted by N’s for the other taxa. All tRNAUAALeu sequences used in the analysis were sequenced in this work, while the 16S rDNA sequences are from Rudi et al. (32), with the addition of Nostoc commune and Nostoc flagelliforme (EMBL accession no. y12687 and y12688, respectively).
Separate phylogenetic trees were constructed with the neighbor-joining method (33) from the Trecon software package (39), the maximum-parsimony method (10) from Phylogenetic Analysis Using Parsimony (PAUP; version 3.1.1) and the minimal-evolution method from PAUP* 4.0 developed by D. L. Swofford (Illinois Natural History Survey, Champaign), and finally the maximum-likelihood method (8) using the Phylogeny Inference Package (PHYLIP; version 3.5) developed by J. Felsenstein (Department of Genetics, University of Washington, Seattle). The Kimura two-parameter model (16), with a transversion: transition weight of 2:1, was used to compute the distance matrices for the neighbor-joining analysis. The minimal evolution tree was constructed by using LogDet distances (19a). A heuristic tree search on the LogDet distances was conducted with parameters set to default values. For the maximum-parsimony analysis, the heuristic search algorithm implemented in the PAUP package was used to find the shortest trees. The rescaled consistency index was calculated for each position in the alignment, and this criterion was then used in the tree construction. To investigate the phyletic structure of the data, the tree length skewness was also determined for 100,000 randomly generated trees and compared to critical values given by Hillis and Huelsenbeck (14). In the maximum-likelihood analysis, we used a transition: transversion ratio of 2 and empirically determined base frequencies. To infer the confidence levels of the branch points in the constructed tree, bootstrap analysis (9) was used. Consensus trees were constructed from 500 bootstrap replicates for the neighbor-joining analysis and from 100 replicates for both the maximum-parsimony analysis and the tRNAUAALeu intron maximum-likelihood and minimal-evolution analyses. Because of the extensive computational requirements, only 40 replicates were used for the 16S rDNA maximum-likelihood analysis. Unfortunately, we could not use the minimal-evolution analysis with LogDet distances for 16S rDNA because of the extensive computational requirements.
We also tested whether the tree topologies generated with the 16S rDNA alignment and the tRNAUAALeu intron data for the same taxa were significantly different. This analysis was done with the Templeton-Felsenstein test (38) for user-defined trees, as implemented in the DNAPARS program of the PHYLIP package.
RESULTS
The characterization of tRNAUAALeu group I introns was done both by screening for the presence or absence of introns by PCR and Southern hybridization analysis and by comparative sequence analysis of intron and 16S rDNA evolution.
Intron distribution determined by PCR amplification.
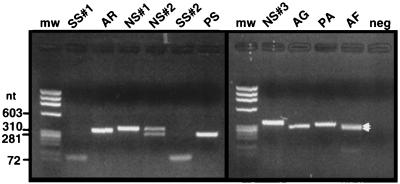
The PCR primer pair (CA-CB) complementary to the tRNAUAALeu exon generated PCR products with sizes corresponding to tRNA genes both with (270 to 365 nt) and without (approximately 60 nt) introns. In addition, double bands suggest the presence of two intron-containing genes in the strains Aphanizomenon flos-aquae NIVA-CYA 142 and Nostoc sp. strain NIVA-CYA 308 (Fig. 1). Strains belonging to the Microcystis category gave amplification products with the predicted lengths of genes both with and without introns, as previously reported by Rudi and Jakobsen (30). All strains generating bands corresponding to uninterrupted genes were also screened with the primer pairs CA-CR and CS-CB (primers CR and CS are complementary to the highly conserved intron regions R and P, respectively), enabling selective amplification of intron-containing genes. No amplification products were obtained with these primer pairs for the strains without introns, while the strains verified to contain introns (by amplification with the CA-CB primer pair) also gave amplification products with CA-CR and CS-CB primers (results not shown).
FIG. 1.
Amplification products with the tRNAUAALeu exon-specific primer pair CA-CB. The PCR products were electrophoresed in an ethidium bromide-stained 1.5% agarose gel for 30 min at 100 V. Twenty percent of the amplification product was loaded in each lane. Strain abbreviations: SS#1, Spirulina subsalsa NIVA-CYA 164; AR, Arthrospira fusiformis NIVA-CYA 136/2; NS#1 and NS#2, Nostoc sp. strains NIVA-CYA 194 and 308; SS#2, Spirulina subsalsa NIVA-CYA 163; PS, Phormidium sp. strain NIVA-CYA 202; NS#3, Nostoc sp. strain NIVA-CYA 124; AG, Aphanizomenon gracile NIVA-CYA 103; PA, P. agarthii NIVA-CYA 29; AF, A. flos-aquae NIVA-CYA 142. The two intron bands amplified for A. flos-aquae NIVA-CYA 142 (see arrows) are not properly separated in this gel. mw, molecular weight standard; neg, negative control.
Identification of tRNAUAALeu genes.
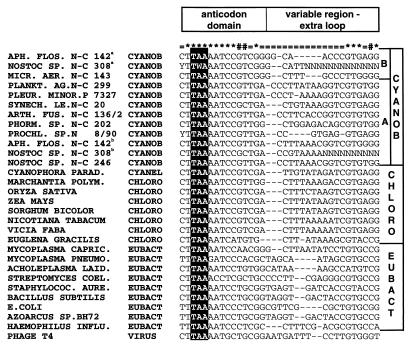
For all sequenced PCR products, the exons flanking the introns contain both the anticodon and the conserved sequences 5′CTTGAAATCCGT3′ in the anticodon loop and stem typical for cyanobacterial tRNAUAALeu genes (Fig. 2) (36). The UAA anticodon was also identified by sequencing of the amplification products for the strains lacking introns (results not shown). We found two putative clusters of tRNAUAALeu genes in the cyanobacterial radiation. In the following discussion, these clusters are designated types A and B, respectively (Fig. 2).
FIG. 2.
Compilation of tRNAUAALeu exon sequences. All the tRNAUAALeu sequences in the tRNA database (36) were compiled and compared to the partial exon sequences identified in this work. The figure includes the two putative classes A and B of tRNAUAALeu genes found for the cyanobacteria (CYANOB) in this work. ∗, conserved position (conserved in 90% or more of the species); #, position conserved only for cyanobacteria and chloroplasts (CHLORO), i.e., conserved in 90% or more of the cyanobacteria and chloroplasts and 40% or less conserved for the other species; =, variable position (position 80% or less conserved for all species); a, interrupted by cluster II intron; b, interrupted by cluster I intron. EUBACT, eubacteria. Species abbreviations: APH. FLOS., Aphanizomenon flos-aquae; MICR. AER., Microcystis aeruginosa; PLANKT. AG., Planktothrix agarhii; PLEUR. MINOR., Pleurocapsa minor; SYNECH. LE., Synechococcus leopoliensis; ARTH. FUS., Arthrospira fusiformis; PHORM., Phormidium; PROCHL., Prochlorothrix; CYANOPHORA PARAD., Cyanophora paradoxa; MARCHANTIA POLYM., Marchantia polymorpha; MYCOPLASMA CAPRIC., Mycoplasma capricolum; MYCOPLASMA PNEUMO., Mycoplasma pneumoniae; ACHOLEPLASMA LAID., Acholeplasma laidlawii; STREPTOMYCES COEL., Streptomyces coelicolor; STAPHYLOCOC. AURE., Staphylococcus aureus; E.COLI, Escherichia coli; HAEMOPHILUS INFLU., Haemophilus influenzae.
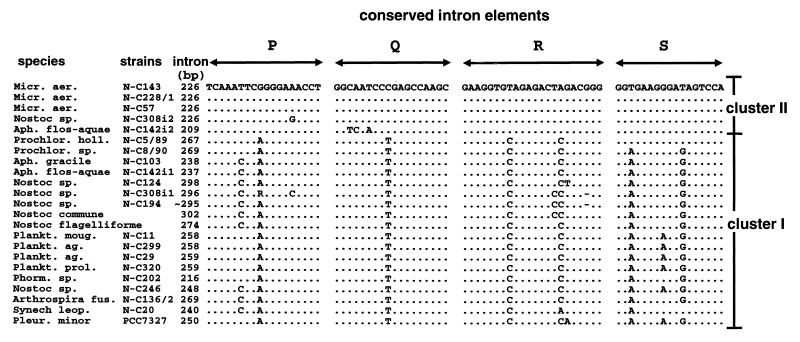
Identification of intron sequences.
All of the inserted sequences have the regions corresponding to P, Q, R, and S (Fig. 3), which are generally conserved among group I introns (20). Our data set defines two clear intron clusters (clusters I and II), as judged from the conserved intron regions. The introns belonging to cluster I are inserted into the type A tRNA genes, while introns belonging to cluster II are inserted into type B genes. In each of the strains shown to have two intron-containing tRNAUAALeu genes, A. flos-aquae NIVA-CYA 142 and Nostoc sp. strain NIVA-CYA 308, both cluster I and cluster II introns are present.
FIG. 3.
Conserved intron elements. The intron elements P, Q, R, and S, generally conserved among group I introns (20), are shown for the cyanobacterial introns characterized in this work. The two introns present in A. (Aph.) flos-auae NIVA-CYA 142 and Nostoc sp. strain NIVA-CYA 308 are annotated with i1 and i2, respectively. Dots indicate identity to M. aeruginosa (Micr. aer.) NIVA-CYA 143, while lines indicate gaps in the alignment. Prochlor. holl., Prochlorothrix hollandica; Plankt. moug., Planktothrix mougeotii; Plankt. ag., P. agardhii; Plank. prol., P. prolifica; Phorm., Phormidium; Arthrospira fus., Arthrospira fusiformis; Synech leop., Synechococcus leopoliensis; Pleur., Pleurocapsa.
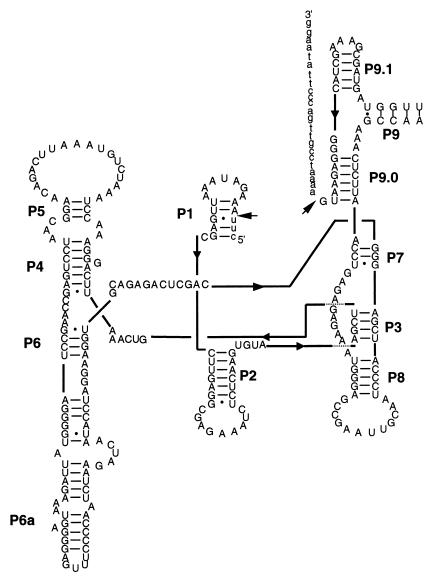
The intron belonging to cluster I for the strain Planktothrix agardhii NIVA-CYA 29 has a secondary structure resembling the characteristic structure of group I introns (Fig. 4). The secondary structure of the intron sequences belonging to cluster II has previously been reconstructed for Microcystis aeruginosa NIVA-CYA 57 (30). Both of these structures resemble what is expected for group I introns belonging to the IC3 subgroup (20).
FIG. 4.
Secondary structure of the P. agardhii NIVA-CYA 29 intron (cluster I). The structure is shown in the format described by Cech et al. (5), with marked secondary structure elements (P1 to P9). The sequences in lowercase letters represent exon sequence, while arrows indicate splice sites.
Distribution of introns in the Nostoc category determined by Southern hybridization.
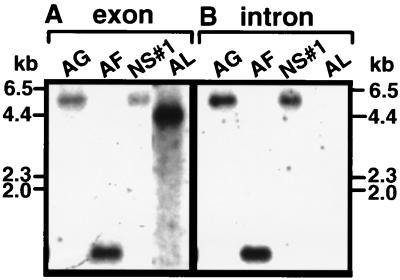
Selected strains belonging to the Nostoc category with both intron-containing genes and genes without introns (as determined by PCR) were used in Southern hybridization analysis. Only a single hybridizing band was identified for all strains with a tRNAUAALeu exon probe generated from Anabaena lemmermannii NIVA-CYA 266/1 (Fig. 5A). This suggests that the two tRNA genes identified by PCR in Nostoc sp. strain NIVA-CYA 308 and in A. flos-aquae NIVA-CYA 142, respectively, are located on the same restriction fragments. All Nostoc strains containing introns gave hybridizing bands with a intron probe generated from Nostoc sp. strain NIVA-CYA 194 (Fig. 5B), while Anabaena lemmermannii NIVA-CYA 266/1 did not give a hybridizing band with this probe. This finding, together with the PCR results, shows that Anabaena lemmermanii NIVA-CYA 266/1 has an uninterrupted tRNAUAALeu gene.
FIG. 5.
Southern hybridization using a tRNAUAALeu exon (A) or an intron (B) probe for selected Nostoc strains. The exon probe was generated from CA-CB primer amplification products for Anabaena lemmermannii NIVA-CYA 266/1 (AL; a strain confirmed to have an uninterrupted tRNAUAALeu gene). The intron probe was obtained from an internal fragment of the intron from the strain Nostoc sp. strain NIVA-CYA 194 amplified with primer pair CR-CS. Genomic DNA digested with HindIII was separated, blotted, and hybridized as described in Materials and Methods. The same membrane was used for each of the hybridization experiments, providing exact assignment of the hybridizing bands from different experiments. The molecular weight standard is HindIII-digested λ DNA. Strain abbreviations are as for Fig. 1.
Comparison of 16S rDNA and tRNAUAALeu intron evolution.
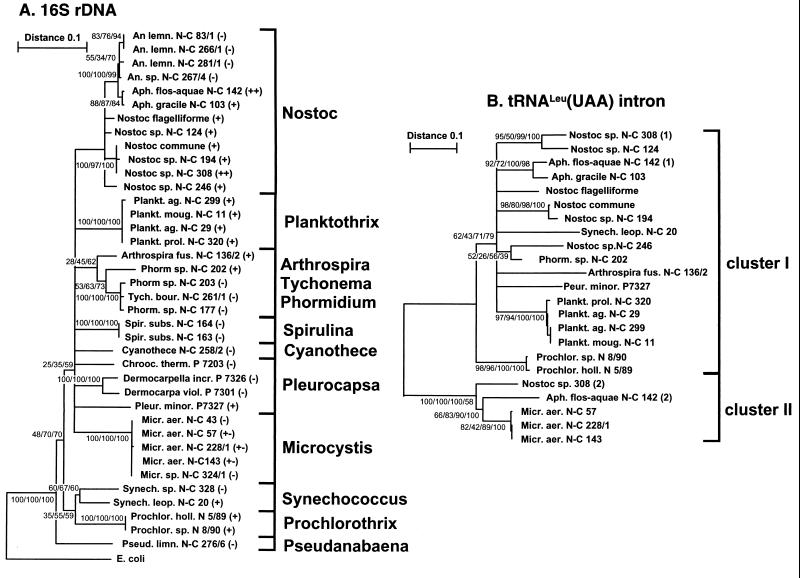
The tree length distribution skewness (g1 = −0.557) shows that the 16S rDNA evolutionary tree contains significant phylogenetic signals (P < 0.01) (11). We could identify eight statistically supported groups of strains (Nostoc, Planktothrix, Phormidium/Tychonema, Spirulina, Dermocarpa/Dermocarpella, Microcystis, Synecococcus, and Prochlorothrix) (Fig. 6A). None of the strains in the Phormidium/Tychonema, Spirulina, and Dermocarpa/Dermocarpella groups contain introns (as inferred from PCR). In the Nostoc, Synechococcus, and Microcystis groups, on the other hand, there are both intron-containing strains and strains without introns. All strains in the Planktothrix and Prochlorothrix groups contain introns.
FIG. 6.
Evolutionary trees for 16S rDNA (A) and tRNAUAALeu introns sequences (B). The trees were built using the neighbor-joining, maximum-parsimony, and maximum-likelihood methods. The trees shown in panels A and B (based on 489 and 346 aligned positions, respectively) are consensus trees for the branches supported by ≥25% of the bootstrap trees in all of the phylogenetic methods tested. The genetic distances between two strains are Kimura distances expressed in substitutions per nucleotide in the neighbor-joining tree. Numbers at the nodes (minimal evolution [for the intron tree]/maximum likelihood/maximum parsimony/neighbor joining) indicate the percentage of the bootstrap trees in which the cluster descending from the node was found. In panel A, the presence and absence of introns are indicated with (+) and (−), respectively. The respective clone numbers (N-C, NIVA-CYA; N, NIVA; P, Pasteur) are given for each branch. Species abbreviations: An. lemn., Anabaena lemmermannii; Tych. bour., Tychonema bourrellyi; Spir. subs., Spirulina subsalsa; Cyanothece, Cyanothece aeruginosa; Chrooc. therm., Chroococcidiopsis thermalis; Dermocarpella incr., Dermocarpella incrassata; Dermocarpella viol., Dermocarpella violacea; Pseud. limn., Pseudanabaena limnetica. All other species are abbreviated as in Fig. 2 and 3.
The tRNAUAALeu intron data (346 aligned positions) provide significant phylogenetic signals (g1 = −0.90) (P < 0.01) (11). In general, the minimal evolution analysis with LogDet distances gave the tree with the best statistical support. There is a large base composition bias for the different introns; the LogDet algorithm corrects for such biases (19a). We were able to identify statistically supported groups for the introns in Aphanizomenon, Nostoc sp. strain NIVA-CYA 308/124, and N. commune/Nostoc sp. strain NIVA-CYA 194 in the Nostoc category, in addition to the group formed by Nostoc sp. strain NIVA-CYA 246/Phormidium sp. strain NIVA-CYA 202. We also identified the introns found in Planktothrix, Prochlorothrix and Microcystis as phylogenetic groups (Fig. 6B). The phylogenetic reconstruction also confirm the clustering (cluster I and II) defined from the conserved intron regions (compare Fig. 3 and 6B).
The intron groups belonging to cluster I for the Nostoc strains are relatively distantly related to each other (Fig. 6B), while all Nostoc strains are clustered in the 16S rDNA evolutionary tree (Fig. 6A). Furthermore, the intron located in the strain Nostoc sp. strain NIVA-CYA 246 is more closely related to the intron in strain Phormidium sp. NIVA-CYA 202 (88% identity) compared to the other Nostoc introns belonging to cluster I (85 to 86% identity). In contrast, Nostoc sp. strain NIVA-CYA 246 is approximately 95% identical to the other Nostoc strains for 16S rDNA, while for this locus NIVA-CYA 246 shows only 88.5% identity to Phormidium sp. NIVA-CYA 202 (Fig. 6A). This discrepancy is also supported by a phylogenetic reconstruction involving 346 aligned intron positions (Fig. 6B) and by an analysis involving 204 unambiguously aligned positions where only the Nostoc introns, Phormidium sp. strain NIVA-CYA 202, and Synechococcus leopoliensis NIVA-CYA 20 (outgroup) were considered. In the last analysis, NIVA-CYA 246 and 202 formed a separate clade from the other Nostoc introns, with bootstrap values of 66, 75, and 79% for the maximum-likelihood, maximum-parsimony, and neighbor-joining analyses, respectively (data not shown). The introns in Nostoc sp. strains NIVA-CYA 308 and 124 also form a clade in the phylogenetic reconstruction displayed in Fig. 6B, while for 16S rDNA these organisms are located on separate branches. The introns in Prochlorothrix (NIVA-CYA 8/90 and 5/89) and S. leopoliensis NIVA-CYA 20 are in separate clades, while for 16S rDNA these organisms are located in the same clade. Finally, the intron belonging to cluster II for A. flos-aquae NIVA-CYA 142 is closer to the Microcystis introns compared to the Nostoc sp. NIVA-CYA 308 intron (Fig. 6B).
The evolutionary patterns for the introns seem dissimilar in the distinct lineages as defined from 16S rDNA phylogenetic reconstruction (Fig. 6A). There are both strains without introns and strains with evolutionarily divergent introns in the clustered Nostoc lineage. In the tightly clustered Planktothrix group, on the other hand, all strains contain evolutionarily closely related introns. Finally, in the Microcystis group, closely related introns are present in some Microcystis strains, while other strains contain no introns (30).
We used the Templeton-Felsenstein test (38) implemented in the DNAPARS program in the PHYLIP software package to further test whether the 16S rDNA and tRNAUAALeu intron topologies are significantly different for strains containing introns belonging to cluster I. In this comparison, we used unambiguous alignments of 479 positions for 16S rDNA and 204 positions for introns belonging to cluster I. The DNAPARS program was used to generate the tree topologies for the 18 strains with introns belonging cluster I. The shortest trees generated with both data sets were used as user-defined trees for the tRNAUAALeu data. The 16S rDNA tree required 246 steps to explain the data, while the tRNAUAALeu tree required 221 steps. The variance in step differences as determined by the step differences at individual positions for the two trees is 7.37, which is a significant difference according to the Templeton-Felsenstein test.
DISCUSSION
Two tRNAUAALeu genes.
Since the strains used in our study are confirmed to be unialgal (see Materials and Methods), there still may be a low level of commensals in the cultures. Hypothetically, the two clusters of group I introns could reside in tRNAUAALeu genes in different bacteria. However, this is unlikely because the PCR primers used in this work were designed for selective amplification of cyanobacterial and chloroplast tRNAUAALeu genes, excluding other known eubacterial genes (36). Both the type A and B tRNAUAALeu genes identified in this work group with the chloroplast genes, confirming a cyanobacterial origin of these gene clusters (Fig. 2). Furthermore, no introns have yet been identified in tRNAUAALeu genes from other eubacteria (26). Taken together, this is strong evidence that the intron-containing tRNAUAALeu genes are of cyanobacterial origin. Two divergent elongator tRNACAUMet genes (73% identity) have also been identified in Methanococcus jannaschii, a methanogenic archaeon (2). Furthermore, studies of a tRNA operon in gamma purple bacteria suggests that tRNA operons can be hot spots for rearrangements and that gene duplications and deletions are common evolutionary events (12).
Introns belonging to each of the intron clusters I and II are inserted in the A and B tRNAUAALeu genes, respectively (Fig. 2). As shown by Rudi and Jakobsen (30), introns belonging to cluster II are closer related to introns located in tRNACCUArg and tRNACAUIle genes (21a, 27) than to the tRNAUAALeu introns belonging to cluster I. This finding suggests ancient lateral transfer of the intron between different tRNA genes.
Possible mechanisms for intron evolution.
There are strains with and without introns in both the evolutionarily tightly clustered (as defined from 16S rDNA) Microcystis group (introns belonging to cluster II) and the Nostoc group (introns belonging to cluster I). This finding can be interpreted as relatively recent gain and/or loss of the introns in these groups. Furthermore, there are four examples where topologies are different for the 16S rDNA and the intron trees (compare Fig. 6A and B). The tree topology generated for the introns belonging to cluster I is also significantly different from that of the 16S rDNA topology.
There are different evolutionary patterns for group I introns located in the distinct clustered groups defined from 16S rDNA (Fig. 6), which may indicate variance in the stability of introns located in the different lineages. Group I introns without open reading frames (ORF’s) can possibly transpose through reverse transcription and splicing (19, 21, 29, 40, 42). The reverse transcriptase activity in prokaryotes can be provided from a retron element (37), for example. Retron elements are of special interest because they are mobile and not universally distributed among closely related strains (15, 28), which could explain a possible difference in evolutionary patterns for the different lineages. Experiments addressing whether lateral transfer for tRNA group I introns is correlated with reverse transcriptase activity are thus called for.
Homing endonucleases may be mobile elements, e.g., through invasion of group I introns (18, 34). Because only one of seven identified tRNACAUfMet introns contains a putative homing endonuclease ORF (1), this ORF might be mobile. Indeed, the current tRNAUAALeu introns distribution can also be explained assuming a mobile homing endonuclease for this intron, although no ORFs have yet been detected. Hypothethically, homing endonucleases conferring mobility to introns might also be encoded in trans. Thus, further work is needed to address whether there exists a homing endonuclease for the tRNAUAALeu intron.
Is lateral transfer a main force in the evolution of tRNA group I introns in cyanobacteria?
It has been suggested that the evolution of tRNAUAALeu group I introns is fundamentally different from that of tRNACAUfMet introns (1, 26). The tRNAUAALeu introns were anticipated to be stable and of ancient origin (1, 17, 26, 41), while the tRNACAUfMet introns were suggested as recent invaders of the genomes (1, 26).
An intron stability scenario for tRNAUAALeu group I introns is, according to Paquin et al. (26), more parsimonious than a mobility scenario. They explain the distribution of group I introns in the cyanobacterial radiation (26) by four intron losses and suggest that the intron mobility theory will require at least 13 insertions into each intron-containing strain. On the other hand, they also noted that the intron distribution could be explained through four insertions at earlier evolutionary stages into the intron-containing lineages. However, Paquin et al. (26) preferred the stability scenario, arguing that that an intron loss is more likely than an intron gain.
Paquin et al. (26) assume in their evolutionary model, based on the parsimony principle, that the data contain no homoplasy, that is, several events of gain or loss in a single branch. Such an assumption cannot be justified statistically without knowing the actual likelihood of these events. To our knowledge, the likelihood of intron gain or loss in the cyanobacterial radiation has never been calculated.
Several putative events of tRNAUAALeu intron loss and/or gain could be traced in the cyanobacterial radiation in our data. Notably, we identified intron-negative strains in both the Nostoc lineage (confirmed by Southern hybridization) and the Synechococcus lineage. According to Paquin et al. (26), these two lineages contain only intron-positive strains. We also identified intron-positive strains in the Microcystis lineage, which the same authors found to contain only intron-negative strains. Thus, our data do not support a model involving only a few events of loss of an ancient intron (26). Our data may in fact indicate homoplasy, with events of intron gain and/or loss in several of the cyanobacterial lineages. The intron also seems vertically inherited in some of the clustered groups, so that the balance between intron gain and/or loss is still unknown. Finally, our data suggested no fundamental differences in the tRNAUAALeu evolutionary patterns compared to the structurally similar tRNACAUfMet group I introns.
An argument for the intron stability view for tRNAUAALeu introns is that the cyanobacterial ancestor of chloroplasts, engulfed by an eukaryote more than 1 billion years ago, contained a tRNAUAALeu and not a tRNACAUfMet intron (26). There is fairly good evidence for a monophyletic origin of chloroplasts (6, 22). Thus, the presence of tRNAUAALeu introns and the absence of the intron in tRNACAUfMet genes in chloroplasts may simply be explained by that the chloroplast ancestor coincidentally contained a tRNAUAALeu gene with an intron and a tRNACAUfMet gene without intron, as seen for many of the current cyanobacterial species. The reason for the apparent stability of the intron in chloroplasts may be that the introns require a bacterial environment for mobility, e.g., reverse transcriptase activity or homing endonucleases.
With the data presented here, the evolution of the intron located in the tRNAUAALeu gene is considerably more complex than previous studies have found to be the case. Our data suggest that an evolutionary model involving both lateral transfer and differential loss of the intron should be considered. However, experimental data demonstrating the properties of the introns are probably needed to resolve the tRNAUAALeu intron stability-versus-intron mobility controversy.
ACKNOWLEDGMENTS
This work was supported by grant 107622/420 from the Norwegian Research Council to K.S.J.
We give special thanks to Olav M. Skulberg and Randi Skulberg for providing the cyanobacterial strains used in this work. Furthermore, we thank Camilla L. Nesbø for help with the phylogenetic analysis and Heidi Rudi for critically reading the manuscript. Finally, we thank Kamran Shalchian-Tabrizi for conducting the LogDet analysis and for bringing the base composition bias between the introns to our attention.
REFERENCES
- 1.Biniszkiewicz D, Cesnaviciene E, Shub D A. Self-splicing group I intron in cyanobacterial initiator methionine tRNA: evidence for lateral transfer of introns in bacteria. EMBO J. 1994;13:4629–4635. doi: 10.1002/j.1460-2075.1994.tb06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bult C J, et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 3.Burke J M. Molecular genetics of group I introns: RNA structure and protein factors required for splicing. Gene. 1988;73:273–294. doi: 10.1016/0378-1119(88)90493-3. [DOI] [PubMed] [Google Scholar]
- 4.Castenholz R W, Waterbury J B. Group I cyanobacteria. In: Staley J T, Bryant M P, Pfenning N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 1710–1728. [Google Scholar]
- 5.Cech T R, Damberger S H, Gutell R R. Representation of the secondary structure of group I introns. Nat Struct Biol. 1994;1:273–280. doi: 10.1038/nsb0594-273. [DOI] [PubMed] [Google Scholar]
- 6.Delwiche C F, Palmer J D. The origin of plastids and their spread via secondary symbiosis. Plant Syst Evol Suppl. 1997;11:53–86. [Google Scholar]
- 7.Espelund M, Stacy R A P, Jakobsen K S. A simple method for generating single-stranded DNA probes labeled to high activities. Nucleic Acids Res. 1990;18:1657–1658. doi: 10.1093/nar/18.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 9.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Fitch W M. On the problem of discovering the most parsimonious tree. Am Nat. 1977;111:223–257. [Google Scholar]
- 11.Galau G A, Huges D W, Dure L., III Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol Biol. 1986;7:155–170. doi: 10.1007/BF00021327. [DOI] [PubMed] [Google Scholar]
- 12.Giroux S, Cedergren R. Evolution of a tRNA operon in gamma purple bacteria. J Bacteriol. 1989;171:6446–6454. doi: 10.1128/jb.171.12.6446-6454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 14.Hillis D M, Huelsenbeck J P. Signal, noise, and reliability in molecular phylogenic analyses. J Hered. 1992;83:189–195. doi: 10.1093/oxfordjournals.jhered.a111190. [DOI] [PubMed] [Google Scholar]
- 15.Inouye S, Sunshine M G, Six E W, Inouye M. Retronphage phi R73: an E. coli phage that contains a retroelement and integrates into a tRNA gene. Science. 1991;252:969–971. doi: 10.1126/science.1709758. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M. A simple method for estimating evolutionary rates of base substitutions trough comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 17.Kuhsel M G, Strickland R, Palmer J D. An ancient group I intron shared by eubacteria and chloroplasts. Science. 1990;250:1570–1573. doi: 10.1126/science.2125748. [DOI] [PubMed] [Google Scholar]
- 18.Lambowitz A M. Infectious introns. Cell. 1989;56:323–326. doi: 10.1016/0092-8674(89)90232-8. [DOI] [PubMed] [Google Scholar]
- 19.Lambowitz A M, Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- 19a.Lockhart P J, Steel M A, Hendy M D, Penny D. Recovering evolutionary trees under a more realistic model of sequence evolution. Mol Biol Evol. 1994;11:605–612. doi: 10.1093/oxfordjournals.molbev.a040136. [DOI] [PubMed] [Google Scholar]
- 20.Michel F, Westhof E. Modelling the three-dimensional arcitecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990;216:585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- 21.Mohr G, Lambowitz A M. Integration of a group I intron into a ribosomal RNA sequences promoted by a tyrosyl-tRNA synthetase. Nature. 1991;354:164–167. doi: 10.1038/354164a0. [DOI] [PubMed] [Google Scholar]
- 21a.Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988;336:179–81. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- 22.Nelissen B, De Peer Y V, Wilmotte A, De Wachter R. An early origin of plastids within the cyanobacterial divergence is suggested by evolutionary trees based on complete 16S rRNA sequences. Mol Biol Evol. 1995;12:1166–1173. doi: 10.1093/oxfordjournals.molbev.a040289. [DOI] [PubMed] [Google Scholar]
- 23.Norwegian Institute for Water Research. Culture collection of algae. Catalogue of strains. Oslo, Norway: Norwegian Institute for Water Research; 1990. [Google Scholar]
- 24.Paerl H W. A comparison of cyanobacterial bloom dynamics in freshwater, estuarine and marine environments. Phycologia. 1996;35(Suppl. 6):25–35. [Google Scholar]
- 25.Palmer J D, Logsdon J M. The recent origin of introns. Curr Opin Genet Dev. 1991;1:470–477. doi: 10.1016/s0959-437x(05)80194-7. [DOI] [PubMed] [Google Scholar]
- 26.Paquin B, Kathe S D, Nierzwicki-Bauer S A, Shub D A. Origin and evolution of group I introns in cyanobacterial tRNA genes. J Bacteriol. 1997;179:6798–6806. doi: 10.1128/jb.179.21.6798-6806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhold-Hurek B, Shub D A. Self-splicing introns in tRNA genes of widely divergent bacteria. Nature. 1992;357:173–176. doi: 10.1038/357173a0. [DOI] [PubMed] [Google Scholar]
- 28.Rice S A, Bieber J, Chun J Y, Stacey G, Lampson B C. Diversity of retron elements in a population of rhizobia and other gram-negative bacteria. J Bacteriol. 1993;175:4250–4254. doi: 10.1128/jb.175.13.4250-4254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman J, Woodson S A. Integration of the Tetrahymena group I intron into bacterial rRNA by reverse splicing in vivo. Proc Natl Acad Sci USA. 1998;95:2134–2139. doi: 10.1073/pnas.95.5.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudi K, Jakobsen K S. Cyanobacterial tRNALeu(UAA) group I introns have polyphyletic origin. FEMS Microbiol Lett. 1997;156:293–298. doi: 10.1016/s0378-1097(97)00446-1. [DOI] [PubMed] [Google Scholar]
- 31.Rudi K, Kroken M, Dahlberg O J, Deggerdal A, Jakobsen K S, Larsen F. Rapid, universal method to isolate PCR-ready DNA using magnetic beads. BioTechniques. 1997;22:506–511. doi: 10.2144/97223rr01. [DOI] [PubMed] [Google Scholar]
- 32.Rudi K, Skulberg O M, Larsen F, Jakobsen K S. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl Environ Microbiol. 1997;63:2593–2599. doi: 10.1128/aem.63.7.2593-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 34.Sharma M, Ellis R L, Hinton D M. Identification of a family of bacteriophage T4 genes encoding proteins similar to those present in group I introns of fungi and phage. Proc Natl Acad Sci USA. 1992;89:6658–6662. doi: 10.1073/pnas.89.14.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shub D A. The antiquity of group I introns. Curr Opin Genet Dev. 1991;1:478–484. doi: 10.1016/s0959-437x(05)80195-9. [DOI] [PubMed] [Google Scholar]
- 36.Sprinzl M, Steegborn C, Hübel F, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1996;24:68–72. doi: 10.1093/nar/24.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Temin H M. Reverse transcriptases. Retrons in bacteria. Nature. 1989;339:254–255. doi: 10.1038/339254a0. [DOI] [PubMed] [Google Scholar]
- 38.Templeton A R. Phylogenic inference from restriction endonuclease cleavage site maps with particular reference to the evolution of humans and the apes. Evolution. 1983;37:221–244. doi: 10.1111/j.1558-5646.1983.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 39.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 40.Woodson S A, Cech T R. Reverse self-splicing of the Tetrahymena group I intron: implication for the directionality of splicing and for intron transposition. Cell. 1989;57:335–345. doi: 10.1016/0092-8674(89)90971-9. [DOI] [PubMed] [Google Scholar]
- 41.Xu M-Q, Kathe S D, Goodrich-Blair H, Nierzwicki-Bauer S A, Shub D A. Bacterial origin of a chloroplast intron: conserved self-splicing group I intron in cyanobacteria. Science. 1990;250:1566–1570. doi: 10.1126/science.2125747. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Zimmerly S, Perlman P S, Lambowitz A M. Efficient integration of an intron RNA into double-stranded DNA by reverse splicing. Nature. 1996;381:332–335. doi: 10.1038/381332a0. [DOI] [PubMed] [Google Scholar]