Abstract
The bacterium Sphingomonas sp. strain RW1 is able to use dibenzo-p-dioxin, dibenzofuran, and several hydroxylated derivatives as sole sources of carbon and energy. We have determined and analyzed the nucleic acid sequence of a 9,997-bp HindIII fragment downstream of cistrons dxnA1A2, which encode the dioxygenase component of the initial dioxygenase system of the corresponding catabolic pathways. This fragment contains 10 colinear open reading frames (ORFs), apparently organized in one compact operon. The enzymatic activities of some proteins encoded by these genes were analyzed in the strain RW1 and, after hyperexpression, in Escherichia coli. The first three ORFs of the locus, designated dxnC, ORF2, and fdx3, specify a protein with a low homology to bacterial siderophore receptors, a polypeptide representing no significant homology to known proteins, and a putative ferredoxin, respectively. dxnD encodes a 69-kDa phenol monooxygenase-like protein with activity for the turnover of 4-hydroxysalicylate, and dxnE codes for a 37-kDa protein whose sequence and activity are similar to those of known maleylacetate reductases. The following gene, dxnF, encodes a 33-kDa intradiol dioxygenase which efficiently cleaves hydroxyquinol, yielding maleylacetate, the ketoform of 3-hydroxy-cis,cis-muconate. The heteromeric protein encoded by dxnGH is a 3-oxoadipate succinyl coenzyme A (succinyl-CoA) transferase, whereas dxnI specifies a protein exhibiting marked homology to acetyl-CoA acetyltransferases (thiolases). The last ORF of the sequenced fragment codes for a putative transposase. DxnD, DxnF, DxnE, DxnGH, and DxnI (the activities of most of them have also been detected in strain RW1) thus form a complete 4-hydroxysalicylate/hydroxyquinol degradative pathway. A route for the mineralization of the growth substrates 3-hydroxydibenzofuran and 2-hydroxydibenzo-p-dioxin in Sphingomonas sp. strain RW1 thus suggests itself.
The isolation from the river Elbe of a bacterium able to use unchlorinated dibenzo-p-dioxin and dibenzofuran as sole sources of carbon and energy was reported by Wittich et al. (42). This strain, Sphingomonas sp. strain RW1, is also able to metabolize some mono- and dichlorinated congeners (40). The degradative pathways for the unchlorinated compounds in this bacterium have been elucidated, and several key enzymes have been purified, such as a three-component initial dioxygenase (8), an extradiol dioxygenase (18), and two hydrolases (9). The initial step of the degradation of dibenzofuran and dioxin, a stereospecific angular dioxygenation of one of the aromatic rings, is carried out by dioxin dioxygenase acting with a specific reductase and an electron carrier. This initial reaction leads to the formation of cyclic hemiacetals, which spontaneously cleave to yield 2,2′,3-trihydroxybiphenyl for dibenzofuran and 2,2′,3-trihydroxydiphenyl ether for dibenzo-p-dioxin. The hydroxylated rings of these two molecules are then meta cleaved by extradiol dioxygenase activity to pro-duce 2-hydroxy-6-oxo-6-(2-hydroxyphenyl)hexa-2,4-dienoic acid and 6-(2-hydroxyphenoxy)-2-hydroxy-muconic acid, respectively. The first compound is further converted by a hydrolase to 2-hydroxypenta-2,4-dienoic acid and salicylic acid, which is subsequently converted to gentisic acid, whereas the second compound is hydrolyzed to catechol and 2-hydroxymuconic acid. The next steps of the pathway concerning the conversion of catechol to Krebs cycle intermediates are thought to proceed through a typical meta cleavage pathway.
Sphingomonas sp. strain RW1 is also able to utilize several hydroxylated dibenzo-p-dioxins and dibenzofurans as sole carbon sources. However, the corresponding pathways have not yet been analyzed. We have recently cloned and characterized the cistrons encoding the heteromeric dioxin dioxygenase, dxnA1A2, by means of a PCR-based strategy (3), as well as those encoding a ferredoxin (6) and a reductase (7), which have been shown in vitro to interact with the dioxin dioxygenase. The dxnA1A2 cistrons are located on cosmid pAJ114, a member of a Sphingomonas sp. strain RW1 pLAFR3-based cosmid library. Here, we report the analysis of a 9,997-bp HindIII fragment located downstream of the dxnA1A2 cistrons and carrying 10 open reading frames (ORFs) apparently organized in a operon. Their predicted amino acid sequences were compared with the sequences of other degradative enzymes. The activities of some of the encoded proteins were analyzed in Escherichia coli and also in the Sphingomonas sp. strain RW1 wild type. This led to the identification of a pathway for the breakdown of 3-hydroxydibenzofuran via 4-hydroxysalicylate and hydroxyquinol, whereas the degradative pathway in Sphingomonas sp. strain RW1 for 2-hydroxydibenzo-p-dioxin is directly channeled in this converging route from the hydroxyquinol step.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains, plasmids, and media used in this study have been described previously (3, 7), as well as the conditions for growth of strain RW1 (42).
Nucleotide sequence determination and computer analysis.
Plasmid templates for DNA sequencing were prepared by means of a Qiawell-8 kit (Qiagen) from overnight-grown cultures as described by the supplier. Primers designed on the basis of the known sequence were synthesized on an Applied Biosystem model 381A DNA synthesizer, desalted, and used without further purification in a walking-sequencing procedure. DNA sequencing was performed on double-stranded templates in the presence of 5% dimethyl sulfoxide, with an Applied Biosystem DNA sequencing kit based on Taq DNA polymerase-initiated cycle sequencing reactions with fluorescence-labeled dideoxynucleotide terminators. Samples were processed on a model 373 Stretch Applied Biosystem automated sequencer. Sequence analysis and phylogenetic unrooted tree drawing were done essentially as previously described (7). Analysis of the transmembrane topology of DxnC was carried out using the resources from the Swiss Institute for Experimental Cancer Research (20a).
DNA manipulations and construction of expressing plasmids.
Plasmid and cosmid DNA isolation procedures were carried out with Qiagen kits, as recommended by the supplier. DNA manipulations such as subcloning, digestion, ligation, and transformation were performed according to standard procedures (29). PCR conditions were similar to those described by Armengaud et al. (2, 4), except that vent polymerase from New England Biolabs was used in order to minimize nucleotide misincorporation during the synthesis. The cloning of the dxnD, dxnE, dxnF, dxnGH, and dxnI genes in vector pET9a was carried out mainly as described previously for fdx1 (6). Briefly, dxnD-spanning nucleotides 14,991 to 16,883, dxnE-spanning nucleotides 16,883 to 17,953, dxnF-spanning nucleotides 17,978 to 18,877, dxnGH-spanning nucleotides 18,874 to 20,246, and dxnI-spanning nucleotides 20,265 to 21,449 were PCR amplified by using the oligonucleotides listed in Table 1. The expected fragments were cloned into pCR-Script SK(+) vector (Stratagene), thereby generating plasmids pAJ122, pAJ140, pAJ124, pAJ142, and pAJ144, respectively. The inserts were sequenced in order to check the integrity of the nucleotide information and then subcloned into plasmid pET9a, to produce expression plasmids pAJ123, pAJ141, pAJ125, pAJ143, and pAJ145, respectively.
TABLE 1.
Oligonucleotides used in this study for the construction of hyperexpressing vectors
| Primer | Sequencea | Hybridization region |
|---|---|---|
| AJ352 | cgggcatATGTCGATATGCGAGAGGA | 5′ end of dxnD |
| NdeI | ||
| AJ353 | gcgcggatccTTACAGAGCCCCAGCC | 3′ end of dxnD |
| BamHI | ||
| AJ440 | cgggcatATGTCGGTCGGGCGCTTCATT | 5′ end of dxnE |
| NdeI | ||
| AJ441 | gcccagaTCTCCTGCGAATGTTACATCT | 3′ end of dxnE |
| BglII | ||
| AJ350 | cgggcatATGACCTCTGAGGAAGAGA | 5′ end of dxnF |
| NdeI | ||
| AJ351 | gcgcagatctTCATGCAACGTGCGCC | 3′ end of dxnF |
| BglII | ||
| AJ442 | cgggcatATGAGCAAAAAGATTTACTCG | 5′ end of dxnGH |
| NdeI | ||
| AJ443 | gcccggatCCTCTGTCATGCAACCAGGC | 3′ end of dxnGH |
| BamHI | ||
| AJ444 | cgggcatATGCCGTCCGTACACCCATCG | 5′ end of dxnI |
| NdeI | ||
| AJ445 | gcccggatCCTTGAAGGCGACCTCCTCA | 3′ end of dxnI |
| BamHI |
Relevant recognition sequences for the restriction enzymes are underlined, and lowercase characters indicate the sequences not homologuous to the corresponding DNA region from Sphingomonas sp. strain RW1.
Enzyme assays and analytical methods.
The products of genes dxnD, dxnE, dxnF, dxnGH, and dxnI were hyperexpressed in bacteria of derivatives of E. coli BL21(DE3)(pLysS) carrying the above expression plasmids, under conditions similar to those described by Armengaud et al. (4). Cell extracts were prepared by disruption of biomass samples with a French press (pressure, 10,000 lb/in2) and centrifugation at 10,000 × g. Standard analytical procedures, such as determination of protein concentration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), were performed as described previously (5). Photometric measurements were carried out with a UV-visible recording spectrophotometer (UV-2100; Shimadzu), equipped with a CPS temperature controller. Monooxygenase and catechol 1,2-dioxygenase activities were measured as oxygen uptake with an oxygen electrode (Bachofer), as described by Happe et al. (18). Activities of degradative enzymes were also determined spectrophotometrically according to the method of Wittich et al. (42). Maleylacetate reductase, 4-hydroxysalicylate hydroxylase, and hydroxyquinol 1,2-dioxygenase activities were assayed according to the methods of Seibert et al. (33), Stolz and Knackmuss (34), and Latus et al. (22), respectively. Substrate-dependent oxidation of NADH was used to monitor monooxygenase activities. The initial rate of oxidation of NADH was determined by recording the decrease in A340, using an ɛ340 of 6,220 M−1 · cm−1. Reactions were performed in 1.0-ml quartz cuvettes with a 1-cm light path. Activities were assayed at 25°C by adding 10 μl of 100 mM substrate to 0.49 ml of 100 mM sodium phosphate buffer at pH 8.0, containing 0.2 mM NADH, 0.1 mM FAD, and various cell extract quantities. Values were corrected for oxidation of NADH in the absence of substrate, and activities were expressed in units defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of NADH per min. The 3-oxoadipate succinyl coenzyme A (succinyl-CoA) transferase activity was first assayed as described by Yeh and Ornston (44). The 0.5-ml enzyme assay mixture contained 50 mM Tris-HCl buffer at pH 7.3, 2 mM 3-oxoadipate, 0.1 mM succinyl-CoA, and cell extract. Samples were taken at intervals of 10 min and assayed directly or stopped by addition of an equal amount of ice-cold methanol and chilling on ice until analysis. The transferase activity was subsequently assayed by direct quantification of substrate decrease and product formation, according to a modified method described previously by Corkey et al. (11). The latter procedure involved measurement of individual compounds by reversed-phase high-performance liquid chromatography (HPLC) (Merck-Hitachi chromatograph system). Separations were achieved on an analytical SC column (125 by 4.6 mm; Lichrospher 100 RP8 5.0 μm [granulometry of the resin inside the column]; Lichrospher, Bischof, Germany) by elution with 50 mM potassium phosphate, buffered at pH 5.3, in water containing 5% methanol (vol/vol) at a flow rate of 1 ml per min. The column effluent was monitored by measuring the absorption at 260 nm, which corresponds to the UV maxima of CoA, succinyl-CoA, 3-oxodipyl-CoA, and acetyl-CoA. The molecular extinction coefficient of all these compounds is identical, due to the shared chromophore. Retention times for CoA, succinyl-CoA, 3-oxoadipyl-CoA, and acetyl-CoA (all purchased from Sigma) were 11.4, 16.5, 19.7, and 38 min, respectively. Further investigations were carried out by HPLC-mass spectrometry (MS) with a Hewlett-Packard HPLC system interfaced to a Perkin-Elmer electrospray ionization mass spectrometer. After injection, the sample was eluted with a 20-min linear gradient from 0 to 20% acetonitrile in 10 mM ammonium acetate buffer, pH 6.7.
Nucleotide sequence accession number.
The nucleotide sequence described in this article is deposited in the EMBL/DDBJ/GenBank database under accession no. X72850, which corresponds to an update of the sequence described by Happe et al. (18) and previously updated by Armengaud et al. (3).
RESULTS
Subcloning strategy and sequence of the cluster.
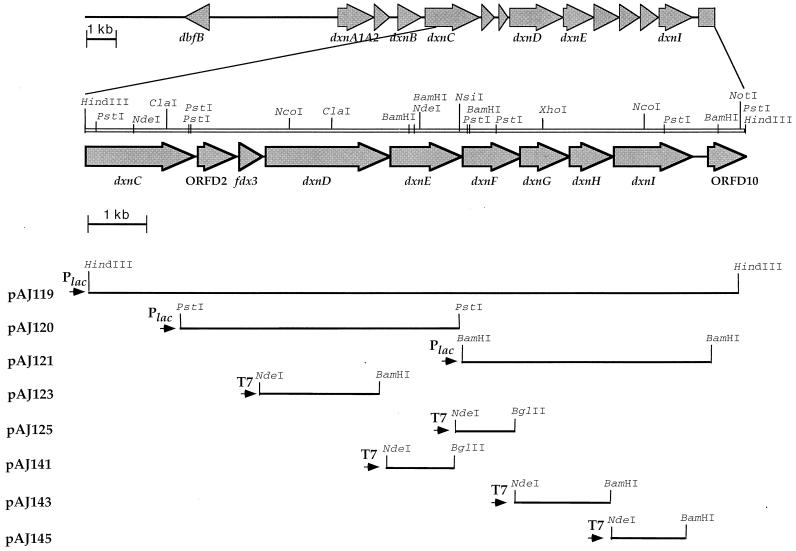
A map showing relevant restriction enzyme sites of the 9,997-bp HindIII fragment of cosmid pAJ114 was constructed (Fig. 1). In order to rapidly identify the genes located on this fragment, we subcloned into pBluescript II KS(+) various fragments generated by digestion of cosmid pAJ114 with the single restriction endonucleases BamHI, PstI, NsiI, and HindIII, as indicated in Fig. 1, and sequenced them partially by using universal primers. This strategy, developed previously for complex sequencing projects (10), allowed us to identify several genes of interest along the 10-kb fragment downstream of dxnA1A2. We completed the nucleotide sequencing by a walking strategy with oligonucleotides priming the determined sequences. Relevant subclones constructed for this purpose are shown in Fig. 1. The nucleotide sequence of the HindIII fragment revealed the presence of 10 ORFs, all in the same orientation. Since no obvious transcription terminator was detected in the sequence, the ORFs may be part of the same transcription unit. The 5′ upstream region of this gene cluster contains the dxnA1A2 cistrons encoding the two subunits of the dioxin dioxygenase, as well as the dxnB gene, which specifies the hydrolase involved in the third step of the dibenzofuran/dibenzo-p-dioxin pathway. The downstream ORFs (ORFD1 to ORFD10) were therefore designated dxnC-dxnI. All of the ORFs identified by computer analysis start with the canonical ATG start codon sequence and are preceded by a putative Shine-Dalgarno sequence located 4 to 10 bp upstream of the methionine initiation codon. The G+C content of these cistrons ranged from 49% for fdx3 to 59% for dxnF, values significantly differing from G+C contents (62 to 67%) found for genomes of Sphingomonas species (43), except for dxnI and dxnE, which exhibit a 62% G+C content. This indicates that some or all of these genes might have been acquired from another organism.
FIG. 1.
Genetic organization of the 10-kb HindIII fragment of plasmid pAJ119 and subcloning strategies. Cosmid pAJ114 was previously identified in a pLAFR3 library of Sphingomonas sp. strain RW1 genomic DNA by hybridization with a probe specific for the dxnA1A2 cistrons. A 9,997-bp HindIII fragment was subcloned from this cosmid into pBluescript, yielding pAJ119. The positions and orientations of the different ORFs detected within the locus are shown by large arrows on the top of the figure. The orientation of the vector promoter in each construct designed for hyperexpression is shown by small black arrows. Relevant restriction sites present on the fragment are indicated, as well as sites artificially introduced by PCR.
dxnC codes for a putative receptor protein.
A partial ORF was detected spanning nucleotides 1 to 1,686 (nucleotides 12,028 to 13,923 in the sequence X72850). This nucleotide sequence corresponds to the 3′ end of dxnC (Fig. 1), previously identified downstream of dxnA1A2 (3). Comparison of the deduced sequence of this 66,917-Da polypeptide with protein sequences in the EMBL/DDBJ/GenBank and SwissProt databases revealed only minute similarities with restricted portions of some bacterial siderophore receptors (15, 28). Four potential transmembrane helices were found in DxnC. The dxnC gene is followed by a possible ORF, ORFD2, spanning nucleotides 13,992 to 14,444, whose deduced polypeptide sequence does not show any similarities with known protein sequences.
Identification of a new putative ferredoxin gene.
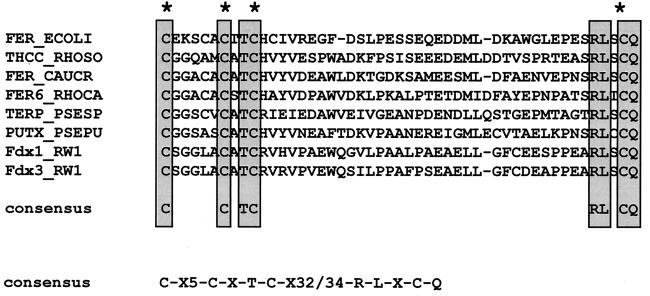
ORFD3, which spans nucleotides 14,621 to 14,941, encodes a putative 106-amino-acid polypeptide whose sequence presents 65% identity with small electron carriers (Table 2) and contains 5 cysteinyl residues, four of which are arranged in a pattern (Cys-X5-Cys-X2-Cys-Xn-Cys) typical of putidaredoxin-type [2Fe-2S] cluster ligands (Fig. 2). A homology search using the Blitz software facilities offered by the European BioInformatics Institute revealed the highest similarity scores with Fdx1, another ferredoxin of RW1, which was recently genetically and biochemically characterized (6), and putidaredoxin, a ferredoxin acting with a monooxygenase involved in the hydroxylation of camphor (27). The comparison of Fdx1 and ORFD3 sequences suggested that these two proteins are isoferredoxins, probably derived from a common ancestor. Fdx1 was shown to be able to donate electrons to the dioxin dioxygenase (8) and thus is considered to be the in vivo electron donor of this enzyme. We have also identified another gene (ORFG3) encoding a ferredoxin (Fdx2) in the same organism (3), but the corresponding protein should differ significantly from Fdx1 and this new putative ferredoxin, as its sequence contains the typical signature of a Rieske-type [2Fe-2S] cluster. We therefore designated this third putative ferredoxin gene fdx3. Further biochemical studies will be necessary to demonstrate the presence of Fdx3 in RW1 cells and to define its function.
TABLE 2.
Genes and gene products of the dxn locus
| Gene | Position in sequence (nt)c | Probable function or product | Calculated molecular mass (Da) | Protein with homologous sequence | Source | % Identitya | Reference for homologous proteinb |
|---|---|---|---|---|---|---|---|
| dxnC | 12028–13923 | Unknown | 66,917 | FyuA-YEREN | Yersinia enterolitica | 5 | P46360 |
| FyuA-YERPE | Yersinia pestis | 5 | P46359 | ||||
| ViuA-VIBCH | Vibrio cholerae | 5 | Q00964 | ||||
| ORF2d | 13992–14444 | Unknown | 15,866 | ||||
| fdx3 | 14621–14941 | Electron carrier | 11,379 | Fdx1-RW1 | Sphingomonas sp. strain RW1 | 45 | Y13118 |
| PutX-PSESP | Pseudomonas putida | 44 | P00259 | ||||
| FdVI-RHOCA | Rhodobacter capsulatus | 36 | P80306 | ||||
| dxnD | 14991–16883 | 4-Hydroxysalicylate hydroxylase | 68,999 | HbpA-HPB1 | Pseudomonas azelaica | 28 | U73900 |
| PheA-PSESP | Pseudomonas sp. strain EST1001 | 13 | P31020 | ||||
| HydL-STRHA | Streptomyces halstechii | 12 | Q05355 | ||||
| TfdB-ALCEU | Ralstonia eutropha | 11 | P27138 | ||||
| dxnE | 16883–17953 | Maleylacetate reductase | 36,723 | TcbF-PSESP | Pseudomonas sp. strain P51 | 55 | P27101 |
| TfdF-ALCEU | Ralstonia eutropha | 51 | P27137 | ||||
| dxnF | 17978–18877 | Hydroxyquinol 1,2-dioxygenase | 33,080 | HadC-DTP0602 | Ralstonia pickettii | 44 | D86544 |
| TftH-AC1100 | Burkholderia cepacia | 44 | U19883 | ||||
| ClcA-PSEPU | Pseudomonas putida | 24 | P11451 | ||||
| CatA-ACICA | Acinetobacter calcoaceticus | 24 | P07773 | ||||
| TfdC-pJP4 | Ralstonia eutropha | 23 | P05413 | ||||
| dxnG | 18874–19584 | 3-Oxoadipate succinyl-CoA transferase (α-subunit) | 25,914 | YxjD-BACSU | Bacillus subtilis | 58 | D83026 |
| PcaJ-PSEPU | Pseudomonas putida | 43 | Q01103 | ||||
| AtoD-HAEIN | Haemophilus influenzae | 35 | P44875 | ||||
| dxnH | 19584–20246 | 3-Oxoadipate succinyl-CoA transferase (β-subunit) | 22,986 | YxjE-BACSU | Bacillus subtilis | 60 | D83026 |
| PcaJ-PSEPU | Pseudomonas putida | 57 | Q01104 | ||||
| CtfB-CLOAB | Clostridium acetobutylicum | 51 | P14611 | ||||
| dxnI | 20265–21449 | Acetyl-CoA acetyltrans-ferase (thiolase) | 41,723 | PcaF-PRS2000 | Pseudomonas putida | 52 | U10895 |
| PcaF-ADP1 | Acinetobacter sp. strain ADP1 | 51 | L05770 | ||||
| ThiL-HAEIN | Haemophilus influenzae | 34 | Q57190 | ||||
| ThiL-ALCEU | Ralstonia eutropha | 32 | P14611 | ||||
| ORF10 | 21689–22264 | Transposase | >21,133 | Tra3-RHIME | Rhizobium meliloti | 61 | P80011 |
| Tra-CORDI | Corynebacterium diphtheriae | 31 | P35879 | ||||
| Tra5-LACLA | Lactococcus lactis | 24 | P35881 |
Percentage of amino acids that are identical when sequences are aligned with sequences listed in the GenBank database by using the algorithm of Needleman and Wunsch via the Blitz program of the European BioInformatic Institute facilities.
Accession number in the SwissProt and EMBL/DDBJ/GenBank formats.
Accession no. X72850. nt, nucleotide.
This gene’s product showed no similarity to known proteins.
FIG. 2.
Conserved sequences that characterize putidaredoxin-type [2Fe-2S] ferredoxins. Well-conserved fingerprint sequence regions in putidaredoxin-type ferredoxins are shown. Cysteines implicated in binding the [2Fe-2S] cluster are indicated with an asterisk. A consensus sequence for the whole family of this type of ferredoxin is also indicated. The sequences were compiled and aligned by using GeneWorks software (version 2.5N) from IntelliGenetics. The proteins are labeled by trivial abbreviations. Their accession codes in the GenBank/EMBL/DDBJ databases and their origins are as follows: P25528 for ferredoxin from E. coli (FER_ECOLI), P43493 for ferredoxin from Rhodococcus erythropolis sp. strain NI86/21 (THCC_RHOSO), P37098 for ferredoxin from Caulobacter crescentus (FER_CAUCR), P80306 for ferredoxin FdVI from Rhodobacter capsulatus (FER6_RHOCA), P33007 for ferredoxin from Pseudomonas sp. (TERP_PSESP), P00259 for ferredoxin from P. putida (PUTX_PSEPU), Y13118 for ferredoxin Fdx1 from Sphingomonas sp. RW1 (Fdx1_RW1), and X72850 for ferredoxin Fdx3 from Sphingomonas sp. RW1 (Fdx3_RW1).
dxnD specifies a 4-hydroxysalicylate hydroxylase.
The 572-amino-acid protein specified by dxnD shows not high but significant similarity over the whole sequence with a 2-hydroxybiphenyl 3-monooxygenase characterized recently (30, 35) and some weak similarity to monooxygenases involved in the degradation of phenolic compounds (E.C. 1.14.13.7), with no more than 28% of the residues being conserved in the best case (Table 2). In order to assess the functional role of the DxnD putative monooxygenase, a hyperexpression system for its gene was constructed, and the activity of the gene product with various substrates was tested. The dxnD gene was cloned into plasmid pET9a, downstream of the φ10 promoter, which is strongly induced by IPTG (isopropyl-β-d-thiogalactopyranoside) in the E. coli strain BL21(DE3)(pLysS). Induced recombinant bacteria containing plasmid pAJ123 hyperproduced the expected 70-kDa polypeptide (Fig. 3, lane 2). Cell extracts obtained by French press disruption were assayed for NADH oxidation with various aromatic compounds, such as phenol, 2-hydroxybiphenyl, 2,2′-dihydroxybiphenyl, 2,3-dihydroxybiphenyl, 4-hydroxybenzoate, salicylate, and 4-hydroxysalicylate, a possible precursor of hydroxyquinol as discussed below. No detectable activity was found with these compounds, except with 4-hydroxysalicylate; in the presence of this compound NADH was oxidized with a specific activity of 44 nmol/min/mg (Table 3), and the compound was identified as a growth substrate for RW1.
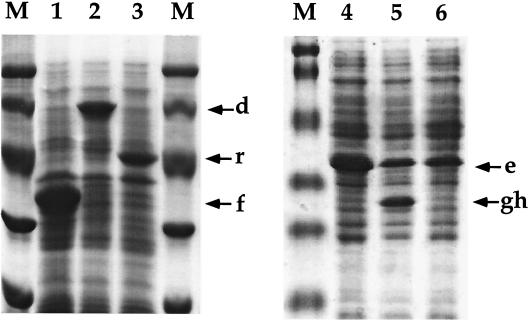
FIG. 3.
SDS-PAGE analysis of the hyperexpression products of dxnD, dxnE, dxnF, dxnGH, and dxnI. E. coli cells were treated with lysis buffer and subjected to electrophoresis on a 10% (left gel) or 15% (right gel) glycine polyacrylamide gel, and the gels were subsequently stained with Coomassie blue. Protein standards (lysozyme 14.4 kDa; trypsin inhibitor, 21.5 kDa; carbonic anhydrase, 31 kDa; ovalbumin, 45 kDa; serum albumin, 66.2 kDa; and phosphorylase B, 97.4 kDa) (lanes M) and induced whole cells of E. coli BL21(DE3)(pLysS) containing plasmids pAJ125, pAJ123, pAJ111, pAJ141, pAJ143, and pAJ145 (lanes 2 to 6, respectively) were loaded on the gels. Construct pAJ111, designed for the hyperexpression of reductase RedA2 from RW1 (25), was used as a control. The hyperproduced polypeptides are indicated by arrows: DxnF (f), DxnD (d), RedA2 (r), DxnE (e), DxnG (g), and DxnH (h).
TABLE 3.
Specific activities of conversion by Sphingomonas sp. strain RW1 and constructs expressed in E. colia
| Enzyme (substrate) | Sp act of:
|
|||||
|---|---|---|---|---|---|---|
| Constructs expressed in E. coli BL21(DE3)
|
Conversion of Sphingomonas sp. strain RW1 cultured on:
|
|||||
| pAJ123 | pAJ125 | pAJ141 | Dibenzofuran (early growth phaseb) | Dibenzofuran (later growth phaseb) | 4-Hydroxy-salicylate | |
| 4-Hydroxysalicylate hydroxylase [4-hydroxysalicylate (2,4-dihydroxybenzoate)] | 44 | 2.1 | <1 | 60 | ||
| Hydroxyquinol dioxygenase [hydroxyquinol (4-hydroxycatechol)] | 600 | 2.8 | 1.6 | 6.3 | ||
| Maleylacetate reductase (maleylacetate) | 399 | 1.9 | <1 | 27 | ||
Results represent means of at least two independently performed experiments. Specific activities are expressed in nanomoles of substrate transformed per minute per milligram of protein present in the crude extract.
Cells were collected at an A600 of 0.3 (early growth phase) or 0.8 (later growth phase).
DxnE is a functional maleylacetate reductase.
The sequence of the putative protein encoded by the fifth ORF is similar to the sequences of several (chloro)maleylacetate reductases, with similarities reaching 55% for TcbF from Pseudomonas sp. strain P51 (Table 2). Such proteins are involved in ortho cleavage pathways for the degradation of chlorocatechols produced from various chloroaromatics (24), more specifically in the NADH-dependent conversion of (chloro)maleylacetate, formed from (chloro)catechol by (chloro)catechol 1,2-dioxygenase and subsequent enzyme activities, to furnish (chloro)-3-oxoadipate (in the case of halomaleylacetate, two successive reactions are needed to yield the corresponding 3-oxoadipate [21]).
To determine whether ORF3 encodes a functional maleylacetate reductase, dxnE was hyperexpressed as a 38-kDa polypeptide from plasmid pAJ141 (Fig. 3, lane 4). A maleylacetate reduction activity of 399 nmol/min/mg was measured in bacteria carrying this expression system, which confirmed the function of DxnE (Table 3).
DxnF is a hydroxyquinol 1,2-dioxygenase.
The 299-amino-acid polypeptide specified by dxnF shows significant amino acid identity with several chlorocatechol and catechol 1,2-dioxygenases that are involved in the metabolism of chlorinated or nonchlorinated aromatics (Table 2). More detailed sequence comparison revealed that the dxnF-specified polypeptide exhibited the highest similarities with hydroxyquinol 1,2-dioxygenases (Fig. 4). Cells of E. coli BL21(DE3)(pLysS)(pAJ125), when induced with IPTG, hyperproduced a 33-kDa polypeptide (Fig. 3, lane 1), whose molecular mass closely matched the predicted molecular mass of the dxnF gene product (33.1 kDa). Cell extracts prepared from these bacteria exhibited high ring cleavage activity for hydroxyquinol (4-hydroxycatechol, 600 nmol/min/mg) and lower activity for catechol (270 nmol/min/mg).
FIG. 4.
Phylogenetic tree obtained by alignment of DxnF with related dioxygenases. The sequences were compiled by using the software mentioned in the legend to Fig. 2; the multiple alignment analysis was performed with the Phylips package programs. The phylogenetic unrooted tree was drawn by using TreeView. The horizontal bar indicates the percent divergence (distance). The numbers on some of the branches refer to the confidence (percent) estimated by bootstrap analysis (100 replications). The proteins are labeled by trivial abbreviations. Their accession codes in the GenBank/EMBL/DDBJ databases and their origins are as follows: D86544 for hydroxyquinol 1,2-dioxygenase from Ralstonia pickettii strain DTP0602 (HadC-DTP0602), U19883 for hydroxyquinol 1,2-dioxygenase from B. cepacia AC1100 (TftH-AC1100), AF003948 for chlorocatechol 1,2-dioxygenase from Rhodococcus opacus 1CP (ClcA-1CP), AF044314 for chlorocatechol 1,2-dioxygenase from Variovorax paradoxus TV1 (TfdC-TV1), U32188 for the so-called 3,5-dichlorocatechol 1,2-dioxygenase from P. putida EST4011 (TfdC-EST4011), M57629 for chlorocatechol 1,2-dioxygenase II from Pseudomonas sp. strain P51 (TcbC-P51), M35097 for chlorocatechol 1,2-dioxygenase I from plasmid pJP4 of Ralstonia eutropha JMP134 (TfdC-pJP4), M36279 for chlorocatechol 1,2-dioxygenase II from plasmid pJP4 of R. eutropha JMP134 (TfdCII-pJP4), M16964 for chlorocatechol 1,2-dioxygenase from plasmid pAC27 (ClcA-pAC27), AJ006307 for chlorocatechol 1,2-dioxygenase from Ralstonia sp. strain JS705 (ClcA-JS705), D16356 for the so-called 3,5-dichlorocatechol 1,2-dioxygenase from B. cepacia CSV90 (TfdC-CSV90), AF003947 for the α-subunit of protocatechuate 3,4-dioxygenase from R. opacus 1CP (PcaG-1CP), P15110 for the β-subunit of protocatechuate 3,4-dioxygenase from B. cepacia (PcxB-cepacia), L14836 for the β-subunit of protocatechuate 3,4-dioxygenase from P. putida (PcaH-putida), L23213 for the α-subunit of protocatechuate 3,4-dioxygenase from P. putida (PcaG-putida), L05770 for the β-subunit of protocatechuate 3,4-dioxygenase from Acinetobacter sp. ADP1 (PcaH-ADP1), P20372 for the β-subunit of protocatechuate 3,4-dioxygenase from Acinetobacter calcoaceticus (PcaH-acine), X99622 for the dioxygenase-like enzyme from R. opacus (Dle-1CP), U77658 for catechol 1,2-dioxygenase I from Acinetobacter lwoffii K24 (CatA1-K24), Z36909 for catechol 1,2-dioxygenase from A. calcoaceticus NCIB8250 (ORF7-NCBI8250), U77659 for catechol 1,2-dioxygenase II from Acinetobacter lwoffii K24 (CatA2-K24), P07773 for catechol 1,2-dioxygenase from A. calcoaceticus BD413 (CatA-BD413), U12557 for catechol 1,2-dioxygenase from P. putida PRS1 (CatA-PRS1), D37783 for catechol 1,2-dioxygenase from P. putida C-1 (CatA-C1), D37782 for catechol 1,2-dioxygenase from P. putida mt2 (CatA-mt2), P31019 for catechol 1,2-dioxygenase from Pseudomonas sp. strain EST1001 (PheB-EST1001), M57500 for catechol 1,2-dioxygenase from plasmid pEST1226 of Pseudomonas sp. strain EST1001 (PheB-pEST1226), M94318 for catechol 1,2-dioxygenase from Arthrobacter sp. strain mA3 (CatA-mA3), X99622 for catechol 1,2-dioxygenase from R. opacus (CatA-1CP), D83237 for catechol 1,2-dioxygenase from R. erythropolis AN-13 (CatA-AN13), and AF043741 for catechol 1,2-dioxygenase from Rhodococcus rhodochrous NCIMB 13259 (CatA-NCIMB13259).
Cistrons dxnGH code for the two subunits of a 3-oxoadipate succinyl-CoA transferase.
Translation of ORF7 and ORF8 predicts protein products of 26 and 23 kDa, respectively (Table 2). The deduced amino acid sequences of these two ORFs, named dxnG and dxnH, respectively, were found to be 43 to 60% identical to the two subunits of the 3-oxoadipate succinyl-CoA transferase which carries out the penultimate step of the so-called 3-oxoadipate pathway of Bacillus subtilis (45) and Pseudomonas putida (25). This enzyme converts 3-oxoadipate, an intermediate produced during degradation of protocatechuate and catechol, to 3-oxoadipyl-CoA, which is further transformed upon thiolase activity to succinyl-CoA and acetyl-CoA. Coexpression of the two cistrons was carried out in E. coli BL21(DE3)(pLysS)(pAJ143) cells. SDS-PAGE analysis revealed the production of 25-kDa polypeptides, which may correspond to the two expected polypeptides (Fig. 3, lane 5). Cell extracts prepared from induced cells converted 3-oxoadipate to 3-oxoadipyl-CoA with a specific activity of 8.1 nmol/min/mg, calculated upon HPLC-based analysis of the transformation of oxoadipate to oxoadipyl-CoA. The structure of the latter compound was confirmed by HPLC-MS analysis. The mass 910.6 atomic mass units (MH+) was found in the appropriate peak as well as in scanning of the whole separation in the single ion monitoring mode of the Perkin-Elmer mass spectrometer. The predominant base peak at 258.5 atomic mass units was also found in the scans of the peaks originating from reduced CoA and succinyl-CoA.
dxnI specifies a thiolase.
The penultimate ORF identified in the HindIII fragment encodes a protein of 394 amino acids, with a calculated molecular mass of 42 kDa (Table 2). The sequence of the corresponding polypeptide is closely related to the sequences of acetyl-CoA acetyltransferases, also known as thiolases. These enzymes play a key role in bacterial metabolism. They control the flow of carbon by catalyzing the condensation of two molecules of acetyl-CoA to produce acetoacetyl-CoA (anabolic thiolases [26]) or the disruption of 3-oxoadipyl-CoA into succinyl-CoA and acetyl-CoA (catabolic thiolases [14, 20]). Attempts to express this gene by using the plasmid pAJ145 did not result in the production of the expected polypeptide (Fig. 3, lane 6).
ORFD10 encodes a putative transposase.
Downstream of dxnI and extending to the end of the sequenced fragment, is a partial ORF 210 codons long. The polypeptide sequence of this ORF exhibits some marked similarities with several transposases (Table 2), with the highest score (61% of identical residues) being recorded for a transposase from Rhizobium meliloti (39). The presence of such a gene within the dxn locus suggests that at least part of the dioxin catabolic pathway may be located on a transposable element. RW1 readily loses its capacity to grow on dibenzofuran and dibenzo-p-dioxin as sole sources of carbon during several subcultures in rich medium, which indicates that at least some of the genes involved in this catabolism are encoded by an unstable genetic element. A stable strain, a derivative of RW1, was isolated and shown to stably retain the catabolic capacity (27a). It will be interesting to analyze the transposase gene and its target sequences in this stable strain.
RW1 is able to grow on hydroxyquinol and 4-hydroxysalicylate as sole sources of carbon.
As hydroxyquinol is toxic for RW1, growth of the strain on this substrate was tested on minimal medium plates within a substrate gradient. Incubation at 30°C for 4 days produced visible colonies of RW1 in the tolerable substrate concentration zone. RW1 was also able to grow on 4-hydroxysalicylate as the sole energy and carbon source, with a generation time of approximately 12 h. Furthermore, RW1 could be grown on minimal medium plates within a substrate gradient of 2-hydroxydibenzo-p-dioxin and 3-hydroxydibenzofuran as sole carbon and energy sources. Some growth in liquid culture was achieved only in fed-batch experiments due to the toxicity of these substrates at concentrations above 0.2 mM. Higher activities of transformation of 4-hydroxysalicylate, hydroxyquinol, and maleylacetate were measured for cells grown on 4-hydroxysalicylate than for cells grown on dibenzofuran. The specific activities, however, were higher when dibenzofuran-grown cells were collected at an early growth phase rather than in a later stage (Table 3). For cells pregrown on dibenzofuran (to an A600 of about 0.3) and with the subsequent addition of 0.1 mM 3-hydroxydibenzofuran as a potential inducer over a period of up to 3 h prior to collection (A600 of 0.5) and workup, no further increase of activities was noticed.
DISCUSSION
Several bacterial strains able to degrade diaryl ethers have been isolated during the last few years, mostly belonging to the new genus Sphingomonas (16, 19, 31, 32, 42) which was established by Yabuuchi et al. (43). Among them, Sphingomonas sp. strain RW1 is physiologically, biochemically, and genetically the best characterized (1, 3, 6–9, 18, 23, 40, 42). The recent cloning of the genes specifying the dioxin dioxygenase and its electron transport system will open new perspectives: a more complete description of the genetics, biochemistry, and regulation of the different proteins involved in the catabolism of diaryl ethers is an essential basis needed for the development of rational strategies to improve the limited catabolic activities of RW1 (40) toward the breakdown and mineralization of more highly chlorinated derivatives, which represent environmentally critical pollutants.
Genes specifying the biodegradation of aromatic compounds are usually clustered on the same genomic locus. They may be well organized and divided into two parts, namely, (i) an upstream segment, involving the initial steps of the pathway, leading to the ring cleavage substrate catechol or its chlorinated derivatives, and (ii) a downstream (in the pathway and on the genetic level) segment, dedicated to the channeling of the ring cleavage substrate towards Krebs cycle intermediates. Such a genetic organization was not, however, found for the dioxin/dibenzofuran pathway of RW1, whose degradative genes were found to be scattered over the chromosome (3). Other genes specifying yet-uncharacterized polypeptides exhibiting sequence homology to various catabolic enzymes are loosely clustered with the genes for dioxin/dibenzofuran degradation (3, 6). It was therefore of interest to further characterize the dxnA1A2 locus. Sequencing the 8.9-kb region upstream of dxnA1 revealed no relevant information, except for the presence of divergently transcribed dbfB, a meta cleavage enzyme involved in the dibenzofuran pathway (3). We therefore focused our attention on the region downstream of dxnA1A2 and sequenced a 10-kb HindIII fragment carrying this region. Ten ORFs were identified on this fragment, each of which is located only a few nucleotides downstream of the previous one, suggesting that they may be cotranscribed. Consistent with this possibility was the lack within the fragment of an obvious stable stem-loop structure able to act as a transcription terminator.
The function of the products of each ORF was tentatively deduced from sequence comparisons with known catabolic enzymes and, in some cases, by enzymatic assays of cell extracts containing cloned and hyperexpressed gene products. Unlike the situation with the other ORFs within the gene cluster, analysis of the sequence of the first two ORFs did not point to any obvious function for the corresponding individual polypeptides. The putative amino acid sequence of the protein specified by dxnC showed minor, but significant similarity with those of ferric iron-siderophore receptor proteins found in diverse bacteria (15, 28), which raises the possibility that it might be involved in substrate uptake of polar compounds or sensing in the context of chemotaxis (3-hydroxysalicylate iron chelator versus 4-hydroxysalicylate transporter). Diaryl ethers such as dioxins and dibenzofurans are thought to diffuse freely through the bacterial membrane, and no system of specific uptake of these, or similar, compounds has so far been reported. Other genes of unknown function encoding membrane proteins are sometimes clustered with degradative genes, such as todX, which is associated with the toluene degradation pathway of P. putida F1 (37). Inactivation of dxnC in RW1 and analysis of the phenotype of the resulting mutant may provide information on the function of DxnC.
Another gene, namely, dxnD, codes for a putative monooxygenase exhibiting weak similarities (11 to 28%) with phenol- and 2-hydroxybiphenyl monooxygenases. The latter enzyme isolated from Pseudomonas azelaica HPB1 (30, 35) is active against 2-hydroxybiphenyl, a widely used fungicide, and 2,2′-dihydroxybiphenyl, forming 2,3-dihydroxybiphenyl and 2,2′,3-trihydroxybiphenyl, respectively, which are also produced during metabolism of biphenyl and dibenzofuran, respectively. The dxnD product was shown here to exhibit NADH oxidation activity with 4-hydroxysalicylate as the substrate, but no activity was detectable with salicylate. The transformation product of 4-hydroxysalicylate could not be clearly identified but is presumed to be hydroxyquinol. The latter compound is known as a highly unstable chemical (34) and therefore may not accumulate due to the relatively low transformation rate of its precursor.
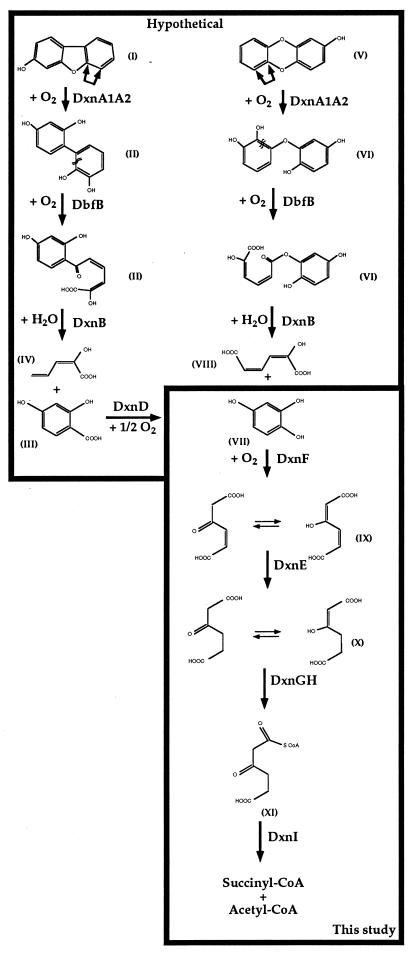
In this report, we have provided strong evidence for a 4-hydroxysalicylate/hydroxyquinol degradative pathway in RW1. Hydroxyquinol, the putative product of the decarboxylating monooxygenation of 4-hydroxysalicylate by DxnD, is cleaved by DxnF dioxygenase to 3-hydroxy-cis,cis-muconate (maleylacetate), which is then reduced by the DxnE maleylacetate reductase to 3-oxoadipate. We showed that this compound is then converted by DxnGH to 3-oxoadipyl-CoA, which is probably further transformed by DxnI to succinyl-CoA and acetyl-CoA (Fig. 5). A similar pathway was identified in Burkholderia cepacia AC1100 as the lower pathway of 2,4,5-trichlorophenoxyacetic acid catabolism (12, 13, 46) and is thought to be active in pentachlorophenol catabolism (17, 36).
FIG. 5.
Proposed converging pathways for the degradation of 3-hydroxydibenzofuran and 2-hydroxydibenzo-p-dioxin through 4-hydroxysalicylate and hydroxyquinol in Sphingomonas sp. strain RW1. The unstable compounds (hemiacetals) formed by angular dioxygenation by DnxA1A2, which spontaneously decay, are not indicated in order to simplify the pathway. The double-arrows indicate the position of possible dioxygenolytic attack by the initial dioxin dioxygenase. Chemical designations: I, 3-hydroxydibenzofuran; II, 2,2′,3,4′-tetrahydroxybiphenyl; III, 2-hydroxy-6-oxo-6-(2,4-dihydroxyphenyl)-hexa-2,4-dienoic acid; IV, 2-hydroxypenta-2,4-dienoic acid; V, 2-hydroxydibenzo-p-dioxin; VI, 2,2′,3,5′-tetrahydroxydiphenyl ether; VII, 6-(2,5-dihydroxyphenyl)-ester of 2-hydroxy-cis,cis-muconic acid; VIII, 2-hydroxy-cis,cis-muconic acid; IX, 4-hydroxysalicylic acid; X, hydroxyquinol; XI, 3-hydroxy-cis,cis-muconic acid (maleylacetic acid); XII, 3-oxoadipic acid (and its enol); XIII, 3-oxoadipyl-CoA.
The fact that the dxnEFGHI genes, specifying the hydroxyquinol pathway, are located just downstream of the dxnA1A2B locus, which encodes the upper degradative pathway for dibenzo-p-dioxin and dibenzofuran, is probably not fortuitous and raises the question of the possible role of hydroxyquinol as a central intermediate in the degradation of derivatives of such bicyclic compounds. Such a possible role has been already evocated by Seibert et al. (33), concerning the degradation of 2,7-dichlorodibenzo-p-dioxin by the basidiomycete Phanerochaete chrysosporium. As suggested in Fig. 5, hydroxyquinol may arise in RW1 by dioxygenation of 2-hydroxydibenzo-p-dioxin, followed by meta cleavage, and subsequent hydrolysis of the formed ester, or through decarboxylating monooxygenation by DxnD of 4-hydroxysalicylate, itself a possible product of the angular dioxygenation of 3-hydroxydibenzofuran, followed by ring cleavage and hydrolysis. The latter compound has been shown to be converted by another microorganism, Sphingomonas sp. strain HH69 (19), to 4-hydroxysalicylate and salicylate, which were found in this case as major metabolites. Complete degradation of 3-hydroxydibenzofuran and 2-hydroxydibenzo-p-dioxin by RW1 would require the action of the upper dioxin and dibenzofuran pathways and the 4-hydroxysalicylate/hydroxyquinol degradative pathway. This is reflected at the genetic level by a clustering of the genes specifying these activities. Expression of the two pathways is not necessarily coregulated, if at all, as the hydroxyquinol pathway is not required for dibenzo-p-dioxin and dibenzofuran degradation. We observed that the activities of the enzymes of this lower pathway are quite low in cells grown on dibenzofuran but slightly higher if cells are collected at an early growth phase. This correlates well with high expression of the dioxin dioxygenase system in the exponential growth phase with salicylate as the substrate (8). Growth with crystalline dibenzofuran is only exponential at a very early growth phase and then linear, due to the reduced bioavailability of the substrate (41). In addition, no increase of the level of these activities was noticed when 3-hydroxydibenzofuran was added to cells growing on dibenzofuran. The relatively high enzyme activities during growth of Sphingomonas sp. strain RW1 on 4-hydroxysalicylate (Table 3) are obviously mainly due to the exponential growth, which, in fact, is only linear on dibenzofuran because of the relatively low bioavailability as mentioned above. Therefore, these data tend to indicate that expression of the two pathways may be coregulated, although any regulatory elements such as transcriptional activators have been identified on the 40.1 kb of genomic sequence available from RW1 (3) and on a 10.7-kb fragment of Sphingomonas sp. strain RW5 (38) harboring a gentisate degradative pathway. The construction of specific knockout mutants of the genes of the two pathways is necessary in order to ascertain that no other isofunctional enzymes contribute to the wild-type activities.
The clustering of the genetic elements of the two pathways may indicate the possible origins of degradative genes coding for catabolic pathways for dibenzofuran and dibenzo-p-dioxin. Hydroxyl-substituted molecules containing structural elements similar to dibenzo-p-dioxins and dibenzofurans have been produced by algae, fungi, lichens, and other organisms over millions of years (41) and therefore have been present in the environment over a long evolutionary period, whereas unsubstituted dibenzo-p-dioxin obviously has not. Thus, microbial enzyme systems for the breakdown of these hydroxylated structures possibly evolved long before anthropogenic contamination of the environment.
ACKNOWLEDGMENTS
We gratefully acknowledge Silke Backhaus, Annette Krüger, and Carsten Strömpel for assistance with sequencing gel runs; Tschong-Hun Löhnert for assistance with some of the enzyme assays; and our collaborative partners at the University of Hamburg, Heinz Wilkes and Wittko Francke, for the kind gift of 3-hydroxydibenzofuran and 2-hydroxydibenzo-p-dioxin. We also thank Heinrich Steinmetz for analyzing our samples by HPLC-MS.
Jean Armengaud was initially supported by a long-term grant from the FEBS and thanks the persons acting generously in this federation. This research was funded in part by the German Ministry for Education and Research (BMBF grant 0318896C). Kenneth Timmis gratefully acknowledges the generous support from the Fonds der Chemischen Industrie.
REFERENCES
- 1.Arfmann H-A, Timmis K N, Wittich R-M. Mineralization of 4-chlorodibenzofuran by a consortium consisting of Sphingomonas sp. strain RW1 and Burkholderia sp. strain JWS. Appl Environ Microbiol. 1997;63:3458–3462. doi: 10.1128/aem.63.9.3458-3462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armengaud J, Gaillard J, Forest E, Jouanneau Y. Characterization of a 2[4Fe-4S] ferredoxin obtained by chemical insertion of the Fe-S clusters into the apoferredoxin II from Rhodobacter capsulatus. Eur J Biochem. 1995;231:396–404. doi: 10.1111/j.1432-1033.1995.tb20712.x. [DOI] [PubMed] [Google Scholar]
- 3.Armengaud J, Happe B, Timmis K N. Genetic analysis of dioxin dioxygenase of Sphingomonas sp. strain RW1: catabolic genes dispersed on the genome. J Bacteriol. 1998;180:3954–3966. doi: 10.1128/jb.180.15.3954-3966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armengaud J, Meyer C, Jouanneau Y. Recombinant expression of the fdxD gene of Rhodobacter capsulatus and characterization of its product, a [2Fe-2S] ferredoxin. Biochem J. 1994;300:413–418. doi: 10.1042/bj3000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armengaud J, Meyer C, Jouanneau Y. A [2Fe-2S] ferredoxin (FdVI) is essential for growth of the photosynthetic bacterium Rhodobacter capsulatus. J Bacteriol. 1997;179:3304–3309. doi: 10.1128/jb.179.10.3304-3309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armengaud J, Timmis K N. Molecular characterization of Fdx1, a putidaredoxin-type [2Fe-2S] ferredoxin able to transfer electrons to the dioxin dioxygenase of Sphingomonas sp. RW1. Eur J Biochem. 1997;247:833–842. doi: 10.1111/j.1432-1033.1997.00833.x. [DOI] [PubMed] [Google Scholar]
- 7.Armengaud J, Timmis K N. The reductase RedA2 of the multi-component dioxin dioxygenase system of Sphingomonas sp. RW1 is related to class-I cytochrome P450-type reductases. Eur J Biochem. 1998;253:437–444. doi: 10.1046/j.1432-1327.1998.2530437.x. [DOI] [PubMed] [Google Scholar]
- 8.Bünz P V, Cook A M. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J Bacteriol. 1993;175:6467–6475. doi: 10.1128/jb.175.20.6467-6475.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bünz P V, Falchetto R, Cook A M. Purification of two isofunctional hydrolases (EC 3.7.1.8) in the degradative pathway for dibenzofuran in Sphingomonas sp. strain RW1. Biodegradation. 1993;4:171–178. doi: 10.1007/BF00695119. [DOI] [PubMed] [Google Scholar]
- 10.Claverie J M. A streamlined random sequencing strategy for finding coding exons. Genomics. 1994;23:575–581. doi: 10.1006/geno.1994.1545. [DOI] [PubMed] [Google Scholar]
- 11.Corkey B E, Brandt M, Williams R J, Williamson J R. Assay of short-chain acyl coenzyme A intermediates in tissue extracts by high-pressure liquid chromatography. Anal Biochem. 1981;118:30–41. doi: 10.1016/0003-2697(81)90152-4. [DOI] [PubMed] [Google Scholar]
- 12.Daubaras D L, Hershberger C D, Kitano K, Chakrabarty A M. Sequence analysis of a gene cluster involved in metabolism of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia AC1100. Appl Environ Microbiol. 1995;61:1279–1289. doi: 10.1128/aem.61.4.1279-1289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daubaras D L, Saido K, Chakrabarty A M. Purification of hydroxyquinol 1,2-dioxygenase and maleylacetate reductase: the lower pathway of 2,4,5-trichlorophenoxyacetic acid metabolism by Burkholderia cepacia AC1100. Appl Environ Microbiol. 1996;62:4276–4279. doi: 10.1128/aem.62.11.4276-4279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doten R C, Ngai K L, Mitchell D J, Ornston L N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987;169:3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fetherston J D, Lillard J W, Jr, Perry R D. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortnagel P, Harms H, Wittich R-M, Francke W, Krohn S, Meyer H. Cleavage of dibenzofuran and dibenzodioxin ring systems by a Pseudomonas bacterium. Naturwissenschaften. 1989;76:222–223. doi: 10.1007/BF00627694. [DOI] [PubMed] [Google Scholar]
- 17.Golovleva L A, Zaborina O, Pertsova R, Baskunov B, Schurukhin Y, Kuzmin S. Degradation of polychlorinated phenols by Streptomyces rochei 303. Biodegradation. 1991;2:201–208. doi: 10.1007/BF00124494. [DOI] [PubMed] [Google Scholar]
- 18.Happe B, Eltis L D, Poth H, Hedderich R, Timmis K N. Characterization of 2,2′,3-trihydroxybiphenyl dioxygenase, an extradiol dioxygenase from the dibenzofuran- and dibenzo-p-dioxin-degrading bacterium Sphingomonas sp. strain RW1. J Bacteriol. 1993;175:7313–7320. doi: 10.1128/jb.175.22.7313-7320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms H, Wilkes H, Wittich R-M, Fortnagel P. Metabolism of hydroxydibenzofurans, methoxydibenzofurans, acetoxydibenzofurans, and nitrodibenzofurans by Sphingomonas sp. strain HH69. Appl Environ Microbiol. 1995;61:2499–2505. doi: 10.1128/aem.61.7.2499-2505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harwood C S, Nichols N N, Kim M K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Hofmann, K., and W. Stoffel. TMPRED program. [Online.] Swiss Institute for Experimental Cancer Research, Epalinges s/ Lausanne, Switzerland. http://www.isrec.isb-sib.ch/software/TMPRED_form.html. [25 April 1999, last date accessed.]
- 21.Kaschabek S R, Reineke W. Maleylacetate reductase of Pseudomonas sp. strain B13: dechlorination of chloromaleylacetates, metabolites in the degradation of chloroaromatic compounds. Arch Microbiol. 1992;158:412–417. doi: 10.1007/BF00276301. [DOI] [PubMed] [Google Scholar]
- 22.Latus M, Seitz H-J, Eberspächer J, Lingens F. Purification and characterization of hydroxyquinol 1,2-dioxygenase from Azotobacter sp. strain GP1. Appl Environ Microbiol. 1995;61:2453–2460. doi: 10.1128/aem.61.7.2453-2460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Megharaj M, Wittich R-M, Blasco R, Pieper D H, Timmis K N. Superior survival and degradation of dibenzo-p-dioxin and dibenzofuran in soil by soil-adapted Sphingomonas sp. strain RW1. Appl Microbiol Biotechnol. 1997;48:109–114. [Google Scholar]
- 24.Muller C, Petruschka L, Cuypers H, Burchhardt G, Herrmann H. Carbon catabolite repression of phenol degradation in Pseudomonas putida is mediated by the inhibition of the activator protein PhlR. J Bacteriol. 1996;178:2030–2036. doi: 10.1128/jb.178.7.2030-2036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parales R E, Harwood C S. Characterization of the genes encoding β-ketoadipate: succinyl-coenzyme A transferase in Pseudomonas putida. J Bacteriol. 1992;174:4657–4666. doi: 10.1128/jb.174.14.4657-4666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peoples O P, Sinskey A J. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding β-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem. 1989;264:15293–15297. [PubMed] [Google Scholar]
- 27.Peterson J A, Lorence M C, Amarneh B. Putidaredoxin reductase and putidaredoxin. Cloning, sequence determination, and heterologous expression of the proteins. J Biol Chem. 1990;265:6066–6073. [PubMed] [Google Scholar]
- 27a.Poth, H., and B. Averhoff. Unpublished results.
- 28.Rakin A, Saken E, Harmsen D, Heesemann J. The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol Microbiol. 1994;13:253–263. doi: 10.1111/j.1365-2958.1994.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 30.Schmid A. Der Metabolismus von 2-Hydroxybiphenyl-Verbindungen in Pseudomonas azelaica HBP1. Ph.D. thesis. Stuttgart, Germany: University of Stuttgart; 1997. [Google Scholar]
- 31.Schmidt S, Wittich R-M, Erdmann D, Wilkes H, Francke W, Fortnagel P. Biodegradation of diphenyl ether and its monohalogenated derivatives by Sphingomonas sp. strain SS3. Appl Environ Microbiol. 1992;58:2744–2750. doi: 10.1128/aem.58.9.2744-2750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt S, Wittich R-M, Fortnagel P, Erdmann D, Francke W. Metabolism of 3-methyldiphenyl ether by Sphingomonas sp. SS31. FEMS Microbiol Lett. 1992;75:253–258. doi: 10.1016/0378-1097(92)90413-i. [DOI] [PubMed] [Google Scholar]
- 33.Seibert V, Stadler-Fritzsche K, Schlömann M. Purification and characterization of maleylacetate reductase from Alcaligenes eutrophus JMP134(pJP4) J Bacteriol. 1993;175:6745–6754. doi: 10.1128/jb.175.21.6745-6754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolz A, Knackmuss H-J. Degradation of 2,4-dihydroxybenzoate by Pseudomonas sp. BN9. FEMS Microbiol Lett. 1993;108:219–224. doi: 10.1111/j.1574-6968.1993.tb06102.x. [DOI] [PubMed] [Google Scholar]
- 35.Suske W A, Held M, Schmid A, Fleischmann T, Wubbolts M G, Kohler H-P. Purification and characterization of 2-hydroxybiphenyl 3-monooxygenase, a novel NADH-dependent, FAD-containing aromatic hydroxylase from Pseudomonas azelaica HBP1. J Biol Chem. 1997;272:24257–24265. doi: 10.1074/jbc.272.39.24257. [DOI] [PubMed] [Google Scholar]
- 36.Uotila J S, Kitunen V H, Coote T, Saastamoinen T, Salkinoja-Salonen M, Apajalahti J H. Metabolism of halohydroquinones in Rhodococcus chlorophenolicus PCP-1. Biodegradation. 1995;6:119–126. doi: 10.1007/BF00695342. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Rawlings M, Gibson D T, Labbe D, Bergeron H, Brousseau R, Lau P C. Identification of a membrane protein and a truncated LysR-type regulator associated with the toluene degradation pathway in Pseudomonas putida F1. Mol Gen Genet. 1995;246:570–579. doi: 10.1007/BF00298963. [DOI] [PubMed] [Google Scholar]
- 38.Werwath J, Arfmann H-A, Pieper D H, Timmis K N, Wittich R-M. Biochemical and genetic characterization of a gentisate 1,2-dioxygenase from Sphingomonas sp. strain RW5. J Bacteriol. 1998;180:4171–4176. doi: 10.1128/jb.180.16.4171-4176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheatcroft R, Laberge S. Identification and nucleotide sequence of Rhizobium meliloti insertion sequence ISRm3: similarity between the putative transposase encoded by ISRm3 and those encoded by Staphylococcus aureus IS256 and Thiobacillus ferrooxidans IST2. J Bacteriol. 1991;173:2530–2538. doi: 10.1128/jb.173.8.2530-2538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkes H, Wittich R-M, Timmis K N, Fortnagel P, Francke W. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by Sphingomonas sp. strain RW1. Appl Environ Microbiol. 1996;62:367–371. doi: 10.1128/aem.62.2.367-371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittich R-M, editor. Biodegradation of dioxins and furans. Heidelberg, Germany: Springer-Verlag; 1998. pp. 1–28. [Google Scholar]
- 42.Wittich R-M, Wilkes H, Sinnwell V, Francke W, Fortnagel P. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl Environ Microbiol. 1992;58:1005–1010. doi: 10.1128/aem.58.3.1005-1010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yabuuchi E, Yano I, Oyaizu H, Hashimoto Y, Ezaki T, Yamamoto H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol Immunol. 1990;34:99–119. doi: 10.1111/j.1348-0421.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 44.Yeh W K, Ornston L N. Evolutionarily homologous α2β2 oligomeric structures in beta-ketoadipate succinyl-CoA transferases from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1981;256:1565–1569. [PubMed] [Google Scholar]
- 45.Yoshida K, Shindo K, Sano H, Seki S, Fujimura M, Yanai N, Miwa Y, Fujita Y. Sequencing of a 65kb region of the Bacillus subtilis genome containing the lic and cel loci, and creation of a 177 kb contig covering the gnt-sacXY region. Microbiology. 1996;142:3113–3123. doi: 10.1099/13500872-142-11-3113. [DOI] [PubMed] [Google Scholar]
- 46.Zaborina O, Daubaras D L, Zago A, Xun L, Saido K, Klem T, Nikolic D, Chakrabarty A M. Novel pathway for conversion of chlorohydroxyquinol to maleylacetate in Burkholderia cepacia AC1100. J Bacteriol. 1998;180:4667–4675. doi: 10.1128/jb.180.17.4667-4675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]