Keloids are a common type of pathological scar as a result of skin healing, which are extremely difficult to prevent and treat without recurrence. The pathological mechanism of keloids is the excessive proliferation of fibroblasts, which synthesize more extracellular matrices (ECMs), including type I/III collagen (COL-1/3), mucopolysaccharides, connective tissue growth factor (CTGF, also known as cellular communication network factor 2 (CCN2)), and fibronectin (FN) in scar tissue, mostly through the abnormal activation of transforming growth factor-β (TGF-β)/Smads pathway (Finnson et al., 2013; Song et al., 2018). Genetic factors, including race and skin tone, are considered to contribute to keloid formation. The reported incidence of keloids in black people is as high as 16%, whereas white people are less affected. The prevalence ratio of colored people to white people is 5:1–15:1 (Rockwell et al., 1989; LaRanger et al., 2019). In addition, keloids have not been reported in albinism patients of any race, and those with darker skin in the same race are more likely to develop this disease (LaRanger et al., 2019). Skin melanocyte activity is significantly different among people with different skin tones. The more active the melanocyte function, the more melanin is produced and the darker the skin. Similarly, in the same individual, the incidence of keloids increases during periods when melanocytes are active, such as adolescence and pregnancy. Keloids rarely appear in areas where melanocytes synthesize less melanin, such as in the palms and soles. Thus, the formation of keloids seems to be closely related to melanocyte activity.
In the skin, melanocytes are located in the basal layer of the epidermis, while fibroblasts are found in the dermis and fascia. The basement membrane is broken when a wound appears, thus enabling fibroblasts that migrate from the fascia to the edge of the wound to communicate with the melanocytes (Correa-Gallegos et al., 2019). Previous in vitroexperiments showed that both the supernatants of cultured melanocytes and co-culturing melanocytes with fibroblasts significantly stimulated proliferation and DNA synthesis in fibroblasts (Gao et al., 2013). Herein, we observed that melanocytes in keloids are more active in vivo compared to normal scars, since there are more mel
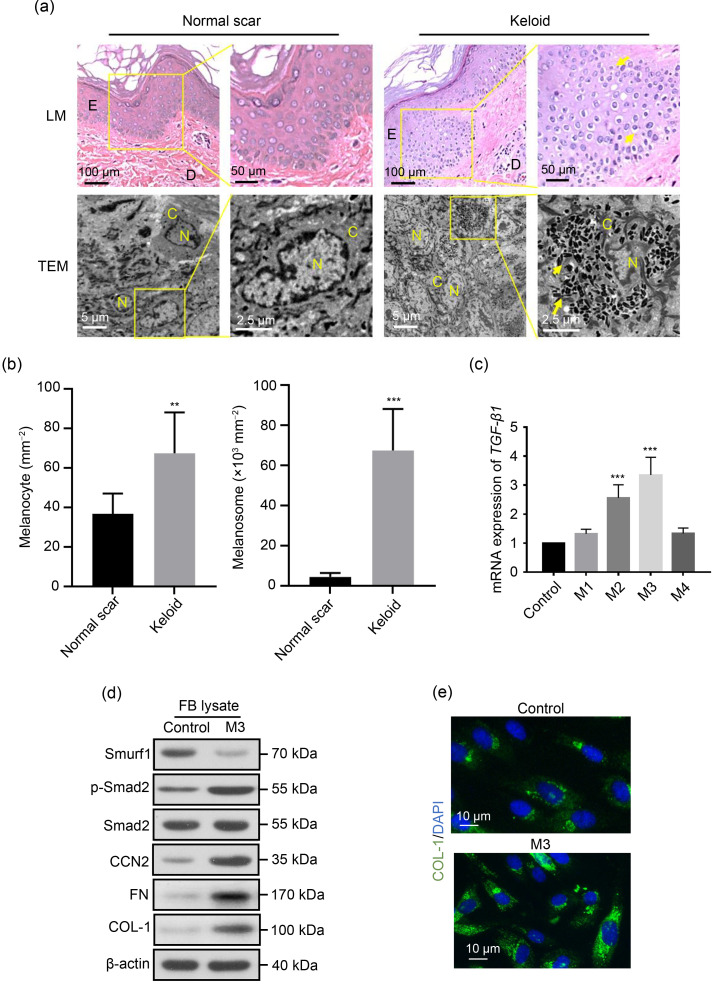
anocytes and melanosomes in keloid tissue (Figs. 1a and 1b). During keloid formation, the TGF-β type I receptor (TβR-I) directly phosphorylates the downstream substrate Smad2/3, and phosphorylated Smad2/3 (p-Smad2/3) binds to the mediator-type Smad4 to form a trimeric transcription factor that enters the nucleus and participates in promoting the proliferation of fibroblasts and ECM synthesis (Wang et al., 2012). Moreover, we found that the melanocyte culture supernatant in a suitable concentration can upregulate the TGF-β/Smads transduction levels of fibroblasts (Figs. 1c and 1d) and ECM synthesis in vitro (Figs. 1d and 1e). Therefore, functional melanocytes may release some secretions to stimulate fibroblasts during wound healing and promote excessive fibrosis, thereby contributing to the occurrence and development of keloids.
Fig. 1. Activation of transforming growth factor-β (TGF-β) signaling by melanocyte secretions in fibroblasts. (a) Light microscopy (LM) and transmission electron microscopy (TEM) observation of normal and keloid scar tissues. The presence of more melanocytes and melanosomes in keloid tissue is highlighted. The yellow arrows indicate melanocytes in LM and melanosomes in TEM. E, epidermis; D, dermis; N, nucleus; C, cytoplasm. (b) Quantitative analyses of the numbers of melanocytes and melanosomes in normal scars and keloids from patients. (c‒e) The supernatant of cultured human primary melanocytes upregulates the TGF-β/Smads transduction levels of fibroblasts and promotes the synthesis of extracellular matrices. Fibroblasts were cultured in a normal culture medium (control) or an M1–M4-conditioned medium for 72 h. M1–M4 indicate the culture supernatants of melanocytes diluted in a normal medium at different concentrations (volume ratio): M1, 1:10; M2, 1:4; M3, 1:2; M4, 3:4. (c) Effect of the conditioned medium at different concentrations (M1–M4) on the TGF-β1 messenger RNA (mRNA) expression in fibroblasts, as determined by quantitative real-time polymerase chain reaction (PCR). (d) Comparison of the type I collagen (COL-1), Smad specific E3 ubiquitin protein ligase 1 (Smurf1), phosphorylated Smad2 (p-Smad2), Smad2, connective tissue growth factor (CTGF, also known as cellular communication network factor 2 (CCN2)), and fibronectin (FN) protein contents in fibroblasts between M3 and the control groups by western blot. (e) Comparison of COL-1 synthesis content in fibroblasts between M3 and the control groups based on fluorescent staining. DAPI: 4',6-diamidino-2-phenylindole. Data are expressed as mean±standard deviation (SD), n=6. ** P<0.01, *** P<0.001, vs. normal scar or control.
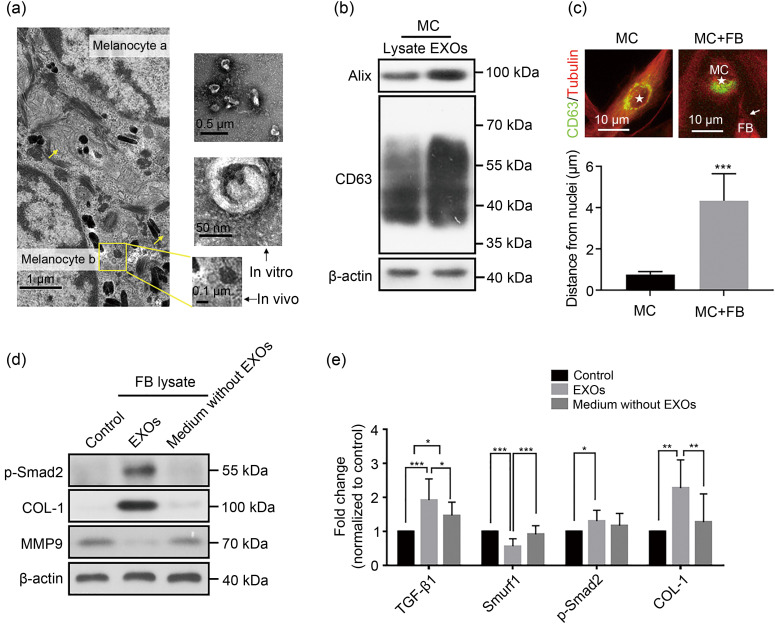
Intercellular communication can be carried out through soluble molecules or extracellular vesicles. Exosomes are common types of extracellular vesicles secreted by most cells, which carry the molecular information of parental cells, such as DNA, RNA, and proteins involved in a variety of physiological and pathological processes (Raposo and Stoorvogel, 2013; Chen et al., 2020; Geng et al., 2021; Liu et al., 2021). We observed a typical exosomal structure in and around melanocytes in vivo (Fig. 2a). Exosomes from the supernatant of human primary melanocytes were isolated through differential centrifugation and identified by transmission electron microscopy (TEM) observation (Fig. 2a) and western blot analysis (Fig. 2b). The results of nanoparticle tracking analysis confirmed that the sizes of isolated extracellular vesicles (EVs) were in the range of 30–150 nm. Interestingly, when melanocytes were co-cultured with fibroblasts for a short period, the distribution of exosomes within the melanocytes shifted from around the nucleus to the side near the fibroblasts toward the contact site of cells (Fig. 2c), as quantified by the increased distance of exosomes from the nuclei (Fig. 2c, bottom panel). Similar redistributions have been reported in other exosomal secretion systems (Mittelbrunn et al., 2011; lo Cicero et al., 2015). To verify whether exosomes are the key to melanocytes affecting fibroblasts, eventually leading to keloid formation by activating the TGF-β pathway, we carried out further investigation. Exosomes obtained by differential ultracentrifugation of the melanocyte supernatant were resuspended in phosphate-buffered saline (PBS) to obtain an exosome suspension, and the supernatant obtained in the last step of exosome purification was the culture supernatant depleted of melanocyte exosomes. We found that melanocyte-derived exosome suspension could upregulate the TGF-β/Smads transduction levels of fibroblasts, promote collagen synthesis, and prevent collagen degradation by inhibiting the secretion of matrix metalloproteinases (MMPs) (Figs. 2d and 2e), whereas the culture supernatants depleted of melanocyte exosomes had no such effect, thereby excluding the role of soluble molecules. Therefore, we speculated that melanocytes could act on adjacent fibroblasts through exosomes and the TGF-β signaling pathway to regulate the occurrence and development of keloids.
Fig. 2. Activation of the transforming growth factor-β (TGF-β)/Smads pathway by melanocyte-derived exosomes in fibroblasts. (a) Transmission electron microscopy (TEM) observation of a keloid tissue in vivo and exosomes from the melanocyte culture supernatant through differential centrifugation. The yellow arrows and box indicate exosomes of 30‒150 nm size with a saucer-like structure. (b) Western blot showing exosomal characteristic proteins from the melanocyte culture supernatant through differential centrifugation. (c) Redistribution of exosomes in melanocytes. The exosomes (marked by CD63) were originally distributed around the nucleus in melanocytes. The distance of CD63+ compartments from the center of the corresponding nucleus was quantified in melanocytes in mono- or co-culture with fibroblasts (bottom panel). Tubulin is a cytoskeleton protein; ★ indicates the melanocyte nucleus. After co-culture with fibroblasts, the melanocyte-derived exosomes migrate to the side near the fibroblasts toward the contact site of cells (indicated by arrow). MC: melanocyte; FB: fibroblast. (d) Comparison of the type I collagen (COL-1), phosphorylated Smad2 (p-Smad2), and matrix metalloproteinase 9 (MMP9) protein contents in fibroblasts among normal culture group (control), exosome group (EXOs), and exosome-depleted culture supernatant group (medium without EXOs) by western blot. (e) Changes of TGF-β/Smads transduction levels of fibroblasts in the control, EXOs, and medium without EXOs by polymerase chain reaction (PCR). Smurf1: Smad specific E3 ubiquitin protein ligase 1. Data are expressed as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01, *** P<0.001.
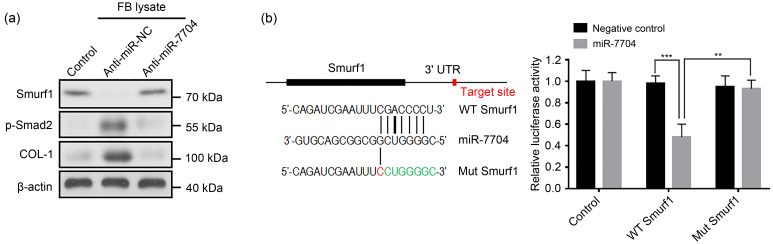
Exosomes can carry microRNAs (miRNAs) to achieve the intracellular transfer of their own RNAs; most miRNAs that play a role in circulation take exosomes as carriers (Thomou et al., 2017). For example, macrophage exosomes deliver miR-223 to ovarian cancer cells to induce chemotherapy resistance (Zhu et al., 2019), and bone marrow mesenchymal stem cell exosomes transport miR-29b-3p to regulate aging-related insulin resistance (Su et al., 2019). Melanocyte activity is enhanced by ultraviolet B (UVB) irradiation. In our previous study, RNA sequencing showed that 15 miRNAs levels were higher in UVB-irradiated melanocyte-derived exosomes than in the non-irradiated melanocyte-derived exosomes (Shen et al., 2020). Among them, miR-7704, miR-320a-3p, miR-423-5p, miR-375-3p, and miR-1246 were predicted to target multiple molecules in the TGF-β/Smads signaling pathway, including Smad-specific E3 ubiquitin protein ligase 1 (Smurf1), bone morphogenetic protein receptor type 1a (Bmpr1a), Smad9, repulsive guidance molecule A (Rgma), bone morphogenetic protein 8b (Bmp8b), Bmpr1b, gremlin 2 (Grem2), and cysteine-rich transmembrane BMP regulator 1 (Crim1). In addition, miR-423-5p (Feng et al., 2019), miR-375-3p (Yang et al., 2019), and miR-1246 (Liu et al., 2019) were reported to be closely related to fibrosis. Notably, miR-7704 is the top upregulated miRNA among the five selected ones, of which the predicted target Smurf1 is one of the key negative regulators of the TGF-β/Smads pathway, which has not been reported in the current literature. Smurf proteins (Smurf1/2) can recruit Smad7 to the plasma membrane, thus inhibiting the phosphorylation of Smad2/3 and downregulating the TGF-β pathway. We found that Smurf1, as a key negative regulator, was downregulated by both melanocyte supernatant and exosome suspension (Figs. 1d and 2e). Moreover, melanocyte-derived exosomes obtained after transfection with anti-miR-7704 into melanocytes no longer upregulated the TGF-β/Smads transduction levels and collagen synthesis by fibroblasts (Fig. 3a). The dual-luciferase reporter assay verified the target relationship between miR-7704 and Smurf1 in fibroblasts (Fig. 3b). The results indicated that melanocyte exosomes may secrete miR-7704 to target and inhibit Smurf1 in fibroblasts, thereby achieving the regulation of the TGF-β/Smads pathway.
Fig. 3. Inhibition of Smurf1 in fibroblasts by melanocyte-derived exosomal miR-7704. (a) The effects of melanocyte-derived exosomes obtained after transfection with anti-miR-7704 on Smad-specific E3 ubiquitin protein ligase 1 (Smurf1), type I collagen (COL-1), and phosphorylated Smad2 (p-Smad2) expression levels in fibroblasts by western blot. (b) Activity of the luciferase gene linked to the 3' untranslated region (UTR) of Smurf1 and the schematic representation of miR-7704 target sequence with the 3' UTR of Smurf1. FB: fibroblast; WT: wild type; Mut: mutation. Data are expressed as mean±standard deviation (SD), n=3. ** P<0.01, *** P<0.001.
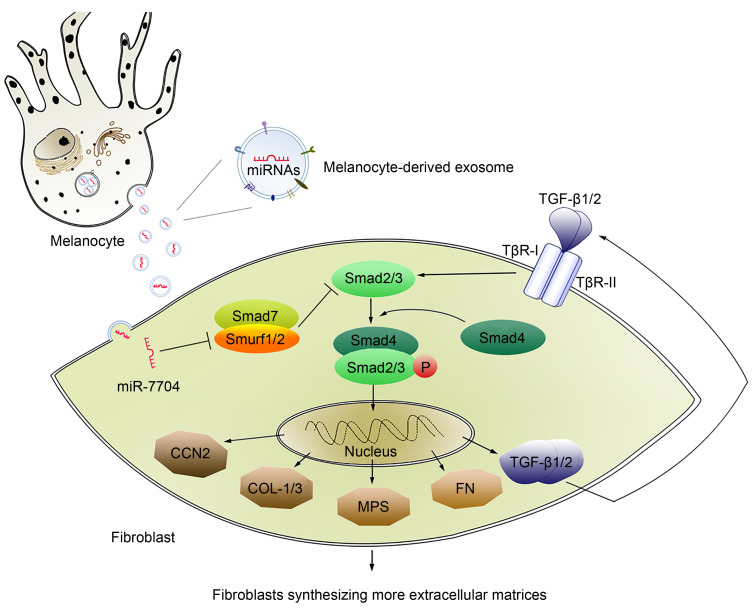
This study demonstrated that melanocyte-derived exosomal miR-7704 promotes keloid formation by inhibiting Smurf1 in fibroblasts and activating the TGF-β/Smads pathway (Fig. 4). Our findings not only collectively highlight a novel mode of communication between melanocytes and fibroblasts, attributing the novel function for exosomes to the regulation of keloid formation, but also contribute to revealing the biological nature of keloids prevalent in dark-skinned people from the microenvironmental perspective.
Fig. 4. Role of exosomal miR-7704 in the crosstalk between melanocytes and fibroblasts. CCN2: cellular communication network factor 2, also known as connective tissue growth factor (CTGF); COL-1/3: type I/III collagen; FN: fibronectin; miRNA: microRNA; MPS: mucopolysaccharide; P: phosphorylation; Smurf 1/2: Smad-specific E3 ubiquitin protein ligase 1/2; TGF-β: transforming growth factor-β; TβR-I: TGF-β type I receptor.
Our preliminary study provides data on the function of melanocytes of releasing exosomes during the formation of keloids. All changes in this process, especially the mechanism of exosomes and exosomal miR-7704, need to be further studied. Given this innovative discovery, we look forward to further breakthroughs in this field, as the reassessment of crosstalk between melanocytes and fibroblasts is expected to provide new opportunities for the prevention and treatment of scars and keloids.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
This work is supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ22H150005) and the National Natural Science Foundation of China (No. 81873937).
Author contributions
Jiaqi SUN performed the experimental research and data analysis. Zeren SHEN designed the experiments and wrote this article. Jinjin SHAO and Jinghong XU gave some good suggestions on the revision of the manuscript, polished English, and checked the final version. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Zeren SHEN, Jinjin SHAO, Jiaqi SUN, and Jinghong XU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this article.
References
- Chen C, Liu JM, Luo YP, 2020. MicroRNAs in tumor immunity: functional regulation in tumor-associated macrophages. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(1): 12-28. 10.1631/jzus.B1900452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Gallegos D, Jiang DS, Christ S, et al. , 2019. Patch repair of deep wounds by mobilized fascia. Nature, 576(7786): 287-292. 10.1038/s41586-019-1794-y [DOI] [PubMed] [Google Scholar]
- Feng MH, Li JW, Sun HT, et al. , 2019. Sulforaphane inhibits the activation of hepatic stellate cell by miRNA-423-5p targeting suppressor of fused. Hum Cell, 32(4): 403-410. 10.1007/s13577-019-00264-2 [DOI] [PubMed] [Google Scholar]
- Finnson KW, McLean S, di Guglielmo GM, et al. , 2013. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Adv Wound Care (New Rochelle), 2(5): 195-214. 10.1089/wound.2013.0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FL, Jin R, Zhang L, et al. , 2013. The contribution of melanocytes to pathological scar formation during wound healing. Int J Clin Exp Med, 6(7): 609-613. [PMC free article] [PubMed] [Google Scholar]
- Geng H, Zhou QC, Guo WH, et al. , 2021. Exosomes in bladder cancer: novel biomarkers and targets. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(5): 341-347. 10.1631/jzus.B2000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRanger R, Karimpour-Fard A, Costa C, et al. , 2019. Analysis of keloid response to 5-fluorouracil treatment and long-term prevention of keloid recurrence. Plast Reconstr Surg, 143(2): 490-494. 10.1097/prs.0000000000005257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Liao YW, Hsieh PL, et al. , 2019. miR-1246 as a therapeutic target in oral submucosa fibrosis pathogenesis. J Formos Med Assoc, 118(7): 1093-1098. 10.1016/j.jfma.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Liu CY, Chen SX, Zhang HF, et al. , 2021. Bioinformatic analysis for potential biological processes and key targets of heart failure-related stroke. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(9): 718-732. 10.1631/jzus.B2000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- lo Cicero A, Delevoye C, Gilles-Marsens F, et al. , 2015. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun, 6: 7506. 10.1038/ncomms8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. , 2011. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun, 2: 282. 10.1038/ncomms1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W, 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol, 200(4): 373-383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell WB, Cohen IK, Ehrlich HP, 1989. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg, 84(5): 827-837. [DOI] [PubMed] [Google Scholar]
- Shen ZR, Sun JQ, Shao JJ, et al. , 2020. Ultraviolet B irradiation enhances the secretion of exosomes by human primary melanocytes and changes their exosomal miRNA profile. PLoS ONE, 15(8): e0237023. 10.1371/journal.pone.0237023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KX, Liu S, Zhang MZ, et al. , 2018. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 19(11): 853-862. 10.1631/jzus.B1800132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Xiao YZ, Xiao Y, et al. , 2019. Bone marrow mesenchymal stem cells-derived exosomal miR-29b-3p regulates aging-associated insulin resistance. ACS Nano, 13(2): 2450-2462. 10.1021/acsnano.8b09375 [DOI] [PubMed] [Google Scholar]
- Thomou T, Mori MA, Dreyfuss JM, et al. , 2017. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature, 542(7642): 450-455. 10.1038/nature21365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YW, Ren JH, Xia K, et al. , 2012. Effect of mitomycin on normal dermal fibroblast and HaCat cell: an in vitro study. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 13(12): 997-1005. 10.1631/jzus.B1200055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YZ, Zhao XJ, Xu HJ, et al. , 2019. Magnesium isoglycyrrhizinate ameliorates high fructose-induced liver fibrosis in rat by increasing miR-375-3p to suppress JAK2/STAT3 pathway and TGF-β1/Smad signaling. Acta Pharmacol Sin, 40(7): 879-894. 10.1038/s41401-018-0194-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XL, Shen HL, Yin XM, et al. , 2019. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res, 38: 81. 10.1186/s13046-019-1095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.