Abstract
Cerebral microvascular disease has been reported as a central feature of the neurological disorders in patients with SARS-CoV-2 infection that may be associated with an increased risk of ischemic stroke. The main pathomechanism in the development of cerebrovascular injury due to SARS-CoV-2 infection can be a consequence of endothelial cell dysfunction as a structural part of the blood-brain barrier (BBB), which may be accompanied by increased inflammatory response and thrombocytopenia along with blood coagulation disorders. In this review, we described the properties of the BBB, the neurotropism behavior of SARS-CoV-2, and the possible mechanisms of damage to the CNS microvascular upon SARS-CoV-2 infection.
Keywords: SARS-CoV-2, Neurological disorders, Cerebral microvascular, Blood-brain barrier, Inflammatory response, Neurotropism
Graphical Abstract
1. Introduction
In December 2019, several cases of pneumonia with an unknown etiology were reported in Wuhan, China [1]. The disease was later named as coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [2]. COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly around the world and sparked widespread concern [3], [4]. Although the initial clinical sign of COVID-19 was pneumonia, several reports also described that the pathogenesis of the virus can be accompanied by neurological complications in patients with COVID-19, which confirms the neurotropism behavior of this respiratory virus [5], [6], [7]. In this regard, increasing evidence from clinical reports indicates that vascular endothelium and leukocyte migration through the blood-brain barrier (BBB) are important pathways for the neuro-invasion of SARS-CoV-2 [8]. It has been shown that angiotensin-converting enzyme 2 (ACE2), which is potentiated with several cofactors such as neuropilin-1 (NRP-1), is involved in the SARS-CoV-2 entry into the microvascular endothelial cells [9], [10], [11]. Although several SARS-CoV-2 vaccines are currently being developed, new variants of SARS-CoV-2 make this fact that we will still be involved with the COVID-19 pandemic [12]. In this case, understanding the pathophysiological mechanisms of this virus is an important key to creating a significant strategy for effective treatment of SARS-CoV-2 infection. Generally, most direct evidence of cerebral microvascular dysfunction in COVID-19 derives from humans, whereas data from in vitro and animal models is relatively limited. Therefore, it is essential to have a clear view of the microvascular and BBB function to understand the pathological mechanism of COVID-19 in the CNS.
2. The CNS microvascular and the BBB
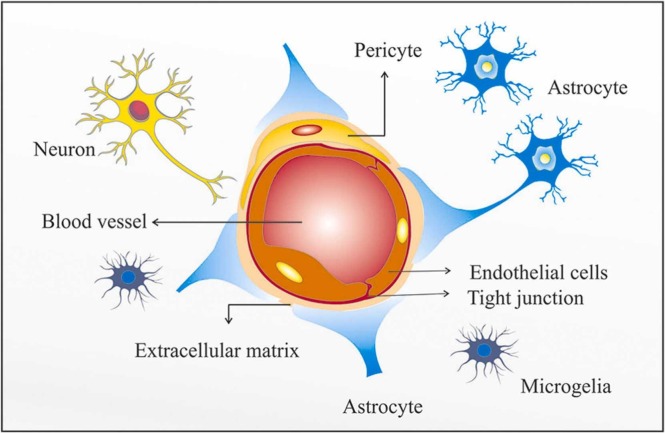
The cerebral microvascular network is made up of the arterioles, capillaries, and venules, which have a diameter of about 1 mm. However, it is necessary to consider that the term “microvascular” mainly refers to the capillaries that constitute the extensive surface area of the central nervous system (CNS) microvascular [13], [14], [15], [16]. In particular, the cerebral capillaries with their special features display the differential functions in the brain, such as regulating the influx and efflux of ions and nutrients among the walls between the circulation and the CNS, and sustaining the BBB [17], [18], [19]. The BBB is a dynamic system and demonstrates the important properties of the cerebral microvascular. This structure acts as a selective barrier to maintain the neuroparenchymal microenvironment and regulate molecular trafficking in the brain [20], [21]. Actually, three cell types involved in the construction of the capillaries are endothelial cells (ECs), pericytes, and astrocytic end-feet, and critical interactions between these cells are remarkable and important for representing the molecular mechanisms involved in the BBB function [17], [18] ( Fig. 1). The endothelium is known for a wide variety of its critical roles in all aspects of vascular homeostasis and pathophysiological processes such as inflammation or thrombosis [22], [23]. ECs are simple squamous epithelial cells that arise from the mesoderm and create a single layer which surrounds the capillary lumen [16], [24]. The tight junctions (TJ) seal the intercellular space among adjacent ECs and form a high-resistance paracellular barrier to the transport of hydrophilic compounds between the blood and brain and preserve cell polarity across the apical and basal domains of the ECs [15], [20], [25], [26]. While strong evidence emphasizes the important role of microvascular ECs in the BBB, pericytes are necessary for regulating brain endothelial hemostasis [27]. In the brain, pericytes may act as the first line of immunologic defense by expression of macrophage scavenger receptors, as well as cell phagocytosis [28], [29], [30]. In the capillary bed, astrocytes have antiviral effects against many neurotropic pathogens targeting the CNS. It has been shown that astrocytes perform this action via expressing several subgroups of pattern recognition receptors (PRRs) and differential activation of antiviral signaling pathways, for example, type I interferon (IFN) signaling, as well as restricting virus replication in the CNS [31], [32], [33]. However, the CNS is protected against pathogens via strong immune responses and the specialized structure of the BBB. But in certain situations, several respiratory viruses can infect the CNS and induce inflammatory injury in the brain. The Nipah (NiV) and Hendra viruses (HeV) are the pathogens which cause severe respiratory illness and also result in encephalitis via moving into CNS parenchymal cells in animals and humans [34], [35]. The SARS-CoV-2 is another case of these respiratory viruses that can target the CNS and cause a variety of neurological damage.
Fig. 1.
The CNS microvascular and the blood-brain barrier (BBB).
3. SARS-Cov-2 infection
Coronaviruses (CoVs) belong to the order Nidovirales and the family Coronaviridae and subfamily Coronavirinae. Phylogenetically, Coronaviridae are grouped into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus [36], [37]. The coronavirus is a significant pathogen that leads to respiratory and enteric illness in both humans and animals [38], [39]. In this case, it has been suggested that beta genera of coronaviruses may periodically predominate in human societies due to cross-species infections and cause severe disease [3], [40]. Severe acute respiratory syndrome (SARS)-CoV and the Middle East respiratory syndrome (MERS)-CoV are two important strains of the beta-CoVs which previously caused a global pandemic via their special features, such as a wide range of genomic variability and a high potential for genetic recombination [38], [39]. Interestingly, SARS-CoV-2 is a new betacoronavirus family that causes infection of the respiratory tract and is genetically similar to (SARS)-CoV and (MERS)-CoV [41]. It is an enveloped, positive-sense single-stranded RNA viruse (ss-RNA viruse) with a non-segmented genome of 30-kilobase (kb) length [42], [43], [44]. In addition, the four major structural proteins are encoded in the SARS-CoV-2 genome, i.e., membrane (M), envelope (E), nucleocapsid (N) and spike (S) proteins [44], [45]. In this case, the spike proteins (S) that are located on the external surface of the virus play a crucial role in an infection as it mediate virus entry into host cells with viral binding to the cell surface receptor and facilitate the membrane fusion [46], [47]. The findings obtained from the prior human coronavirus (SARS-CoV and MERS-CoV) showed that SARS-CoV-2 displays more enhanced pathogenicity for the lower respiratory tract and can cause acute respiratory distress syndrome (ARDS) as a life-threatening lung injury [48], [49]. The common signs reported in patients with COVID-19 are difficulty breathing, fever, dry cough, shortness of breath, muscle aches, and loss of smell and taste [42], [50]. Even though pneumonia, severe acute respiratory syndrome, and renal failure seem to be the most prominent symptoms in severe cases of COVID-19 infection, increasing evidence from clinical reports suggests that the pathogenesis of the virus can potentially involve the brain and nervous system [51], [52]. In fact, SARS-CoV-2 may invade the CNS in the early steps of infection, which shows the neurotropic capabilities of it [51].
4. SARS-Cov-2 infection and CNS
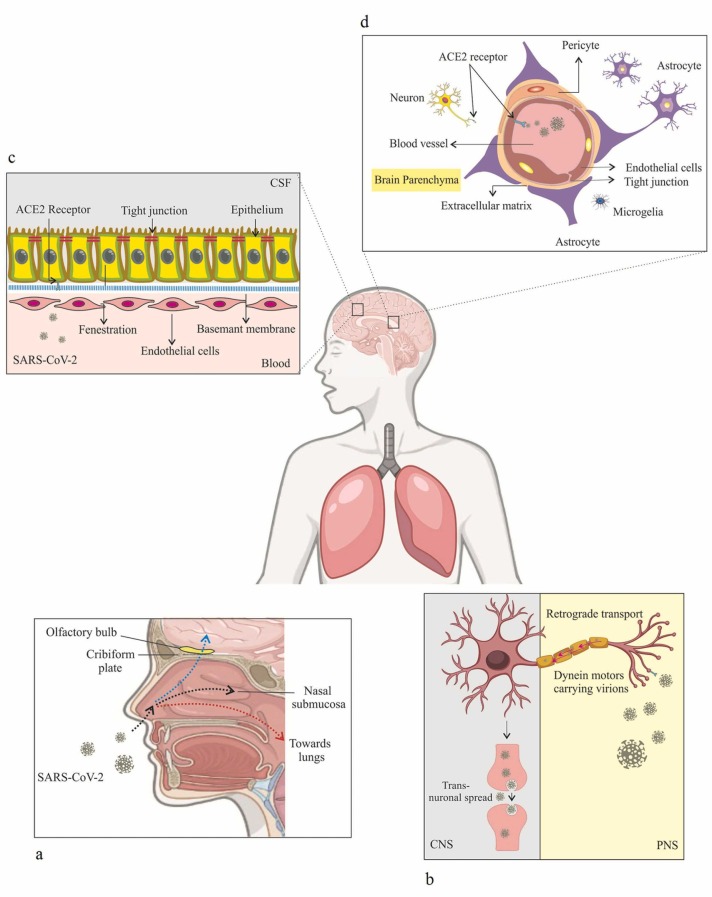
Multiple neurological symptoms such as loss of smell (anosmia), loss of coordination of movement (ataxia), headache, dizziness, increased vascular inflammation, encephalitis, and stroke have been observed following infection with SARS-CoV-2, which may indicate viral tropism for brain cells [52]. Recently, it has been reported that the SARS-CoV-2 genome was detected in the cerebrospinal fluid (CSF) and several reports suggest that respiratory viruses like SARS-Cov-2 may reach the CNS through various routes [53], [54]. The olfactory pathway is one of the strongest candidate routes that contains several nerves [55], [56]. In this case, a recent study on 417 COVID-19 patients revealed that olfactory dysfunction was observed among 85.6 % of patients [57]. This fact has shown that olfactory nerves provide a direct pathway for entry of Coronaviridae families into the brain. Olfactory nerves are bipolar neurons composed of dendrites and numerous unmyelinated or myelinated axons that link the nasal epithelium to different regions of the CNS [58], [59]. In fact, this pathway begins at the olfactory epithelium, which is located in the superior part of the nasal cavity and mainly ends at the olfactory bulb. Due to these anatomical features, viruses can infiltrate the brain by attaching to the nasal cavity and infecting the olfactory neurons [56] ( Fig. 2a). Furthermore, it has been proposed that other cranial nerves and hematogenous routes are two additional major pathways that may be responsible for virus penetration into neuronal cells. In the first route, the virus infects neurons in the periphery nervous system and undergoes retrograde axonal transport until it reaches the CNS [60], [61]. Local peripheral nerves which are localized in the gastrointestinal tract, vagal nerve afferents, and also trigeminal nerves that innervate the nasal cavity could be possible routes for this transmission of SARS-CoV-2 to the CNS [62] (Fig. 2b). In the hematogenous route, viruses can spread into the circulatory system and then from there reach the brain and thus infect epithelial cells of the blood-cerebrospinal fluid barrier in the choroid plexus or ECs of the BBB [38], [61], [63]. The three important mechanisms, which have been proposed to describe the spread of the virus through the BBB, are the Trojan horse model, paracellular migration, and transcellular migration [64]. During the Trojan horse mechanism, SARS-CoV-2 uses circulating immune cells (leukocytes and myeloid cells) as a viral source to diffuse into the CNS [62]. Recently Elena Percivalle et al. in an in vitro study demonstrated that the monocytes (MN) and monocyte-derived macrophages have been infected with the SARS-CoV-2 without the virus replication. They also suggested that these cells may act as “Trojan horses” and were able to transfer the viral infection throughout the body [65]. In paracellular migration, the virus passes through the BBB with the destruction of TJ complexes. During transcellular migration, it can be hypothesized that SARS-CoV-2 could infect the endothelial cells and then be transported across the BBB [64]. In the vascular endothelium, the human receptor ACE2 has been identified as the binding target receptor for SARS-CoV-2 and mediates virus entry into the cells [66] (Fig. 2c,d).
Fig. 2.
Proposed possible mechanisms of SARS-CoV-2 invasion into the CNS. a Olfactory route. b Retrograde route. c Blood-brain barrier route. d Hematogenous route following infection with coronavirus.
5. The significance of angiotensin-converting enzyme 2 (ACE2) in the CNS during SARS-CoV-2 infection
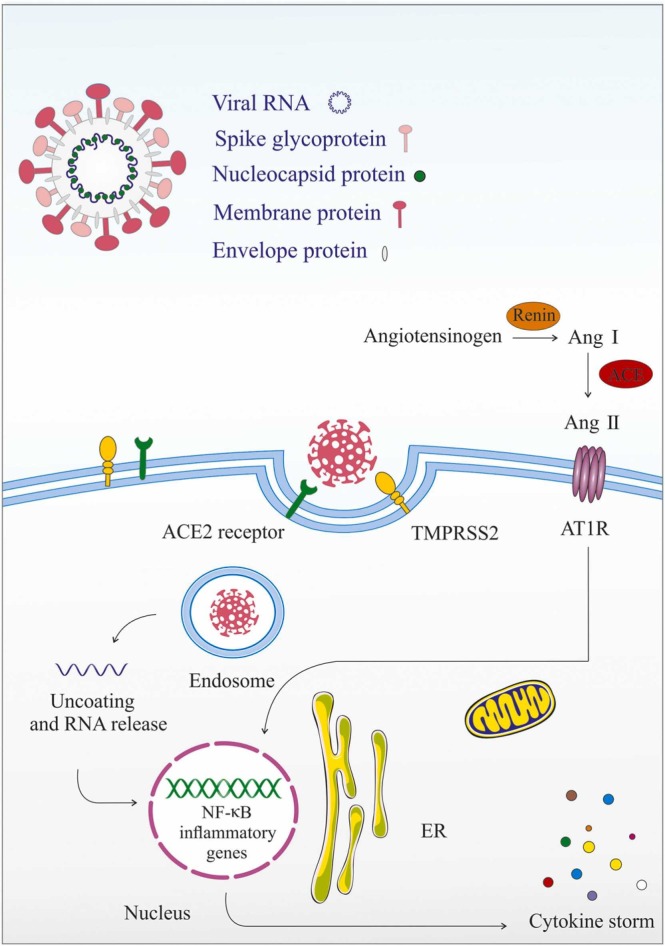
ACE2 is a transmembrane receptor with specific enzymatical properties and is expressed in epithelial cells of various human organs, including the lung, heart, kidney, intestine, brain, and also endothelium [67], [68], [69]. Studies have shown that this receptor promotes viral replication of SARS-CoV in several organs and confirmed the presence of human ACE2 in cerebrovascular endothelium [70]. It has been suggested that SARS-CoV-2, similar to the other CoVs, uses the ACE2 receptor for entry into host cells [71]. In the brain, the S-glycoproteins on the surface of the SARS-CoV-2 via van der Waals contacts bind to the enzymatic domain of the ACE2 receptor and attack the target cell via endocytosis or membrane fusion [10], [68], [72], [73]. The spike (S) protein of SARS-CoV-2 consists of S1 and S2 subunits. The S1 subunit has a primary role in adherence of the viral to the host cells [74], [75]. In this case, the receptor-binding domain (RBD) is a class-I membrane-fusion protein located at the S1 subunit and is accountable for the fusion of the ACE-2 receptor with viral membranes [76]. Following the interaction between RBD and ACE2, the spike protein is cleaved along its S1/S2 cleavage site by transmembrane serine protease 2 (TMPRSS2), a process which facilitates the membrane fusion [77], [78]. Furin has been proposed as another protease involved in this pattern, particularly when the low levels of TMPRSS2 present on the cell surface [64]. The results of an in vivo study illustrate a critical role for the furin cleavage site in COVID-19 infection and report that in the absence of this site, the replication of SARS-CoV-2 can be attenuated [79]. In addition, recently, it has been suggested that neuropilin-1 (NRP1) is another cofactor, which can facilitate the interaction of SARS-CoV-2 spike protein S1 subunit with ACE2 and promote infection into cells [80], [81], [82]. However, despite the presence of proteases to regulate the process of membrane fusion, the virus usually prefers to enter the cell through endocytosis [77]. Ultimately, the SARS-CoV-2 virus gains entry into the host cell cytoplasm and the sub-genomic RNA transcription of the virus begins [78]. Because ACE2 regulates several roles of the renin-angiotensin-aldosterone system (RAAS), it is to be expected that activation of ACE2 in turn may result in dysfunction of the RAAS, which can contribute to the pathogenesis of SARS-CoV-2 [78], [83]. The RAAS system includes enzymatic cascades that stimulate the conversion of angiotensinogen into angiotensin I. This process is then completed via the conversion of Ang-I to Ang-II by the action of ACE. Although Ang-II signaling through its receptor AT1R mediates several physiological processes, it also has the potential to contribute to the pathogenesis of detrimental conditions such as enhancing inflammation [78]. Furthermore, ACE2, a homolog of ACE, cleaves angiotensin II into the angiotensin fragments 1–7, which bind to its Mas receptor (MasR) and can act as an effector substance in the RAS system via the decrease of hypertension and the inhibition of inflammation [84], [85], [86], [87]. Although in normal physiological status, the activities of the ACE/Ang II/AT1R axis and the ACE2/Ang-(1−7)/Mas receptor axis are in a dynamic equilibrium condition, upon the infection of SARS-CoV-2, they can also be disturbed [88]. Relatedly, internalization of the ACE2-binding SARS-CoV-2 by endocytosis into the host cell downregulates the ACE2 on the cell surface and promotes a significant inflammatory response through the overactivation of the ACE/Ang II/AT1R pathway and the deactivation of the ACE2/Ang-(1−7)/Mas-R pathway [88], [89]. This is generally gained by activation of the NF-κB pathway, which causes to an enhancement in the formation of proinflammatory cytokines [78]. ( Fig. 3). Surprisingly, it has been suggested that integrin may have a key role in SARS-CoV-2 transmission into cells.
Fig. 3.
The possible interaction between Angiotensin-converting enzyme 2 (ACE2) and SARS-CoV-2 during CNS infection.
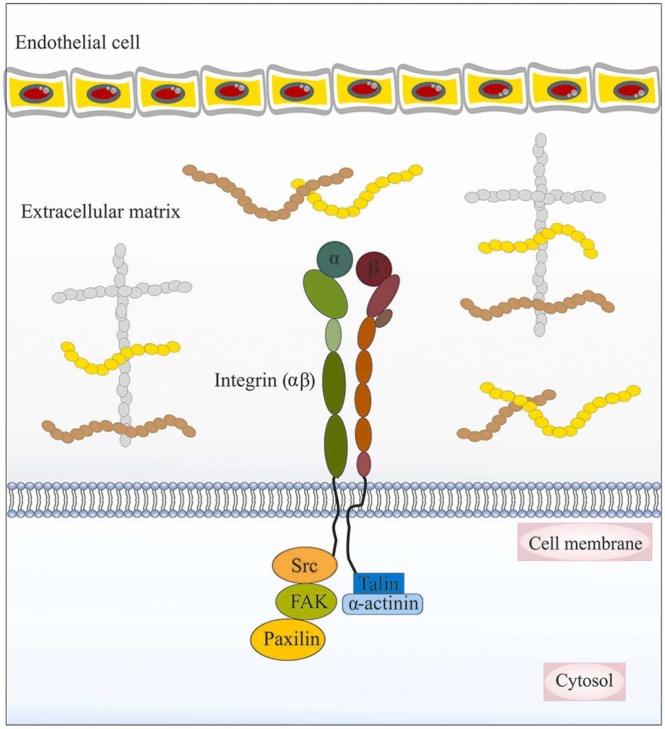
6. Integrin’s role of in SARS-CoV-2 virus transmission
Integrins are an important family of cell surface adhesion receptors that belong to a group of heterodimeric transmembrane receptors. They are made up of noncovalently linked α and β subunits (18 different α and 8 different β subunits) and are important regulators of cell adhesion and migration [90], [91], [92] ( Fig. 4). Several studies have reported that various pathogens can enter cells through binding to integrins. Hussein et al. proposed an important role for the Arg-Gly-Asp acid (RGD) motif, which is known for its role in helping the virus to infect the host cells via linking to integrin subunits including αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, α5β1, α8β1 and αIIbβ3 [93]. The precise role of integrin in SARS-CoV-2 transmission to cells is unknown. Recently, Sigrist et al. suggested a model that SARS-CoV-2 may use integrins to reach the cells, through an integrin-binding RGD motif as mentioned above. SARS-CoV-2 can attach to the surface of target cells through binding their spike protein to the enzymatic domain of the ACE2 receptor. They reported that the RGD motif is located adjacent to the surface of the spike protein of SARS-CoV-2 and subsequent ACE2 binding to the virus. A conformational change may expose the RGD motifs to integrin and, therefore, integrin via binding to RGD motifs may enhance the ability of virus transmission into host cells [94]. However, one study on a patient with SARS-CoV-2 who suffered from multiple sclerosis suggested that treatment with natalizumab as an antibody which blocks integrin α4 binding may have a protective role against this infection [95]. Furthermore, integrin β1 and β3 are two major subunits that can be involved in SARS-CoV-2 transmission [96], [97]. In this regard, the transmembrane glycoprotein neuropilin 1 (NRP1), which is localized at specific adhesion sites, has been considered as a receptor for SARS-CoV-2 infection and facilitates the endocytosis of activated α5β1 integrin into the cytoplasm [98]. The short linear motifs (SLiMs) are sequences known are recognized for their ability to activate of the NRP1, which can be found in the cytoplasmic tails of ACE2 or integrin β3, and can connect SARS-CoV-2 host cell receptors binding to endocytosis and autophagy mediators [99], [100]. In a recent study, Peter Simons et al. demonstrated that talin interaction with integrin β-subunit cytoplasmic tail (β-CTs) can lead to integrin extension and increase the binding affinity of the integrin ectodomain for SARS-CoV-2, thereby promoting viral entry into cells [98]. Therefore, the use of integrin antagonists might have a potential role as an antiviral drug against SARS-CoV-2, which needs further research [101]. Overall, some reports indicate that the cytotoxicity induced by the neurotropic behavior of the SARS-CoV-2 in ECs of cerebral microvascular can cause various neurological disorders and may have severe and fatal consequences [102], [103].
Fig. 4.
The association between integrins and the extracellular matrix (ECM) in the cerebral microvasculature.
7. Clinical manifestations of COVID-19 infection in the CNS microvascular system
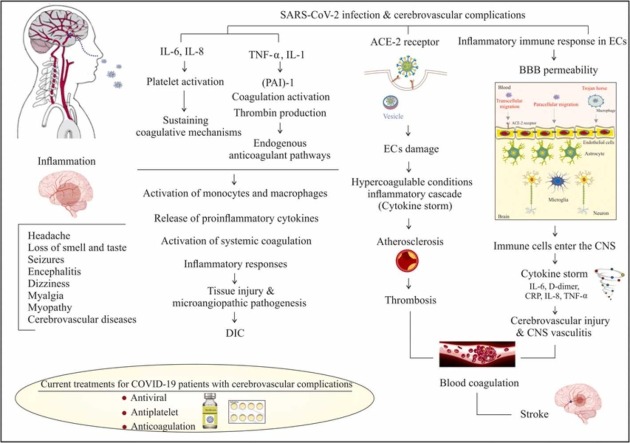
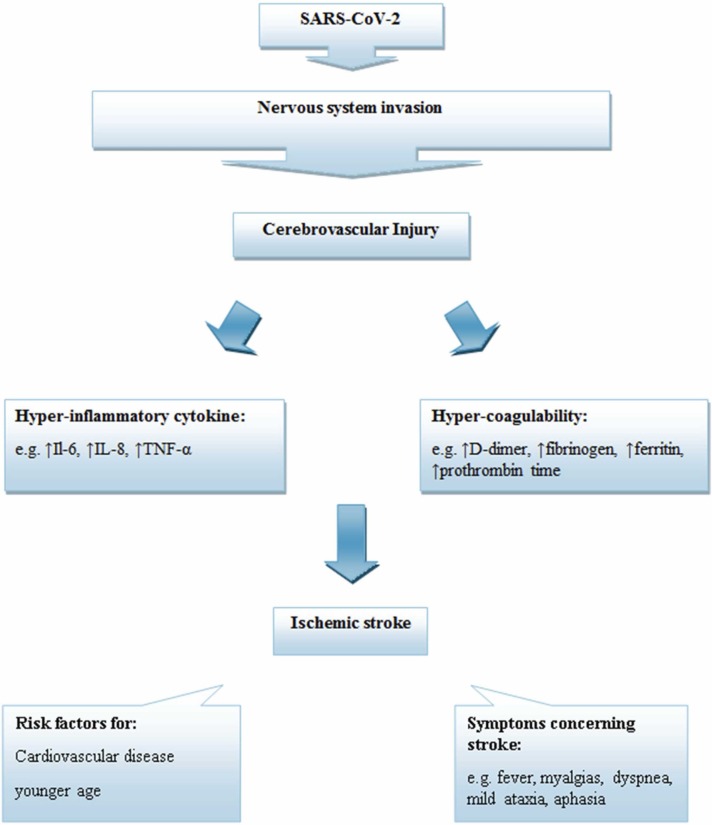
Since the first reports of neurologic manifestations of hospitalized patients with COVID-19 [7], numerous studies have focused on identifying these injuries. One study on 841 patients hospitalized with COVID-19 has described that up to 57.4 % of patients suffered from some neurological complications, which were considered to be the main cause of patient death in 4.1 % of all cases. These neurological complications were associated with some symptoms, including disorders of consciousness (19.6 %), myalgia (17.2 %), headache (14.1 %), dizziness (6.1 %), anosmia (4.9 %), dysgeusia (6.2 %), myopathy (3.1 %), dysautonomia (2.5 %), cerebrovascular diseases (1.7 %), seizures (0.7 %), movement disorders (0.7 %), etc. They also reported cerebrovascular disorders associated with SARS-CoV-2 in 11 (1.3 %) patients suffering from ischemic stroke, and 3 (0.4 %) patients having intracranial hemorrhage [104]. Albeit, it should be noted that new clinical observations have reported that, in addition to ischemic stroke and intracranial hemorrhage, cerebral venous sinus thrombosis and posterior reversible encephalopathy syndrome are other cerebrovascular complications associated with SARS-CoV-2 in the pandemic [105], [106]. In a retrospective observational cohort study of 14,483 hospitalized patients with SARS-CoV-2, it has been demonstrated that acute cerebrovascular events (CVEs) were observed in 1.13 % of all patients (1.08 % acute ischemic stroke, 0.19 % intracranial hemorrhages and 0.02 % cortical vein and/or sinus thrombosis). About 40.5 % of patients who suffered from CVE were female, and 55.8 % of them were between the ages of 60 and 79 years old. The author also reported that during hospitalization, the mortality rate for COVID-19 patients with acute stroke or intracranial hemorrhages was 38.1 % and 58.3 %, respectively [107]. However, it appears that patients with hypertension, diabetes mellitus, hyperlipidemia, smoking, coronary artery disease, or previous stroke history have a higher risk of developing cerebrovascular disorders [108], [109]. In a case series including 219 patients with COVID-19 infection from Wuhan, it was reported that acute cerebrovascular disease (CVD) was found in 11 (5.0 %) patients (90.9 % ischemic stroke), while the elderly patients compared to younger subjects represented a high potential for CVD. Furthermore, they reported a high level of D-dimer as a hypercoagulation marker, as well as elevated level of several inflammatory factors in these patients [110]. In fact, most studies have suggested that the main pathomechanism in the development of cerebrovascular diseases during COVID-19 infection are increased inflammatory markers, cytokine storm and thrombocytopenia as well as coagulation disorders, which may be associated with a fundamentally increased risk of ischemic stroke [7], [111].
7.1. Inflammation
During the SARS-CoV-2 infection, increased levels of inflammatory immune response are a main pathway that could involve ECs as a structural part of the BBB and therefore lead to increased BBB permeability. Following the BBB break down, immune cells can enter the CNS and initiate significant inflammatory processes associated with cytokine storms, which can cause several pathological conditions and increase the severity of COVID-19 [112], [113]. An interesting recent study reported that low-level expression of ACE2 was detected in human ECs. Therefore, via increasing evidence, it has been proposed that other CNS perivascular cells may have an implication in mediating the inflammatory response in cerebrovascular disorders associated with SARS-CoV-2 infection [114], [115], [116]. In line with this evidence, pericyte are one type of perivascular cells that has high level expression of ACE2 and plays a major role in immune response and maintenance of the BBB. Due to SARS-CoV-2 infection, a malfunction in the immunoregulatory role of pericytes can account for increased NF-κB phosphorylation, production of several cytokine and chemokines such as stromal cell-derived factor (SDF)− 1, interleukin (IL)18, plasminogen activator inhibitor (PAI)− 1, and macrophage migration inhibitory factor (MIF), and may eventually lead to leukocyte trafficking into vascular tissue [114]. Clinical evidence of brain inflammation during SARS-CoV-2 infection confirmed that the majority of patients with neurological conditions associated with SARS-CoV-2 had elevated levels of inflammatory markers including IL-6, D-dimer, and C-reactive protein (CRP) [117], [118]. High levels of IL-6 can be considered as the predominant trigger for the cytokine storm phenomenon associated with increased levels of numerous pro-inflammatory factors such as interleukin (IL)− 8 and TNF-α, and may have a fundamental role in the enhanced risk of incident stroke in those patients who suffered from COVID-19 [119], [120], [121], [122]. Interestingly, several antibodies, which were produced against SARS-CoV-2 accompanied the infected immune cells’ passage into brain parenchyma. These immune cells and the targeting of the EC antigens by antibodies can result in disrupting the endothelial barrier function as well as cerebral edema or autoimmune disorder [10], [112], [123]. This inflammatory response against SARS-CoV-2 infection might have contributed to the elevation of plasma D-dimer levels. However, as will be explained later, D-dimer reflects a hypercoagulable state, and it has been suggested that patients with D-dimer levels greater than 2.0 µg/ml have a higher risk of death with COVID-19 [124]. Interestingly, the lymphocyte counts are lower in patients with CNS symptoms caused by SARS-CoV-2 (especially in the severe subgroup) compared to patients without CNS symptoms. This phenomenon led support to the hypothesis that immunosuppression can occur in patients with COVID-19 with CNS symptoms [125]. In fact, several studies have indicated that lymphopenia is a hallmark of SARS-CoV-2 infection severity and can mainly be related to T and B cell apoptosis [126], [127]. However, the inflammatory disorder associated with SARS-CoV-2 infection may have a key role in different CNS manifestations. Recently, it has been reported that Kawasaki syndrome, which is a systemic vasculitis of children, can be caused by SARS-CoV-2 infection and may be involved in the triggering of acute encephalopathy complications [128], [129]. Moreover, the cerebrovascular system is another important part of the CNS that is affected by inflammation caused by SARS-CoV-2. It has been shown that during SARS-CoV-2 infection, neuroinflammation has a high potential to contribute to the pathogenesis of vascular coagulation and stroke.
7.2. SARS-CoV-2 and coagulation disorders
ECs via several components such as glycosaminoglycans (GAGs) and thrombomodulin (TM) have powerful anticoagulant and antifibrinolytic routes. However this function during several pathological conditions may be destroyed [130], [131]. A hypercoagulable condition occurs following dysfunction of endothelial cells due to infection that may be accompanied by blocked fibrinolysis and elevated thrombin formation [132]. New evidence of laboratory findings of cerebrovascular disorder associated with SARS-CoV-2 has shown increased values of coagulopathy factors including D-dimer, fibrinogen, ferritin, platelet count, prothrombin time, partial thromboplastin time, and antiphospholipid antibody [133], [134]. However, D-dimer is a more important coagulopathy marker, which may become significantly higher in severe patients with SARS-CoV-2 compared to non-severe [134]. In a study of 191 patients with SARS-CoV-2 reported, D-dimer levels higher than 1 μg/ml could be attributed to increased rates of mortality [135]. A D-dimer is generated from cross-linked fibrin breakdown by plasmin in the final steps of clot construction and acts as a marker of endogenous fibrinolysis [134], [136], [137]. Following endothelial cell damage, increased D-dimer levels often occur, with a consequent increase in inflammation and coagulation in SARS-CoV-2 infection. It has been suggested that greater elevations in D-dimer levels have been associated with more severe inflammatory responses and mortality [138], [139]. Increased concentrations of cytokines, such as TNF-α and IL-1, for example, in severe SARS-CoV-2, can mediate the suppression of endogenous anticoagulant pathways, such as upregulation of plasminogen activator inhibitor (PAI)− 1, as well as coagulation activation and thrombin production [140], [141]. In addition, it has been identified that pro-inflammatory cytokines such as IL-6 and IL-8 via platelet activation could be involved in the sustainment of coagulative mechanisms [142]. In the SARS-CoV-2 cases, elevated serum levels of ferritin have been reported, which can also act as a marker for inflammatory responses. Hyperferritinemia by participating in antiphospholipid syndrome is linked to thrombosis and contributes to the coagulopathy disorder caused by SARS-CoV-2 [143], [144], [145]. Interestingly, hypoxia can regulate thrombus and therefore result in coagulation through the activation of several pathways. In hypo-oxygenation, enhanced expression of hypoxia-inducible factors (HIFs) can result in promoting thrombogenesis via targeting several genes, which are involved in the regulation of blood coagulation, such as plasminogen activator inhibitor (PAI). Hypoxia-responsive signaling pathways that can stimulate thrombus formation through induction of TNF-α and interleukin (IL) 1 are also involved in the development of the coagulation process during hypoxia conditions [146]. Recently, it has been reported that disseminated intravascular coagulation (DIC) disease is an important outcome in patients with critical conditions due to SARS-CoV-2 infection and is associated with high patient mortality [147], [148], [149]. In patients with severe illness (DIC disease), infectious complications are typically associated with high activation of systemic coagulation, inflammatory responses, activation of monocytes and macrophages, and stimulation of inflammatory response and release of proinflammatory cytokines, which can result in tissue injury and microangiopathic pathogenesis [137], [148]. On the other hand, several studies have reported that stroke via brain damage is the main outcome of the coagulation disorder associated with SARS-CoV-2 [149], [150], [151].
8. SARS-CoV-2 and coagulation disorders and stroke complication
Multiple population health studies have been published on patients with stroke as a main feature of the COVID-19 and suggest that the risk incidence of stroke during this pandemic is around 4.67 %, which is mostly present in younger patients [130], [152], [153]. Interestingly, COVID-19-related ischemic strokes are far more common than hemorrhagic strokes, and most of those patients who suffered from ischemic stroke needed critical care support [130], [154]. One study demonstrated that ischemic stroke usually occurs within about 2 weeks after SARS-CoV-2 infection and the risk of ischemic stroke was strongly correlated with the incidence of cardiovascular disease risk factors [118], [155]. In a multicenter retrospective cohort study of 216 COVID-19 patients with acute ischemic stroke, it was observed that 68.1 % of these patients were older than 60 years, and the mortality at discharge was approximately 39.1 %. They also reported that the three comorbidities in ischemic stroke patients associated with SARS-CoV-2 infection were hypertension (80.0 %), hyperlipidemia (47.8 %) and diabetes mellitus (47.6 %) [156]. In another study among 1875 patients, 50 patients with a history of ischemic stroke were identified. Those patients with a history of stroke had higher neutrophil levels, cardiac troponin I, D-dimers levels, NT pro-brain natriuretic peptide, IL-6, and lower lymphocyte and platelet counts in contrast to those without a history of stroke [157]. On the other hand, it has been demonstrated that the majority of patients with ischemic strokes have a hypercoagulability state induced by SARS-CoV-2 infection [157], [158]. Other markers that indicate coagulopathy in patients with COVID-19 infection, in addition to D-dimer levels, include a slight increase in prothrombin-time (PT) activity, international normalized ratio (INR), partial thromboplastin time (PTT), platelet counts, thrombocytopenia, elevated lactate dehydrogenase (LDH), and fibrinogen [159], [160], [161]. Interestingly, sometimes thrombotic events are present only in the CNS of patients with COVID-19-related ischemic strokes without any evidence to confirm that these events also involve other organs [162]. These studies also defined the various mechanisms for ischemic stroke and its complications, claiming that during SARS-CoV-2 infection, direct endothelial damage mediated by the ACE2 receptor initiates hypercoagulable conditions and inflammatory cascades (cytokine storm), both of which play a critical role in the induction of ischemic stroke [154]. On the other hand, activation of inflammatory pathways contributes to atherosclerosis and affects plaque stability, which acts as the best source of thrombosis [134], [163]. Furthermore, mitochondrial dysfunction due to enhanced the inflammatory and oxidative conditions might also lead to platelet destruction and an increased risk of death [164]. Consistent with these findings, several reports have been shown that patients with COVID-19-related ischemic stroke not only have developed large vessel occlusions but also small vessel occlusions can be associated with ischemic stroke injury, which often involves older patients [110], [165], [166]. These patients are often admitted to hospital with several symptoms concerning stroke, such as fever, myalgias, dyspnea, mild ataxia, aphasia, hemiparesis, and, unfortunately, many patients do not improve [134], [167], [168]. Unfortunately, with the progression of vaccination for SARS-CoV-2, very rare cases of vaccine-induced immune thrombotic thrombocytopenia (VITT) have been reported in COVID-19 patients. This syndrome, which is associated with thrombocytopenia, thromboembolic events, and antibodies against PF4, might present with ischemic stroke as an early symptom of it [169]. Therefore, it is essential to find the most effective form of treatment to cure the cerebrovascular disease associated with SARS-CoV-2 and thus ischemic stroke as a main outcome of these injuries ( Fig. 5).
Fig. 5.
Proposed mechanism of ischemic stroke due to SARS-CoV-2 invasion to CNS.
9. Current treatments for patients with cerebrovascular complications associated with COVID-19
Several kinds of antiviral drugs have exhibited good therapeutic effects against SARS-CoV-2 infection, but no very safe or efficacious drugs have been submitted for improvement in mortality [110], [170], [171]. However, many physicians have tried to improve some complications of SARS-CoV-2 infection via administering a number of different drugs, which has been successful in several cases. Table 1 presents the main drugs administered to the patients with cerebrovascular disease associated with COVID-19, with focusing on their antiviral, antiplatelet, and anticoagulation therapies.
Table 1.
Current treatments and their potential for improving patients with cerebrovascular disease following COVID-19.
| Category | Patient population and characteristics | Treatment by | Implications for Therapy | Ref. |
|---|---|---|---|---|
| Anticoagulation therapy | 6 patients with COVID-19-related ischemic strokes | low molecular weight heparin (LMWH), apixaban or intravenous thrombolysis | One mortality in patients who were treated with LMWH and thromboembolism was reduced in other patients | [172] |
| Anticoagulation therapy | 3 patients with COVID-19-related ischemic strokes | 1#: apixaban and angiography 2#: enoxaparin and angiography 3#: heparin infusion |
In all patients the thrombosis had resolved | [173] |
| Anticoagulation therapy | 3 patients with COVID-19-related Intracranial hemorrhage | Apixaban or enoxaparin and switched to therapeutic UFH | All patients had brain-dead | [174] |
| Anticoagulation therapy | One patient with COVID-19-related cerebral venous Sinus Thrombosis | LMWH followed by apixaban | The patient was discharged from the hospital | [175] |
| Anticoagulation therapy | 2 patients with COVID-19 related Intraparenchymal Hemorrhage | Combination of heparin and extracorporeal Membrane Oxygenation | Patients were comfortably extubated and expired after 20 days | [176] |
| Anti-inflammatory therapy | One patient with COVID-19-related ischemic strokes | Hydroxychloroquine, tocilizumab and methylprednisolone | The patient was admitted to the ICU. | [177] |
| Anti-inflammatory and anticoagulation therapy | One patient with covid-19 vaccine-induced immune thrombotic thrombocytopenia Related Ischemic Stroke | intravenous immunoglobulin, dexamethasone, argatroban followed by fondaparinux, warfarin | the patient clinically improved and platelet count returned to normal range. | [178] |
| Anti-inflammatory and anticoagulation therapy | One patient with covid-19 vaccine-induced immune thrombotic thrombocytopenia Related Ischemic Stroke | intravenous immunoglobulin, fondaparinux and heparin (In addition to thrombectomy) | Patient's state improved and discharged to a rehabilitation unit | [179] |
| Anti inflammatory, Antiviral and Antimicrobial therapy | Three COVID-19 patient with Posterior reversible encephalopathy syndrome | 1#: azithromycin, hydroxychloroquine, ceftriaxone and hydrocortisone 2#: hydroxychloroquine, methylprednisolone, ceftriaxone and azithromycin 3#: dexamethasone, Remdesivir, ceftriaxone and azithromycin |
All patients were discharged from hospital but in #1 the signs of visual dysfunction have been reminded | [180] |
| Antimicrobial and anticoagulation therapy | 2 patients with COVID-19-related ischemic strokes | 1#: rivaroxaban unfractionated heparin, hydroxychloroquine, and ceftriaxone 2#: ceftriaxone Hydroxychloroquine and apixaban |
1# was discharged from hospital after 17 days and 2# had deid (day 18) | [162] |
| Antimicrobial and anticoagulation therapy | 2 patients with COVID-19-related catastrophic intracranial hemorrhages | Combination of vancomycin and zosyn, following via heparin and sarilumab or ceftriaxone, azithromycin, plaquenil and heparin | All patients had died | [181] |
| Antiplatelet and anti inflammatory therapy | 4 patients with COVID-19-related acute ischemic strokes | Combination of Aspirin/low dose LMWH or Aspirin/Klopidogrel | Two patients were discharged and two patients were bedridden. | [167] |
| Anticoagulation, antiviral and anti inflammatory therapy | One patient with COVID-19-related ischemic strokes | Oseltamivir and ribavirin, moxifloxacin and dexamethasone as anti inflammatory drugs, clopidogrel and atorvastatin as anticoagulant drugs | Improved walking and talking in patient. After 12 days and patient was discharged | [182] |
| Antiplatelet,anticoagulation and anti hypertensive therapy | 6 patients with COVID-19-related ischemic strokes | Combination of aspirin, warfarin, enoxaparin, clopidogrel, ramipril, enalapril, hydrochlorothiazide, telmisartan, | After 14 days neurological conditions were incompletely improved in one patients who were treated with aspirin and warfarin but all other patients had died | [183] |
| Anticoagulation and antiplatelet therapy |
5 patients with COVID-19-related ischemic strokes | 1#: apixaban 2#: clot retrieval, apixaban 3#: clot retrieval, aspirin 4#: intravenous t-PA, clot retrieval, hemicraniectomy, aspirin 5# Clot retrieval, stent, aspirin, clopidogrel |
1# and 5# were discharged 2#: was discharged home 3#: was admitted to the ICU 4#: was admitted to stroke unit |
[184] |
| Antiplatelet and anticoagulation therapy | 10 Severe and non-severe patients with COVID-19-related ischemic strokes | Aspirin, clopidogrel and enoxaparin | 50 % mortality in treatment with aspirin or clopidogrel as compared with 25 % in patient treated with anticoagulant | [110] |
| Antidiabetic therapy | 42 COVID-19 patients with type 2 diabetes mellitus who presented with acute ischemic stroke | Metformin or glibenclamide and pioglitazone | Ferritin serum,CRP, LDH, and D-dimer serum levels were lower in metformin-treated patients | [185] |
| Anti-interleukin therapy | One COVID-19 patient with Posterior Reversible Encephalopathy Syndrome | Anakinra, tocilizumab | Patient had died | [186] |
| Anticoagulation, anti-interleukin anti-inflammatory and antiviral therapy | 19 patients with COVID-19-related hemorrhagic strokes | Different combination of warfarin, heparin, enoxaparin as anticoagulation and anakinra, tocilizumab as anti-interleukin and methylprednisone, lopinavir/ritonavir, hydroxychloroquine as anti-inflammatory and azithromycin, nitazoxanide as antiviral | Mortality rate at hospital discharge was 84.6 %. | [187] |
| Antihypertensive therapy | One COVID-19 patient with reversible cerebral vasoconstriction syndrome | verapamil | The complete recovery of the patient's functional was achieved following three months. | [188] |
| Anti-cholesterol, antiplatelet, anti-inflammatory, antihypertensive and antiviral thrapy | Three COVID-19 patients related to ischemic stroke | 1#: hydroxychloroquine, lopinavir/ ritonavir, azithromycin, intravenous immunoglobulin, clopidogrel, and atorvastatin 2#: hydroxychloroquine, lopinavir/ ritonavir, azithromycin, interferon beta-1a, clopidogrel and atorvastatin. 3#: aspirin, clopidogrel, carvedilol, digoxin, hydroxychloroquine, LPV/RTV, interferon beta-1a, and azithromycin |
1# had deid, 2# and 3# had no improvement of respiratory and neurological problems during hospitalization. | [189] |
| Antiviral, anti-inflammatory, Acid suppressive and vitamin Therapy | One COVID-19 patient related to ischemic stroke | Vitamin C, zinc, vitamin D, azithromycin, proton pump inhibitor (PPI), Dexamethason | the improvement of respiratory, neurological and hemorrhage symptoms were very satisfied | [190] |
10. Conclusion
We summarized recent findings about following SARS-CoV-2 infection, cerebrovascular endothelial damage initiates hypercoagulable conditions and inflammatory cascades, both of which play a role in the induction of ischemic stroke. In fact, ischemic stroke in the setting of COVID-19 is the main outcome of this infection, which is mostly present in younger patients, therefore it is essential to be more understood. It seems likely that the antiplatelet and anticoagulation drugs may be effective for the treatment of cerebrovascular disease, especially ischemic stroke related to COVID-19. This research has led to an increase in our knowledge of CNS microvascular diseases associated with SARS-CoV-2 and we suggest that prevention and treatment for the cerebrovascular damage could be important for the recovery of COVID-19 patients.
CRediT authorship contribution statement
Neda Omidian: Writing – original draft, Investigation, Methodology. Pantea Mohammadi: Software, Validation. Mona Sadeghalvad: Visualization, Writing – review & editing. Hamid-Reza Mohammadi-Motlagh: Conceptualization, Supervision, Data curation.
Conflict of Interest Statement
The authors certify that no actual or potential conflict of interest in relation to this study exists.
Acknowledgements
We thank the Vice Chancellor for Research & Technology of Kermanshah University of Medical Sciences (Kermanshah, Iran) for financial support for this study.
Data Availability
Data will be made available on request.
References
- 1.Bogoch I.I., Watts A., Thomas-Bachli A., Huber C., Kraemer M.U.G., Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J. Travel. Med. 2020;27(2):taaa008. doi: 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S., Shi Z., Shu Y., Song J., Gao G.F., Tan W., Guo D. A distinct name is needed for the new coronavirus. Lancet. 2020;395(10228):949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang Bo, Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus investigating and research team, a novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China medical treatment expert group for Covid-19, clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonardel C., Bonnerot M., Ludwig M., Vadot W., Beaune G., Chanzy B., Cornut L., Baysson H., Farines M., Combes I., Macheda G., Bing F. Bilateral posterior cerebral artery territory infarction in a SARS-Cov-2 infected patient: discussion about an unusual case. J. Stroke Cerebrovasc. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.1050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luan J., Lu Y., Gao S., Zhang L. A potential inhibitory role for integrin in the receptor targeting of SARS-CoV-2. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Xue Q., Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox. Res. 2020;38(1):1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karuppan M.K.M., Devadoss D., Nair M., Chand H.S., Lakshmana M.K. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism. Mol. Neurobiol. 2021;58(6):2465–2480. doi: 10.1007/s12035-020-02245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M., James W., Gordon S., Pepper M.S. SARS-CoV-2 variants, vaccines, and host immunity. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannistraro R.J., Badi M., Eidelman B.H., Dickson D.W., Middlebrooks E.H., Meschia J.F. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146–1156. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macdonald J.A., Murugesan N., Pachter J.S. Endothelial cell heterogeneity of blood‐brain barrier gene expression along the cerebral microvasculature. J. Neurosci. Res. 2010;88(7):1457–1474. doi: 10.1002/jnr.22316.17. [DOI] [PubMed] [Google Scholar]
- 15.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 16.Daneman R., Prat A. The blood–brain barrier. Cold. Spring Harb. Perspect. Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farkas E., Luiten P.G. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64(6):575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 18.Coomber B.L., Stewart P.A. Morphometric analysis of CNS microvascular endothelium. Microvasc. Res. 1985;30(1):99–115. doi: 10.1016/0026-2862(85)90042-1. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y.-M., Zhang C.-L., Chen A.-Q., Wang H.-L., Zhou Y.-F., Li Y.-N., Hu B. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.678744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolburg H., Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vasc. Pharm. 2002;38(6):323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 21.Takata F., Nakagawa S., Matsumoto J., Dohgu S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michiels C. Endothelial cell functions. J. Cell. Physiol. 2003;196(3):430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 23.Huang X., Hussain B., Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci. Ther. 2021;27(1):36–47. doi: 10.1111/cns.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aird W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 25.Wolburg H. Wiley-VCH. Verlag. GmbH & Co. KGaA; Weinheim. Germany: 2006. Blood-Brain Barriers-From Ontogeny to Artificial Interfaces. [Google Scholar]
- 26.Cardoso F.L., Kittel A., Veszelka S., Palmela I., Tóth A., Brites D., Deli M.A., Brito Maria A. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS. One. 2012;7(5) doi: 10.1371/journal.pone.0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J.A., Tran N.D., Li Z., Yang F., Zhou W., Fisher M.J. Brain endothelial hemostasis regulation by pericytes. J. Cereb. Blood. Flow. Metab. 2006;26(2):209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- 28.Thomas W.E. Brain macrophages: on the role of pericytes and perivascular cells. Brain. Res. Brain. Res. Rev. 1999;31(1):42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 29.Balabanov R., Dore‐Duffy P. Role of the CNS microvascular pericyte in the blood‐brain barrier. J. Neurosci. Res. 1998;53(6):637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Guillemin G.J., Brew B.J. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J. Leukoc. Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 31.Bansode Y.D., Chattopadhyay D., Saha B. Transcriptomic analysis of interferon response in toll-like receptor 2 ligand-treated and herpes simplex virus 1-infected neurons and astrocytes. Viral Immunol. 2021;34(4):256–266. doi: 10.1089/vim.2020.0238. [DOI] [PubMed] [Google Scholar]
- 32.Daniels B.P., Jujjavarapu H., Durrant D.M., Williams J.L., Green R.R., White J.P., Lazear H.M., Jr M.G., Diamond M.S., Klein R.S. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J. Clin. Invest. 2017;127(3):843–856. doi: 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindqvist R., Mundt F., Gilthorpe J.D., Wölfel S., Gekara N.O., Kröger A., Överby A.K. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflamm. 2016;13(1):277. doi: 10.1186/s12974-016-0748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escaffre O., Borisevich V., Carmical J.R., Prusak D., Prescott J., Feldmann H., Rockx B. Henipavirus pathogenesis in human respiratory epithelial cells. J. Virol. 2018;87(6):3284–3294. doi: 10.1128/JVI.02576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Amaya M., Addetia A., Dang H.V., Reggiano G., Yan L., Hickey A.C., DiMaio F., Broder C.C., Veesler D. Architecture and antigenicity of the Nipah virus attachment glycoprotein. Science. 2022;375(6587):1373–1378. doi: 10.1126/science.abm55612022;375(6587):1373-8. [DOI] [PubMed] [Google Scholar]
- 36.Mansourabadi A.H., Sadeghalvad M., Mohammadi-Motlagh H.-R., Rezaei N. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life. Sci. 2020;258 doi: 10.1016/j.lfs.2020.118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alquisiras-Burgos I., Peralta-Arrieta I., Alonso-Palomares L.A., Zacapala-Gómez A.E., Salmerón-Bárcenas E.G., Aguilera P. Neurological complications associated with the blood-brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Mol. Neurobiol. 2021;58(2):520–535. doi: 10.1007/s12035-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennisi M., Lanza G., Falzone L., Fisicaro F., Ferri R., Bella R. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int. J. Mol. Sci. 2020;21(15):5475. doi: 10.3390/ijms21155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozma M.A., Maroufi P., Khodadadi E., Köse Ş., Esposito I., Ganbarov K., Dao S., Esposito S., Dal T., Zeinalzadeh E., Kafil H.S. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez. Med. 2020;28(2):153–165. [PubMed] [Google Scholar]
- 40.Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M., James W., Gordon S., Pepper M.S. SARS-CoV-2 variants, vaccines, and host immunity. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannan S., Ali P.S.S., Sheeza A., Hemalatha K. COVID-19 (Novel Coronavirus 2019)-recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4):2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 42.Vallamkondu J., John A., Wani W.Y., Ramadevi S.P., Jella K.K., Reddy P.H., Kandimalla R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim. Biophys. Acta Mol. Basis. Dis. 2020;1866(10) doi: 10.1016/j.bbadis.2020.165889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Li J.Z. SARS-CoV-2 Virology. Infect. Dis. Clin. North. Am. 2022;36(2):251–265. doi: 10.1016/j.idc.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Troyano-Hernáez P., Reinosa R., Holguín Á. Evolution of SARS-CoV-2 envelope, membrane, nucleocapsid, and spike structural proteins from the beginning of the pandemic to september 2020: a global and regional approach by epidemiological week. Viruses. 2021;13(2):243. doi: 10.3390/v13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng W., Xiang Y., Wu L., Chen Z., Li Q., Chen J., Guo Y., Xia D., Chen N., Zhang L., Zhu S., Zhao K.-N. Nucleocapsid protein of SARS-CoV-2 is a potential target for developing new generation of vaccine. J. Clin. Lab. 2022;36(6) doi: 10.1002/jcla.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27(6):951–961. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes Q., Inchakalody V.P., Merhi M., Mestiri S., Taib N., El-Ella D.M.A., Bedhiafi T., Raza A., Al-Zaidan L., Mohsen M.O., Al-Nesf M.A.Y., Hssain A.A., Yassine H.M., Bachmann M.F., Uddin S., Dermime S. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022;54(1):524–540. doi: 10.1080/07853890.2022.2031274.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swenson K.E., Swenson E.R. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit. Care. Clin. 2021;37(4):749–776. doi: 10.1016/j.ccc.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rofail D., McGale N., Podolanczuk A.J., Rams A., Przydzial K., Sivapalasingam S., Mastey V., Marquis P. Patient experience of symptoms and impacts of COVID-19: a qualitative investigation with symptomatic outpatients. B. M. J. Open. 2022;12(5) doi: 10.1136/bmjopen-2021-055989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karuppan M.K.M., Devadoss D., Nair M., Chand H.S., Lakshmana M.K. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanis. Mol. Neurobiol. 2021;58(6):2465–2480. doi: 10.1007/s12035-020-02245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukiw W.J., Pogue A., Hill J.M. SARS-CoV-2 infectivity and neurological targets in the brain. Cell. Mol. Neurobiol. 2022;42(1):217–224. doi: 10.1007/s10571-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viszlayová D., Sojka M., Dobrodenková S., Szabó S., Bilec O., Turzová M., Ďurina J., Baloghová B., Borbély Z., Kršák M. SARS-CoV-2 RNA in the cerebrospinal fluid of a patient with long COVID. Ther. Adv. Infect. Dis. 2021;8 doi: 10.1177/20499361211048572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galea M., Agius M., Vassallo N. Neurological manifestations and pathogenic mechanisms of COVID-19. Neurol. Res. 2022;44(7):571–582. doi: 10.1080/01616412.2021.2024732. [DOI] [PubMed] [Google Scholar]
- 56.DosSantos M.F., Devalle S., Aran V., Capra D., Roque N.R., Coelho-Aguiar Jd.M., Spohr T.E., Subilhaga J.G., Pereira C.M., Meira I.D., Filho P.N.S., Moura-Neto V. Neuromechanisms of SARS-CoV-2: a review. Front. Neuroanat. 2020;14:37. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechien J.R., Chiesa-Estomba C.M., Siati D.R.D., Horoi M., Bon S.D.L., Rodriguez A., Dequanter D., Blecic S., Afia F.E., Distinguin L., Chekkoury-Idrissi Y., Hans S., Lopez Delgado I., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., Filippis C.D., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koyuncu O.O., Hogue I.B., Enquist L.W. Virus infections in the nervous system. Cell. Host. Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) J. Med. Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desforges M., Coupanec A.L., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Virus. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lima M., Siokas V., Aloizou A.-M., Liampas I., Mentis A.-F.A., Tsouris Z., Papadimitriou A., Mitsias P.D., Tsatsakis A., Bogdanos D.P., Baloyannis S.J., Dardiotis E. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr. Treat. Options Neurol. 2020;22(11):37. doi: 10.1007/s11940-020-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Chen X., Jia L., Zhang Y. Potential mechanism of SARS-CoV-2-associated central and peripheral nervous system impairment. Acta Neurol. Scand. 2022 doi: 10.1111/ane.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achar A., Ghosh C. COVID-19-Associated neurological disorders: the potential route of CNS invasion and blood-brain barrier relevance. Cells. 2020;9(11):2360. doi: 10.3390/cells9112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Percivalle E., Sammartino J.C., Cassaniti I., Arbustini E., Urtis M., Smirnova A., Concardi M., Belgiovine C., Ferrari A., Lilleri D., Piralla A., Baldanti F. Macrophages and monocytes: "Trojan Horses" in COVID-19. Viruses. 2021;13(11):2178. doi: 10.3390/v13112178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lukiw W.J., Pogue A., Hil J.M. SARS-CoV-2 infectivity and neurological targets in the brain. Cell. Mol. Neurobiol. 2022;42(1):217–224. doi: 10.1007/s10571-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caillet-Saguy C., Wolff N. PDZ-containing proteins targeted by the ACE2 receptor. Viruses. 2021;13(11):2281. doi: 10.3390/v13112281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., Goor Hv. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. Acs. Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 72.(a) Lukiw W.J., Pogue A., Hill J.M. SARS-CoV-2 infectivity and neurological targets in the brain. Cell. Mol. Neurobiol. 2022;42(1):217–224. doi: 10.1007/s10571-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Khan S., Gomes J. Neuropathogenesis of SARS-CoV-2 infection. ELife. 2020;9 doi: 10.7554/eLife.59136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo Li, Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grzesiak J., Fellner L., Grünewald K., Kölbl C., Walter A., Horlacher R., Duschek F. Fluorescence signatures of SARS-CoV-2 spike S1 proteins and a human ACE-2: excitation-emission maps and fluorescence lifetimes. J. Biomed. Opt. 2022;27(5) doi: 10.1117/1.JBO.27.5.050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y., Yang C., Xu X.-F., Xu W., Liu S.-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fenrich M., Mrdenovic S., Balog M., Tomic S., Zjalic M., Roncevic A., Mandic D., Debeljak Z., Heffer M. SARS-CoV-2 dissemination through peripheral nerves explains multiple organ injury. Front. Cell. Neurosci. 2020;14:229. doi: 10.3389/fncel.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haidar M.A., Shakkour Z., Reslan M.A., Al-Haj N., Chamoun P., Habashy K., Kaafarani H., Shahjouei S., Farran S.H., Shaito A., Saba E.S., Badran B., Sabra M., Kobeissy F., Bizri M. SARS-CoV-2 involvement in central nervous system tissue damage. Neural Regen. Res. 2022;17(6):1228–1239. doi: 10.4103/1673-5374.327323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson B.A., Xie X., Bailey A.L., Kalveram B., Lokugamage K.G., Muruato A., Zou J., Zhang X., Juelich T., Smith J.K., Zhang L., Bopp N., Schindewolf C., Vu M., Vanderheiden A., Winkler E.S., Swetnam D., Plante J.A., Aguilar P., Plante K.S., Popov V., Lee B., Weaver S.C., Suthar M.S., Routh A.L., Ren P., Ku Z., An Z., Debbink K., Diamond M.S., Shi P.-Y., Freiberg A.N., Menachery V.D. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature. 2021;591(7849):293–299. doi: 10.1038/s41586-021-03237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simonetti B., Daly J.L., Simón-Gracia L., Klein K., Weeratunga S., Antón-Plágaro C., Tobi A., Hodgson L., Lewis P.A., Heesom K.J., Shoemark D.K., Davidson A.D., Collins B.M., Teesalu T., Yamauchi Y., Cullen P.J. ESCPE-1 mediates retrograde endosomal sorting of the SARS-CoV-2 host factor Neuropilin-1. Proc. Natl. Acad. Sci. U. S. A. 2022;119(25) doi: 10.1073/pnas.2201980119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., v.d. Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius Ari, Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Y.Yamauchi Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alharthy A., Faqihi F., Memish Z.A., Karakitsos D. Fragile endothelium and brain dysregulated neurochemical activity in COVID-19. ACS Chem. Neurosci. 2020;11(15):2159–2162. doi: 10.1021/acschemneuro.0c00437. [DOI] [PubMed] [Google Scholar]
- 84.Abiodun O.A., Ola M.S. Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J. Biol. Sci. 2020;27(3):905–912. doi: 10.1016/j.sjbs.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mali S.N., Thorat B.R., Chopade A.R. A viewpoint on angiotensin-converting enzyme 2, anti-hypertensives and coronavirus disease 2019 (COVID-19) Infect. Disord. Drug. Targets. 2021;21(3):311–313. doi: 10.2174/1871526520666200511005546. [DOI] [PubMed] [Google Scholar]
- 86.Santos R.A. Angiotensin-(1–7) Hypertension. 2014;63(6):1138–1147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 87.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98(3):1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furuhashi M., Moniwa N., Takizawa H., Ura N., Shimamoto K. Potential differential effects of renin-angiotensin system inhibitors on SARS-CoV-2 infection and lung injury in COVID-19. Hypertens. Res. 2020;43(8):837–840. doi: 10.1038/s41440-020-0478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zangbar H.S., Gorji A., Ghadiri T. A review on the neurological manifestations of covid-19 infection: a mechanistic view. Mol. Neurobiol. 2021;58(2):536–549. doi: 10.1007/s12035-020-02149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clegg D.O., Wingerd K.L., Hikita S.T., Tolhurst E.C. Integrins in the development, function and dysfunction of the nervous system. Front. Biosci. 2003;8:d723–d750. doi: 10.2741/1020. [DOI] [PubMed] [Google Scholar]
- 91.Hynes R.O. Cell adhesion: old and new questions. Trends Cell. Biol. 1999;9(12):33–37. [PubMed] [Google Scholar]
- 92.Fagerholm S.C., Hilden T.J., Gahmberg C.G. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends Biochem. Sci. 2004;29(9):504–512. doi: 10.1016/j.tibs.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Hussein H.A.M., Walker L.R., Abdel-Raouf U.M., Desouky S.A., Montasser A.K.M., Akula S.M. Beyond RGD: virus interactions with integrins. Arch. Virol. 2015;160(11):2669–2681. doi: 10.1007/s00705-015-2579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sigrist C.J., Bridge A., Mercier P.L. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aguirre C., Meca-Lallana V., Barrios-Blandino A., Río B.Del, Vivancos J. Covid-19 in a patient with multiple sclerosis treated with natalizumab: May the blockade of integrins have a protective role? Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nader D., Fletcher N., Curley G.F., Kerrigan S.W. SARS-CoV-2 uses major endothelial integrin αvβ3 to cause vascular dysregulation in-vitro during COVID-19. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park E.J., Myint P.K., Appiah M.G., Darkwah S., Caidengbate S., Ito A., Matsuo E., Kawamoto E., Gaowa A., Shimaoka M. The spike glycoprotein of SARS-CoV-2 binds to β1 integrins expressed on the surface of lung epithelial cells. Viruses. 2021;13(4):645. doi: 10.3390/v13040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simons P., Rinaldi D.A., Bondu V., Kell A.M., Bradfute S., Lidke D.S., Buranda T. Integrin activation is an essential component of SARS-CoV-2 infection. Sci. Rep. 2021;11(1):20398. doi: 10.1038/s41598-021-99893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kliche J., Kuss H., Ali M., Ivarsson Y. Cytoplasmic short linear motifs in ACE2 and integrin β3 link SARS-CoV-2 host cell receptors to mediators of endocytosis and autophagy. Sci. Signal. 2021;14(665):eabf1117. doi: 10.1126/scisignal.abf1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mészáros B., Sámano-Sánchez H., Alvarado-Valverde J., Čalyševa J., Martínez-Pérez E., Alves R., Shields D.C., Kumar M., Rippmann F., Chemes L.B., Gibson T.J. Short linear motif candidates in the cell entry system used by SARS-CoV-2 and their potential therapeutic implications. Sci. Signal. 2021;14(665):eabd0334. doi: 10.1126/scisignal.abd0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Makowski L., Olson-Sidford W., W-Weisel J. Biological and clinical consequences of integrin binding via a rogue RGD motif in the SARS CoV-2 spike protein. Viruses. 2021;13(2):146. doi: 10.3390/v13020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Kuraishy H.M., Al-Gareeb A.I., Monteiro M.C., Al-Saiddy H.J. Brain injury and SARS-CoV-2 infection: Bidirectional pathways. Curr. Med. Drug. Res. 2020;4:207. [Google Scholar]
- 103.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., Razmpour R., Hale J.F., Galie P.A., Potula R., Andrews A.M., Ramirez S.H. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Valle-Pérez J.A.D., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ioan P., Ribigan A.C., Rusu O., Bratu I.F., Badea R.S., Antochi F. Posterior reversible encephalopathy syndrome - a pathology that should not be overlooked in the era of COVID-19. Am. J. Emerg. Med. 2022;56:393.e5–393.e8. doi: 10.1016/j.ajem.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaur I., Vyas C., Mughal M., Gandhi H., Du D. Cerebral venous sinus thrombosis in COVID-19: an unusual presentation. Cureus. 2021;13(3) doi: 10.7759/cureus.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siegler J.E., Cardona P., Arenillas J.F., Talavera B., Guillen A.N., Chavarría-Miranda A., de Lera M., Khandelwal P., Bach I., Patel P., Singla A., Requena M., Ribo M., Jillella D.V., Rangaraju S., Nogueira R.G., Haussen D.C., Vazquez A.R., Urra X., Chamorro Á., Román L.S., Thon J.M., Then R., Sanborn E., Ossa N.Pdl, Millàn M., Ruiz I.N., Mansour O.Y., Megahed M., Tiu C., Terecoasa E.O., Radu R.A., Nguyen T.N., Curiale G., Kaliaev A., Czap A.L., Sebaugh J., Zha A.M., Liebeskind D.S., Ortega-Gutierrez S., Farooqui M., Hassan A.E., Preston L., Patterson M.S., Bushnaq S., Zaidat O., Jovin T.G. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int. J. Stroke. 2021;16(4):437–447. doi: 10.1177/1747493020959216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nannoni S., Groot Rd, Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int. J. Stroke. 2021;16(2):137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munhoz R.P., Pedroso J.L., Nascimento F.A., Almeida S.Md, Barsottini O.G.P., Cardoso F.E.C., Teive H.A. Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arq. Neuropsiquiatr. 2020;78(5):290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- 110.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., Wang D., Mao L., Jin H., Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc. Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020;70(9):311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 112.Alexopoulos H., Magira E., Bitzogli K., Kafasi N., Vlachoyiannopoulos P., Tzioufas A., Kotanidou A., Dalakas M.C. Anti–SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(6) doi: 10.1212/NXI.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., Zhang S., Cao T., Yang C., Li M., Guo G., Chen X., Chen Y., Lei M., Liu H., Zhao J., Peng P., Wang C.-Y., Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khaddaj-Mallat R., Aldib N., Bernard M., Paquette A.-S., Ferreira A., Lecordier S., Saghatelyan A., Flamand L., ElAli A. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via spike protein. Neurobiol. Dis. 2021;161 doi: 10.1016/j.nbd.2021.105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muus C., Luecken M.D., Eraslan G., Sikkema L., Waghray A., Heimberg G., Kobayashi Y., Vaishnav E.D., Subramanian A., Smillie C., Jagadeesh K.A., Duong E.T., Fiskin E., Triglia E.T., Ansari M., Cai P., Lin B., Buchanan J., Chen S., Shu J., Haber A.L., Chung H., Montoro D.T., Adams T., Aliee H., Allon S.J., Andrusivova Z., Angelidis I., Ashenberg O., Bassler K., Bécavin C., Benhar I., Bergenstråhle J., Bergenstråhle L., Bolt L., Braun E., Bui L.T., Callori S., Chaffin M., et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 2021;27(3):546–559. doi: 10.1038/s41591-020-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bocci M., Oudenaarden C., Sàenz-Sardà X., Simrén J., Edén A., Sjölund J., Möller C., Gisslén M., Zetterberg H., Englund E., Pietras K. Infection of Brain Pericytes Underlying Neuropathology of COVID-19 Patients. Int. J. Mol. Sci. 2021;22(21):11622. doi: 10.3390/ijms222111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nori S., Moran A., Franolich J., Patel J., Stern M. COVID-19 and neurologic manifestations: an experience at major New York city hospitals. Cureus. 2022;14(4) doi: 10.7759/cureus.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radmard S., Epstein S.E., Roeder H.J., Michalak A.J., Shapiro S.D., Boehme A., Wilson T.J., Duran J.C., Bain J.M., Willey J.Z., Thakur K.T. Inpatient neurology consultations during the onset of the SARS-CoV-2 New York City pandemic: a single center case series. Front. Neurol. 2020;11:805. doi: 10.3389/fneur.2020.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ronconi G., Teté G., Kritas S.K., Gallenga C.E., Caraffa A., Ross R., Conti P. COVID-19 induced by SARS-CoV-2 causes Kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2020;34(3):767–773. doi: 10.23812/EDITORIAL-RONCONI-E-59. [DOI] [PubMed] [Google Scholar]
- 120.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;12 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reddy S.T., Garg T., Shah C., Nascimento F.A., Imran R., Kan P., Bowry R., Gonzales N., Barreto A., Kumar A., Volpi J., Misra V., Chiu D., Gadhia R., Savitz S.I. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep. Neurol. 2020;12(2):199–209. doi: 10.1159/000508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. COVID-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann. Neurol. 2020;88(4):860–861. doi: 10.1002/ana.25855. [DOI] [PubMed] [Google Scholar]
- 123.Bodro M., Compta Y., Llansó L., Esteller D., Doncel-Moriano A., Mesa A., Rodríguez A., Sarto J., Martínez-Hernandez E., Vlagea A., Egri N., Filella X., Morales-Ruiz M., Yagüe J., Soriano Á., Graus F., García F. “Hospital Clínic Infecto-COVID-19” and “Hospital Clínic Neuro-COVID-19” groups, Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2–associated encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J. Thromb. Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mao L., Wang M., Chen S., He Q., Chang J., Hong C., Zhou Y., David Wang, Li Y., Jin H., Hu B. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv. 2020 doi: 10.1101/2020.02.22.20026500. [DOI] [Google Scholar]
- 126.André S., Picard M., Cezar R., Roux-Dalvai F., Alleaume-Butaux A., Soundaramourty C., Cruz A.S., Mendes-Frias A., Gotti C., Leclercq M., Nicolas A., Tauzin A., Carvalho A., Capela C., Pedrosa J., Castro A.G., Kundura L., Loubet P., Sotto A., Muller L., Lefrant J.-Y., Roger C., Claret P.-G., Duvnjak S., Tran T.-A., Racine G., Zghidi-Abouzid O., Nioche P., Silvestre R., Droit A., Mammano F., Corbeau P., Estaquier J. T cell apoptosis characterizes severe Covid-19 disease. Cell Death Differ. 2022;29(8):1486–1499. doi: 10.1038/s41418-022-00936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cizmecioglu A., Cizmecioglu H.A., Goktepe M.H., Emsen A., Korkmaz C., Tasbent F.E., Colkesen F., Artac H. Apoptosis-induced T-cell lymphopenia is related to COVID-19 severity. J. Med. Virol. 2021;93(5):2867–2874. doi: 10.1002/jmv.26742. [DOI] [PubMed] [Google Scholar]
- 128.Esper F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Association between a novel human coronavirus and Kawasaki disease. J. Infect. Dis. 2005;191(4):499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tabarki B., Mahdhaoui A., Selmi H., Yacoub M., Essoussi A.S. Kawasaki disease with predominant central nervous system involvement. Pediatr. Neurol. 2001;25(3):239–241. doi: 10.1016/s0887-8994(01)00290-9. [DOI] [PubMed] [Google Scholar]
- 130.Jin R.C., Voetsch B., Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 2005;12(3):247–258. doi: 10.1016/s0887-8994(01)00290-9. [DOI] [PubMed] [Google Scholar]
- 131.Esmon C.T. Coagulation inhibitors in inflammation. Biochem. Soc. Trans. 2005;33(Pt 2):401–405. doi: 10.1042/BST0330401. [DOI] [PubMed] [Google Scholar]
- 132.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV. J. Thromb. Thrombolysis. 2021;51(4):1107–1110. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hong L.-Z., Shou Z.-X., Zheng D.-M., Jin X. The most important biomarker associated with coagulation and inflammation among COVID-19 patients. Mol. Cell. Biochem. 2021;476(7):2877–2885. doi: 10.1007/s11010-021-04122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goldberg M.F., Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular disease in COVID-19. AJNR Am. J. Neuroradiol. 2020;41(7):1170–1172. doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou F., Yu T., Du R., Fan G., Liu Yi, Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao Bin. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rizal S., Joshi B.R., Regmi S. Raised D-dimer level among COVID-19 patients in a tertiary care hospital: a descriptive cross-sectional study. JNMA J. Nepal. Med. Assoc. 2022;60(247):259–262. doi: 10.31729/jnma.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wells P.S., Anderson D.R., Rodger M., Forgie M., Kearon C., Dreyer J., Kovacs G., Mitchell Michael, Lewandowski Bernard, Kovacs Michael J. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 2003;349(13):1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 138.Nemec H.M., Ferenczy A., Christie B.D., 3rd, Ashley D.W., Montgomery A. Correlation of D-dimer and outcomes in COVID-19 patients. Am. Surg. 2022 doi: 10.1177/00031348221091940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cesari M., Pahor M., Incalzi R.A. Plasminogen activator inhibitor‐1 (PAI‐1): a key factor linking fibrinolysis and age‐related subclinical and clinical conditions. Cardiovasc. Ther. 2010;28(5):e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vidali S., Morosetti D., Cossu E., Luisi M.L.E., Pancani S., Semeraro V., Consales G. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open. Res. 2020;6(2) doi: 10.1183/23120541.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rosário C., Zandman-Goddard G., Meyron-Holtz E.G., D'Cruz D.P., Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nayer A., Ortega L.M. Catastrophic antiphospholipid syndrome: a clinical review. J. Nephropathol. 2014;3(1):9–17. doi: 10.12860/jnp.2014.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17):e38.146. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gupta N., Zhao Y.-Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 147.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lillicrap D. Disseminated intravascular coagulation in patients with 2019–nCoV pneumonia. J. Thromb. Haemost. 2020;18(4):786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Soliman S., Ghaly M. Ischemic stroke and bilateral pulmonary embolism in COVID-19: COVID-associated coagulopathy or heparin-induced thrombocytopenia. J. Hematol. 2022;11(1):40–44. doi: 10.14740/jh956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Biembengut Í.V., de Souza Td.A.C. Coagulation modifiers targeting SARS-CoV-2 main protease Mpro for COVID-19 treatment: an in silico approach. Mem. Inst. Oswaldo. Cruz. 2020;115 doi: 10.1590/0074-02760200179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aggarwal G., Lippi G., Henry B.M. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int. J. Stroke. 2020;15(4):385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 153.Kakarla V., Kaneko N., Nour M., Khatibi K., Elahi F., Liebeskind D.S., Hinman J.D. Pathophysiologic mechanisms of cerebral endotheliopathy and stroke due to SARS-CoV. J. Cereb. Blood Flow Metab. 2021;41(6):1179–1192. doi: 10.1177/0271678X20985666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spence J.D., de Freitas G.R., Pettigrew L.C., Ay H., Liebeskind D.S., Kase C.S., Brutto O.H.D., Hankey G.J., Venketasubramanian N. Mechanisms of stroke in COVID-19. Cereb. Dis. 2020;49(4):451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou Y., Li W., Wang D., Mao L., Jin H., Li Y., Hong C., Chen S., Chang J., He Q., Wang M., Hu B. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc. Neurol. 2020;5(2):177–179. doi: 10.1136/svn-2020-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dmytriw A.A., Dibas M., Phan K., Efendizade A., Ospel J., Schirmer C., Settecase F., Heran M.K.S., Kühn A.L., Puri A.S., Menon B.K., Sivakumar S., Mowla A., Vela-Duarte D., Linfante I., Dabus G.C., Regenhardt R.W., D'Amato S., Rosenthal J.A., Zha A., Talukder N., Sheth S.A., Hassan A.E., Cooke D.L., Leung L.Y., Malek A.M., Voetsch B., Sehgal S., Wakhloo A.K., Goyal M., Wu H., Cohen J., Ghozy S., Turkel-Parella D., Farooq Z., Vranic J.E., Rabinov J.D., et al. North American Neurovascular COVID-19 (NAN-C) Consortium & Society of Vascular and Interventional Neurology (SVIN) investigators, acute ischaemic stroke associated with SARS-CoV-2 infection in North America. J. Neurol. Neurosurg. Psychiatry. 2022;93(4):360–368. doi: 10.1136/jnnp-2021-328354. [DOI] [PubMed] [Google Scholar]
- 157.Qin C., Zhou L., Hu Z., Yang S., Zhang S., Chen M., Yu H., Tian D.-S., Wang W. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke. 2020;51(7):2219–2223. doi: 10.1161/STROKEAHA.120.030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Choudhry H., Klingensmith J., Dalton L.Br, Myers M., Mercado E. Large middle cerebral artery ischemic stroke in a therapeutically anticoagulated patient with severe SARS-CoV-2 infection. Neurologist. 2022;27(4):218–221. doi: 10.1097/NRL.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Choi J.K., Prabhakaran K., Latifi R., Smiley A., Klein J., Lombardo G., Rhee P. Serial rotational thromboelastography (ROTEM) in mechanically ventilated patients with COVID-19 demonstrates hypercoagulopathy despite therapeutic heparinization. Trauma Surg. Acute Care Open. 2022;7(1) doi: 10.1136/tsaco-2020-000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Katz J.M., Libman R.B., Wang J.J., Filippi C.G., Sanelli P., Zlochower A., Gribko M., Pacia S.V., Kuzniecky R.I., Najjar S., Azhar S. COVID-19 severity and stroke: correlation of imaging and laboratory markers. AJNR Am. J. Neuroradiol. 2021;42(2):257–261. doi: 10.3174/ajnr.A6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee S.G., Fralick M., Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21) doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zayet S., Klopfenstein T., Kovẚcs R., Stancescu S., Hagenkötter B. Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg. Infect. Dis. 2020;26(9):2258–2260. doi: 10.3201/eid2609.201791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hartmann P., Schober A., Weber C. Chemokines and microRNAs in atherosclerosis. Cell Mol. Life Sci. 2015;72(17):3253–3266. doi: 10.1007/s00018-015-1925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chavda V., Chaurasia B., Fiorindi A., Umana G.E., Lu B., Montemurro N. Ischemic stroke and SARS-CoV-2 infection: the bidirectional pathology and risk morbidities. Neurol. Int. 2022;14(2):391–405. doi: 10.3390/neurolint14020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tiwari A., Berekashvili K., Vulkanov V., Agarwal S., Khaneja A., Turkel-Parella D., Liff J., Farkas J., Nandakumar T., Zhou T., Frontera J., Kahn D.E., Kim S., Humbert K.A., Sanger M.D., Yaghi S., Lord A., Arcot K., Dmytriw A.A. Etiologic subtypes of ischemic stroke in SARS-CoV-2 patients in a cohort of New York City hospitals. Front. Neurol. 2020;11:1004. doi: 10.3389/fneur.2020.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moshayedi P., Ryan T.E., Mejia L.L.P., Nour M., Liebeskind D.S. Triage of acute ischemic stroke in confirmed COVID-19: large vessel occlusion associated with coronavirus infection. Front. Neurol. 2020;11:353. doi: 10.3389/fneur.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tun A., ÜnlÜbaŞ Y., Alemdar M., AkyÜz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J. Clin. Neurosci. 2020;77:227–229. doi: 10.1016/j.jocn.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Viguier A., Delamarre L., Duplantier J., Olivot J.-M., Bonneville F. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J. Neuroradiol. 2020;47(5):393–394. doi: 10.1016/j.neurad.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.De Michele M., Kahan J., Berto I., Schiavo O.G., Iacobucci M., Toni D., Merkler A.E. Cerebrovascular complications of COVID-19 and COVID-19 vaccination. Circ. Res. 2022;130(8):1187–1203. doi: 10.1161/CIRCRESAHA.122.319954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vegivinti C.T.R., Evanson K.W., Lyons H., Akosman I., Barrett A., Hardy N., Kane B., Keesari P.R., Pulakurthi Y.S., Sheffels E., Balasubramanian P., Chibbar R., Chittajallu S., Cowie K., Karon J., Siegel L., Tarchand R., Zinn C., Gupta N., Kallmes K.M., Saravu K., Touchette J. Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials. BMC Infect. Dis. 2022;22(1):107. doi: 10.1186/s12879-022-07068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li S.-R., Tang Z.-J., Li Z.-Ha, Liu X. Searching therapeutic strategy of new coronavirus pneumonia from angiotensin-converting enzyme 2: the target of COVID-19 and SARS-CoV. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1021–1026. doi: 10.1007/s10096-020-03883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fara M.G., Stein L.K., Skliut M., Morgello S., Fifi J.T., Dhamoon M.S. Macrothrombosis and stroke in patients with mild Covid‐19 infection. J. Thromb. Haemost. 2020;18(8):2031–2033. doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ghani M.U., Kumar M., Ghani U., Sonia F., Abbas S.A. Intracranial hemorrhage complicating anticoagulant prophylactic therapy in three hospitalized COVID-19 patients. J. Neurovirol. 2020;26(4):602–604. doi: 10.1007/s13365-020-00869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur. J. Case Rep. Intern. Med. 2020;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heman-Ackah S.M., Su Y.S., Spadola M., Petrov D., Chen H.I., Schuster J., Lucas T. Neurologically devastating intraparenchymal hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation: a case series. Neurosurgery. 2020;87(2):E147–E151. doi: 10.1093/neuros/nyaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gunasekaran K., Amoah K., Rajasurya V., Buscher M.G. Stroke in a young COVID-19 patient. QJM. 2020;113(8):573–574. doi: 10.1093/qjmed/hcaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rizzo A.C., Giussani G., Agostoni E.C. Ischemic stroke and vaccine-induced immune thrombotic thrombocytopenia following COVID-19 vaccine: a case report with systematic review of the literature. Cerebrovasc. Dis. 2022:1–13. doi: 10.1159/000524290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kenda J., Lovrič D., Škerget M., Milivojević N. Treatment of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia related acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2021;30(11) doi: 10.1016/j.jstrokecerebrovasdis.2021.106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hixon A.M., Thaker A.A., Pelak V.S. Persistent visual dysfunction following posterior reversible encephalopathy syndrome due to COVID-19: Case series and literature review. Eur. J. Neurol. 2021;28(10):3289–3302. doi: 10.1111/ene.14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Carroll E., Lewis A. Catastrophic intracranial hemorrhage in two critically ill patients with COVID-19. Neurocrit. Care. 2021;34(1):354–358. doi: 10.1007/s12028-020-00993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhai P., Ding Y., Li Y. The impact of COVID-19 on ischemic stroke. Diagn. Pathol. 2020;15(1):78. doi: 10.1186/s13000-020-00994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morassi M., Bagatto D., Cobelli M., D'Agostini S., Gigli G.L., Bnà C., Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J. Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20):e60.1. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Al-Kuraishy H.M., Al-Gareeb A.I., Alblihed M., Cruz-Martins N., Batiha G.E.-S. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type ii diabetes mellitus: the anti-inflammatory role of metformin. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Llansó L., Urra X. Posterior reversible encephalopathy syndrome in COVID-19 disease: a case-report. SN Compr. Clin. Med. 2020;2(10):1900–1902. doi: 10.1007/s42399-020-00470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kvernland A., Kumar A., Yaghi S., Raz E., Frontera J., Lewis A., Czeisler B., Kahn D.E., Zhou T., Ishida K., Torres J., Riina H.A., Shapiro M., Nossek E., Nelson P.K., Tanweer O., Gordon D., Jain R., Dehkharghani S., Henninger N., de Havenon A., Grory B.M., Lord A., Melmed K. Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a New York healthcare system. Neurocrit. Care. 2021;34(3):748–759. doi: 10.1007/s12028-020-01077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Srinivasan A., Wilson B.C., Bear M., Hasan A., Ezzeldin O., Alim S., Elfallal S., Fang X., Ezzeldin M. Intracerebral hemorrhage and reversible cerebral vasoconstriction syndrome in a patient With COVID-19. Cureus. 2021;13(8) doi: 10.7759/cureus.17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sharifi-Razavi A., Karimi N., Zarvani A., Cheraghmakani H., Baghbanian S.M. Ischemic stroke associated with novel coronavirus 2019: a report of three cases. Int. J. Neurosci. 2021;131(12):1243–1247. doi: 10.1080/00207454.2020.1782902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rajae A., Manal M., Ghizlane E.A., Amine B., Zaid I., Houssam B., Yassine M., Brahim H. Ischemic stroke revealing COVID-19 infection: case report. Ann. Med. Surg. (Lond.) 2021;71 doi: 10.1016/j.amsu.2021.102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.