Abstract
Two proteins of Escherichia coli, termed Wzc and Wzb, were analyzed for their capacity to participate in the reversible phosphorylation of proteins on tyrosine. First, Wzc was overproduced from its specific gene and purified to homogeneity by affinity chromatography. Upon incubation in the presence of radioactive ATP, it was found to effectively autophosphorylate. Two-dimensional analysis of its phosphoamino acid content revealed that it was modified exclusively at tyrosine. Second, Wzb was also overproduced from the corresponding gene and purified to homogeneity by affinity chromatography. It was shown to contain a phosphatase activity capable of cleaving the synthetic substrate p-nitrophenyl phosphate into p-nitrophenol and free phosphate. In addition, it was assayed on individual phosphorylated amino acids and appeared to dephosphorylate specifically phosphotyrosine, with no effect on phosphoserine or phosphothreonine. Such specificity for phosphotyrosine was confirmed by the observation that Wzb was able to dephosphorylate previously autophosphorylated Wzc. Together, these data demonstrate, for the first time, that E. coli cells contain both a protein-tyrosine kinase and a phosphotyrosine-protein phosphatase. They also provide evidence that this phosphatase can utilize the kinase as an endogenous substrate, which suggests the occurrence of a regulatory mechanism connected with reversible protein phosphorylation on tyrosine. From comparative analysis of amino acid sequences, Wzc was found to be similar to a number of proteins present in other bacterial species which are all involved in the synthesis or export of exopolysaccharides. Since these polymers are considered important virulence factors, we suggest that reversible protein phosphorylation on tyrosine may be part of the cascade of reactions that determine the pathogenicity of bacteria.
In eukaryotes, a plethora of protein-tyrosine kinases and phosphotyrosine-protein phosphatases that catalyze the reversible phosphorylation of proteins on tyrosine residues have been detected and shown to play a key role in the regulation of various important biological functions, including signal transduction, growth control, and malignant transformation (15, 22). In prokaryotes, the presence of protein-tyrosine kinase activities was suggested, much later than in eukaryotes, by the finding of phosphotyrosine, first in the proteins of Escherichia coli (9) and then in the proteins of a series of other bacterial species (10, 11, 24). On the other hand, the occurrence of phosphotyrosine-protein phosphatases was recently reported for a few examples, such as the IphP protein of Nostoc commune UTEX 584 (20), the YopH protein of Yersinia pseudotuberculosis (4, 19), and the PtpA protein of Streptomyces coelicolor (26). However, in bacteria, the biological significance of reversible protein phosphorylation on tyrosine is still unclear, essentially because for a long time, no individual protein-tyrosine kinase was characterized and no endogenous protein substrate for a phosphotyrosine-protein phosphatase was identified. The only exception so far reported concerns two proteins of Acinetobacter johnsonii that harbor opposing activities: the Ptk protein, which has been recently demonstrated to autophosphorylate on several tyrosine residues (14), and the Ptp protein, which has been identified as a phosphotyrosine-protein phosphatase (18). Moreover, in vitro experiments have shown that Ptp is able to specifically dephosphorylate Ptk, which constitutes the first evidence for a reversible protein phosphorylation reaction on tyrosine in bacteria. From these observations, it seemed interesting to determine whether such a reversible tyrosine phosphorylation system was unique and restricted to the bacterial genus Acinetobacter or was applicable to other types of bacteria as well.
For that purpose, we analyzed comparatively two proteins of E. coli, Wzc and Wzb (33), which exhibit striking sequence similarity with proteins Ptk and Ptp of A. johnsonii, respectively, and we checked whether such sequence relationships were linked to functional homologies. Wzc and Wzb are known to participate in the export of the extracellular polysaccharide colanic acid from the cell to medium (33). Wzc is an inner membrane protein that possesses an ATP-binding domain and three predicted transmembrane segments, while Wzb has an amino acid sequence homologous to that of acid phosphatases. The corresponding genes, wzc and wzb, are adjacent at 46 min on the E. coli chromosome and located at the second and third positions, respectively, in order of transcription, within the colanic acid cluster that comprises a total of 19 different genes (33).
In this work, Wzc was overproduced, purified to homogeneity, and shown to autophosphorylate on tyrosine. Wzb, also overproduced and purified, was found to exhibit a protein phosphatase activity with a strict specificity for phosphotyrosine. The functional properties of these two proteins were analyzed, and the phosphorylated form of Wzc was shown to be sensitive to dephosphorylation by Wzb, thus indicating that the Wzc-Wzb pair of E. coli is homolog of the Ptk-Ptp pair of A. johnsonii.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli JM109 was used as template for PCR amplification of the wzc and wzb genes. E. coli XL1-Blue was used to propagate plasmids in cloning experiments. E. coli BL21(pREP4-groESL), used for expression experiments, was previously described (1); it was a gift from I. Martin-Verstraete (Pasteur Institute, Paris, France). Plasmid vectors pQE30 and pGEX-KT were purchased from Qiagen.
Culture media and growth conditions.
E. coli strains were grown in LB or 2YT medium at 37°C. In the case of strains carrying drug resistance genes, the antibiotics kanamycin, ampicillin, and tetracycline were added to the medium at concentrations of 25, 50, and 15 μg ml−1, respectively. Growth was monitored by measuring the A600.
DNA manipulation.
Small- and large-scale plasmid isolations were carried out by the alkaline lysis method, and plasmids were purified by using cesium chloride-ethidium bromide gradients (23). Genomic DNA from E. coli was prepared as described elsewhere (31). All restriction enzymes, calf intestine phosphatase, T4 DNA ligase, and Taq DNA polymerase were used as recommended by the manufacturer (Promega). Transformation of E. coli cells was performed as previously reported (12).
Construction of the wzc and wzb expression plasmids.
Total DNA from E. coli JM109 served as the template in PCR amplification for preparing the wzc and wzb genes with appropriate restriction sites at both ends.
For wzc gene cloning, the sequences of the two primers were 5′-GCGGGATCCACAGAAAAAGTAAAACAACATGCCGCTCCGG-3′ at the N terminus (the BamHI site is italicized; the second codon of wzc is underlined) and 5′-CCGGAATTCTTATTTCGCATCCGACTTATATTCG-3′ at the C-terminus (the EcoRI site is italicized; the stop codon of wzc is underlined). The amplified fragment was digested with restriction enzymes BamHI and EcoRI and ligated into pGEX-KT vector, opened with the same enzymes, to yield plasmid pGEX-wzc.
For wzb gene amplification, the sequences of the primers used were 5′-TATGGATCCTTTAACAACATCTTAGTTGTCTGTGTCGGC-3′ at the N terminus (the BamHI site is italicized; the second codon of wzb is underlined) and 5′-CGGGGTACCTTATACCTGCTCTGCGTTCAATGC-3′ at the C terminus (the KpnI site is italicized; the stop codon of wzb is underlined). The synthesized DNA was restricted by BamHI and KpnI and ligated into pQE30 vector, opened with the same enzymes. The resulting plasmid was termed pQE30-wzb.
In each case, the nucleotide sequence of the synthesized gene was checked by dideoxynucleotide sequencing (32).
Purification of protein Wzc.
E. coli BL21(pREP4-groESL) cells were transformed with plasmid pGEX-wzc. Cells from this strain were used to inoculate 1 liter of 2YT medium supplemented with ampicillin and kanamycin and were incubated at 37°C under shaking until the A600 reached 0.8. Isopropyl-β-d-thiogalactopyranoside (IPTG) was then added at a final concentration of 0.1 mM, and growth was continued for 2 h at 30°C under shaking. Cells were harvested by centrifugation at 3,000 × g for 10 min and suspended in 12 ml of buffer A (10 mM sodium phosphate [pH 7.4], 150 mM NaCl, 1 mM EDTA, 10% glycerol) containing 1 mM phenylmethylsulfonyl fluoride plus DNase I and RNase A, each at a final concentration of 100 μg ml−1. Cells were disrupted in a French pressure cell at 16,000 lb/in2. The resulting suspension was supplemented with Triton X-100 at a final concentration of 1% and centrifuged at 4°C for 30 min at 30,000 × g. The supernatant was incubated for 30 min at 4°C with glutathione-Sepharose 4B matrix (Pharmacia Biotech), suitable for purification of glutathione S-transferase (GST) fusion proteins. The protein-resin complex was packed into a column for washing and elution. The column was washed with 50 ml of buffer A containing 1% Triton X-100. Protein elution was carried out with buffer B (50 mM Tris-HCl [pH 8.0], 5 mM MgCl, 10% glycerol) containing 0.1% Triton X-100 and 10 mM glutathione. Eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (25). Fractions containing GST-Wzc were pooled and dialyzed against buffer C (20 mM Tris-HCl [pH 8.8], 1 mM EDTA, 10% glycerol) supplemented with 20 mM NaCl. This protein solution was then loaded onto a column of Q-Sepharose High Performance matrix (Pharmacia Biotech). Proteins were eluted with buffer C containing 0.1% Triton X-100 and NaCl varying from 150 to 500 mM. The GST-Wzc fusion protein was eluted at a concentration of 250 mM. Fractions containing the purified GST-Wzc protein were dialyzed against buffer B and stored at −20°C.
Purification of protein Wzb.
E. coli BL21(pREP4-groESL) cells were transformed with plasmid pQE30-wzb. Cells from this strain were used to inoculate 100 ml of LB medium supplemented with ampicillin and kanamycin and were incubated at 37°C under shaking until the A600 reached 0.7. IPTG was then added at a final concentration of 0.5 mM, and growth was continued for 2 h at 20°C under shaking. Cells were harvested by centrifugation at 3,000 × g for 10 min and suspended in 1 ml of buffer D (50 mM Tris-HCl [pH 7.4], 500 mM NaCl, 10% glycerol) containing DNase I and RNase A, each at a final concentration of 100 μg ml−1. Cells were disrupted in a French pressure cell at 16,000 lb/in2. The resulting suspension was centrifuged at 4°C for 30 min at 30,000 × g. The supernatant was loaded onto a Zn2+-immobilized matrix (Boehringer Mannheim), suitable for purification of fusion proteins carrying a polyhistidine tag. The column was washed first with buffer D and then with 50 mM imidazole in the same buffer for 5 min. Protein elution was monitored at 280 nm, and eluted fractions were analyzed by SDS-PAGE (25). His-tagged Wzb was eluted at a concentration of 100 mM imidazole. Fractions containing purified Wzb were applied to a Hi.Trap desalting column (Pharmacia) and stored in a buffer made of 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA, 20% glycerol, and 5 mM dithiothreitol (DTT) at −20°C.
In vitro phosphorylation assay.
In vitro phosphorylation of about 3 μg of purified GST-Wzc protein was performed at 30°C in 10 μl of a buffer containing 25 mM Tris-HCl (pH 7.0), 1 mM DTT, 5 mM MgCl2, 1 mM EDTA, and 10 μM ATP with 200 μCi of [γ-32P]ATP ml−1. After 10 min of incubation, the reaction was stopped by addition of an equal volume of 2× sample buffer, and the mixture was heated at 100°C for 5 min. One-dimensional gel electrophoresis was performed as previously described (25). In an alternative procedure used for two-dimensional gel analysis, after 10 min of incubation, the protein was precipitated with 5 vol of acetone for 30 min at −20°C and centrifuged for 5 min at 30,000 × g before dissolution in the loading buffer (29). After electrophoresis, gels were soaked in 16% trichloroacetic acid (TCA) for 10 min at 90°C. They were stained with Coomassie blue, and radioactive proteins were visualized by autoradiography.
Analysis of the phosphoamino acid content of proteins.
Protein samples were separated by one-dimensional gel electrophoresis (25) and then electroblotted onto an Immobilon polyvinylidene difluoride (PVDF) membrane. Phosphorylated proteins bound to the membrane fraction were detected by autoradiography. The 32P-labeled protein bands were excised from the Immobilon blot and hydrolyzed in 6 M HCl for 1 h at 110°C. The acid-stable phosphoamino acids thus liberated were separated by electrophoresis in the first dimension at pH 1.9 (800V · h) in 7.8% acetic acid–2.5% formic acid, followed by ascending chromatography in the second dimension in 2-methyl-1-propanol–formic acid–water (8:3:4). After migration, radioactive molecules were detected by autoradiography. Authentic phosphoserine, phosphothreonine, and phosphotyrosine were run in parallel and visualized by staining with ninhydrin.
Phosphatase assay.
Acid phosphatase activity was monitored at 37°C by using a continuous method based on the detection of p-nitrophenol formed from p-nitrophenyl phosphate (PNPP). Rates of dephosphorylation were determined at 405 nm in a reaction buffer containing 100 mM sodium citrate (pH 6.5), 1 mM EDTA, 0.1% β-mercaptoethanol, and PNPP at a concentration varying from 0.5 to 40 mM. The amount of p-nitrophenol released was estimated by using a molar extinction coefficient ɛ405 of 18,000 M−1 cm−1 (8). The assay was optimized with respect to protein concentration, time, and pH.
Phosphotyrosine phosphatase (PTPase) activity was assayed at 37°C in a 50-μl reaction volume containing 10 mM O-phosphotyrosine as the substrate, 1 mM EDTA, 100 mM sodium citrate (pH 6.5), and 1 μg of purified Wzb. After 15 min of incubation, the reaction was stopped by adding 150 μl of 25% TCA and then 50 μl of bovine serum albumin (10 mg ml−1). The precipitated protein was removed by centrifugation, and the supernatant was used for measurement of released inorganic phosphate by using 1 volume of a mixture containing 1.2 M sulfuric acid, 0.5% ammonium molybdate, and 2% ascorbic acid. Samples were heated at 56°C for 15 min, and the A750 was measured (7, 28).
Wzc dephosphorylation assay.
In vitro phosphorylation of about 0.1 μg of purified Wzc protein was performed as described above. After 10 min of incubation, a dephosphorylation assay of Wzc was carried out with 0.1 μg of purified Wzb at 37°C for 2 to 30 min in 30 μl of buffer consisting of 100 mM sodium citrate (pH 6.5) and 1 mM EDTA. The reaction was stopped by addition of an equal volume of 2× sample buffer, and the mixture was heated at 100°C for 5 min. The Wzc protein was separated by gel electrophoresis, treated with TCA, and analyzed by autoradiography. The radioactive bands were excised, and their radioactivity was counted in a liquid scintillation spectrometer.
RESULTS
The starting point of this study was the comparative analysis of the amino sequence deduced from the nucleotide sequence of the ptk gene of A. johnsonii (17) with the different amino acid sequences deduced from the E. coli genome (3). By using the Swissprot database, we detected a striking sequence similarity between protein Ptk and the previously described (33) E. coli protein Wzc. Indeed, the best-fit sequence alignments showed that these two proteins exhibit over 36% identity and 61% similarity (Fig. 1). Since Ptk is known to autophosphorylate on multiple tyrosine residues (14), it was of interest to assay also Wzc for phosphorylation. For that purpose, it was first necessary to overproduce and purify this protein.
FIG. 1.
Comparison of proteins Wzc and Ptk. Alignment of the amino acid sequence of Wzc with that of the prokaryotic protein-tyrosine kinase Ptk from A. johnsonii is presented. Identical amino acids are indicated by asterisks, and high similarity is indicated by double dots.
Overproduction and purification of Wzc.
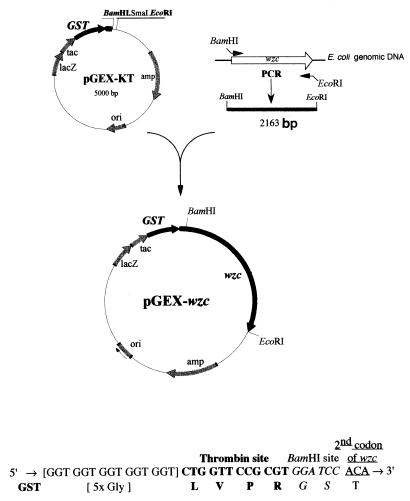
The wzc gene lacking the start codon was synthesized by PCR, by using oligonucleotide primers deduced from the wzc gene sequence (33). The amplified DNA was cloned in plasmid pGEX-KT previously digested with restriction enzymes BamHI and EcoRI. The resulting plasmid, termed pGEX-wzc, expressed a fusion protein consisting of Wzc with GST at its N terminus (Fig. 2). This construct was used to transform competent cells from E. coli BL21(pREP4-groESL). This strain overproduces the two chaperone proteins GroES and GroEL and is suitable for the overproduction of proteins that possess a high degree of hydrophobicity and thus a tendency to aggregate, such as Wzc. Upon induction by IPTG, efficient overexpression of a 105-kDa protein, consistent with the calculated molecular mass of the fusion protein, was obtained in the soluble fraction of cells.
FIG. 2.
Construction of plasmid pGEX-wzc. The wzc gene, with BamHI and EcoRI restriction sites at both ends, was synthesized by PCR and cloned into plasmid pGEX-KT, previously digested with the same restriction enzymes, to yield plasmid pGEX-wzc. The N-terminal part of the recombinant protein with the thrombin site is shown at the bottom.
The GST-Wzc fusion protein was then purified to homogeneity in a two-step chromatographic procedure consisting of an affinity chromatography on glutathione-Sepharose 4B matrix followed by an anion-exchange chromatography on a Q-Sepharose column. In these conditions, about 1 mg of GST-Wzc protein was obtained from 1 liter of bacterial culture.
Autophosphorylation of Wzc at tyrosine.
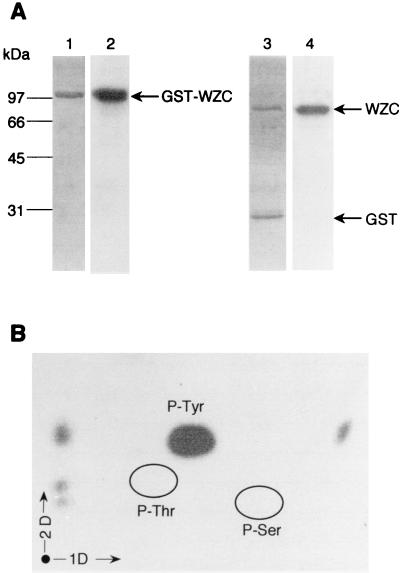
For comparison with Ptk, the GST-Wzc protein was assayed for phosphorylation. It was observed that purified GST-Wzc was significantly labeled in vitro in the presence of [γ-32P]ATP (Fig. 3A). The ability of GST-Wzc to phosphorylate in these conditions indicated that it contains an intrinsic protein kinase activity that catalyzes its autophosphorylation. As a control, the phosphorylated fusion protein was submitted to proteolysis by thrombin to cleave Wzc from the linked GST, and the location of the bound radioactivity was determined. It was observed that the radioactive labeling of the fusion protein was due exclusively to the phosphorylation of the Wzc protein, while no radioactivity was present on GST (Fig. 3A).
FIG. 3.
GST-Wzc autophosphorylation assay. About 3 μg of purified GST-Wzc was incubated with [γ-32P]ATP. The protein was analyzed by SDS-PAGE; gels were soaked in 16% TCA and either stained with Coomassie blue (lane 1) or submitted to autoradiography (lane 2). The protein was then hydrolyzed by thrombin and analyzed by SDS-PAGE. The products of hydrolysis were revealed by Coomassie blue staining (lane 3) or autoradiography (lane 4). (B) Phosphoamino acid content of GST-Wzc. GST-Wzc labeled with [γ-32P]ATP was analyzed by SDS-PAGE, electroblotted onto an Immobilon PVDF membrane, excised, and hydrolyzed in acid. The phosphoamino acids thus liberated were separated by electrophoresis in the first dimension (1D) and ascending chromatography in the second dimension (2D). After migration, radioactive molecules were detected by autoradiography. Authentic phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) were run in parallel and visualized by ninhydrin staining.
The phosphoamino acid content of the labeled protein was determined after acid hydrolysis and two-dimensional analysis. In these conditions, only acid-resistant phosphoamino acids were analyzed since a number of other phosphorylated compounds, such as phosphohistidine, phosphoarginine, or phosphoaspartate, are known to be labile in acid (13). Only phosphotyrosine was revealed on the corresponding autoradiogram (Fig. 3B), which indicated that GST-Wzc was modified exclusively at tyrosine residues. To obtain more information on the phosphorylation state of GST-Wzc, the purified protein was phosphorylated in vitro and then analyzed by two-dimensional gel electrophoresis. Interestingly, this gel, stained with Coomassie blue, and the corresponding autoradiogram revealed a series of spots with the same molecular mass and a different isoelectric point, which likely correspond to a varying degree of phosphorylation of the protein (data not shown) as previously observed for Ptk (14). Wzc, like Ptk and other Wzc homologs, contains a relatively large number of tyrosine residues (20 in total) especially in its C-terminal part, but the precise number and the location of the phosphorylation sites are still unknown.
To characterize further the Wzc protein, different attempts were made to obtain the Wzc protein in its native state, i.e., without GST at its N terminus, after cleavage by thrombin. The fusion protein was efficiently hydrolyzed but the native Wzc protein thus obtained had no more autophosphorylating activity. This loss of activity might be related to the aggregation of the Wzc protein, due to its high degree of hydrophobicity. The fusion protein GST-Wzc was therefore used in all subsequent experiments.
Overproduction and purification of Wzb.
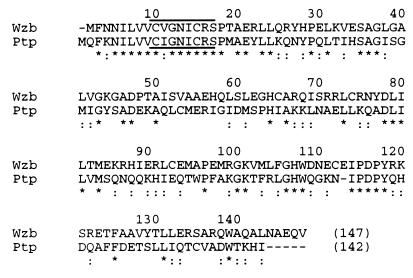
Further searches in the Swissprot database revealed, on the other hand, a high similarity between the phosphotyrosine-protein phosphatase Ptp of Acinetobacter and a protein, termed Wzb, from E. coli. The comparative analysis of the amino acid sequences of these two proteins showed that they were 33% identical and 58% similar over their entire lengths (Fig. 4). In particular, they both appeared to contain the CX5R(S/T) motif which is found in the N-terminal parts of numerous low-Mr acid PTPases, namely, eukaryotic phosphatases, and which is considered to be the major signature of this type of enzyme (8, 35).
FIG. 4.
Comparison of proteins Wzb and Ptp. Alignment of the amino acid sequence of Wzb with that of the prokaryotic phosphotyrosine-protein phosphatase Ptp from A. johnsonii is presented. Identical amino acids are indicated by asterisks, and high similarity is indicated by double dots. The phosphatase specific motif CX5R(S/T) is overlined in Wzb and underlined in Ptp.
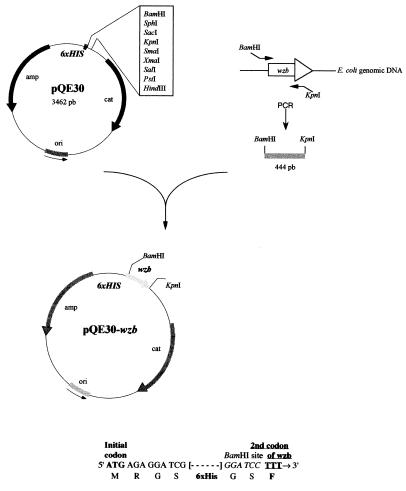
From this observation, it seemed worthwhile to analyze Wzb, especially its enzymatic activity on dephosphorylatable substrates, in more detail. For this, it was first necessary, as previously done for Wzc, to overproduce and purify the protein. The oligonucleotide primers corresponding to the 5′ and 3′ ends of the wzb gene (33) were prepared with the appropriate restriction sites at both ends. The wzb gene lacking the start codon ATG was then synthesized by PCR and cloned in the expression vector pQE30 from E. coli, previously digested with the restriction enzymes BamHI and KpnI. The resulting plasmid pQE30-wzb allowed production of the Wzb protein with an N-terminal addition of 11 amino acids, including 6 histidines (Fig. 5). It was used to transform competent cells of E. coli BL21(pREP4-groESL). Upon induction with 0.5 mM IPTG, a relatively high level of a 19-kDa protein, consistent with the calculated molecular mass of the fusion protein His6-Wzb, was obtained in the soluble fraction of cells. The fusion protein was then purified to homogeneity in a single-step chromatographic procedure by using a Zn2+-immobilized matrix generally used for purifying His-tagged proteins. In these conditions, about 1 mg of pure protein was obtained from 100 ml of bacterial culture.
FIG. 5.
Construction of plasmid pQE30-wzb. The wzb gene with BamHI and KpnI restriction sites at both ends was synthesized by PCR and cloned into pQE30, previously digested with the same restriction enzymes. The N-terminal part of the recombinant protein is shown at the bottom. pb, base pairs.
Phosphotyrosine-protein phosphatase activity of Wzb.
The phosphatase activity of His6-Wzb was first assayed for its ability to cleave PNPP. It was observed that the protein could efficiently hydrolyze this synthetic substrate at an optimum pH value of 6.5. The corresponding kinetic constants, Km and Vmax, measured at 37°C, were 1 mM and 4.6 μmol min−1 mg−1, respectively. These values are in the same range as those previously reported for eukaryotic low-Mr PTPases such as bovine heart phosphatase (34).
Further experiments were performed to measure the in vitro activity of Wzb on individual phosphorylated amino acids. Wzb was shown to quantitatively release inorganic phosphate from phosphotyrosine but had no effect on either phosphoserine or phosphothreonine. This result is consistent with a strict specificity of the dephosphorylating activity of Wzb for phosphotyrosine, which is a general property of the low-Mr PTPases of eukaryotic cells (8, 35).
Endogenous substrate for Wzb.
At this stage, two proteins of E. coli harboring opposing activities had been identified: the Wzc protein, which is able to autophosphorylate on tyrosine residues, and the Wzb protein, which possesses the characteristics of a phosphotyrosine-protein phosphatase. In view of a possible regulation of bacterial physiology by reversible protein phosphorylation on tyrosine, it was then of special interest to check whether Wzb could utilize Wzc as an endogenous substrate and catalyze its dephosphorylation.
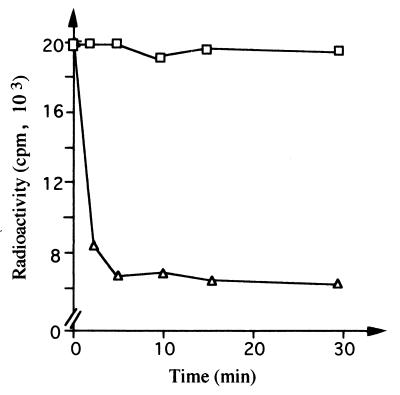
For this, the purified Wzc protein was first radioactively labeled in the presence of [γ-32P]ATP and then incubated in the presence of Wzb. The results presented in Fig. 6 clearly indicate that in these conditions, Wzc was rapidly and extensively dephosphorylated by Wzb. These data provide evidence that Wzb can use Wzc as an endogenous substrate and support the concept that the enzymatic activity of the phosphorylatable kinase Wzc is regulated by the dephosphorylating activity of Wzb.
FIG. 6.
Dephosphorylation of Wzc by Wzb. Purified Wzc was phosphorylated in vitro with [γ-32P]ATP. The labeled protein was incubated without (□) or with (▵) Wzb for various times as indicated, then separated by gel electrophoresis, treated with 16% TCA, and revealed by autoradiography. The amount of radioactivity incorporated in Wzc was counted in a liquid scintillation spectrometer.
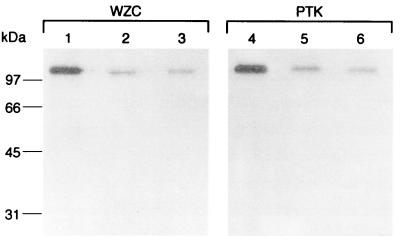
Considering the high similarity between, on the one hand, the phosphorylatable proteins Ptk and Wzc and, on the other hand, the phosphotyrosine-protein phosphatases Ptp and Wzb, it was interesting to see whether these proteins could cross-react. For that purpose, Wzc from E. coli and Ptk from A. johnsonii were labeled in vitro in the presence of [γ-32P]ATP and then assayed for dephosphorylation by using either Wzb from E. coli or Ptp from A. johnsonii as the protein phosphatase. It appeared that Wzb could dephosphorylate protein Ptk (Fig. 7, lane 5) with the same efficiency as Ptp (Fig. 7, lane 6). Conversely, Ptp protein could catalyze the extensive dephosphorylation of Wzc (Fig. 7, lane 3) as well as Wzb (Fig. 7, lane 2).
FIG. 7.
Protein dephosphorylation assay. GST-Wzc and GST-Ptk were first phosphorylated with [γ-32P]ATP. Each phosphoprotein was incubated in a dephosphorylation buffer at 37°C for 30 min either in the absence (lanes 1 and 4) or in the presence of 5 μg of purified Wzb (lanes 2 and 5) or Ptp (lanes 3 and 6). Proteins were then analyzed by SDS-PAGE, gels were soaked in 16% TCA, and radioactive bands were revealed by autoradiography.
DISCUSSION
The main result of this study is the demonstration that two proteins of E. coli, Wzc and Wzb, carry an autophosphorylating protein-tyrosine kinase activity and a phosphotyrosine-protein phosphatase activity, respectively. The presence of a protein-tyrosine kinase activity in E. coli had been previously suggested by the original finding of phosphotyrosine in an acid hydrolysate prepared from the total protein fraction of this bacterium (27), and it was further documented by the detection of a phosphoprotein of unknown function, termed TypA, modified selectively at tyrosine (16). But no evidence had been adduced for the occurrence of a specific kinase responsible for such modification of proteins. Our results now show, for the first time, that a phosphorylating enzyme of this type, Wzc, is indeed present in E. coli cells. Similarly, our data show that E. coli harbors a phosphotyrosine-protein phosphatase, Wzb, with the same biochemical characteristics as those of several low-Mr acid phosphotyrosine-protein phosphatases, namely, of eukaryotic origin, previously described by other authors (8, 35). Here again, this is the first evidence for an enzyme of this type in E. coli cells. Of particular interest is the further finding that Wzb can dephosphorylate in vitro Wzc, which thus appears as a specific endogenous substrate for Wzb. This observation supports the existence, to be tested, of a regulatory mechanism of bacterial physiology operating by reversible protein phosphorylation on tyrosine.
Interestingly, the same possibility was previously envisaged for A. johnsonii. Indeed, we have recently identified two genes, ptk and ptp, which are located next to each other in a gene cluster and which encode a protein-tyrosine kinase and a low-Mr phosphotyrosine-protein phosphatase, respectively (14, 17, 18). As in the case of the Wzc-Wzb couple, it has been shown that Ptp can actively dephosphorylate Ptk. Furthermore, the two proteins of E. coli possess the same biochemical characteristics as Ptk and Ptp from A. johnsonii. Thus, the capacity of Wzc to autophosphorylate is identical to that observed for Ptk. Also, the Wzb protein dephosphorylates the synthetic substrate PNPP with the same kinetic constant values as those measured for Ptp, and the optimum hydrolysis of this substrate is obtained in each case at pH 6.5. The functional similarity between the Ptk-Ptp and Wzc-Wzb proteins is reinforced by the observation that these different proteins can cross-react; i.e., Wzb can dephosphorylate Ptk, and Ptp is able to dephosphorylate Wzc.
The finding that the Wzc-Wzb pair of proteins of E. coli is a homolog of the Ptk-Ptp pair of A. johnsonii proteins confirms that similar activities can be predicted from sequence relationships. Therefore, one can expect that comparable pairs of proteins acting in the same dual manner would exist in other bacterial species as well. Indeed, some genes similar to wzc and wzb have been detected in various bacteria, including amsA and amsI in Erwinia amylovora (5, 6), epsB and epsP in Pseudomonas solanacearum (21), and orf6 and orf5 in Klebsiella pneumoniae (2). They all belong to gene clusters involved in the synthesis or transport of exopolysaccharides and are present only in these clusters, but their specific functions are unknown. It would be worthwhile to check for the protein-tyrosine kinase and phosphotyrosine-protein phosphatase activities of the proteins encoded by these different genes and thus to assess the general nature of the relationship between reversible tyrosine phosphorylation of proteins and production of polysaccharides. It has been widely demonstrated that cell surface polysaccharides play a critical role in a number of important biological processes, including adherence, resistance to specific and non specific host immunity, and prevention of desiccation (30). Exopolysaccharides also mediate direct interaction between bacteria and their immediate environment and, for that reason, are considered an important factor in the virulence of many pathogens. On this basis, we suggest that protein tyrosine phosphorylation may be part of the cascade of reactions that determine the pathogenicity of bacteria.
ACKNOWLEDGMENTS
The expert assistance of Mylène Riberty is gratefully acknowledged.
This work was supported by the CNRS (UPR 412), the Université de Lyon, the Région Rhône-Alpes (contract Emergence), and the Institut Universitaire de France.
REFERENCES
- 1.Amreim K E, Takacs B, Stieger M, Molnos J, Flint N A, Burn P. Purification and characterization of recombinant human p50csk protein-tyrosine kinase from an Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc Natl Acad Sci USA. 1995;92:1048–1052. doi: 10.1073/pnas.92.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Otha M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain chedid. J Bacteriol. 1995;177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunket III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Wayne Davis N, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bliska J B, Guan K, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugert P, Geider K. Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol Microbiol. 1995;15:917–933. doi: 10.1111/j.1365-2958.1995.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 6.Bugert P, Geider K. Characterization of the amsI gene product as a low molecular weight acid phosphatase controlling exopolysaccharide synthesis of Erwinia amylovora. FEBS Lett. 1997;400:252–256. doi: 10.1016/s0014-5793(96)01398-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen P S, Toribara T Y, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 8.Cirri P, Chiarugi P, Camici G, Manao G, Raugei G, Cappugi G, Ramponi G. The role of Cys12, Cys17 and Arg18 in the catalytic mechanism of low-Mr cytosolic phosphotyrosine protein phosphatase. Eur J Biochem. 1993;214:647–657. doi: 10.1111/j.1432-1033.1993.tb17965.x. [DOI] [PubMed] [Google Scholar]
- 9.Cortay J C, Duclos B, Cozzone A J. Phosphorylation of a bacterial protein at tyrosine. J Mol Biol. 1986;187:305–308. doi: 10.1016/0022-2836(86)90236-6. [DOI] [PubMed] [Google Scholar]
- 10.Cozzone A J. ATP-dependent protein kinases in bacteria. J Cell Biochem. 1993;51:7–13. doi: 10.1002/jcb.240510103. [DOI] [PubMed] [Google Scholar]
- 11.Cozzone A J. Diversity and specificity of protein-phosphorylating systems in bacteria. Folia Microbiol. 1997;42:165–170. doi: 10.1007/BF02818973. [DOI] [PubMed] [Google Scholar]
- 12.Dagert M, Ehrlich S D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 13.Duclos B, Marcandier S, Cozzone A J. Chemical properties and separation of phosphoamino acids by thin-layer chromatography and/or electrophoresis. Methods Enzymol. 1991;201:10–21. doi: 10.1016/0076-6879(91)01004-l. [DOI] [PubMed] [Google Scholar]
- 14.Duclos B, Grangeasse C, Vaganay E, Riberty M, Cozzone A J. Autophosphorylation of a bacterial protein at tyrosine. J Mol Biol. 1996;259:891–895. doi: 10.1006/jmbi.1996.0366. [DOI] [PubMed] [Google Scholar]
- 15.Fantl W J, Johnson D E, Williams L T. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 16.Freestone P, Trinei M, Clarke S C, Nystrom T, Norris V. Tyrosine phosphorylation in Escherichia coli. J Mol Biol. 1998;279:1045–1051. doi: 10.1006/jmbi.1998.1836. [DOI] [PubMed] [Google Scholar]
- 17.Grangeasse C, Doublet P, Vaganay E, Vincent C, Deleage G, Duclos B, Cozzone A J. Characterization of a bacterial gene encoding an autophosphorylating protein tyrosine kinase. Gene. 1997;204:259–265. doi: 10.1016/s0378-1119(97)00554-4. [DOI] [PubMed] [Google Scholar]
- 18.Grangeasse C, Doublet P, Vincent C, Vaganay E, Riberty M, Duclos B, Cozzone A J. Functional characterization of the low-molecular-mass phosphotyrosine-protein phosphatase of Acinetobacter johnsonii. J Mol Biol. 1998;278:339–347. doi: 10.1006/jmbi.1998.1650. [DOI] [PubMed] [Google Scholar]
- 19.Guan K, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 20.Howell L D, Griffiths C, Slade L W, Potts M, Kennelly P J. Substrate specificity of IphP, a cyanobacterial dual-specificity protein phosphatase with MAP kinase phosphatase activity. Biochemistry. 1996;35:7566–7572. doi: 10.1021/bi9600409. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Schell M. Molecular characterization of the eps gene cluster of Pseudomonas solanacearum and its transcriptional regulation at a single promoter. Mol Microbiol. 1995;16:977–989. doi: 10.1111/j.1365-2958.1995.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 22.Hunter T. Protein kinases and phosphatases: the Yin and Yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 23.Ish-Horowicz D, Burke J F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981;9:2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennelly P J, Potts M. Fancy meeting you here! a fresh look at prokaryotic protein phosphorylation. J Bacteriol. 1996;178:4759–4764. doi: 10.1128/jb.178.16.4759-4764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Strohl W R. Cloning, purification, and properties of a phosphotyrosine protein phosphatase from Streptomyces coelicolor. J Bacteriol. 1996;178:136–142. doi: 10.1128/jb.178.1.136-142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manaï M, Cozzone A J. Characterization of the amino acids phosphorylated in E. coli proteins. FEMS Microbiol Lett. 1983;17:87–91. [Google Scholar]
- 28.Mustelin T, Coggeshall K M, Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci USA. 1989;86:6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts I S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson G, Andrianopoulos K, Hobbs M, Reeves P R. Organization of the Escherichia coli K-12 gene cluster responsible of the extracellular polysaccharide colanic acid. J Bacteriol. 1996;178:4885–4893. doi: 10.1128/jb.178.16.4885-4893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z Y, Van Etten R L. Purification and characterization of a low-molecular-weight acid phosphatase, a phosphotyrosyl-protein phosphatase from bovine heart. Arch Biochem Biophys. 1990;282:39–49. doi: 10.1016/0003-9861(90)90084-c. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z Y, Zhou G, Denu J M, Wu L, Tang X, Mondesert O, Russel P, Butch E, Guan K L. Purification and characterization of the low-molecular-weight tyrosine phosphatase, Stp1, from the fission yeast Schizosaccharomyces pombe. Biochemistry. 1995;34:10560–10568. doi: 10.1021/bi00033a031. [DOI] [PubMed] [Google Scholar]