Abstract
Both Pseudomonas aeruginosa and the phytopathogen P. syringae produce the exopolysaccharide alginate. However, the environmental signals that trigger alginate gene expression in P. syringae are different from those in P. aeruginosa with copper being a major signal in P. syringae. In P. aeruginosa, the alternate sigma factor encoded by algT (ς22) and the response regulator AlgR1 are required for transcription of algD, a gene which encodes a key enzyme in the alginate biosynthetic pathway. In the present study, we cloned and characterized the gene encoding AlgR1 from P. syringae. The deduced amino acid sequence of AlgR1 from P. syringae showed 86% identity to its P. aeruginosa counterpart. Sequence analysis of the region flanking algR1 in P. syringae revealed the presence of argH, algZ, and hemC in an arrangement virtually identical to that reported in P. aeruginosa. An algR1 mutant, P. syringae FF5.32, was defective in alginate production but could be complemented when algR1 was expressed in trans. The algD promoter region in P. syringae (PsalgD) was also characterized and shown to diverge significantly from the algD promoter in P. aeruginosa. Unlike P. aeruginosa, algR1 was not required for the transcription of algD in P. syringae, and PsalgD lacked the consensus sequence recognized by AlgR1. However, both the algD and algR1 upstream regions in P. syringae contained the consensus sequence recognized by ς22, suggesting that algT is required for transcription of both genes.
The exopolysaccharide alginate is a copolymer of O-acetylated β-1,4-linked d-mannuronic acid and its C-5 epimer, l-guluronic acid (46). Alginate biosynthesis has been extensively studied in Pseudomonas aeruginosa, where it functions as a major virulence factor in strains infecting the lungs of cystic fibrosis patients (45). In P. aeruginosa, genes that encode the biosynthesis and regulation of alginate map to four chromosomal locations. With the exception of algC, which is located at 10 min, the structural genes are clustered within an 18-kb region located at 34 min (18, 46). Structural genes that have been characterized in this region include algA, which encodes a bifunctional enzyme which functions as a phosphomannose isomerase and a GDP-mannose pyrophosphorylase (54); algG, which encodes a C-5 epimerase (7); algF, algI, and algJ, which are involved in acetylation of the alginate polymer (16, 17, 55); and algD, which encodes GDP-mannose dehydrogenase (11). This region also contains algE and algK, which encode proteins with putative roles in polymer export and synthesis, respectively (1, 9, 22), and algL, which encodes alginate lyase (6, 49). Other genes which map within this region include alg44, alg8, and algX (alg60) (33, 41, 60); however, the functional role of the proteins encoded by these genes remains unclear. Chitnis and Ohman (8) postulated that the alginate biosynthetic gene cluster in P. aeruginosa is organized as an operon with transcription initiating at the algD promoter.
A region mapping at 68 min on the P. aeruginosa chromosome harbors a gene cluster consisting of algT (algU), mucA, mucB (algN), mucC, and mucD. These genes modulate the conversion to constitutive alginate production; at the head of this regulatory hierarchy is algT (algU). The alternative sigma factor encoded by algT, ς22, is required for transcription of algD, algT, and algR1 (21, 51). mucA is a negative regulator of algT transcription and encodes an antisigma factor with affinity for ς22 (52, 62). Mutations in mucA inactivate the MucA protein and result in the Alg+ phenotype; however, these mutations are unstable and spontaneous reversion to the Alg− phenotype often occurs due to suppressor mutations in algT (14, 50, 52). The remaining muc genes also modulate the expression of algT and have been described elsewhere (19, 34, 52, 62).
Other genes controlling the regulation of alginate production include algR1 (algR), algR2 (algQ), algR3 (algP), and algB (20, 53). AlgR1 functions as a response regulator member of the two-component signal transduction system and binds to multiple sites upstream of algC and algD (12, 24, 39, 65). Both the algD and algR1 promoters show a consensus sequence at the −35/10 region which is consistent with recognition by ς22, suggesting that an RNA polymerase-ς22 complex binds to both promoters and positively regulates transcription (51).
Like P. aeruginosa, phytopathogenic strains of P. syringae are normally nonmucoid in vitro. Kidambi et al. (28) previously showed that exposure to copper ions stimulated alginate production in selected strains of P. syringae. Furthermore, an indigenous plasmid designated pPSR12 conferred constitutive alginate production to P. syringae pv. syringae FF5. pPSR12 does not contain homologs of the biosynthetic or regulatory genes which control alginate production in P. aeruginosa; instead this plasmid presumably contains regulatory genes which remain uncharacterized (28). Mutagenesis of FF5(pPSR12) with Tn5 resulted in the isolation of alginate-defective (Alg−) mutants, including FF5.31 and FF5.32 (28). The Tn5 insertion in FF5.31 was located in algL, which encodes alginate lyase. Alginate production in FF5.31 was restored by pSK2, a cosmid clone containing homologues of algD, alg8, alg44, algG, algX, algL, algF, and algA. The order and arrangement of the alginate structural gene cluster were virtually identical to those previously described for P. aeruginosa. Complementation analyses, however, indicated that the structural gene clusters in P. aeruginosa and P. syringae were not functionally interchangeable when expressed from their native promoters (44).
In the present study, the Alg− mutant FF5.32 was shown to contain a Tn5 insertion in algR1. Unlike P. aeruginosa, expression from the P. syringae algD promoter (PsalgD) did not require a functional copy of algR1. Nucleotide sequence analysis indicated that PsalgD did not contain recognizable AlgR1 binding sites, which helps explain the differential regulation of alginate gene expression in P. aeruginosa and P. syringae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Table 1 lists the bacterial strains and plasmids used in the present study. Pseudomonas spp. were routinely maintained at 28°C on King’s medium B (29), mannitol-glutamate (MG) medium (25), or MG medium supplemented with yeast extract at 0.25 g/liter (MGY); Escherichia coli strains were grown on Luria-Bertani (LB) medium (36) at 37°C. Antibiotics were added to the media at the following concentrations: ampicillin, 100 μg/ml; tetracycline, 25 μg/ml; kanamycin, 25 μg/ml; spectinomycin, 25 μg/ml; streptomycin, 25 μg/ml; piperacillin, 250 μg/ml; and chloramphenicol, 25 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Escherichia coli | ||
| DH5α | 48 | |
| Pseudomonas syringae pv. syringae | ||
| FF5 | Cus; no detectable plasmids, nonmucoid | 28 |
| FF5.31 | Cur Kmr; contains pPSR12, nonmucoid, algL::Tn5 | 44 |
| FF5.32 | Cur Kmr; contains pPSR12, nonmucoid, algR1::Tn5 | 28 |
| Plasmids | ||
| pPSR12 | Cur Smr; 200 kb, confers constitutive alginate production to P. syringae pv. syringae FF5 | 28 |
| pSK2 | Tcr; contains alginate biosynthetic cluster from P. syringae pv. syringae FF5 in pRK7813 | 44 |
| pRK2013 | Kmr; helper plasmid | 15 |
| pRK415 | Tcr; RK2-derived cloning vector | 26 |
| pRK7813 | Tcr; cosmid vector | 23 |
| pCP13 | Tcr; cosmid vector | 10 |
| pBluescript SK(+) | Apr; ColE1 origin, cloning vehicle | Stratagene |
| pRG960sd | Smr Spr; contains promoterless uidA with start codon and Shine-Dalgarno sequence | 58 |
| pBBR1MCS | Cmr; 4.7-kb broad-host-range cloning vector | 30 |
| pBBR.Gus | Cmr; 6.6-kb promoter probe vector containing uidA in pBBR1MCS | 43 |
| pCR2.1 | Apr Kmr; 3.9-kb cloning vector | Invitrogen |
| pADP | Apr Kmr; contains PsalgD as a 2.7-kb PCR product in pCR2.1 | This study |
| pAPDP | Cmr; 7.4-kb contains PsalgD as a 2.7-kb HindIII-EcoRV fragment in pBBR.Gus | This study |
| pAPDPΔ15 | Cmr; derivative of pAPDP containing a 1.5-kb deletion from the 5′ end of PsalgD | This study |
| pAPDPΔ23 | Cmr; derivative of pAPDP containing a 2.3-kb deletion from the 5′ end of PsalgD | This study |
| pSK3 | Smr Spr; contains a 1.0-kb fragment from PsalgD in pRG960sd in the transcriptionally active orientation | 44 |
| pSK4 | Smr Spr; contains a 1.0-kb fragment from PsalgD in pRG960sd in the transcriptionally inactive orientation | 44 |
| pAP32 | Tcr Kmr; contains Tn5-inactivated alginate genes from FF5.32 in pRK7813 | This study |
| pAP32.1 | Apr Kmr; contains a 5.3-kb BamHI fragment consisting of 2.8 kb from Tn5 and 2.5 kb of FF5.32 DNA in pBluescript SK(+) | This study |
| pMF4 | Tcr; cosmid clone from FF5(pPSR12) in pRK7813 | This study |
| pMF6 | Tcr; cosmid clone from FF5(pPSR12) in pRK7813 | This study |
| pMF6.1 | Apr; contains a 2.7-kb EcoRI fragment from pMF6 | This study |
| pMF6.2 | Apr; contains a 2.0-kb PstI fragment from pMF6 | This study |
| pMF6.21 | Tcr; 2.0-kb PstI fragment from pMF6.2 in pRK415 in the transcriptionally active orientation with respect to lacZ and algR1 | This study |
| pMF6.22 | Tcr; 2.0-kb PstI fragment from pMF6.2 in pRK415 in the transcriptionally inactive orientation with respect to lacZ and algR1 | This study |
| pAD1039 | Tcr; contains algR1 from P. aeruginosa in pCP13 | V. Kapatral |
Molecular genetic techniques.
Plasmid DNA was isolated from Pseudomonas spp. by alkali lysis (48). Restriction enzyme digests, agarose gel electrophoresis, Southern transfers, and isolation of DNA fragments from agarose gels were performed by standard methods (48). Genomic DNA was isolated from P. syringae by established procedures (56), and a total genomic library of FF5.32 was constructed in pRK7813 as described previously (2). Clones were mobilized into nonmucoid recipient strains by using a triparental mating procedure and the mobilizer plasmid pRK2013 (4).
DNA fragments were isolated from agarose gels by electroelution (48) and labelled with digoxigenin (Genius labelling and detection kit; Boehringer Mannheim, Indianapolis, Ind.) or with [α-32P]dCTP by using the Rad Prime DNA Labeling System (Gibco BRL, Gaithersburg, Md.). Hybridizations and posthybridization washes were conducted under high-stringency conditions (57).
Isolation and quantitation of alginate.
Selected strains were inoculated by dilution streaking to MGY agar (three plates per strain) and incubated at 28°C for 72 h. Each plate was handled separately for quantification of alginate. The cells were washed from each plate and resuspended in 0.9% NaCl. Removal of cellular material from the mucoid growth and estimation of the alginate content and total cellular protein were performed as described previously (35). Alginic acid from seaweed (Macrocystis pyrifera; Sigma Chemical Co., St. Louis, Mo.) was used as a standard in these experiments. Mean values of three replicate determinations were expressed as micrograms of alginate per milligram of protein.
Construction of transcriptional fusions.
PsalgD was initially cloned in pCR2.1 as a 2.7-kb PCR product. Plasmid pSK2 was used as template, and the following oligonucleotides were used as primers: forward primer, 5′ TGGTGCTGGAAATATCCACACC (located 100 bp downstream of the presumed translational start site of algD [P1 in Fig. 1A]); and reverse primer, 5′ AATTCTGCCAGTCCAGCCACTGAC (P2 in Fig. 1A). Following amplification of the 2.7-kb PCR product, ligation in pCR2.1, and transformation into E. coli DH5α, plasmid pAPD was recovered. The promoter probe construct, pBBR.Gus, which contains a promoterless glucuronidase gene (uidA) downstream of the polylinker in pBBR1MCS (43), was used to more precisely define the promoter region upstream of algD. pAPD was digested with HindIII and EcoRV, and the 2.7-kb insert was isolated, end-filled with Klenow, and ligated into pBBR.Gus. Transformants were selected on LB agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and chloramphenicol, and pAPDP was found to contain the algD::uidA fusion in the transcriptionally active orientation.
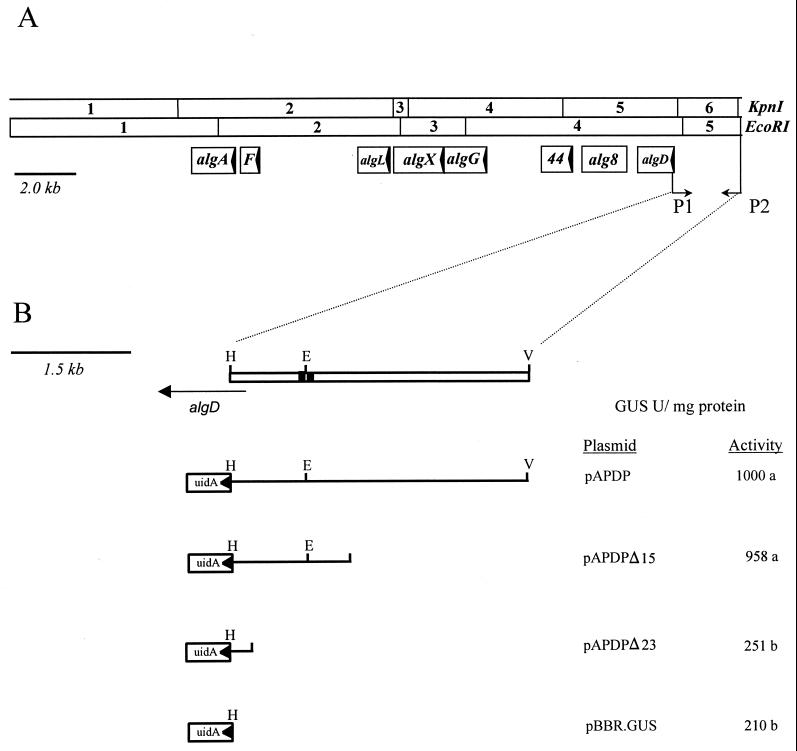
FIG. 1.
(A) Physical and functional map of the alginate structural gene cluster in Pseudomonas syringae pv. syringae FF5. The arrows within each open reading frame indicate the direction of translation. The locations of the primers (P1 and P2) used to amplify the algD promoter region are indicated. Abbreviations: F, algF; 44, alg44. (B) Expanded view of the region amplified with primers P1 and P2. The location and orientation of the coding region for algD are shown (horizontal arrow). The black boxes flanking the EcoRI site indicate the consensus sequence recognized by AlgT (ς22). The location and orientation of the algD::uidA transcriptional fusions are indicated; GUS activity is shown in the column adjacent to each construct. Values followed by the same letter were not significantly different (P = 0.01). Abbreviations: E, EcoRI; H, HindIII; V, EcoRV.
Exonuclease III (ExoIII) was used to determine the minimal size of the PsalgD promoter. pAPDP was digested with ClaI and ApaI, which generate ExoIII-sensitive and ExoIII-resistant sites, respectively. Staggered deletions in the PsalgD promoter region were generated by following the protocols supplied with the Erase-a-Base kit (Promega, Madison, Wis.). Transcriptional fusions were then mobilized into FF5(pPSR12) and assayed for glucuronidase activity as described below.
GUS assays.
Transcriptional activity was initially screened by spotting bacterial suspensions (absorbance at 600 nm of 0.1) on MG agar medium amended with spectinomycin and 20 μg of X-Gluc (5-bromo-4-chloro-3-indolylglucuronide) per ml; the plates were then incubated at 28°C for 24 to 72 h. Glucuronidase (GUS) activity was quantified by fluorometric analysis of cells grown for 18 to 20 h in 3 ml of MG medium. Fluorescence was monitored with a Fluoroscan II version 4.0 microplate reader (ICN Biomedicals, Inc., Costa Mesa, Calif.) in 96-well microtiter plates. GUS activity was expressed in units per milligram of protein, with 1 U being equivalent to 1 nmol of methylumbelliferone formed per min. Values presented for GUS activity represent the average of three replicates per experiment. When significant differences in GUS activity were detected, the experiment was repeated.
DNA sequencing and analysis.
Nucleotide sequencing reactions were performed by the dideoxynucleotide method with AmpliTaq DNA polymerase (Perkin-Elmer, Foster City, Calif.). Automated DNA sequencing was performed with an ABI 373A apparatus and the ABI PRISM Dye Primer cycle-sequencing kit (Perkin-Elmer). Automated sequencing was provided by the Oklahoma State University Recombinant DNA/Protein Resource Facility. The Tn5 insertion in FF5.32 was localized by sequencing the DNA flanking the transposon by using the oligonucleotide 5′ GGTTCCGTTCAGGACGCTAC, which is derived from the border region of IS50. Sequence data were aligned and homology searches were executed by using the University of Wisconsin Genetics Computer Group (UWGCG) sequence analysis package, version 9.0. Sequences associated with ς22 and AlgR1 binding were located by using the MOTIFS program included with the UWGCG software.
Nucleotide sequence accession numbers.
The nucleotide sequences described in this study were deposited in GenBank under accession no. AF131199 (fimS-algR1-hemC) and AF131068 (PsalgD).
RESULTS
Location of Tn5 insertion in FF5.32.
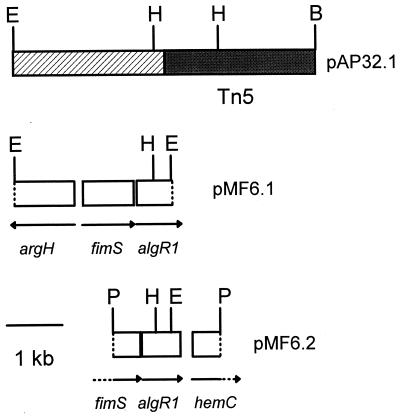
A genomic library of FF5.32 was constructed in pRK7813, and a clone containing the Tn5 insertion from FF5.32 was recovered and designated pAP32. The internal BamHI site in Tn5 and 2.5 kb of FF5.32 DNA were cloned from pAP32 into pBluescript SK(+), resulting in a clone named pAP32.1 (Fig. 2). A primer specific for the border region of IS50 was used to sequence approximately 300 bp of FF5.32 DNA flanking the Tn5 insertion site. This sequence showed 76% nucleotide identity to algR1 from P. aeruginosa, and the Tn5 insertion was located at nucleotide 51 of algR1 from P. aeruginosa (12).
FIG. 2.
Constructs used for the cloning and sequencing of algR1 from P. syringae pv. syringae FF5. pAP32.1 is a subclone containing Tn5 (shaded region) and flanking DNA from P. syringae pv. syringae FF5.32 (hatched region). The HindIII-EcoRI fragment in pAP32.1 was used as a probe for algR1 in the current study. pMF6.1 and pMF6.2 are subclones derived from pMF6, a cosmid which complemented FF5.32 for alginate production. The 2.0-kb PstI fragment in pMF6.2 was sequenced on both strands and shown to contain an intact copy of algR1. Abbreviations: B, BamHI; E, EcoRI; H, HindIII; P, PstI.
Genomic DNA from FF5(pPSR12) and FF5.32 was digested with EcoRI and analyzed by Southern blotting with the 2.3-kb HindIII-EcoRI fragment from pAP32.1 as a probe (Fig. 2). The probe hybridized to 2.7- and 8.4-kb EcoRI fragments in FF5(pPSR12) and FF5.32, respectively (data not shown). These results indicated that the region associated with algR1 was located in a 2.7-kb EcoRI fragment, and the 2.7-kb fragment was inactivated by Tn5 (5.7 kb) in FF5.32.
Cloning of algR1 from P. syringae.
A genomic library of P. syringae pv. syringae FF5(pPSR12) was previously constructed in pRK7813 (44). In the current study, the 2.3-kb HindIII-EcoRI fragment from pAP32.1 (Fig. 2) was used to screen the library for clones containing the complete algR1 coding region. Seven cosmid clones hybridized with the probe; two clones designated pMF4 and pMF6 were chosen for further study and contained a 2.7-kb EcoRI fragment which hybridized with the probe. This fragment was subcloned from pMF6 in pBluescript SK(+), resulting in pMF6.1 (Fig. 2). Sequence information for pMF6.1 was generated with the T7 and T3 primers and indicated that this fragment contained DNA homologous to argH, fimS, and algR1. In previous studies, the fimS gene showed relatedness to sensor kinases of two-component systems and mapped immediately upstream of algR1 in P. aeruginosa (61). It is important to note that fimS, which was also named algZ (63), is distinct from the algZ described by Baynham and Wozniak (3). To avoid further confusion in nomenclature, the name “fimS” will be used hereafter to describe the sensor kinase which maps adjacent to algR1. In P. syringae, argH, which encodes arginosuccinate lyase, mapped adjacent to fimS; in P. aeruginosa, argH was divergently transcribed with respect to both fimS and algR1 (37, 63). Sequence analysis of pMF6.1 indicated that this arrangement is conserved in P. syringae (Fig. 2).
Sequence analysis indicated that pMF6.1 contained 560 bp of algR1 but lacked approximately 180 bp located at the 3′ end. Southern blot analysis of pMF6 and pMF6.1 suggested that the intact algR1 was probably contained in a 2.0-kb PstI fragment; this was subcloned in pBluescript SK(+) and designated pMF6.2 (Fig. 2). pMF6.2 was completely sequenced on both strands and shown to contain DNA homologous to the 3′ end of fimS (585 bp), an intact copy of algR1 (747 bp), and the 5′ end of hemC (432 bp). In P. aeruginosa, hemC encodes porphobilinogen deaminase and maps adjacent to algR1 (40). The P. syringae homologues showed a high degree of relatedness to the corresponding P. aeruginosa genes; for example, nucleotide identity between fimS, algR1, and hemC in the two species was 88, 84, and 80%, respectively. Furthermore, the algR1 homologue in P. syringae showed extensive relatedness (86 to 88% nucleotide identity) to algR from Azotobacter vinelandii (42) and to pprA, an algR1 homologue in P. putida (59). In P. aeruginosa, AlgR1 contains two aspartate residues (D54 and D85) which have been suggested to function as phosphorylation sites (32, 61); both aspartate residues were present in the predicted translation product of algR1 from P. syringae. A consensus sequence for ς22 was located 108 bp upstream of the algR1 translational start site, a location which is also conserved in P. aeruginosa (63).
Complementation experiments.
pMF4 and pMF6, the cosmid clones containing argH, fimS, algR1, and hemC, were evaluated for their ability to complement P. syringae pv. syringae FF5.32 for alginate production. Transconjugants of FF5.32 containing pMF4 or pMF6 were visibly mucoid and produced significantly more alginate than the mutant FF5.32 did (Table 2). Since Tn5 frequently causes polar mutations on downstream genes, the 2.0-kb PstI fragment in pMF6.2 was used to investigate whether the Alg− phenotype in FF5.32 was caused by the mutation in algR1. pMF6.2 contains an intact copy of algR1 with the cognate ς22 recognition site and truncated copies of fimS and hemC (Fig. 2). The 2.0-kb PstI fragment in pMF6.2 was subcloned in pRK415 to form pMF6.21 and pMF6.22, which contain algR1 in the transcriptionally active and inactive orientations with respect to the lac promoter (Table 1). Both pMF6.21 and pMF6.22 restored alginate production to FF5.32 (Table 2), indicating that the Alg− phenotype of FF5.32 was caused by the Tn5 insertion in algR1. FF5.32 was complemented with both clones irrespective of the orientation of the lac promoter and without the addition of isopropyl-β-d-thiogalactopyranoside (IPTG), indicating that a functional promoter for algR1 was present on the 2.0-kb PstI fragment. To further confirm that FF5.32 was indeed an algR1 mutant, we investigated whether this mutant could be complemented by algR1 from P. aeruginosa. Plasmid pAD1039, which contains algR1 from P. aeruginosa (Table 1), complemented FF5.32 and restored alginate production in the mutant to a level equivalent to FF5(pPSR12) (data not shown).
TABLE 2.
Alginate production by derivatives of P. syringae pv. syringae FF5
| Strain | Alginate production (μg/mg of protein)a |
|---|---|
| FF5(pPSR12) | 3,791a |
| FF5.32 | 401b |
| FF5.32(pMF4) | 2,635a |
| FF5.32(pMF6) | 2,619a |
| FF5.32(pMF6.21) | 3,450a |
| FF5.32(pMF6.22) | 3,804a |
Mean values followed by the same letter are not significantly different at P = 0.05 by Duncan’s multiple-range test.
Expression of the PsalgD promoter does not require AlgR1.
In P. aeruginosa, AlgR1 is required for expression of the algD promoter (PalgD) and has been shown to bind PalgD at several conserved sites (24, 39). A portion of PsalgD was previously cloned as a 1-kb fragment in the promoter probe vector, pRG960sd, creating pSK3 (PsalgD::uidA; transcriptionally active orientation) and pSK4 (uidA::PsalgD; transcriptionally inactive) (44). In the present study, we investigated whether PsalgD was transcriptionally active in FF5.32, the algR1 mutant. GUS activities in FF5(pPSR12) and FF5.32(pSK3) were not significantly different (Table 3), indicating that a functional copy of algR1 was not required for transcription of algD in P. syringae.
TABLE 3.
GUS activity for P. syringae pv. syringae FF5 and FF5.32 containing various promoter constructs with the algD upstream region
| Straina | GUS activity (U/mg of protein)b in strains containing:
|
||
|---|---|---|---|
| pSK3 | pSK4 | pRG960sd | |
| FF5(pPSR12) | 537a | 88b | 66b |
| FF5.32 | 398a | 82b | 64b |
FF5(pPSR12) is the wild type, and FF5.32 is an algR1 mutant derived from FF5(pPSR12).
Mean values followed by the same letter are not significantly different at P = 0.05 by the Student-Newman Keuls test. pSK3 contains the algD promoter in the transcriptionally active orientation, pSK4 contains algD in the transcriptionally inactive orientation, and pRG960sd is the vector used for construction of pSK3 and pSK4. FF5(pPSR12, pSK3) and FF5.32(pRG960sd) were regarded as positive and negative controls for the GUS assay, respectively.
Analysis of the PsalgD promoter.
To more fully characterize the minimum sequence necessary for algD expression in P. syringae, we constructed a series of deletions from the 5′ (EcoRV) end of the PsalgD promoter (Fig. 1B). A new construct, pAPDP (Fig. 1B), was designed for this purpose since the pBBR.Gus polylinker was more amenable to deletion analysis than was the multicloning site in pRG960sd, the vector used for construction of pSK3. Two deletion derivatives of pAPDP, pAPDPΔ15 and pAPDPΔ23, proved useful for delineating the algD promoter region; sequence analysis indicated that these two constructs lacked 1.5 and 2.3 kb of DNA downstream of the EcoRV site, respectively. FF5(pPSR12, pAPDPΔ15) (Fig. 1B) retained the full level of GUS activity exhibited by FF5(pPSR12, pAPDP) (Fig. 1B), suggesting that the 1.5-kb region downstream of the EcoRV site was dispensable for promoter activity. However, GUS activity in FF5(pPSR12, pAPDPΔ23) was 3.8-fold lower than in FF5(pPSR12, pAPDPΔ15), demonstrating that deletion of an additional 0.8 kb from the 5′ end of pAPDPΔ15 virtually eliminated PsalgD promoter activity (Fig. 1B).
Sequence analysis of the PsalgD promoter in pAPDPΔ15 indicated that it contained a putative AlgT (ς22) recognition site 508 bp upstream of the predicted algD translational start site (Fig. 3). In this respect, PsalgD is similar to the algD promoter in P. aeruginosa where a long, untranslated leader sequence is located between the algD translational start site and the ς22 binding region (11, 51). However, PsalgD lacked the AlgR1 binding sites, which are located upstream of the algD transcriptional start in P. aeruginosa (Fig. 3) (24, 38). The absence of these conserved motifs for AlgR1 binding could explain why the P. syringae algD promoter does not require a functional copy of algR1 for transcriptional activity.
FIG. 3.
Alignment of the algD promoter sequences from P. syringae pv. syringae FF5 (Ps algD) and P. aeruginosa (Pa algD). The P. aeruginosa algD promoter was previously reported (24, 39); the nucleotides for this sequence are shown on the left, with +1 (asterisk) corresponding to the transcriptional start site. Nucleotides for the P. syringae pv. syringae algD promoter are shown on the right. The EcoRI site in the P. syringae sequence corresponds to the left border of EcoRI fragment 5 in Fig. 1A. Gaps (––) were used to maximize the alignment, and identical bases are shaded. The AlgR1 binding sites (ABS) in the P. aeruginosa algD promoter are shown in bold and double-underlined. The ς22 recognition sequence in both species is indicated in bold and single-underlined. The algD translational start site and coding region are shown in bold (algD–→).
DISCUSSION
The AlgR1 mutant characterized in the present study, FF5.32, was previously shown to be completely defective in alginate synthesis (28), thereby demonstrating that AlgR1 is absolutely required for alginate production in P. syringae. However, the role of AlgR1 in P. syringae is unclear, since this protein is not required for algD expression; it remains possible that AlgR1 is required for transcriptional activation of algC in P. syringae, which is true in P. aeruginosa (65). Alternatively, AlgR1 may function differently in P. syringae, perhaps as part of a signal transduction cascade which controls alginate production. A complex regulatory network for alginate synthesis in P. syringae seems plausible, since plasmid-encoded regulatory genes are known to mediate the constitutive production of alginate in the P. syringae strains which harbor them (28).
The organization of the region flanking AlgR1 is conserved in both P. aeruginosa and P. syringae (argH-fimS-algR1-hemC). In both species, the ς22 recognition site preceding algR1 is located within the 3′ end of fimS (63). FimS shows relatedness to the histidine protein kinases which function as environmental sensors, and both AlgR1 and FimS are required for twitching motility in P. aeruginosa, a process mediated by type IV pili. Although type IV pili have been identified in P. syringae (47), our efforts to demonstrate twitching motility in P. syringae pv. syringae FF5 were completely unsuccessful; therefore, the involvement of AlgR1 in twitching motility in P. syringae remains unclear. It has also been proposed that FimS may function as the cognate sensor kinase for AlgR1, but the exact role of FimS in alginate production remains unclear (61, 63). Interestingly, phosphorylation of AlgR1 was not required for alginate production in P. aeruginosa (32).
Sequence analysis of the algR1 and algD upstream regions in P. syringae revealed the presence of ς22 recognition sites (Fig. 3). The ς22 recognition site identified in the algR1 upstream region was identical to that identified in P. aeruginosa, whereas the ς22 recognition sequence in PsalgD differed from the corresponding sequence in P. aeruginosa by a single nucleotide (51). Although the transcriptional start sites for algR1 and algD were not identified in P. syringae, the positions of the ς22 recognition sites relative to the translational start site are conserved in both species. The conservation of ς22 recognition sequences upstream of algR1 and algD strongly suggests that transcriptional activation of these genes requires a functional copy of algT. An algT homologue in P. syringae has recently been identified, and the role of algT in the transcriptional activation of algD and algR1 in P. syringae is under investigation (27).
The percent nucleotide identity in the algD coding region of P. syringae pv. syringae and P. aeruginosa ranged from 80 to 90% (Fig. 3 and data not shown); however, upstream of the translational start site, the relatedness between the two species diverged and nucleotide identity decreased to approximately 20% (Fig. 3). This divergence is consistent with the absence of specific sequences in PsalgD which are known to be involved in transcriptional activation of algD in P. aeruginosa. These include the consensus sequences for binding AlgR1 (24), integration host factor (38), and cyclic AMP receptor protein (13). Although some signals for activation of the algD promoter are conserved in P. aeruginosa and P. syringae (5, 31, 44), the algD promoter in P. syringae is stimulated by exposure to copper ions (44) and does not require a functional copy of AlgR1 for transcriptional activation. Recently, Yu et al. (64) provided the first genetic evidence for the role of alginate in the virulence and epiphytic fitness of P. syringae. Consequently, the differential regulation of algD expression in P. syringae and CF isolates of P. aeruginosa and the marked divergence in their algD promoter regions probably reflect their adaptation to plant and human hosts, respectively.
It remains possible that some unknown regulatory protein binds to PsalgD and that this regulator recognizes different signals (such as copper) and activates the algD promoter in P. syringae. Perhaps this putative DNA binding protein was recruited during the evolutionary divergence of P. aeruginosa and P. syringae to accommodate a different signal and perhaps another activator. The algD::uidA transcriptional fusion described in the present investigation could be used to screen for mutants lacking the unknown activator. Such experiments are under way and will probably reveal additional differences in the regulation of alginate biosynthesis in human and phytopathogenic bacteria.
ACKNOWLEDGMENTS
M.F. and A.P.V. contributed equally to this paper, and both should be regarded as first authors.
M.F. acknowledges financial support from the Egyptian government for his dissertation research. C.B. acknowledges support from the Oklahoma Agricultural Experiment Station and Public Health Service grant AI 43311-01 from the National Institutes of Health. A.M.C. acknowledges support by NIH grant AI 16790-18.
We thank V. Rangaswamy and F. Alarcón-Chaidez for help with graphics and sequence analysis and V. Kapatral for providing pAD1039.
REFERENCES
- 1.Aarons S J, Sutherland I W, Chakrabarty A M, Gallagher M P. A novel gene, algK, from the alginate biosynthetic cluster of Pseudomonas aeruginosa. Microbiology. 1997;143:641–652. doi: 10.1099/00221287-143-2-641. [DOI] [PubMed] [Google Scholar]
- 2.Barta T M, Kinscherf T G, Willis D K. Regulation of tabtoxin production by the lemA gene in Pseudomonas syringae. J Bacteriol. 1992;174:3021–3029. doi: 10.1128/jb.174.9.3021-3029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baynham P J, Wozniak D J. Identification and characterization of AlgZ, an AlgT-dependent DNA-binding protein required for Pseudomonas aeruginosa algD transcription. Mol Microbiol. 1996;22:97–108. doi: 10.1111/j.1365-2958.1996.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 4.Bender C L, Young S A, Mitchell R E. Conservation of plasmid DNA sequences in coronatine-producing pathovars of Pseudomonas syringae. Appl Environ Microbiol. 1991;57:993–999. doi: 10.1128/aem.57.4.993-999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry A, DeVault J D, Chakrabarty A M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989;171:2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd A, Ghosh M, May T B, Shinabarger D, Keogh R, Chakrabarty A M. Sequence of the algL gene of Pseudomonas aeruginosa and purification of its alginate lyase product. Gene. 1993;131:1–8. doi: 10.1016/0378-1119(93)90662-m. [DOI] [PubMed] [Google Scholar]
- 7.Chitnis C E, Ohman D E. Cloning of Pseudomonas aeruginosa algG, which controls alginate structure. J Bacteriol. 1990;172:2894–2900. doi: 10.1128/jb.172.6.2894-2900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitnis C E, Ohman D E. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Mol Microbiol. 1993;8:583–590. doi: 10.1111/j.1365-2958.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 9.Chu L, May T B, Chakrabarty A M, Misra T K. Nucleotide sequence and expression of the algE gene involved in alginate biosynthesis by Pseudomonas aeruginosa. Gene. 1991;107:1–10. doi: 10.1016/0378-1119(91)90290-r. [DOI] [PubMed] [Google Scholar]
- 10.Darzins A, Chakrabarty A M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984;159:9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deretic V, Gill J F, Chakrabarty A M. Pseudomonas aeruginosa infection in cystic fibrosis: nucleotide sequence and transcriptional regulation of the algD gene. Nucleic Acids Res. 1987;15:4567–4581. doi: 10.1093/nar/15.11.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic V, Kishit R, Konyecsni W M, Chakrabarty A M, Misra T K. The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes. J Bacteriol. 1989;171:1278–1283. doi: 10.1128/jb.171.3.1278-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVault J D, Hendrickson W, Kato J, Chakrabarty A M. Environmentally regulated algD promoter is responsive to the cAMP receptor protein in Escherichia coli. Mol Microbiol. 1991;5:2503–2509. doi: 10.1111/j.1365-2958.1991.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 14.DeVries C A, Ohman D E. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternative sigma factor, and shows evidence for autoregulation. J Bacteriol. 1994;176:6677–6687. doi: 10.1128/jb.176.21.6677-6687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figurski D, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin M J, Ohman D E. Identification of algF in the alginate biosynthetic gene cluster of Pseudomonas aeruginosa which is required for alginate acetylation. J Bacteriol. 1993;175:5057–5065. doi: 10.1128/jb.175.16.5057-5065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin M J, Ohman D E. Identification of algI and algJ in the Pseudomonas aeruginosa alginate biosynthetic gene cluster which are required for alginate O acetylation. J Bacteriol. 1996;178:2186–2195. doi: 10.1128/jb.178.8.2186-2195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gacesa P. Bacterial alginate biosynthesis—recent progress and future prospects. Microbiology. 1998;144:1133–1143. doi: 10.1099/00221287-144-5-1133. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg J B, Gorham W B, Flynn J L, Ohman D E. A mutation in algN permits trans activation of alginate production by algT in Pseudomonas species. J Bacteriol. 1993;175:1303–1308. doi: 10.1128/jb.175.5.1303-1308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershberger C D, Ye R W, Parsek M R, Xie Z-D, Chakrabarty A M. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (ςE) Proc Natl Acad Sci USA. 1995;92:7941–7945. doi: 10.1073/pnas.92.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Ohman D E. Deletion of algK in mucoid Pseudomonas aeruginosa blocks alginate polymer formation and results in uronic acid secretion. J Bacteriol. 1998;180:634–641. doi: 10.1128/jb.180.3.634-641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones J D G, Gutterson N. An efficient mobilizable cosmid vector, pRK7813, and its use in a rapid method for marker exchange in Pseudomonas fluorescens strain HV37a. Gene. 1987;61:299–306. doi: 10.1016/0378-1119(87)90193-4. [DOI] [PubMed] [Google Scholar]
- 24.Kato J, Chakrabarty A M. Purification of the regulatory protein AlgR1 and its binding in the far upstream region of the algD promoter in P. aeruginosa. Proc Natl Acad Sci USA. 1991;88:1760–1764. doi: 10.1073/pnas.88.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keane P J, Kerr A, New P B. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust J Biol Sci. 1970;23:585–595. [Google Scholar]
- 26.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 27.Keith, L. M. W., and C. L. Bender. Unpublished results.
- 28.Kidambi S P, Sundin G W, Palmer D A, Chakrabarty A M, Bender C L. Copper as a signal for alginate synthesis in Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 1995;61:2172–2179. doi: 10.1128/aem.61.6.2172-2179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King E O, Ward M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 30.Kovach M E, Phillips R W, Elzer P H, Roop III R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 31.Leitáo J H, Fialho A M, Correia I S. Effects of growth temperature on alginate synthesis and enzymes in Pseudomonas aeruginosa variants. J Gen Microbiol. 1992;138:605–610. doi: 10.1099/00221287-138-3-605. [DOI] [PubMed] [Google Scholar]
- 32.Ma S, Selvaraj U, Ohman D E, Quarless R, Hassett D J, Wozniak D J. Phosphorylation-independent activity of the response regulators AlgB and AlgR in promoting alginate biosynthesis in mucoid Pseudomonas aeruginosa. J Bacteriol. 1998;180:956–968. doi: 10.1128/jb.180.4.956-968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maharaj R, May T B, Wang S-K, Chakrabarty A M. Sequence of the alg8 and alg44 genes involved in the synthesis of alginate by Pseudomonas aeruginosa. Gene. 1993;136:267–269. doi: 10.1016/0378-1119(93)90477-k. [DOI] [PubMed] [Google Scholar]
- 34.Mathee K, McPherson C J, Ohman D E. Posttranslational control of the algT (algU)-encoded ς22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN) J Bacteriol. 1997;179:3711–3720. doi: 10.1128/jb.179.11.3711-3720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May T B, Chakrabarty A M. Isolation and assay of Pseudomonas aeruginosa alginate. Methods Enzymol. 1994;235:295–304. doi: 10.1016/0076-6879(94)35148-1. [DOI] [PubMed] [Google Scholar]
- 36.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 37.Mohr C D, Deretic V. Gene-scrambling mutagenesis: generation and analysis of insertional mutations in the alginate regulatory region of Pseudomonas aeruginosa. J Bacteriol. 1990;172:6252–6260. doi: 10.1128/jb.172.11.6252-6260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr C D, Deretic V. In vitro interactions of the histone-like protein IHF with the algD promoter, a critical site for control of mucoidy in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1992;189:837–844. doi: 10.1016/0006-291x(92)92279-7. [DOI] [PubMed] [Google Scholar]
- 39.Mohr C D, Leveau J H J, Krieg D P, Hibler N S, Deretic V. AlgR-binding sites within the algD promoter make up a set of inverted repeats separated by a large intervening segment of DNA. J Bacteriol. 1992;174:6624–6633. doi: 10.1128/jb.174.20.6624-6633.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohr C D, Sonsteby S K, Deretic V. The Pseudomonas aeruginosa homologs of hemC and hemD are linked to the gene encoding the regulator of mucoidy AlgR. Mol Gen Genet. 1994;242:177–184. doi: 10.1007/BF00391011. [DOI] [PubMed] [Google Scholar]
- 41.Monday S R, Schiller N L. Alginate synthesis in Pseudomonas aeruginosa: the role of AlgL (alginate lyase) and AlgX. J Bacteriol. 1996;178:625–632. doi: 10.1128/jb.178.3.625-632.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunez C E, Moreno S, Soberon-Chavez G, Espin E G. GenBank accession no. AF077237. 1998. [Google Scholar]
- 43.Peñaloza-Vázquez A, Bender C L. Characterization of CorR, a transcriptional activator which is required for biosynthesis of the phytotoxin coronatine. J Bacteriol. 1998;180:6252–6259. doi: 10.1128/jb.180.23.6252-6259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peñaloza-Vázquez A, Kidambi S P, Chakrabarty A M, Bender C L. Characterization of the alginate biosynthetic gene cluster in Pseudomonas syringae pv. syringae. J Bacteriol. 1997;179:4464–4472. doi: 10.1128/jb.179.14.4464-4472.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pier G B. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 46.Rehm B H A, Valla S. Bacterial alginates: biosynthesis and applications. Appl Microbiol Biotechnol. 1997;48:281–288. doi: 10.1007/s002530051051. [DOI] [PubMed] [Google Scholar]
- 47.Roine E, Raineri D M, Romantschuk M, Wilson M, Nunn D N. Characterization of type IV pilus genes in Pseudomonas syringae pv. tomato DC3000. Mol Plant-Microbe Interact. 1998;11:1048–1056. doi: 10.1094/MPMI.1998.11.11.1048. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 49.Schiller N L, Monday S R, Boyd C M, Keen N T, Ohman D E. Characterization of the Pseudomonas aeruginosa alginate lyase gene (algL): cloning, sequencing, and expression in Escherichia coli. J Bacteriol. 1993;175:4780–4789. doi: 10.1128/jb.175.15.4780-4789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schurr M J, Martin D W, Mudd M H, Deretic V. Gene cluster controlling conversion of alginate-overproducing phenotypes in Pseudomonas aeruginosa: functional analysis in a heterologous host and role in the instability of mucoidy. J Bacteriol. 1994;176:3375–3382. doi: 10.1128/jb.176.11.3375-3382.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schurr M J, Yu H, Boucher J C, Hibler N S, Deretic V. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (ςE) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J Bacteriol. 1995;177:5670–5679. doi: 10.1128/jb.177.19.5670-5679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schurr M J, Yu H, Martinez-Salazar J M, Boucher J C, Deretic V. Control of AlgU, a member of the ςE-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol. 1996;178:4997–5004. doi: 10.1128/jb.178.16.4997-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shankar S, Ye R, Schlictman D, Chakrabarty A M. Exopolysaccharide alginate synthesis in Pseudomonas aeruginosa: enzymology and regulation of gene expression. Adv Enzymol. 1995;70:221–255. doi: 10.1002/9780470123164.ch4. [DOI] [PubMed] [Google Scholar]
- 54.Shinabarger D, Berry A, May T B, Rothmel R, Fialho A, Chakrabarty A M. Purification and characterization of phosphomannose isomerase-guanosine diphospho-d-mannose pyrophosphorylase. J Biol Chem. 1991;266:2080–2088. [PubMed] [Google Scholar]
- 55.Shinabarger D, May T B, Boyd A, Ghosh M, Chakrabarty A M. Nucleotide sequence and expression of the Pseudomonas aeruginosa algF gene controlling acetylation of alginate. Mol Microbiol. 1993;9:1027–1035. doi: 10.1111/j.1365-2958.1993.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 56.Staskawicz B J, Dahlbeck D, Keen N T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc Natl Acad Sci USA. 1984;81:6024–6028. doi: 10.1073/pnas.81.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sundin G W, Bender C L. Ecological and genetic analysis of copper and streptomycin resistance in Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 1993;59:1018–1024. doi: 10.1128/aem.59.4.1018-1024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van den Eede G, Deblaere R, Goethals K, Montagu M V, Holsters M. Broad host range and promoter selection vectors for bacteria that interact with plants. Mol Plant-Microbe Interact. 1992;5:228–234. doi: 10.1094/mpmi-5-228. [DOI] [PubMed] [Google Scholar]
- 59.Venturi V, Otten M, Korse V, Brouwer B, Leong J, Weisbeek P. Alginate regulatory and biosynthetic gene homologs in Pseudomonas putida WCS358: correlation with the siderophore regulatory gene pfrA. Gene. 1995;155:83–88. doi: 10.1016/0378-1119(94)00868-s. [DOI] [PubMed] [Google Scholar]
- 60.Wang S-K, Correia I S, Darzins A, Chakrabarty A M. Characterization of the Pseudomonas aeruginosa alginate (alg) gene region II. J Gen Microbiol. 1987;133:2303–2314. doi: 10.1099/00221287-133-8-2303. [DOI] [PubMed] [Google Scholar]
- 61.Whitchurch C B, Alm R A, Mattick J S. The alginate regulator AlgR and an associated sensor FimS are required for twitching motility in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1996;93:9839–9843. doi: 10.1073/pnas.93.18.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Z, Hershberger C D, Shankar S, Ye R W, Chakrabarty A M. Sigma factor–anti-sigma factor interaction in alginate synthesis: inhibition of AlgT by MucA. J Bacteriol. 1996;178:4990–4996. doi: 10.1128/jb.178.16.4990-4996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu H, Mudd M, Boucher J C, Schurr M J, Deretic V. Identification of the algZ gene upstream of the response regulator algR and its participation in control of alginate production in Pseudomonas aeruginosa. J Bacteriol. 1997;179:187–193. doi: 10.1128/jb.179.1.187-193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J, Peñaloza-Vázquez A, Bender C L. Role of the exopolysaccharide alginate in the pathogenicity of Pseudomonas syringae pv. syringae. Phytopathology. 1998;88:S102. [Google Scholar]
- 65.Zielinski N A, Maharaj R, Roychoudhury S, Danganan C E, Hendrickson W, Chakrabarty A M. Alginate synthesis in Pseudomonas aeruginosa: environmental regulation of the algC promoter. J Bacteriol. 1992;174:7680–7688. doi: 10.1128/jb.174.23.7680-7688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]