Abstract
Aquaglyceroporins (AQGPs), including AQP3, AQP7, AQP9, and AQP10, are transmembrane channels that allow small solutes across biological membranes, such as water, glycerol, H2O2, and so on. Increasing evidence suggests that they play critical roles in cancer. Overexpression or knockdown of AQGPs can promote or inhibit cancer cell proliferation, migration, invasion, apoptosis, epithelial–mesenchymal transition and metastasis, and the expression levels of AQGPs are closely linked to the prognosis of cancer patients. Here, we provide a comprehensive and detailed review to discuss the expression patterns of AQGPs in different cancers as well as the relationship between the expression patterns and prognosis. Then, we elaborate the relevance between AQGPs and malignant behaviors in cancer as well as the latent upstream regulators and downstream targets or signaling pathways of AQGPs. Finally, we summarize the potential clinical value in cancer treatment. This review will provide us with new ideas and thoughts for subsequent cancer therapy specifically targeting AQGPs.
Keywords: Aquaglyceroporin, Cancer, Expression pattern, Regulator, Signaling pathway
Introduction
Water is the source of life. Water entering and leaving cells are a basic process of metabolism. In the very beginning, simple diffusion was considered to be the major route of water, but membrane water permeability shown by some epithelia was too high to just be explained by simple diffusion, which inspired explorations on the existence of water-specific channels [1]. Until the historic discovery of a novel 28 kDa integral membrane protein (CHIP28) in human erythrocytes [2, 3], people gradually uncovered the veils of water channel proteins. Then, CHIP28 was named aquaporin 1(AQP-1) after its water transport functions were proven by Peter Agre et al. in Xenopus oocytes [4–7].
Aquaporins exert a profound influence on the regulation of water homeostasis by providing selective pores for the rapid movement of water across diverse cell membranes and regulating cell volume [8]. To date, 13 aquaporins have been found in mammals. Among them, orthodox aquaporins are permeable to water. However, aquaglyceroporins (AQGPs), another subclass of aquaporins, including AQP3, AQP7, AQP9, and AQP10, are capable of facilitating the transport of some small molecules across the membrane, especially glycerin and urea, in addition to water. They were noted to be genetically close to the known E.coli glycerol transport protein GlpF [9], and thus, they were also classified as “the GlpF group.”
Cancer is a threat to human health. Lipid metabolism is receiving much attention in cancer research today. Cancer cells rely on abnormal lipid metabolism to proliferate, metastasize, and adapt to the tumor microenvironment (TME) [10]. Moreover, aberrant uptake, storage, synthesis, and utilization of lipids have been detected in many cancers, and directly exacerbated tumorigenicity and malignancy [11]. Emerging evidence also shows that the functions of immune cells in the TME are closely related to abnormal lipid metabolism [12]. Aquaglyceroporins, as channels for glycerol, determine glycerol trafficking in and out of cells and subsequent lipid metabolism. Research has demonstrated that silencing AQP3 contributes to proliferation impairment and apoptosis via decreased glycerol uptake and lipid synthesis in gastric cancer cells [13, 14]. Hara-Chikuma and Verkman found that glycerol permeability via AQP3 is required for epidermal cell proliferation and tumorigenesis, as cellular glycerol is a key determinant of cellular ATP energy [15]; also, AQP3/PLD2 signaling module may be involved in the process of converting glycerol to phosphatidylglycerol in squamous cell carcinoma and basal cell carcinoma [16]. In mouse breast cancer models, lipid accumulation in Aqp7 KD tumors was detectable by Oil Red O staining [17]. Moreover, AQP9 participates in hepatic glycerol metabolism reprogramming in early rat liver cancer [18]. Therefore, in this review, we focus on aquaglyceroporins, which are not only channels for glycerin and water transportation, but also important biomarkers for predicting tumor prognosis and affecting malignant behaviors.
To date, no systematic review has further explored the relationship between aquaglyceroporins and cancer. In this review, we analyze how AQGPs affect the malignant behaviors of cancer by investigating the expression patterns of AQGPs and their relationship with cancer prognosis in hope of some new ideas beneficial to cancer treatment.
Structure
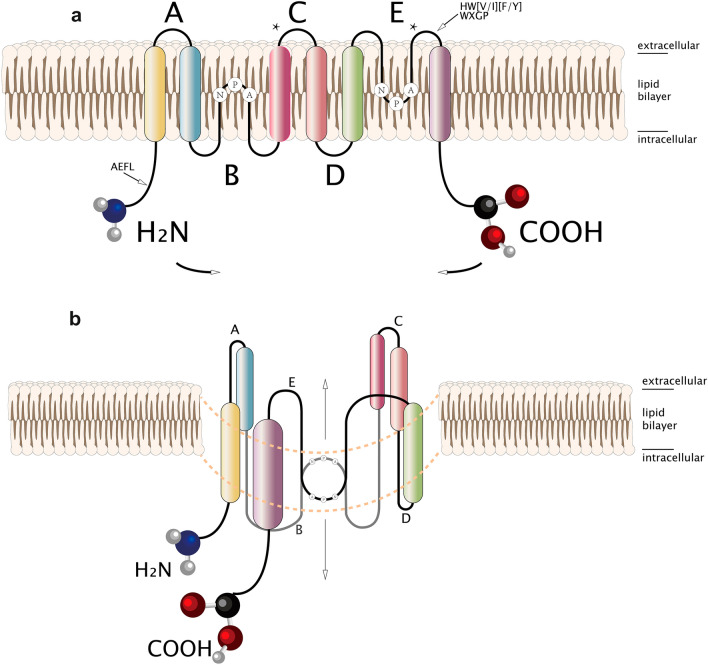
Members of the AQGPs show similar topology, including six nonpolar membrane-spanning domains of sufficient length, five connected loops consisting of three extracellular loops (A, C, E), and two intracellular loops (B, D), cytoplasmic-facing NH2 and COOH termini, and highly conserved motifs covering two tandem repeat Asp-Pro-Ala sequences (NPA box) located in loops B and E, respectively, one “AEFL” and one “HW[V/I][F/Y]WXGP” sequence[19–21]. The three-dimensional “hourglass model” is composed of homotetramers, and each monomer of the homotetramers has a functional water channel [22].
Surprisingly, the presence of two additional peptide spans, one in loop C and the other in loop E after the second NPA motif, was observed in all aquaglyceroporins but not in orthodox aquaporins[9, 19, 23]. Although the structural explanation for the functional difference between aquaporins and aquaglyceroporins has not reached a consensus today, these distinctive domains may be the key [9, 19] (See Fig. 1)
Fig. 1.
a Locations of NPA boxes, membrane-spanning domains, cytoplasmic-facing terminus are shown, and two additional peptide spans are denoted by asterisks; b Hourglass model for aquaglyceroporin membrane topology
Expression patterns and relationship with cancer prognosis
AQP3
AQP3 was the first aquaglyceroporin known and studied in humans and is expressed in a variety of tissues, including the renal collecting duct [24], respiratory epithelium [25], breast [26], stomach [27], and prostate [28]. Recently, an increasing number of researchers have pointed out that AQP3 is inclined to be of considerable importance in cancer development, which indicates that it may serve as a biomarker of cancer prognosis.
A recent study provided insight into the possible etiological theory that positive AQP3 expression was related to lymph node metastasis, invasion, and high TNM stage in patients with pancreatic ductal adenocarcinoma (PDAC) [29]. In addition, AQP3 expression was reinforced in later and more aggressive stages of PDAC [30]. Another study suggested that AQP3, regulated by estrogen, might be adopted as a diagnostic biomarker for the early detection of ovarian cancer [31]. Furthermore, enhanced expression of AQP3 was also correlated with lymph node metastasis in patients with colon and gastric cancer [32, 33]. In addition, a Chinese research team highlighted that preoperative serum AQP3 levels were significantly elevated in patients diagnosed with colon cancer, demonstrating its clinical value for the early screening of colon cancer [34]. Protein or mRNA expression levels of AQP3 are related to the TNM stage, lymph node status, relapse, metastasis, and some other clinical indicators, which ultimately contribute to cancer outcomes. See Table 1 for the relationship between AQP3 expression levels and cancer prognosis in detail.
Table 1.
Relationship between AQP3 expression levels and cancer prognosis
| Cancer types | Expression types | Expression and prognosis |
|---|---|---|
| Gastric carcinoma [35, 36] | mRNA | Higher expression is correlated with better OS |
| Pancreatic ductal adenocarcinoma [29] | protein | Higher expression is correlated with worse OS |
| Esophageal squamous cell carcinoma [37] | protein | Co-expression of AQP3 and AQP5 shows worse OS + DFS |
| Triple-negative breast cancer [38] | protein | Higher AQP3 and AQP5 expression shows worse OS + DFS |
| Breast cancer [39] | mRNA | Higher expression is correlated with worse RFS |
| HER2-positve early breast cancer [40] | protein | 40.3% positive expression shows worse DFS |
| HER2-positive early breast cancer [41] | mRNA | Positive expression shows worse RFS |
| Hepatocellular carcinoma [42] | protein | Higher expression is correlated with worse OS + DFS |
| Endometrioid carcinoma [43] | protein | Positive expression shows better OS + PFS |
| Urothelial carcinoma [44–47] | protein | High expression in CIS: usually poor prognosis MIBC: higher expression means better PFS |
| Ovarian cancer[48] | mRNA | Higher expression is correlated with better OS |
OS overall survival, DFS disease-free survival, RFS relapse-free survival, PFS progression-free survival, HER2 human epidermal growth factor receptor 2, CIS carcinoma in situ, MIBC muscle-invasive bladder cancer
As shown in Table 1, AQP3 is also expressed in many cancer tissues and cells, but its expression patterns differ from those of cancers. Given its relationship with cancer prognosis, we delve into its expression patterns in different cancers hoping for some new discoveries. Table 2 shows the expression levels of AQP3 in cancer tissues or cells and corresponding normal tissues or cells.
Table 2.
Expression levels of AQP3 in cancer tissues or cells and corresponding normal tissues or cells
| Cancer types | Tissues/cell lines | Methods | Expression of tumors (T) and normal tissues or cells (N) |
|---|---|---|---|
| GC [33] | tissues | RT-PCR, IF, WB | T > N |
| GC [49] | tissues | WB | T > N |
| GC [50, 51] | tissues | IHC | T > N |
| GC [14] | tissues | RT-PCR | T > N |
| GC [36] | tissues | TCGA database | T < N |
| HCC [42] | tissues | IHC | T > N |
| HCC [52] | tissues | qRT-PCR, WB, IHC | T > N |
| HCC [53] | tissues | qRT-PCR, WB | T > N |
| HCC [54] | tissues | qRT-PCR, WB, IHC | T > N |
| Breast cancer [38] | tissues | IHC | T > N |
| Breast cancer [39] | tissues | Oncomine database | T < N |
| Breast cancer [55] | tissues | qRT-PCR | T > N |
| PDAC [29] | tissues | WB, IHC | T > N |
| PDAC [30] | tissues | IHC | T > N |
| SCC [16] | tissues (skin) | IHC | T > N |
| SCC [56] | tissues (esophagus, oral) | IHC | T > N |
| SCC [57] | tissues (oral) | IHC | T > N |
| SCC [37] | tissues (esophagus) | IHC | T > N |
| NSCLC [58] | tissues, cell lines | qRT-PCR, WB | T > N |
| Colorectal carcinoma [59] | tissues | IHC | T > N |
| Prostate cancer [60] | cell lines | qRT-PCR, WB | T > N |
| Osteosarcoma [61] | tissues, cell lines | qRT-PCR | T > N |
| Ovarian carcinoma(hen) [31] | tissues | qRT-PCR, IHC | T > N |
| Nonmelanoma skin cancer [62] | tissues (skin) | IHC | BCC < SCC < N |
GC gastric carcinoma, HCC hepatocellular carcinoma, PDAC pancreatic ductal adenocarcinoma, SCC squamous cell carcinoma, NSCLC non-small cell lung carcinoma, BCC basal cell carcinoma
RT-PCR reverse transcription PCR, IF immunofluorescence, WB western blot, IHC immunohistochemistry, qRT-PCR quantitative real-time PCR
From Table 2, the expression level of AQP3 in most cancers is higher than that in the corresponding normal tissues or cells, particularly at the protein level, except for nonmelanoma skin cancer. From Table 1, at the protein level, overexpression of AQP3 or AQP3-positive often contributes to a worse prognosis except for endometrioid carcinoma and MIBC, indicating that AQP3 frequently acts as a villain in cancer.
The expression patterns of AQP3 in thyroid cancer, breast cancer, and prostate cancer are exceptional. In the thyroid, AQP3 expression was positive only in parafollicular cells (C cells). Nevertheless, in thyroid cancer, AQP3 mRNA and protein were only identified in medullary thyroid cancer derived from C cells [63], which might be interpreted as stimulation by hormones secreted by C cells such as calcitonin. In breast cancer, the highest level of expression of AQP3 was observed in endocrine-sensitive (YS1.2) breast cancer cells, followed by endocrine -resistant (pII) breast cancer cells, and the weakest expression was found in normal breast epithelial cells (MCF10A) [64], implicating that estrogen might act as an upstream regulator of AQP3. For prostate cancer (PC), our team demonstrated that AQP3 was primarily expressed in the membranes in the normal prostate epithelia, but in prostate cancer epithelia, AQP3 was often located in the cytoplasm [28]. Insang Hwang et al. achieved similar results [65]. It is worth noting that another study showed that AQP3 was expressed in the membrane and cytoplasm of LNCaP cells, an androgen-dependent cell line, and mainly in the cytoplasm of PC3 and Du145 cells, which are androgen-independent [66]. In summary, we observed an interesting phenomenon. In normal prostate epithelia, AQP3 is mainly found in the membrane. However, as the disease progresses to androgen-dependent PC, AQP3 often lies in the membrane and cytoplasm. When the disease progresses to the castration resistance stage, it mainly appears in the cytoplasm. Since the key to the pathological progression of prostate cancer is androgen, we hypothesize that androgen may also be responsible for the tendency of AQP3 to translocate from the cell membrane to the cytoplasm as prostate cancer progresses. Unfortunately, little work has been performed on our conjecture thus far. It seems reasonable that AQP3 can be regulated by hormones, including androgen, estrogen, and calcitonin. However, whether other hormones in the body have an influence and how they work is an issue that urgently needs to be verified. All of the above results show that AQP3, as a functional protein, is important for forecasting the prognosis of some cancers and indirectly indicates its feasibility as a therapeutic target.
AQP7 and AQP10
Studies have shown that in addition to its rich expression in fatty cells, AQP7 is also expressed in other tissues, such as kidney, testis, heart, muscle, pancreas, and small intestines, to varying degrees [27, 67–70], and its main function is transporting water and glycerol.
AQP7 has different expression levels between tumors and corresponding normal tissues, implying that it may affect the prognosis of cancer. As shown in Table 3, we determined that the expression level of AQP7 mRNA in cancer tissues was often lower than that in the corresponding normal tissues, but protein-level evidence still needs to be discovered. Research suggests that the protein expression level of AQP7 in HCC and ovarian carcinoma tissues is significantly different from that in normal tissues, but its clinical significance remains to be explored.
Table 3.
AQP7 expression levels in cancer tissues or cells and corresponding normal tissues or cells and relationship between AQP7 expression levels and cancer prognosis
| Cancer types | Tissues/cell lines | Expression types | Expression of tumors (T) and normal tissues or cells (N) | Expression and prognosis |
|---|---|---|---|---|
| Low-grade glioma [48] | TCGA database | mRNA | T < N | Lower expression means better OS |
| ccRCC [71] | TCGA database | mRNA | T < N | Lower expression means worse OS |
| Breast cancer[39] | Oncomine database | mRNA | T < N | Lower expression means worse OS (in Grade 1) |
| Breast cancer [17] | TCGA database | mRNA | —— | Lower expression means better OS |
| Breast cance [17] (mouse) | qRT-PCR IHC | mRNA, protein | T < N | —— |
| PDAC [72] | GEO database | mRNA | T < N | Lower expression means worse OS |
| HCC [73] | qRT-PCR WB IHC | mRNA, protein | T < N | —— |
| Ovarian carcinoma [74] | WB | protein | T > N | —— |
ccRCC clear cell renal cell carcinoma
AQP10, permeable to water, glycerol, and urea, is expressed in the digestive tract [75–77]. However, present studies have not been particularly informative about its role in cancer. Despite being a part of the AQGP family, its function remains unknown. Until now, AQP10 mRNA has been found in several cancers, such as breast cancer and ovarian cancer [78, 79]. A study of ovarian cancer demonstrated that higher AQP10 mRNA expression meant a better OS [79], and Lizhe Zhu et al. found that increased AQP10 mRNA expression in breast cancer was associated with better RFS [39]. In contrast, another study obtained the opposite result that AQP10 mRNA expression was relevant to poor OS [35].
The relationship between the expression levels of AQP7 and AQP10 and the prognosis of cancer remains ambiguous because there have been only a few attempts to examine AQP7 and AQP10 in cancer, and existing research was limited to the mRNA level. The identification and location of AQP7 and AQP10 at the protein level may be of considerable significance.
AQP9
AQP9 is widely distributed in the body, including the nerve, digestive, and reproductive systems [80–84]. Although its molecular structure and water permeability are closely analogous to those of other aquaglyceroporins, relatively little is known about its specific physiological functions. AQP7 of adipocytes transports the glycerol produced by fat mobilization to the blood. After the blood enters the liver through the portal vein, AQP9 expressed in the liver facilitates the uptake of glycerol, and then, glucose is produced by gluconeogenesis [85]. In addition, it also plays a role in tumorigenesis, progression, and even metastasis. Similarly, we explored its expression levels in different cancer tissues or cells and corresponding normal tissues or cells. According to Table 4, the expression levels of AQP9 in hepatocellular carcinoma, lung cancer, and laryngeal cancer are lower than those in corresponding normal tissues, but the opposite result is observed in other cancers. Then, we compared the expression levels of AQP9 with cancer prognosis (Table 5). It is reasonable that AQP9 promotes cancer except for hepatocellular carcinoma.
Table 4.
Expression levels of AQP9 in different tumor tissues or cells and corresponding normal tissues or cells
| Cancer types | Tissues/cell lines | Methods | Expression of tumors (T) and normal tissues or cells (N) |
|---|---|---|---|
| HCC [86, 87] | tissues, cell lines | qRT-PCR, WB, IHC | T < N |
| HCC [88] | tissues, cell lines | qRT-PCR, WB, IHC | T < N |
| HCC [54] | tissues | qRT-PCR, IHC | T < N |
| HCC [89] | cell lines | qRT-PCR, WB, IHC | T < N |
| HCC [90] | tissues | qRT-PCR, WB, IHC | T < N |
| ccRCC [91–93] | tissues | ICGC database, qRT-PCR, IHC | T > N |
| Breast cancer [39, 94] | tissues | Oncomine database | T > N |
| NSCLC [95] | tissues | qRT-PCR, IHC | T > N |
| Lung cancer [94] | tissues | Oncomine database | T < N |
| Prostate cancer [96] | tissues | GEO database | T > N |
| Glioma [97] | tissues (human, mouse) | IHC | T > N |
| Ovarian cancer [74] | tissues | WB | T > N |
| Colorectal cancer [94] | tissues | Oncomine database | T > N |
| Colon cancer [94] | tissues | Oncomine database | T > N |
| Gastric cancer [94] | tissues | Oncomine database | T > N |
| Astrocytic tumor [98] | tissues | RT-PCR, WB | T > N |
| Laryngeal cancer [99] | tissues, cell lines | qRT-PCR, IHC | T < N |
Table 5.
AQP9 expression and prognosis of cancer
| Cancer types | Expression types | Expression and prognosis |
|---|---|---|
| HCC [54, 87] | mRNA, protein | Higher expression shows better OS |
| Breast cancer [94] | mRNA | Higher expression shows worse OS, RFS |
| Breast cancer [39] | mRNA | Higher expression shows worse RFS |
| ccRCC [91, 93] | mRNA, protein | Higher expression shows worse OS |
| ccRCC [92] | mRNA, protein | Higher expression shows worse OS, PFS |
| Colorectal cancer [94] | mRNA | Higher expression shows worse DFS |
| NSCLC [95] | mRNA, protein | Higher expression shows worse OS, DFS |
| Colon cancer [94] | mRNA | Higher expression shows worse DFS |
| Gastric cancer [94] | mRNA | Higher expression shows worse OS, PFS |
| Lung cancer [94] | mRNA | Higher expression shows worse OS, PFS |
| Laryngeal cancer [99] | mRNA | Higher expression shows worse OS |
Functions in cancer
AQP3
AQP3 is multifaceted in cancer. As a member of the AQGP family, it is universally acknowledged that AQP3 works as a channel for water and glycerol. Impaired glycerol transport and lipid synthesis due to AQP3 knockdown promoted apoptosis and inhibited the proliferation of gastric cancer cells [13, 14], AQP3-facilitated glycerol, a major source of ATP, participates in epidermal proliferation and tumor formation [15].
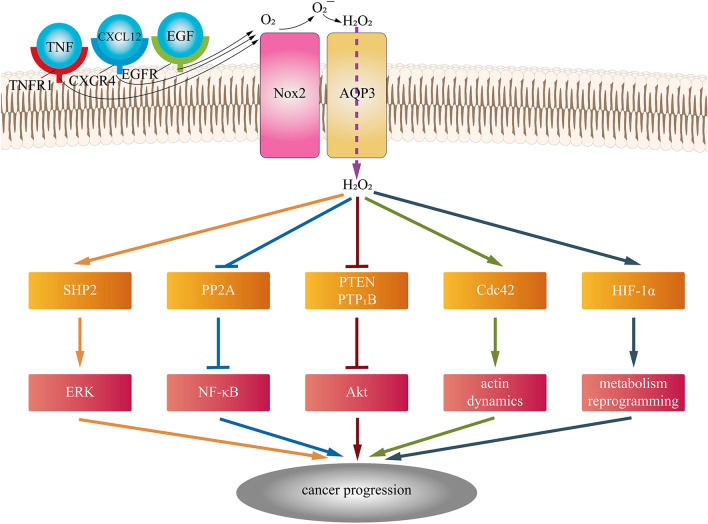
In addition to transporting water and glycerol, it can also transport H2O2, an important second messenger in cellular activities [100], which makes the role of AQP3 in cancer more significant. Extracellular H2O2, synthesized by NADPH oxidase 2 (Nox2), which responds to various stimuli, including TNF-α, EGF, and CXCL12, is delivered intracellularly through AQP3, and then, H2O2 inactivates protein phosphatase 2A (PP2A) followed by the regulation of IKKβ and NF-κB/p65 [101]. AQP3-mediated H2O2 oxidized PTEN and protein tyrosine phosphatase 1B (PTP1B) and activated the Akt pathway in breast cancer cells and lung adenocarcinoma cells [102, 103]. Moreover, AQP3-facilitated H2O2 engaged in Cdc42 activation, a GTPase of the Rho family and subsequent actin dynamics [104]. In addition, AQP3 was involved in the EGF-induced ERK pathway in cancer, in which AQP3-mediated H2O2 modulated SHP2, an indispensable part of the downstream MAPK signaling cascade [105, 106]. Moreover, HIF-1α could be upregulated by ROS transported by AQP3, which made a difference in reprogramming cancer metabolism [107, 108]. Figure 2 shows AQP3-mediated H2O2 in cancer.
Fig. 2.
AQP3-mediated H2O2 promotes cancer progression via tumor-related signaling pathways
AQP3 functions as a functional protein molecule, and knockdown of AQP3 inhibits cancer cell proliferation, invasion, and migration as well as promotes apoptosis [16, 55, 64, 109].
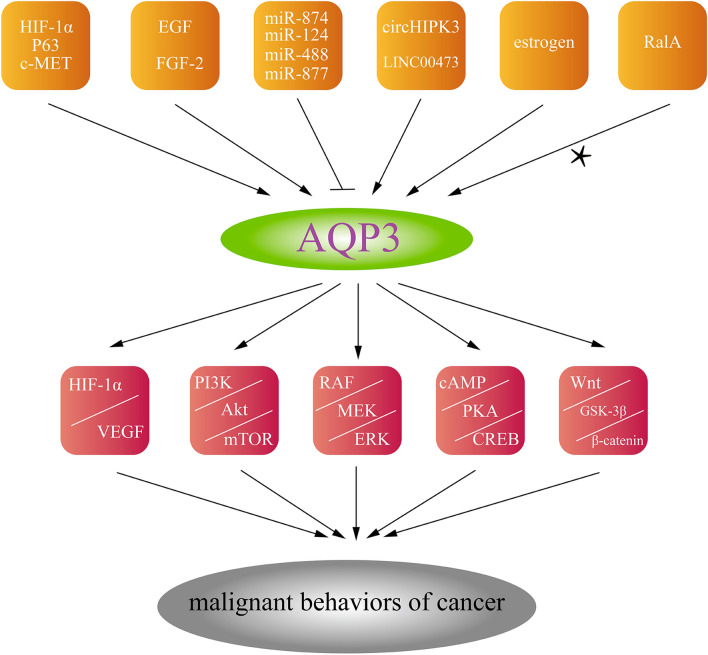
There are many transcription factors, cytokines, microRNAs, and other regulators that affect AQP3 in cancer. Likewise, AQP3 can regulate the malignant behaviors of cancer cells through several signaling pathways. Here, we summarize the upstream regulators and the downstream activated tumor-related signaling pathways of AQP3 in different cancers, hoping to provide some basis for AQP3 as a target for cancer treatment (see Fig. 3, for more details).
Fig. 3.
The upstream regulators of AQP3 and the downstream activated tumor-related signaling pathways [13, 49–53, 56, 58–61, 107, 110–124]
Growing evidence shows that some metal compounds modulating the expression of AQP3 exhibit different anticancer properties, such as antiproliferative and proapoptotic properties. In vivo, Auphen could regulate the expression of AQP3 to inhibit tumor growth and promote apoptosis [54]. P2W18, a polyoxotungstate, showed the ability to suppress cancer cell migration mainly by affecting AQP3, implying the potential of AQP3 as an anticancer agent in tumors with high AQP3 expression [125]. Some natural compounds also show anticancer ability to some degree. Curcumin, which regulates AQP3 gating [126], exerted an inhibitory effect on EGF-induced AQP3 upregulation and ovarian cancer cell migration through the PI3K/Akt and MEK/ERK pathways [127]. Similarly, Manuka honey accelerated epithelial cancer cell apoptosis by maintaining the high permeability of AQP3-induced H2O2 [128].
AQP3 can interact with certain chemotherapy drugs or participate in certain cancer treatments. AQP3 gave rise to chemoresistance to cisplatin in gastric cancer and facilitated chemoresistance to arsenite in melanoma [129, 130]. Meanwhile, AQP3 also participates in the cytotoxic effect exerted by nucleoside-derived drugs, including 5-fluorouracil and gemcitabine, in breast cancer and colon cancer [131]. Cryotherapy is gradually becoming an alternative treatment for the early stage of the neoplastic process, in which AQP3 plays a role in gilding. Breast cancer cells and prostate cancer cells treated with AQP3-siRNA were more sensitive to cryoinjury than control-siRNA [132, 133]. Thus, inhibition of AQP3 may be a potential adjunct to cryotherapy for breast and prostate cancer patients.
AQP7 and AQP10
AQP7 is regarded as a gateway for water and glycerol transportation, but little work has been performed on its involvement in tumor cell lipid metabolism. Nevertheless, AQP7, which serves as an important target for arsenite uptake in mammals [134], may provide us with novel perceptions of its chemotherapeutic efficacy in acute promyelocytic leukocytes. AQP7 regulated multiple metabolic pathways, including lipid metabolism, urea metabolism, and carbohydrate metabolism and activated p38, EGFR, and mTOR signaling cascades. In addition, AQP7 made cells more sensitive to the oxidative environment [17]. In other words, AQP7, as a critical regulator, might eventually lead to the development of more effective therapeutics in breast cancer.
Studies have shown that silencing AQP7 in adipose cells could increase the glycerol content, strengthen the activity of the Gyk enzyme, and promote the accumulation of triglycerides [135]. When the body needs energy, triglycerides are hydrolyzed into free fatty acids (FFAs) and glycerol, glycerol is delivered to the liver to participate in gluconeogenesis, FFAs are transported to mitochondria where energy is produced, and AQP7 functions as the glycerol gateway during the process [136]. Another study demonstrated low glycerol and ATP contents in the hearts of KO-AQP7 mice [137]. Therefore, we infer that low expression of AQP7, which leads to an increased content of triglycerides, impaired glycerol and FFA transport, and reduced energy, inhibits the malignant behaviors of tumor cells. Moreover, the role of AQP10 in cancer has never been satisfactorily elucidated, which means that more research regarding AQP7 and AQP10 in cancer is needed.
AQP9
The involvement of AQP9 in glycerol transportation continues to draw attention from researchers, and now it has been extended to cancer research. In several cancer cell lines, the expression of AQP9 was related to the uptake of [14C]-labeled glycerol [138]. Another experiment in a rat hepatocellular carcinoma model found that the expression of AQP9 was present at a low level before tumorigenesis, while it was significantly increased in the early stage of hepatocellular carcinoma. This indicates a transition of glycerol metabolism during the stage [18].
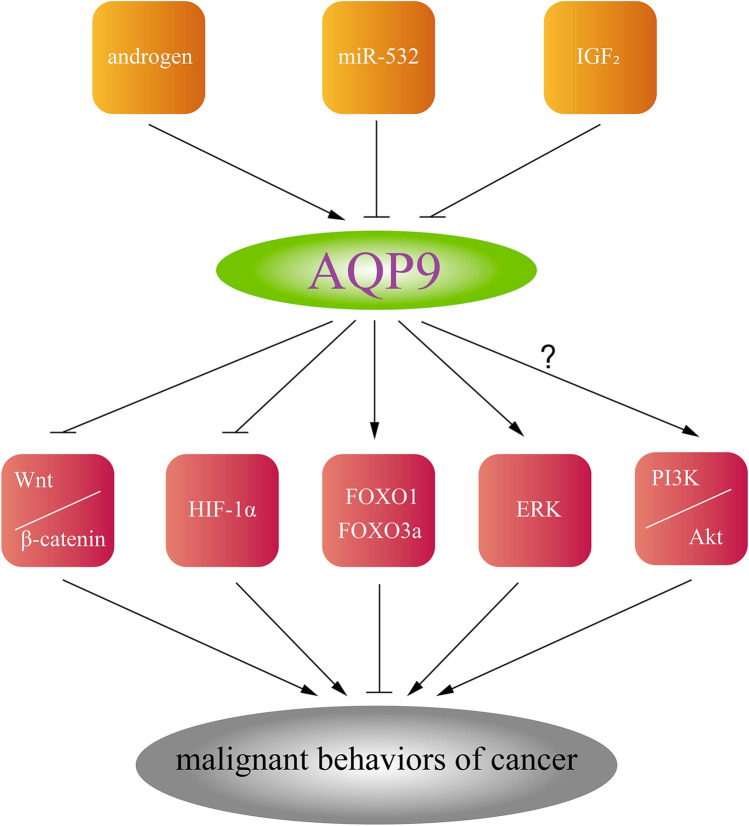
Our team found that AQP9 plays an extraordinary role in the prostate. First, we proved the positive regulatory effect of androgen on AQP9 in the prostate in vitro and in vivo [139]. In addition, knockdown of AQP9 inhibited proliferation, migration, and invasion as well as promoted apoptosis in androgen-independent prostate cancer, which is involved in the ERK pathway [96]. We can conclude that AQ9 accelerates prostate cancer progression in combination with the relatively high expression level of AQP9 in prostate cancer tissues compared with normal prostate tissues. Specific targeted therapy with AQP9 might exert far-reaching significance in prostate cancer treatment.
In renal cell carcinoma, Yasutaka Yamada et al. found that AQP9 was regulated by miR-532, silencing AQP9 could affect the oncological behaviors of renal cancer cells [91], and a cancer-promoting effect via the Akt pathway was also found in astrocytoma [140].
For hepatocellular carcinoma, AQP9 suppresses hepatocellular carcinoma cell growth and metastasis via distinct pathways, including HIF-1α, PI3k/Akt, Wnt/β-catenin, and FOXO1 [86–88, 90, 141]. Further findings from another study announced the role of AQP9 in H2O2 transport, as Sachiko Watanabe et al. reported [142]. In this study, the author proved that AQP9 mediated by insulin-like growth factor 2 (IGF2), inhibited liver cancer stem cell stemness through ROS/β-catenin/FOXO3a [143]. Figure 4 shows the upstream regulators of AQP9 and the downstream activated tumor-related signaling pathways.
Fig. 4.
The upstream regulators of AQP9 and the downstream activated tumor-related signaling pathways
With respect to additional functions of AQP9, it regulated arsenic transportation and affected As2O3 sensitivity [134, 144, 145]. The expression level of AQP9 was related to sensitivity to As2O3 in acute promyelocytic leukemia [146], and azacytidine upregulated AQP9 to make acute myeloid leukemia cells more sensitive to As2O3 [147].
AQP9 is also involved in the chemotherapy effects of a variety of solid tumors. AQP9 enhanced the chemotherapy response and alleviated the chemotherapy resistance of arsenic during the treatment of lung cancer [148, 149]. In contrast, AQP9 fostered the chemotherapy resistance of melanoma to arsenite [129]. 5-FU chemotherapy possesses a better curative effect in mice with colorectal cancer because of cell cycle arrest caused by AQP9 [150]. The expression of AQP9 at a low level in patients with stage III colorectal cancer who do not respond to chemotherapy, makes AQP9 a potential prognostic indicator [151]. Moreover, the functions of AQP9 in glycerol transportation and differential expression between tumors and normal tissues make AQP9 a promising scientific hot button for the treatment of various tumors.
Conclusions and perspectives
During the past decade, great achievements have been witnessed in the research of aquaporins, from the location of genetic information, distribution and function to the transport mechanism, drug mechanism, etc. Aquaglyceroporins, as a special group from the aquaporin family, have been rooted in researchers’ minds.
AQP3 and AQP9, which are permeable to glycerol and H2O2, often contribute to the malignant behaviors of cancer. However, AQP3 plays an opposite role in endometrioid carcinoma and MIBC, as well as AQP9 in hepatocellular carcinoma. AQP7 is involved in multiple metabolic pathways in breast cancer while its functions in other cancers remain to be explored. In addition, they all facilitate arsenic transportation or affect its chemotherapy effect, making them hopeful therapeutic targets in cancer treatments. However, further analysis at the protein level is needed, especially for AQP7 and AQP10.
This review shows that AQGPs behave as double-edged swords in different tumors. They have different distributions and expression patterns from each other in cancers, and they are linked to different oncological behaviors of tumor cells, including proliferation, migration, invasion, apoptosis, epithelial–mesenchymal transition, metastasis, etc.
To the best of our knowledge, this is the first review on aquaglyceroporins that combines clinical and basic research. We calculated the relationship between the expression levels of AQGPs and prognostic indicators in different tumors in the published literature. We also summarized the upstream and downstream regulators and signaling pathways involved, which may provide some references for subsequent further research on AQGPs and drug treatments specifically targeting AQGPs. Despite these findings, we still cannot provide a systematic and complete explanation of the mechanism of AQGPs in cancer; further basic researches about what role AQPs may play and how they regulate the reprogramming of lipid metabolism in tumor cells are needed.
Acknowledgements
RW and XW wrote the first draft of the manuscript; JZ and JJ edited the English language; WF, XZ, and QC designed the figures; BZ, LL, and KQ generated the tables; LZ and JW reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This work was funded by the grant from a National Natural Science Foundation of China (No.81372761).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui Wang and Xuejian Wang have contributed equally to this work.
Contributor Information
Liang Zhu, Email: zhuliang0210@sina.com.
Jianbo Wang, Email: wangjianbo@dmu.edu.cn.
References
- 1.King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- 2.Agre P, Saboori AM, Asimos A, et al. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem. 1987;262(36):17497–17503. doi: 10.1016/S0021-9258(18)45408-7. [DOI] [PubMed] [Google Scholar]
- 3.Denker BM, Smith BL, Kuhajda FP, et al. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263(30):15634–15642. doi: 10.1016/S0021-9258(19)37635-5. [DOI] [PubMed] [Google Scholar]
- 4.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preston GM, Carroll TP, Guggino WB, et al. Appearance of water channels in xenopus oocytes expressing red cell CHIP28 protein. Science (New York, N.Y.) 1992;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 6.Agre P, Sasaki S, Chrispeels MJ. Aquaporins: a family of water channel proteins. Am J Physiol. 1993;265(3 Pt 2):F461. doi: 10.1152/ajprenal.1993.265.3.F461. [DOI] [PubMed] [Google Scholar]
- 7.Agre P, Preston GM, Smith BL, et al. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- 8.Day RE, Kitchen P, Owen DS, et al. Human aquaporins: regulators of transcellular water flow. Biochem Biophys Acta. 2014;1840(5):1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Borgnia M, Nielsen S, Engel A, et al. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 10.Corbet C, Feron O. Emerging roles of lipid metabolism in cancer progression. Curr Opin Clin Nutr Metab Care. 2017;20(4):254–260. doi: 10.1097/MCO.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C, Geng F, Cheng X, et al. Lipid metabolism reprogramming and its potential targets in cancer. Cancer commun (London, England) 2018;38(1):27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleve A, Durante B, Sica A, et al. Lipid metabolism and cancer immunotherapy: immunosuppressive myeloid cells at the crossroad. Int j mol sci. 2020 doi: 10.3390/ijms21165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Li B, Zhang L, et al. The proliferation impairment induced by AQP3 deficiency is the result of glycerol uptake and metabolism inhibition in gastric cancer cells. Tumour Biol. 2016;37(7):9169–9179. doi: 10.1007/s13277-015-4753-8. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Li Z, Zhang Q, et al. Silencing of AQP3 induces apoptosis of gastric cancer cells via downregulation of glycerol intake and downstream inhibition of lipogenesis and autophagy. Onco Targets Ther. 2017;10:2791–2804. doi: 10.2147/OTT.S134016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28(1):326–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Tao C, Yuan C, et al. AQP3 small interfering RNA and PLD2 small interfering RNA inhibit the proliferation and promote the apoptosis of squamous cell carcinoma. Mol Med Rep. 2017;16(2):1964–1972. doi: 10.3892/mmr.2017.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai C, Charlestin V, Wang M, et al. Aquaporin-7 regulates the response to cellular stress in breast cancer. Can Res. 2020;80(19):4071–4086. doi: 10.1158/0008-5472.CAN-19-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzetti F, Capiglioni AM, Marinelli RA, et al. Hepatic glycerol metabolism is early reprogrammed in rat liver cancer development. Biochimie. 2020;170:88–93. doi: 10.1016/j.biochi.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki S, Ishibashi K, Marumo F. Aquaporin-2 and -3: representatives of two subgroups of the aquaporin family colocalized in the kidney collecting duct. Annu Rev Physiol. 1998;60:199–220. doi: 10.1146/annurev.physiol.60.1.199. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen S, Frøkiaer J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82(1):205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 21.Echevarría M, Ilundáin AA. Aquaporins. J Physiol Biochem. 1998;54(2):107–118. [PubMed] [Google Scholar]
- 22.Jung JS, Preston GM, Smith BL, et al. Molecular structure of the water channel through aquaporin CHIP the hourglass model. J biol chem. 1994;269(20):14648–14654. doi: 10.1016/S0021-9258(17)36674-7. [DOI] [PubMed] [Google Scholar]
- 23.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278(1):F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 24.Ishibashi K, Sasaki S, Fushimi K, et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA. 1994;91(14):6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreda SM, Gynn MC, Fenstermacher DA, et al. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24(3):224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 26.Mobasheri A, Barrett-Jolley R. Aquaporin water channels in the mammary gland: from physiology to pathophysiology and neoplasia. J mammary gland boil neoplasia. 2014 doi: 10.1007/s10911-013-9312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu C, Chen Z, Jiang Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int j mol sci. 2016 doi: 10.3390/ijms17091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Tanji N, Kikugawa T, et al. Expression of aquaporin 3 in the human prostate. In J Urol. 2007 doi: 10.1111/j.1442-2042.2007.01901.x. [DOI] [PubMed] [Google Scholar]
- 29.Zou W, Yang Z, Li D, et al. AQP1 and AQP3 Expression are associated with severe symptoms and poor-prognosis of the pancreatic ductal adenocarcinoma. Appl immunohistochemistry mol morphol. 2019;27(1):40–47. doi: 10.1097/PAI.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 30.Direito I, Paulino J, Vigia E, et al. Differential expression of aquaporin-3 and aquaporin-5 in pancreatic ductal adenocarcinoma. J Surg Oncol. 2017;115(8):980–996. doi: 10.1002/jso.24605. [DOI] [PubMed] [Google Scholar]
- 31.Yang C, Lim W, Bae H, et al. Aquaporin 3 is regulated by estrogen in the chicken oviduct and is involved in progression of epithelial cell-derived ovarian carcinomas. Domest anim endocrinol. 2016 doi: 10.1016/j.domaniend.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Kang BW, Kim JG, Lee SJ, et al. Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncology. 2015;88(6):369–376. doi: 10.1159/000369073. [DOI] [PubMed] [Google Scholar]
- 33.Shen L, Zhu Z, Huang Y, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed pharmacother. 2010;64(5):313–318. doi: 10.1016/j.biopha.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y, Chen Z, Li N, et al. Prognostic value of serum aquaporin-1, aquaporin-3 and galectin-3 for young patients with colon cancer. Ann Clin Biochem. 2020;57(6):404–411. doi: 10.1177/0004563220961752. [DOI] [PubMed] [Google Scholar]
- 35.Thapa S, Chetry M, Huang K, 2018. Significance of aquaporins’ expression in the prognosis of gastric cancer. Biosci rep. [DOI] [PMC free article] [PubMed]
- 36.Zhang X, Zheng P, Li Z, et al. The somatic mutation landscape and RNA prognostic markers in stomach adenocarcinoma. Onco Targets Ther. 2020;13:7735–7746. doi: 10.2147/OTT.S263733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Zhang S, Jiang H, et al. Co-expression of AQP3 and AQP5 in esophageal squamous cell carcinoma correlates with aggressive tumor progression and poor prognosis. Medi oncol (Northwood, London, England) 2013;30(3):636. doi: 10.1007/s12032-013-0636-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Z, Jiao L, Li T, et al. Expression of AQP3 and AQP5 as a prognostic marker in triple-negative breast cancer. Oncol Lett. 2018;16(2):2661–2667. doi: 10.3892/ol.2018.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L, Ma N, Wang B, et al. Significant prognostic values of aquaporin mRNA expression in breast cancer. Cancer manag res. 2019;11:1503–1515. doi: 10.2147/CMAR.S193396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S, Chae YS, Lee SJ, et al. Aquaporin 3 expression predicts survival in patients with HER2-positive early breast cancer. Anticancer Res. 2015;35(5):2775–2782. [PubMed] [Google Scholar]
- 41.Jia L, Ling Y, Li K, et al. A 10-gene signature for predicting the response to neoadjuvant trastuzumab therapy in HER2-positive breast cancer. Clin breast cancer. 2021 doi: 10.1016/j.clbc.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Guo X, Sun T, Yang M, et al. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. BioMed res int. 2013 doi: 10.1155/2013/206525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Sato K, Kono T, et al. Aquaporin 3 expression in endometrioid carcinoma of the uterine body correlated with early stage and lower grade. Pathol oncol res. 2020;26(4):2247–2253. doi: 10.1007/s12253-020-00813-3. [DOI] [PubMed] [Google Scholar]
- 44.Breyer J, Otto W, Burger M, et al. Aquaporin 3 expression loss in urothelial carcinoma: association with tumor invasion depth, but not with grading? Bladder cancer (Amsterdam, Netherlands) 2017;3(1):31–34. doi: 10.3233/BLC-160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubenwolf P, Thomas C, Denzinger S, et al. Loss of AQP3 protein expression is associated with worse progression-free and cancer-specific survival in patients with muscle-invasive bladder cancer. World J Urol. 2015;33(12):1959–1964. doi: 10.1007/s00345-015-1574-8. [DOI] [PubMed] [Google Scholar]
- 46.Rubenwolf PC, Otto W, Denzinger S, et al. Expression of aquaporin water channels in human urothelial carcinoma: correlation of AQP3 expression with tumour grade and stage. World J Urol. 2014;32(4):991–997. doi: 10.1007/s00345-013-1153-9. [DOI] [PubMed] [Google Scholar]
- 47.Otto W, Rubenwolf PC, Burger M, et al. Loss of aquaporin 3 protein expression constitutes an independent prognostic factor for progression-free survival: an immunohistochemical study on stage pT1 urothelial bladder cancer. BMC Cancer. 2012;12:459. doi: 10.1186/1471-2407-12-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chetry M, Li S, Liu H, 2018. Prognostic values of mRNA expression in human ovarian cancer. Biosci rep. [DOI] [PMC free article] [PubMed]
- 49.Jiang B, Li Z, Zhang W, et al. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J Gastroenterol. 2014;49(6):1011–1025. doi: 10.1007/s00535-013-0851-9. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Wang T, Zhou Y-C, et al. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp clin cancer res. 2014;33:38. doi: 10.1186/1756-9966-33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Wang Y, Wen J, et al. Aquaporin 3 promotes the stem-like properties of gastric cancer cells via Wnt/GSK-3β/β-catenin pathway. Oncotarget. 2016;7(13):16529–16541. doi: 10.18632/oncotarget.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen G, Shi Y, Liu M, et al. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018;9(2):175. doi: 10.1038/s41419-017-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Wu G, Fu X, et al. Aquaporin 3 maintains the stemness of CD133+ hepatocellular carcinoma cells by activating STAT3. Cell Death Dis. 2019;10(6):465. doi: 10.1038/s41419-019-1712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng R, Zhao G-X, Li J, et al. Auphen and dibutyryl cAMP suppress growth of hepatocellular carcinoma by regulating expression of aquaporins 3 and 9 in vivo. World J Gastroenterol. 2016;22(12):3341–3354. doi: 10.3748/wjg.v22.i12.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arif M, Kitchen P, Conner MT, et al. Downregulation of aquaporin 3 inhibits cellular proliferation, migration and invasion in the MDA-MB-231 breast cancer cell line. Oncol Lett. 2018;16(1):713–720. doi: 10.3892/ol.2018.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kusayama M, Wada K, Nagata M, et al. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011;102(6):1128–1136. doi: 10.1111/j.1349-7006.2011.01927.x. [DOI] [PubMed] [Google Scholar]
- 57.Lekshmy MS, Sivakumar TT, Joseph AP, et al. Expression of transmembrane protein aquaporin-3 in oral epithelial dysplasia and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(2):202–208. doi: 10.1016/j.oooo.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Wu Y, Yang S, et al. miR-874 directly targets AQP3 to inhibit cell proliferation, mobility and EMT in non-small cell lung cancer. Thoracic cancer. 2020;11(6):1550–1558. doi: 10.1111/1759-7714.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li A, Lu D, Zhang Y, et al. Critical role of aquaporin-3 in epidermal growth factor-induced migration of colorectal carcinoma cells and its clinical significance. Oncol Rep. 2013;29(2):535–540. doi: 10.3892/or.2012.2144. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Wang Z, Xu D, et al. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol Med Rep. 2015;11(4):2882–2888. doi: 10.3892/mmr.2014.3097. [DOI] [PubMed] [Google Scholar]
- 61.Qiu J, Zhang Y, Chen H, et al. MicroRNA-488 inhibits proliferation, invasion and EMT in osteosarcoma cell lines by targeting aquaporin 3. Int J Oncol. 2018;53(4):1493–1504. doi: 10.3892/ijo.2018.4483. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Seleit I, Bakry OA, Al Sharaky D, et al. Evaluation of aquaporin-3 Role in nonmelanoma skin cancer: an immunohistochemical study. Ultrastruct Pathol. 2015;39(5):306–317. doi: 10.3109/01913123.2015.1022241. [DOI] [PubMed] [Google Scholar]
- 63.Niu D, Kondo T, Nakazawa T, et al. Differential expression of aquaporins and its diagnostic utility in thyroid cancer. PLoS ONE. 2012;7(7):e40770. doi: 10.1371/journal.pone.0040770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmad AE, Khajah MA, Khushaish S, et al. Aquaporin expression in breast cancer and their involvement in bleb formation, cell motility and invasion in endocrine resistant variant cells. Int J Oncol. 2020;56(4):1014–1024. doi: 10.3892/ijo.2020.4976. [DOI] [PubMed] [Google Scholar]
- 65.Hwang I, Jung S-I, Hwang E-C, et al. Expression and localization of aquaporins in benign prostate hyperplasia and prostate cancer. Chonnam Med J. 2012;48(3):174–178. doi: 10.4068/cmj.2012.48.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Almeida A, Parthimos D, Dew H, et al. Aquaglyceroporin-3’s expression and cellular localization is differentially modulated by hypoxia in prostate cancer Cell lines. Cells. 2021 doi: 10.3390/cells10040838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skowronski MT, Lebeck J, Rojek A, et al. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am J Physiol Renal Physiol. 2007;292(3):F956–F965. doi: 10.1152/ajprenal.00314.2006. [DOI] [PubMed] [Google Scholar]
- 68.Saito K, Kageyama Y, Okada Y, et al. Localization of aquaporin-7 in human testis and ejaculated sperm: possible involvement in maintenance of sperm quality. J Urol. 2004;172(5 Pt 1):2073–2076. doi: 10.1097/01.ju.0000141499.08650.ab. [DOI] [PubMed] [Google Scholar]
- 69.Lebeck J, Søndergaard E, Nielsen S. Increased AQP7 abundance in skeletal muscle from obese men with type 2 diabetes American journal of physiology. Endocrinol metabol. 2018;315(3):E367–E373. doi: 10.1152/ajpendo.00468.2017. [DOI] [PubMed] [Google Scholar]
- 70.da Silva IV, Cardoso C, Méndez-Giménez L, et al. Aquaporin-7 and aquaporin-12 modulate the inflammatory phenotype of endocrine pancreatic beta-cells. Arch Biochem Biophys. 2020;691:108481. doi: 10.1016/j.abb.2020.108481. [DOI] [PubMed] [Google Scholar]
- 71.Xu W-H, Xu Y, Wang J, et al. Prognostic value and immune infiltration of novel signatures in clear cell renal cell carcinoma microenvironment. Aging. 2019;11(17):6999–7020. doi: 10.18632/aging.102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magouliotis DE, Tasiopoulou VS, Dimas K, et al. Transcriptomic analysis of the aquaporin (AQP) gene family interactome identifies a molecular panel of four prognostic markers in patients with pancreatic ductal adenocarcinoma. Pancreatology. 2019;19(3):436–442. doi: 10.1016/j.pan.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Chen X-F, Li C-F, Lü L, et al. Expression and clinical significance of aquaglyceroporins in human hepatocellular carcinoma. Mol Med Rep. 2016;13(6):5283–5289. doi: 10.3892/mmr.2016.5184. [DOI] [PubMed] [Google Scholar]
- 74.Yang JH, Yan CX, Chen XJ, et al. Expression of aquaglyceroporins in epithelial ovarian tumours and their clinical significance. J Int Med Res. 2011;39(3):702–711. doi: 10.1177/147323001103900302. [DOI] [PubMed] [Google Scholar]
- 75.Li H, Kamiie J, Morishita Y, et al. Expression and localization of two isoforms of AQP10 in human small intestine. Biol Cell. 2005;97(11):823–829. doi: 10.1042/BC20040091. [DOI] [PubMed] [Google Scholar]
- 76.Hatakeyama S, Yoshida Y, Tani T, et al. Cloning of a new aquaporin (AQP10) abundantly expressed in duodenum and jejunum. Biochem Biophys Res Commun. 2001;287(4):814–819. doi: 10.1006/bbrc.2001.5661. [DOI] [PubMed] [Google Scholar]
- 77.Mobasheri A, Shakibaei M, Marples D. Immunohistochemical localization of aquaporin 10 in the apical membranes of the human ileum: a potential pathway for luminal water and small solute absorption. Histochem Cell Biol. 2004;121(6):463–471. doi: 10.1007/s00418-004-0657-1. [DOI] [PubMed] [Google Scholar]
- 78.Shi Z, Zhang T, Luo L, et al. Aquaporins in human breast cancer: identification and involvement in carcinogenesis of breast cancer. J Surg Oncol. 2012;106(3):267–272. doi: 10.1002/jso.22155. [DOI] [PubMed] [Google Scholar]
- 79.Xuejun C, Weimin C, Xiaoyan D, et al. Effects of aquaporins on chemosensitivity to cisplatin in ovarian cancer cells. Arch Gynecol Obstet. 2014;290(3):525–532. doi: 10.1007/s00404-014-3216-6. [DOI] [PubMed] [Google Scholar]
- 80.Yang M-H, Dibas A, Tyan Y-C. 2013. Changes in retinal aquaporin-9 (AQP9) expression in glaucoma. Biosci rep. [DOI] [PMC free article] [PubMed]
- 81.Carbrey JM, Gorelick-Feldman DA, Kozono D, et al. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA. 2003;100(5):2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castro-Parodi M, Szpilbarg N, Dietrich V, et al. Oxygen tension modulates AQP9 expression in human placenta. Placenta. 2013;34(8):690–698. doi: 10.1016/j.placenta.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 83.Pastor-Soler N, Bagnis C, Sabolic I, et al. Aquaporin 9 expression along the male reproductive tract. Biol Reprod. 2001;65(2):384–393. doi: 10.1095/biolreprod65.2.384. [DOI] [PubMed] [Google Scholar]
- 84.Xiang T, Ge S, Wen J, et al. The possible association between AQP9 in the intestinal epithelium and acute liver injury-induced intestinal epithelium damage. Mol Med Rep. 2018;18(6):4987–4993. doi: 10.3892/mmr.2018.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lebeck J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J Mol Endocrinol. 2014;52(2):R165–R178. doi: 10.1530/JME-13-0268. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W-G, Li C-F, Liu M, et al. Aquaporin 9 is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Cancer Lett. 2016;378(2):111–119. doi: 10.1016/j.canlet.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Liao S, Chen H, Liu M, et al. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging. 2020;12(2):1527–1544. doi: 10.18632/aging.102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jablonski EM, Mattocks MA, Sokolov E, et al. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250(1):36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng R, Zhang Y, Zhao GX, et al. Differential regulation of the expression of aquaporins 3 and 9 by auphen and dbcAMP in the SMMC-7721 hepatocellular carcinoma cell line. Biotechnic histochemistry. 2016;91(5):333–341. doi: 10.3109/10520295.2016.1168525. [DOI] [PubMed] [Google Scholar]
- 90.Li C-F, Zhang W-G, Liu M, et al. Aquaporin 9 inhibits hepatocellular carcinoma through up-regulating FOXO1 expression. Oncotarget. 2016;7(28):44161–44170. doi: 10.18632/oncotarget.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamada Y, Arai T, Kato M, et al. Role of pre- (and) in regulation of gene expression and molecular pathogenesis in renal cell carcinoma. Am j clin exp urol. 2019;7(1):11–30. [PMC free article] [PubMed] [Google Scholar]
- 92.Xu W-H, Shi S-N, Xu Y, et al. Prognostic implications of aquaporin 9 expression in clear cell renal cell carcinoma. J Transl Med. 2019;17(1):363. doi: 10.1186/s12967-019-2113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jing J, Sun J, Wu Y, et al. AQP9 Is a prognostic factor for kidney cancer and a promising indicator for M2 TAM polarization and CD8+ T-cell recruitment. Front Oncol. 2021;11:770565. doi: 10.3389/fonc.2021.770565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X, Xu Q, Li Z, et al. Integrated analysis identifies AQP9 correlates with immune infiltration and acts as a prognosticator in multiple cancers. Sci Rep. 2020;10(1):20795. doi: 10.1038/s41598-020-77657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen P, Li Q, Zhou Y, et al. Clinical implication of aquaporin 9 in non-small cell lung cancer patients: its expression and relationship with clinical features and prognosis. Irish J Med Sci. 2021 doi: 10.1007/s11845-021-02523-4. [DOI] [PubMed] [Google Scholar]
- 96.Chen Q, Zhu L, Zheng B, et al. Effect of AQP9 expression in androgen-Independent prostate cancer cell PC3. Int J Mol Sci. 2016 doi: 10.3390/ijms17050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warth A, Mittelbronn M, Hülper P, et al. Expression of the water channel protein aquaporin-9 in malignant brain tumors. Appl immunohistochemistry mol morphol. 2007;15(2):193–198. doi: 10.1097/01.pai.0000213110.05108.e9. [DOI] [PubMed] [Google Scholar]
- 98.Tan G, Sun SQ, Yuan DL. Expression of the water channel protein aquaporin-9 in human astrocytic tumours: correlation with pathological grade. J Int Med Res. 2008;36(4):777–782. doi: 10.1177/147323000803600420. [DOI] [PubMed] [Google Scholar]
- 99.Ren L, Li P, Li Z, et al. AQP9 and ZAP70 as immune-related prognostic biomarkers suppress proliferation, migration and invasion of laryngeal cancer cells. BMC Cancer. 2022;22(1):465. doi: 10.1186/s12885-022-09458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3(12):1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 101.Hara-Chikuma M, Satooka H, Watanabe S, et al. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat Commun. 2015;6:7454. doi: 10.1038/ncomms8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Satooka H, Hara-Chikuma M. Aquaporin-3 controls breast cancer Cell Migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol Cell Biol. 2016;36(7):1206–1218. doi: 10.1128/MCB.00971-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Chen D, Liu Y, et al. AQP3-mediated H(2) O(2) uptake inhibits LUAD autophagy by inactivating PTEN. Cancer Sci. 2021;112(8):3278–3292. doi: 10.1111/cas.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hara-Chikuma M, Chikuma S, Sugiyama Y, et al. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J Exp Med. 2012;209(10):1743–1752. doi: 10.1084/jem.20112398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hara-Chikuma M, Watanabe S, Satooka H. Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem Biophys Res Commun. 2016;471(4):603–609. doi: 10.1016/j.bbrc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 106.Mainardi S, Mulero-Sánchez A, Prahallad A, et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat Med. 2018;24(7):961–967. doi: 10.1038/s41591-018-0023-9. [DOI] [PubMed] [Google Scholar]
- 107.Wen J, Wang Y, Gao C, et al. Helicobacter pylori infection promotes aquaporin 3 expression via the ROS-HIF-1α-AQP3-ROS loop in stomach mucosa: a potential novel mechanism for cancer pathogenesis. Oncogene. 2018;37(26):3549–3561. doi: 10.1038/s41388-018-0208-1. [DOI] [PubMed] [Google Scholar]
- 108.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiong G, Chen X, Zhang Q, et al. RNA interference influenced the proliferation and invasion of XWLC-05 lung cancer cells through inhibiting aquaporin 3. Biochem Biophys Res Commun. 2017;485(3):627–634. doi: 10.1016/j.bbrc.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Malale K, Fu J, Qiu L, et al. Hypoxia-Induced aquaporin-3 changes hepatocellular carcinoma cell sensitivity to sorafenib by activating the PI3K/Akt signaling pathway. Cancer Manag Res. 2020;12:4321–4333. doi: 10.2147/CMAR.S243918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Wang G, Gao F, Zhang W, et al. Involvement of aquaporin 3 in helicobacter pylori-related gastric diseases. PLoS ONE. 2012;7(11):e49104. doi: 10.1371/journal.pone.0049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gu X, Coates PJ, Boldrup L, et al. p63 contributes to cell invasion and migration in squamous cell carcinoma of the head and neck. Cancer Lett. 2008;263(1):26–34. doi: 10.1016/j.canlet.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 113.Wang J, Gui Z, Deng L, et al. c-Met upregulates aquaporin 3 expression in human gastric carcinoma cells via the ERK signalling pathway. Cancer Lett. 2012;319(1):109–117. doi: 10.1016/j.canlet.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 114.Huang Y, Zhu Z, Sun M, et al. Critical role of aquaporin-3 in the human epidermal growth factor-induced migration and proliferation in the human gastric adenocarcinoma cells. Cancer Biol Ther. 2010;9(12):1000–1007. doi: 10.4161/cbt.9.12.11705. [DOI] [PubMed] [Google Scholar]
- 115.Liu W, Wang K, Gong K, et al. Epidermal growth factor enhances MPC-83 pancreatic cancer cell migration through the upregulation of aquaporin 3. Mol Med Rep. 2012;6(3):607–610. doi: 10.3892/mmr.2012.966. [DOI] [PubMed] [Google Scholar]
- 116.Cao X-C, Zhang W-R, Cao W-F, et al. Aquaporin3 is required for FGF-2-induced migration of human breast cancers. PLoS ONE. 2013;8(2):e56735. doi: 10.1371/journal.pone.0056735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang X, Huang L, Shao M. Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling. Biochem Biophys Res Commun. 2017;486(4):1097–1102. doi: 10.1016/j.bbrc.2017.03.168. [DOI] [PubMed] [Google Scholar]
- 118.Zhu H, Wu Y, Kang M, et al. MiR-877 suppresses gastric cancer progression by downregulating AQP3. J Int Med Res. 2020;48(6):300060520903661. doi: 10.1177/0300060520903661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhuo S, Sun M, Bai R, et al. Long intergenic non-coding RNA 00473 promotes proliferation and migration of gastric cancer via the miR-16-5p/CCND2 axis and by regulating AQP3. Cell Death Dis. 2021;12(5):496. doi: 10.1038/s41419-021-03775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang Y-T, Zhou J, Shi S, et al. Identification of estrogen response element in aquaporin-3 gene that mediates estrogen-induced cell migration and invasion in estrogen receptor-positive breast cancer. Sci Rep. 2015;5:12484. doi: 10.1038/srep12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Q, Zhu L, Zong H, et al. Subcellular localization of aquaporin 3 in prostate cancer is regulated by RalA. Oncol Rep. 2018;39(5):2171–2177. doi: 10.3892/or.2018.6308. [DOI] [PubMed] [Google Scholar]
- 122.Hou S-Y, Li Y-P, Wang J-H, et al. Aquaporin-3 inhibition reduces the growth of NSCLC cells induced by hypoxia. Cellular physiol biochem. 2016;38(1):129–140. doi: 10.1159/000438615. [DOI] [PubMed] [Google Scholar]
- 123.Xia H, Ma Y-F, Yu C-H, et al. Aquaporin 3 knockdown suppresses tumour growth and angiogenesis in experimental non-small cell lung cancer. Exp Physiol. 2014;99(7):974–984. doi: 10.1113/expphysiol.2014.078527. [DOI] [PubMed] [Google Scholar]
- 124.Xu H, Xu Y, Zhang W, et al. Aquaporin-3 positively regulates matrix metalloproteinases via PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J Exp clin cancer res. 2011;30:86. doi: 10.1186/1756-9966-30-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pimpão C, da Silva IV, Mósca AF, et al. The aquaporin-3-inhibiting potential of polyoxotungstates. Int J mol sci. 2020 doi: 10.3390/ijms21072467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pellavio G, Rui M, Caliogna L, et al. Regulation of aquaporin functional properties mediated by the antioxidant effects of natural compounds. Int j mol sci. 2017 doi: 10.3390/ijms18122665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ji C, Cao C, Lu S, et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother Pharmacol. 2008;62(5):857–865. doi: 10.1007/s00280-007-0674-6. [DOI] [PubMed] [Google Scholar]
- 128.Martinotti S, Pellavio G, Patrone M, et al. Manuka honey induces apoptosis of epithelial cancer cells through aquaporin-3 and calcium signaling. Life (Basel, Switzerland) 2020 doi: 10.3390/life10110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao L, Gao Y, Li X, et al. Aquaporins mediate the chemoresistance of human melanoma cells to arsenite. Mol Oncol. 2012;6(1):81–87. doi: 10.1016/j.molonc.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dong X, Wang Y, Zhou Y, et al. Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin autophagy. Cell death discovery. 2016;2:16087. doi: 10.1038/cddiscovery.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trigueros-Motos L, Pérez-Torras S, Casado FJ, et al. Aquaporin 3 (AQP3) participates in the cytotoxic response to nucleoside-derived drugs. BMC Cancer. 2012;12:434. doi: 10.1186/1471-2407-12-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ismail M, Bokaee S, Morgan R, et al. Inhibition of the aquaporin 3 water channel increases the sensitivity of prostate cancer cells to cryotherapy. Br J Cancer. 2009;100(12):1889–1895. doi: 10.1038/sj.bjc.6605093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alkhalifa H, Mohammed F, Taurin S, et al. Inhibition of aquaporins as a potential adjunct to breast cancer cryotherapy. Oncol Lett. 2021;21(6):458. doi: 10.3892/ol.2021.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Z, Shen J, Carbrey JM, et al. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA. 2002;99(9):6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hibuse T, Maeda N, Funahashi T, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA. 2005;102(31):10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Funahashi T, Nagasawa A, Hibuse T, et al. Impact of glycerol gateway molecule in adipocytes. Cell mol biol. 2006;52(7):40–45. [PubMed] [Google Scholar]
- 137.Hibuse T, Maeda N, Nakatsuji H, et al. The heart requires glycerol as an energy substrate through aquaporin 7, a glycerol facilitator. Cardiovasc Res. 2009;83(1):34–41. doi: 10.1093/cvr/cvp095. [DOI] [PubMed] [Google Scholar]
- 138.Saito Y, Furukawa T, Obata T, et al. Molecular imaging of aquaglycero-aquaporins: its potential for cancer characterization. Biol Pharm Bull. 2013;36(8):1292–1298. doi: 10.1248/bpb.b13-00079. [DOI] [PubMed] [Google Scholar]
- 139.Wang J, Tanji N, Sasaki T, et al. Androgens upregulate aquaporin 9 expression in the prostate. Int J urol. 2008;15(10):936–941. doi: 10.1111/j.1442-2042.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 140.Lv Y, Huang Q, Dai W, et al. AQP9 promotes astrocytoma cell invasion and motility via the AKT pathway. Oncol Lett. 2018;16(5):6059–6064. doi: 10.3892/ol.2018.9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qian Y, Liu F, Zhang W, et al. AQP9 suppresses hepatocellular carcinoma cell invasion through inhibition of hypoxia-inducible factor 1α expression under hypoxia. J Gastroenterol Hepatol. 2020;35(11):1990–1997. doi: 10.1111/jgh.15023. [DOI] [PubMed] [Google Scholar]
- 142.Watanabe S, Moniaga CS, Nielsen S, et al. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem Biophys Res Commun. 2016;471(1):191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 143.Zheng X, Li C, Yu K, et al. Aquaporin-9, mediated by IGF2, suppresses liver cancer stem cell properties via augmenting ROS/β-catenin/FOXO3a signaling. Molr cancer res. 2020 doi: 10.1158/1541-7786.MCR-19-1180. [DOI] [PubMed] [Google Scholar]
- 144.Bhattacharjee H, Carbrey J, Rosen BP, et al. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem Biophys Res Commun. 2004;322(3):836–841. doi: 10.1016/j.bbrc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 145.Leung J, Pang A, Yuen W-H, et al. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109(2):740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 146.Iriyama N, Yuan B, Yoshino Y, et al. Aquaporin 9, a promising predictor for the cytocidal effects of arsenic trioxide in acute promyelocytic leukemia cell lines and primary blasts. Oncol Rep. 2013;29(6):2362–2368. doi: 10.3892/or.2013.2388. [DOI] [PubMed] [Google Scholar]
- 147.Chau D, Ng K, Chan TS-Y, et al. Azacytidine sensitizes acute myeloid leukemia cells to arsenic trioxide by up-regulating the arsenic transporter aquaglyceroporin 9. J Hematol Oncol. 2015;8:46. doi: 10.1186/s13045-015-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y, Yin J-Y, Li X-P, et al. The association of transporter genes polymorphisms and lung cancer chemotherapy response. PLoS ONE. 2014;9(3):e91967. doi: 10.1371/journal.pone.0091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miao Z-F, Chang EE, Tsai F-Y, et al. Increased aquaglyceroporin 9 expression disrupts arsenic resistance in human lung cancer cells. Toxicol in vitro. 2009;23(2):209–216. doi: 10.1016/j.tiv.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 150.Huang D, Feng X, Liu Y, et al. AQP9-induced cell cycle arrest is associated with RAS activation and improves chemotherapy treatment efficacy in colorectal cancer. Cell Death Dis. 2017;8(6):e2894. doi: 10.1038/cddis.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dou R, Deng Y, Huang L, et al. Multi-microarray identifies lower AQP9 expression in adjuvant chemotherapy nonresponders with stage III colorectal cancer. Cancer Lett. 2013;336(1):106–113. doi: 10.1016/j.canlet.2013.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Thapa S, Chetry M, Huang K, 2018. Significance of aquaporins’ expression in the prognosis of gastric cancer. Biosci rep. [DOI] [PMC free article] [PubMed]
- Chetry M, Li S, Liu H, 2018. Prognostic values of mRNA expression in human ovarian cancer. Biosci rep. [DOI] [PMC free article] [PubMed]
- Yang M-H, Dibas A, Tyan Y-C. 2013. Changes in retinal aquaporin-9 (AQP9) expression in glaucoma. Biosci rep. [DOI] [PMC free article] [PubMed]