Abstract
Introduction
For 30 years synapse loss has been referred to as the major pathological correlate of cognitive impairment in Alzheimer's disease (AD). However, this statement is based on remarkably few patients studied by autopsy or biopsy. With the recent advent of synaptic vesicle glycoprotein 2A (SV2A) positron emission tomography (PET) imaging, we have begun to evaluate the consequences of synaptic alterations in vivo.
Methods
We examined the relationship between synaptic density measured by [11C]UCB‐J PET and neuropsychological test performance in 45 participants with early AD.
Results
Global synaptic density showed a significant positive association with global cognition and performance on five individual cognitive domains in participants with early AD. Synaptic density was a stronger predictor of cognitive performance than gray matter volume.
Conclusion
These results confirm neuropathologic studies demonstrating a significant association between synaptic density and cognitive performance, and suggest that this correlation extends to the early stages of AD.
Keywords: Alzheimer's disease, cognition, synaptic density, synaptic vesicle glycoprotein 2A, [11C]UCB‐J
1. INTRODUCTION
For 30 years, synapse loss has been referred to as the major pathological correlate of cognitive impairment in Alzheimer's disease (AD). 1 , 2 , 3 However, this statement is based on remarkably few patients studied by autopsy or biopsy in limited brain regions, largely at the moderate to severe stages of disease. The earliest efforts to correlate synapse loss with cognitive impairment in AD came from a single brain biopsy study 1 and a single autopsy study, 2 , 3 both conducted primarily in participants with moderate to severe dementia. A subsequent clinicopathological investigation incorporated individuals with mild cognitive impairment (MCI) and focused on the hippocampus, 4 demonstrating that synapse number in the dentate gyrus (outer molecular layer) correlated with ante mortem cognitive performance in a pooled sample, including patients with advanced dementia and normal controls. However, these results derived partially from the inclusion of cognitively normal (CN) and neuropathologically confirmed controls and thus may not apply to synapse loss within the AD continuum.
With the recent advent of synaptic positron emission tomography (PET) imaging, we have begun to evaluate synaptic alterations in vivo. Synaptic vesicle glycoprotein 2A (SV2A) is expressed in virtually all synapses and is located in synaptic vesicles at presynaptic terminals. 5 [11C]UCB‐J was recently developed as a PET tracer for SV2A and advanced for human studies. 6 In our recent study of [11C]UCB‐J PET, we observed widespread reductions of SV2A binding in medial temporal and neocortical brain regions in early AD compared to CN participants. 7 However, initial attempts using PET imaging to associate synaptic density with cognitive performance have been hindered by the use of limited cognitive measures. 7 , 8 , 9
In this study, we examined the relationship between synaptic density and cognitive performance in early AD using [11C]UCB‐J PET and an extensive neuropsychological test battery. We aimed to test the hypothesis that synaptic density, as assessed by [11C]UCB‐J in brain regions that are typically affected by AD, is associated with neuropsychological function globally and in individual cognitive domains. We also examined the effects of tissue loss on these associations—compared to previous clinicopathological studies. Finally, we examined the relationship between gray matter (GM) volume—compared to synaptic density—and neuropsychological function in this sample.
RESEARCH IN CONTEXT
Systematic review: Synapse loss has been referred to as the major pathological correlate of cognitive impairment in Alzheimer's disease (AD). With the recent advent of synaptic vesicle glycoprotein 2A (SV2A) positron emission tomography (PET) imaging, we have begun to evaluate the consequences of synaptic alterations in vivo.
Interpretation: In 45 participants with early AD, global synaptic density showed a significant positive association with global cognition and performance on five individual cognitive domains. These results confirm neuropathologic studies, demonstrating a significant association between synaptic density and cognitive performance and suggest that this correlation extends to the early stages of AD.
Future directions: These results further support the use of synaptic imaging as a potential surrogate biomarker outcome for therapeutic trials that is well‐correlated with clinical measures. Longitudinal studies are needed to relate change in synaptic density as measured by [11C]UCB‐J PET with change in cognitive performance.
2. METHODS
Detailed methods are in supporting information (Supplement).
2.1. Study participants and design
Participants aged 50 to 85 years were screened for eligibility as previously described. 7 Individuals with dementia met diagnostic criteria for probable AD dementia, 10 had a Clinical Dementia Rating global score (CDR‐global) of 0.5 to 1.0, and a Mini‐Mental State Examination (MMSE) score of ≤ 26. Participants with MCI met diagnostic criteria for amnestic MCI (aMCI), 11 had a CDR‐global score of 0.5, and a MMSE score of 24 to 30, inclusive. Participants with dementia and MCI were required to demonstrate impaired episodic memory, as evidenced by a Logical Memory (LM) II score 1.5 standard deviations (SD) below an education‐adjusted norm. Older CN participants were enrolled solely to provide additional normative data for neuropsychological test scores and were required to have a CDR‐global score of 0, a MMSE score of > 26, and a normal education‐adjusted LMII score. All participants received a PET scan with [11C]Pittsburgh compound B ([11C]PiB) to assess for the accumulated presence of brain amyloid beta (Aβ), and a PET scan with [11C]UCB‐J to measure synaptic density. Participants with dementia and MCI were required to be Aβ+ and CN participants were required to be Aβ–. 7 All participants provided written informed consent as approved by the Yale University Human Investigation Committee.
Validated neuropsychological tests were administered to assess performance in five cognitive domains: verbal memory (LMI and II, Rey Auditory Verbal Learning Test [RAVLT] total words recalled across trials 1–5, RAVLT delayed recall), language (Boston Naming Test, Category Fluency), executive function (Stroop Color Word, Trail Making Test‐Part B, Letter Fluency), processing speed (Stroop Word, Trail Making Test‐Part A, Wechsler Adult Intelligence Scale [WAIS]‐III Digit Symbol Substitution), and visuospatial ability (Rey‐Osterrieth Complex Figure, WAIS‐III Block Design, WAIS‐3 Picture Completion). Raw scores from each test were converted to z‐scores using means and SDs of the entire sample (CN and AD). Cognitive domain scores were generated for each AD participant by averaging the z‐scores for the tests in each domain. A global cognition score was generated for each participant by averaging all five cognitive domain scores.
2.2. Brain imaging
T1‐weighted magnetic resonance imaging (MRI) was performed to define regions of interest (ROI) and to perform partial volume correction (PVC) using the iterative Yang (IY) approach. 12 , 13 PET scans were performed on the HRRT (207 slices, resolution < 3 mm full width half max). 14 List‐mode data were reconstructed using the MOLAR algorithm 15 with event‐by‐event motion correction based on an optical detector (Vicra, NDI Systems). 16 Dynamic [11C]PiB scans were acquired for 90 minutes after a bolus of up to 555 MBq of tracer 17 and dynamic [11C]UCB‐J scans were acquired for 60 or 90 minutes after administration of a bolus of up to 740 MBq. 18 Software motion correction was applied to the dynamic PET images using a mutual‐information algorithm (FSL‐FLIRT) to perform frame‐by‐frame registration to a summed image (0 to 10 minutes). A summed motion‐corrected PET image was registered to each MRI. Cortical reconstruction and volumetric segmentation was performed using FreeSurfer (version 6.0). 19 Regions defined by the FreeSurfer segmentation were used for both PET and MRI analyses in native participant space. Brain volume was normalized using estimated total intracranial volume. 20 A composite ROI of AD‐affected regions (prefrontal, lateral temporal, medial temporal, lateral parietal, anterior cingulate, posterior cingulate, precuneus, and lateral occipital) was defined (Table S1 in supporting information).
2.3. Tracer kinetic modeling
For [11C]PiB image analysis, parametric images of BP ND were generated using a simplified reference tissue model‐2 step (SRTM2) 21 as previously described. 7 , 17 For [11C]UCB‐J image analysis, parametric images of BP ND were generated using a SRTM2 from 0 to 60 minutes 21 with the centrum semiovale (CS) as the reference region. 22 , 23 Distribution volume ratio (DVR) using a whole cerebellum reference was computed as (BP ND +1)/(BP ND[cerebellum]+1). 7
2.4. Statistical analyses
Statistical methods are detailed in supporting information. Group comparisons were performed using χ2 tests for categorical variables and unpaired t‐tests for continuous variables. Multiple variable linear regression analyses used synaptic density or GM volume as the main explanatory variables and cognitive scores as outcomes and controlled for age, sex, and years of education. The Benjamini‐Hochberg procedure was used to control the false discovery rate (FDR) for multiple comparisons (five cognitive domains). Pearson's r (effect size) maps were created with the voxels in each region set uniformly to the calculated effect size. P < 0.05 was used as a threshold for significance.
3. RESULTS
3.1. Participant characteristics
The study sample consisted of 64 participants—45 with AD (28 with mild dementia, 17 with aMCI), and 19 who were CN and provided normative neuropsychological data—whose characteristics are shown in Table 1. The sample had substantial overlap with those of our previous reports 7 , 8 , 24 and included seven additional participants (three aMCI and four mild dementia). CN and AD groups were balanced for age and sex, but the CN group had a higher education level. Neuropsychological testing was performed an average of 1.6 (SD 6.5) weeks after synaptic density PET with a range of 16.1 weeks before to 15.6 weeks after the PET scan. As expected, the AD group had lower MMSE scores, higher CDR‐global scores, and significantly lower performance on a composite of global cognition, as well as in all cognitive domains. As in our previous analyses of a largely overlapping sample, synaptic density in both a composite of AD‐affected regions, as well as in the hippocampus were significantly lower in the AD group.
TABLE 1.
Participant characteristics
| Cognitively normal | Alzheimer's disease | P | |
|---|---|---|---|
| Participants (n) | 19 | 45 (mild dementia: 28, MCI: 17) | – |
| Sex (M/F) | 9/10 | 23/22 | 0.78 |
| Age (years) | 70.84 (7.78) (59–82) | 70.82 (7.48) (50–83) | 0.99 |
| Education (years) | 17.74 (2.00) (12–20) | 16.13 (2.31) (12–20) | 0.0093 |
| CDR‐global | 0 (0) (0) | 0.76 (.25) (0.5–1) | < 0.00001 |
| CDR‐SB | 0.00 (0.00) (0) | 4.11 (1.81) (0.5–9.0) | < 0.00001 |
| MMSE | 29.21 (1.06) (27–30) | 23.64 (3.37) (14–30) | < 0.00001 |
| Global cognition | 0.93 (0.25) (0.44–1.25) | –0.41 (0.62) (–1.68–0.69) | < 0.00001 |
| Executive functions | 0.85 (0.40) (0.20–1.71) | –0.42 (0.77) (–1.74–1.00) | < 0.00001 |
| Processing speed | 0.75 (0.28) (0.38–1.41) | –0.32 (0.87) (–2.32–1.00) | < 0.00001 |
| Language | 0.89 (0.32) (0.29–1.90) | –0.38 (0.79) (–2.16–0.85) | < 0.00001 |
| Visuospatial ability | 0.85 (0.41) (–0.15–1.54) | –0.37 (0.78) (–2.05–1.07) | < 0.00001 |
| Verbal memory | 1.29 (0.52) (0.29–2.01) | –0.54 (0.42) (–1.19–0.51) | < 0.00001 |
| Amyloid ± | 0/19 | 45/0 | < 0.00001 |
| Composite synaptic density (DVR) | 1.57 (0.08) (1.44–1.73) | 1.45 (0.11) (1.27–1.70) | 0.00004 |
| Hippocampal synaptic density (DVR) | 1.03 (0.08) (0.86–1.20) | 0.88 (0.12) (0.66–1.17) | < 0.00001 |
| APOE ε4 copy number (n) | |||
| 2 copies | 0 | 12 (26.7%) | – |
| 1 copy | 4 (21%) | 23 (51.1%) | – |
| 0 copies | 15 (79%) | 10 (22.2%) | – |
Abbreviations: APOE, apolipoprotein E: CDR‐global, Clinical Dementia Rating global score; CDR‐SB, Clinical Dementia Rating Sum of Boxes; DVR, distribution volume ratio of [11C]UCB‐J calculated with a whole cerebellum reference region; MMSE, Mini‐Mental State Examination; SD, standard deviation.
Notes: Data are mean (SD) (range). Scores for cognitive measures are z‐scores. P‐values are for unpaired t‐tests (continuous variables) or χ2 (categorical variables).
3.2. Association between synaptic density (DVR) and cognition in AD
The primary analysis investigated the association between global synaptic density (DVR) in a composite of AD‐affected regions and global cognition within the AD group. A multiple linear regression model with DVR as the predictor and global cognition as the outcome was significant (F[4, 40] = 6.19, P = 0.001, R 2 = 0.38) and synaptic density was a significant predictor of global cognition (β = 3.21, η2 = 0.29, P = 0.0001, Figure 1A, Table S2a in supporting information).
FIGURE 1.
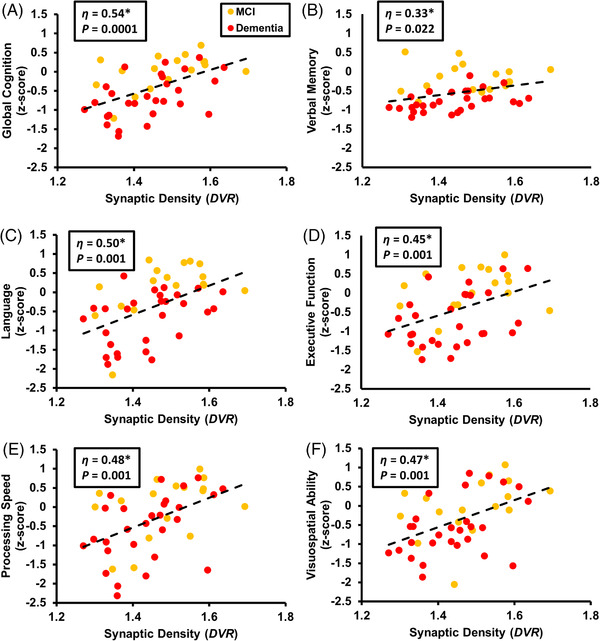
Correlation of synaptic density (DVR) and cognition in participants with AD. Global synaptic density in a composite of AD‐affected regions, represented as DVR, was plotted with (A) global cognition, (B) verbal memory, (C) language, (D) executive function, (E) processing speed, or (F) visuospatial ability. Multiple variable linear regression analysis included predictors of synaptic density, years of education, age, and sex. η is displayed for the main explanatory variable of synaptic density as it contributes to the overall model (*P < 0.05). Data points and line of best fit (dotted line) are unadjusted values. AD, Alzheimer's disease; DVR, distribution volume ratio of [11C]UCB‐J calculated with a whole cerebellum reference region
To investigate the association between synaptic density and performance in specific cognitive domains, separate models were fit with performance in each of the five domains (verbal memory, language, executive function, processing speed, and visuospatial ability) as the outcome (Table S2b). Each model was significant and synaptic density was a significant predictor of performance in every domain (Table S2b, Figure 1B‐1F).
Additional exploratory analyses assessed the association between synaptic density in all brain regions and global cognition. Pearson's r was calculated for the correlation between synaptic density and global cognition (Figure 2A, Table S3 in supporting information). Synaptic density had a significant positive association with global cognition across many prefrontal, temporal, parietal, and occipital cortical regions. Similar regional patterns were observed for specific cognitive domain scores, including language, executive function, processing speed, and visuospatial ability (Figure 3). By contrast, associations between synaptic density and verbal memory performance were more restricted within temporal, parietal, and occipital cortical regions. Interestingly, synaptic density in hippocampus and entorhinal cortex was not significantly correlated with global cognition (Figure 2A, Table S3) or domain‐specific cognitive performance (Figure 3).
FIGURE 2.
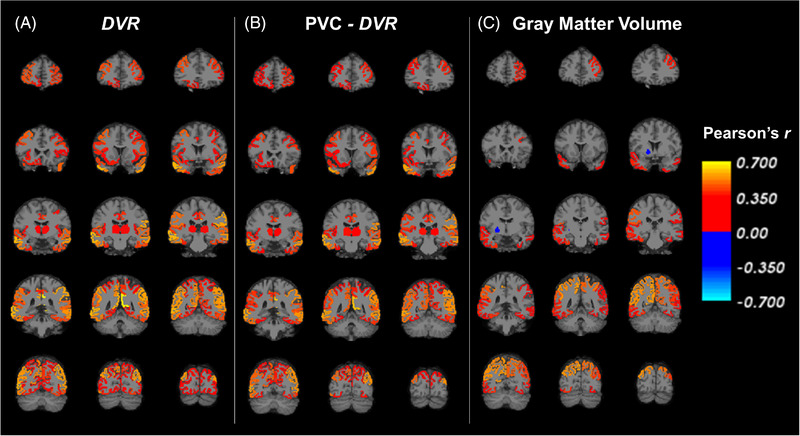
Correlation maps of synaptic density (DVR) and global cognition in all regions for participants with AD. (A) Pearson's r was calculated for the correlation between synaptic density ([11C]UCB‐J DVR) and global cognition in all FreeSurfer regions. A similar analysis was conducted (B) after PVC of [11C]UCB‐J PET images, and (C) with gray matter volume. Brain maps were created by producing images with the voxels in each FreeSurfer region set uniformly to the calculated Pearson's r for that region and overlaid on an MNI template T1 MRI. The color scale represents Pearson's r, which is displayed only for regions that had an uncorrected P < 0.05. AD, Alzheimer's disease; DVR, distribution volume ratio of [11C]UCB‐J calculated with a whole cerebellum reference region; MNI, Montreal Neurological Institute; MRI, magnetic resonance imaging; PET, positron emission tomography; PVC, partial volume correction
FIGURE 3.

Correlation maps of synaptic density (DVR) and domain specific cognitive performance in all regions for participants with AD. Pearson's r was calculated for the correlation between synaptic density ([11C]UCB‐J DVR) and (A) verbal memory, (B) language, (C) executive function, (D) processing speed, and (E) visuospatial ability domain scores. Brain maps were created by producing images with the voxels in each FreeSurfer region set uniformly to the calculated Pearson's r for that region and overlaid on an MNI template T1 MRI. The color scale represents Pearson's r, which is displayed only for regions that had an uncorrected P < 0.05. AD, Alzheimer's disease; DVR, distribution volume ratio of [11C]UCB‐J calculated with a whole cerebellum reference region; MNI, Montreal Neurological Institute; MRI, magnetic resonance imaging
3.3. Association between partial volume corrected synaptic density (PVC‐DVR) and cognition in AD
Because volume loss related to AD can lead to underestimation of synaptic density, we repeated the previous analyses after PVC. A multiple linear regression model with global PVC‐DVR as the predictor and global cognition as the outcome was significant (F[4, 40] = 4.93, P = 0.003, R 2 = 0.33) and synaptic density was a significant predictor of global cognition (β = 2.16, η2 = 0.23, P = 0.001, Figure 4A, Table S4a in supporting information).
FIGURE 4.

Correlation of synaptic density with PVC (PVC–DVR) and cognition in participants with AD. PVC was applied to [11C]UCB‐J PET images. Global synaptic density in a composite of AD‐affected regions, represented as DVR, was plotted with (A) global cognition, (B) verbal memory, (C) language, (D) executive function, (E) processing speed, or (F) visuospatial ability. Multiple variable linear regression analysis included predictors of synaptic density, years of education, age, and sex. η is displayed for the main explanatory variable of synaptic density as it contributes to the overall model (*P < 0.05). Data points and line of best fit (dotted line) are unadjusted values. AD, Alzheimer's disease; DVR, distribution volume ratio of [11C]UCB‐J calculated with a whole cerebellum reference region; PET, positron emission tomography; PVC, partial volume correction
Separate models were fit with performance on each of the five cognitive domains as the outcomes (Table S4b). Each model was significant, and synaptic density was a significant predictor of performance in every domain (Table S4b, Figure 4B‐4F).
The correlation between regional synaptic density (PVC‐DVR) and global cognition was again assessed (Figure 2B, Table S3). Similar to the analysis without PVC, synaptic density had a significant positive association with global cognition across many prefrontal, temporal, parietal, and occipital cortical regions. Compared to the analysis using global cognition, similar regional patterns were present with significant positive associations between regional synaptic density and language, executive function, processing speed, and visuospatial ability (Figure S1 in supporting information).
3.4. Association between GM volume and cognition in AD
As a comparator to synaptic density, we examined the relationship between GM volume and cognition in this sample. A multiple linear regression model with GM volume as the predictor and global cognition as the outcome was significant (F[4, 40] = 3.57, P = 0.014, R 2 = 0.26) and GM volume was a significant predictor of global cognition (β = 0.012, η2 = 0.17, P = 0.005, Figure S2A, Table S5a in supporting information).
Separate models were again fit with performance in each of the five cognitive domains as the outcomes (Table S5b). In contrast to synaptic density, GM volume was only a significant predictor of language and executive function, but not the other cognitive domains (Table S5b). In addition, the effect sizes for correlations between GM volume and cognition (Figure S2) were generally smaller than the effect sizes for correlations between synaptic density and cognition (Figure 1 and Figure 4).
When regional GM volume was correlated with global cognition, there were positive associations (Figure 2C, Table S3), but with fewer significant regions compared to synaptic density (Figure 2A, B). When regional GM volume was correlated with individual cognitive domains, similar regional patterns as for global cognition were observed, with significant positive associations between regional GM volume and language, executive functions, processing speed, and visuospatial ability (Figure S3 in supporting information).
4. DISCUSSION
In this study we examined the relationship between synaptic density and cognitive performance in early AD using [11C]UCB‐J PET and an extensive neuropsychological test battery. In a multiple linear regression model controlling for age, sex, and education, global synaptic density ([11C]UCB‐J DVR) was a significant predictor of global cognitive performance in participants with AD. Synaptic density was also a significant predictor of performance in all five cognitive domains: language, executive function, processing speed, visuospatial ability, and verbal memory. The relatively weak association with verbal memory likely resulted from floor effects on the cognitive measures that comprised this domain. The observed associations between synaptic density and global cognition remained significant after correction for partial volume effects, and synaptic density was a stronger predictor of cognitive performance than GM volume.
4.1. Comparison with post mortem and biopsy specimen human studies
These results confirm neuropathologic studies demonstrating a significant association between synaptic density and cognitive performance, and suggest that this correlation extends to the mild and prodromal stages of AD. The early evidence for synapse loss as the major pathological correlate of cognitive impairment in AD 1 came from a single brain biopsy study and a single autopsy study 2 both conducted largely at the moderate to severe stages of disease. DeKosky and Scheff first reported synapse loss in eight individuals with AD who had undergone frontal cortex biopsies. 1 They found that in eight AD patients with MMSE scores ranging from 12 to 20, synapse counts correlated with MMSE performance. 1 The next year, Terry et al. 2 reported on autopsy data from 15 patients with AD and nine neuropathologically confirmed controls and found that within the AD group (MMSE = 3–20), synapse density in midfrontal and inferior parietal regions was correlated with ante mortem cognitive performance on the Blessed Information‐Memory‐Concentration Test, the MMSE, and the Mattis Dementia Rating Scale. Masliah et al. then investigated a subset of the cases in Terry et al. (AD: 9, neuropathologically normal controls: 4) and demonstrated that the strongest correlation was between synapse density and Blessed score of cognitive impairment. 3
In 2006 Scheff et al. reported for the first time on synaptic data in the hippocampus 4 and found that synapse number in dentate gyrus (outer molecular layer) correlated with ante mortem cognitive performance in a pooled sample, including normal controls (MMSE = 8–30). The AD sample included nine with dementia and nine with MCI. However, the inclusion of 10 cognitively normal and neuropathologically confirmed controls in the analyses left unclear the extent to which associations were with synapse loss along the AD continuum as opposed to the presence or absence of AD. Another limitation of that study—as with most post mortem studies—was that the overall AD sample had a mean age of 88, and 87 in the MCI group. These individuals were likely prodromal for dementia onset in their 90s and perhaps similar to very old AD samples, which have been shown to have unique characteristics, particularly multiple neuropathologies. 25 , 26 , 27 Therefore, they are not necessarily representative of the broad samples of early AD that are studied in vivo.
4.2. Comparison with previous human synaptic density imaging studies
With the recent advent of synaptic PET imaging, we have begun to evaluate synaptic alterations in vivo. However, initial attempts using PET imaging to associate synaptic density with cognitive performance in AD have been hindered by the use of limited cognitive measures. In our preliminary report, 8 we observed that hippocampal SV2A‐specific binding (BPND ) was correlated with a composite episodic memory score and a measure of global functioning (CDR‐Sum of Boxes [SB]) in a pooled sample of participants who had early AD or were cognitively normal. In a follow‐up study of a larger sample (19 CN and 35 early AD, a subset of the present sample of 45), in which we demonstrated widespread synaptic loss in AD using a more robust SV2A outcome measure (DVR with cerebellum as reference region), we again examined the relationship between synaptic density and clinical measures. 7 Specifically, we investigated the association of DVR in either hippocampus or a composite ROI of AD‐affected regions (identical to those in the present study) with episodic memory or CDR‐SB. In the overall sample, statistically significant correlations were observed between DVR in either the hippocampus or the composite region and either episodic memory or CDR‐SB. However, none of these correlations were significant within the AD or CN groups alone.
We believe that the negative results using more limited cognitive and clinical measures may relate to at least two factors. The memory score is comprised of difficult memory tasks that produce “floor effects” in the participants with early AD and a lack of dynamic range. The verbal memory score in the present study incorporated a measure with more range (the total of trials 1 through 5 on the RAVLT and LMI [immediate recall], instead of only the delayed recall score). However, the correlation between global SV2A and verbal memory remained the weakest correlation between global SV2A and any cognitive domain. Moreover, the brain regions that are most likely to correlate with verbal memory are those that already demonstrate marked degeneration in early AD, thus yielding a lack of dynamic range in both the memory and the SV2A imaging variables.
Apart from our own work, Bastin et al., using a different SV2A ligand ([18F]UCB‐H with partial volume correction), reported in 24 participants with AD that reduced hippocampal uptake was related to cognitive decline (MMSE score) and unawareness of memory problems. 9 Coomans et al. explored cognitive associations and synaptic density in a small sample of seven AD participants and found that MMSE score was not associated with a global measure of synaptic density using [11C]UCB‐J BPND but was strongly associated with a global measure of tau deposition using [18F]flortaucipir BPND. 28
4.3. Limitations and future directions
This study has a number of important limitations. First, we cannot comment on the relative strength or spatial patterns of association with different cognitive domains, as these comparisons are limited by test selection. Specifically, individual tests may differ in relative difficulty, and some may exhibit floor effects, thus hindering our ability to evaluate associations with PET measures. Second, the cross‐sectional nature of this study is susceptible to many confounding factors that may result in considerable inter‐individual variability in synaptic density and neuropsychological performance. Longitudinal studies will better enable us to test directly the hypothesis that synaptic loss is associated with a decline in cognitive performance. In particular, longitudinal studies that enroll participants at pre‐symptomatic stages of disease may capture the early emergence of both synaptic loss and cognitive symptoms. Longitudinal studies—by minimizing inter‐individual sources of variance—will also confer greater statistical power to detect more specific associations between regional synaptic loss and the decline in specific cognitive domains. If synaptic loss is the major pathological correlate of cognitive impairment in AD, then the heterogeneity of decline in cognitive domains should be related to the regional patterns of synaptic loss.
4.4. Conclusion
These results confirm neuropathologic studies demonstrating a significant association between synaptic density and cognitive performance, and suggest that this correlation extends to the mild and prodromal stages of AD. They further support the use of synaptic imaging as a potential surrogate biomarker outcome for therapeutic trials that is well‐correlated with clinical measures. Longitudinal studies are needed to relate change in synaptic density as measured by [11C]UCB‐J PET with change in cognitive performance.
CONFLICTS OF INTEREST
APM, REC, and CHvD report grants from National Institutes of Health (NIH) for the conduct of the study. APM, ESS, TT, BCV, AFTA, YH, and REC report grant support from the NIH for work not related to this manuscript. APM reports grants for clinical trials from Genentech, Eisai, and Eli Lilly outside the submitted work. MKC reports research support from the Dana Foundation and Eli Lilly outside the submitted work. YH reports research grants from UCB and Eli Lilly outside the submitted work. REC reports grants from Bristol Myers Squibb, Cerevel Therapeutics, Invicro, and UCB outside the submitted work. CHvD reports grants for clinical trials from Biogen, Novartis, Eli Lilly, Eisai, Biohaven, and the Alzheimer's Association outside the submitted work. YH, REC, and NBN have a patent for a newer version of the SV2A tracer. MKC reports consulting fees from Eisai and Actinum. AFTA reports consulting fees from Vallon, Supernus, and Ludbeck. CHvD reports consulting fees from Roche, Esai, and Ono Pharmaceuticals. APM received honoraria for presentations at University of Connecticut and Stanford University. NBN received honoraria for presentations from UCLA Semel Institute for Neuroscience & Human Behavior. AFTA received honoraria for presentations at McGill University, Killam Institute, Montreal Neurological Institute, Harvard, Massachusetts General Hospital, Western Connecticut State University, and the University of Massachusetts. APM received support from ACTC/ATRI for travel to ACTC/ATRI meetings. TT received travel support from the conference for the 2019 Brain and Brain PET meeting. AFTA received support from the conference for travel to give a presentation at ACNP. APM is a member of the ISTAART Neuroimaging PIA executive committee. NN receives royalties from MD Anderson Cancer Center. AFTA and Yale receive royalties from Shire/Takada from USA sales of Intuniv. ERB, HHB, WZ, SL, NGD, and MN have nothing to disclose.
AUTHOR CONTRIBUTIONS
Dr. Mecca had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Mecca, Sharp, Chen, O'Dell, Carson, van Dyck. Acquisition, analysis, or interpretation of data: Mecca, Sharp, O'Dell, Banks, Bartlett, Naganawa, Diepenbrock, Lipior, Zhao, Toyonaga, Nabulsi, Arnsten, Huang, Carson, van Dyck. Drafting of the manuscript: Mecca, Sharp, O'Dell, Banks, Bartlett, Arnsten, Carson, van Dyck. Critical revision of the manuscript for important intellectual content: Mecca, Sharp, O'Dell, Banks, Bartlett, Naganawa, Diepenbrock, Lipior, Zhao, Toyonaga, Nabulsi, Arnsten, Huang, Carson, van Dyck. Statistical analysis: Mecca, O'Dell, Banks, Bartlett, Carson, van Dyck. Obtained funding: van Dyck, Carson, Mecca, Chen. Administrative, technical, or material support: Mecca, Sharp, O'Dell, Banks, Bartlett, Naganawa, Toyonaga, Diepenbrock, Lipior, Nabulsi, Huang, Chen, Carson, van Dyck. Study supervision: Mecca, Chen, Carson, van Dyck.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We wish to thank the research participants for their contributions, and the staff of the Yale AD Research Unit and PET Center for their excellent technical assistance. We also thank UCB for providing the [11C]UCB‐J radiolabeling precursor and the unlabeled reference standard. This research was supported by the National Institute on Aging (P30AG066508, P50AG047270, K23AG057784, R01AG052560, R01AG062276, RF1AG057553, and P30AG021342), The American Brain Foundation (APM), and The Dana Foundation (MKC), the Thomas P. Detre Fellowship Award in Translational Neuroscience Research in Psychiatry (RSO), and the Ruth L. Kirschstein National Research Service Award, Clinical Neuroscience Research Training in Psychiatry (T32MH019961). This publication was made possible by CTSA Grant Number UL1TR000142 from the National Center for Advancing Translational Science (NCATS), a component of NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Mecca AP, O'Dell RS, Sharp ES, et al. Synaptic density and cognitive performance in Alzheimer's disease: A PET imaging study with [11C]UCB‐J. Alzheimer's Dement. 2022;18:2527–2536. 10.1002/alz.12582
REFERENCES
- 1. DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457‐464. [DOI] [PubMed] [Google Scholar]
- 2. Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572‐580. [DOI] [PubMed] [Google Scholar]
- 3. Masliah E, Ellisman M, Carragher B, et al. Three‐dimensional analysis of the relationship between synaptic pathology and neuropil threads in Alzheimer's disease. J Neuropathol Exp Neurol. 1992;51:404‐414. [DOI] [PubMed] [Google Scholar]
- 4. Scheff SW, DA Price, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372‐1384. [DOI] [PubMed] [Google Scholar]
- 5. Bajjalieh SM, Peterson K, Linial M, Scheller RH. Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci U S A. 1993;90:2150‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finnema SJ, Nabulsi NB, Eid T, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. [DOI] [PubMed] [Google Scholar]
- 7. Mecca AP, Chen MK, O'Dell RS, et al. In vivo measurement of widespread synaptic loss in Alzheimer's disease with SV2A PET. Alzheimers Dement. 2020;16:974‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen MK, Mecca AP, Naganawa M, et al. Assessing synaptic density in Alzheimer's disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 2018;75:1215‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bastin C, Bahri MA, Meyer F, et al. In vivo imaging of synaptic loss in Alzheimer's disease with [18F]UCB‐H positron emission tomography. Eur J Nucl Med Mol Imaging. 2020;47:390‐402. [DOI] [PubMed] [Google Scholar]
- 10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erlandsson K, Buvat I, Pretorius PH, Thomas BA, Hutton BF. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys Med Biol. 2012;57:R119‐R159. [DOI] [PubMed] [Google Scholar]
- 13. Shidahara M, Thomas BA, Okamura N, et al. A comparison of five partial volume correction methods for Tau and Amyloid PET imaging with [18F]THK5351 and [11C]PIB. Ann Nucl Med. 2017;31:563‐569. [DOI] [PubMed] [Google Scholar]
- 14. de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA. Performance evaluation of the ECAT HRRT: an LSO‐LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol. 2007;52:1505‐1526. [DOI] [PubMed] [Google Scholar]
- 15. Carson R, Barker W, Liow J, Adler S, Johnson C, Design of a motion‐compensation OSEM list‐mode algorithm for resolution‐recovery reconstruction of the HRRT. IEEE Nuclear Sciences Symposium. Portland, OR, USA: 2003. [Google Scholar]
- 16. Jin X, Mulnix T, Gallezot JD, Carson RE. Evaluation of motion correction methods in human brain PET imaging–a simulation study based on human motion data. Medical physics. 2013;40:102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mecca AP, Barcelos NM, Wang S, et al. Cortical beta‐amyloid burden, gray matter, and memory in adults at varying APOE epsilon4 risk for Alzheimer's disease. Neurobiol Aging. 2017;61:207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finnema SJ, Nabulsi NB, Mercier J, et al. Kinetic evaluation and test‐retest reproducibility of [(11)C]UCB‐J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2018;38:2041‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischl B. FreeSurfer. Neuroimage. 2012;62:774‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas‐based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724‐738. [DOI] [PubMed] [Google Scholar]
- 21. Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440‐1452. [DOI] [PubMed] [Google Scholar]
- 22. Rossano S, Toyonaga T, Finnema SJ, et al. Assessment of a white matter reference region for (11)C‐UCB‐J PET quantification. J Cereb Blood Flow Metab. 2020;40:1890‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mertens N, Maguire RP, Serdons K, et al. Validation of parametric methods for [(11)C]UCB‐J PET imaging using subcortical white matter as reference tissue. Mol Imaging Biol. 2020;22:444‐452. [DOI] [PubMed] [Google Scholar]
- 24. O'Dell RS, Mecca AP, Chen MK, et al. Association of Abeta deposition and regional synaptic density in early Alzheimer's disease: a PET imaging study with [(11)C]UCB‐J. Alzheimers Res Ther. 2021;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson JL, Molina‐Porcel L, Corrada MM, et al. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer's disease in the oldest‐old. Brain. 2014;137:2578‐2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest‐old: the 90+ Study. Neurology. 2015;85:535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corrada MM, Sonnen JA, Kim RC, Kawas CH. Microinfarcts are common and strongly related to dementia in the oldest‐old: the 90+ study. Alzheimers Dement. 2016;12:900‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coomans EM, Schoonhoven DN, Tuncel H, et al. In vivo tau pathology is associated with synaptic loss and altered synaptic function. Alzheimers Res Ther. 2021;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


