Abstract
Background
Early hypotension following severe traumatic brain injury (sTBI) is associated with increased mortality and poor long-term outcomes. Current guidelines suggest the use of intravenous vasopressors, commonly norepinephrine and phenylephrine, to support blood pressure following TBI. However, guidelines do not specify vasopressor type, resulting in variation in clinical practice. We describe early vasopressor utilization patterns in critically ill patients with TBI and examine the association between utilization of norepinephrine, compared to phenylephrine, with hospital mortality following sTBI.
Methods
We conducted a retrospective cohort study of United States hospitals participating in the Premier Healthcare Database between 2009–2018. We examined adult patients (>17 years) with a primary diagnosis of sTBI who received care in an intensive care unit (ICU) following injury. The primary exposure was vasopressor choice (phenylephrine versus norepinephrine) within the first two days of hospital admission. The primary outcome was in-hospital mortality. Secondary outcomes examined included hospital length of stay (LOS) and intensive care unit LOS. We conducted a post-hoc subgroup analysis in all patients with ICP monitor placement. Regression analysis was used to assess differences in outcomes between patients exposed to phenylephrine versus norepinephrine, with propensity-matching to address selection bias due to the non-random allocation of treatment groups.
Results
From 2009–2018, 24,718 (37.1%) of 66,610 sTBI patients received vasopressors within the first two days of hospitalization. Among these patients, 60.6% (n=14991) received only phenylephrine, 10.8% (n=2668) received only norepinephrine, 3.5% (n=877) received other vasopressors, and 25.0% (n=6182) received multiple vasopressors. In that time period, use of all vasopressors following sTBI increased. A moderate degree of variation in vasopressor choice was explained at the individual hospital level (23.1%). In propensity-matched analysis, use of norepinephrine, compared to phenylephrine, was associated with an increased risk of in-hospital mortality (OR 1.65, CI 1.46–1.86, p <0.0001).
Conclusions
Early vasopressor utilization among critically ill patients with sTBI is common, increasing over the last decade, and varies across hospitals caring for TBI patients. Norepinephrine, compared to phenylephrine, was associated with increased risk of in-hospital mortality in propensity-matched analysis. Given the wide variation in vasopressor utilization and possible differences in efficacy, our analysis suggests the need for randomized controlled trials to better inform vasopressor choice for patients with sTBI.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and long-term disability in the United States (US) and globally1–3. With over 252,000 annual hospitalizations and 56,600 deaths attributable to TBI in the US, treatment of critically ill patients with head injury is a significant challenge for healthcare providers. In recent years, greater understanding of the systemic effects of severe TBI (sTBI) has resulted in strategies for multi-organ system management aimed at reducing secondary brain injury in addition to primary brain injury4–6. In particular, maintenance of blood pressure following TBI allows adequate cerebral perfusion pressure (CPP) to the injured brain6. Unfortunately, it is estimated that around 20% to 66% of patients with moderate to severe head injury have at least one episode of early hypotension following TBI7–10. Early hypotension after TBI can trigger cerebral ischemia, compromise cerebral hemodynamics, and is associated with increased mortality and worse clinical outcomes7,11–15. Therefore, blood pressure management and avoidance of hypotension with vasopressors are vital components of clinical TBI management.
Current TBI patient care guidelines support maintenance of systolic blood pressure (SBP) >100 mm Hg for patients 50–69 years and >110 mm Hg for patients 15–49 or >70 years, but do not include specific recommendations on choice of vasopressor4,16,17. Intravenous (IV) vasopressors, often phenylephrine or norepinephrine, are commonly used to augment SBP and CPP following sTBI, though their full impact on cerebral hemodynamics remains unclear6,18,19. Previous studies analyzing utilization patterns and efficacy of vasopressors following TBI vary in their results and are limited by small sample size18–22. To address this gap, we conducted a large multicenter study to: 1) Describe early vasopressor utilization patterns in patients with acute sTBI in the US and 2) Examine the association between utilization of norepinephrine, compared to phenylephrine, with hospital outcomes following sTBI.
Methods
Database and Study Design
We conducted a retrospective cohort study of adult patients in the Premier Healthcare Database from 2008–2018. Premier is a large, US hospital-based, service-level, all-payer database that contains information on inpatient discharges from more than 1,041 geographically diverse non-profit, non-governmental and community and teaching hospitals and health systems from rural and urban areas23. Premier data includes standard hospital discharge administrative files as well as date-stamped diagnostic, pharmacy, and laboratory charges from hospitals across all geographic regions of the US. This study was exempt from institutional review board (IRB) review at Duke University, given the fully de-identified nature of the Premier Healthcare Database.
Study Population
We examined adult (age>17 years) patients with a primary diagnosis of sTBI based on ICD-9 and ICD-10 diagnosis codes (Supplemental Table 1) and Head/Neck Abbreviated Injury Scale (AIS) values of 3 (serious), 4 (severe), and 5 (critical)24. We included those patients with Emergent, Urgent, or Trauma admission to the hospital and had charge codes for the intensive care unit (ICU) on the initial hospital day. AIS and Injury Severity Score (ISS) were calculated using the International Classification of Disease Program for Injury Classification and R statistical software (ICDPIC-R) with the General Equivalency Mapping (GEM) method25, which generate AIS scores, stratified by body region26. Vasopressors (norepinephrine, phenylephrine, vasopressin, epinephrine, dopamine, dobutamine) within the first two days of admission were identified using hospital charge codes. These hospital charge codes on the Premier database are day-level charges rather than hour-level charges from the Electronic Health Record. We chose to examine early vasopressor utilization (within the first 2 days of hospital admission) to increase the likelihood that vasopressor treatments were used for injury-induced hypotension, rather than from subsequent hospital complications (such as septic shock or pulmonary embolism), and during a period when the brain is most sensitive to secondary injury7.
Exposure, Outcomes, and Covariates
To determine early vasopressor utilization after acute sTBI, the primary exposures were demographics, clinical characteristics, facility characteristics, and time (calendar year). The primary outcome was use of early vasopressors, categorized as: None, Norepinephrine, Phenylephrine, and Other (vasopressin, epinephrine, dopamine, dobutamine). To examine early mixed vasopressor therapies, we separated patients who received more than one early vasopressor into a Multiple Vasopressor category.
To define associations between norepinephrine use (compared to phenylephrine) with hospital outcomes, the primary exposure was vasopressor choice (phenylephrine versus norepinephrine) within the first two days of hospitalization. The decision to consider only norepinephrine and phenylephrine as primary exposures was decided a priori and supported by the study team’s clinical and subject matter expertise, as these two vasopressors were deemed to likely represent the most commonly used vasopressors for management of hypotension following TBI in current clinical practice18,19. The primary outcome was in-hospital mortality. Secondary outcomes examined included hospital length of stay (LOS) and intensive care unit LOS. We conducted a post-hoc subgroup analysis in all patients with ICP monitor placement.
Covariates examined in our model included data on patient demographic and clinical characteristics (age, gender, race, ethnicity, insurance status), medical co-morbidities (29 co-morbidities based on the Elixhauser scheme27), co-treatments within 2 days of admission (central line placement, mannitol, hypertonic saline, tranexamic acid, and intracranial pressure monitoring), injury severity score (ISS), and hospital characteristics (bed size, teaching status, and rural location). Final model covariates were selected based on literature review, the subject matter expertise of the authorship team, and creation of a directed acyclic graph.
Statistical Analysis
Descriptive statistics were used to examine demographic and clinical characteristics, hospital characteristics, vasopressors, and utilization patterns over time. Categorical variables were reported as number (percentage) and continuous variables were reported as mean and standard deviation. For our first research objective, mixed-effects logistic regression models were used to identify predictors of vasopressor use (phenylephrine vs norepinephrine) including covariates described above as fixed effects. A random intercept was included for each hospital to model the variation of vasopressor choice to cluster at the hospital level. The intraclass correlation (ICC) was computed to examine the amount of variation in vasopressor choice explained at the hospital level (random effect), above and beyond patient-level factors (fixed effects)28.
For our second research objective, we examined the association of vasopressor choice with hospital outcomes using a propensity score matched analysis. Among patients receiving phenylephrine and norepinephrine within the first two days of hospitalization, we built propensity scores for treatment (choice of phenylephrine versus norepinephrine) using a logistic regression model and adjusted based on the following covariates: age, gender, race, ethnicity, insurance status, 29 Elixhauser co-morbidities, co-treatments within 2 days of admission (central line placement, mannitol, hypertonic saline, tranexamic acid, and intracranial pressure monitoring), injury severity score (ISS), and hospital characteristics (bed size, teaching status, and rural location). Then, patients exposed to phenylephrine were matched to patients exposed to norepinephrine using greedy propensity score techniques (in a 1:1 fashion) without replacement and a caliper width of 0.1029. After matching, standardized mean differences (SMD) were used to test the balance of covariates with a value greater than 0.1 or less than −0.1 indicating significant imbalance. Propensity score overlap was visually inspected. Lastly, univariable logistic regression models with robust standard errors for binary outcomes and Cox proportional hazard models for hospital discharge and intensive care unit discharge (with death treated as a censored observation) were used to determine the association between vasopressor use and clinical outcomes in the fully matched cohort, as well as in a subgroup analysis restricting the population to patients that received ICP monitoring. We chose the cause-specific Cox model over the Fine-Gray or sub-distribution hazard model because we were interested in the direct association between treatment and length of stay, since it compares the hazard of discharge for all patients who had survived to a given time point30. We conducted a sensitivity analysis for primary and secondary outcomes by restricting the cohort to survivors of at least two days of hospitalization, to demonstrate stability of our risk estimates with restriction due to the potential for immortal time bias. All analyses were conducted using SAS version 9.4 (SAS Institute; Cary, NC).
Results
Demographic and Clinical Characteristics of Study Population
We identified 66,610 patients that met inclusion and exclusion criteria (Figure 1). Details on the complete demographic and clinical characteristics of the study population may be found in Supplement Table 2. Of the entire study population, 37.1% (n=24,718) patients received IV vasopressors within two days of admission. The mean age of all patients was 59.0 ± 21.2 years. Males were 68.5% and Caucasian patients were 72.3%. Patients with Medicare made up the largest share of payors (45.3%). Most patients received care at teaching hospitals (65.5%) and large volume centers [>500 beds] (56.1%). The study population had a mean ISS of 20.1 ± 8.3. Early co-treatment utilization in the entire study population was common, including mannitol use (21.9%), hypertonic saline (11.3%), central line placement (12.1%), and ICP monitor use (4.8%).
Figure 1:
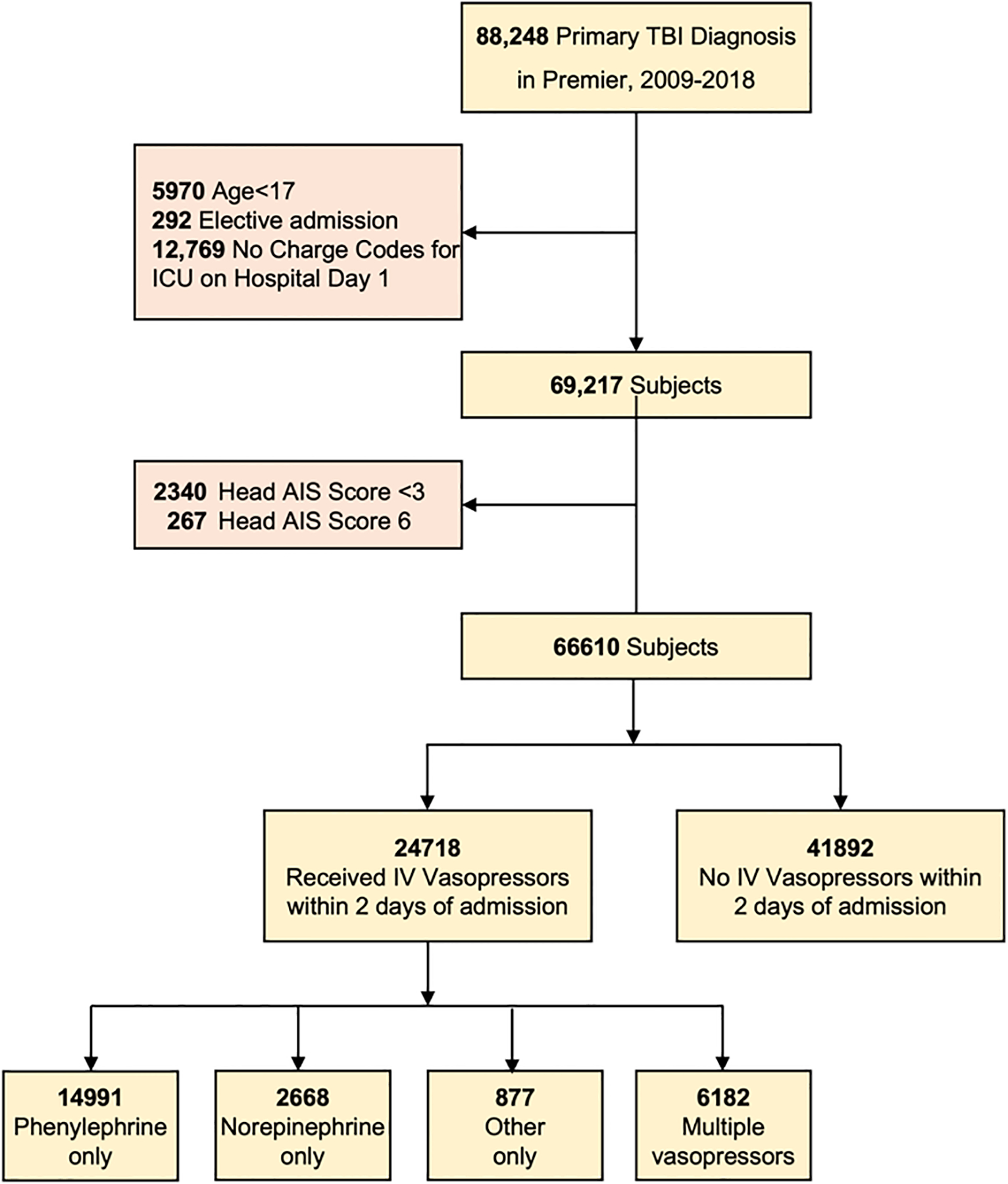
STROBE Diagram
Vasopressor Utilization Patterns
Of the 24,718 patients who received IV vasopressors within two days of admission, 60.6% (n=14991) received only phenylephrine and 10.8% (n=2668) received only norepinephrine. 3.5% (n=877) received other vasopressors, including dopamine, epinephrine, vasopressin, and dobutamine. Lastly, 25.0% (n=6182) of patients received more than one vasopressor during the first two days of hospital admission. Figure 2 demonstrates vasopressor utilization over the study period for all critically ill patients with a primary diagnosis of TBI (n= 66,610). Of the total sample, phenylephrine utilization increased from 16.0% in 2009 to a maximum of 26.7% in 2015; norepinephrine utilization increased from 3.1% in 2009 to 5.9% in 2018; and use of multiple vasopressors increased from 8.3% in 2009 to 13.2% 2018. Conversely, the proportion of the study population receiving no IV vasopressors decreased each year from 70.1% in 2009 to 54.4% in 2018. Figure 3 shows the number of IV vasopressors (one, two, three, four) utilized for each patient within two days of admission in the study population over time. The greatest share of increased vasopressor utilization was the use of single vasopressors, which increased from 21.5% in 2009 to 32.2% in 2018.
Figure 2:
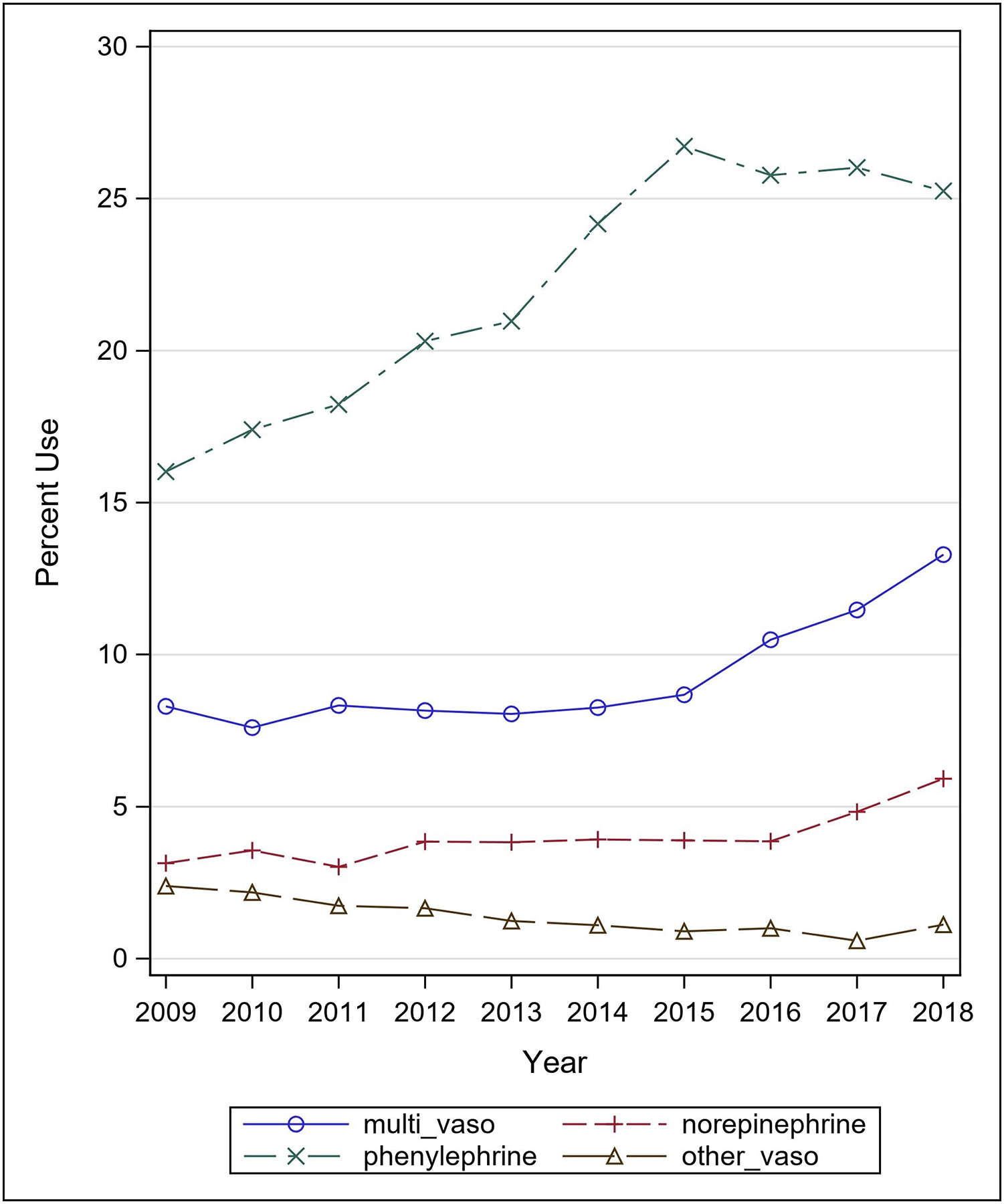
Initial choice of vasopressor, 2009–2018
Figure 3:
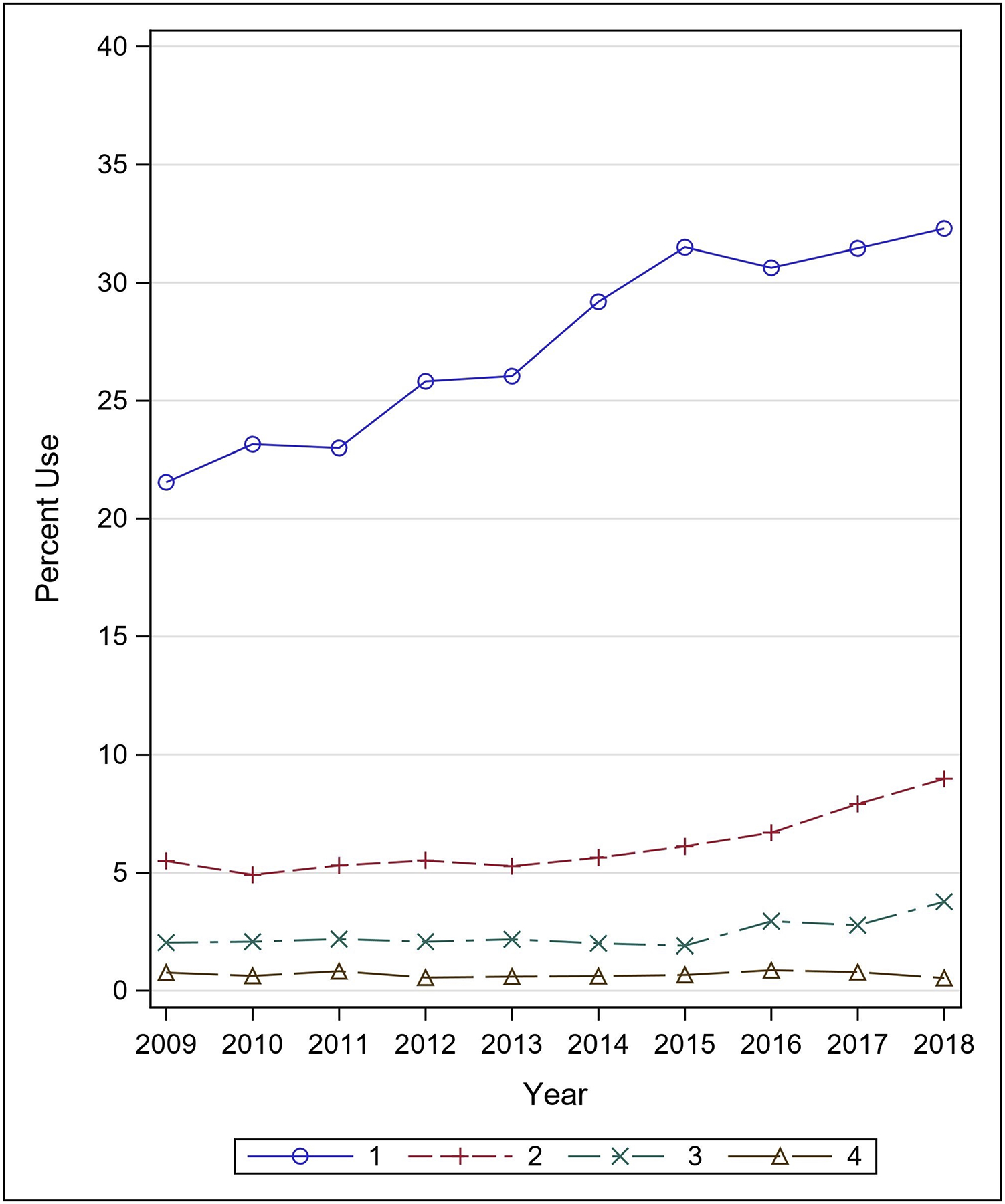
Multiple vasopressor utilization, 2009–2018
Associations with Vasopressor Choice
Supplemental Table 3 shows demographic, clinical, and facility characteristics significantly associated with choice of norepinephrine compared to phenylephrine. Use of phenylephrine was significantly associated with age <30 years (OR 0.73, 95% CI 0.63–0.89), age >80 years (OR 0.79, 95% CI 0.64–0.99). Injury Severity Score (ISS) was significantly associated with choice of norepinephrine (OR 1.04, 95% CI 1.03–1.04). Several co-treatments within two days of admission were associated with choice of norepinephrine over phenylephrine, including use of ICP monitor (OR 1.22, 95% CI 1.02–1.46), central line placement (OR 2.22, 95% CI 1.92–2.51), and hypertonic saline therapy (OR 2.29, 95% CI 2.02–2.60). Our analysis demonstrated an intraclass correlation coefficient (ICC) of 0.231 (SE 0.12), indicating that 23.1% of the variability in choice of norepinephrine compared to phenylephrine was explained by hospital-level differences, above and beyond patient factors.
Clinical Outcomes
Table 1 shows primary and secondary clinical outcomes among patients exposed to early norepinephrine versus phenylephrine. Patients who received phenylephrine as their only vasopressor had crude in-hospital mortality of 15.3%, compared to 32.4% in patients who received norepinephrine. After propensity matching, good overlap in treatment groups was identified, with adequate covariate balance (Supplemental Figure 1, Supplemental Table 4). In propensity-matched analysis, exposure to norepinephrine was associated with increased risk of in-hospital mortality (OR 1.65, CI 1.46–1.86, p <0.0001). Choice of norepinephrine, compared to phenylephrine, was also associated with a lower hazard ratio of hospital discharge (HR 0.76, CI 0.65–0.84, p <0.0001), and intensive care unit discharge (HR 0.68, CI 0.63–0.73, p <0.0001).
Table 1:
Outcomes associated with initial vasopressor choice, after propensity weighting
| p-value | ||||
|---|---|---|---|---|
| Number | N=2665 | N=2665 | ||
| ORi (95% CI) | ||||
| Hospital mortality [no. (%)] | 593 (22.4) | 855 (32.4) | 1.65 (1.46–1.86) | <0.0001 |
| HRii (95% CI) | ||||
| Hospital LOS, days [mean ± SD] | 17.7 ± 20.4 | 19.4 ± 20.9 | 0.76 (0.65–0.84) | <0.0001 |
| ICU days [mean ± SD] | 9.9 ± 9.4 | 12 ± 10.2 | 0.68 (0.63–0.73) | <0.0001 |
OR>1 means the odds of in-hospital mortality is higher in norepinephrine group compared to phenylephrine
HR<1 means the hazard of discharge alive is lower in norepinephrine compared to phenylephrine
In a sub-group analysis of propensity-matched patients with ICP monitor placement, we observed similar trends to those of the entire group (Supplemental Table 5). Exposure to norepinephrine was associated with increased risk of in-hospital mortality (OR 1.58, CI 1.08–2.33, p <0.0187) and lower hazard ratio of intensive care unit discharge (HR 0.79, CI 0.64–0.97, p <0.023) compared to phenylephrine. Results of sensitivity analyses (Supplemental Table 6 & 7) demonstrated stable risk estimates.
Discussion
We conducted a multicenter retrospective study to describe early vasopressor utilization patterns in patients with sTBI and association between utilization of norepinephrine compared to phenylephrine with clinical outcomes following injury. We found 1) significant variation in vasopressors utilization associated with both patient-level and hospital-level characteristics, 2) choice of norepinephrine, compared to phenylephrine, was associated with increased in-hospital mortality.
Following TBI, vasopressors are commonly used for restoring and maintaining adequate CPP by increasing mean arterial pressure (MAP) and thus optimizing cerebral blood flow (CBF) to meet metabolic demands4. Normally, cerebral circulation is maintained in homeostasis through a complex and poorly understood cerebral autoregulatory system involving cardiovascular, respiratory, and neural mechanisms31–33. Following sTBI, if autoregulation remains intact, a drop in blood pressure triggers autoregulatory vasodilation in an attempt to maintain adequate brain perfusion. This results in increased CBF which may elevate ICP. If autoregulation is not intact, cerebral perfusion can become passively dependent on SBP, which require augmentation to prevent secondary ischemia14,15,34. Phenylephrine, a selective alpha-1 agonist, is used as an arterial vasoconstrictor to increase MAP. Norepinephrine has predominantly alpha-1 and some beta-1 agonist properties, causing arterial vasoconstriction in addition to inotropic and chronotropic effects35. Both norepinephrine and phenylephrine appear to increase MAP and CPP but with variable effects on cerebral tissue oxygenation6,35–37. A recent systematic review comparing vasopressor use with clinical outcomes in TBI patients found no evidence favoring norepinephrine over phenylephrine in augmenting CPP or any clinical outcomes21. However, only two studies met inclusion criteria for the review and were limited to single institutions. Furthermore, prior small studies on utilization of vasopressors in TBI patients are consistent with variation observed in the present study18,19.
The utilization patterns described in this study present wide variability of use based on demographics, clinical characteristics, facility characteristics, and over time. First and foremost, use of vasopressors for early hemodynamic management of sTBI is common, with 37.1% of the study population receiving at least one vasopressor. We observed temporal variation in the use of vasopressors for critically ill patients with TBI, which increased from 2009 to 2018. During this study period, the most commonly utilized vasopressor for early hemodynamic management was phenylephrine, the use of which increased substantially from 2009 to 2018. Increased utilization of vasopressors may be related to updated guidelines for SBP thresholds from the Brain Trauma Foundation in 20154. Previously defined as >90 mm Hg, hypotension thresholds were increased to >100 mm Hg for patients 50–69 years and >110 mm Hg for patients 15–49 or >70 years following new evidence to suggest benefits to mortality and outcomes4,15,16. Our results also suggest that norepinephrine may be preferred in sicker patients. Choice of norepinephrine over phenylephrine was significantly associated with ISS and a number of co-treatments, including use of ICP monitor, central line, hyperosmolar therapy, which are employed for patients with more severe injury who exhibit increased ICP upon admission. Norepinephrine was also more common for certain comorbidities, such as congestive heart failure. Because of norepinephrine’s beta-agonism, it may be preferred for patient who are more susceptible to concurrent declines in systolic function38,39. Finally, our analysis indicates that a moderate degree of variability in the choice between the most commonly used vasopressors, norepinephrine and phenylephrine, was based on the individual hospital, above and beyond patient-specific characteristics. Therefore, hospital culture continues to be an important contributor to the choice of vasopressor for hemodynamic management of sTBI patients.
Despite the reported variation in use of vasopressors, our analysis showed that after controlling for patient- and facility-level characteristics, choice of phenylephrine was associated with reduced risk of hospital mortality and reduced hazard ratio of hospital discharge (hospital and ICU), compared to norepinephrine. The reduced risk of hospital mortality with phenylephrine was also present in the sub-group analysis of patients with ICP monitoring. While the retrospective nature of this study limits our ability to make definitive claim of superiority of specific vasopressors, we considered these findings as exploratory and hypothesis generating. Our data clearly demonstrate wide variability in use of vasopressor and possible mortality benefit with use of phenylephrine. Therefore, these results confirm the need for future studies to better understand the impact of different vasopressor therapies on reducing secondary brain injury, including multicenter randomized controlled trials that include personalized measures of hemodynamic status and cerebrovascular reactivity to identify optimal vasopressor therapy40,41.
While this may be the largest study examining vasopressor utilization following TBI, there are several limitations. First, while detailed information on pharmaceutical exposures was available, information on underlying mechanisms for hypotension was not; thus, type of shock state could not be assessed and may contribute to clinical decision-making and thus bias in the analysis. Second, we could not adequately assess any dose effect as a measure of degree of exposure. Third, lack of randomization into vasopressor groups introduces potential confounding by indication (i.e., sicker patients potentially received norepinephrine); our study addressed this by employing propensity-matching analytic methods, but this may not account for unobserved variables. Additionally, given the administrative nature of the dataset, we were not able to match based on patient-level physiologic measures. Without physiologic variables for each patient, we were limited in our ability to make a definitive claim to the superiority of one vasopressor over another and ascertain the mechanism by which phenylephrine reduces mortality following sTBI. Fourth, due to limitations in the Premier dataset, we were not able to collect long-term functional outcomes on patients such as the Disability Rating Scale or Glasgow Outcome Scale Extended. We are therefore unable to draw conclusions whether management involving phenylephrine versus norepinephrine results in improved quality of life or long-term function following injury. Lastly, despite the extensive clinical variables collected in Premier, our observational study remains at significant risk for residual confounding.
Early vasopressor utilization among critically ill patients with sTBI is common, increasing over the last decade, and varies across hospitals caring for TBI patients. Norepinephrine, compared to phenylephrine, was associated with increased risk of in-hospital mortality in propensity-matched analysis. Given the wide variation in vasopressor choice and possible differences in efficacy, our analysis suggests the need for randomized controlled trials to better inform vasopressor choice for patients with sTBI.
Supplementary Material
Supplemental Figure 1: Propensity scores distribution
Supplemental Table 1: ICD-9 and ICD-10 Diagnosis Codes for TBI
Supplemental Table 2: Demographic, clinical, and facility characteristics of the study population
Supplemental Table 3: Demographic and clinical characteristics significantly associated with choice of norepinephrine versus phenylephrine
Supplemental Table 4: Standardized mean differences (SMD) and group characteristics, pre- and post- propensity matching
Supplemental Table 5: ICP monitoring sub-group analysis, outcomes associated with vasopressor choice
Supplemental Table 6: Sensitivity analysis restricting to survivors of two days after admission among entire cohort
Supplemental Table 7: Sensitivity analysis restricting to survivors of two days after admission among patients with ICP monitoring
Key Points.
Question:
How are vasopressors utilized following severe TBI and is their use associated with clinical outcomes?
Findings:
Early vasopressor utilization among critically ill patients with severe TBI is common, increasing over the last decade, and varies across hospitals caring for TBI patients, with propensity-matched analysis demonstrating increased risk of in-hospital mortality with norepinephrine, compared to phenylephrine.
Meaning:
Given the variation in use and potential benefit of phenylephrine over norepinephrine, our analysis confirms the need for randomized control trials.
Financial Disclosure & Conflicts of Interest:
We have no conflicts of interests to disclose. This manuscript was supported by the National Institute of Neurological Disorders and Stroke (K23 NS109274, PI: Krishnamoorthy).
Glossary of Terms
- US
United States
- TBI
Traumatic brain injury
- sTBI
Severe traumatic brain injury
- CPP
Cerebral perfusion pressure
- SBP
Systolic blood pressure
- IV
Intravenous
- IRB
Institutional review board
- AIS
Abbreviated injury scale
- ICU
Intensive care unit
- ISS
Injury severity score
- ICDPIC-R
International Classification of Disease Program for Injury Classification and R statistical software
- GEM
General Equivalency Mapping
- LOS
Length of stay
- ICP
Intracranial pressure
- ICC
Intraclass correlation
- SMD
Standardized mean differences
- MAP
Mean arterial pressure
- CBF
Cerebral blood flow
Footnotes
IRB: This study was exempt from institutional review board (IRB) review at Duke University, given the fully de-identified nature of the Premier Healthcare Database.
References
- 1.Prevention CfDCa. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths-United States, 2014. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services;2019. [Google Scholar]
- 2.Dewan MC, Rattani A, Gupta S, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018:1–18. [DOI] [PubMed] [Google Scholar]
- 3.Oberholzer M, Muri RM. Neurorehabilitation of Traumatic Brain Injury (TBI): A Clinical Review. Med Sci (Basel). 2019;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. [DOI] [PubMed] [Google Scholar]
- 6.Thorup L, Koch KU, Upton RN, Ostergaard L, Rasmussen M. Effects of Vasopressors on Cerebral Circulation and Oxygenation: A Narrative Review of Pharmacodynamics in Health and Traumatic Brain Injury. J Neurosurg Anesthesiol. 2020;32(1):18–28. [DOI] [PubMed] [Google Scholar]
- 7.Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg. 2001;136(10):1118–1123. [DOI] [PubMed] [Google Scholar]
- 8.Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Manez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care. 2012;16(2):R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schirmer-Mikalsen K, Vik A, Gisvold SE, Skandsen T, Hynne H, Klepstad P. Severe head injury: control of physiological variables, organ failure and complications in the intensive care unit. Acta Anaesthesiol Scand. 2007;51(9):1194–1201. [DOI] [PubMed] [Google Scholar]
- 10.Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54(2):312–319. [DOI] [PubMed] [Google Scholar]
- 11.Vavilala MS, Lee LA, Lam AM. Cerebral blood flow and vascular physiology. Anesthesiol Clin North Am. 2002;20(2):247–264, v. [DOI] [PubMed] [Google Scholar]
- 12.Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. J Neurosurg. 2001;95(5):756–763. [DOI] [PubMed] [Google Scholar]
- 13.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. JAMA. 1978;240(5):439–442. [PubMed] [Google Scholar]
- 14.Butcher I, Maas AI, Lu J, et al. Prognostic value of admission blood pressure in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):294–302. [DOI] [PubMed] [Google Scholar]
- 15.Brenner M, Stein DM, Hu PF, Aarabi B, Sheth K, Scalea TM. Traditional systolic blood pressure targets underestimate hypotension-induced secondary brain injury. J Trauma Acute Care Surg. 2012;72(5):1135–1139. [DOI] [PubMed] [Google Scholar]
- 16.Berry C, Ley EJ, Bukur M, et al. Redefining hypotension in traumatic brain injury. Injury. 2012;43(11):1833–1837. [DOI] [PubMed] [Google Scholar]
- 17.Shibahashi K, Sugiyama K, Okura Y, Tomio J, Hoda H, Hamabe Y. Defining Hypotension in Patients with Severe Traumatic Brain Injury. World Neurosurg. 2018;120:e667–e674. [DOI] [PubMed] [Google Scholar]
- 18.Sookplung P, Siriussawakul A, Malakouti A, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. 2011;15(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhillon NK, Huang R, Mason R, et al. Vasopressors in traumatic brain injury: Quantifying their effect on mortality. Am J Surg. 2020;220(6):1498–1502. [DOI] [PubMed] [Google Scholar]
- 20.Di Gennaro JL, Mack CD, Malakouti A, Zimmerman JJ, Armstead W, Vavilala MS. Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci. 2010;32(5–6):420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Donald P, Spencer W, Cheng J, et al. In adult patients with severe traumatic brain injury, does the use of norepinephrine for augmenting cerebral perfusion pressure improve neurological outcome? A systematic review. Injury. 2020;51(10):2129–2134. [DOI] [PubMed] [Google Scholar]
- 22.Ract C, Vigue B. Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients. Intensive Care Med. 2001;27(1):101–106. [DOI] [PubMed] [Google Scholar]
- 23.Premier Healtcare Database White Paper: Data that informs and performs. Premier Inc.; March 2, 2020. 2020. [Google Scholar]
- 24.Gennarelli TA, Wodzin E. AIS 2005: a contemporary injury scale. Injury. 2006;37(12):1083–1091. [DOI] [PubMed] [Google Scholar]
- 25.Clark DE, Black AW, Skavdahl DH, Hallagan LD. Open-access programs for injury categorization using ICD-9 or ICD-10. Inj Epidemiol. 2018;5(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker SP, O’Neill B, Haddon W Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 27.Thompson NR, Fan Y, Dalton JE, et al. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care. 2015;53(4):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donald H, Gibbons RD. Longitudinal Data Analysis. John Wiley & Sons; 2006. [Google Scholar]
- 29.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brassard P, Tymko MM, Ainslie PN. Sympathetic control of the brain circulation: Appreciating the complexities to better understand the controversy. Auton Neurosci. 2017;207:37–47. [DOI] [PubMed] [Google Scholar]
- 32.ter Laan M, van Dijk JM, Elting JW, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth. 2013;111(3):361–367. [DOI] [PubMed] [Google Scholar]
- 33.Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32(2):264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75(5):685–693. [DOI] [PubMed] [Google Scholar]
- 35.Jentzer JC, Coons JC, Link CB, Schmidhofer M. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther. 2015;20(3):249–260. [DOI] [PubMed] [Google Scholar]
- 36.Brassard P, Seifert T, Secher NH. Is cerebral oxygenation negatively affected by infusion of norepinephrine in healthy subjects? Br J Anaesth. 2009;102(6):800–805. [DOI] [PubMed] [Google Scholar]
- 37.Meng L, Cannesson M, Alexander BS, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth. 2011;107(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnamoorthy V, Rowhani-Rahbar A, Chaikittisilpa N, et al. Association of Early Hemodynamic Profile and the Development of Systolic Dysfunction Following Traumatic Brain Injury. Neurocrit Care. 2017;26(3):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamper G, Havel C, Arrich J, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2016;2:CD003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16(2):258–266. [DOI] [PubMed] [Google Scholar]
- 41.Depreitere B, Citerio G, Smith M, et al. Cerebrovascular Autoregulation Monitoring in the Management of Adult Severe Traumatic Brain Injury: A Delphi Consensus of Clinicians. Neurocrit Care. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Propensity scores distribution
Supplemental Table 1: ICD-9 and ICD-10 Diagnosis Codes for TBI
Supplemental Table 2: Demographic, clinical, and facility characteristics of the study population
Supplemental Table 3: Demographic and clinical characteristics significantly associated with choice of norepinephrine versus phenylephrine
Supplemental Table 4: Standardized mean differences (SMD) and group characteristics, pre- and post- propensity matching
Supplemental Table 5: ICP monitoring sub-group analysis, outcomes associated with vasopressor choice
Supplemental Table 6: Sensitivity analysis restricting to survivors of two days after admission among entire cohort
Supplemental Table 7: Sensitivity analysis restricting to survivors of two days after admission among patients with ICP monitoring


