Abstract
Cardiogenic shock (CS) remains a leading cause of morbidity and mortality among patients with cardiovascular disease. In the past, acute myocardial infarction was the leading cause of CS. However, in recent years, other etiologies, such as decompensated chronic heart failure, arrhythmia, valvular disease, and post-cardiotomy, each with distinct hemodynamic profiles, have risen in prevalence. The number of treatment options, particularly with regard to device-mediated therapy has also increased. In this review, we sought to survey the medical literature and provide an update on current practices.
Keywords: Heart failure, Cardiogenic shock, Mechanical circulatory support, Hemodynamics, Inotrope
Key Summary Points
| Cardiogenic shock (CS) remains a leading cause of death among patients with acute myocardial infarction. |
| Although acute myocardial infarction has historically been the leading cause of cardiogenic shock, decompensated heart failure is increasing in prevalence and dominates recent registries. |
| Cardiogenic shock is a dynamic and hemodynamically heterogeneous condition, with diagnosis based primarily on clinical evaluation with few standard criteria. This heterogeneity has made study design and data interpretation difficult. |
| In the absence of evidence-based standards, current practices in the treatment of CS are driven mainly by clinician preference and experience; thus, a common language and principles is needed to facilitate the acquisition of high-quality data. |
| The Society for Cardiovascular Angiography and Interventions (SCAI) has devised a system for classifying and staging the severity of cardiogenic shock based on clinical variables; validation studies have demonstrated a convincing correlation between the SCAI shock stages and risk of mortality. |
Cardiogenic shock (CS) continues as a leading cause of morbidity and mortality among patients with acute and chronic cardiac disease, accompanying up to 10–12% of cases of acute myocardial infarction (AMI), and accounting for 2–5% of acute heart failure presentations [1–4]. Despite notable advances in percutaneous coronary interventions (PCI), availability of temporary mechanical circulatory support (MCS), and widespread recognition that early revascularization is key to survival [5, 6], improvement in acute CS mortality has plateaued in the 20 years since the landmark SHOCK (SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK) trial [2–4]. Part of the difficulty of treating CS lies in the diversity of presentations and hemodynamic profiles as non-AMI causes for CS have risen in prevalence. Changes in patient demographics and comorbidities, as well as improvements in medical therapy, have led to a decline in the Incidence of AMI, and an increase in the prevalence of heart failure [1, 4]. Consequently, there has been a gradual shift away from AMI-related CS (AMI-CS) to causes such as heart failure decompensation with cardiogenic shock (HF-CS), right ventricular failure, valvular disease, arrhythmia, myocarditis, and post-cardiotomy CS [1, 4]. An analysis of the United States National Inpatient Sample, revealed that the incidence of AMI-CS had fallen from 39.2% in 2004 to 28.5% in 2018 [7]. Similarly in a recent review of CS admissions across 16 North American coronary care units, 30% were due to AMI-CS and 46% were related to ischemic and non-ischemic cardiomyopathy [4, 8].
Estimates for CS mortality vary depending on the study and the patient population, with consensus settling at approximately 50% acutely, and at 1 year [1, 4, 8]. Osman et al. found that mortality for AMI-CS fell from 43 to 34% and from 52 to 37% for non-AMI-CS over the years from 2004 to 2018 [7]. Compared to non-AMI-CS, AMI-CS has the advantages of standardized care objectives, quantitative diagnostics such as troponin levels, and validated performance metrics such as “door-to-balloon time” and prompt introduction of MCS. Conversely, HF-CS can be challenging to recognize in the absence of hemodynamic data, since the physiologic adaptations that allow for baseline tolerance of lower cardiac output and blood pressure can create a more insidious onset and presentation [9]. Additionally, while guidelines advocate a multidisciplinary approach to managing CS [3], a review of current clinical practices demonstrates that consultations by advanced heart failure specialists are often late in the disease process, with patients already requiring inotropic or mechanical support [10]. Early involvement of heart failure specialists is particularly beneficial when advanced mechanical circulatory support is employed, since these “bridge” therapies require longitudinal consideration of realistic exit strategies if recovery is not achieved.
Regardless of etiology, the hallmark of CS is hypotension unresponsive to fluid resuscitation, with evidence of end-organ hypoperfusion in the setting of suspected cardiac dysfunction [2, 3]. Although reduced cardiac output/cardiac index (CO/CI) is a finding common to CS, other physiologic parameters such as central venous pressure (CVP) and congestion, pulmonary capillary wedge pressure (PCWP), systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) may differ according to the etiology and underlying pathophysiology of CS. Classically, CS has been described as presenting over a range of phenotypic profiles which can be used to infer hemodynamic parameters, such as a patient’s volume (wet or dry) and perfusion (warm or cold), which in turn stand as surrogates for PCWP and CO/CI, respectively [3, 11, 12]. Patients with classical CS are “cold and wet,” representing a low CI, increased SVR, and increased PCWP [2, 3]. Cold and dry (“euvolemic”) CS represents reduced CI, increased SVR, and a normal PCWP [2, 3]. These patients are less likely to have AMI-CS and are more likely to have HF-CS [2]. HF-CS and CS associated with RHF can be associated with long-term physiologic adaptations that can confound “classic” physical signs, in addition, hemodynamic profiles may change over the course of acute illness [10]. Furthermore, the concordance between physician clinical assessment and hemodynamic profiles derived from PA catheters is low. While the overall goal in CS is to preserve arterial pressure, ensure adequate tissue perfusion, and prevent deterioration, the varying profiles require different approaches and diagnostic accuracy is essential.
Beyond early revascularization, addressing CS-mediated hypotension and hypoperfusion becomes more challenging, as attempts to improve the outcomes of each, individually, can be counterproductive. Vasopressors increase peripheral vasoconstriction to increase mean arterial pressure and therefore increase cardiac afterload, wall stress, and myocardial oxygen consumption (MVO2) [12–14]. Vasopressors may also impair microcirculation and worsen peripheral ischemia [12, 13]. Conversely, inotropes can augment CO but can worsen hypotension, particularly in the settings of hypovolemia and/or systemic vasodilation [14, 15]. While pulmonary artery catheterization (PAC) is not required in all CS cases, current guidelines recommend use when CS is refractory to initial therapy or the mechanism of shock is unclear [1, 16, 17]. Although historical studies such as ESCAPE (Evaluation Study of Congestive heart failure And Pulmonary artery catheterization Effectiveness) suggested limited utility and possible harm with the use of PAC, many, including ESCAPE, actively excluded patients with CS [18]. More recently, observational studies of large cohorts have revealed that PAC in CS is associated with more aggressive therapy [19, 20] and improved survival [20, 21]. PAC as a diagnostic tool can lend greater precision to hemodynamic profiling and contribute to expediting decision-making and rapid stabilization, all of which contribute to improved clinical outcomes.
Selecting an inotrope or vasopressor is primarily based on the theoretical outcomes of addressing the underlying pathophysiology in order to preserve arterial pressure and ensure adequate tissue perfusion. In a survey of 839 physicians conducted by the European Society of Intensive Care Medicine, 84% of respondents used dobutamine as their first-line inotrope, with 44% reporting CO as their primary therapeutic target [22]. Inotrope options include dobutamine, milrinone, and levosimendan, which all increase contractility by increasing intracellular calcium within cardiac myocytes [14]. Dobutamine acts primarily through β1-receptors on the myocardium, while also activating β2 and α1-receptors peripherally [14, 15]. The net effect is increased heart rate, stroke volume (SV), contractility, and mixed vasodilation and vasoconstriction [15]. Advantages of dobutamine include its short half-life, thereby quickly reaching steady-state, and that it can be used with renal disease. However, caution must be used among patients who are already tachycardic or have a history of arrhythmia, as dobutamine is significantly pro-arrhythmic [14, 15, 23]. Milrinone is a phosphodiesterase-3 inhibitor which, by further downstream mechanisms, leads to increased intracellular calcium to increase contractility and decrease SVR and PVR [14, 15]. It is a first-line agent for acute decompensated heart failure, causes less tachycardia than dobutamine, and is useful in cases of pulmonary hypertension and RV failure [14, 15, 23]. The utility of milrinone in CS is somewhat limited by its longer half-life, nearly exclusive renal excretion, and the relative contraindication of hypotension [15]. Milrinone has the added benefit that patients can remain on beta-blockade, which has been associated with improved outcomes in cases of decompensated heart failure [15]. While beta-blockers are frequently discontinued with dobutamine, recent studies suggest improved outcomes with their continued use [23, 24]. Finally, levosimendan is a myofilament calcium sensitizer, available only in Europe and the United Kingdom, with a hemodynamic profile and limitations similar to milrinone [14, 15].
Like inotropes, vasopressors also increase intracellular calcium, albeit peripherally to cause vasoconstriction [14]. Norepinephrine, epinephrine, dopamine (as a precursor to the former two agents), and phenylephrine exert their effect by directly acting on α1 receptors, while vasopressin and angiotensin-II have their own dedicated receptors [14, 15]. While vasopressors are typically thought of primarily as vasoconstrictors, norepinephrine, epinephrine, and dopamine all have some degree of β1 agonism; however, debate remains on their degree of inotropic augmentation and proarrhythmia [14, 15]. Dopamine also has the unique property of exerting different hemodynamics at different doses: at up to 5 mg/kg/min, it primarily increases renal blood flow; between 5 and 10 mg/kg/min, inotropic augmentation predominates; and at doses > 10 mg/kg/min, it is a primary vasoconstrictor [14]. Phenylephrine, as a synthetic catecholamine, primarily causes vasoconstriction and is particularly useful among hypotensive patients with arrhythmia (e.g., atrial fibrillation) [14]. Similarly, vasopressin and angiotensin-II are primary vasoconstrictors, but are most useful as adjunctive agents to sensitize response to other vasopressors, the latter of which is especially beneficial in cases of vasodilatory CS [14].
There is a paucity of high-quality evidence supporting such decision-making, since available data are marred by heterogeneity in design and patient populations [3, 22, 23, 25]. As such, guidelines differ on which vasopressor or inotrope to initiate as first-line therapy [1, 3]. The ESC recommends norepinephrine as a first-line vasopressor with secondary addition of dobutamine if needed [1]. The AHA suggests that norepinephrine can be considered a first-line vasopressor, but acknowledges the scant evidence supporting an affirmative recommendation, while further suggesting that vasopressor selection be individualized to the patient, based on clinical markers of adequate end-organ perfusion while counterbalancing any deleterious effects [3]. Of note, the use of norepinephrine as first-line therapy in heart failure is controversial because of the generally counterproductive increase in heart rate and SVR [15]. The FRENSHOCK (FREnch observatory on the management of cardiogenic SHOCK) registry, in which over 60% of cases were nonischemic in etiology, norepinephrine use was one of six independent risk factors for 30-day mortality identified by multivariate analysis (OR 2.55, 95% CI 1.69–3.84) [26] (Table 1). In the DOREMI (DObutamine compaREd with MIlrinone) trial, a single-center, double-blind study of 192 patients randomized to either milrinone or dobutamine, there were no significant differences in major adverse cardiovascular events or need for renal replacement therapy (RRT) between the groups [23]. Separate studies evaluating levosimendan compared to placebo or dobutamine have demonstrated inconsistent results on its effect on mortality [14, 15].
Table 1.
Summary of major studies of inotropes and vasopressors for treatment of cardiogenic shock
| Major studies of inotrope or vasopressor effect on outcome in cardiogenic shock | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Interventions and objective | Design | n | Etiology | Findings |
| De Backer et al. (SOAP II) [30] | 2010 | Dopamine vs. norepinephrine in any form of shock; subgroup analysis of CS | Multicenter RCT | 1679; 280 with CS | Any | Norepinephrine was associated with improved CS survival at 28 days (0.5 vs. 0.4 in Kaplan–Meier analysis, respectively; p = 0.03) |
| Levy et al. (OptimaCC) [31] | 2018 | Safety and efficacy of norepinephrine vs. epinephrine | Multicenter RCT | 57 | AMI-CS | No difference in improvement in cardiac index or mortality to 28 days (p = 0.11). Epinephrine associated with higher incidence of refractory CS (37% vs. 7%, p = 0.011) |
| Mathew et al. (DOREMI) [24] | 2021 | Dobutamine vs. milrinone in treatment of CS | Multicenter RCT | 192 | Any | Composite primary outcome (all cause in hospital mortality, resuscitated cardiac arrest, cardiac transplant, or mechanical circulatory support, nonfatal myocardial infarction, transient ischemic attack, or stroke, or initiation of RRT) occurred in 54% of dobutamine recipients compared to 49% of the milrinone cohort (RR 0.90, 95% CI 0.69–1.19 |
| Delmas et al. (FRENSHOCK) [27] | 2022 | Registry comparing interventions and outcomes for 30-day CS survivors vs. non-survivors | Multicenter cohort, retrospective review | 772 | Any; 36.3% with ischemic CS | Multivariate analysis showed that norepinephrine (OR 2.55, 95% CI 1.69–3.84, p < 0.001), was associated with higher 30-day mortality |
| Pirracchio et al. [32] | 2013 | Comparison of inopressor alone (norepinephrine, epinephrine, or dopamine) vs. inopressor with inodilator (dobutamine, levosimendan, or phosphodiesterase-3 inhibitors) | Multicenter retrospective propensity-matched cohort | 1272 | Any | Patients who received inodilator with inopressor had improved 30-day survival (HR 0.61, 95% CI 0.52–0.71, p < 0.05) compared to inopressor alone |
| Schumann et al. [26] | 2018 | Review of vasodilators and inotropes | Systematic review/meta-analysis, RCTs | 2001 | Any | Levosimendan may reduce short-term mortality compared to dobutamine (RR 0.60, 95% CI 0.37–0.95, p < 0.05), but its effect on long-term mortality and its effect compared to placebo is undefined due to lack of statistical power |
| Karami et al. [28] | 2020 | Comparative meta-analysis of inotropes and vasopressors | Systematic review/meta-analysis | 2478 | AMI-CS | Levosimendan trended towards improved mortality up to 90 days, but this was not significant (RR 0.69, 95% CI 0.47–1.00). There were otherwise no differences in mortality between therapies |
In a meta-analysis of 19 studies and 2478 patients evaluating the effect of dobutamine, milrinone, levosimendan, epinephrine, norepinephrine, vasopressin, or dopamine on mortality in AMI-CS, Karami et al. found no differences between any agent [27]. From the SOAP II trial (Sepsis Occurrence in Acutely Ill Patients), a pre-specified subgroup analysis of CS patients demonstrated norepinephrine in comparison to dopamine, was associated with improved survival and fewer arrhythmias [3, 28, 29]. In a small, prospective, multi-center, double-blind, randomized study, Levy et al. found that among patients with AMI-CS, norepinephrine and epinephrine were equivalent in improving CI and other hemodynamic parameters. At the same time, the latter was associated with a higher incidence of refractory CS (37% vs. 7%, p = 0.011) [30]. Finally, Pirracchio et al. found that 30-day mortality was higher among CS patients treated with a vasopressor alone (epinephrine, norepinephrine, or dopamine) than with a vasopressor plus an inotrope [31].
Medications may not offer sufficient support, and recent evidence suggests that early initiation of MCS, especially with AMI-CS, is associated with improved survival [2]. The goal of MCS is to augment CO and reduce MVO2 through ventricular unloading and enhanced circulatory support [12]. Selecting an MCS device depends on patient-specific factors and comorbidities, including RV function, valvular or structural heart disease, arrhythmias, and systemic factors, especially with respect to the potential for limb ischemia and bleeding [32–34]. Determining when and which MCS to implement for specific CS hemodynamic profiles has not been established [2, 3]. All forms of MCS may increase the risk of thromboembolic events, cause some degree of thrombocytopenia and hemolysis, and are relatively or absolutely contraindicated in cases of peripheral vascular disease (PVD) and aortic insufficiency [35].
The intra-aortic balloon pump (IABP) is a fixed, counter-pulsation, pneumatic pump placed in the descending thoracic aorta that inflates during diastole, increasing coronary perfusion, and deflates during systole, generating a pressure sink that reduces left ventricular (LV) afterload [12, 32, 34]. The net effect of the IABP is that preload, afterload, and LV wall stress are decreased, and therefore also MVO2, while simultaneously increasing both SV and CO [32, 34]. Its dependence on intrinsic cardiac function limits the IABP, and it is less effective in the setting of arrhythmia and reduced LV functional reserve [12, 32, 35]. Studies evaluating IABP use in AMI-CS have demonstrated mixed results regarding mortality benefits, with IABP: randomized controlled trials not demonstrating any survival benefit, while retrospective cohort studies have, albeit marked by high heterogeneity in study design [34, 36–39] (Table 2). However, the hemodynamic profile of AMI-CS differs from HF-CS, as the latter begins with congestion and compensated CO [3]. As such, there may yet be an undefined role for IABP use outside of AMI-CS, such as in earlier shock stages or as a bridge to alternative MCS [32], but there have not yet been any large, randomized studies evaluating such approaches [12].
Table 2.
Summary of major studies of temporary mechanical support in cardiogenic shock
| Major studies of device intervention and outcome in cardiogenic shock | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Interventions and objective | Design | n | Etiology | Findings |
| IABP | ||||||
| Thiele et al. (IABP-SHOCK II) [37] | 2012 | PCI with IABP vs. without in AMI-CS | Multicenter RCT | 600 | AMI-CS | Routine use of IABP did not reduce mortality at 30 days (RR 0.96, 95% CI 0.79–1.17) |
| Sjauw et al. [38] | 2008 | ST-elevation myocardial infarction outcomes with/ without CS treated with/without IABP | Meta-analysis of RCTs compared to cohort studies | 11,538; 10,529 with CS | AMI-CS | Among RCTs, IABP was not associated with mortality at 30 days (OR 0.01, 95% CI − 0.03 to 0.04; I2 = 0%). Among cohort studies, IABP was associated with improved 30-day mortality (OR − 0.11, 95% CI − 0.13 to − 0.19; I2 = 93.6%) |
| Unverzagt et al. [40] | 2011 | AMI-CS outcomes with IABP vs. other or no cardiac assist devices | Meta-analysis, RCTs | 190 | AMI-CS | IABP was not associated with improvement in 30-day mortality (HR 1.04, 95% CI 0.62–1.73) |
| Bahekar et al. [39] | 2012 | AMI outcomes with or without CS treated with or without IABP | Meta-analysis, RCTs and cohort studies | 11,778; 5272 with CS | AMI-CS | IABP was associated with improved inpatient mortality (RR 0.72, 95% CI 0.60–0.86; I2 = 58.9%) |
| TandemHeart | ||||||
| Thiele et al. [41] | 2001 | Hemodynamics after placement among patients with AMI-CS | Case series | 18 | AMI-CS | Cardiac index improved by 41% on average with concomitant reduction in PCWP, CVP, and pulmonary artery pressure |
| Burkhoff et al. [43] | 2006 | Comparison of CS outcomes with IABP vs. TandemHeart | Multicenter RCT | 42 | Any; 70% with AMI-CS | TandemHeart improved cardiac index by 0.5 compared to only 0.2 for IABP (p < 0.05). 30-day mortality was 36% in the IABP cohort vs. 47% for TandemHeart (p > 0.05) |
| Kar et al. [44] | 2011 | Outcomes in ischemic vs. non-ischemic patients | Single-center retrospective cohort | 117 | Any; 4% with AMI | Improved survival with non-ischemic vs. ischemic CS (0.6 vs. 0.4 in Kaplan–Meier analysis, up to 1400 days (p < 0.05) |
| Ni hIci et al. [48] | 2020 | Impella or TandemHeart vs. IABP | Meta-analysis, RCTs | 162 | Any | No difference in 30-day mortality with Impella or TandemHeart individually compared to IABP (RR 1.01, 95% CI 0.76–1.35) |
| IMPELLA | ||||||
| O’Neill et al. [49] | 2014 | AMI-CS outcomes of Impella 2.5 pre- vs. post-PCI | Retrospective database review | 154 | AMI-CS | Pre-PCI patients had more complete revascularization and better survival to discharge (65.1% vs. 40.7%, p = 0.003) |
| Miyashita et al. [50] | 2021 | Comparison of AMI-CS pre- vs. post-PCI | Meta-analysis, cohort studies | 432 | AMI-CS | Improved mortality acutely (RR 0.62, 95% CI 0.50–0.76), and at 6 months (HR 0.66, 95% CI 0.44–0.97; I2 = 0% for all) Pre-PCI patients |
| Iannaccone et al. [51] | 2020 | Evaluation of CS outcomes following Impella implant; no comparator group | Meta-analysis, cohort studies | 2210 | Any; 75.9% with AMI | With placement of an Impella, 30-day mortality was 47.8% |
| Chung et al. [52] | 2020 | Evaluation of CS outcomes (recovery, LVAD, or transplant) Impella 5.0; no comparator group | Single-center retrospective cohort | 100 | Any | Overall survival 64%, 50% for patients without definitive advanced heart failure therapy, 48% for patients who underwent durable LVAD, and 81% for patients who underwent transplant |
Additional MCS devices include rotary-flow pumps categorized as either axial-flow or centrifugal-flow, including the TandemHeart device (TandemLife, LivaNova, London, UK) and veno-arterial extracorporeal membrane oxygenation (VA-ECMO) [12, 32, 34]. VA-ECMO is a venous-to-arterial pump that can be used for full cardiopulmonary support in the setting of fulminant biventricular failure, whereby the outflow cannula is placed in the central venous system and inflow cannula in the peripheral arterial system [12, 32, 33]. By its design, VA-ECMO increases afterload and does not significantly reduce LV volume; thus LV pressure, wall stress, myocardial work, and MVO2 are increased [12]. The increase in afterload can be mitigated by way of LV “venting” via atrial septostomy (to allow left-to-right shunting), a surgically placed LV vent, or by the simultaneous placement of an IABP or Impella (AbioMed, Danvers, MA, USA) [33]. VA-ECMO is absolutely contraindicated in cases of aortic insufficiency, intolerance to anticoagulation, and severe PVD, the last of which may be overcome by centrally inserting the inflow cannula into the thoracic aorta [35]. There have not been any randomized clinical studies or meta-analyses evaluating VA-ECMO with a mortality endpoint [2, 34].
Alternatively, the TandemHeart device can also provide full cardiac support, with the added benefit of reducing LV preload by drawing blood directly from the left atrium, thereby reducing volume and pressure, wall stress, workload, and MVO2 when compared to VA-ECMO [33]. The inflow cannula is placed peripherally in either femoral artery; thus, similar limitations in use apply, as for other forms of MCS. The main limiting factor to TandemHeart use is the transeptal puncture, a technically demanding procedure [32, 35]. Multiple small, randomized studies have shown that in the setting of AMI-CS, TandemHeart improves acute hemodynamics, but without a significant difference in 30-day mortality compared to IABP [32, 34, 40–43]. Other studies have demonstrated the feasibility of TandemHeart as a bridge to more definitive therapy [32, 44–46], but studies evaluating TandemHeart use beyond AMI-CS are lacking [47].
The Impella is a continuous axial flow pump that is placed transvalvular across the aortic valve, and is available in 3 different levels of CO augmentation, up to 2.5 L/min (Impella 2.5), 3.7 L/min (Impella CP), or 5.0 L/min (Impella 5.0) [32–34]. The former two can be placed percutaneously, while the Impella 5.0 requires a surgical cutdown of either the femoral or axillary arteries [32, 34]. The Impella is dependent on adequate RV function to supply enough preload to the LV, and is absolutely contraindicated in cases of metallic aortic valves, ventricular septal defects, left ventricular thrombus, and severe PVD [35]. Unlike the IABP, the Impella can be used in the setting of tachyarrhythmias or electromechanical disassociation [32, 35]. The Impella is advantageous in decreasing myocardial energetics: blood is removed directly from the LV and displaced into the aorta independent of the aortic valve, and wall stress and workload are decreased [33, 34]. Simultaneously, peripheral arterial pressure increases, and therefore coronary perfusion is also improved [33].
The Impella has been demonstrated to be most beneficial when initiated early in the setting of AMI-CS. In a retrospective analysis of the USpella registry, O’Neill et al. demonstrated that treatment of AMI-CS with early initiation of hemodynamic support with the Impella 2.5 prior to PCI was associated with more complete revascularization and less inpatient mortality (absolute risk reduction 24.4%, p = 0.003; OR = 0.37, p = 0.01) [48]. In a separate meta-analysis of 5 observational studies evaluating outcomes of Impella implant pre- versus post-PCI among 432 patients with AMI-CS, the pre-PCI group had lower in-hospital, 30-day, and 6-month mortality compared to patients in the post-PCI group [49]. Studies with definitive survival endpoints of Impella in non-AMI-CS are lacking [50]. In a single-center, retrospective analysis of 100 patients who presented with either HF-CS (84%) or AMI-CS (16%), Chung et al. found that the Impella 5.0 was beneficial to support patients to recovery (n = 30), durable left ventricular assist device implantation (LVAD; n = 23), or heart transplantation (n = 47), with survival rates of 50%, 48%, and 81%, respectively [51]. In the recovery group, 11 had AMI-CS, 6 had HF-CS, and the remainder from other causes; all patients had some form of mechanical or pharmacological support prior to Impella 5.0 insertion [51]. Eighteen patients were weaned from the Impella, and 14 survived to hospital discharge. Patient distributions among those who underwent durable LVAD implantation or transplantation, and outcome differentiation between those with HF-CS and AMI-CS, were not described [51].
Congestion, a central factor contributing to poor outcomes in HF-CS, may persist despite adequate circulatory support with MCS and vasoactive therapies [12, 20]. Acute kidney injury (AKI) in this setting is not uncommon, and is associated with poor outcomes [3]. Up to 20% of patients with CS will require renal replacement therapy (RRT), which is associated with higher short- and long-term mortality and the need for permanent dialysis [3, 52]. Angiotensin-II infusion in this patient population has been associated with decreased mortality and greater success of liberating from RRT [14]. Despite inherent risks, the AHA agrees with the Kidney Disease Improving Global Outcomes group that RRT can be considered for CS-related stage 2 or worse AKI [3].
Given the increased heterogeneity of shock presentations, the expanding armamentarium and the time-sensitive nature of CS management, multidisciplinary shock teams have emerged as an effective strategy for optimizing care [53]. While the constituent members of shock teams may vary depending on the institution, teams typically include intensivists, advanced heart failure and transplant cardiologists, interventional cardiologists, and cardiothoracic surgeons. Teams are generally activated when defined CS shock criteria are met, and, while team composition may differ across institutions, the goals are quite consistent, and include rapid triage, early diagnosis and phenotyping, and timely implementation and escalation of effective therapies. The use of shock teams is included among the most promising interventions designed to improve outcomes, including survival, in patients with CS [53].
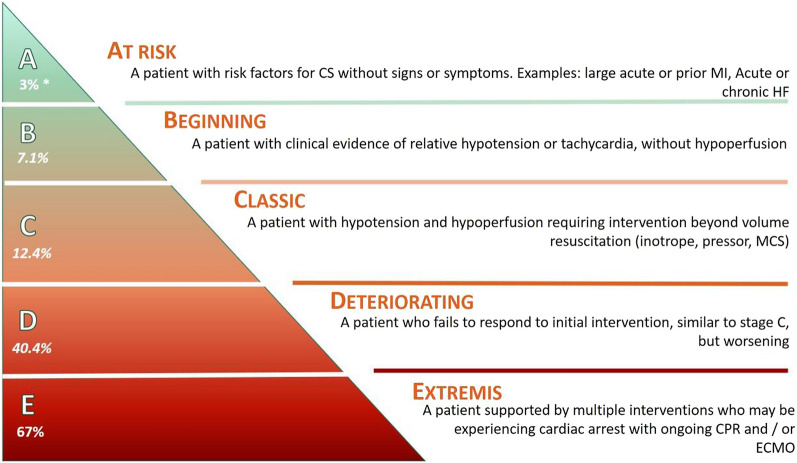
The lack of uniform criteria defining CS has been a hindrance to clinical trial design in CS, an impediment to the interpretation of data, and an obstacle to the dissemination of knowledge [54]. The recently developed SCAI shock staging system has demonstrated promise in framing and defining CS for future investigation and therapeutic guidance. The SCAI classification system was developed by a multidisciplinary panel of experts, in an effort to standardize communication between clinicians by employing a multi-domain classification system (physical examination, biochemical, and hemodynamic criteria) for definition and risk stratification (Table 3) [54]. Stage A represents patients “at risk” for CS (e.g., have AMI or pre-existing heart failure), who are normotensive with normal laboratory values and no physical examination findings of volume overload [54]. Stage B patients have “beginning CS”, such that vital signs and physical examination findings are consistent with compromised cardiac output, but with minimal laboratory derangements (i.e., preserved perfusion) [9, 54]. Stage C is “classic CS”, with many of the signs, symptoms, and abnormal objective diagnostic criteria described in the SHOCK and IABP-SHOCK II (Intra-Aortic Balloon Pump in cardiogenic SHOCK II) trials: these patients are hypo-perfused with impaired renal function, a lactate level of ≥ 2 mmol/L, and require at least one form of pharmacotherapy or MCS to augment CO [9, 54]. Stage D patients are “deteriorating” stage C patients, who require additional or escalating forms of hemodynamic support [9, 54]. Finally, stage E represents CS patients in “extremis” and “trying to die”: lactate is ≥ 8 mmol/L, hemodynamically significant ventricular arrhythmia may be present, and perfusion cannot be restored despite multiple MCS, drugs, or increasing doses of pressors [9, 54].
Table 3.
SCAI Shock Stage Classification.
Adapted from SCAI Clinical Expert Consensus Statement on the Classification of Cardiogenic Shock
| Stage | Description | Physical examination | Biomarkers | Hemodynamics |
|---|---|---|---|---|
| A |
“At risk” for CS without signs and symptoms Large AMI Prior MI Acute HF |
Normal JVP Clear lungs Warm and well perfused Strong distal pulses Normal mentation |
Normal labs Normal renal function Normal lactate |
Normotensive SBP ≥ 100 or normal for patient Hemodynamics CI ≥ 2.5 CVP < 10 PA sat ≥ 65% |
| B | Relative hypotension or tachycardia without hypoperfusion |
Elevated JVP Rales in lungs Warm and well perfused Strong distal pulses Normal mentation |
Normal lactate Mildly impaired renal function Elevated BNP |
Hypotensive SBP < 90 OR MAP < 60 OR > ↓ 30 from baseline Pulse ≥ 100 Hemodynamics CI ≥ 2.2 PA sat ≥ 65% |
| C |
Relative hypotension Hypoperfusion requiring intervention beyond volume resuscitation Inotropes MCS |
Any of the following: Unwell appearing Volume overload Extensive rales Killip class 3 or 4 Mechanical ventilation Cold, clammy Acute AMS Urine output < 30-ml/h |
Any of the following: Lactate ≥ 2 May be normal in chronic HF Creatinine ↑ × 2 OR > 50% ↓ GFR Increased LFTs Elevated BNP |
Any of below: Hypotensive SBP < 90 OR MAP < 60 OR > ↓ 30 from baseline AND requires drugs/device to maintain BP Hemodynamics CI < 2.2 PCWP > 15 RAP/PCWP ≥ 0.8 PAPI < 1.85 CPO ≤ 0.6 |
| D | Worsening Stage C and failure to respond to initial interventions | Any of Stage C AND worsening signs and symptoms of hypoperfusion |
Any of Stage C AND deteriorating Lactate rising |
Any of Stage C AND requiring multiple pressors, escalating pressor doses, OR MCS to maintain perfusion |
| E |
Ongoing cardiac arrest Requires support by multiple interventions or ECMO |
Near pulselessness Cardiac collapse Mechanical ventilation Defibrillator used |
CPR pH ≤ 7.2 Lactate ≥ 8 |
NO SBP without resuscitation PEA or refractory VT/VF Hypotension despite maximal support |
The SCAI shock classification system has been validated to correlate with acute and short-term mortality [9]. In a single-center, retrospective analysis of 10,004 patients, Jentzer et al. demonstrated that the initial SCAI stage directly correlated with inpatient all-cause mortality, whereby SCAI CS stages A through E had mortalities of 3.0%, 7.1%, 12.4%, 40.4%, and 67.0% (p < 0.001), respectively (Fig. 1) [55]. These results were consistent among patients with AMI-CS and HF-CS [55]. In a prospective analysis of 1414 patients with HF-CS (50.4%), AMI-CS (34.9%), or other causes of CS (14.7%) across 8 American tertiary care centers, Thayer et al. also demonstrated that SCAI shock stage correlated with acute inpatient all-cause mortality [56]. All stage B patients survived their hospitalization; among patients with HF-CS compared to stage C, stages D and E patients had respective increased mortality odds of 3.5 (95% CI 2.0–6.1) and 10.0 (95% CI 4.7–21.0) [56]. Mortality odds for AMICS-related were similar [56]. Finally, in a single-center, prospective analysis of 166 patients, Baran et al. demonstrated that initial CS stage upon patient presentation and CS stage improvement within 24 h both correlated directly with all-cause mortality at 30 days [57]. It is anticipated that establishing this common language will springboard future investigations and improve the interpretability of clinical studies.
Fig. 1.
Mortality risk by SCAI stages [54]. *Unadjusted mortality risk by SCAI stage at ICU admission from a retrospective analysis of 10,000 unique patients from a single center [55]
In conclusion, CS is a dynamic condition representing a major health challenge. A review of the literature demonstrates that a one-size-fits all approach to CS is inadequate for addressing the needs of this grievously ill patient population. While there is a relative abundance of evidence informing treatment of AMI-CS, there are scarce data involving optimal treatment of non-AMI-CS. Prior investigation has also been marred by heterogeneous definitions and the lack of a unified system to codify the diversity of hemodynamic profiles. The SCAI shock classification system has shown great promise in this regard. Its multi-domain methodology allows for flexibility in incorporating the diversity of presentation. This and other emerging tools will enable more precise evaluation of therapeutic interventions, which can, in turn, define future evidence-based practice. For now, cardiogenic shock remains among the most daunting clinical challenges. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Neal Olarte – writing of original draft, data curation; Nina Thakkar-Rivera – figure design, review and editing, validation, formal analysis; Luanda Grazette – writing of original draft, review and editing, methodology, validation, formal analysis, conceptualization, supervision.
Disclosures
Neal Olarte, Luanda Grazette, and Nina Thakkar-Rivera all have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(8):1315–1341. doi: 10.1002/ejhf.1922. [DOI] [PubMed] [Google Scholar]
- 2.Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8(8):e011991. doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 4.Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care. 2021;27(4):401–408. doi: 10.1097/MCC.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley D, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 7.Osman M, Syed M, Patibandla S, Sulaiman S, Kheiri B, Shah MK, et al. Fifteen-year trends in incidence of cardiogenic shock hospitalization and in-hospital mortality in the United States. J Am Heart Assoc. 2021;10(15):021061. doi: 10.1161/JAHA.121.021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM et al. Epidemiology of shock in contemporary cardiac intensive care units. Circulation: Cardiovasc Qual Outcomes. 2019;12(3). [DOI] [PMC free article] [PubMed]
- 9.Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Soc Cardiovasc Angiogr Interv. 2022;1(1):100008. doi: 10.1016/j.jscai.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herr JJ, Ravichandran A, Sheikh FH, Lala A, Chien CV, Hsiao S, et al. Practices of referring patients to advanced heart failure centers. J Cardiac Fail. 2021;27(11):1251–1259. doi: 10.1016/j.cardfail.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Brenner MI, Rosenblum HR, Burkhoff D. Pathophysiology and advanced hemodynamic assessment of cardiogenic shock. Methodist Debakey Cardiovasc J. 2020;16(1):7–15. doi: 10.14797/mdcj-16-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapur NK, Esposito ML. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000 Res. 2017;6:737. doi: 10.12688/f1000research.11150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 14.Jentzer JC, Hollenberg SM. Vasopressor and inotrope therapy in cardiac critical care. J Intensive Care Med. 2021;36(8):843–856. doi: 10.1177/0885066620917630. [DOI] [PubMed] [Google Scholar]
- 15.Nandkeolyar S, Ryu R, Mohammad A, Cordero-Caban K, Abramov D, Tran H, et al. A review of inotropes and inopressors for effective utilization in patients with acute decompensated heart failure. J Cardiovasc Pharmacol. 2021;78(3):336–345. doi: 10.1097/FJC.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. 2022;79(17):e263–421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Saxena A, Garan AR, Kapur NK, O’Neill WW, Lindenfeld J, Pinney SP, et al. Value of hemodynamic monitoring in patients with cardiogenic shock undergoing mechanical circulatory support. Circulation. 2020;141(14):1184–1197. doi: 10.1161/CIRCULATIONAHA.119.043080. [DOI] [PubMed] [Google Scholar]
- 18.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. J Am Med Assoc. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 19.Sionis A, Rivas-Lasarte M, Mebazaa A, Tarvasmäki T, Sans-Roselló J, Tolppanen H, et al. Current use and impact on 30-day mortality of pulmonary artery catheter in cardiogenic shock patients: results from the cardshock study. J Intensive Care Med. 2020;35(12):1426–1433. doi: 10.1177/0885066619828959. [DOI] [PubMed] [Google Scholar]
- 20.Ranka S, Mastoris I, Kapur NK, Tedford RJ, Rali A, Acharya P, et al. Right heart catheterization in cardiogenic shock is associated with improved outcomes: insights from the Nationwide Readmissions Database. J Am Heart Assoc. 2021;10(17):e019843. doi: 10.1161/JAHA.120.019843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, et al. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Cardiac Fail. 2019;25(5):364–371. doi: 10.1016/j.cardfail.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Scheeren TWL, Bakker J, Kaufmann T, Annane D, Asfar P, Boerma EC, et al. Current use of inotropes in circulatory shock. Ann Intensive Care. 2021;11(1):21. doi: 10.1186/s13613-021-00806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew R, di Santo P, Jung RG, Marbach JA, Hutson J, Simard T, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. 2021;385(6):516–525. doi: 10.1056/NEJMoa2026845. [DOI] [PubMed] [Google Scholar]
- 24.Grazette L, Tran JS, Zawadzki NK, Zawadzki RS, McLeod JM, Fong MW, et al. Geographic variation in the use of continuous outpatient inotrope infusion therapy and beta blockers. IJC Heart Vasc. 2022;39:100948. doi: 10.1016/j.ijcha.2021.100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumann J, Henrich EC, Strobl H, Prondzinsky R, Weiche S, Thiele H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. doi: 10.1002/14651858.CD009669.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delmas C, Roubille F, Lamblin N, Bonello L, Leurent G, Levy B, et al. Baseline characteristics, management, and predictors of early mortality in cardiogenic shock: insights from the FRENSHOCK registry. ESC Heart Fail. 2022;9(1):408–419. doi: 10.1002/ehf2.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karami M, Hemradj VV, Ouweneel DM, den Uil CA, Limpens J, Otterspoor LC, et al. Vasopressors and inotropes in acute myocardial infarction related cardiogenic shock: a systematic review and meta-analysis. J Clin Med. 2020;9(7):2051. doi: 10.3390/jcm9072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Diepen S. Norepinephrine as a first-line inopressor in cardiogenic shock: oversimplification or best practice? J Am Coll Cardiol. 2018;72(2):183–186. doi: 10.1016/j.jacc.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 29.de Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 30.Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173–182. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Pirracchio R, Parenica J, Resche Rigon M, Chevret S, Spinar J, Jarkovsky J, et al. The effectiveness of inodilators in reducing short term mortality among patient with severe cardiogenic shock: a propensity-based analysis. PLoS ONE. 2013;8(8):e71659. doi: 10.1371/journal.pone.0071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandawat A, Rao SV. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv. 2017;10(5):e004337. doi: 10.1161/CIRCINTERVENTIONS.116.004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2016;66(23):2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35(3):156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 35.Wong ASK, Sin SWC. Short-term mechanical circulatory support (intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): a review. Ann Transl Med. 2020;8(13):829–829. doi: 10.21037/atm-20-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 37.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Jr, Koch KT, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 38.Bahekar A, Singh M, Singh S, Bhuriya R, Ahmad K, Khosla S, et al. Cardiovascular outcomes using intra-aortic balloon pump in high-risk acute myocardial infarction with or without cardiogenic shock: a meta-analysis. J Cardiovasc Pharmacol Ther. 2012;17(1):44–56. doi: 10.1177/1074248410395019. [DOI] [PubMed] [Google Scholar]
- 39.Unverzagt S, Machemer MT, Solms A, Thiele H, Burkhoff D, Seyfarth M, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Prondzinsky R, editor. Cochrane Database Syst Rev. 2011;7:CD007398. doi: 10.1002/14651858.CD007398.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104:2917–2922. doi: 10.1161/hc4901.100361. [DOI] [PubMed] [Google Scholar]
- 41.Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 42.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.e1–469.e8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011;57(6):688–696. doi: 10.1016/j.jacc.2010.08.613. [DOI] [PubMed] [Google Scholar]
- 44.Velez-Martinez M, Rao K, Warner J, Dimaio J, Ewing G, Mishkin JD, et al. Successful use of the TandemHeart percutaneous ventricular assist device as a bridge to recovery for acute cellular rejection in a cardiac transplant patient. Transplant Proc. 2011;43(10):3882–3884. doi: 10.1016/j.transproceed.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 45.Gregoric ID, Jacob LP, la Francesca S, Bruckner BA, Cohn WE, Loyalka P, et al. The TandemHeart as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge) Tex Heart Inst J. 2008;35(2):125–129. [PMC free article] [PubMed] [Google Scholar]
- 46.Bruckner BA, Jacob LP, Gregoric ID, Loyalka P, Kar B, Cohn WE, et al. Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Tex Heart Inst J. 2008;35(4):447–450. [PMC free article] [PubMed] [Google Scholar]
- 47.NihIci T, Boardman HM, Baig K, Stafford JL, Cernei C, Bodger O, et al. Mechanical assist devices for acute cardiogenic shock. Cochrane Database Syst Rev. 2020;200(6):CD013002. doi: 10.1002/14651858.CD013002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill WW, Schreiber T, Wohns DHW, Rihal C, Naidu SS, Civitello AB, et al. The current use of impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27(1):1–11. doi: 10.1111/joic.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyashita S, Banlengchit R, Marbach JA, Chweich H, Kawabori M, Kimmelstiel CD, et al. Left ventricular unloading before percutaneous coronary intervention is associated with improved survival in patients with acute myocardial infarction complicated by cardiogenic shock: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2021;39:28–35. doi: 10.1016/j.carrev.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Iannaccone M, Albani S, Giannini F, Colangelo S, Boccuzzi GG, Garbo R, et al. Short term outcomes of Impella in cardiogenic shock: a review and meta-analysis of observational studies. Int J Cardiol. 2021;324:44–51. doi: 10.1016/j.ijcard.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 51.Chung JS, Emerson D, Ramzy D, Akhmerov A, Megna D, Esmailian F, et al. A new paradigm in mechanical circulatory support: 100-patient experience. Ann Thorac Surg. 2020;109(5):1370–1377. doi: 10.1016/j.athoracsur.2019.08.041. [DOI] [PubMed] [Google Scholar]
- 52.Lauridsen MD, Gammelager H, Schmidt M, Rasmussen TB, Shaw RE, Bøtker HE, et al. Acute kidney injury treated with renal replacement therapy and 5-year mortality after myocardial infarction-related cardiogenic shock: a nationwide population-based cohort study. Crit Care. 2015;19(1):452. doi: 10.1186/s13054-015-1170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brusca SB, Caughron H, Njoroge JN, Cheng R, O’Brien CG, Barnett CF. The shock team: a multidisciplinary approach to early patient phenotyping and appropriate care escalation in cardiogenic shock. Curr Opin Cardiol. 2022;37(3):241–249. doi: 10.1097/HCO.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 54.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 55.Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 56.Thayer KL, Zweck E, Ayouty M, Garan AR, Hernandez-Montfort J, Mahr C, et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9):e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: Single center analysis. Catheter Cardiovasc Interv. 2020;96(7):1339–1347. doi: 10.1002/ccd.29319. [DOI] [PMC free article] [PubMed] [Google Scholar]