Abstract
Background
Cervical cancer is marked by the uncontrolled proliferation and division of cells making up the cervix. Because of its enormous population, Asia accounts for more than half of the cervical cancer cases and deaths in the world. Cervical cancer is the major cause of death from cancer in women in rural as well as urban areas in India. In most cases, persistent infection with highly infectious types of human papillomavirus (HPV) such as HPV 16 and 18 is believed to be the cause of the disease. The HPV virus is primarily reported to invade cervical epithelial cells and then goes through a non-viremic infection cycle under the influence of various potent viral oncogenic proteins, namely E6 and E7. Among several other risk factors, increased oxidative stress, hyperactivation of inflammatory pathways, and immunological factors play a key role in cervical cancer pathogenesis. Although, standardized screening services in developed countries have substantially reduced the prevalence of cervical cancer but there are numerous drawbacks to cytology-based screening. Advances in understanding the virology of the human papillomavirus have prompted the discovery of several novel biomarkers of different categories such as protein-based, DNA-based as well as stem cell-based markers. The incorporation of biomarker information will assist in recognizing efficacious therapy systems as well as improve the prognosis of cervical malignancy.
Conclusions
The review discussed the role of HPV in the development of cervical cancer and its pathogenesis. Further, summarized the potential therapeutic biomarkers for the prevention and treatment of cervical cancer.
Keywords: Cervical cancer, Human papillomavirus, Pathogenesis, Biomarkers, HPV infection cycle, HPV virology
Introduction
Cervical cancer is a major cause of mortality and globally it is the fourth most common cancer among women after lung cancer (0.73MM), colorectal cancer (0.79MM), and breast cancer (2.09MM cases). Underdeveloped or emerging nations account for > 85% of cervical cancer related deaths, in which 35% of the global burden is covered by India and China [1, 2]. In 2018, India witnessed as many as 570,000 cases and 311,000 deaths while China reported 106,000 cases of cervical cancer with 48,000 deaths [2]. Most patients can now be diagnosed and treated early with the popularization of cervical cancer screening and the implementation of the HPV vaccine. However, the effect of treatment is not significant for advanced or recurrent cervical cancer [3]. According to IARC studies, the prevalence of HPV varies by roughly 20 times in different regions. Central Asia, Western Asia, and Europe had HPV prevalence rates of 8–9%, whereas Africa, Oceania, North America, South and Central America had prevalence rates of more than 20%. Globally, a crude prevalence estimate of 31% was reported among women, with Eastern Asia, including China and Korea, having the highest estimate of 57% and Europe having the lowest prevalence estimate of 3.7%. When compared to developed nations (22.6%), the HPV prevalence was found greater in developing countries (42.2%) [2, 4]. The prevalence of HPV in Indian research ranged from 2.3 to 36.9%. According to hospital-based research, the prevalence of benign cervical cytology ranged from 9.9 to 16.6% [5]. The prevalence was greater in high-risk groups such as commercial sex workers at 25% and urban slums in Mumbai at 32.3% [6, 7]. A prospective research in Delhi revealed that high-risk HPV types had a greater likelihood of persistence, with HPV types 16, 45, 67, 31, 51, and 59 having the highest prevalence of persistence [8]. The HPV infection rate was reported to be 5 per 1,000 woman among healthy women in a Delhi slum [8]. The cumulative incidence of a first HPV infection in young, HPV-negative women is expected to be 32% at 24 months and 43% at 36 months [8]. According to Bhatla et al. meta-analysis, there is no significant variation in the prevalence of HPV infection between North and South India. HPV-16 and 45, on the other hand, found to be much more widespread in North India, whereas HPV-35 showed to be more prevalent in South India [9]. In Maharashtra, high-risk HPV was connected with poor education level, early age at first sexual intercourse, cumulative age, manual labour, and widowhood/separation. In eastern India, married women were shown to be at a greater risk of HPV infection [10].
Cervical cancer is a big concern today since the currently available drugs give barely any positive results. Thus, the exploration of pathological pathways and targeted therapies can drive progress in the development of novel therapeutic drugs against this dreaded disease. Chronic carcinogenic human papillomavirus infection (HPV) has shown to be the primal cause of invasive cervical cancer. In nearly 95% of cervical cancer lesions, the HPV genome has been identified including cervical squamous cell carcinoma (SCC) and adenocarcinoma (AC) histotypes [11]. Most of the HPV infections are spontaneously cleansed and are transitory. Persistent infection with certain subtypes of HPV, can lead to the development of a premalignant condition known as cervical neoplasia. Almost always, squamous intraepithelial lesions (SILS) caused by HPV infection precede cervical SCC. Other etiological factors are also implicated in the development of cervical carcinoma. These include sexual acquisition of HPV, immune dysfunction, mutagen exposure, and hormone-related factors. Early-onset of sexual activity especially with multiple partners, susceptibility to other sexually transmitted infections, tobacco smoking, oral contraceptives, HIV infection, and immunosuppressive medications are also among the most important risk factors. Hereditary assumes a little role in the event of cervical malignant growth [12].
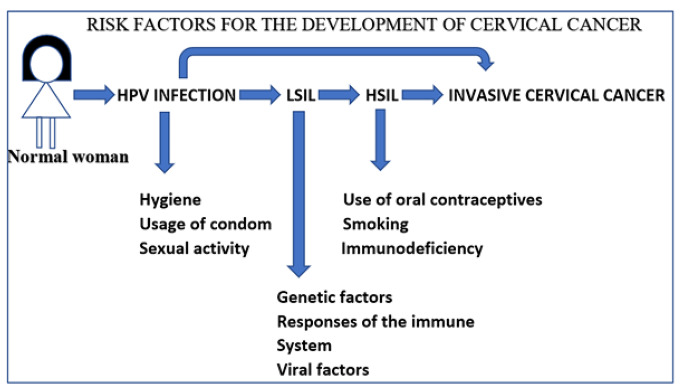
Low education levels and low financial status are among the two significant social risk factors of cervical malignancy as it leads to poor access to disease screening services and resistance against screening visits. This additionally adversely influences the execution of HPV immunizations because of its significant expense. However, the most crucial element for the growth of invasive cervical cancer remains continual infection with human papillomavirus [12]. An illustration of the various etiological factors involved in the development of cervical cancer is depicted in Fig. 1.
Fig. 1.
Risk factors associated with the development of cervical cancer
Human papillomavirus (HPV)
Types of HPV and their role in cervical cancer
HPV’s are tiny, non-enveloped DNA tumor viruses. The genome is ~ 8 kb, covalently enclosed and circular, and encoded by genes from the open reading frames E1, E2, E4, E5, E6, E7, L1, and L2 (ORFs). The nucleosomes which is covered by the genome is protected by a 60 nm T = 7 capsid [13]. Human papillomavirus developed alongside humans over numerous centuries and infects humans productively. Assessments of different forms of HPV have indicated a traditional family tree including host-specific variants that have a propensity to infect the skin or mucosal basal epithelial cells, primarily the genital organs and oropharynx [13, 14]. Condyloma acuminata as well as invasive cervical cancers are caused by very different forms of HPV [15, 16].
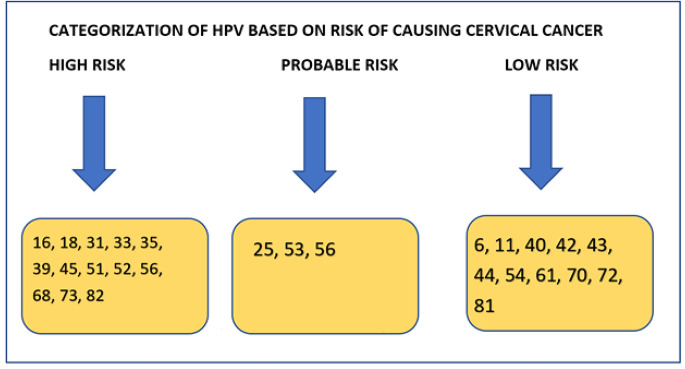
Human papillomaviruses are phylogenetically divided into α, β, γ, δ, and µ genera based on the variation of at least 10%in the nucleotide sequence of their L1 open reading frame. The alpha class particularly infects the genital and oropharyngeal mucosal membranes and comprises the tumorigenic forms of HPV associated with malignant cervical carcinoma. In their L1 configurations, HPV “variants” vary by < 3%, and express a single-nucleotide variation for a particular tissue or lesion [14, 17]. This implies that premalignant and cancerous cervical lesions are linked to distinct HPV strains than those seen in benign lesions. The diverse kinds of HPV can also be characterized dependent on their clinical “oncogenic potential’’ as “low risk” and “high risk” HPV types [11]. It is noteworthy that a vast majority of the intrusive cervical cancers are the squamous type (~ 90%), while the frequency of them being adenocarcinomas is less than 5%. Both forms of cancers are caused by different kinds of HPV. A few rare histological subclasses make up the rest of the cervical tumors. HPV 16 and 18 are the two essential HPV types that cause genital diseases. The originally reported genital form of HPV was HPV6, usually found in benign condyloma acuminata, while the 11 and 16 variants were consistently isolated from laryngeal papillomas and condyloma acuminata. Thus, in benign genital wart lesions most of the times HPV 6 and 11 are usually seen and are considered to be low-risk types [15]. In contrast, types 16 and 18 of HPV are established as high-risk carcinogenic types present in high-grade cervical dysplastic lesions in addition to precancerous lesions and most intrusive and progressive cervical tumors. HPV 16 and 18 are also liable to develop from high-grade pre-cancerous cervical lesions to intrusive cervical carcinoma [11, 15, 16, 18]. HPV16 is divided into 4 main lineages and 9 variant sublineages [A (A1 to A4), B (B1-B2), C, and D (D1 to D2)]. The largest study conducted by Mirabello et al reported HPV16 to cause cancer in 3200 women [19]. HPV 31, 33, 45, 52, and 58 are also perceived to possess tumorigenic potential in addition to the above-mentioned forms but are less commonly expressed in cervical cancer. The 16 and 18 types of HPV are the most prevalent types in both symptomatic and asymptomatic women by a wide margin and are associated with the majority of the invasive cervical cancer cases detected around the globe [12, 15, 16, 18]. The classification of HPV based on its risk of causing cervical cancer is illustrated in Fig. 2.
Fig. 2.
Categorization of HPV
In the clinical environment, it is rather normal to find different forms of HPV in the same patient. There can be multiple or concurrent genital diseases occurring in the patient, which makes it difficult to differentiate persistent infections from newly developed infections caused by distinct subclasses of HPV, especially in young adolescent girls [20]. Since the transition of the cells of the cervix to a malignant state is propelled by many different variables and several unexplained molecular phenomena, persistent infection with an oncogenic HPV type remains the primary driving force for the development of carcinogenesis. This is thought to happen as a result of the incorporation of the viral genome into the host cell causing epithelial cell genetic instability during its prolonged course of infection [15, 21].
The infection cycle of HPV
As referenced beforehand, HPV has a propensity towards cervical epithelial cells which are stratified into a non-differentiated basal monolayer and a non-proliferating suprabasal differentiated epidermis. Over the basement membrane, beneath which is the cervical stromal plate is situated, lies the basal layer. As per the normal procedure of epithelial development, dividing immature basal cells travel upwards to the epidermal layer and are cast-off thereafter. Exposure of the immature basal layer cells to HPV can occur due to minute abrasions that occur during sexual intercourse [22, 23].
The entrance of the virus into cells is not yet properly known, but it is agreed to be regulated by various receptors [24, 25]. HPV replication is based on and uses the typical reproductive machinery of the cervical cells that is corrupted by viral proteins, E1 and E2. The virus is typically kept at ~ 100 episomal duplicates per basal cell, and up to this stage, primary infection induces a viral replication eruption. HPV-infected basal cells continue to divide, creating two daughter cells in each of them with viral genomic material. One cell out of the two daughter cells stays in the basal layer and keeps hold of its splitting ability, serving as a storehouse for viral duplication requiring dynamic cellular division to sustain progression through the normal life cycle. Conversely, via the suprabasal layer, the second daughter cell continues upwards where it separates and is finally discarded from the epithelial surface [24, 26]. Early HPV proteins are expressed to make sure that cervical cells are sustained in a condition of consistent growth and differentiation, such as through the activities of the genes E5, E6, and E7. The viral genome is multiplied to about 10,000 or more copies/cell after cell division in the suprabasal sheet and subsequently, the production of the viral genes E4, L1, and L2 is activated. The capsid enclosure covering the viral genome consists of the L1 (major) and L2 (minor) proteins. When this is accomplished inside the cells, then during the final shedding process, mature viral particles are released from the epithelial surface [24, 26, 27]. It is assumed that the viral protein E4 promotes the liberation and dissemination of HPV from the keratin cage to the inside of the keratinocytes by disruption of the keratin filaments in the dying squames. It must be remembered that cancer development is not associated with the typical life cycle of HPV, but instead is a non-productive part that is only linked to a minor subtype of infections and happens after a prolonged infection period only [11].
HPV Virology
Role of E1 and E2
E1 and E2 are known to be the early genes that are expressed as soon as the virus gains entry into the cell and integrates its genome in the nucleus of the cell. The expression of these genes activates viral replication. E2 binds to the proximal sites of the promoter which causes recruitment of E1 to the HPV origin of replication, which enhances stable binding to AT-rich sequences within the origin of replication through the development of a binding complex. Through alternate splicing, E2 can both activate and repress its target proteins in HPV. It is also rather interesting that the expression of E6 and E7 oncogenes are down-regulated by E2. Thus, E2 has a critical role in the modulation of the genes E1, E6, and E7 [26, 27]. When the HPV virus enters carcinogenic progress, this regulation process is distributed and leads to the following events: - Due to loss of E2 expression, E6 and E7 are relieved of their repressive effect resulting in the rise in the level of these viral oncogenes. The viral copy number per cell in the basal epithelia is maintained by the suppression of an early promoter by E2, however as the differentiation of keratinocytes progresses, this control is lost allowing uncontrolled production of several copies per cell. This is followed by the enclosure of the viral genomes attached to the nucleosome through the expression of capsid antigen [26, 27].
Role of E4
During the viral replication cycle, E4 was shown to be most abundant in cells located in the middle and upper layers of the epithelial layer and appears in granules inside the cytoplasm of infected host cells. These E4-containing granules were suggested to be one of the primary factors to cytopathic effect [28]. As a result of mRNA splicing, the HPV E4 protein is synthesized as an E1˄E4 fusion protein which maintains similar functionality and mechanisms of action in HPV1, HPV16, HPV18, and HPV31. E1E4 protein is undetectable within the host cell throughout the early viral replication cycle. E1˄E4 expression occurs as the host cell undergoes late viral promoter activation [29]. Currently, there is no complete information on function of E1˄E4 in early stage of replication is incomplete. However, scientists demonstrated that the E4 protein, in the form of E1˄E4 to have a ‘leucine cluster’ pattern near the protein’s N-terminus, which is essential for protein interaction with keratin for viral release. The interaction of the leucine structure inside E1˄E4 protein from multiple HPV strains with the C-terminal domain of other E4 protein, enables E4 to create structures that resemble amyloid fibres, allowing manipulation of host cell organisation, including the cytokeratin network and cause post-translational modification via phosphorylation. The ability of E4 to alter keratin organisation implies a role for E4 in viral release. Although the precise role of E4 in the viral replication cycle has yet to be discovered, scientists speculate that E4 may play a significant role in cell cycle arrest. Cell growth arrest in G2 was seen in epithelial cells expressing E4 from HPV types 16 and 18, as well as HPV1. This was attributable to the suppression of nuclear accumulation of Cyclin/Cdk1 protein in the nucleus. The suppression of E6 and E7 function when cells transit from the basal to the mid layer of the epithelia may be connected with E4-associated activation of cell cycle arrest in high risk HPV type 16 [28, 29].
Role of E5
The E5 gene is expressed as a part of the initial replication cycle. E5 is a tiny, hydrophobic protein that interacts with the receptor of growth factor derived from platelets and epidermal growth factor receptor to promote cell development [27]. Although it is known that it has oncogenic potential, its expression in cancerous cervical cells has not been found. This has contributed to the conclusion that E5 is not necessary for malignancy-causing carcinogenic epithelial transformation. However, it is interesting to note that some studies have linked E5 expression with decreased MHC-I expression which may help the virus evade cellular immunity [30].
Role of E6
E6 is an oncoprotein that along with HPV E7 triggers cellular transformation but E6 alone has weak transforming potential. P53 is a core cell cycle regulator and has an antiproliferative function. HPV E6 attaches to the central region of P53 via the formation of an E6AP trimer which is an E3 ubiquitin ligase. E6AP promotes proteasomal destruction of cellular proteins. The development of this complex degrades P53 which results in the proliferation of the cervical cells at an unparalleled rate. Continuous binding of E6 to E6AP also causes the degradation of another tumor suppressor protein named blk, resulting in unchecked proliferation. E6 also targets Bak, a BCL2 family proapoptotic gene that holds cell division under regulation. It functions by blocking the mitochondrial apoptotic mechanism in which a mitochondrial pore is created due to the release of cytochrome C, which then stimulates the caspase cascade, eventually contributing to cell death. The HPVE6 binding to Bak protein stops the above-mentioned cycle and avoids apoptosis of the growing cervical cells [31–34]. Besides targeting the intrinsic apoptotic mechanism mediated by BaK, BlK, high-risk HPV type E6 disrupts the extrinsic apoptosis mechanism by binding to TNFR1, Fas, CD95 ligand which blocks caspases 8 and 10 prevents the development of death-inducing signaling complex (DISC) providing an advantage to the proliferating virus [35].
There are various other pathways by which HPV E6 metamorphosizes cells and activates carcinogenic development. As stated earlier, the basal epithelial cells rest on the extracellular matrix which descends upwards and divides into various forms of cells and is eventually discarded. As stated earlier, the extracellular matrix holds the basal epithelial cells, which descend upwards and divide into various forms of cells and are eventually discarded. The signal to halt growth and divide is closely controlled. E6 interrupts this signal and causes the normal cells to transform into cancerous cells. Besides, adhesion molecules such as paxillin and zyxin, which anchor the basal cells to the ECM on E6 binding, are disrupted and contribute to epithelial cell detachment, which thus creates a favorable atmosphere for continuous viral duplication within constantly separating epithelial cells [11].
The high-risk HPV type E6 protein contains a PDZ-binding motif which is essential for cell conversion by linking cell proteins with PDZ residues. Some proteins, such as hScribble and hDLG (tumor suppressors), MUPP1, and PATJ (tighten junction proteins), were detected in vitro studies. The deterioration of these proteins by E6 binding dysregulates growth and interferes with cell junctions. For carcinogenesis, PDZ binding is an essential activity, as E6 oncoproteins found in almost all invasive cervical cancers have the PDZ binding motif [36].
Role of E7
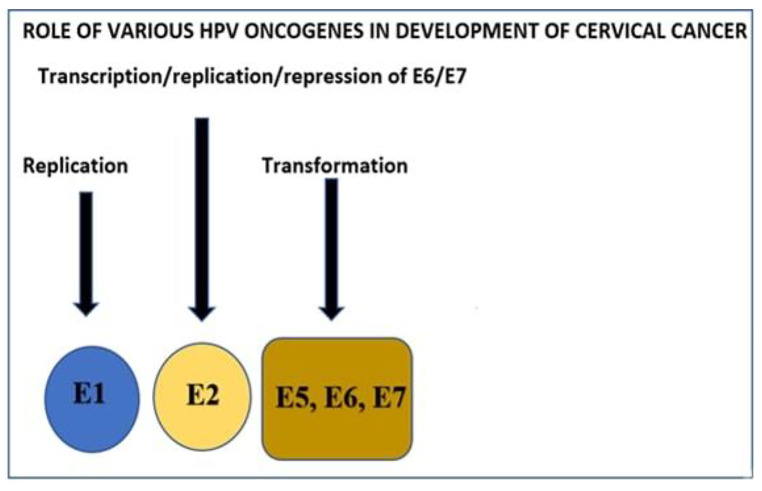
E7 comprises around a hundred amino acids and, as the E6 does, lacks endogenous enzyme activity [37, 38]. E7 exhibits its oncogenic influence by binding to the proteins of retinoblastoma (pRb) and its associated pocket proteins p107 and p130. By controlling entry into the G1/S stage of the cell cycle, these proteins control cell growth and this is accomplished by modulating E2F transcriptional factors. Prb binds to low-risk as well as high-risk HPV E7. Nonetheless, owing to a mono-amino acid substitution in the binding region, the low-risk HPV E7 has a greatly reduced affinity [37, 39]. Binding of E7 to E2F guarantees that the epithelial cell retains transcriptional function such that it can go through the S stage of the cell cycle indefinitely. E2F controls the synthetic stage of the cell cycle through modulation of specific cyclins such as cyclin A and cyclin E. E7 leads to cell multiplication along with increased levels of cyclins and also blocks cyclin-dependent kinase inhibitors (CKI), p21, and p27 activities. This is accomplished due to inhibition of pRb and direct E2F activation [39]. The association of E7 with pRb is also correlated with the c-jun expression, which is essential for the regulation of cell differentiation and development. Besides, the suppression of pRb as well as c-jun, ensures that the cell is retained in the S stage continually. Likewise, E7 also interacts with histone deacetylase (HDACs), which results in enhanced E2F-mediated transcription of the epithelial cells in the S-phase. Besides causing dysregulation of the cell cycle, E7 facilitates numerous structural modifications in the genomic structure of the epithelial cells, especially in extra chromosomal material, anaphase, aneuploidy, and polar mitoses. For instance, E7 detaches the production of centriole from the cell cycle control and induces an aberrant number of centrioles, and causes mis aggregation of chromosomes. The cdk2 activation by E7 is held partially accountable for these shifts, and in vitro studies have shown that cdk2 inhibition is consistent with the reduction in the incidences of chromosomal alteration [40]. The major roles of various HPV oncogenes are shown in Fig. 3.
Fig. 3.
Role of various HPV oncogenes in the development of cervical cancer
Role of HPV and inflammation
About 20% of human malignant tumors are linked to chronic inflammation brought about by infectious agents, immune system disorders, prolonged exposure to irritants, and different toxic substances [1]. Over-activation of the inflammatory cascade assumes a critical role in carcinogenesis, prompting the advancement of the low-grade lesion to high-grade invasive cervical cancer lesions [41]. It is predicted that an excessive inflammatory reaction is the cause of at least 15–20% of all cancer deaths around the globe [42]. In contrast to the basic role of inflammation as in the facilitation of healing of wounds and killing foreign agents entering the body, inflammation continues throughout the cancerous phase to develop a pathological chronic disease and does not go through a healing mechanism resulting in a prolonged infection. The development of persistent inflammation is followed by the release of other entities from the immune response cells that help the pathogen in the host organism to survive superiorly. It increases the integration of the tumorigenic genetic material into the host machinery through the virus [43]. A deeper understanding of inflammation-related pathways and tumor advancement in terms of pathogen survival, tumor growth, advancement, and its spread can contribute to developing a revolutionary strategy for cancer care [43].
Inflammation involves re-modeling of tissues caused by modifications in the vascular, epithelial and immune cell function. Such events occur through the intervention of reactive oxygen species (ROS), tumor necrosis factor-alpha (TNF-Alpha), cytokines, interleukin-1 (IL-1), interleukin-8 (IL-8), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), matrix metalloproteinase-9 (MMP9), cyclooxygenase (COX), growth factors, chemokines, and lipid mediators as well as through the induction of transcription factors such as nuclear factors- STAT3 and HIF-1 [43]. This can either lead to acute inflammation which causes tumor suppression or chronic inflammation can occur which helps in tumor survival and progression. The elimination of the cancer lesions usually takes place in the early stages of most HPV infections, failure of which can give HPV the ability to bypass the immune system of the host and incorporate them into the host’s genome. In the end, this entire cycle leads to the occurrence of inflammation during an HPV infection. The primary transmission of the HPV virus to the cervix is through the semen of an infected partner during sexual intercourse, during which the virus lives in the epidermal mucosa with a complete cut off from the connective tissue, and is thus able to conceal itself from the host’s immune system. Throughout this time, the virus can induce mechanisms to circumvent the host immune system by dysregulating different pathways involving pattern recognition receptors namely the toll-like receptor (TLR-9) by the host [33]. Toll-like receptor 4 (TLR4) is a pattern recognition receptor often associated with tumor growth and cervical cell inflammation. The signaling of TLR4 triggers cell activation, inflammation, and development of tumors through the signaling pathways associated with NF-κB. Reduced expression of TLR4 was found to be one of the reasons for apoptosis in SiHa CC cells [44].
NF-κB is a transcription factor involved in the stimulation of immune cells and chronic inflammatory responses. HPV-16 E5 and E7 oncoproteins cause induction of the NF-κB pathway, which is associated with CC carcinogenesis advancement. In response to inflammatory stimuli, COX-2 is also activated along with cytokines, growth factors, and mitogens. NF-κB is known to be a positive COX-2 regulator [45]. The cyclooxygenase- prostaglandin (COX-PG) pathway is an important process implicated in cervical cancer inflammation. HPV-16 induces COX transcription. The expression of COX-2 increases after infection with HPV and its introduction into the cells of the cervix. This up-regulates the synthesis of PGE2 and E-series prostanoid G-protein coupled receptors (PTGER). PGE2 then directs the tumor functions through PTGER [33]. NF-κB can also be activated by Caspase-1, which splits pro-IL-β to mature IL-1β activator. Intriguingly, it is established that the NF-Κb-cox-2/ caspase-1 pathway is important for cell proliferation, inflammation, and anti-apoptosis [45].
Cytokine production is a notable feature of human cancers caused by viruses [43]. Cytokine production by tumor cells engages inflammatory cells that bring about tumor development and advancement. Numerous evidences confirm these processes taking place during cervical cancer development [1, 46]. Latest research has indicated an association between deregulation of cytokines, and the development of cervical precancerous lesions (low-and high grade squamous intraepithelial lesions), advancement from precancer lesions to malignant growth, further invasiveness, and the last stage of metastasis. More than 33 cytokines have been distinguished as of now, and it is now known that different cytokines or their receptors are essentially modified in tumorigenesis and metastasis of CC. Thus, Cytokines namely IL-6, IL-8, IL12, IL-4R, IL-4, IL-10, and vascular endothelial growth factor (VEGF), have been utilized as markers for evaluating the risk of invasive malignant growth and metastasis [47–49]. IL-1α facilitates the proliferation of cancer cells in the cervix. IL-1 and IL-6 play a role in the growth of cells, metastasis, and also in tumor progression. Increased expression of IL-8 induced by cell cycle and apoptosis regulator 2 under conditions of oxidative stress has been proposed to play a role in inflammation and CC progression [43].
Abnormal STAT3 signaling promotes growth, invasion, metastasis, and inflammation of the tumor cells. Its continual stimulation is known to be a marker of poor prognosis in CC [41]. Also, the number of inflammatory cells of T-helper type 2 (Th2) is increased during the advancement of cervical lesions caused by HPV. The levels of the most known cytokine of the Th2 type, IL-10, are elevated in cervical tissues and sera from patients diagnosed with HPV and is associated with high-grade lesions. [50]. Researchers have shown that up-regulated IL-10 secretions are likely to suppress the immune response of the host to the infection in the initial cervical lesions, whereas up-regulated TNF-α and disturbed cytokine secretions (the imbalance between Th1 and Th2 cytokines) could be contributing to the weak immune response in the late-stage lesions. Measurement of IL-10 and TNF-α may thus provide a valuable measure of the local autoimmune response to HPV lesions during cervical secretions. [51]. TNF has a decisive function to play in any inflammation caused by infection. TNFs are primarily implicated in the modulation of the inflammatory process by binding to their cell-specific TNF receptors (TNFR1 or TNFR2) to produce signaling pathways against viral invasion, cause apoptosis, growth arrest, or assist in cell proliferation [43]. During viral entry and initiation of inflammation, tumor necrosis factor-alpha type serves as a dominant keratinocyte receptor, eliciting extrinsic apoptotic pathways. The extrinsic cascade is activated by the binding of these cytokines to receptors mainly TNFR1 or TRAIL. Further, studies suggest that E6 high-risk proteins bind to TNFR1 or TRAIL and obstruct the downstream pathways. To stimulate the expression of p21CIP1/WAF1, a well-established cyclin-dependent kinase (Cdk) inhibitor, TNF stimulates the NF-κB pathway. Additionally, TNF also plays a key role in down regulating other proteins in the cell cycle, such as cyclin A, cyclin B, cdc2, respectively [43]. The increased expression of receptors of the epidermal growth factor (EGFR) also leads to unwanted coupling between other signaling pathways engaged in releasing growth factors, cytokines, and inflammatory mediators. Over-expression of EGFR was shown to be consistent with inflammatory activation and poor prognosis in patients with CC [43]. The complex interaction between HPV particles and inflammation is thus well established. Increased knowledge of inflammatory and tumor micro-environments is essential for designing novel clinical approaches focused on the origin, progression, and metastasis of tumor growth [43].
Role of HPV and oxidative stress
The existence of elevated concentrations of oxidized biomolecules is correlated with alterations in aerobic metabolism, inflammatory response, UV radiation exposure, hypoxia, anomalous cell proliferation, and viral infections [52–54]. In recent years, oxidative stress has also been identified as a causal factor in the development of numerous cancers [55–57]. Reportedly, it has a key role to play in cervical cancer pathogenesis and may also be responsible for resistance to chemo/radiotherapy. Additionally, oxidative stress is also recognized to play a major role in CC caused by HPV.
Oxidative stress is induced by the disrupted oxidant-antioxidant balance in favor of oxidants, triggering immoderate production of free radicals, especially reactive oxygen species (ROS) and thus eventually resulting in biological damage [58]. Hydrogen peroxide (H2O2), Superoxide anion (O2.-), and hydroxyl radical (.OH) are types of ROS created by the partial atmospheric reduction of O2. ROS is critical in controlling metabolic pathways and immune defense against infectious agents. Moreover, excessive generation of ROS, which may be due to the influence of endogenous or exogenous factors, is harmful to cellular structures and the function of proteins, carbohydrates, nucleic acids as well as lipids [59]. Damage to these macromolecules plays a critical role in cancer progression, influencing numerous cellular activities such as cell multiplication, migration, angiogenesis, apoptosis, and also treatment resistance [59, 60]. Therefore, the body is loaded with nonenzymatic (glutathione [GSH], vitamins, and carotenes) as well as antioxidant defense mechanisms (thioredoxin system, superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferases (GST), and catalase) [61, 62]. The antioxidant system regulates the oxidative cycle within anatomical boundaries, avoiding injuries that may contribute to irreparable systemic damage and diseases [63]. A variety of clinical and preclinical studies have suggested the role of dysregulated antioxidant system in the pathogenesis of CC. Decreased levels of GSH, GPx, GST, SOD, and lower amounts of non-enzymic antioxidants such as vitamin C and vitamin E in cervical cancer patients relative to the controls were observed in a case-control test. Naidu et al. recorded elevation in serum levels of NO, Cu, and malondialdehyde (MDA), (a predictor of lipid peroxidation), while the production of vitamin C Zinc (Zn), and RBS-SOD were greatly decreased in cervical cancer patients when compared to healthy controls [64]. Palan et al. found that plasma levels of dietary antioxidants, such as β- carotene and α tocopherol, were reduced dramatically in women with cervical dysplasia or carcinoma. Possible causes proposed for decreased quantities of enzymatic and nonenzymatic modulators of the redox system are an oxidative injury to enzymes, lack of vital trace elements, metabolic deficiencies, and enhanced usage of antioxidants to help prevent the accumulation of ROS [65].
There has been a thorough study of the link between oxidative damage and HPV infection as being a key cause of cervical cancer disease. Inflammation and oxidative stress can promote HPV penetration, which is a critical step in HPV carcinogenesis [66, 67]. Stimulation of inflammatory reactions is a defensive mechanism activated when HPV infects the cervical cells that stimulate white blood corpuscles and releases various inflammatory mediators into the circulation. This inflammatory condition brought about by HPV, as a result, can enhance ROS production from granular leukocytes and macrophages [68, 69]. ROS then induces damage to the DNA leading to double-stranded DNA breakages, which are important for the incorporation of the HPV genome into the cell’s DNA and also for the initiation of carcinogenesis [70].
E6 has a crucial role to play in the modulation of various transcription factors participating in the modulation of the expression of antioxidant enzymes. It can thus be inferred that modification of transcriptional factors is a potential mechanism through which E6 controls the expression of antioxidant enzymes [71]. DNA repair mechanisms can also be impaired by HPV oncoproteins contributing to the inactivation of essential gene classes such as base excision repair (BER), nucleotide excision pair (NER), DNA mismatch repair (DMR), microhomology-mediated end joining (MMEJ), Fanconi anemia (FA), ataxia- telangiectasia mutated (ATM), and the ATM and RAD3 (ATR) related genes. Such pathways are implicated in the pathogenesis of cervical cancer regulated by high-risk HPV [72, 73]. Thus, in the light of these results, few antioxidant molecules including natural compounds can be useful as protective or medicinal alternatives.
Immunological aspects of HPV infection and cervical cancer
A considerable majority of the HPV infections are cleared up during a three-year treatment course by the patient’s immune system, a few become chronic whereas only 1% of the cases lead to cervical cancer. The infection is usually asymptomatic but may manifest into genital lesions and eventually result in progressive and invasive carcinoma [74]. A compromised immune system favors the transition of a low-grade squamous intraepithelial lesion (LSIL) into the high-grade squamous intraepithelial lesion (HSIL) and even invasive cervical carcinoma [75]. HPV viruses are strikingly capable of avoiding interaction with the human immune system. The specific characteristics endow the virus with this essential capability. Firstly, since there is no viremic stage in the HPV infection, the cells of the immune system present in the circulation become unable to easily access the viral particles. The primary site of infection by the virus is the basement membrane where only Langerhans cells prevail and that too in small numbers. Also, human papillomaviruses do not cause substantial harm to the host cervical cells, for instance, destruction of infected cells, reducing inflammation and consequent signaling, allowing the virus to silently replicate [76, 77]. The third escape factor is the virus’s cautious gene expression behavior. The expression of the viral oncogenes during the preliminary stages of infection are maintained at low levels and since the extremely immunogenic agents such as L1, L2 capsid proteins are produced distinctively in the epithelium’s peripheral surfaces, the virus can successfully bypass the host’s immune system [78, 79].
Role of Innate Immunity
Natural immunity is the nonspecific component of the immunological system. Innate immunity is regulated by an epithelial barrier, the complement system, and a range of cells that induce phagocytosis of the antigens and destroy them or transfer them to other cells. In contrast to the decreased numbers of LC’s present in the transition zone, a slightly increased number of LC’s are noticed in SIL’s (squamous intraepithelial lesions) but their function remains unclear [80, 81]. Due to lack of adequate co-stimulative microenvironment, even when there is no injury present, Langerhans cells of the epidermis fail to generate a proper T-cell response. As a result, Langerhans cells are not able to induce a positive immunological response and may then be responsible for viral tolerance [82].
Macrophages come from monocytes and are found in tissues. They come under the class of phagocytes and play a significant role in natural immunity, activating acquired immunity, digesting pathogenic material, and also triggering lymphocytes and other cells of the immune system. Particular proteins such as monocyte chemotactic protein-1 (MCP1) and the inflammatory protein macrophage (MIPa3) support macrophage agglomeration. These proteins tend to be downregulated directly or indirectly by HPV [83, 84]. Available data imply that tumors are invaded by enormous numbers of macrophages that migrate to the site as a result of the identification of cancerous cells as foreign cells [85]. In contrast to the conventional proinflammatory and antitumor response, macrophages within the solid tumor microenvironment may contribute to the progression of the disease. Tumor-associated macrophages (TAM’s) facilitate the proliferation and migration of cancer cells, angiogenesis, and inhibition of immune responses against cancerous cells [86]. This is brought about by two different phenotypes of the macrophage: M1 the proinflammatory macrophage and M2: the immunomodulatory macrophage. In normal conditions, M2 evokes overexpression of vascular endothelial growth factor (VEGF) and metalloprotease-9 to assist in tissue repair, but when stimulated by the tumor, it destroys the basement membrane, development of tumors, and metastasis [85, 87, 88]. As cervical lesions grow, the number of M2 macrophages, are increased which are often seen in HPV-associated tumors [89, 90]. It also facilitates the differentiation of immature T cells into T-regulatory cells via IL10 and thus promotes the growth of tumors [90, 91]. TAM depletion can be considered as a potential target for immunotherapy.
Natural killer cells (NK cells) are also an integral component of the innate immune response to viral attacks. They are lymphocytes that react immediately to stressed cells which are under viral or carcinogenic attacks, without the necessity of a major histocompatibility complex (MHC) system. Recently it was found that the NK-activating receptors NKp30 and NKp45 are significantly downregulated in HSILS and cervical carcinoma caused by HPV 16, affecting its cytolytic functionality [92].
Acquired immunity
Acquired immunity, the specific immune response to pathogens, is made up of B cells and T cells. B cells are involved in humoral immune response and T cells, which are classified into helper T cells, cytotoxic T cells (CTLs), and regulatory T cells (Tregs), also perform several other tasks. The T cell response among women with different stages of cervical malignancy depicts a distinct pattern and thus it is proposed that T helper cells might be the dominant response required to clean up an HPV infection [93]. When T helper cells mature, they are differentiated into two phenotypic populations, namely Th1 and Th2. Recent studies support the fact that the equilibrium between these two phenotypes is disrupted in HPV lesions. Generally, both Th1 and Th2 phenotypes are suppressed in HPV lesions, most notably Th1, possibly due to T regulatory cell activity. T-regulatory cells are a type of T cells that express CD4, CD25, and Foxp3 transcription factor that is usually essential for tolerance induction, but an increase in their numbers can obstruct the immune response and block the antitumor activity by inhibiting cytokines. Their activation, which is caused by TGF-β and IL2, leads to a poor prognosis of cancer. This coordinated Treg immunity suppression is still not completely understood. Tregs is believed to reduce the T-helper and cytotoxic T cell proliferation [94–96]. B-cells exhibit a humoral response that neutralizes viral agents and opsonizes them. Humoral immunity is triggered by cell antigen and Th2 cytokine pattern and relies on CD4 helper T cells that aid in the maturation of B cells as well as in the development of antibodies against a particular epitope [97].
Even though weak antibodies against E2, E6, E7, and L2 have been developed, the antibodies against HPV target mainly the L1 capsid protein. A substantial proportion of such antibodies are IgG1 class, a typical response against viral immunogenic products. Two kinds of neutralizing L1 antibodies are known. The first type impedes attachment of the virus particles to the cytomembrane while the second type avoids attachment to the basement membrane. Both seem to hamper viral integration either by direct attachment or by obstructing the required structural changes. It is possible to estimate seroconversion and the neutralizing antibodies after eight to nine months of initial infection but their concentrations are small and not evident in all women [98]. Knowing the immunological aspects of the infection and its microenvironment can be a valuable method to implement new treatment options.
Biomarkers
Generally, early stage cervical cancer does not cause any symptoms instantly such as scratching, burning, or vaginal discharge although such symptoms may be experienced by some women [99]. Detection and prognosis of asymptomatic, invasive diseases are inadequate but the management of pre-invasive lesions is highly successful [100]. It is well known that there is no single form of screening available which is highly sensitive, precise, and affordable as well as practical [101]. Historically, several screening methods including pap smears and colposcopy have efficiently decreased fatality rate utilizing early-stage identification. Regardless of these advancements, issues of misdiagnosis, poor specificity of different markers, lack of adherence, and insufficient analytical resources to discover novel strategies of detection have afflicted the field of detection. Therefore, to detect cancerous cells in tissues, there is a need to identify biomarkers that could supplement standard cyto/histopathology assessments. Although several efforts have been made over the years, there is always a need to identify and establish new biomarkers for the detection of cervical neoplasia [102].
According to the working group of the United States National Institute of Health (NIH) and the consortium of biomarkers, “A biomarker is a characteristic that can be objectively measured as an indicator of normal pathogenic processes or a pharmacological response to a therapeutic intervention” [103]. The principal aim of biomarker establishment is not just to create new therapeutic avenues but also to focus on improved strategies to evaluate the individual patient’s risk for the development of cancer at an early stage when they can be treated more efficiently [104].
The PAP test
A Pap smear test is considered the main screening method for the identification of precancerous cervical intraepithelial neoplasia and also the early stages of invasive cervical cancer [105]. The principle of the Pap test is mainly the morphological evaluation of cervical cancer biomarkers based on biomolecular changes in the nuclei of basal squamous epithelial cells in samples collected from the peripheral surface of the cervix. In the majority of the cases, carcinogenic transformation results from chromosomal instability, which occurs spontaneously in specific somatic cells during a phase generally called cancer initiation. As the cervical epithelium progresses towards carcinogenic transformation, ultimately resulting in intrusive cancer, aneuploidy of the nuclei of the respective cells becomes prevalent by hyperchromatic nuclei, morphological changes of the nuclear membrane, condensation, and irregular decondensation of distinct chromosomes. Hence the shifts in the DNA content and the process of aneuploidy have been used as the main diagnostic criteria in cervical cytopathology which ultimately resulted in the success of Pap test-based screening for cervical cancer diagnosis. However, morphological changes are distinct and challenging to differentiate from non-specific morphological modifications of cells that are not related to carcinogenic transformation, especially in the early stages of the transformation phase. Along with the inconsistencies in morphological assessment, the Pap test also fails to detect high-grade cervical neoplasia effectively as initial alterations may be neglected due to individual error. These technical limitations pose a huge challenge to the patients affected and are also a major cost factor. Thus, there is a growing need for the development of improved biomarkers that can help address the existing ambiguities of cervical cytopathology [105].
FISH
Fluorescent in situ hybridization (FISH) technology is widely accepted as a useful method of assessing cervical dysplasia [106, 107]. Research shows that among the most significant chromosomal anomalies observed in the cervical tumor is the acquirement of chromosome arm 3q, which is found in around 70% of cervical carcinomas [108]. These additional copies contribute to the acquirement of the human telomerase RNA gene (TERC) situated in the 3q2 section. Its genetic product, telomerase, is implicated in the regulation of chromosomes by providing telomerase stability and by controlling telomere length. FISH analysis for 3q could be useful as a biomarker [109].
The ProExC Test
The ProExC test is a newly designed immunocytochemical assay to detect minichromosome maintenance proteins (MCMs) as a predictor of abnormal S-phase initiation and the inherent high-grade dysplasia in cervical cytology slides. MCMs are necessary for the initiation of DNA duplication and are observed to be highly expressed in high-grade cervical dysplasia and carcinoma [110–112]. Although, ProExC test is found positive in HSILs and negative in regular cytological samples consistently [113]. It is not widely used to aid in diagnosis since MCMs are also found to be expressed in certain benign squamous lesions and glandular cells. Further validation of this test as a diagnostic supplement for cervical cancer will rely on the findings of large-scale studies including biopsy results and correlation of clinical outcomes [114, 115].
The OncoE6 TM cervical test
The OncoE6 TM Cervical Test detects levels of E6 oncoproteins which are known to increase during epithelial cell transformation. Therefore, the detection of E6 proteins appears to be an enticing, disease-specific biomarker of viral oncogenic behavior in populations with high-risk HPV infection and aid in the administration of appropriate treatment accordingly. The OncoE6 TM Cervical Test does not require complex tools or special training of the operator. This facilitates its use in low resource settings. Previous results have demonstrated a very high positive predictive value (PPV) of the OncoE6 cervical test. These findings suggest that it is an accurate tool for detecting the presence of precancerous as well as cancerous cervical lesions as compared to other HPV DNA tests. However, its poor sensitivity to detect early epithelial alterations limits its use [116].
Molecular markers
The key pathological phenomenon in cervical tumorigenesis is the incorporation of high-risk HPV viral DNA into the host chromosomal DNA, which stimulates the development of pre-malignant cells via the appearance of cell clones with dysregulated production of viral oncogenes in basal and parabasal cell layers, eventually resulting in progressive cervical cancer. Therefore, DNA- based assays can be developed to identify the existence of the DNA accountable for cervical cancer [117]. HPV DNA is the only molecular marker that was established for cervical cancer detection. HPV DNA testing alone is used as a principal screening tool. The detection of CIN2 and CIN3 by HPV DNA testing is far more sensitive than by cervical cytology. Using this technique, mutated circulating DNA can be detected in stage I itself in most women affected with cervical cancer and its concentration helps determine the severity of cancer. Serum HPV DNA could also be a valuable marker for early diagnosis of cervical cancer relapse [118].
The Digene HPV test
The Digene HPV test was the first licensed HPV test. This test is a solution-phase hybridization assay using RNA probes complementary to HPV DNA, leading to amplification of the signal. The test identifies the existence of 13 types of high-risk HPV [16/18/31/33/35/39/45/51/52/56/58/59/68] and 5 types of low risk [119–123]. Typically, the assay requires only the high-risk HPV probe set, as low-risk HPV is medically irrelevant [124]. The benefit is that since a negative report eliminates the risk of HPV-related illness in future years, treatment periods in such patients can be safely extended to three to five years. Other benefits are good interlaboratory reproducibility and user-friendliness. However, it also holds certain drawbacks, the major one being the inability of the test to differentiate between the individual types of HPV. It is only known to detect the existence of at least one of the high risk or low-risk HPV types which poses a major disadvantage as with the emergence of human papillomavirus vaccines, HPV genotyping is becoming exceedingly important [125].
The Cervista HPV HR test
The Cervista HPV HR test measures the presence of 14 high-risk HPV forms, including 16/18/31/33/36/45/51/52/56/58/59/66/68. This technique detects specific nucleic acid sequences through signal amplification. The process consists of two isothermal reactions: the main reaction on the specific sequence of the target DNA and a secondary reaction that generates a fluorescent signal to aid in visual detection. The Cervista assay exhibits greater sensitivity than the Digene HPV test in detecting CIN2 and CIN3 lesions. Therefore, one can say HPV DNA testing has a sensitivity above 90% for the identification of CIN + lesions, but unfortunately fails to accurately detect the underlying clinically relevant lesions, because a majority of positive cases reflect only temporary infections rather than cervical mucosal transformation [126].
DNA aneuploidy
Infection with HPV may result in hypermethylation of DNA, disturbance of the normal cell cycle, and chromosomal aberrations. These events together contribute to alterations in the DNA content. Studies found major variations in the pattern of aneuploidy between HSILs and LSILs: 79% aneuploid versus 4% respectively [127]. Repeated experiments have demonstrated a significant link between extremely aneuploid squamous cells and high-risk HPV [128]. The combined use of high-risk HPV testing and measurement of DNA ploidy on ASCUS (Atypical squamous cells of undetermined significance) cytology specimens could perhaps help in better management of patients undergoing colposcopy and also to recognize patients with ASC-H (High grade atypical squamous cells) who are at a greater risk for developing CIN2 lesions or even cervical cancer [129]. Thus, DNA image cytometry has become a standardized, increasingly used, and reproducible test in the diagnosis of cervical lesions [130].
Protein based biomarkers
With the growing popularity of the applications of proteomics in the field of diagnostics and prognostics, biomarkers based on proteins have also caught the attention of many researchers. Protein biomarkers could enable early identification of the disease at a phase where the chances of recovering are fairly high. It may also help the doctor to differentiate patients who respond well to certain forms of treatment strategies from those who don’t [131].
Squamous cell carcinoma antigen (SCC-Ag)
Squamous cell carcinomas make up the majority of the cases of cervical cancer and high levels of squamous cell carcinoma antigen (SCC-Ag) have been reported in these types of carcinomas [132]. SCC concentrations are linked to the stage of the disorder, tumor size, and extent of the stromal invasion which further helps define early treatment strategies [133]. Thus, elevated levels of SCC play a predictive role in prognosis and it is also suggested that it could explain the response to the therapy administered as well as determine the possibility of recurrence of the disease as it has been shown that elevated serum SCC precedes the clinical diagnosis of the disease’s recurrence [134–136].
Serum fragments of cytokeratin (CYFRA)
The cytoskeleton of epithelial cells is primarily made up of cytokeratins which belong to the category filamentous proteins. CYFRA detects the serum levels of cytokeratin 19 in epithelial cells which is an acidic subunit of cytokeratin known to be expressed in normal as well as in carcinogenic conditions of the cervix. In 42–52% of patients with squamous cell carcinoma of the uterine cervix, high concentrations of cytokeratin fragments were detected and therefore this aspect can be used to determine the stage of the disease, tumor size, the extent of the stromal invasion, the involvement of the lymph-vascular area, and lymph node metastasis. However, although an enticing biomarker, CYFRA appears to be a poor prognostic indicator of cervical cancer [137].
Carcinoma embryonic antigen (CEA)
Interestingly, Carcinoma embryonic antigen (CEA), a glycoprotein was first used as a colon cancer marker and subsequent research found that elevated CEA levels exist in many other cancers including gynecological cancers (ovarian/cervical cancer). Unfortunately, increased levels of this antigen are also observed in non-cancerous lesions and therefore it cannot be used as a screening procedure. However, it can aid in the management of the disease because its levels are known to increase gradually as the disease progresses from stage I to stage II (26-88%) in patients with invasive cervical squamous cell carcinoma, suggesting that CEA as a biomarker may hold good prognostic value in cervical cancer. CEA levels are also reported to serve as a prognostic predictor in adenocarcinoma of cervical cancer for disease management strategies [137].
Immunosuppressive acidic protein (IAP)
Immunosuppressive acidic protein (IAP) is another novel biomarker whose levels are found to be elevated in 43–51% of cervical cancer cases including squamous cell carcinoma and adenocarcinoma. IAP levels can be correlated with the disease stage and lymph node metastasis, therefore it can also be used in diagnosis, prognosis, and validation of the selected treatment strategy. However, the true importance of evaluating IAP as a screening tool depends on what stage of the disease the serum levels of IAP show an increase [138].
Cyclooxygenase
[COX]-2 is the main enzyme involved in the synthesis of prostaglandins from arachidonic acid and in the control of many cellular processes in cell cycle control, apoptosis, extracellular matrix deposition, and angiogenesis [139]. COX-1 is a housekeeping gene whose expression remains essentially constant. Conversely, COX-2 is an immediate-early response gene that can be activated by growth factors, tumor promoters, oncogenes, and carcinogens [140–143]. There is a significant body of evidence suggesting that COX-2 is essential in carcinogenesis. In 12 out of 13 cases of cervical cancer, COX-2 was found to be expressed in high concentrations but was undetectable in the normal cervix. In cervical cancer, the EGFR is also usually overexpressed. Activation of this EGFR pathway can also lead to enhanced COX-2 expression in cervical cancer. Over COX-2 expression is said to indicate metastatic invasiveness in cervical carcinoma. It is also considered a good indicator of poor response to radiotherapy or chemoradiation, independent of the histological type. Over COX-2 expression is said to indicate metastatic invasiveness in cervical carcinoma. It is also considered a good indicator of poor response to radiotherapy or chemoradiation, independent of histology [144].
Soluble CD44 [sCD44]
CD44 is a fundamental membrane protein involved in carcinogenesis. They function as both cell adhesion molecules and lymphocyte homing mechanism (both cell-cell and cell-matrix) and are also involved in the growth, spread, and invasion of tumors [145]. The presence of the standard CD44 protein, including its variant CD44v1-9, is reported to be detected in the tissue and serum of patients with cervical neoplasia [146]. In most of the cases of cervical intraepithelial neoplasia [CIN] and microinvasive carcinoma (MIC), a substantial rise in CD44 levels was reported, and it was also noticed that sCD44 staining displayed a gradual reduction in its expression levels as lesions progressed from CIN 1 to CIN 3 and then to MIC [147]. Thus, soluble protein marker CD44 could be used to differentiate cases of premalignant cervical lesions from cases of malignant lesions in cervical cancer [148].
HPV mRNA
DNA-based HPV tests can only distinguish between the presence and absence of an HPV-specific DNA target sequence but cannot specify if an infection is active or potentially transforming [149]. As mentioned earlier, the expression of HPV E6 / HPV E7 results in cellular transformation with unchecked cell-cycle progression due to deterioration of p53 and Rb respectively [150]. Overexpression of the E6 and E7 proteins can be identified by screening for the E6 / E7 mRNA transcripts which in turn can give insights into any possible risks of carcinogenic progression of the disease. HR-HPV E6 / E7mRNA test is therefore a promising non-invasive biomarker for the identification of high-grade cervical lesion (CIN2 +) that not only allows the identification of HPV infection but at the same time predicts any alterations in the cervical lesions. This is because the persistent expression of HR-HPV oncogenes E6 / E7 is essential to establish and sustain the dysplastic phenotype [151].
The PreTect HPV-Proofer assay
The PreTect HPV-Proofer assay uses nucleic acid sequence-based amplification (NASBA), a sensitive transcription-based amplification system (TAS) for mRNA-specific in vitro replication [152]. This assay detects E6/E7 mRNA from five HR-HPV types (16, 18, 31, 33, and 45). A positive result for the HPV-Proofer test is representative of E6 / E7 incorporation and indicates a high risk of persistent infection. The HPV-Proofer assay is sensitive but more accurate to identify underlying high-grade lesions than the HPV PCR. It is less sensitive than the APTIMA test, however is said to be more specific as the PreTect HPV-Proofer detects only the mRNA of the five most common forms of HPV (HPV16, 18, 31, 33 and 45) unlike APTIMA that detects more genotypes of mRNAs [151].
The APTIMA HPV assay
The APTIMA® HPV Assay is a target amplification assay that uses transcription-mediated amplification (TMA) to detect the viral polycistronic E6 / E7 mRNA from 14 types of HPV. Furthermore, the APTIMA® HPV 16 18/45 Genotype Assay also uses the TMA technique to identify HPV types 16 and 18/45 specific to type. The Aptima HPV assay is performed in three sequential steps that take place in a single tube: target capture specimen processing; target amplification using transcription-mediated amplification (TMA), and identification of the amplification products by hybridization protection assay (HPA). A positive HPV-Proofer test suggests E6 / E7 integration and recognizes any high risk of chronic infection. In comparison with other HPV tests, the AHPV test consistently has comparable clinical sensitivity, but greater specificity for cervical disease detection (CIN2+/CIN3 +) [152].
HPV L1 capsid protein
L1 capsid protein is an integral HPV capsid protein that occupies almost 90% of the total protein on the virus surface. It is highly conserved and is the most important target for the body’s immune response against HPV infections [153, 154]. Generally, it is detectable during the proliferative phase of the HPV infection cycle. L1 is abundant in active infections. In the later stages, it is gradually lost. It is reported that the expression of the L1 capsid protein tends to decrease with increasing lesion severity. It is reported that the expression of the L1 capsid protein tends to decrease with increasing lesion severity. This is the reason why the positive rate in LSIL is higher than in HSIL and cancers, and HSIL positive rate is greater than cancers. A few studies have evaluated the prognostic importance of L1 levels; although the studies are comparatively limited, it was proposed that The L1 levels could be useful in predicting the progression of the disease. However, a recent study by Galgano et al. showed that L1 was neither sensitive nor specific for the detection of CIN2/3 lesions [155].
p16INK4a
Gene products that are over-expressed in HPV-infected cells as a direct result of the production of the transforming viral oncoproteins E6 and E7 could prove to be more useful as compared to multiplication-associated proteins. The p16INK4a protein functions as a cyclin-dependent kinase inhibitor in normal cells and is an important part of the regulation of the cell cycle [156]. P16INK4a blocks cdk4-and cdk6-mediated pRb phosphorylation to inhibit E2F-dependent transcription and prevents cell-cycle advancement. The physiological inactivation of pRb by HPV E7 in most cervical carcinomas results in the overexpression and aggregation of p16INK4a in the cells. The biological result of this is the halt of the cell cycle and senescence induction [157]. Some studies indicate that the immortalization of HPV oncogenes in cervical epithelial cells results in increased expression of p16INK4a. Based on these experimental results, it is hypothesized that increased production of the papillomavirus oncogenes could also contribute to significant over-production of p16INK4a in cells over-expressing the HPV E7 gene product in the transition phase of the HPV infection cycle [158]. P16INK4a is therefore considered as a surrogate marker for HPVE7-mediated pRb catabolism which provides evidence of cervical mucosal transformation. Thus, P16INK4a has been successfully used to identify HPV-related diseases for a variety of reasons: (A) The expression of p16INK4a is closely related to the oncogenic activity of HPV, as uninterrupted production of E7 is a prerequisite to preserve the malignant phenotype. (B) The production of p16INK4a is not dependent on the HPV type and thus genotyping is not necessary. (C) The p16INK4a expression by cycling cells is a key marker of HPV-E7 overproduction or other events which inactivate Rb.
One drawback in the usage of p16INK4a as a biomarker for cervical carcinoma is that it is also possible to observe focal and occasionally diffuse expression of p16INK4a in benign endocervical intercalated columnar cells, tuboendometrial metaplasia, and cervical endometriosis [157].
Ki-67
Ki-67 is said to be a proliferation marker known for its prognostic role in the development of a tumor. It is usually expressed during all the active phases of the cell cycle (G1, S, G2, M) in the upper layers of the cervical epithelia which are of considerable importance for the detection of benign lesions that may resemble cancer. Both qualitative and quantitative approaches such as immunohistochemical assays, electron microscopy, ELISA, flow cytometry, and immunocytochemistry have been used to detect Ki-67 in the stratified squamous epithelium presenting cervical intraepithelial neoplastic lesions. In healthy cervical squamous mucosa is found mainly in parabasal epithelial layers [the major source of cell renewal] and also in some basal layers. Ki-67 protein may also act as a proliferative biomarker and give insights on the progressive potential of normal as well as dysplastic implications. Moreover, multiple studies have proposed that Ki-67 could be a sensitive biological predictor of progression of the disease independent of age and menopause status. Ki-67 is linked with invasive carcinoma of the cervix, primarily squamous keratinizing histological type, while enhanced expression of the biomarker is associated with lymphatic invasion. Despite advancements in the understanding of the function of Ki-67 in detecting cervical dysplastic lesions, there is still disagreement over its prognostic importance in CC. While some authors have not shown any association between Ki-67 and CC prognosis, others have indicated the significance of Ki-67 in cell kinetics assessment in response to radiation therapy. Early variations in the levels of Ki-67 during radiotherapy may indicate metastatic invasion [159].
Pin1
Pin1, a member of the family of peptidylprolyl isomerase [PPIase], is over-expressed in many cancers and linked to poor prognosis. Some proteins containing phosphorylated Ser / Thr-Pro motifs, namely c-Jun, β-catenin, and NF-ÿB, are specific Pin1 target substrates. The prolyl isomerase Pin1 is a key catalyst driving proline-directed phosphorylation resulting in tumor progression. By interfering with the equilibrium between oncogenes and tumor suppressors, Pin1 facilitates tumorigenesis and stimulates several cancer-initiating pathways. Pin1 participates in the development and growth of cancer by the modulation of several oncogenes, such as cyclinD1, Notch1, and mutp53. Genetic knockdown of pin1 greatly decreases CCC proliferation. Pin1 is also involved in the regulation of the plasticity, structure, and migratory ability of cancer cells. Pin1-positive LSILs have a greater chance to progress to HSIL compared to a Pin1-negative LSIL. Pin1 production has been strongly linked with metastasis of the lymph node and c-jun expression in tissues of human cervical cancer. KPT-6566 is a recently synthesized inhibitor specific to Pin1 which binds covalently to its catalytic site and aims for degradation of Pin1. KPT-6566 is capable of directly inhibiting the productivity of cancer cells that over-express Pin1 while not affecting normal cells. Human CCCs treated with KPT-6566 reported a substantial reduction in the accumulation of Pin1 and its downstream oncoproteins, including c-Jun, cyclin D1, β-catenin, ERK1/2, p-ERK, AKT, and p-AKT473. These findings suggest that KPT-6566 inhibition of Pin1 contributes to the inhibition of several carcinogenic pathways. Pin1 can therefore be successfully used as a biomarker for determining the chances of the transformation of LSIL to HSIL, and KPT-6566 can thus be established as a potential therapeutic candidate for cervical cancer that inhibits Pin1 [160].
Melanoma-associated antigen-A (MAGE-A)
Melanoma-associated antigen A3 (MAGEA3) is a gene that has been indicated in wide categories of malignancies, such as melanoma, breast, colorectal, stomach, lung, and pancreatic cancer. Recent evidence have indicated that MAGEA3 aberrant expression has been shown to correlate with poor clinical outcome in many tumor types. The MAGEA3 expression level was significantly linked to cervical cancer clinical-stage, pathologic grade, and lymphatic metastasis. It is observed that the silencing of MAGEA3 and MAGEC1 / C7 by siRNA transfection results in the induction of apoptosis in malignant plasma cells [161]. Elevated expression of MAGE-A3 is associated with poor prognosis in CC patients. MAGE-A3 overexpression promotes cell motility, proliferation, EMT, and promotes the activation of the Wnt signaling pathway in CC, suggesting that MAGE-A3 acts as a mediator during malignant transformation of cervical cells. Hence, MAGE-A3 may prove to be a promising therapeutic target for CC patients as well as a good prognostic marker [162].
ERBB2 mutations
ERBB2 mutations are considered as oncogenic factors and therapeutic targets in many cancers, and multiple treatments for the treatment of breast, lung, and gastric cancer have been approved based on this notion. ERBB2 (HER2) a representative of the human epidermal growth factor receptor family, is a transmembrane protein consisting of an extracellular domain (ECD), a transmembrane domain (TM), and an intracellular tyrosine kinase domain (KD). Somatic ERBB2 mutations have been found in a huge variety of solid tumors, such as breast, lung, colorectal, urinary, and stomach cancers. Specific ERBB2 mutations are clustered in exon 8 of the extracellular domain kinase or exon 19–21, and most of the somatic mutations of ERBB2 trigger mutations that are likely to induce tumorigenesis. ERBB2 mutations present distinct pathological features of cervical cancer and numerous studies have indicated that ERBB2-targeted TKIs boost the outcome of ERBB2-bearing cancers when delivered as monotherapy or in conjunction with chemotherapy [163].
Brd4
The cellular bromodomain protein Brd4 22 is yet another potential biomarker that is associated with nearly every phase of the HPV life cycle including multiplication and expression of E6 / E7. Brd4 protein belongs to the BET family (bromodomain and extra-terminal domain) which comprises Brd2, Brd3, Brd4, and testis-specific protein Brdt. Brd4 associates with the viral E2 protein in HPV-positive cells and this association is essential for the transcription regulating activities of E2, and also for the genome segregation of papillomavirus genomes during mitosis and viral DNA replication in a type-dependent manner. In transcriptional regulation of E6 and E7-expression, Brd4 tends to play a key role. Inhibition of Brd4 can be useful as a therapy for cervical cancer in which E6 expression and cisplatin response of resistant cervical tumor cells tend to be modulated. However, to show the usefulness of Brd4 inhibitors in vivo, further studies are recommended [164].
MICRO RNA as a cervical cancer biomarker
MiRNAs have been identified as one of the key epigenetic regulators among different molecules and networks involved in cervical cancer pathogenesis in regulating various vital processes such as growth, differentiation, angiogenesis, and development. The deregulation of these molecules can be attributed to the initial steps of cervical cancer. Microarray studies have shown that some types of microRNA’s are upregulated while others may be downregulated in pathological conditions. Therefore, in the treatment of cervical cancer, miRNAs tend to have the practical ability to be applied as prognostic, diagnostic, and therapeutic biomarkers. The deregulation of these molecules can be attributed to the initial steps of cervical cancer. Therefore, in the treatment of cervical cancer, miRNAs tend to have the practical ability to be applied as prognostic, diagnostic, and therapeutic biomarkers. Besides, it has been suggested that these molecules could be used to monitor patients who have received chemotherapy agents [165]. Recently, Babion et al. (2020) studies association of HPV infection and miRNA expression profile and identified about 160 mature miRNAs differentially expressed through the different cell passages. Among them, “miR-15b-5p, miR-100-5p, miR-103a-3p, miR-125b-5p” were found upregulated and only “miR-221-5p” showed downregulation [166]. Further, Norouzi et al. identified higher expression of “miR-16, miR-21, miR-34a, and miR-143” in intraepithelial lesions from women infected with HPV-16 [167]. In another study by Tong et al. identified 32 miRNAs in HPV positive, in which 5 viz. “miR-92b-5p, miR-92b-3p, miR-193a-5p, miR-193b-5p, and miR-1306-5p” were up-regulated and “miR-548a-3p, miR-1269b, miR-3129-5p, miR-3180-5p, and miR-3180-3p” were down-regulated. Further, in HPV+ cells 70 miRNAs were expressed including “miR-34a-5p, miR-199b-5p, miR-193a-3p, miR-193a-5p, and miR-365b-5p”, while “miR-431-5p, miR-432-5p, miR-816a-5p, miR-3180-5p, and miR-3180-3p” were down-regulated [168]. All these findings suggested a potential contribution of miRNA expression deregulation association to HPV cervical cancer. A bioinformatics study identified different HPV types pre-miRNAs, in which HPV16 showed the coding potential for HPV16-miR-1, HPV16-miR-2, and HPV16-miR-3 located at E6, E1, and L2 ORFs, respectively. ARID5B, ZEB2, THBS1, STAT5B genes were predicted as targets of HPV16-miR-1. RID5B, ZEB2, THBS1 were responsible for cell motility and migration, and STAT5B for cell adhesion. SYNE1, PDE1B, GATA6, and GULP1 were predicted as targets of HPV16-miR-3, responsible of cell death. However, the targets of HPV16-miR-2 i.e. “AFF3, FRMD7, IGDCC4, MYRIP, NRN1, PMP22, RBPMS” were predicted to not responsible for cervical cancer progression. The data suggests that, viral miRNAs may aid in the evasion of the host immune response by promoting the latent phase of the HPV life cycle, hence raising the risk of cancer progression [169].
Cervical stem cells as biomarker
Latest findings speculate that cervical cancer grows in the transformation zone of the cervical opening infected with HPV from “stem-like cells” [170]. PSCA, PIWIL1, HPV16 E7, and transcriptional factor TBX2 are genes associated with stem cells shown to be expressed in cervical cancer stem cells (CCSC). This junction may be a potential CCSC niche. Increased expression of PSCA (60%), PIWIL1 (70%), and TBX2 (50%) was seen in CCSC as compared to normal cervix cells. PSCA and PIWIL1 exhibited increased levels, and this remained consistent with invasions of CCSC. TBX2 expression was, on the other hand, upregulated and had a favorable correlation with lymph node metastasis. Thus, a strong association was seen between PIWIL1, PSCA, TBX2, and HPV16. A Piwil2-like (PL2L) protein that is produced in human primary and metastatic cervical cancers in combination with nuclear factor kappa beta (NF-Xb) known to induce carcinogenesis has recently been identified. In vitro or in vivo models have been used to indicate the definitive role of HPV16 in PL2L, PSCA, PIWIL1, and TBX2 over-expression [171–174].
Nucleostemin, Musashi1, nanog and p63
The undifferentiated embryonic stem cells produce, in large concentrations, SC proteins such as Nanog, nucleostemin (NS), and Musashi1 (Msi1). Such proteins in a negative feedback system are known to control SC differentiation and proliferation. In tissue samples of cervical cancer and cervical dysplasia, immunohistochemical experiments have demonstrated significantly increased expression of Nanog in contrast to the normal cervical epithelium, validating its involvement in carcinogenesis of cervical cancer disease and the advancement of the disease [174]. In reserve cell hyperplasia and CIN lesions (I to III), expression of p63 (nuclear) and CK17 (cytoplasmic) have been noticed. Several other studies affirm the production of p63 in metaplastic squamous cells and sub columnar reserve cells of TZ. Expression of p63 inversely correlates with squamous cell maturation and non-squamous cell differentiation. In turn, this causes multiplication of cancer cells, support-dependent growth, and clonogenicity, suggesting that p63 is a CSC marker in the epithelial tissue (a possible tumor-initiating agent in the cancer cell) [175].
Aldehyde dehydrogenase
Yet another valuable CCSC marker is aldehyde dehydrogenase (ALDH1), an enzyme that is active in retinol oxidation and the initial phases of stem cell differentiation [176]. Cervical cancer cells that express ALDH1 are linked with a high degree of cell multiplication and carcinogenesis. In vivo experiments have demonstrated that cells that express increased levels of ALDH have an increased capacity for carcinogenicity, suggesting that it functions as a stemness factor in cervical cancer. Studies have reported that ALDH1 is linked with poorer clinical results [177, 178]. ALDH1 is classified as a cervical stem cell biomarker which suggests that cervical neoplasia has a group of cells that have increased levels of ALDH which shares functions with CSC [178].
CD49F
CD49F, a cell surface protein, is expressed in mesenchymal, developmental, and hemopoietic SCs [179]. Experiments have demonstrated that CD49F is overexpressed in breast cancer, as well as in other types of cancer, such as gastric, bowel, and prostate cancers, and it preserves the stemness of these cancer cells. Also, cervical CSC tumor spheroids with elevated CD49F levels have been reported to be resistant to radiation therapy. These discoveries indicate that CCSCs are actively involved in tumorigenesis, and these cells may prove to be useful as a therapeutic target, but several issues remain unresolved as stem cell therapy is a relatively growing field of research [176–182].
OCT4 and SOX2
SOX2, is a member of the SOX gene family, plays a role in cell fate and differentiation by encoding transcription factors that are expressed in embryonic development. The difference in expression levels of SOX2 in normal and pathological tissues i.e., in tumorspheres proves that it plays a significant role in carcinogenesis, indicating that it may be a potential biomarker. Also, OCT4 (POU5fl), a POU-domain transcription factor, is another pluripotency-related marker that is known to be overexpressed in cervical cancer stem cells. High OCT4 expression of cervical cancer cells is associated with low cervical cancer cell differentiation and possible metastasis of the lymph nodes. Overexpression of OCT4 is consistent with tolerance to chemo-radio resistance and is a specific risk factor for the survival of patients having cervical cancer disease [179].
ABCG2
An ATP-binding cassette (ABC) subfamily G member 2 known as ABCG2 is another potential marker for cervical cancer lesions. It functions as a drug efflux membrane transporter for the ABC family and is known to have a critical role in multidrug resistance in different malignancies because it can expel out several cell chemicals. Nrf2, a redox sensing factor, is majorly involved in the transcriptional modulation of ABCG2 in cervical cancer. In cells that have stem-like properties, such as indefinite cell multiplication, survival, and prevention of cell death, the upregulation of Nrf2 and ABCG2 is noticed. Cells’ capacity to develop resistance to several medicines is also mediated by ATP-binding cassette (ABC) transporter overexpression that eliminates drugs from the cells and lowers the levels of intracellular drugs. The gene and protein levels of ABCG2 are known to be regulated by the expression of SOX4. Thus, ABCG2 may be a possible marker in cervical cancer [170].
Latest studies have shown that cervical stem cells may have important implications in cervical cancer treatment and therapy. These biomarkers may help to identify patients who are likely to develop the disease and hence facilitate early detection and thus help in choosing the right care and also in tracking the response of patients. It is rather an emerging field of research and is at its initial stage due to challenges such as resistance of CSC to radiation and its heterogenic nature. However, owing to the advancement in the field of molecular sciences that enables scientists to detect tumor cells, the existence of CSCs is becoming increasingly persuasive [170].
Conclusions
Cervical cancer is linked with significant morbidity and mortality around the globe. Early diagnosis and cure of pre-malignant lesions can discourage the development of cervical cancer and reduce fatal consequences. Cytological screening of cervical cells has mainly been used to detect precancerous lesions. However, cellular anomalies may be ignored or may not be properly distinguished, and higher-grade diseases may be detected only by subsequent colposcopy and biopsy especially in patients with borderline or moderate dyskaryotic cytomorphology. The need for novel diagnostic and therapeutic approaches for cervical cancer is demonstrated by the data indicating the occurrence and high mortality rates in cervical cancer. The establishment of effective biomarkers is necessary for cervical cancer screening to detect women at risk of contracting cancer at a point that still allows for effective treatment before an invasive tumor develops. The study of the main molecular events implicated in cervical carcinogenesis has led to the development of some promising biomarkers such as those based on chromosomal irregularity. Numerous protein biomarkers have also been identified for the diagnosis of cervical cancer disease. Many of these proteins are implicated in the modulation of the cell cycle, signal transduction, replication of DNA, and proliferation of cells. It is estimated that about 90% of human genes are regulated by miRNAs. In nearly any biological process, MiRNAs can have remarkable effects on gene expression and several studies suggest that miRNAs are actively engaged in cancer pathogenesis; hence measuring their level of expression can be useful to distinguish between the different stages of cancer and its treatment. Cervical cancer grows in the transformation zone of the cervical cavity infected with HPV from “stem-like cells”. Stem cells have a property of cancer self-renewability, and thus comparatively fewer mutations are required for neoplastic conversion than their mature counterparts. The standard treatment for early-stage cervical cancer varies from lesion removal to absolute hysterectomy but it is harder to handle later-stage tumors which have spread to other organs. Thus, a big problem in cancer research has been finding efficient therapies for later-stage cancers. In addition to introducing novel screening methods, evaluating their adherence and effectiveness over time will help to optimize the benefits of cancer prevention strategies.
Acknowledgements
The authors thank Mr. Akash Saxena and the Principal of KLE College of Pharmacy, KLE Academy of Higher Education and Research, Belagavi for encouragement of the work.
Author contributions
PSO and ST are the main workers, contributed equally in conceptualization, literature review and manuscript writing. MMM supervised the study, provided inputs and critically revised the manuscript. VSP co-supervised the study, literature review, provided inputs, and critically revised the manuscript. All the authors read and finalized the manuscript.
Funding
Not applicable.
Availability of data and material
Not applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Prachi S. Ojha and Siddarth Tubachi authors contributed equally to this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Prachi S. Ojha, Email: pojha875@gmail.com
Meenaxi M. Maste, Email: menaimm@gmail.com, Email: meenaximaste@klepharm.edu
Siddarth Tubachi, Email: siddarthtubachi18@gmail.com.
Vishal S. Patil, Email: vishalpatil6377@gmail.com
References
- 1.Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, Mirzaei H, Pourhanifeh M, Bokharaei F, Mirzaei H, Hamblin M. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. Int J Cancer. 2020;15(2):305–20. doi: 10.1002/ijc.32688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbyn M, Weiderpass E, Bruni L, Sanjosé S, Saraiya M, Ferlay J, Bray F Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. The Lancet. February 1, 2020;8(2):E19.1–E203, DOI: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed]
- 3.Yang H-J, Xue J‐M, Li J, Wan L‐H, Zhu Y‐X. Identification of key genes and pathways of diagnosis and prognosis in cervical cancer by bioinformatics analysis. Mol Genet Genomic Med. 2020;8:e1200. doi: 10.1002/mgg3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda GA, Zhou Y, Jin T. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Public Health Front 2021, 1003. 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed]
- 5.Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405–14. doi: 10.2147/IJWH.S50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahasrabuddhe VV, Ramesh AB, Smita NJ, et al. Prevalence and predictors of colposcopic-histopathologically confirmed cervical intraepithelial neoplasia in HIV-infected women in India. PLoS ONE. 2010;5(1):e8634. [DOI] [PMC free article] [PubMed]
- 7.Kamalesh S, Reshmi P, Baishali B, Bibhuti S, Subhasish B. Oncogenic HPV among HIV infected female population in West Bengal, India. BMC Infect Dis. 2011;11:72. doi: 10.1186/1471-2334-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta P, Bhatla N, Pandey RM, et al. Type-specific incidence and persistence of HPV infection among young women: a prospective study in North India. Asian Pac J Cancer Prev. 2012;13(3):1019–24. doi: 10.7314/APJCP.2012.13.3.1019. [DOI] [PubMed] [Google Scholar]
- 9.Bhatla N, Lal N, Bao YP, et al. A meta-analysis of human papillomavirus type-distribution in women from South Asia: implications for vaccination. Vaccine. 2008;26(23):2811–7. doi: 10.1016/j.vaccine.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Sauvaget C, Nene B, Jayant K, et al. Prevalence and determinants of high-risk human papillomavirus infection in middle-aged Indian women. Sex Transm Dis. 2011;38(10):902–6. doi: 10.1097/OLQ.0b013e318223be5f. [DOI] [PubMed] [Google Scholar]
- 11.Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther. 2011;1(3):295–306. doi: 10.4161/cbt.11.3.14686. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan S, Jaggi M, Bell M, Verma M, Kumar D. Epidemiology of human papillomavirus in cervical mucosa. Methods Mol Bio. 2009;471:439–56. doi: 10.1007/978-1-59745-416-2_22. [DOI] [PubMed] [Google Scholar]
- 13.Lizano M, Berumen J, Garcia-Carranca A. HPV-related carcinogenesis: Basic concepts, viral types and variants. Arch Med Res. 2009;40:428–34. doi: 10.1016/j.arcmed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.De Villiers E, Fauquet C, Broker T, Bernard H. Zur Hausen, H. Classification of papillomaviruses. Virol. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Zur Hausen H. Papillomaviruses in the causation of human cancers—a brief historical account. Virol. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman M, Rodriguez A, Chen Z, Wacholder S, Herrero R, Hildesheim A, Desalle R, Befano B, Yu K, Safaeian M, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, Solomon D, Castle PE, Burk R. D. A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence and cervical neoplasia. Cancer Res. 2010;70:3159–69. doi: 10.1158/0008-5472.CAN-09-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einstein M, Schiller J, Viscidi R, Strickler H, Coursaget P, Tan T, Halsey N, Jenkins D. Clinician’s guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis. 2009;9:347–56. doi: 10.1016/S1473-3099(09)70108-2. [DOI] [PubMed] [Google Scholar]
- 19.Mirabello L, Yeager M, Cullen M, Boland JF, Chen Z, Wentzensen N, et al. HPV16 sublineage associations with histology-specific cancer risk using HPV whole-genome sequences in 3200 women. J Natl Cancer Inst. 2016;JNCI(9):djw100. doi: 10.1093/jnci/djw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho G, Bierman R, Beardsley L, Chang C, Burk R. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 21.Lehoux M, D’Abramo C, Archambault J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Pub Health Genomics. 2009;12:268–80. doi: 10.1159/000214918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci. 2006;110:525–41. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 23.Hebner C, Laimins L. Human papillomaviruses: Basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16:83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- 24.Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virol J. 2010;7:1–7. doi: 10.1186/1743-422X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giroglou T, Florin L, Schafer F, Streeck R, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–7. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamid N, Brown C, Gaston K. The regulation of cell proliferation by the papillomavirus early proteins. Cell Mol Life Sci. 2009;66:1700–17. doi: 10.1007/s00018-009-8631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubenrauch F, Laiminis L. Human papillomavirus life cycle: active and latent phases. Sem Cancer Biol. 1999;9:379–86. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- 28.Yajid AI, Zakariah MA, Zin AA, Othman NH. Potential role of E4 protein in human papillomavirus screening: A review. APJCP. 2017;18(2):315. doi: 10.22034/APJCP.2017.18.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell I, Martin A, Roberts S. The E1circumflexE4 protein of human papillomavirus interacts with the serine-arginine-specific protein kinase SRPK1. J Virol. 2007;81(11):5437–48. doi: 10.1128/JVI.02609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullen A, Reid R, Campion M, Lörincz AT. Analysis of the physical state of different human papillomavirus RNAs in intraepithelial and invasive cervical neoplasia. J Virol. 1991;65:606–12. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz E, Freese U, Gissman L, Mayer W, Roggenbuck B, Stremlau A, Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–4. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 32.Howie H, Katzenellenbogen R, Galloway D. Papillomavirus E6 proteins. Virol. 2009;384:324–34. doi: 10.1016/j.virol.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 34.Wise-Draper T, Wells S. Papillomavirus E6 and E7 proteins and their cellular targets. Front Biosci. 2008;13:1003–17. doi: 10.2741/2739. [DOI] [PubMed] [Google Scholar]
- 35.Scheffner M, Huibregtse J, Vierstra R, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. [DOI] [PubMed]
- 36.Martinez-Lagunas A, Madrid-Marina V, Gariglio P. Modulation of apoptosis by early human papillomavirus proteins in cervical cancer. Biochim Biophys Acta. 2010;1805:6–16. doi: 10.1016/j.bbcan.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin-Drubin M, Munger K. The human papillomavirus E7 oncoprotein. Virol. 2009;384:335–44. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem. 2006;281:578–86. doi: 10.1074/jbc.M508455200. [DOI] [PubMed] [Google Scholar]
- 39.Ganguly N, Parihar S. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci. 2009;34:113–23. doi: 10.1007/s12038-009-0013-7. [DOI] [PubMed] [Google Scholar]
- 40.Mammas I, Sourvinos G, Giannoudis A, Spandidos D. Human papillomavirus (HPV) and host cellular interactions. Pathol Oncol Res. 2008;14:345–54. doi: 10.1007/s12253-008-9056-6. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Deivendran S, Marzook KH, Radhakrishna Pillai M. The role of inflammation in Cervical Csancer. Adv Exp Med Bio. 2014;816:377–99. doi: 10.1007/978-3-0348-0837-8_15. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Chen GT, Wang YQ, Xian S, Zhang L, Zhu SM, Pan F, Cheng YX. TLR4 promotes the expression of HIF-1α by triggering reactive oxygen species in cervical cancer cells in vitro-implications for therapeutic intervention. Mol Med Rep. 2018;17:2229–38. doi: 10.3892/mmr.2017.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng L, Zhen Y, Chen Y, Zou L, Zhang Y, Hu F, Feng J, Shen J, Wei B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NFκB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int J Oncol. 2014;45:1929–36. doi: 10.3892/ijo.2014.2617. [DOI] [PubMed] [Google Scholar]
- 46.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–64. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 47.Paradkar PH, Joshi JV, Mertia PN, Agashe SV, Vaidya RA. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac J Cancer Prev. 2014;15:3851–64. doi: 10.7314/APJCP.2014.15.9.3851. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Zhang J, Cui ZM, Zhao J, Zheng Y. Expression of the CXCL12/CXCR4 and CXCL16/CXCR6 axes in cervical intraepithelial neoplasia and cervical cancer. Chin J Cancer. 2013;32:289–96. doi: 10.5732/cjc.012.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayshree RS, Sreenivas A, Tessy M, Krishna S. Cell intrinsic and extrinsic factors in cervical carcinogenesis. Indian J Med Res. 2009;130:286–95. [PubMed] [Google Scholar]
- 50.Feng Q, Wei H, Morihara J, Stern J, Yu M, Kiviat N, Hellstrom I, Hellstrom KE. Th2 type inflammation promotes the gradual progression of HPV-infected cervical cells to cervical carcinoma. Gynecol Oncol. 2012;127:412–9. doi: 10.1016/j.ygyno.2012.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim W, Pyo J, Noh B-J, Jeong JW, Lee J, Kim JE. CCAR2 negatively regulates IL-8 production in cervical cancer cells. Oncotarget. 2018;9:1143. doi: 10.18632/oncotarget.23199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Y, Fang F, Dhar SK, Bosch A, St Clair WH, Kasarskis EJ, St Clair DK. Mutations in the SOD2 promoter reveal a molecular basis for an activating protein 2-dependent dysregulation of manganese superoxide dismutase expression in cancer cells. Mol Cancer Res. 2008;6(12):1881–93. doi: 10.1158/1541-7786.MCR-08-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallagher CJ, Ahn K, Knipe AL, Dyer AM, Richie JP, Lazarus P, Muscat J. Association between haplotypes of manganese superoxide dismutase (SOD2), smoking, and lung cancer risk. Free Radic Biol Med. 2009;46(1):20–4. doi: 10.3892/br.2020.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorestani S, Hashemy SI, Mojarad M, Shahrestanaki M, Bahari A, Asadi M, Avval F. Increased glutathione reductase expression and activity in colorectal cancer tissue samples: an investigational study in Mashhad, Iran. Middle East J Cancer. 2018;9:99–104. doi: 10.30476/mejc.2018.42111. [DOI] [Google Scholar]
- 56.Pakfetrat A, Dalirsani Z, Hashemy SI, Ghazi A, Mostaan LV, Anvari K, Pour AM. Evaluation of serum levels of oxidized and reduced glutathione and total antioxidant capacity in patients with head and neck squamous cell carcinoma. J Cancer Res Ther. 2018;14:428–31. doi: 10.4103/0973-1482.189229. [DOI] [PubMed] [Google Scholar]
- 57.Sobhani M, Taheri AR, Jafarian AH, Hashemy SI. The activity and tissue distribution of thioredoxin reductase in basal cell carcinoma. J Cancer Res Clin Oncol. 2016;142:2303–7. doi: 10.1007/s00432-016-2242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kruk J, Aboul-Enein HY. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev Med Chem. 2017;17:904‐919, doi: 10.2174/1389557517666170228115324. [DOI] [PubMed]
- 59.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashemy SI. The human thioredoxin system: modifications and clinical applications. Iran J Basic Med Sci. 2011;14:191–204. doi: 10.1074/jbc.M710133200. [DOI] [Google Scholar]
- 62.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 63.Silva GÁF, Nunes RAL, Morale MG, Boccardo E, Aguayo F, Termini L. Oxidative stress: therapeutic approaches for cervical cancer treatment. Clin (Sao Paulo). 2018;73, doi:10.6061/clinics/2018/e548s. [DOI] [PMC free article] [PubMed]
- 64.Naidu MSK, Suryakar AN, Swami SC, Katkam RV, Kumbar KM. Oxidative stress and antioxidant status in cervical cancer patients. Indian J Clin Biochem. 2007;22:140–4. doi: 10.1007/BF02913333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palan PR, Mikhail MS, Basu J, Romney SL. 1991. Plasma levels of antioxidant β-carotene and α‐tocopherol in uterine cervix dysplasias and cancer. Nutrition and Cancer. 1991;15(1), 13–20. [DOI] [PubMed]
- 66.De Marco F. Oxidative stress and HPV carcinogenesis. Viruses. 2013;5:708–31. doi: 10.3390/v5020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV‐ DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol. 2011;6:45‐57. doi: 10.2217/fvl.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 69.Ponath V, Kaina B. Death of monocytes through oxidative burst of macrophages and neutrophils: killing in trans. PLoS ONE. 2017;12:e0170347. doi: 10.1371/journal.pone.0170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Wongworawat Y, Filippova M, Williams VM, Filippov V, Duerksen-Hughes PJ. Chronic oxidative stress increases the integration frequency of foreign DNA and human papillomavirus 16 in human keratinocytes. Am J Cancer Res. 2016;6:764‐780. [PMC free article] [PubMed] [Google Scholar]
- 71.Surh YJ. Transcriptional regulation of cellular antioxidant defense mechanisms. In: Surh Y-J, Packer L, editors. Oxidative stress, inflammation and health. Boca Raton: CRC Press LLC; 2005. p. 21‐40. [Google Scholar]
- 72.Peitsaro P, Hietanen S, Johansson B, Lakkala T, Syrjänen S. Single copy heterozygote integration of HPV 33 in chromosomal band 5p14 is found in an epithelial cell clone with selective growth advantage. Carcinogenesis. 2002;23(6):1057–64. doi: 10.1093/carcin/23.6.1057. [DOI] [PubMed] [Google Scholar]
- 73.Pett MR, Herdman MT, Palmer RD, Yeo GS, Shivji MK, Stanley MA, Coleman N. Selection of cervical keratinocytes containing integrated HPV16 associates with episome loss and an endogenous antiviral response. Proc Natl Acad Sci U S A. 2006;103(10):3822–7. doi: 10.1073/pnas.0600078103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao C, Hughes JP, Kiviat N, Kuypers J, Lee SK, Adam DE, Koutsky LA. Clinical findings among young women with genital human papillomavirus infection. Am J Obstet Gynecol. 2003;188(3):677–84. doi: 10.1067/mob.2003.164. [DOI] [PubMed] [Google Scholar]
- 75.Ault KA Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect Dis Obstet Gynecol, 2006;2006:5 pages, doi: 10.1155/IDOG/2006/40470. [DOI] [PMC free article] [PubMed]
- 76.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7:79–89. doi: 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- 77.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;30:16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32:7–15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(2):15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Giannini SL, Hubert P, Doyen J, Boniver J, Delvenne P. Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix. Int J Cancer. 2002;97(5):654–9. doi: 10.1002/ijc.10084. [DOI] [PubMed] [Google Scholar]
- 81.Tay SK, Jenkins D, Maddox P. Subpopulations of Langerhans’ cells in cervical neoplasia. Br J Obstet Gynaecol. 1987;94(1):10–5. doi: 10.1111/j.1471-0528.1987.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 82.Fausch SC, Da Silva DM, Kast WM. Differential uptake and cross-presentation of human papillomavirus virus like particles by dendritic cells and Langerhans cells. Cancer Res. 2003;63(13):3478–82. [PubMed] [Google Scholar]
- 83.Hacke K, Rincon-Orozco B, Buchwalter G, Siehler S, Wasylyk B, Wiesmuller L, Rosl F. Regulation of MCP-1 chemokine transcription by p53. Mol Cancer. 2010;9:82. doi: 10.1186/1476-4598-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3α production. J Virol. 2005;79(23):14852–62. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 86.Mazibrada J, Ritta M, Mondini M, Andrea M, Azzimonti B, Borgogna C, Ciotti M, Orlando A, Surico N, Chiusa L, Landolfo S, Gariglio M. Interaction between inflammation and angiogenesis during different stages of cervical carcinogenesis. Gynecol Oncol. 2008;108(1):112–20. doi: 10.1016/j.ygyno.2007.08.095. [DOI] [PubMed] [Google Scholar]
- 87.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4(4):376–89. [PMC free article] [PubMed] [Google Scholar]
- 88.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138(2):93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, Syrjänen KJ, Cunha-Filho JS. Macrophages, inflammation and risk of cervical intraepithelial neoplasia (CIN) progression-Clinicopathological correlation. Gynecol Oncol. 2007;105(1):157–65. doi: 10.1016/j.ygyno.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 90.Lepique AP, Kaghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. 2009;15(13):4391–400. doi: 10.1158/1078-0432.CCR-09-0489. [DOI] [PubMed] [Google Scholar]
- 91.Bolpetti A, Silva JS, Villa LL, Lepique AP. Interleukin10 production by tumor infiltrating macrophages plays a role in Human Papillomavirus 16 tumor growth. BMC Immunol. 2010;11:27. doi: 10.1186/1471-2172-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Iglesias, Toro-Arreola T, Albarran-Somoza B, Toro-Arreola S, Sanchez-Hernandez P, Ramirez-Duenas M, Balderas-Pena LMA, Bravo-Cuellar A, Ortiz-Lazareno P, Daneri-Navarro A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steele JC, Mann CH, Rookes S, Rollason T, Murphy D, Freeth MG, Gallimore PH, Roberts T-cell responses to human papillomavirus type 16 among women with different grades of cervical neoplasia. Br J Cancer. 2005;93(2):248–59. doi: 10.1038/sj.bjc.6602679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Correll A, Tuettenberg A, Becker C, Jonuleit H. Increased regulatory T-cell frequencies in patients with advanced melanoma correlate with a generally impaired T-cell responsiveness and are restored after dendritic cell-based vaccination. Experimental Dermatology. 2010;19(8):e213–21. doi: 10.1111/j.1600-0625.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 95.French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3 + regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metabolism. 2010;95(5):2325–33. doi: 10.1210/jc.2009-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 97.Viscidi RP, Schiffman M, Hildesheim A, Herrero R, Castle PE, Bratti MC, Rodriguez AC, Sherman ME, Wang S, Clayman B, Burk RD. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13(2):324–7. doi: 10.1158/1055-9965.EPI-03-0166. [DOI] [PubMed] [Google Scholar]
- 98.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 99.Mao C, Hughes JP, Kiviat N, Kuypers J, Lee SK, Adam DE, Koutsky LA. Clinical findings among young women with genital human papillomavirus infection. Am J Obstet Gynecol. 2003;188:677–84. doi: 10.1067/mob.2003.164. [DOI] [PubMed] [Google Scholar]
- 100.Dewar MA, Hall K, Perchalski J. Cervical cancer screening. Past success and future challenge. Prim Care. 1992;19:589–606. doi: 10.1016/S0095-4543(21)00940-4. [DOI] [PubMed] [Google Scholar]
- 101.Gheit T, Vaccarella S, Schmitt M, Pawlita M, Franceschi S, Sankaranarayanan R, Sylla BS, Tommasino M, Gangane N. Prevalence of human papillomavirus types in cervical and oral cancers in central India. Vaccine. 2009;27:636–9. doi: 10.1016/j.vaccine.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 102.Bast RC, Jr, Lilja H, Urban N, Rimm D, Fritsche H, Gray J, Veltri R, Klee G, Allen A, Kim N, Gutman S, Rubin M, Hruszkewycz A. Translational crossroads for biomarkers. Clin Cancer Res. 2005;11:6103–8. doi: 10.1158/1078-0432.CCR-04-2213. [DOI] [PubMed] [Google Scholar]
- 103.Wang E, Panelli MC, Marincola FM. Genomic analysis of cancer. Princ Pract Oncol. 2003;17:1–16. [Google Scholar]
- 104.Upender M, Rashmi-Gopal S, Sudhir S. Recent advances in biomarkers for cancer diagnosis and treatment. Drug Discov Today. 2005;10:965–72. doi: 10.1016/S1359-6446(05)03487-2. [DOI] [PubMed] [Google Scholar]
- 105.Guo X, Hao Y, Kamilijiang M, Hasimu A, Yuan J, Wu G, Reyimu H, Kadeer N, Abudula A. Potential predictive plasma biomarkers for cervical cancer by 2D-DIGE proteomics and Ingenuity Pathway Analysis. Tumour Biol. 2015 Mar;36(3):1711–20. [DOI] [PubMed]
- 106.Wang X, Zheng B, Li S, Zhang R, Mulvihill J, Chen W, Liu H. Automated detection and analysis of fluorescent in situ hybridization spots depicted in digital microscopic images of Pap-smear specimens. J Biomed Opt. 2009;14:2. doi: 10.1117/1.3081545. [DOI] [PubMed] [Google Scholar]
- 107.Heselmeyer-Haddad K, Sommerfeld K, White NM, Chaudhri N, Morrison L, Palanisamy N, Wang Z, Auer G, Steinberg W, Ried W. Genomic amplification of the human telomerase gene (TERC) in Pap smears predicts the development of cervical cancer. Am J Pathol. 2005;166(4):1229–38. doi: 10.1016/S0002-9440(10)62341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allen DG, White DJ, Hutchins AM, Scurry JP, Tabrizi SN, Garland SM, Armes JE. Progressive genetic aberrations detected by comparative genomic hybridization in squamous cell cervical cancer. Br J Cancer. 2000;83(12):1659–63. doi: 10.1054/bjoc.2000.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caraway NP, Khanna A, Dawlett M, Guo M, Guo N, Lin E, Katz R. Gain of the 3q26 region in cervicovaginal liquid-based pap preparations is associated with squamous intraepithelial lesions and squamous cell carcinoma. Gynecologic Oncol. 2008;110(1):37–42. doi: 10.1016/j.ygyno.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 110.Santin AD, Zhan F, Bignotti E, Siegel E, Cané E, Bellone S, Palmieri M, Anfossi S, Thomas M, Burnett A, Kay H, Roman J, O’Brien T, Tian E, Cannon M, Shaughnessy J, Pecorelli S. Gene expression profiles of primary HPV16- and HPV18-infected early-stage cervical cancers and normal cervical epithelium: identification of novel candidate molecular markers for cervical cancer diagnosis and therapy. Virology. 2005;331(2):269–91. doi: 10.1016/j.virol.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 111.Murphy N, Ring M, Heffron CC, King B, Killalea AG, Hughes C, Guinness E, Sheils O, Leary, J.J. p16INK4A, CDC6, and MCM5: predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. Journal of Clinical Pathology. 2005;58(5):525–534. [DOI] [PMC free article] [PubMed]
- 112.Malinowski DP. Molecular diagnostic assays for cervical neoplasia: emerging markers for the detection of high-grade cervical disease. BioTechniques. 2005;17–23. [DOI] [PubMed]
- 113.Shroyer KR, Homer P, Heinz D, Singh M. Validation of a novel immunocytochemical assay for topoisomerase IIα and minichromosome maintenance protein 2 expression in cervical cytology. Cancer. 2006;108(5):324–30. doi: 10.1002/cncr.22171. [DOI] [PubMed] [Google Scholar]
- 114.Oberg TN, Kipp BR, Vrana JA, Bartholet MK, Fales CJ, Garcia R, cDonald AN, Rosas BL, Henry MR, Clayton AC. Comparison of p16INK4a and ProEx C immunostaining on cervical ThinPrep cytology and biopsy specimens. Diagn Cytopathol. 2010;38(8):564–72. doi: 10.1002/dc.21251. [DOI] [PubMed] [Google Scholar]
- 115.Conesa-Zamora P, Domenech-Peris A, Orantes-Casado FJ, Ortiz-Reina S, Sahuquillo-Frías L, Acosta-Ortega J, García-Solano J, Pérez-Guillermo M. Effect of human papillomavirus on cell cycle-related proteins p16, Ki-67, cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: a tissue microarray study. Am J Clin Pathol. 2009;132(3):378–90. doi: 10.1309/AJCPO0WY1VIFCYDC. [DOI] [PubMed] [Google Scholar]
- 116.Valdez M, Jeronimo J, Bansil P, Qiao YL, Zhao FH, Chen W, Zhang X. Effectiveness of novel, lower cost molecular human papillomavirus-based tests for cervical cancer screening in rural china. Int J Cancer. 2016;15(6):1453–61. doi: 10.1002/ijc.29877. [DOI] [PubMed] [Google Scholar]
- 117.Wentzensen N, Bergeron C, Cas F, Eschenbach D, Vinokurova S, von Doeberitz K. Evaluation of a nuclear score for p16INK4a-stained cervical squamous cells in liquid-based cytology samples. Cancer. 2005;105:461–7. doi: 10.1002/cncr.21378. [DOI] [PubMed] [Google Scholar]
- 118.Syrjanen KJ. Immunohistochemistry in assessment of molecular pathogenesis of cervical carcinogenesis. Eur J Gynaecol Oncol. 2005;26:118–24. [PubMed] [Google Scholar]
- 119.Schiffman MH, Bauer HM, Hoover RN, Glass A, Cadell D, Rush B, Scott D, Sherman M, Kurman R, Wacholder S, Stanton C, Manos M. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. JNCI: J Natl Cancer Inst. 1993;85(12):958–64. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 120.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 121.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2536–45. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212–422. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tringler B, Gup CJ, Singh M, Groshong S, Shroyer L, Heinz D, Shroyer K. Evaluation of p16INK4a and pRb expression in cervical squamous and glandular neoplasia. Hum Pathol. 2004;35(6):689–96. doi: 10.1016/j.humpath.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS) Arch Pathol Lab Med. 2003;127(8):946–9. doi: 10.5858/2003-127-946-FTDFTA. [DOI] [PubMed] [Google Scholar]
- 125.Davies P, Kornegay J, Iftner T. Current methods of testing for human papillomavirus. Best Pract Res Clin Obstet Gynaecol. 2001;15(5):677–700. doi: 10.1053/beog.2001.0214. [DOI] [PubMed] [Google Scholar]
- 126.Johnson LR, Starkey CR, Palmer J, Taylor J, Stout S, Holt S, Hendren R, Bock B, Waibel E, Tyree G, Miller G. A comparison of two methods to determine the presence of high-risk HPV cervical infections. Am J Clin Pathol. 2008;130(3):401–8. doi: 10.1309/4DXEAFG2JXYF34N3. [DOI] [PubMed] [Google Scholar]
- 127.Bollmann R, Bollmann M, Henson DE, Bodo M. DNA cytometry confirms the utility of the Bethesda System for the classification of Papanicolaou smears. Cancer. 2001;93(3):222–8. doi: 10.1002/cncr.9033. [DOI] [PubMed] [Google Scholar]
- 128.Bollmann R, Mehes G, Torka R, Speich N, Schmitt C, Bollmann M. Human papillomavirus typing and DNA ploidy determination of squamous intraepithelial lesions in liquid-based cytologic samples. Cancer. 2003;99(1):57–62. doi: 10.1002/cncr.10953. [DOI] [PubMed] [Google Scholar]
- 129.Lorenzato M, Caudroy S, Nou J, Dalstein V, Joseph K, Bellefqih S, Durlach A, Thil C, Dez F, Bouttens D, Clavel C, Birembaut P. Contribution of DNA ploidy image cytometry to the management of ASC cervical lesions. Cancer. 2008;114:263–9. doi: 10.1002/cncr.23638. [DOI] [PubMed] [Google Scholar]
- 130.Grote HJ, Nguyen HV, Leick AG, Bocking A. Identification of progressive cervical epithelial cell abnormalities using DNA image cytometry. Cancer. 2004;102(6):373–9. doi: 10.1002/cncr.20644. [DOI] [PubMed] [Google Scholar]
- 131.Dasari S, Shouri RND, Rajendra W, Valluru L. Effect of concurrent radiochemotherapy and chemotherapy on serum proteins for prospective predictors of patients with HPV induced cervical cancer. Biomed Pharmacother. 2014;68:315–20. doi: 10.1016/j.biopha.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 132.Di Saia PJ, Creasman WT Clinical Gynecologic Oncology. 6th ed. St. Louis, MO, USA: Mosby. 2002:53–112.
- 133.Takeda M, Sakuragi N, Okamoto K, Todo Y, Minobe S, Nomura E, Negishi H, Oikawa M, Yamamoto R, Fujimoto S. Preoperative serum SCC, CA125 and CA19-9 levels and lymph node status in squamous cell carcinoma of the uterine cervix. Acta Obstet Gynecol Scand. 2002;81:451–7. doi: 10.1034/j.1600-0412.2002.810513.x. [DOI] [PubMed] [Google Scholar]
- 134.Gaarenstroom KN, Kenter GG, Bonfrer JMG, Korse C, Vijver M, Fleuren G-J, Trimbos B. Can initial serum CYFRA 21–1, SCC antigen and TPA levels in squamous cell cervical cancer predict lymph node metastases or prognosis? Gynecol Oncol. 2000;77:164–70. doi: 10.1006/gyno.2000.5732. [DOI] [PubMed] [Google Scholar]
- 135.Chou CY, Wang ST, Kuo HC, Tzeng CC, Yao BL. Serum level of squamous cell carcinoma antigen and tumor size is useful to identify preoperatively patients at high risk of cervical cancer. Cancer. 1994;74:2497–501. doi: 10.1002/1097-0142(19941101)74:9<2497::AID-CNCR2820740917>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 136.Bolli JA, Doering DL, Bosscher JR, Day T, Rao CV, Owens K, Kelly B, Goldsmith J. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–73. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- 137.Dasari S, Wudayagiri R, Valluru L. Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim Acta. 2015;445:7–11. doi: 10.1016/j.cca.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 138.Battaglia F, Scambia G, Panici PB, Castelli M, Ferrandina G, Foti E, Amoroso M, D’Andrea G, Mancuso S. Immunosuppressive acidic protein (IAP) and squamous cell carcinoma antigen (SCC) in patients with cervical cancer. Gynecol Oncol. 1994;53:176–82. doi: 10.1006/gyno.1994.1112. [DOI] [PubMed] [Google Scholar]
- 139.Gadducci A, Elena GM, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol. 2013;86:104–29. doi: 10.1016/j.critrevonc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 140.Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, Dannenberg AJ. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–9. [PubMed] [Google Scholar]
- 141.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/ cyclooxygenase homologue. J Biol Chem. 1991;266:12866–72. doi: 10.1016/S0021-9258(18)98774-0. [DOI] [PubMed] [Google Scholar]
- 142.DuBois RN, Awad J, Morrow J, Roberts LJ, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-a and phorbol ester. J Clin Investig. 1994;93:493–8. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, Dannenberg AJ. Retinoids suppress epidermal growth factor-induced transcription of cyclooxygenase-2 in human oral squamous carcinoma cells. Cancer Res. 1997;57:2890–5. [PubMed] [Google Scholar]
- 144.Kulkarni S, Rader J, Zhang F, Liapis H, Koki A, Jaime L. Cyclooxygenase-2 Is Overexpressed in Human Cervical Cancer. Clin Cancer Res February. 2001;1:429–34. [PubMed] [Google Scholar]
- 145.Uhl-Steidl M, Muller-Holzner E, Zeimet AG, Adolf GR, Daxenbichler G, Marth C, Dapunt O. Prognostic value of CD44 splices variant expression in ovarian cancer. Oncology. 1995;52:400–6. doi: 10.1159/000227497. [DOI] [PubMed] [Google Scholar]
- 146.Zeimet AG, Widschwendter M, Uhl-Steidl M, Muller-Holzner M, Daxenbichler G, Marth C, Dapunt O. High serum level of soluble CD44 variant isoform v5 are associated with favorable clinical outcome in ovarian cancer. Br J Cancer. 1997;76:1646–51. doi: 10.1038/bjc.1997.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kainz C, Sliutz G, Mustafa G, Bieglmayr C, Koelbl H, Reinthaller A, Gitsch G. Cytokeratin subunit 19 measured by CYFRA 21–1 assay in follow-up of cervical cancer. Gynecol Oncol. 1995;56:402–25. doi: 10.1006/gyno.1995.1071. [DOI] [PubMed] [Google Scholar]
- 148.Subramanyam D, Rajendra W, Lokanatha V. Evaluation of soluble CD44 protein marker to distinguish the benign and squamous cell carcinoma cases in cervical cancer Patients. Med Oncol. 2014;31:1–7. doi: 10.1007/s12032-014-0139-9. [DOI] [PubMed] [Google Scholar]
- 149.Haedicke J, Iftner J. A review of the clinical performance of the Aptima HPV assay. J Clin Virol. 2015:1386–6532. [DOI] [PubMed]
- 150.Hudson JB, Bedell MA, McCance DJ, Laimins LA. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64(2):519–26. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Derbie A, Mekonnen D, Woldeamanuel Y, Ostade X, Abebe T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): a systematic review. Infect Agents Cancer. 2020;15(9). [DOI] [PMC free article] [PubMed]
- 152.Haedicke J, Iftner T. A review of the clinical performance of the Aptima HPV assay. J Clin Virol. 2016;76(1):40–8. doi: 10.1016/j.jcv.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 153.Bachtiary PBirner;B, Dreier B, Monika S, Elmar J, Gerhard B, Georg O.Signal-amplified colorimetric in situ hybridization for assessment of human papillomavirus infection in cervical lesions. Mod Pathol. 2001;14(7):702–9. doi: 10.1038/modpathol.3880375. [DOI] [PubMed] [Google Scholar]
- 154.Kaul PMelsheimer;S, Dobeck S, G, Bastert Immunocytochemical detection of HPV high-risk type L1 capsid proteins in LSIL and HSIL as compared with detection of HPV L1 DNA. Acta Cytol. 2003;47(2):124–8. doi: 10.1159/000326491. [DOI] [PubMed] [Google Scholar]
- 155.Huiyan Hu, Wen Yu, Junwei Z, Zhewei W, Lin J, Yunyun Yu, Lingfei H, Lu W, Fang. Li.Human papillomavirus DNA, HPV L1 capsid protein and p16INK4a protein as markers to predict cervical lesion progression. Arch Gynecol Obstet. 2019:141–149. [DOI] [PubMed]
- 156.Doeberitz M, Reuschenbach M, Schmidt D, Bergeron C. Biomarkers for cervical cancer screening: the role of p16INK4a to highlight transforming HPV infections. Expert Rev Proteomics. 2012;9(2):149–63. doi: 10.1586/epr.12.13. [DOI] [PubMed] [Google Scholar]
- 157.Hwang SJ, Shroyer KR. Biomarkers of cervical dysplasia and carcinoma. J Oncol. 2012, 507286. [DOI] [PMC free article] [PubMed]
- 158.Von Doeberitz K, Reuschenbach M, Schmidt M, Bergeron D. Biomarkers for cervical cancer screening: the role of p16INK4ato highlight transforming HPV infections. Expert Rev Proteomics. 2012;9(2):149–63. doi: 10.1586/epr.12.13. [DOI] [PubMed] [Google Scholar]
- 159.Ancuţa E, Ancuţa C, Cozma LG, Iordache C, Anghelache-Lupaşcu I, Anton E. Tumor biomarkers in cervical cancer: focus on Ki-67 proliferation factor and E-cadherin expression. Rom J Morphol Embryol. 2009;50(3):413–8. [PubMed] [Google Scholar]
- 160.Guo YT, Lu Y, Jia YY, Qu HN, Qi D, Wang XQ. Predictive Value of Pin1 in Cervical Low-Grade Squamous Intraepithelial Lesions and Inhibition of Pin1 Exerts Potent Anticancer Activity against Human Cervical Cancer. Aging Dis. 2020;1(11):44–59. doi: 10.14336/AD.2019.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao X, Li Q, Chen G, He H, Ma Y. MAGEA3 promotes proliferation and suppresses apoptosis in cervical cancer cells by inhibiting the KAP1/p53 signaling pathway. Am J Transl Res. 2020;12(7):3596–612. [PMC free article] [PubMed] [Google Scholar]
- 162.Gao X, Chen G, Cai H, Wang X, Song K, Liu L. Aberrantly enhanced melanoma-associated antigen (MAGE)-A3 expression facilitates cervical cancer cell proliferation and metastasis via actuating Wnt signaling pathway. Biomed Pharmacother. 2020;122:109710. doi: 10.1016/j.biopha.2019.109710. [DOI] [PubMed] [Google Scholar]
- 163.Xiang L, Jiang W, Ye S, He T, Pei X, Li J. ERBB2 mutation: A promising target in non-squamous cervical cancer. Gynecol Oncol. 2018;148(2):311–6. doi: 10.1016/j.ygyno.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 164.Rataj O, Haedicke-Jarboui J, Stubenrauch F, Iftner T. Brd4 inhibition suppresses HPV16 E6 expression and enhances chemoresponse: A potential new target in cervical cancer therapy. Int J Cancer. 2019;1(9):2330–8. doi: 10.1002/ijc.31986. [DOI] [PubMed] [Google Scholar]
- 165.Nahand JS, Taghizadeh-Boroujeni S, Karimzadeh M, Borran S, Pourhanifeh MH, Moghoofei M, microRNAs: New prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J Cell Physiol. 2019, Aug;234(10):17064–17099. [DOI] [PubMed]
- 166.Babion I, Miok V, Jaspers A, Huseinovic A, Steenbergen RD, van Wieringen WN, Wilting SM. Identification of deregulated pathways, key regulators, and novel miRNA-mRNA interactions in HPV-mediated transformation. Cancers. 2020;12(3):700. doi: 10.3390/cancers12030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Norouzi S, Farhadi A, Farzadfard E, Akbarzade-Jahromi M, Ahmadzadeh N, Nasiri M, et al. MicroRNAs Expression Changes Coincide with Low or High Grade of Squamous Intraepithelial Lesion Infected by HPV-16. Gene Rep. 2021;23:101186. doi: 10.1016/j.genrep.2021.101186. [DOI] [Google Scholar]
- 168.Tong F, Andress A, Tang G, Liu P, Wang X. Comprehensive Profiling of Extracellular RNA in HPV-Induced Cancers Using an Improved Pipeline for Small RNA-Seq Analysis. Sci Rep. 2020;10:19450. doi: 10.1038/s41598-020-76623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weng SL, Huang KY, Weng JT, Hung FY, Chang TH, Lee TY. Genome-wide discovery of viral microRNAs based on phylogenetic analysis and structural evolution of various human papillomavirus subtypes. Brief Bioinf. 2018;19(6):1102–14. doi: 10.1093/bib/bbx046. [DOI] [PubMed] [Google Scholar]
- 170.Sudhalkar N, Rathod NP, Mathews A, Supriya C, Harshini S, Shyam K;Shrivastava, Jayant G. Potential role of cancer stem cells as biomarkers and therapeutic targets in cervical cancer. Cancer Rep. 2019;2:e1144. doi: 10.1002/cnr2.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Suzuki R, Honda S, Kirino Y. PIWI expression and function in cancer. Front Genet. 2012, 3. [DOI] [PMC free article] [PubMed]
- 172.Liu W-K, Jiang X‐Y, Zhang Z‐X. Expression of PSCA, PIWIL1 and TBX2 and its correlation with HPV16 infection in formalin‐fixed, paraffin‐ embedded cervical squamous cell carcinoma specimens. 2010 May;155(5):657–663. [DOI] [PubMed]
- 173.Sasaki T, Shiohama A, Minoshima S, Shimizu N. Identification of eight members of the Argonaute family in the human genome. Genomics. 2003, Sep;82(3):323–330. [DOI] [PubMed]
- 174.Ye Y, Yin D-T, Chen L, Quansheng Z, Rulong S, Gang H, Qingtao Y, Zhenyu T, Andrew I, Charles S, Sanford B, Haifan L, Jian-Jian L, Jian-Xin G. Identification of Piwil2‐like (PL2L) proteins that promote tumorigenesis. PLoS One. 2010 Jan;5(10):e13406. [DOI] [PMC free article] [PubMed]
- 175.Ye F, Zhou C, Cheng Q, Shen J, Chen H. Stem-cell‐abundant proteins Nanog, nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC Cancer, 2008, Dec 18;8(1):108. [DOI] [PMC free article] [PubMed]
- 176.Liu S, Zheng P. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget, 2013, Dec 26;4(12). [DOI] [PMC free article] [PubMed]
- 177.Yao T, Wu Z, Liu Y, Rao Q, Lin Z. Aldehyde dehydrogenase 1 (ALDH1) positivity correlates with poor prognosis in cervical cancer. J Int Med Res. 2014;14(42(4):1038–42. doi: 10.1177/0300060514527060. [DOI] [PubMed] [Google Scholar]
- 178.Qun-Xian R, Ting‐Ting Y, Bing‐Zhong Z, Lin R-C, Chen Z-L, Zhou H, Wang L-J, Lu H-W, Chen Q, Di N, Lin Z-Q. Expression and functional role of ALDH1 in cervical carcinoma cells. Asian Pac J Cancer Prev. 2012;13(4):1325‐1331. doi: 10.7314/apjcp.2012.13.4.1325. [DOI] [PubMed] [Google Scholar]
- 179.Yu K-R, Yang S‐R, Jung J‐W, Kim H, Ko K, Han D-W, Park S-B, Choi S-W, Kang S-K, Scholer H, Kang K-S. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells. 2012;30(5):876‐887. doi: 10.1002/stem.1052. [DOI] [PubMed] [Google Scholar]
- 180.Haraguchi N, Ishii H, Mimori K, Ohta K, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Yamamoto H, Doki Y, Mori M. CD49f-positive cell population efficiently enriches colon cancer-initiating cells. Int J Oncol. 2013;43(2):425–30. doi: 10.3892/ijo.2013.1955. [DOI] [PubMed] [Google Scholar]
- 181.Fukamachi H, Seol HS, Shimada S, Funasaka C, Baba K, Kim JH, Park YS, Kim MJ, Kato K, Inokuchi M, Kawachi H, Yook JH, Eishi Y, Kojima K, Kim WH, Jang SJ, Yuasa Y. CD49fhigh cells retain sphere- forming and tumor‐initiating activities in human gastric tumors. Zhu W‐G editor PLoS One. 2013 Aug;28(8):e72438. 8. [DOI] [PMC free article] [PubMed]
- 182.Guo H, Xu C, Zhou T, Block TM, Guo J-T. Characterization of the host factors required for hepadnavirus covalently closed circular (ccc) DNA formation. Ryu W‐S, editor. PLoS ONE. 2012 Aug;13(8):e43270. 7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.