Abstract
Gout, the most prevalent inflammatory arthritis, is becoming increasingly prevalent in the United States and across the world, and it adversely impacts people’s quality of life and their health. Few studies have focused on the relationship between daily dietary quality and gout, so the topic requires further exploration. Data were derived from the National Health and Nutrition Examination Survey 2007–2016, and the inclusion criteria of the analytic sample were (1) adults, age ≥20 years, with complete information about HEI-2015, gout, and uric acid; (2) complete information of demographics, lifestyle (BMI, smoking, drinking), and disease history [hypertension, chronic kidney disease (CKD), diabetes]. The quality of the daily diet was reflected using the Healthy Eating Index 2015 (HEI-2015). The baseline features of different groups were examined using the Scott-Rao chi-square tests, and the association between the HEI-2015 score and the risk of gout/hyperuricemia (HUA) was investigated using weighted logistic regression models. The effects of different dietary components in the HEI-2015 on reducing the risk of gout/HUA were evaluated by weighted quantile sum (WQS) regression models. After adjusting for demographic characteristics, behavioral covariates, and disease history, higher HEI-2015 scores were associated with a significantly lower risk of gout (OR: 0.878, 95% CI: 0.876–0.880) and HUA (OR: 0.978, 95% CI: 0.976–0.979) in weighted logistic regression. Dairy, whole grains, plant proteins, and added sugar contributed greatly in HEI-2015 to reducing gout risk (weights of WQS index: 42, 17.18, 16.13, and 7.93%, respectively). Dairy, total fruits, greens and beans, and plant proteins contributed greatly in HEI-2015 to reducing HUA risk (weights of WQS index: 28.9, 17.13, 16.84, and 11.39%, respectively). As the result, adherence to the American Dietary Guidelines may assist to decrease the risk of gout/HUA in American adults, and greater emphasis should be placed on dairy products, whole grains, fruits, legumes, and added sugars.
Keywords: healthy eating index, gout, hyperuricemia, daily diet, weighted quantile sum (WQS) regression, NHANES
Introduction
Gout is inflammatory arthritis due to hyperuricemia (HUA), the most important pathological feature of which is the presence of sodium urate crystals in the joints. Gout episodes are frequently accompanied by excruciating joint pain and are closely linked to long-term conditions including obesity and hypertension (1–3), which impair the quality of life (4) and raise medical and care expenditures (5). There were 41.2 million prevalent cases of gout globally, with 7.4 million incident cases per year and almost 1.3 million years lived with disability (YLD) due to gout in 2017 (6, 7). Gout has become a global public health problem.
Recent research has revealed a strong link between diet and gout. According to a 26-year prospective cohort trial in the Health Professionals Follow-up Study (HPFS), the Dietary Approaches to Stop Hypertension (DASH) diet was beneficial in decreasing the incidence of gout and lowering serum uric acid concentrations in adult males, in comparison with a Western diet (8). Additionally, a clinical trial in Israel found that adherence to a Mediterranean diet can significantly lower serum uric acid and reduce the risk of gout in severely obese patients (9). Numerous studies have also evaluated the connection between particular foods or nutrients and gout, including dairy products (10), whole grains, fruits (8, 11, 12), added sugars (13, 14), and vitamins (12, 13). For instance, dairy products were shown to be beneficial in lowering serum uric acid and reducing the frequency of gout episodes in a randomized controlled study of gout patients (15). Moreover, a prospective cohort study showed that vegetables and fruits exhibited similar impacts to dairy products (11), and a cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brazil) revealed that increased consumption of soft drinks and fructose was positively correlated with the risk of HUA (14).
The Healthy Eating Index (HEI) is a dietary quality indicator based on the Dietary Guidelines for Americans (DGA), and the HEI-2015 was the most recent version of the HEI. Multiple studies have discovered that the HEI score is related to health status, including physiological indicators (16), biochemical indicators (17), and disease risk (18, 19). The daily diet proposed by DGA is more adaptable in terms of food choices and is suited for a wider spectrum of people than the DASH diet and the Mediterranean diet (20). Furthermore, the adoption of the HEI-2015 also renders it possible to apply numerical values to represent how healthy a person’s daily diet is, allowing for a comparison of diet quality.
To the best of our knowledge, no studies have investigated the relationship between HEI-2015 and gout or HUA. Therefore, this study intends to employ the HEI-2015 to estimate the overall health impact of various dietary components, in addition to analyzing the risk of developing gout or HUA in terms of adhering to DGA.
Materials and methods
Study sample
The National Health and Nutrition Examination Survey (NHANES) is a large open database developed to better understand the nutrition and diet of the American population. The database utilized a unique and complex multistage probabilistic design, so that sample weights could be used to reflect the non-institutionalized population of the United States. All subjects received a dietary survey and examination by a professional at mobile exam centers (MECs). The examination includes medical, dental, and physiological measurements, as well as laboratory tests, which were supervised by trained medical personnel. In addition, a variety of modern equipment enables NHANES to collect reliable, high-quality data.
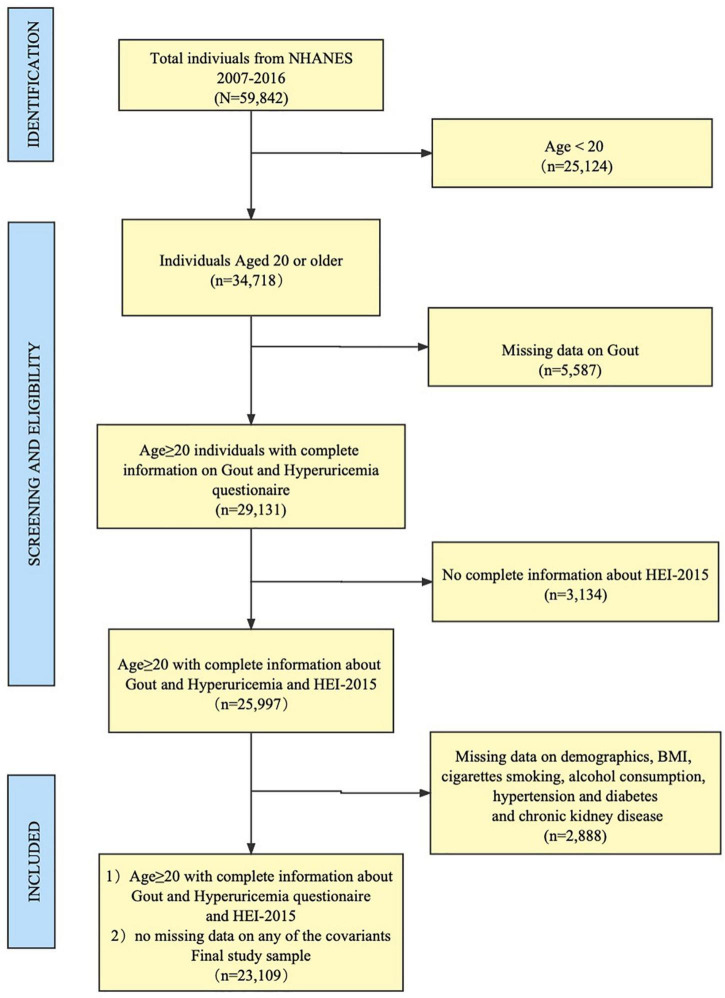
We selected five consecutive survey cycles of NHANES (i.e., 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016), and the overall sample size of adults (age ≥ 20) with no missing data for any variables is 23,109. The sample was weighted to represent a non-institutionalized adult population of 190 million Americans. Additional details of the study design, sampling, and exclusion criteria were illustrated in Figure 1. Only public data were used in the analysis, and ethical approval was not required for this study.
FIGURE 1.
Flowchart of the study population.
Measurements and covariation assessment
Diet quality
HEI-2015 is utilized to assess the degree of adherence to DGA, which consists of 13 kinds of components (21). These 13 components were grouped into two forms, namely, the adequacy components (total vegetables, greens and beans, total fruits, whole fruits, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids) and the moderation components (sodium, refined grains, saturated fats, added sugars). Different maximum scores and weights were assigned to each component, and the overall 13 component scores (HEI-2015 scores) ranged from 0 to 100, with higher HEI scores reflecting better diet quality. Participants who had both two 24-h dietary recalls were included, and their dietary recall status was restricted to be reliable or had satisfied the minimum criteria for each day, which can reduce the bias of the HEI-2015. The 24-h dietary recall data were collected for 2 days, which were conducted by a trained interviewer face-to-face in the MEC on the first day and a follow-up interview 3-10 days later via phone. More details on dietary surveys and quality control can be found in the survey manual.1 The HEI-2015 total and component scores were calculated by the simple HEI scoring algorithm using publicly available SAS macros. For weighted Scott–Rao chi-square tests and weighted logistic regressions, quartiles were used to categorize the HEI-2015 score into four groups, recorded as Q1 (lowest diet quality, reference group), Q2, Q3, and Q4 (highest diet quality), respectively (22).
Gout
All subjects were asked “Has a doctor or other health professional ever told you that you had gout?” and then categorized into non-gout subjects (reference group) and gout subjects according to the answers (23).
Hyperuricemia (HUA)
The level of uric acid in blood, plasma, and urine was determined using a timed endpoint approach. Uric acid is oxidized by uricase to produce allatoin and hydrogen peroxide. The hydrogen peroxide reacts with 4-aminoantipyrine (4-AAP) and 3,5-dichloro-2-hydroxybenzene sulfonate (DCHBS) in a reaction catalyzed by peroxidase to produce a colored product. The system monitors the change in absorbance at 520 nm at a fixed time interval. More quality control details can be found in the documentation (24). The uric acid values were recorded in mg/dl and then converted to μmol/L by multiplying 59.48. A serum urate level of >7.0 mg/dl in men and >5.7 mg/dl in women was considered HUA (23).
Covariates
Sex
Sex was classified as female (reference group) and male.
Age
Age was categorized into four groups, namely, 20–39 years group (reference group), 40–59 years group, 60–79 years group, and 80 years and above group (23).
Race
Race, according to NHANES classification, was categorized into non-Hispanic white (reference group), Mexican American group, non-Hispanic black, and other races (25).
Education
Education level was categorized as less than high school (reference group), high school graduate/GED, some college/AA degree, and college graduate or more (26).
Family income
Family income was categorized as ≤130% (reference group), >130–350%, and >350% by the ratio of family income to poverty (FPL) (27).
BMI status
Body mass index was calculated from measured height and weight as weight/height2 (kg/m2), then categorized into underweight (<18.5), normal (reference group, ≥18.5–24.9), overweight (≥25–29.9), and obese (≥30) (28).
Smoking status
Smoking behavior was measured by the “Smoking: Cigarette Use” questionnaire. The respondent was asked whether he/she had smoked at least 100 cigarettes in his/her life. If the respondent answered “no,” he/she was classified as a never smoker (reference group). If the respondent had smoked at least 100 cigarettes in his/her life and still smokes when he/she answers the questionnaire, he/she is classified as a current smoker. The respondent was classified as a former smoker who has smoked 100 cigarettes in his life and had quit smoking when answering the questionnaire (29).
Drinking status
Drinking behavior was measured in the “alcohol use” questionnaire. In the “alcohol use” questionnaire, each respondent was asked how often he/she had drunk alcoholic drinks in the past 12 months and the average drinks on those days that he/she drank alcoholic beverages. According to these questions, the average number of alcoholic drinks consumed per week in the past 12 months could be calculated. A “drink” was defined as a 12-ounce beer, a 5-ounce glass of wine, or one-and-half ounces of liquor. Then, it was categorized into four strata (0, <1, 1–8, and ≥8 drinks per week) and defined as none (reference group), light, moderate, and heavy alcohol consumption, respectively (30).
Hypertension
All subjects were asked, “Ever been told by a doctor or other health professional that you had hypertension?,” and then categorized into non-hypertension subjects (reference group) and hypertension subjects (31).
Chronic kidney disease
All subjects were asked, “Ever been told by a doctor or other health professional that you had weak or failing kidneys?,” and then categorized into non-CKD subjects (reference group) and chronic kidney disease (CKD) subjects (32).
Diabetes
During the NHANES home interviews, all subjects were asked, “Ever been told by a doctor or other health professional that you had diabetes or sugar diabetes?,” and then categorized into non-diabetic subjects (reference group) and diabetic subjects (33).
Statistical analysis
Analyses were conducted according to the Centers for Disease Control and Prevention (CDC) guidelines for analysis of NHANES data. A full sample 2-year mobile examination centers (MEC) weight was used to calculate the US non-institutionalized population.
Linear regression models were adopted to analyze the trends in the prevalence of gout and HUA in the five consecutive cycles. The baseline features of different groups were examined using the Scott-Rao chi-square tests, and the association between the HEI-2015 score and the risk of gout/HUA was investigated using weighted logistic regression models.
The weighted quartile sum (WQS) (34–37) regression model was used to assess the effects of mixed exposure to thirteen dietary components of HEI-2015. The WQS regression model calculates a weighted regression index that represents the overall dietary health effect for all thirteen dietary components of HEI-2015. The WQS model functions as follows:
where β0 is the intercept; z′ and Φ represent the matrix of covariates and the coefficients of the covariates. c is the number of dietary components considered in the analysis, and 13 dietary components were included in the current analysis. The whole sum of weighted indices (ωi) is equal to 1, with the value of each component varying from 0 to 1 is the regression coefficient of the WQS index. qj represents the quartiles of a dietary component score (= 0, 1, 2, or 3 for the first, second, third, or fourth quartile, respectively). g(μ) is a logit link function, when the outcome of interest is binary (gout or not, HUA or not). The corresponding weight of each dietary component showed how much a specific dietary component contributed to the WQS index. The data were randomly split into two data sets (40% as training set and 60% as validation set).
The corresponding weight of each dietary component showed how much a specific dietary component contributed to the WQS index. The data were randomly split into two data sets (40% as the training set and 60% as the validation set).
For all measures, we calculated 95% confidence intervals (CIs). The receiver operating characteristic curve (ROC) was used to validate the degree of WQS model fit.
All statistical tests were two-sided, and significance was considered at P < 0.05. WQS and ROC were performed with the R (version 4.1.0). RCS was implemented with the R package “rms” (version 6.3-0). WQS was implemented with the R package “gWQS” (version 3.0.4). Additional statistical analyses were performed using the SPSS statistical package (version 23.0; SPSS Inc., Chicago, IL, United States).
Results
Population characteristics between groups
Over the period of a total of five cycles from 2007 to 2016, the prevalence of gout (3.9% in 2007–2008 to 3.8% in 2015–2016, P = 0.519) and HUA (21.6% in 2007–2008 to 20.4% in 2015–2016, P = 0.161) among United States adults remained steady (Table 1).
TABLE 1.
The prevalence of gout and HUA among United States adults from 2007 to 2016.
| 2007–2008 (n = 4831) |
2009–2010 (n = 5006) |
2011–2012 (n = 4150) |
2013–2014 (n = 4620) |
2015–2016 (n = 4502) |
P-value | |
| No. gout | 224 | 223 | 173 | 186 | 216 | |
| The prevalence of gout (Weighted%) | 3.9% | 3.8% | 3.8% | 4.1% | 3.8% | 0.519 |
| No.HUA | 1,115 | 1,084 | 892 | 964 | 928 | |
| The prevalence of HUA (Weighted%) | 21.60% | 21.10% | 20.10% | 19.70% | 20.40% | 0.116 |
HUA, Hyperuricemia.
The baseline characteristics of two groups (adults with/without gout) revealed that adults with gout were more likely to be male, over 60 years old, non-Hispanic black, have less than high school education, have low family income, obese, alcoholic, former smokers and suffer from CKD, hypertension, diabetes, HUA, and have lower HEI scores (Table 2).
TABLE 2.
Characteristics among adults aged 20 years or older by gout.
| Characteristics | Adults without gout (n, %) | Adults with gout (n, %) | P-value |
| Sex no. (Weighted%) | <0.001 | ||
| Female | 11,369 (51.77) | 302 (30.6) | |
| Male | 10,698 (48.23) | 740 (69.4) | |
| Age group no. (Weighted%) | <0.001 | ||
| 20–39 year | 7,296 (35.24) | 54 (6.39) | |
| 40–59 year | 7,416 (37.74) | 264 (34.17) | |
| 60–79 year | 5,966 (22.52) | 557 (47.3) | |
| 80+ year | 1,389 (4.51) | 167 (12.13) | |
| Race no. (Weighted%) | <0.001 | ||
| Non-Hispanic white | 9,615 (68.56) | 560 (77.16) | |
| Mexican American | 3,528 (8.62) | 73 (3.04) | |
| Non-Hispanic black | 4,367 (10.34) | 265 (11.83) | |
| Other | 4,557 (12.48) | 144 (7.97) | |
| Education no. (Weighted%) | <0.001 | ||
| <High school | 5,417 (16.12) | 269 (17.13) | |
| High school/GED | 4,997 (22.09) | 269 (24.27) | |
| College/AA degree | 6,491 (31.86) | 296 (32.01) | |
| College or above | 5,162 (29.94) | 208 (26.6) | |
| Family income no. (Weighted%) | <0.001 | ||
| 0∼130 FPL | 6,496 (19.97) | 309 (20.88) | |
| >130∼350 FPL | 7,550 (33.22) | 368 (32.41) | |
| >350 FPL | 8,021 (46.82) | 365 (46.71) | |
| BMI no. (Weighted%) | <0.001 | ||
| Normal weight | 5,861 (27.78) | 143 (11.59) | |
| Underweight | 330 (1.47) | 6 (0.35) | |
| Overweight | 7,352 (33.63) | 307 (30.87) | |
| Obese | 8,524 (37.11) | 586 (57.19) | |
| Drink level no. (Weighted%) | <0.001 | ||
| None | 7,133 (26.02) | 407 (34.53) | |
| Light | 6,786 (30.65) | 268 (25.86) | |
| Moderate | 7,548 (40.63) | 337 (36.39) | |
| Heavy | 600 (2.69) | 30 (3.22) | |
| Smoke status no. (Weighted%) | <0.001 | ||
| Never smoker | 12,282 (55.65) | 429 (42.83) | |
| Former smoker | 5,214 (24.4) | 446 (42.16) | |
| Current smoker | 4,571 (19.95) | 167 (15.01) | |
| CKD no. (Weighted%) | <0.001 | ||
| No | 21,419 (97.69) | 919 (90.71) | |
| Yes | 648 (2.31) | 123 (9.29) | |
| Diabetes no. (Weighted%) | |||
| No | 19,411 (91.1) | 726 (75.52) | <0.001 |
| Yes | 2,656 (8.9) | 316 (24.48) | |
| Hypertension no. (Weighted%) | <0.001 | ||
| No | 14,432 (69.22) | 261 (30.13) | |
| Yes | 7,635 (30.78) | 781 (69.87) | |
| HUA no. (Weighted%) | <0.001 | ||
| No | 17,577 (80.47) | 549 (53.71) | |
| Yes | 4,490 (19.53) | 493 (46.29) | |
| HEI category no. (Weighted%) | <0.001 | ||
| Q1 | 5,492 (25.04) | 233 (23.75) | |
| Q2 | 5,491 (25.02) | 259 (24.7) | |
| Q3 | 5,590 (24.92) | 294 (27.06) | |
| Q4 | 5 494 (25.02) | 256 (24.49) |
Values are survey-weighted percentages. FPL, family income to poverty; CKD, chronic kidney disease; HUA, Hyperuricemia; HEI, healthy eating index.
Adults with HUA were more likely to be female, over 60 years old, non-Hispanic black, have a middle level of education, originate from low-income families, obese, alcoholics, or former smokers. They were also more likely to have CKD, hypertension, and diabetes, as well as lower HEI scores (Supplementary Table 1).
Higher healthy eating index score is associated with a lower risk of gout
After stepwise adjusting for covariates, a binary logistic regression model revealed that decreased gout risk was related to higher HEI-2015 scores (Table 3). In the model adjusted for age, sex, race/ethnicity, family income, and education, higher diet quality was linked to significantly decreased risks of gout [odds ratio (OR) 0.832 95% confidence intervals (CI): 0.830–0.834 for Q4 compared with Q1, P < 0.001]. Additional adjustments for BMI, smoking, and alcohol consumption did not significantly weaken this connection (OR: 0.886, 95%CI: 0.884–0.888 for Q4 compared with Q1, P < 0.001). The connection between HEI-2015 and gout risk remained significant (OR: 0.878, 95% CI: 0.876–0.880 for Q4 compared with Q1, P < 0.001) after further adjustments for chronic disease characteristics, including hypertension, diabetes, CKD, and HUA.
TABLE 3.
Relationship between HEI-2015 and gout among adults aged 20 years or older.
| Variable | OR (95% CI) | P-value | ||
|
|
||||
| Model 1 | Model 2 | Model 3 | ||
| Sex (reference, female) | <0.001 | |||
| Male | 2.743 (2.739, 2.748) | 2.643 (2.639, 2.648) | 3.027 (3.022, 3.033) | |
| Age group (reference, 20–39 year) | <0.001 | |||
| 40–59 year | 5.204 (5.188, 5.220) | 4.522 (4.508, 4.537) | 3.68 (3.668, 3.692) | |
| 60–79 year | 12.372 (12.334, 12.411) | 9.993 (9.961, 10.025) | 6.234 (6.213, 6.254) | |
| 80+ year | 17.066 (17.002, 17.129) | 15.729 (15.669, 15.790) | 8.465 (8.431, 8.499) | |
| Race (reference, non-Hispanic white) | <0.001 | |||
| Mexican American | 0.430 (0.428, 0.432) | 0.398 (0.396, 0.400) | 0.452 (0.450, 0.454) | |
| Non-Hispanic black | 1.245 (1.242, 1.248) | 1.231 (1.228, 1.234) | 1.022 (1.019, 1.024) | |
| Other | 0.756 (0.753, 0.758) | 0.828 (0.826, 0.830) | 0.809 (0.807, 0.812) | |
| Education (reference, <high school) | <0.001 | |||
| High school/GED | 1.046 (1.044, 1.049) | 1.056 (1.054, 1.059) | 1.098 (1.095, 1.101) | |
| College/AA degree | 1.122 (1.120, 1.125) | 1.113 (1.110, 1.115) | 1.121 (1.118, 1.124) | |
| College or above | 0.932 (0.929, 0.934) | 1.061 (1.059, 1.064) | 1.152 (1.148, 1.155) | |
| Family income (reference,0∼130% FPL) | <0.001 | |||
| >130∼350% FPL | 0.703 (0.702, 0.705) | 0.676 (0.674, 0.677) | 0.722 (0.721, 0.724) | |
| >350% FPL | 0.700 (0.699, 0.702) | 0.681 (0.679, 0.682) | 0.774 (0.772, 0.776) | |
| HEI category (reference, Q1) | <0.001 | |||
| Q2 | 0.910 (0.908, 0.912) | 0.903 (0.901, 0.905) | 0.888 (0.886, 0.890) | |
| Q3 | 0.974 (0.972, 0.976) | 1.01 (1.007, 1.012) | 0.991 (0.989, 0.993) | |
| Q4 | 0.832 (0.830, 0.834) | 0.886 (0.884, 0.888) | 0.878 (0.876, 0.880) | |
| BMI (reference, normal weight) | <0.001 | |||
| Underweight | 0.619 (0.611, 0.627) | 0.689 (0.680, 0.698) | ||
| Overweight | 1.678 (1.674, 1.683) | 1.361 (1.358, 1.365) | ||
| Obese | 3.062 (3.054, 3.069) | 1.907 (1.902, 1.912) | ||
| Drink level (reference, none) | <0.001 | |||
| Light | 0.822 (0.820, 0.823) | 0.871 (0.869, 0.872) | ||
| Moderate | 0.878 (0.876, 0.879) | 0.913 (0.912, 0.915) | ||
| Heavy | 1.314 (1.308, 1.320) | 1.203 (1.197, 1.209) | ||
| Smoke status (reference, never) | <0.001 | |||
| Former | 1.335 (1.332, 1.337) | 1.266 (1.264, 1.268) | ||
| Current | 1.019 (1.017, 1.022) | 1.05 (1.047, 1.052) | ||
| CKD (reference, no) | <0.001 | |||
| Yes | 2.051 (2.045, 2.057) | |||
| Diabetes (reference, no) | <0.001 | |||
| Yes | 1.33 (1.327, 1.332) | |||
| Hypertension (reference, no) | <0.001 | |||
| Yes | 2.304 (2.300, 2.308) | |||
| HUA (reference, no) | <0.001 | |||
| Yes | 2.484 (2.480, 2.488) | |||
FPL, family income to poverty; CI, confidence interval; OR, odds ratio; CKD, chronic kidney disease; Model 1, adjusted for demographic characteristics (sex, age group, race, education, family income); Model 2, adjusted for demographic characteristics (sex, age group, race, education, family income); BMI, smoking, and drinking status; Model 3, adjusted for demographic characteristics (sex, age group, race, education, family income); BMI, smoking, drinking status, hypertension, CKD, diabetes, and hyperuricemia.
By applying the same analytical technique, we also discovered that higher HEI-2015 scores were related to a decreased risk of HUA (Q4 OR: 0.978, 95% CI: 0.976–0.979 for Q4 compared with Q1, P < 0.001, see Supplementary Table 2).
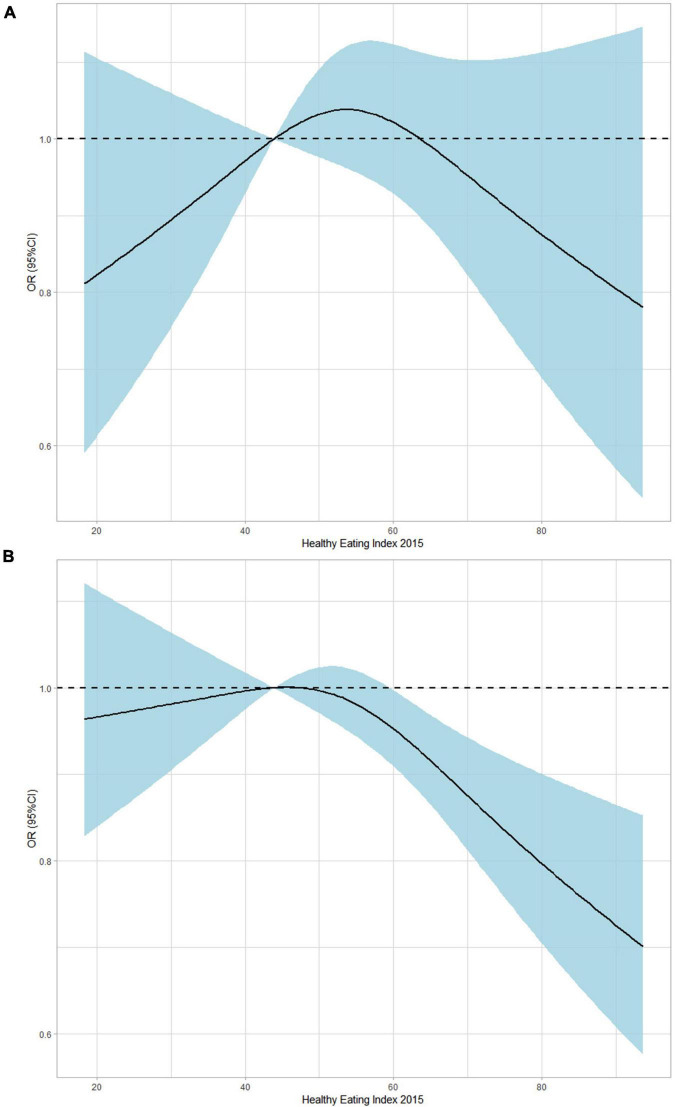
Figure 2 demonstrated the dose-response relationship between gout/HUA and HEI-2015 (as a continuous variable) by applying the restricted cubic splines (RCS) approach. Higher HEI-2015 scores did not demonstrate a protective effect of gout (OR: 0.988, 95% CI: 0.973–1.003, P = 0.1223) and instead showed a declining trend in OR (Figure 2A). Additionally, higher HEI-2015 scores revealed a declining OR trend (OR: 0.992, 95% CI: 0.985–0.999, P = 0.0386) and the preventive effects of HUA (Figure 2B).
FIGURE 2.
Dose-response association between HEI (it continues) and gout/HUA using restricted cubic splines (RCS). The models of gout (A) were adjusted for sex, age group, race, education, family income, BMI, smoking, drinking status, hypertension, CKD, diabetes, and hyperuricemia. The models of HUA (B) were adjusted for sex, age group, race, education, family income, BMI, smoking, drinking status, hypertension, CKD, and diabetes.
Mixed effects of 13 dietary components on gout
The WQS regression was used to evaluate the health contributions of various dietary components to explore the health implications of dietary components in the total diet.
The WQS indices were statistically associated with gout. After adjusting for demographic variables, including age, sex, race, household income, and education level, the WQS index was significantly associated with progressively lower odds of gout (OR: 0.955, 95% CI, 0.930–0.982, P = 0.0009). The WQS index was significantly correlated with decreased risks of gout after further adjustments for BMI, smoking, and alcohol use (OR: 0.957, 95% CI, 0.933–0.983, P = 0.0011). These correlations were not significantly weakened by further adjustments for chronic disease characteristics (hypertension, diabetes, CKD, HUA) (OR: 0.963, 95% CI: 0.937–0.990, P = 0.0067).
Additionally, we discovered that the WQS indices significantly correlated with HUA (Supplementary Table 3). The WQS index was significantly linked with progressively decreased odds of HUA after multiple adjustments (OR: 0.934, 95% CI: 0.919–0.950, P < 0.0001).
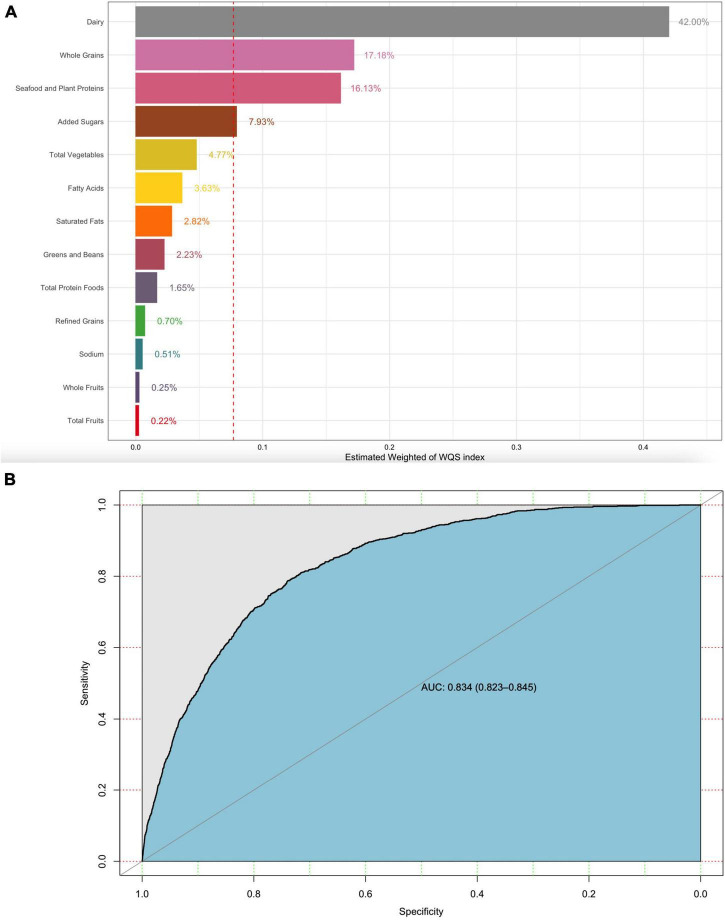
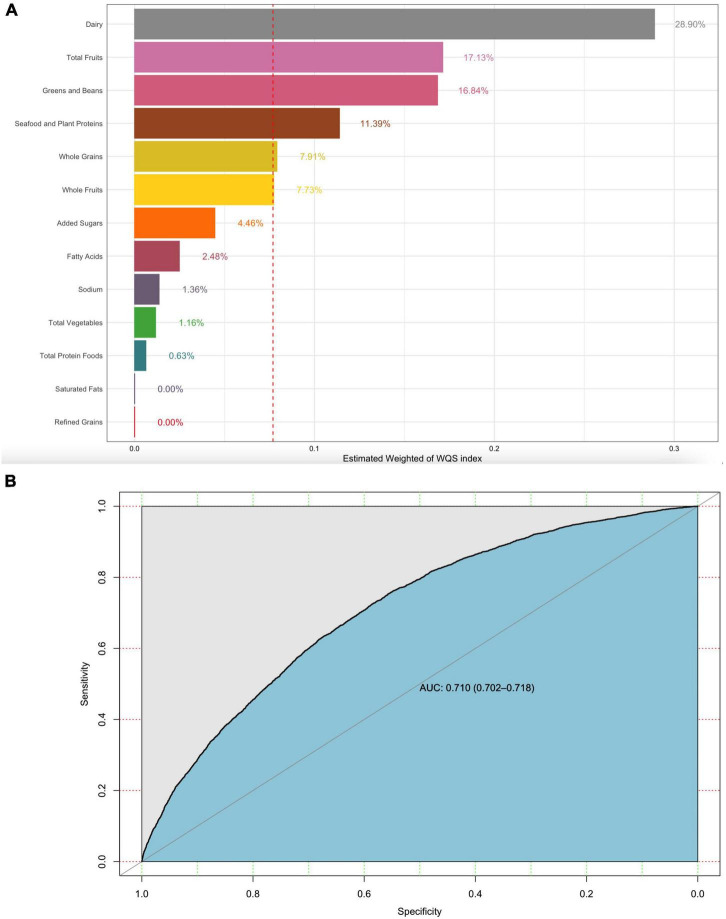
The weight of each dietary component in the WQS regression model represented the contributions of the overall dietary health effects. Dairy, whole grains, and plant proteins were the highest weighted dietary components in the model of gout (42, 17.18, and 16.13%, respectively). Added sugar as a moderation component was also the highest weighted dietary component in the model of gout (7.93%) (Figure 3A). Similarly, dairy, total fruits, greens, beans, and plant proteins were the highest weighted dietary components in the model of HUA (Figure 4A) (28.9, 17.13, 16.84, and 11.39%, respectively). The evaluation of the WQS models showed that the area under the ROC curves (AUCs) was 0.834 in gout (Figure 3B) and 0.710 in HUA (Figure 4B).
FIGURE 3.
WQS model regression index weights for gout (A) and the AUCs of the WQS models (B). Models were adjusted for sex, age group, race, education, family income, BMI, smoking, drinking status, hypertension, CKD, diabetes, and hyperuricemia.
FIGURE 4.
WQS model regression index weights for HUA (A) and the AUCs of the WQS models (B). Models were adjusted for sex, age group, race, education, family income, BMI, smoking, drinking status, hypertension, CKD, and diabetes.
Discussion
In agreement with the American College of Rheumatology report (23), this study discovered that the prevalence of both gout and HUA remained steady from 2007 to 2016. Our stepwise logistic regression models demonstrated that higher HEI-2015 scores were independently related to a decreased risk of gout/HUA. The findings supported the concept of HEI-2015’s recommendations for a healthy diet (20, 21), which noted that a high-quality diet improved the quality of life.
Patients with HUA who followed a Mediterranean diet showed a decrease in blood uric acid concentration of 119 μmol/L over 6 months in a randomized controlled study (P for within-group comparison < 0.001)[9]. The quality of the Mediterranean diet, however, was not measured in this study. After quantifying the quality of the Mediterranean diet, MD Kontogianni et al. found a 70% reduction in the risk of HUA in those scoring in the Q4 of the Mediterranean diet compared with those scoring in the Q1 (OR: 0.30, 95%CI: 0.11–0.82) (38). According to a prospective cohort study of adult men conducted by Rai et al., higher DASH dietary scores were linked to a decreased incidence of gout (OR: 0.68, 95% CI: 0.57–0.80, P< 0.001, Q5 vs. Q1) (8). However, certain studies, such as those on the Mediterranean diet (39, 40) and the DASH diet (41, 42), estimated dietary quality scores based on the frequency of meals. In our study, we determined the consumption of each dietary component and then estimated the HEI-2015 score in the form of nutritional density (43). The HEI-2015 has 13 dietary components, compared with the 11 components of the Mediterranean diet score and the 8 components of the DASH diet, which improves the validity of our findings.
The HEI-2015 score calculation was based on the DGA, which included daily dietary recommendations for the US population and sought to improve dietary quality (20, 44). We concentrated on the contributions of different components to identify the component that contributed the most to offering better dietary guidance to individuals with gout/HUA and at risk of gout/HUA. The results indicated that added sugars, dairy products, whole grains, seafood, and plant proteins contributed the most to lowering the risk of gout in the HEI-2015, implying that increasing dietary intake of dairy products, whole grains, vegetables, and legumes, as well as lowering added sugar intake within the recommended range, may decrease the risk of gout. Additionally, the diet’s ability to protect against HUA was most strongly influenced by dairy products, total fruits, greens and beans, seafood and vegetable protein, whole grains, and whole fruits. It was shown that consuming more dairy products, whole grains, fruits, vegetables, and legumes while consuming added sugars less frequently and within the recommended range can lower the risk of HUA.
Dairy products provide a lot of high-quality protein, and it has been shown that casein and whey protein from milk can lower blood uric acid levels in healthy people (45). Dairy products are also low in purines and contribute less to the purine load associated with other high-quality protein sources, such as meat and seafood (10). Proteins and lipids from dairy products were also found to inhibit inflammatory responses associated with monosodium urate monohydrate (MSU) crystals in vivo/in vitro experiments (46). A study based on NHANES data revealed that a higher ratio of refined grains to whole grains was associated with a greater risk of CKD, and a high intake of whole grains was associated with low serum uric acid levels (47). Whole grains are a rich source of fiber, minerals, vitamins, phenolic compounds, phytoestrogens, and related antioxidants (48). These ingredients are beneficial for disease prevention and management (49–52). The association between fruit and uric acid/gout is disputed, since fructose metabolism generated urates, and fresh fruit is high in fructose (53, 54). Some studies have also found that fruit intake reduces the risk of gout because fruits contribute to urine alkalization and promote uric acid excretion (55). Fruits are rich in various nutrients, such as vitamin C (56, 57), potassium (58, 59), fiber (60), epicatechin, and flavonols (61, 62), which may alter the effects of fructose and urate. Studies on added sugars and gout have also indicated that reducing added sugar intake helps to decrease uric acid and prevent gout.
According to the Dietary Guidelines for Americans 2020–2025 (20), adults in the United States should consume 8 ounces of seafood per week; however, in our study, 79% of participants did not reach this recommended standard. However, we found that the seafood and plant protein components have a healthy contribution to gout/HUA. We assumed that the protective effects exhibited by the seafood and plant protein were due to seafood intake within reasonable limits (63) and the protective effects of plant protein (64, 65). The impact of low-dose seafood consumption and uric acid in healthy individuals required more exploration. Encouragement of greater consumption of dairy products, whole grains, low-fructose fruits, and high-quality protein from plant sources, particularly legumes, is crucial for preventing gout and decreasing blood uric acid.
We discovered additional risk variables for gout/HUA, including male gender, advanced age, non-Hispanic black race, middle education level, low income, obesity, alcohol intake, hypertension, and CKD. Due to estrogen’s stimulation of uric acid excretion, women have always had low rates of HUA and gout (3). Poor education and income levels are risk factors for gout because they are directly associated with socioeconomic position, which affects access to healthcare and treatment compliance (66). Drinking promotes uric acid metabolism, raises blood uric acid levels, and increases the risk of gout/HUA, which was consistent with our results (67). Aging, obesity, hypertension, and CKD are all common gout risk factors, raising the incidence of gout by affecting uric acid metabolism or excretion (3). Interestingly, we found that smoking was a protective factor for HUA, as smoking may lower blood uric acid levels by metabolically interacting with superoxide (68). Another rationale is that since there was only one blood uric acid test implemented in this study, it cannot accurately represent long-term uric acid levels. However, the damage of long-term smoking to overall health also increases the risk of gout (69).
Diabetes was found to be a protective factor for HUA in this investigation, according to the logistic regression results of the HUA model. Diabetes, as a complication of HUA, has a very close relationship with HUA. Our study discovered that the added sugar scores in diabetes patients were substantially higher than those in non-diabetic subjects, indicating a decreased intake of added sugar in diabetic people. Research indicated that added sugar intake may raise serum uric acid levels (70, 71) and raise the risk of developing gout (72). In the liver, the metabolism of fructose, a major source of added sugar in the daily diet, stimulates adenosine monophosphate deaminase which promotes the degradation of adenosine monophosphate to inosine monophosphate. This process could induce insulin resistance (73–75) and promote the production of uric acid in the liver (43), thereby increasing the risk of HUA. Therefore, we assumed that diabetes showed as a protective factor for HUA in the results due to the significantly lower intake of added sugars in diabetic subjects than in non-diabetic subjects. Then, we proposed that restricting added sugar consumption might significantly lower the risk of HUA, including the diabetic population.
The article had several limitations due to the nature of the study. As with any observational study, our estimates were still subject to residual and unmeasured confounders, and no causal relationship can be inferred. Second, self-reported data about disease were subject to recall bias, and lack of adjustment for medication history for disease would affect results. Finally, only one serum uric acid test did not reflect the long-term uric acid level of the subject and thus caused possible erroneous results.
Nonetheless, our study also has some strengths. A major strength is the use of a large, nationally representative database. All dietary interviewers were required to complete professional courses and training and regularly reinforce training annually. Data from two 24-h dietary survey were used to reduce recall bias from the food frequency questionnaire. The HEI was developed and validated using HNANES dietary data, and dietary data were collected in the same manner as the HEI-2015 score. Effective and reasonable quantification of dietary quality is also a strength of this article. Second, the WQS model had been used in fewer nutrition-related studies. We used the WQS model to identify the highest contributing dietary components, and this study was a new application of the WQS model. Through this article, we hope to make the public aware of the benefits of a healthier daily diet for gout and HUA.
Conclusion
We found that people with the highest HEI-2015 score had a reduced risk of gout and HUA by 12.2 and 2.2% compared to people with the lowest HEI-2015 score. Dairy products, whole grains, fruits, and plant protein-related foods contributed the most to the health effects of the daily diet represented by HEI-2015. Therefore, people at high risk of gout or gout sufferers should pay more attention to maintaining a healthy diet and follow the DGA, especially increasing the intake of dairy products, whole grains, fruits, and legumes and reducing the intake of added sugars.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.921550/full#supplementary-material
References
- 1.Richette P, Bardin T. Gout. Lancet (Lond Engl). (2010) 375:318–28. 10.1016/S0140-6736(09)60883-7 [DOI] [PubMed] [Google Scholar]
- 2.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet (Lond Engl). (2016) 388:2039–52. 10.1016/S0140-6736(16)00346-9 [DOI] [PubMed] [Google Scholar]
- 3.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet (Lond Engl). (2021) 397:1843–55. 10.1016/S0140-6736(21)00569-9 [DOI] [PubMed] [Google Scholar]
- 4.Smith E, Hoy D, Cross M, Merriman TR, Vos T, Buchbinder R, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. (2014) 73:1470–6. 10.1136/annrheumdis-2013-204647 [DOI] [PubMed] [Google Scholar]
- 5.Danve A, Neogi T. , Rising global burden of gout: time to act. Arthr Rheumatol (Hoboken N J). (2020) 72:1786–8. 10.1002/art.41453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safiri S, Kolahi AA, Hill C, Smith E, Carson-Chahhoud K, Mansournia MA, et al. Prevalence, deaths, and disability-adjusted life years due to musculoskeletal disorders for 195 countries and territories 1990-2017. Arthr Rheumatol (Hoboken N J). (2021) 73:702–14. 10.1002/art.41571 [DOI] [PubMed] [Google Scholar]
- 7.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16:380–90. 10.1038/s41584-020-0441-1 [DOI] [PubMed] [Google Scholar]
- 8.Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK, et al. The dietary approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ (Clin Res ed). (2017) 357:j1794. 10.1136/bmj.j1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokose C, McCormick N, Rai SK, Lu N, Curhan G, Schwarzfuchs D, et al. Effects of low-fat, mediterranean, or low-carbohydrate weight loss diets on serum urate and cardiometabolic risk factors: a secondary analysis of the dietary intervention randomized controlled trial (DIRECT). Diab Care. (2020) 43:2812–20. 10.2337/dc20-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. (2004) 350:1093–103. 10.1056/NEJMoa035700 [DOI] [PubMed] [Google Scholar]
- 11.Pan W-H, Wu HJ, Yeh CJ, Chuang SY, Chang HY, Yeh NH, et al. Diet and health trends in Taiwan: comparison of two nutrition and health surveys from 1993-1996 and 2005-2008. Asia Pac J Clin Nutr. (2011) 20:238–50. [PubMed] [Google Scholar]
- 12.Towiwat P, Li Z-G. The association of vitamin C, alcohol, coffee, tea, milk and yogurt with uric acid and gout. Int J Rheum Dis. (2015) 18:495–501. 10.1111/1756-185X.12622 [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Harman JL, Coresh J, Köttgen A, McAdams-DeMarco MA, Correa A, et al. The dietary fructose:vitamin c intake ratio is associated with hyperuricemia in african-american adults. J Nutr. (2018) 148:419–26. 10.1093/jn/nxx054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siqueira JH, Mill JG, Velasquez-Melendez G, Barreto SM, Benseñor IM, et al. Sugar-sweetened soft drinks and fructose consumption are associated with hyperuricemia: cross-sectional analysis from the brazilian longitudinal study of adult health (ELSA-Brasil). Nutrients. (2018) 10:981. 10.3390/nu10080981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalbeth N, Wong S, Gamble GD, Mason B, Pool B, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. (2010) 69:1677–82. 10.1136/ard.2009.124230 [DOI] [PubMed] [Google Scholar]
- 16.Xu B, Houston DK, Locher JL, Ellison KJ, Gropper S, Buys DR, et al. Higher Healthy Eating Index-2005 scores are associated with better physical performance. J Gerontol Ser A Biol Sci Med Sci. (2012) 67:93–9. 10.1093/gerona/glr159 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Ajami NJ, El-Serag HB, Hair C, Graham DY, White DL, et al. Dietary quality and the colonic mucosa-associated gut microbiome in humans. Am J Clin Nutr. (2019) 110:701–12. 10.1093/ajcn/nqz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B, Houston D, Locher JL, Zizza C. The association between Healthy Eating Index-2005 scores and disability among older Americans. Age Ageing. (2012) 41:365–71. 10.1093/ageing/afr158 [DOI] [PubMed] [Google Scholar]
- 19.de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB, et al. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. (2011) 34:1150–6. 10.2337/dc10-2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips JA. Dietary guidelines for americans, 2020-2025. Workplace Health Safety. (2021) 69:395. 10.1177/21650799211026980 [DOI] [PubMed] [Google Scholar]
- 21.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo ER, Kim D, Vazquez-Montesino LM, Escober JA, Li AA, Tighe SP, et al. Diet quality and its association with nonalcoholic fatty liver disease and all-cause and cause-specific mortality. Liver Int Off J Int Associat Study Liver. (2020) 40:815–24. 10.1111/liv.14374 [DOI] [PubMed] [Google Scholar]
- 23.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the united states and decadal trends: the national health and nutrition examination survey, 2007-2016. Arthr Rheumatol (Hoboken NJ). (2019) 71:991–9. 10.1002/art.40807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Health and Nutrition Examination Survey. Contract for Difference (CFD). (2016). Available online at: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_MEC_Laboratory_Procedures_Manual.pdf (accessed January 30, 2022). [Google Scholar]
- 25.Tsai J, Homa DM, Gentzke AS, Mahoney M, Sharapova SR, Sosnoff CS, et al. Exposure to secondhand smoke among nonsmokers - United States, 1988-2014. MMWR Morbid Mortal Weekly Report. (2018) 67:1342–6. 10.15585/mmwr.mm6748a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholes S, Bann D. Education-related disparities in reported physical activity during leisure-time, active transportation, and work among US adults: repeated cross-sectional analysis from the National Health and Nutrition Examination Surveys, 2007 to 2016. BMC Public Health (2018) 18:926. 10.1186/s12889-018-5857-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery J, Lu J, Ratliff S, Mezuk B. Food insecurity and depression among adults with diabetes: results from the national health and nutrition examination survey (NHANES). Diabetes Educat. (2017) 43:260–71. 10.1177/0145721717699890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu N, Dubreuil M, Zhang Y, Neogi T, Rai SK, Ascherio A, et al. Gout and the risk of Alzheimer’s disease: a population-based BMI-matched cohort study. Ann Rheum Dis. (2016) 75:547–51. 10.1136/annrheumdis-2014-206917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alharthi SSY, Dubreuil M, Zhang Y, Neogi T, Rai SK, Ascherio A, et al. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90:16–25. 10.1002/JPER.18-0183 [DOI] [PubMed] [Google Scholar]
- 30.Breslow RA, Chen CM, Graubard BI, Jacobovits T, Kant AK. Diets of drinkers on drinking and nondrinking days: NHANES 2003-2008. Am J Clin Nutr. (2013) 97:1068–75. 10.3945/ajcn.112.050161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos D, Dhamoon MS. Trends in antihypertensive medication use among individuals with a history of stroke and hypertension, 2005 to 2016. JAMA Neurol. (2020) 77:1382–89. 10.1001/jamaneurol.2020.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Florea A, Jacobs ET, Harris RB, Klimentidis YC, Thajudeen B, Kohler LN, et al. Chronic kidney disease unawareness and determinants using 1999-2014 National Health and Nutrition Examination Survey Data. J Public Health. (2021) 9:fdab112. 10.1093/pubmed/fdab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saydah SH, Siegel KR, Imperatore G, Mercado C, Gregg EW, et al. The cardiometabolic risk profile of young adults with diabetes in the U.S. Diabetes Care. (2019) 42:1895–902. 10.2337/dc19-0707 [DOI] [PubMed] [Google Scholar]
- 34.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agricult Biol Environ Statist. (2015) 20:100–20. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, et al. Analysis of environmental chemical mixtures and non-hodgkin lymphoma risk in the NCI-SEER NHL Study. Environ Health Perspect. (2015) 123:965–70. 10.1289/ehp.1408630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czarnota J, Gennings C, Wheeler DC. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Informatics. (2015) 14(Suppl. 2):159–71. 10.4137/CIN.S17295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int. (2019) 123:325–36. 10.1016/j.envint.2018.11.076 [DOI] [PubMed] [Google Scholar]
- 38.Kontogianni MD, Panagiotakos DB, Tsetsekou E, Zeimbekis A, Pitsavos C, et al. Adherence to the Mediterranean diet and serum uric acid: the ATTICA study. Scand J Rheumatol. (2012) 41:442–9. 10.3109/03009742.2012.679964 [DOI] [PubMed] [Google Scholar]
- 39.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutrition, metabolism, and cardiovascular diseases. NMCD. (2006) 16:559–68. 10.1016/j.numecd.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 40.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Preventive Med. (2007) 44:335–40. 10.1016/j.ypmed.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 41.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Int Med. (2008) 168:713–20. 10.1001/archinte.168.7.713 [DOI] [PubMed] [Google Scholar]
- 42.Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. (2009) 20:2253–9. 10.1681/ASN.2009030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, et al. Evaluation of the healthy eating index-2015. J Acad Nutr Dietet. (2018) 118:1622–33. 10.1016/j.jand.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snetselaar LG, de Jesus JM, DeSilva DM, Stoody EE. Dietary guidelines for Americans, 2020-2025: understanding the scientific process guidelines, and key recommendations. Nutrit Today. (2021) 56:287–95. 10.1097/NT.0000000000000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrel DR, Verdy M, PetitClerc C, Martin C, Brulé D, Hamet P, et al. Milk- and soy-protein ingestion: acute effect on serum uric acid concentration. Am J Clin Nutr. (1991) 53:665–9. 10.1093/ajcn/53.3.665 [DOI] [PubMed] [Google Scholar]
- 46.Dalbeth N, Gracey E, Pool B, Callon K, McQueen FM, Cornish J, et al. Identification of dairy fractions with anti-inflammatory properties in models of acute gout. Ann Rheum Dis. (2010) 69:766–9. 10.1136/ard.2009.113290 [DOI] [PubMed] [Google Scholar]
- 47.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. A higher ratio of refined grain to whole grain is associated with a greater likelihood of chronic kidney disease: a population-based study. Br J Nutr. (2019) 121:1294–302. 10.1017/S0007114518003124 [DOI] [PubMed] [Google Scholar]
- 48.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ (Clin Res ed.). (2016) 353:i2716. 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liese AD, Roach AK, Sparks KC, Marquart L, D’Agostino RB, Jr., Mayer-Davis EJ, et al. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. (2003) 78:965–71. 10.1093/ajcn/78.5.965 [DOI] [PubMed] [Google Scholar]
- 50.Steffen LM, Jacobs DR, Jr., Murtaugh MA, Moran A, Steinberger J, Hong CP, et al. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am J Epidemiol. (2003) 158:243–50. 10.1093/aje/kwg146 [DOI] [PubMed] [Google Scholar]
- 51.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. (2004) 134:1181–5. 10.1093/jn/134.5.1181 [DOI] [PubMed] [Google Scholar]
- 52.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa T, Lanaspa MA, Johnson RJ. The effects of fruit consumption in patients with hyperuricaemia or gout. Rheumatol (Oxf Engl). (2019) 58:1133–41. 10.1093/rheumatology/kez128 [DOI] [PubMed] [Google Scholar]
- 54.Zou F, Zhao X, Wang F. A review on the fruit components affecting uric acid level and their underlying mechanisms. J Food Biochem. (2021) 45:e13911. 10.1111/jfbc.13911 [DOI] [PubMed] [Google Scholar]
- 55.Kanbara A, Hakoda M, Seyama I. Urine alkalization facilitates uric acid excretion. Nutr J. (2010) 9:45. 10.1186/1475-2891-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger L, Gerson CD, Yü TF. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med. (1977) 62:71–6. 10.1016/0002-9343(77)90351-5 [DOI] [PubMed] [Google Scholar]
- 57.Wu T-K, Wei CW, Pan YR, Cherng SH, Chang WJ, Wang HF, et al. Vitamin C attenuates the toxic effect of aristolochic acid on renal tubular cells via decreasing oxidative stress-mediated cell death pathways. Mol Med Rep. (2015) 12:6086–92. 10.3892/mmr.2015.4167 [DOI] [PubMed] [Google Scholar]
- 58.Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens. (1991) 9:465–73. 10.1097/00004872-199105000-00011 [DOI] [PubMed] [Google Scholar]
- 59.Reungjui S, Roncal CA, Mu W, Srinivas TR, Sirivongs D, Johnson RJ, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. (2007) 18:2724–31. 10.1681/ASN.2007040416 [DOI] [PubMed] [Google Scholar]
- 60.Cronin P, Joyce SA, O’Toole PW, O’Connor EM. Dietary fibre modulates the gut microbiota. Nutrients. (2021) 13:1655. 10.3390/nu13051655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Nair MG, Strasburg GM, Chang YC, Booren AM, Gray JI, et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Products. (1999) 62:802. 10.1021/np990184z [DOI] [PubMed] [Google Scholar]
- 62.Nijveldt RJ, van Nood E, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. (2001) 74:418–25. 10.1093/ajcn/74.4.418 [DOI] [PubMed] [Google Scholar]
- 63.Teng GG, Pan A, Yuan JM, Koh WP. Food sources of protein and risk of incident gout in the singapore chinese health study. Arthr Rheumatol (Hoboken N J). (2015) 67:1933–42. 10.1002/art.39115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sucher S, Markova M, Hornemann S, Pivovarova O, Rudovich N, Thomann R, et al. Comparison of the effects of diets high in animal or plant protein on metabolic and cardiovascular markers in type 2 diabetes: a randomized clinical trial. Diab Obes Metab. (2017) 19:944–52. 10.1111/dom.12901 [DOI] [PubMed] [Google Scholar]
- 65.Belanger MJ, Wee CC, Sacks FM, Miller ER, Appel LJ, Mukamal KJ, et al. Effects of dietary macronutrients on serum urate: results from the OmniHeart trial. Am J Clin Nutr. (2021) 113:1593–9. 10.1093/ajcn/nqaa424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowen-Davies Z, Muller S, Mallen CD, Hayward RA, Roddy E. Gout severity. Arthr Care Res. (2018) 70:1822–8. 10.1002/acr.23562 [DOI] [PubMed] [Google Scholar]
- 67.Nieradko-Iwanicka B. The role of alcohol consumption in pathogenesis of gout. Crit Rev Food Sci Nutr. (2021) 19:1–9. 10.1080/10408398.2021.1911928 [DOI] [PubMed] [Google Scholar]
- 68.Tomita M, Mizuno S, Yokota K. Increased levels of serum uric acid among ex-smokers. J Epidemiol. (2008) 18:132–4. 10.2188/jea.JE2006332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burke BT, Köttgen A, Law A, Grams M, Baer AN, Coresh J, et al. Gout in older adults: the atherosclerosis risk in communities study. J Gerontol Ser A Biol Sci Med Sci. (2016) 71:536–42. 10.1093/gerona/glv120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension (Dallas Tex 1979). (2007) 50:306–12. 10.1161/HYPERTENSIONAHA.107.091041 [DOI] [PubMed] [Google Scholar]
- 71.Johnson RJ, Nakagawa TM, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. (2013) 62:3307–15. 10.2337/db12-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ (Clin Res ed). (2008) 336:309–12. 10.1136/bmj.39449.819271.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DiNicolantonio JJ, O’Keefe JH, Lucan SC. Added fructose: a principal driver of type 2 diabetes mellitus and its consequences. Mayo Clinic Proc. (2015) 90:372–81. 10.1016/j.mayocp.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 74.Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv Nutr (Bethesda Md). (2013) 4:220–5. 10.3945/an.112.002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burner TW, Rosenthal AK. Diabetes and rheumatic diseases. Curr Opini Rheumatol. (2009) 21:50–4. 10.1097/BOR.0b013e32831bc0c4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.