Abstract
Ovarian cancer is the leading cause of mortality due to gynecologic malignancy. The majority of women diagnosed with the most common subtype, high-grade serous ovarian carcinoma (HGSC), develop resistance to conventional therapies despite initial response to treatment. HGSC tumors displaying DNA damage repair (DDR) gene deficiency and high chromosomal instability mainly associate with higher cytotoxic immune cell infiltration and expression of genes associated with these immune pathways. Despite the high level of immune infiltration observed, the majority of patients with HGSC have not benefited from immunomodulatory treatments as the mechanistic basis of this infiltration is unclear. This lack of response can be primarily attributed to heterogeneity at the levels of both cancer cell genetic alterations and the tumour immune microenvironment. Strategies to enhance anti-tumour immunity have been investigated in ovarian cancer, of which interferon activating therapies present as an attractive option. Of the several type I interferon (IFN-1) stimulating therapies, exogenously activating the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway is emerging as a promising avenue. Herein, we highlight our current understanding of how constitutive and induced cGAS-STING pathway activation influences the ovarian tumour microenvironment. We further elaborate on the links between the genomic alterations prevalent in ovarian tumours and how the resultant immune phenotypes can make them more susceptible to exogenous STING pathway activation and potentiate immune-mediated killing of cancer cells. The therapeutic potential of cGAS-STING pathway activation in ovarian cancer and factors implicating treatment outcomes are discussed, providing a rationale for future combinatorial treatment approaches on the backbone of chemotherapy.
Subject terms: Tumour immunology, Ovarian cancer
Introduction
The past decade witnessed several successes with novel therapies that exploit the immune system as a vehicle to treat cancer. A vast majority of these include immune checkpoint blockade (ICB) therapies that block cancer cells’ ability to dampen the magnitude of anti-cancer immune attacks [1, 2]. Among the factors influencing response to ICB or any immunomodulatory therapy, the landscape of the pre-existing tumour immune microenvironment (TIME), which can either co-evolve during carcinogenesis or from treatment induced selective pressures, has emerged to be the most informative [3]. The pre-treatment TIME is defined as a spectrum from ‘inflamed’ to ‘non-inflamed’ based on the density and localization of activated T cell infiltration and interferon (IFN) gene expression patterns, which show distinct associations with treatment outcomes. As such, significant advances have been made to understand factors originating from both host and cancer cells that may contribute to this spectrum of TIME states within solid tumours [4]. It is noteworthy that all these factors converge towards rescuing a non-responsive tumour immune state or host immunity via activation of type I IFN (IFN-1) and its induced chemokines, CXCL9/10/11, to mobilize anti-tumour immune cells to the TIME [5]. Given the paradoxical roles of IFN-1, a balanced exogenous activation commensurate with the magnitude of IFN-1 activation is critical to achieving durable and favorable effects. Exogenously inducing activation of cytosolic innate immune sensing pathways is one strategy towards IFN-1 activation [6].
Among the wide array of cytosolic innate immune sensing pathways that activate IFN-1, the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway has emerged as a significant determinant of distinct pre- and post-treatment TIME states [6]. The molecular underpinnings of the cGAS-STING pathway and its role in eliciting protective immunity against pathogens have been extensively reviewed [6]. More recently, the cGAS-STING pathway has received heightened attention due to its integral and multifaceted role in cancer immunosurveillance — particularly its potential as an immunomodulatory agent in several cancer types [6, 7]. Herein, we focus on how the cGAS-STING pathway might be exploited to inform strategic therapies for the treatment of ovarian cancer.
The cGAS-STING pathway and type I interferon
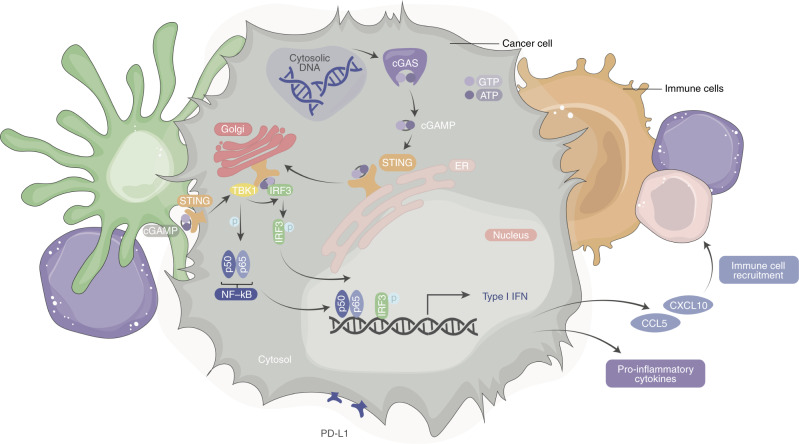
Under physiologically healthy conditions, cell intrinsic DNA is confined primarily to the nucleus and mitochondria and is rarely found in the cytoplasm. In instances where DNA is released into the cytosol from the nucleus or mitochondria, intra- and extracellular ribonucleases and scavenger cells degrade this genetic material [8]. However, when DNA accumulates within the cytosol it can be detected by the protein cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS; Fig. 1) [9, 10]. Activation of cGAS by double-stranded DNA (dsDNA), or to a lesser degree by single-stranded DNA, initiates synthesis of the messenger protein cyclic guanosine monophosphate-adenosine monophosphate (cGAMP; Fig. 1). Apart from intrinsic dsDNA release into the cytosol, extrinsic dsDNA from pathogens, extracellular vesicles, or dying cells can be internalized to the cytosol via several endosomal pathways [11]. Detection of any of the aforementioned pattern recognition receptors (PRRs) by cGAS leads to the synthesis of cGAMP, which serves as a ligand of the Stimulator of Interferon Genes protein (STING; also termed TMEM173, STING1, MITA or ERIS) located on the endoplasmic reticulum [11, 12] and recently identified to localize in the plasma membrane (Fig. 1) [13]. The binding of cGAMP to the STING dimer is made possible via transportation of extracellular cGAMP into the cytosol by SLC19A1, which allows for a conformational change that converts STING to its active conformation [12, 14]. The STING protein is then trafficked from the endoplasmic reticulum (ER) to the Golgi apparatus via translocon-associated protein beta (TRAPβ). STING binds to and activates TANK binding kinase 1 (TBK1) via phosphorylation at the Golgi apparatus (Fig. 1) [15, 16]. Additionally, alternatively spliced STING isoforms on the plasma membrane can use extracellular cGAMP which then promotes dimerization of STING and its subsequent interaction with TBK1 (Fig. 1). The activated STING-TBK1 complex further phosphorylates the transcription factors interferon regulatory factor 3 (IRF3) or nuclear factor kappa B (NF-κB) [16]. The dimerized IRF3 and activated NF-κB then translocate to the nucleus and bind to the IFN-1 promoter to induce transcription and expression of IFN-1 associated genes (Fig. 1) [12]. Though the canonical activation of the STING pathway is mainly discussed due to its relevance to infection and cancer, non-canonical STING activation has implications in the effect of this pathway and is reviewed elsewhere [17–19].
Fig. 1. cGAS-STING pathway.
Schematic of double-stranded DNA (dsDNA) detection and activation by cyclic GMP-AMP synthase (cGAS), which can occur by cellular stress within a cancer cell. Upon binding dsDNA, cGAS is enzymatically activated post-dimerization of GTP and ATP on its surface. This further synthesizes 2’3’ cyclic GMP-AMP (cGAMP). cGAMP binds to stimulator of interferon genes (STING) on the endoplasmic reticulum (ER) or plasma membrane (PM). The STING-cGAMP complex traffics to the Golgi apparatus and recruits tank binding kinase 1 (TBK1). cGAMP also promotes dimerization of PM STING and interaction with TBK1. The phosphorylation of interferon regulatory factor 3 (IRF3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) by TBK1 allows for their translocation into the nucleus and triggers transcription of type I interferon (IFN-1) genes.
IFN-1s are polypeptides that activate intracellular pathways which regulate innate and adaptive immune responses. These molecules differ from type II IFN in their activation by PRRs [20]. PRRs serve as innate cellular sensors that recognize danger signals, which are molecules released by pathogens (pathogen-associated molecular patters, PAMPs) or damaged/dying cells (danger-associated molecular patterns, DAMPs) [21]. These PRRs survey endosomal, cytosolic, and extracellular compartments for signs of stress, that, upon activation, result in cellular stress responses. Although several pathways are involved in the induction of IFN-1 following the activation of PRRs by exogenous and endogenous ligands, recent demonstration of the potent IFN-1 response resulting from cGAS-STING pathway activation and its success in sensitizing tumours to traditional and contemporary therapies has drawn our attention to its anti-cancer therapeutic potential [7].
Recruitment of anti-tumour immune cells including T cells, B cells, natural killer (NK) cells, macrophages, and dendritic cells (DCs), primarily occurs through chemokine gradients such as increased levels of IFN-1 induced CXCL9, CXCL10, and CXCL11. The critical role of cGAS-STING pathway in these processes is now well established [22, 23]. Thus, furthering our current understanding of how these TIME states evolve is critical for evaluating clinical outcomes and response to treatment in ovarian cancer.
The tumour immune microenvironment (TIME) in ovarian cancer
As the most lethal gynaecologic malignancy, ovarian cancer accounts for ~152,000 deaths globally each year [24]. Epithelial ovarian cancers (EOC) compose > 90% of all ovarian cancer cases, of which high-grade serous carcinoma of the ovary (HGSC) is the most common and lethal subtype, with evidence to suggest its origination both from the fallopian tube and ovarian surface epithelium [25]. There are five histologically defined subtypes of EOCs: HGSC, low-grade serous carcinoma (LGSC), mucinous carcinoma (MC), endometroid carcinoma (EC) and clear cell ovarian carcinoma (CCOC) [26]. This review will focus on the implications of STING pathway activation in the context of HGSC, the most fatal subtype, with a dismal 5-year survival rate of 45% [24]. Lack of effective screening practices and nonspecific symptoms lead to late diagnosis, primarily at stage III or IV (> 80%), where the tumour has already spread throughout the peritoneum [24]. Although most HGSC patients initially respond to a combination of platinum and taxane-based chemotherapies, over 70% of cases will relapse due to acquired chemotherapy resistance [24, 26].
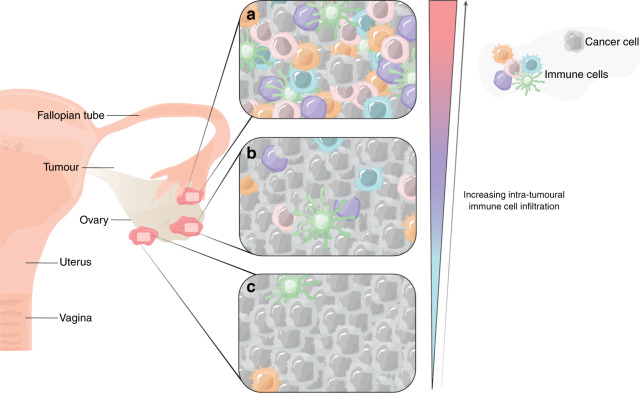
In addition to the broad categorization of ovarian tumours based on the four molecular subtypes (C1 mesenchymal, C2 immunoreactive, C4 differentiated and C5 proliferative) via their transcriptomic signatures [27], the complexity of the disease is further driven by the vast inter- and intratumoural spatial heterogeneity (Fig. 2) [28, 29]. Unlike other solid tumours that are localized and metastasize through the blood, HGSC forms small metastases throughout the peritoneum, resulting in multiple tumours with unique and distinct microenvironments (Fig. 2). To compound this heterogeneity, HGSC is characterized by ascites rich in immunosuppressive factors, which results in its own broader tumour microenvironment [30]. Several studies have revealed the inter-tumoural heterogeneity of HGSC, where underlying genetic alterations and phenotypic differences in the infiltration patterns of cytotoxic T cells can be observed in multiple tumours from the same patient sample [31]. Like other solid tumours, the TIME of HGSC patients at the inter-and intratumoural level has also been associated with cancer progression, metastases, and variable response to therapy (Fig. 2) [32–34]. We, as well as others, have demonstrated the significance of tumour infiltrating lymphocytes (TILs) that associate strongly with clinical outcomes [35]. While the presence of intratumoural cytotoxic T cells correlates with improved clinical outcomes in advanced HGSC [36, 37], these observations only provide a glimpse into the dynamic TIME within. Though limitations exist, these associations have provided the proof of concept that modulating the HGSC TIME may be therapeutically beneficial [38].
Fig. 2. Variable immune cell infiltration patterns within multiple tumours from a single patient.
Representation of the inter-tumoural heterogeneity seen in high grade serous ovarian cancer (HGSC). Distinct immune profiles representing (a) high, (b) intermediate, and (c) poor immune infiltration patterns seen in several tumours from the same patient sample which result, in part, by variable cGAS-STING pathway activation. Unlike other solid tumours, HGSC is further characterized by formation of small metastases throughout the peritoneum, each with a unique tumour immune microenvironment, which has therapeutic implications.
Despite being a highly immunogenic tumour, HGSC has remained difficult to treat due to a higher magnitude of cancer cell intrinsic genomic instability, a hallmark of cancer which imparts both the advantage of evading anti-tumour immunologic responses and the subsequent growth advantage. Recent ICB therapies targeting checkpoints such as indoleamine 2,3-dioxygenase (IDO1) [39, 40], cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [41, 42], and the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) axis have not benefited HGSC patients [41, 42], showing overall response rates of 0–15%, and 0–11%, for CTLA-4 and PD1/PD-L1 ICBs, respectively. Clinical trials assessing IDO1 inhibitors have not reported conclusive findings primarily from their discontinuation due to disease progression [43]. The failure of these immunotherapies may be attributed to their focus on activating the adaptive arm of the immune system, which requires antigen specificity. Treatment with ICB therapy can indeed be challenged by both high genomic instability and intratumoural heterogeneity in HGSC. Indeed, Zhang et al. have demonstrated the dynamic nature of the HGSC TIME and TIL subtypes that closely associate with clonal populations of cancer cells [31]. In line with this finding, a recent single-cell dissection of human ovarian tissues has uncovered potential molecular mechanisms and interactions that underpin the variable immune phenotypes that reflect the refined categories of the inflamed and non-inflamed TIME: immune infiltrated, excluded, and desert tumours [44]. The T cell infiltration patterns seen within these variable phenotypes occur, in part, by immune cell-cancer cell crosstalk via certain chemokine receptor-ligand interactions, such as the CXCR3/CXCL9/10/11 axis and the CXCR6/CXCL16 axis [44]. These latest findings support previous tissue microarray-based studies and emphasize the urgent need to further reveal the genetic and molecular alterations that are key to the evolution of a heterogenous TIME at metastatic sites.
Immune cell spatial heterogeneity in the TIME of HGSC
Resistance to platinum-based chemotherapy remains a major hurdle to improving treatment outcomes in HGSC. In a cohort of 734 HGSC patient tumours, we showed that high CD8+ T cell infiltration and increased expression of IFN genes were associated with chemotherapy sensitivity [45]. Providing additional evidence, a multicenter observational study on a cohort of over 3196 HGSC patient tumours demonstrated a positive correlation between increased CD8+ TILs and HGSC survival [35]. More recently, other lymphocytes have been explored for their prognostic impact, including NK cells and CD20+ B cells. NK cell infiltration has also been associated with improved overall survival in solid cancers [46], including HGSC, as demonstrated in a cohort of 283 HGSC samples [47]. In addition, CD20+ B cell infiltration predicted overall survival in two independent cohorts of chemotherapy naïve HGSC patients [48]. Future investigations evaluating the prognostic benefit of immune cell infiltration should consider paralleled analysis of the suppressive immune populations in the tumour microenvironment as reflected by the expression of regulatory markers and immune checkpoints, such as FoxP3, PD-1, PD-L1, TIGIT and others.
Given that metastasis to the peritoneum is common in HGSC, several reports have explored the contribution of myeloid cells. Specifically, tumour associated macrophages (TAMs) have been reported to prime this pre-metastatic niche, promoting tumour cell extravasation and survival [49]. In a recent analysis of immune cell proportions using tumour transcriptomic profiles from 2000 HGSC patients, an association between high pro-inflammatory M1 and non-activated M0 macrophages with favourable overall survival (OS), was reported [50]. A similar in silico analysis using CIBERSORT approximated immune cell infiltration in tumours and similarly found M1-like macrophages to predict better outcomes, while M2-like immune suppressive macrophages predicted worse outcomes [51]. Furthermore, TAMs, which typically express an M2-like phenotype, can both promote early peritoneal cavity metastasis and upregulate an immunosuppressive environment, hindering the effector functions of CD8+ cytotoxic T cells, NK cells, and DCs [52]. TAMs also play a role in mediating resistance to chemotherapy via induction of epithelial-mesenchymal transition (EMT) and cancer stem cell properties [53]. Further, in contrast to the well-established favourable role of DCs in cross-priming of T cells, myeloid-derived suppressor cells (MDSCs) have been shown to promote an immunosuppressive state in the ID8 syngeneic murine model of HGSC [48, 54].
Overall, findings to date emphasize the importance of activated anti-tumour infiltrating immune cell populations within ovarian tumours. Most importantly, these reports highlight studies evaluating mere infiltration levels of immune cells are limited in their ability to predict survival and require additional insight into the overall tumour state. Further research into the factors that drive variable TIME states induced following activation of IFN-1 in the HGSC TIME is needed.
The role of the cGAS-STING pathway in the TIME of HGSC
Several reports have identified the dominant role of the cytosolic DNA activated cGAS-STING pathway in shaping the TIME, and its potential defects in ovarian cancer cells [6, 55–57]. The mechanism behind how the cGAS-STING pathway orchestrates a pro- or anti-tumour immune response requires additional investigation. This will be crucial to predicting how this pathway influences sensitization to immunomodulatory intervention.
Recent studies have suggested a critical function for STING signalling in lymphocyte recruitment and anti-tumour function. Woo et al. demonstrated impaired spontaneous anti-tumour T-cell priming against tumour antigens in vivo in STING knockout and IRF3 knockout mice [58, 59]. Work exploring T memory stem cells demonstrated the ability for cGAS-STING mediated DNA sensing to maintain CD8+ T cell stemness. This study further supported the key role of IFN-1 signaling in facilitating the maintenance of stem cell-like CD8+ T cells and their role in maintaining the effects of T cell therapy [60]. Confirming the role of STING in enhancing cytotoxic CD8+ T cell recruitment and anti-tumour activity, this provides a strong mechanistic explanation for the known prognostic associations between CD8+ TIL infiltration in HGSC tumours [35, 45]. Furthermore, STING pathway activation can promote TAM re-polarization to a M1 phenotype, enhancing cancer cell apoptosis in lung and gastric cancer; however, this has yet to be described in ovarian cancer [61, 62].
STING signaling may also promote recruitment of suppressive immune populations through the myriad of chemokines it induces. Therefore, developing strategies to selectively recruit anti-tumour immune cells will be critical to the success of STING-based therapies. For example, therapies that induce a potent immunogenic cell death effect, such as platinum-based antineoplastic agents, anthracyclines, or radiotherapy followed by addition of a direct (STING agonists or oncolytic viruses) or indirect (PARP inhibitors or radiation) STING pathway activating therapy may show improved outcomes and further sensitize tumours to ICB [63–66]. Thus, a rational exploitation of this pathway in the neoadjuvant setting, post-chemotherapy, would be an attractive option to potentiate the immune stimulation caused by its initial insult. This intervention is supported by our recent study where we evaluated a combination of carboplatin and STING agonist in the ID8 model and found increased proportions of key anti-tumour immune cells and prolonged overall survival of mice [22]. Leveraging the opportunistic changes to the TIME caused by STING-induced IFN-1 activity may therefore prove beneficial in patients. Importantly, stratifying patients to select those who would benefit from STING immune sensitivity/stimulation becomes essential.
Factors implicating outcomes of STING pathway activation in ovarian cancer
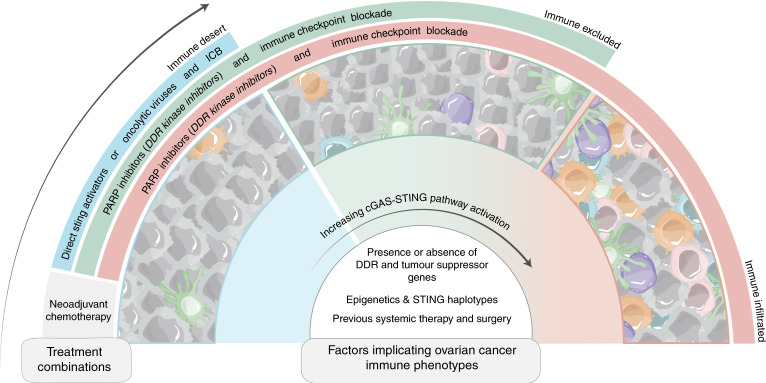
While the cGAS-STING pathway has shown promise as a therapeutic target in pre-clinical ovarian cancer models, there are many factors that can influence STING activation pre- and post-treatment. Au et al. showed that high intratumoural pre-treatment signal transducer and activator of transcription 1 (STAT1) expression, which may be upregulated by cGAS-STING signaling through autocrine signaling, was positively correlated with chemotherapy response and progression free survival (PFS) in HGSC patients [45, 67]. Therapeutically, neoadjuvant chemotherapy (NACT) has been shown to induce local immune activation, potentiating the immunogenicity of tumours [68]. Specifically, evaluation of treatment-naïve and post-NACT HGSC tumours showed a significant increase in NK and T cell infiltration following NACT, demonstrating that irrespective of the high heterogeneity seen in the TIME of advanced HGSC, chemotherapy alone stimulates immune recruitment, even in immune-excluded HGSC tumours (Fig. 3) [68]. Thus, activating the immune system in the neo-adjuvant setting would indeed aid in improving survival outcomes in HGSC.
Fig. 3. Factors altering STING pathway activation in ovarian cancer for potential therapeutic combinations.
The factors influencing activation of the cGAS-STING pathway and treatment response outcomes are not limited to genotype-associated changes such as presence or absence of DNA damage repair (DDR) gene and tumour suppressor gene mutations, epigenetic modifications, and varying STING haplotypes. Additional factors which may influence response to treatment in ovarian cancer include prior treatment exposure and its resulting tumour immune microenvironment (TIME). Understanding and appreciating the several factors which influence cGAS-STING pathway activation can inform rational combinations of traditional and contemporary therapies. The use of neoadjuvant chemotherapy in any case allows for initial immune stimulation further strengthened with various combinations of therapies depending on the pre-existing TIME. An increase in immune cell infiltration is demonstrated with heightened pathway activation, thus, immune desert tumours may see greater benefit from direct STING pathway activating therapies such as STING agonists and oncolytic viruses and may enhance the effects of immune checkpoint blockade (ICB). The combination of indirect activators of the STING pathway, such as PARP inhibitors with chemotherapy may improve outcomes and show promise in further sensitizing tumours to ICB.
Unfortunately, there are many barriers to immune activation intrinsic to tumours including epigenetic modifications. Epigenetic modification has been associated with suppression of STING in a host of cancers (Fig. 3) [69, 70]. In ovarian cancer, Zhang et al. recently showed that USP35 deubiquitinates STING, inactivating it [71]. It was unsurprising that USP35 expression was then associated with reduced CD8+ T cell infiltration, higher tumour grade, and poorer prognosis. Epigenetic silencing of the STING locus was also found to increase sensitivity to oncolytic viral infection in ovarian cancer [57]. Ongoing trials are indeed evaluating the combination of DNA methyltransferase inhibitors and poly-ADP ribose polymerase inhibitors (PARPi) in the treatment of BRCA deficient patients [72].
Differences in STING haplotypes could also alter the effect of STING pathway activation and inform response to treatment (Fig. 3). There are four haplotypes of the STING (TMEM173) gene (REF, HAQ, AQ and Q) in humans that can be inherited as single nucleotide polymorphisms which influence the magnitude of STING pathway activation [73]. Although the HAQ variant is the second most common STING allele and is present in as many as 20% of individuals, it is associated with significantly lower baseline STING pathway activity [74], while the HAQ, R232H and R293Q variants all had reduced IFNβ production in response to a cyclic dinucleotide (CDN) STING agonist in HEK 293T cell [73]. In addition, individuals homozygous for HAQ and R232H were associated with drastically reduced IFN-β production in donor human B cells and mouse models in response to cyclic dinucleotide STING agonists [74]. Future studies surrounding the effect of haplotype on STING pathway activity and response to its activation will be important and hold the potential to serve as biomarkers in HGSC.
Additionally, a variety of immune cell-associated receptors and ligands affect STING pathway activity. For instance, NK cells isolated from the ascites of ovarian cancer patients have decreased KIR2D and NKG2D expression, whose altered expression has previously been shown to be STING-dependent in other cancers [75, 76]. One possible contributor to this phenomenon could be tumor-derived exosomes (TDEs) [77], that contain double-stranded genomic and mitochondrial DNA which can activate the STING pathway in recipient cells to induce an IFN-1 response [78]. While these microvesicles can activate STING signalling, TDEs in patient ascites have been shown to have increased expression of MHC Class I Antigen G, A or HLA-G, which can inhibit NK cell effector functions. TDEs can also suppress the STING-related IFN-1 response through the secretion of cytokines like interleukin 10 (IL-10) and transforming growth factor beta (TGFβ) [79], which have been associated with later disease stage and increased metastasis in ovarian cancer. Taken together, it is possible that differences in cell of origin and cargo of TDEs could affect outcomes of cGAS-STING pathway activation.
Overall, these associations suggest that prior therapy exposure and regimen, epigenetic modifications, differences in STING and cGAS haplotypes, varying immune cell receptors and ligands, and TDEs are all factors that can contribute to the effect of direct or indirect STING activation in ovarian cancer. Indeed, outcome of STING activation influences the pre-existing tumour immune state and the ever-evolving interplay between cancer and immune cells.
Genomic correlates of tumour immune landscape in ovarian cancer and the STING pathway
HGSC tumours exhibit a high magnitude of genomic instability which is a consequence of several copy number alterations and a higher prevalence of DNA damage repair (DDR) gene mutations [28]. Given the critical role of genomic alterations in the regulation of cellular IFN-1 response pathways [80], it is important to integrate these events with associated immune landscapes and correlate with therapeutic outcomes (Fig. 3). Such defects in DDR genes have been reported to prime the IFN-1 response through STING pathway activation, resulting in downstream innate and adaptive immune responses [81]. While HGSC is characterized by ubiquitous TP53 mutations, gene breakages responsible for the inactivation of tumour suppressors RB1, PTEN, NF1, and RAD51B are common and contribute to acquired chemoresistance [28]. Additionally, BRCA1/BRCA2 are inactivated in ~25% of cases, potentially leading to homologous recombination (HR) deficiency and more widespread DDR gene defects [28, 82]. In assessing DDR gene function, the nature of these mutations, with respect to their status as germline versus somatic, may influence the observed outcomes. Consequently, several studies have reported higher immune infiltration and activation, increased chemosensitivity, and more favourable prognosis in ovarian cancer patients with DDR gene function deficiencies such as germline BRCA1/2 loss [83, 84], though the mechanism behind this infiltration is unclear. Despite this commonly known association, tumors with BRCA1/2 deficiency do not always exhibit this phenotype, potentially due to reversion mechanisms. Additionally, loss of the DDR kinase, ATM, results in unrepaired DNA lesions and the subsequent induction of an IFN-1 response in humans and mice by activating cytosolic DNA sensors such as cGAS [85]. Generally, DDR gene deficiency in HGSC associates with longer overall survival [83].
It is well-established that leakage of damaged DNA into the cytosol leads to constitutive activation of the cGAS-STING pathway inducing chemokine secretion and recruitment of immune cells to the TIME [86–88]. The cytotoxic insult and associated immunogenic cell death effect promote further recruitment and killing [89, 90]. As mentioned above, therapies that augment the inhibited repair function caused by these gene deficiencies, such as PARPi, have shown promise in ovarian cancer. Interestingly, although this tumour cell-intrinsic inflammation involving IFN-1 and STING has been reported with BRCA1 loss in ovarian cancer, STING elimination in BRCA1-mutated tumours has been shown to increase CD8+ T cell infiltration and reduce VEGF-A, decreasing tumour neoangiogenesis [91]. Thus, despite the ability of BRCA1 loss to reprogram ovarian tumours toward an inflammatory state, it provides one example, amongst others, that reports a pro-tumoural role of STING. This phenomenon mainly occurs via chronic NF-κB-driven inflammation and should be considered to optimize treatment for patients carrying BRCA1 mutations. Overall, tumours demonstrating deficiencies in genes that result in poor immune infiltration or a “non-inflamed” immune phenotype may therefore benefit from direct STING pathway activating therapies.
Therapeutic potential of STING pathway activation in ovarian cancer
The dichotomous function of the STING pathway must be considered to harness its full potential in the treatment of ovarian cancer. While only one trial assessing the efficacy of direct STING activation using a STING agonist (NCT04609579) in ovarian cancer is on-going, several other combination therapies have demonstrated this feasibility. The PARP inhibitor (PARPi), niraparib, was recently approved for the maintenance treatment of patients with advanced epithelial ovarian cancer who experience complete or partial response to first-line platinum-based chemotherapy [92, 93]. Patients with germline BRCA1/BRCA2 mutations (DDR deficient) and/or patients with a high genomic instability (genomic instability score >42) on this maintenance therapy displayed enhanced PFS [92, 93]. Remarkably, this trial also reported an increased PFS in non-HR deficient patients, which may be attributed to the indirect activation of the STING pathway resulting from PARP inhibition [92]. These improvements in response rates highlight the potential of indirect STING pathway activation as a promising strategy to sensitize ovarian tumours to immunotherapies, even in those that are DDR proficient.
Indeed, PARPi have shown promise in patients with germline BRCA1/BRCA2 mutations [94], but not as a single agent. In addition to inhibiting DNA repair through its typified mode of action rendering cells with underlying DDR deficiencies unable to divide, recent work by Shen et al. demonstrated the ability for PARPi to trigger a STING-dependent immune response. This response was then shown to enhance the therapeutic efficacy of PD-1 targeting ICB, independent of BRCA1/2 status in an ID8 syngeneic murine mouse model [64]. This work demonstrated the ability of PARPi targeting the PD-1/PD-L1 axis to potentiate the therapeutic efficacy of ICB [64]. Thus, immunogenic responses of PARPi can be observed independent of DDR deficiency; however, when present may result in increased STING pathway activity.
Furthermore, heightened STING activity has been accompanied by upregulation of PD-L1 in both cancer and immune cells, providing a promising combinatorial approach [95]. Grabosch et al. demonstrated this phenomenon using high-grade epithelial 2F8 cells [95]. Encouraging results from pre-clinical studies evaluating this combination have driven several phase 1-2 clinical trials (NCT04739800, NCT03330405, NCT02734004, NCT02571725, NCT02484404, NCT02953457) and one phase 3 trial (NCT03598270). PARP inhibitors, used in conjunction with other STING pathway activating therapies such as anthracyclines and platinum-based chemotherapies, offer a rational combinatorial approach with ICB and should be further explored in the context of HGSC (Fig. 3).
Checkpoint therapy is not limited to the immune cell-cancer cell interaction, as seen through the work of Lampert et al., investigating the immune microenvironment of HGSC patient tumours from a phase II clinical trial of the cell cycle checkpoint 1 inhibitor (CHK1i) [96]. Interestingly, the involvement of the cGAS-STING pathway, assessed via expression of TBK1 in biopsies, was associated with improved PFS. Further, whole transcriptome profiling of these tumours using bulk RNA-sequencing analysis revealed an increased infiltration of B and memory T cells following CHK1i therapy, suggesting an enhanced innate and adaptive immune response following this treatment [96].
Another attractive therapeutic that is also a direct activator of the cGAS-STING pathway is oncolytic viral (OV) therapy. A large proportion of ovarian cancer cells display defective STING pathway signaling through the suppression of STING and its upstream sensor, cGAS [57]. However, STING-independent dsRNA-activated cytokine production via the RIG-I/MDA5 pathway was not defective and rendered ovarian cancer cells susceptible to OVs in vitro and in vivo [57]. As mentioned earlier, OVs have a potent immunogenic cell death-inducing effect, which likely underlies the response of tumours in the absence of STING or cGAS in ovarian cancer cells. This work highlights the significance of evaluating cGAS-STING signalling within ovarian cancer cells to predict response and outcome to therapies such as OVs (Fig. 3).
While emerging therapies in the treatment of ovarian cancer focus on immunomodulation, the gold-standard of ovarian cancer treatment is platinum-based chemotherapy. The varying level of immunogenicity induced by different platinum chemotherapeutics as single agents or in combination with others, such as doxorubicin, has been widely explored [97, 98]. However, the involvement of the cGAS-STING pathway in response to the different anti-tumour immune-activating agents in ovarian cancer requires further investigation. Interestingly, both acute and chronic exposure to cisplatin-induced PD-L1 expression, when countered with anti-PD-L1 ICB in either a platinum-sensitive model or in a cisplatin-treated platinum-resistant model of ovarian cancer, increased OS [95]. In line with this, previous work by our group demonstrated this phenomenon with the use of carboplatin in combination with PD-1 ICB [22]. This increased activation of the cGAS-STING pathway using genotoxic agents may not only enhance the immune response alone through an increased IFN-1 response but also sensitize ovarian tumours to ICB, particularly in a neoadjuvant setting (Fig. 3).
Concluding remarks
As a key innate immune sensor, the cGAS-STING pathway is important in controlling tumour growth and progression via anti-tumour immune cell recruitment, priming and activation. Indeed, both indirect and direct STING pathway activators can elevate the efficacy of conventional therapies such as chemotherapeutic agents used in ovarian cancer; however, emerging evidence has described the potential pro-tumorigenic roles of these activators. In order to provide optimal therapeutic combinations, understanding and applying the knowledge of factors that influence STING-associated TIMEs and the underlying biological mechanisms are critical. Further work investigating the factors discussed and their mechanisms and implications on traditional and contemporary therapies is warranted to allow for optimal exploitation of this pathway and to provide improved outcomes in ovarian cancer patients.
Acknowledgements
This work was supported by funding from the Canadian Institutes for Health Research and the Ontario Ministry of Research, Innovation, and Science Early Researcher Award to MK. Fellowship support to NS was provided by the Franklin Bracken Fellowship, Queen’s University, and the Canada Graduate Scholarship program.
Author contributions
MK conceived the manuscript. NS reviewed the literature, drafted the papre and created the illustrations. DL contributed to writing the manuscript. MK, NS, DL, SN, and JWS helped with editing and revising the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 2.Wilky BA. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol Rev. 2019;290:6–23. doi: 10.1111/imr.12766. [DOI] [PubMed] [Google Scholar]
- 3.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–66. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trujillo JA, Sweis RF, Bao R, Luke JJT. Cell-inflamed versus Non-T cell-inflamed tumors: A conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res. 2018;6:990–1000. doi: 10.1158/2326-6066.CIR-18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borden EC. Interferons alpha and beta in cancer: Therapeutic opportunities from new insights. Nat Rev Drug Disco. 2019;18:219–34. doi: 10.1038/s41573-018-0011-2. [DOI] [PubMed] [Google Scholar]
- 6.Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10:26–39. doi: 10.1158/2159-8290.CD-19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger G, Marloye M, Lawler SE. Pharmacological modulation of the STING pathway for cancer immunotherapy. Trends Mol Med. 2019;25:412–27. doi: 10.1016/j.molmed.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Atianand MK, Fitzgerald KA. Molecular basis of DNA recognition in the immune system. J Immunol. 2013;190:1911–8. doi: 10.4049/jimmunol.1203162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L, Liu P. Cytosolic DNA sensing by cGAS: Regulation, function, and human diseases. Signal Transduct Target Ther. 2021;6:170. doi: 10.1038/s41392-021-00554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet. 2019;20:657–74. doi: 10.1038/s41576-019-0151-1. [DOI] [PubMed] [Google Scholar]
- 12.Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21:501–21. doi: 10.1038/s41580-020-0244-x. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhu Y, Zhang X, An X, Weng M, Shi J, et al. An alternatively spliced STING isoform localizes in the cytoplasmic membrane and directly senses extracellular cGAMP. J Clin Invest. 2021;132:1–11. doi: 10.1172/JCI144339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–8. doi: 10.1038/s41586-019-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vashi N, Bakhoum SF. The evolution of STING signaling and its involvement in cancer. Trends Biochem Sci. 2021;46:446–60. doi: 10.1016/j.tibs.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol. 2014;88:5328–41. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–72. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Eyndhoven LC, Singh A, Tel J. Decoding the dynamics of multilayered stochastic antiviral IFN-I responses. Trends Immunol. 2021. [DOI] [PubMed]
- 21.Bai L, Li W, Zheng W, Xu D, Chen N, Cui J. Promising targets based on pattern recognition receptors for cancer immunotherapy. Pharm Res. 2020;159:105017. doi: 10.1016/j.phrs.2020.105017. [DOI] [PubMed] [Google Scholar]
- 22.Ghaffari A, Peterson N, Khalaj K, Vitkin N, Robinson A, Francis JA, et al. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer. 2018;119:440–9. doi: 10.1038/s41416-018-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus A, Mao AJ, Lensink-Vasan M, Wang L, Vance RE, Raulet DH. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49:754–63. doi: 10.1016/j.immuni.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:191–226. doi: 10.6004/jnccn.2021.0007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Dolgalev I, Zhang T, Ran H, Levine DA, Neel BG. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat Commun. 2019;10:5367. doi: 10.1038/s41467-019-13116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 27.Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC, Jr., Beral V, et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668–79. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170:927–38. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stack MS, Nephew KP, Burdette JE, A KM. The tumor microenvironment of high grade serous ovarian cancer. Cancers (Basel). 2018;11:21. [DOI] [PMC free article] [PubMed]
- 31.Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173:1755–69. doi: 10.1016/j.cell.2018.03.073. [DOI] [PubMed] [Google Scholar]
- 32.Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–60. [PubMed] [Google Scholar]
- 33.Westergaard MCW, Milne K, Pedersen M, Hasselager T, Olsen LR, Anglesio MS, et al. Changes in the tumor immune microenvironment during disease progression in patients with ovarian cancer. Cancers (Basel) 2020;12:3828. doi: 10.3390/cancers12123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res. 2017;23:925–34. doi: 10.1158/1078-0432.CCR-16-1433. [DOI] [PubMed] [Google Scholar]
- 35.Ovarian Tumor Tissue Analysis C. Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3:e173290. doi: 10.1001/jamaoncol.2017.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 37.Santoiemma PP, Powell DJ., Jr Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16:807–20. doi: 10.1080/15384047.2015.1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wouters MC, Komdeur FL, Workel HH, Klip HG, Plat A, Kooi NM, et al. Treatment regimen, surgical outcome, and T-cell differentiation influence prognostic benefit of tumor-infiltrating lymphocytes in high-grade serous ovarian cancer. Clin Cancer Res. 2016;22:714–24. doi: 10.1158/1078-0432.CCR-15-1617. [DOI] [PubMed] [Google Scholar]
- 39.Amobi A, Qian F, Lugade AA, Odunsi K. Tryptophan catabolism and cancer immunotherapy targeting IDO mediated immune suppression. Adv Exp Med Biol. 2017;1036:129–44. doi: 10.1007/978-3-319-67577-0_9. [DOI] [PubMed] [Google Scholar]
- 40.Jung KH, LoRusso P, Burris H, Gordon M, Bang YJ, Hellmann MD, et al. Phase I study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in advanced solid tumors. Clin Cancer Res. 2019;25:3220–8. doi: 10.1158/1078-0432.CCR-18-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K, Zhao W, Lou G, Rong Z, Xu H, Wang W, et al. An immunophenotyping of ovarian cancer with clinical and immunological significance. Front Immunol. 2018;9:757. doi: 10.3389/fimmu.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pr. 2016;3:11. doi: 10.1186/s40661-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristeleit R, Davidenko I, Shirinkin V, El-Khouly F, Bondarenko I, Goodheart MJ, et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)-only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol. 2017;146:484–90. doi: 10.1016/j.ygyno.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Hornburg M, Desbois M, Lu S, Guan Y, Lo AA, Kaufman S, et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell. 2021;39:928–44. doi: 10.1016/j.ccell.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Au KK, Le Page C, Ren R, Meunier L, Clement I, Tyrishkin K, et al. STAT1-associated intratumoural TH1 immunity predicts chemotherapy resistance in high-grade serous ovarian cancer. J Pathol Clin Res. 2016;2:259–70. doi: 10.1002/cjp2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nersesian S, Schwartz SL, Grantham SR, MacLean LK, Lee SN, Pugh-Toole M, et al. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl Oncol. 2021;14:100930. doi: 10.1016/j.tranon.2020.100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henriksen JR, Donskov F, Waldstrom M, Jakobsen A, Hjortkjaer M, Petersen CB, et al. Favorable prognostic impact of Natural Killer cells and T cells in high-grade serous ovarian carcinoma. Acta Oncol. 2020;59:652–9. doi: 10.1080/0284186X.2019.1711173. [DOI] [PubMed] [Google Scholar]
- 48.Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer. 2018;6:139. doi: 10.1186/s40425-018-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R, Hu R, Zeng Y, Zhang W, Zhou HH. Tumour immune cell infiltration and survival after platinum-based chemotherapy in high-grade serous ovarian cancer subtypes: A gene expression-based computational study. EBioMedicine. 2020;51:102602. doi: 10.1016/j.ebiom.2019.102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y, Chen L, Cai G, Xiong X, Wu Y, Ma D, et al. Heterogeneity of immune microenvironment in ovarian cancer and its clinical significance: A retrospective study. Oncoimmunology. 2020;9:1760067. doi: 10.1080/2162402X.2020.1760067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etzerodt A, Moulin M, Doktor TK, Delfini M, Mossadegh-Keller N, Bajenoff M, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med. 2020;217. [DOI] [PMC free article] [PubMed]
- 53.Mlynska A, Povilaityte E, Zemleckaite I, Zilionyte K, Strioga M, Krasko J, et al. Platinum sensitivity of ovarian cancer cells does not influence their ability to induce M2-type macrophage polarization. Am J Reprod Immunol. 2018;80:e12996. doi: 10.1111/aji.12996. [DOI] [PubMed] [Google Scholar]
- 54.Baert T, Vankerckhoven A, Riva M, Van Hoylandt A, Thirion G, Holger G, et al. Myeloid derived suppressor cells: Key drivers of immunosuppression in ovarian cancer. Front Immunol. 2019;10:1273. doi: 10.3389/fimmu.2019.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pepin G, Gantier MP. cGAS-STING activation in the tumor microenvironment and its role in cancer immunity. Adv Exp Med Biol. 2017;1024:175–94. doi: 10.1007/978-981-10-5987-2_8. [DOI] [PubMed] [Google Scholar]
- 56.Pu F, Chen F, Liu J, Zhang Z, Shao Z. Immune regulation of the cGAS-STING signaling pathway in the tumor microenvironment and its clinical application. Onco Targets Ther. 2021;14:1501–16. doi: 10.2147/OTT.S298958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Queiroz N, Xia T, Konno H, Barber GN. Ovarian cancer cells commonly exhibit defective STING signaling which affects sensitivity to viral oncolysis. Mol Cancer Res. 2019;17:974–86. doi: 10.1158/1541-7786.MCR-18-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015;36:250–6. doi: 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Lu L, Lu J, Wang X, Yang C, Jin J, et al. cGAS-STING-mediated DNA sensing maintains CD8(+) T cell stemness and promotes antitumor T cell therapy. Sci Transl Med. 2020;12;eaay9013. [DOI] [PubMed]
- 61.Downey CM, Aghaei M, Schwendener RA, Jirik FR. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2'3’-cGAMP, induces M2 macrophage repolarization. PLoS One. 2014;9:e99988. doi: 10.1371/journal.pone.0099988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao L, Qi J, Zhao Q, Wu QN, Wei DL, Wei XL, et al. Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics. 2020;10:498–515. doi: 10.7150/thno.37745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen J, Zhao W, Ju Z, Wang L, Peng Y, Labrie M, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res. 2019;79:311–9. doi: 10.1158/0008-5472.CAN-18-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee EK, Konstantinopoulos PA. Combined PARP and immune checkpoint inhibition in ovarian Cancer. Trends Cancer. 2019;5:524–8. doi: 10.1016/j.trecan.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–41. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 67.Hong C, Tijhuis AE, Foijer F. The cGAS Paradox: Contrasting roles for cGAS-STING pathway in chromosomal instability. Cells. 2019;8:1228. doi: 10.3390/cells8101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jimenez-Sanchez A, Cybulska P, Mager KL, Koplev S, Cast O, Couturier DL, et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat Genet. 2020;52:582–93. doi: 10.1038/s41588-020-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konno H, Yamauchi S, Berglund A, Putney RM, Mule JJ, Barber GN. Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene. 2018;37:2037–51. doi: 10.1038/s41388-017-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14:282–97. doi: 10.1016/j.celrep.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Chen Y, Chen X, Zhang W, Zhao L, Weng L, et al. Deubiquitinase USP35 restrains STING-mediated interferon signaling in ovarian cancer. Cell Death Differ. 2021;28:139–55. doi: 10.1038/s41418-020-0588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLaughlin LJ, Stojanovic L, Kogan AA, Rutherford JL, Choi EY, Yen RC, et al. Pharmacologic induction of innate immune signaling directly drives homologous recombination deficiency. Proc Natl Acad Sci USA. 2020;117:17785–95. doi: 10.1073/pnas.2003499117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi G, Brendel VP, Shu C, Li P, Palanathan S, Cheng Kao C. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 2013;8:e77846. doi: 10.1371/journal.pone.0077846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel S, Blaauboer SM, Tucker HR, Mansouri S, Ruiz-Moreno JS, Hamann L, et al. The Common R71H-G230A-R293Q Human TMEM173 Is a Null Allele. J Immunol. 2017;198:776–87. doi: 10.4049/jimmunol.1601585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nersesian S, Glazebrook H, Toulany J, Grantham SR, Boudreau JE. Naturally killing the silent killer: NK cell-based immunotherapy for ovarian cancer. Front Immunol. 2019;10:1782. doi: 10.3389/fimmu.2019.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Bert N, Lam AR, Ho SS, Shen YJ, Liu MM, Gasser S. STING-dependent cytosolic DNA sensor pathways regulate NKG2D ligand expression. Oncoimmunology. 2014;3:e29259. doi: 10.4161/onci.29259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014;24:766–9. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198:1649–59. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- 79.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42:332–43. doi: 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 82.McCormick A, Donoghue P, Dixon M, O’Sullivan R, O’Donnell RL, Murray J, et al. Ovarian cancers harbor defects in nonhomologous end joining resulting in resistance to rucaparib. Clin Cancer Res. 2017;23:2050–60. doi: 10.1158/1078-0432.CCR-16-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsibulak I, Wieser V, Degasper C, Shivalingaiah G, Wenzel S, Sprung S, et al. BRCA1 and BRCA2 mRNA-expression prove to be of clinical impact in ovarian cancer. Br J Cancer. 2018;119:683–92. doi: 10.1038/s41416-018-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang YW. Association of BRCA1/2 mutations with ovarian cancer prognosis: An updated meta-analysis. Med (Baltim) 2018;97:e9380. doi: 10.1097/MD.0000000000009380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu M, Zhou M, Bao X, Pan D, Jiao M, Liu X, et al. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J Clin Invest. 2021;131:e139333. doi: 10.1172/JCI139333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mowat C, Mosley SR, Namdar A, Schiller D, Baker K. Anti-tumor immunity in mismatch repair-deficient colorectal cancers requires type I IFN-driven CCL5 and CXCL10. J Exp Med. 2021;218: e139333. [DOI] [PMC free article] [PubMed]
- 87.Reislander T, Groelly FJ, Tarsounas M. DNA Damage and Cancer Immunotherapy: A STING in the Tale. Mol Cell. 2020;80:21–8. doi: 10.1016/j.molcel.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 88.Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109:djw199. doi: 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–9. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 90.Petroni G, Buque A, Zitvogel L, Kroemer G, Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2021;39:310–45. doi: 10.1016/j.ccell.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Bruand M, Barras D, Mina M, Ghisoni E, Morotti M, Lanitis E, et al. Cell-autonomous inflammation of BRCA1-deficient ovarian cancers drives both tumor-intrinsic immunoreactivity and immune resistance via STING. Cell Rep. 2021;36:109412. doi: 10.1016/j.celrep.2021.109412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 93.Liu MC, Sutedja J, Tewari KS. Niraparib in the maintenance treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: Safety and efficacy. Expert Rev Anticancer Ther. 2021;21:475–80. doi: 10.1080/14737140.2021.1880326. [DOI] [PubMed] [Google Scholar]
- 94.Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 2018;25:2972–80. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38:2380–93. doi: 10.1038/s41388-018-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lampert EJ, Cimino-Mathews A, Lee JS, Nair J, Lee MJ, Yuno A, et al. Clinical outcomes of prexasertib monotherapy in recurrent BRCA wild-type high-grade serous ovarian cancer involve innate and adaptive immune responses. J Immunother Cancer. 2020;8:e000516. [DOI] [PMC free article] [PubMed]
- 97.Shakfa N, Siemens DR, Koti M. Revisiting immunogenic cell death to improve treatment response in cancer. Biological Mechanisms and the Advancing Approaches to Overcoming Cancer Drug Resistance 2021. p. 65–90.
- 98.Wilkinson RDA, McCabe, N Parkes, EE, Barros, EM, Johnston, DI, Ali, RMM, et al. Topoisomerase II inhibitors induce cGAS-STING dependent inflammation resulting in cytokine induction and immune checkpoint activation. bioRxiv. 2019. 10.1101/764662.